Abstract
Surface proteins of mouse thymus and spleen cells were radioiodinated with lactoperoxidase. After solubilization, the labeled proteins were precipitated by antibodies directed against mouse immunoglobulin chains; the precipitates were analyzed by radioautography after Na dodecyl sulfate-gel electrophoresis. Radioactive μ and L chains were absent from thymocyte extracts and conspicuous in spleen-cell extracts. The following cells were biosynthetically labeled for 4 hr [35S]methionine or 24 hr with [14C]leucine: (1) Thymocytes, (2) cortisoneresistant thymocytes [both treated with rabbit antisera cytotoxic to bone marrow-derived (B) lymphocytes and IgM-containing plasma cells, to kill possible contaminating nonthymus-derived cells], (3) “activated thymocytes” (allogeneic cell cultures of cortisone-resistant thymocytes), (4) human Daudi cells (a B lymphoblastic cell line), and (5) purified mouse B spleen lymphocytes devoid of plasma cells. Again no μ and L chains could be detected in thymocyte or thymus-derived cell extracts by immune precipitation and gel electrophoresis, while these chains were conspicuous in B-cell extracts. “Educated thymocytes,” obtained from spleens of lethally irradiated mice injected with syngeneic thymocytes and antigen, synthesized μ and L chains under similar conditions; this synthesis resulted from contamination of these cells by IgM-containing plasma cells.
Keywords: B lymphocyte, lymphocyte receptor
Full text
PDF
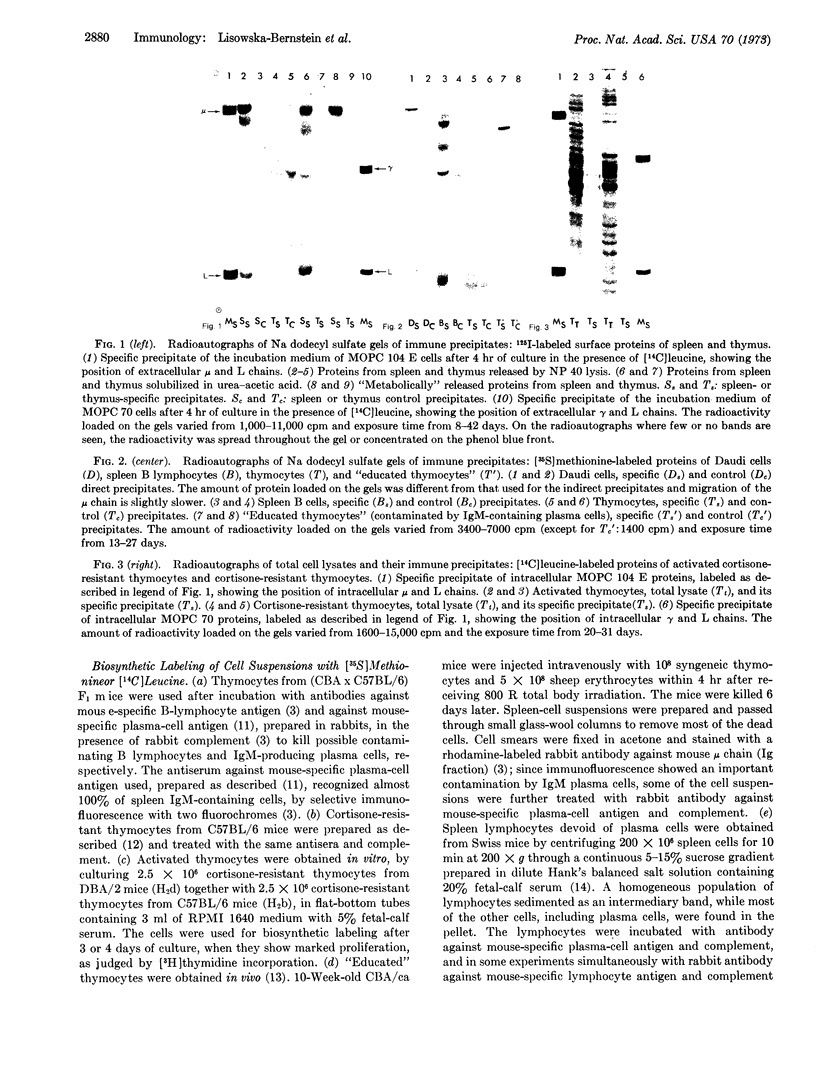



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALLEGRETTI N., VITALE B., DEKARIS D. Plasma-cell proliferation in irradiated rats. Int J Radiat Biol Relat Stud Phys Chem Med. 1962 Feb;4:363–370. doi: 10.1080/09553006214550171. [DOI] [PubMed] [Google Scholar]
- Benacerraf B., McDevitt H. O. Histocompatibility-linked immune response genes. Science. 1972 Jan 21;175(4019):273–279. doi: 10.1126/science.175.4019.273. [DOI] [PubMed] [Google Scholar]
- Blomgren H., Andersson B. Evidence for a small pool of immunocompetent cells in the mouse thymus. Exp Cell Res. 1969 Oct;57(2):185–192. doi: 10.1016/0014-4827(69)90140-2. [DOI] [PubMed] [Google Scholar]
- Cone R. E., Sprent J., Marchalonis J. J. Antigen-binding specificity of isolated cell-surface immunoglobulin from thymus cells activated to histocompatibility antigens. Proc Natl Acad Sci U S A. 1972 Sep;69(9):2556–2560. doi: 10.1073/pnas.69.9.2556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crone M., Koch C., Simonsen M. The elusive T cell receptor. Transplant Rev. 1972;10:36–56. doi: 10.1111/j.1600-065x.1972.tb01538.x. [DOI] [PubMed] [Google Scholar]
- Feldmann M., Basten A. Cell interactions in the immune response in vitro. 3. Specific collaboration across a cell impermeable membrane. J Exp Med. 1972 Jul 1;136(1):49–67. doi: 10.1084/jem.136.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grey H. M., Kubo R. T., Cerottini J. C. Thymus-derived (T) cell immunoglobulins. Presence of a receptor site for IgG and absence of large amounts of "buried" Ig determinants on T cells. J Exp Med. 1972 Nov 1;136(5):1323–1328. doi: 10.1084/jem.136.5.1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson L. I. Bone marrow lymphocytes: their isolation, colony-forming capacity and graft-versus-host potential. I. The isolation and characterization of subpopulations of bone marrow lymphocytes. Proc Soc Exp Biol Med. 1971 Apr;136(4):1277–1283. doi: 10.3181/00379727-136-35475. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lamelin J. P., Lisowska-Bernstein B., Matter A., Ryser J. E., Vassalli P. Mouse thymus-independent and thymus-derived lymphoid cells. I. Immunofluorescent and functional studies. J Exp Med. 1972 Nov 1;136(5):984–1007. doi: 10.1084/jem.136.5.984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchalonis J. J., Cone R. E., Atwell J. L. Isolation and partial characterization of lymphocyte surface immunoglobulins. J Exp Med. 1972 Apr 1;135(4):956–971. doi: 10.1084/jem.135.4.956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchalonis J. J., Cone R. E., Santer V. Enzymic iodination. A probe for accessible surface proteins of normal and neoplastic lymphocytes. Biochem J. 1971 Oct;124(5):921–927. doi: 10.1042/bj1240921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melchers F. Difference in carbohydrate composition and a possible conformational difference between intracellular and extracellular immunoglobulin M. Biochemistry. 1972 May 23;11(11):2204–2208. doi: 10.1021/bi00761a031. [DOI] [PubMed] [Google Scholar]
- NOSSAL G. J. Plasma cell proliferation following whole body irradiation. Aust J Exp Biol Med Sci. 1959 Oct;37:499–504. doi: 10.1038/icb.1959.51. [DOI] [PubMed] [Google Scholar]
- Nossal G. J., Warner N. L., Lewis H., Sprent J. Quantitative features of a sandwich radioimmunolabeling technique for lymphocyte surface receptors. J Exp Med. 1972 Feb 1;135(2):405–428. doi: 10.1084/jem.135.2.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert D. Immunoglobulin biosynthesis. IV. Carbohydrate attachment to immunoglobulin subunits. J Mol Biol. 1970 Jul 28;51(2):287–301. doi: 10.1016/0022-2836(70)90143-9. [DOI] [PubMed] [Google Scholar]
- Sherr C. J., Uhr J. W. Immunoglobulin synthesis and secretion. VI. Synthesis and intracellular transport of immunoglobulin in nonsecretory lymphoma cells. J Exp Med. 1971 Apr 1;133(4):901–920. doi: 10.1084/jem.133.4.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi T., Old L. J., Hsu C. J., Boyse E. A. A new differentiation antigen of plasma cells. Eur J Immunol. 1971 Dec;1(6):478–482. doi: 10.1002/eji.1830010614. [DOI] [PubMed] [Google Scholar]
- Unanue E. R., Grey H. M., Rabellino E., Campbell P., Schmidtke J. Immunoglobulins on the surface of lymphocytes. II. The bone marrow as the main source of lymphocytes with detectable surface-bound immunoglobulin. J Exp Med. 1971 Jun 1;133(6):1188–1198. doi: 10.1084/jem.133.6.1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitetta E. S., Bianco C., Nussenzweig V., Uhr J. W. Cell surface immunoglobulin. IV. Distribution among thymocytes, bone mrrow cells, and their derived populations. J Exp Med. 1972 Jul 1;136(1):81–93. doi: 10.1084/jem.136.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitetta E. S., Uhr J. W., Boyse E. A. Immunoglobulin synthesis and secretion by cells in the mouse thymus that do not bear theta antigen. Proc Natl Acad Sci U S A. 1973 Mar;70(3):834–838. doi: 10.1073/pnas.70.3.834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida T. O., Andersson B. Evidence for a receptor recognizing antigen complexed immunoglobulin on the surface of activated mouse thymus lymphocytes. Scand J Immunol. 1972;1(4):401–408. doi: 10.1111/j.1365-3083.1972.tb03306.x. [DOI] [PubMed] [Google Scholar]



