Abstract
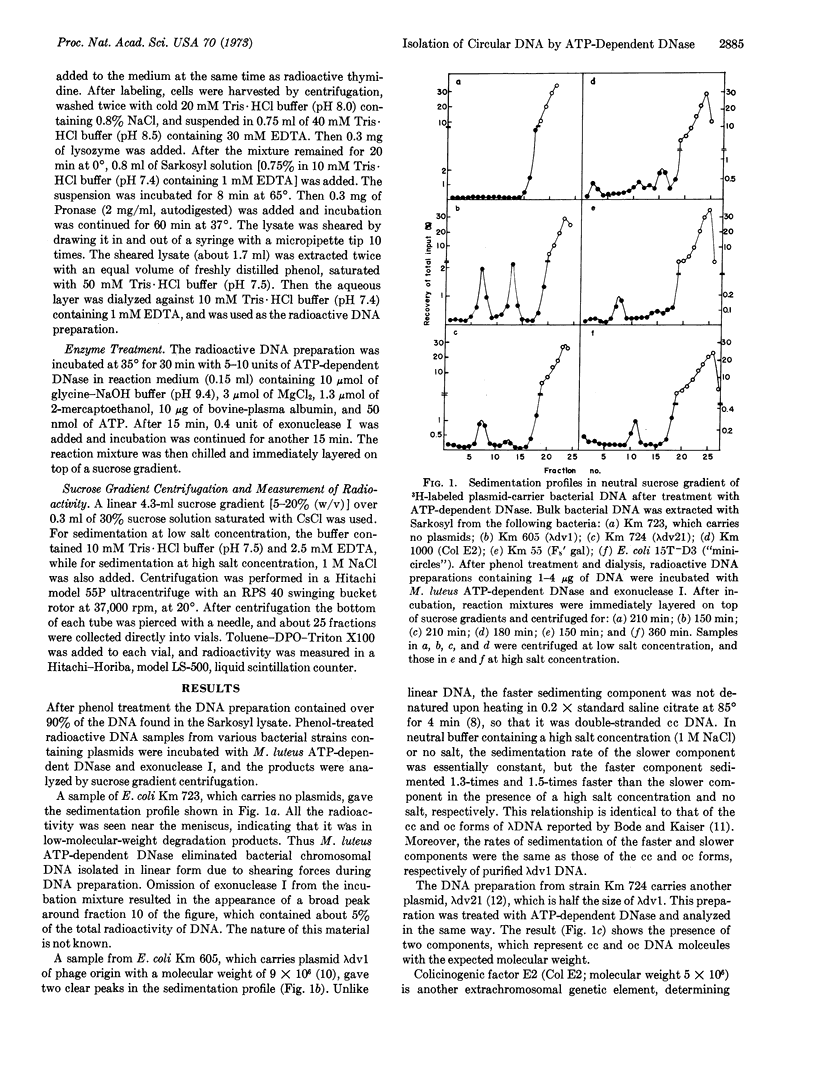
A technique is described for isolation of plasmid DNA in closed and open circular double-stranded forms from bacterial cells, by use of ATP-dependent deoxyribonuclease purified from Micrococcus luteus. This DNase, acting only upon linear DNA molecules, degrades all bacterial chromosomal DNA extracted in the linear form. Circular plasmid DNAs are left intact, and are then separated by sedimentation through a sucrose gradient. Unlike previous techniques for analysis of plasmid DNA, this technique can be used to isolate not only closed circular DNA but also open circular DNA. Several plasmids, such as those from phage (λdv1 and λdv21), a colicinogenic factor (Col E2), a sex factor (F8′ gal), and “minicircles” in Escherichia coli 15, in both the open and closed circular forms, were well separated from chromosomal DNA by this technique.
Keywords: plasmid, open and closed circular DNA, sedimentation
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anai M., Hirahashi T., Takagi Y. A deoxyribonuclease which requires nucleoside triphosphate from Micrococcus lysodeikticus. I. Purification and characterization of the deoxyribonuclease activity. J Biol Chem. 1970 Feb 25;245(4):767–774. [PubMed] [Google Scholar]
- Bazaral M., Helinski D. R. Circular DNA forms of colicinogenic factors E1, E2 and E3 from Escherichia coli. J Mol Biol. 1968 Sep 14;36(2):185–194. doi: 10.1016/0022-2836(68)90374-4. [DOI] [PubMed] [Google Scholar]
- Blair D. G., Clewell D. B., Sheratt D. J., Helinski D. R. Strand-specific supercoiled DNA-protein relaxation complexes: comparison of the complexes of bacterial plasmids ColE1 and ColE2. Proc Natl Acad Sci U S A. 1971 Jan;68(1):210–214. doi: 10.1073/pnas.68.1.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bode V. C., Kaiser A. D. Changes in the structure and activity of lambda DNA in a superinfected immune bacterium. J Mol Biol. 1965 Dec;14(2):399–417. doi: 10.1016/s0022-2836(65)80190-5. [DOI] [PubMed] [Google Scholar]
- CAIRNS J. The bacterial chromosome and its manner of replication as seen by autoradiography. J Mol Biol. 1963 Mar;6:208–213. doi: 10.1016/s0022-2836(63)80070-4. [DOI] [PubMed] [Google Scholar]
- Clowes R. C. Molecular structure of bacterial plasmids. Bacteriol Rev. 1972 Sep;36(3):361–405. doi: 10.1128/br.36.3.361-405.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cozzarelli N. R., Kelly R. B., Kornberg A. A minute circular DNA from Escherichia coli 15. Proc Natl Acad Sci U S A. 1968 Jul;60(3):992–999. doi: 10.1073/pnas.60.3.992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freifelder D., Folkmanis A., Kirschner I. Studies on Escherichia coli sex factors: evidence that covalent circles exist within cells and the general problem of isolation of covalent circles. J Bacteriol. 1971 Mar;105(3):722–727. doi: 10.1128/jb.105.3.722-727.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman E. A., Smith H. O. An adenosine triphosphate-dependent deoxyribonuclease from Hemophilus influenzae Rd. 3. Substrate specificity. J Biol Chem. 1972 May 10;247(9):2859–2865. [PubMed] [Google Scholar]
- Friedman E. A., Smith H. O. Production of possible recombination intermediates by an ATP-dependent DNAase. Nat New Biol. 1973 Jan 10;241(106):54–58. doi: 10.1038/newbio241054a0. [DOI] [PubMed] [Google Scholar]
- Goldmark P. J., Linn S. Purification and properties of the recBC DNase of Escherichia coli K-12. J Biol Chem. 1972 Mar 25;247(6):1849–1860. [PubMed] [Google Scholar]
- Helinski D. R., Clewell D. B. Circular DNA. Annu Rev Biochem. 1971;40:899–942. doi: 10.1146/annurev.bi.40.070171.004343. [DOI] [PubMed] [Google Scholar]
- Matsubara K., Kaiser A. D. Lambda dv: an autonomously replicating DNA fragment. Cold Spring Harb Symp Quant Biol. 1968;33:769–775. doi: 10.1101/sqb.1968.033.01.088. [DOI] [PubMed] [Google Scholar]
- Mukai T., Matsubara K., Takagi Y. A sensitive method for determination of deoxyribonuclease (endonuclease) activity using (lambda-dv) DNA and a nitrocellulose membrane filter. J Biochem. 1973 May;73(5):1107–1109. doi: 10.1093/oxfordjournals.jbchem.a130165. [DOI] [PubMed] [Google Scholar]
- Oishi M., Cosloy S. D. The genetic and biochemical basis of the transformability of Escherichia coli K12. Biochem Biophys Res Commun. 1972 Dec 18;49(6):1568–1572. doi: 10.1016/0006-291x(72)90520-7. [DOI] [PubMed] [Google Scholar]
- Radloff R., Bauer W., Vinograd J. A dye-buoyant-density method for the detection and isolation of closed circular duplex DNA: the closed circular DNA in HeLa cells. Proc Natl Acad Sci U S A. 1967 May;57(5):1514–1521. doi: 10.1073/pnas.57.5.1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takagi Y., Matsubara K., Anai M. A deoxyribonuclease which requires nucleoside triphosphate from Micrococcus lysodeikticus. IV. The mode of DNA hydrolysis. Biochim Biophys Acta. 1972 May 29;269(3):347–353. doi: 10.1016/0005-2787(72)90121-9. [DOI] [PubMed] [Google Scholar]
- Vapnek D., Rupp W. D. Identification of individual sex-factor DNA strands and their replication during conjugation in thermosensitive DNA mutants of Escherichia coli. J Mol Biol. 1971 Sep 28;60(3):413–424. doi: 10.1016/0022-2836(71)90178-1. [DOI] [PubMed] [Google Scholar]
- Vinograd J., Lebowitz J. Physical and topological properties of circular DNA. J Gen Physiol. 1966 Jul;49(6):103–125. doi: 10.1085/jgp.49.6.103. [DOI] [PMC free article] [PubMed] [Google Scholar]


