Abstract
Introduction
Nicotine delivery from smokeless tobacco (ST) products leads to addiction and the use of ST causes pathology that is associated with increased initiation of cigarette smoking. The rapid delivery of nicotine from ST seems to be associated with the pH of the aqueous suspension of the products - high pH is associated with high nicotine absorption. However, early studies compared nicotine absorption from different commercial products that not only differed in pH but in flavoring, nicotine content, and in format-pouches and loose tobacco.
Methods
The present study compared nicotine absorption from a single unflavored referent ST product (pH 7.7) that was flavored with a low level of wintergreen (2 mg/g) and the pH was amended to either high (8.3) or low (5.4) pH with sodium carbonate or citric acid, respectively.
Results
In a within-subject clinical study, the higher pH products delivered more nicotine. No significant differences were seen between perceived product strengths and product experience in all conditions. Heart rate increased by 4 to 6 beats per minute after the high pH flavored and the un-amended product but did not change after the low pH flavored product.
Conclusions
These results indicate that pH is a primary determinant of buccal nicotine absorption. The role of flavoring and other components of ST products in nicotine absorption remain to be determined.
Keywords: Smokeless tobacco, Nicotine, Tobacco, Moist snuff, CRP2
Introduction
There are many forms of oral tobacco products worldwide. Some formulations are chewed (e.g. betel quid, plug, loose leaf or twist varieties of chewing tobacco) whereas other products are placed in between the cheek and the gum (e.g. chimo, dry snuff, maras, or moist snuff) [1]. In the United States (U.S.), products such as chewing tobacco or moist snuff are more common. There are currently over 9 million smokeless tobacco (ST) users in the United States [2]. As many as 6.4% of high school students [3] and 3% of adults [4] use these products. Surveys data suggest that smokeless tobacco use is predominantly a public health problem among men, young adults, and people with lower education, and in certain states (e.g. smokeless tobacco prevalence in 2009 in Wyoming among men was as high as 16.9%) [4]. This is a disturbingly high prevalence because ST use causes significant morbidity, such as oral cancers, precancerous lesions, other dental pathology [2] and heart diseases [5]. Furthermore, the use of ST strongly predicts subsequent cigarette smoking with all of its attendant risks of addiction and pathology [6]. Future ST use may increase because of the continuous introduction of new ST products and marketing strategies that promote ST use as a temporary substitute for cigarettes where smoking is prohibited and as a way to reduce cigarette smoking. The rate and amount of nicotine absorption into the systemic circulation from tobacco and pharmaceutical nicotine products importantly influences their abuse liability and risk for addiction [7]. Products that deliver nicotine rapidly such as cigarettes [8] and other inhaled combustible tobacco products including little cigars [9] cigarillos [10] and e-cigarettes [11] pose a greater risk for addiction than slow release products such as the nicotine patch [12].
As with smoked tobacco products, ST products cause addiction to nicotine that is characterized by intense craving, compelling urges to continue use despite recognized harm, inability to quit, and a withdrawal syndrome on abrupt discontinuation [13]. Nicotine absorption from ST is importantly influenced by the pH at the buccal-product interface. In an alkaline (high) pH, environment nicotine is unionized and rapidly absorbed whereas in an acidic (low) pH, nicotine is ionized and does not cross biological membranes. Absorption of nicotine across the buccal membrane appears to be related to the amount of nicotine present in the unionized “free base” form. Aqueous solution of smokeless tobacco products bracket a wide range of pH from 5.0 to 8.4 with associated concentrations of unionized nicotine between <1 to 70% [14,15].
In addition to pH other factors could modulate nicotine absorption, such as: local blood flow, “wettability” of the product, size and surface area of the tobacco mixture, buffering capacity to hold the pH constant, and the nicotine content of the tobacco. Several lines of evidence suggest that mint flavorings, such as menthol and wintergreen, may increase nicotine absorption [15]. However, this hypothesis has not been systematically tested.
In a previous study, the nicotine absorption was examined of several commercial products [7] with similar but not identical concentrations of nicotine and with differing pH. Products with a higher pH delivered more nicotine than those with a lower pH. A limitation of that study was that the various products may have differed tobacco blends, buffering capacity, levels of flavoring, and matrix composition. The present preliminary study extends that research by measuring nicotine absorption after experimentally manipulating pH and flavorings of a single referent product.
Methods
Participants
The participants were recruited from the metropolitan area of Baltimore, MD, via newspaper advertisements, posters, and word-ofmouth. Participants were smokeless tobacco (ST) users for at least 1 year and have not sought treatment for tobacco dependence. Inclusion criteria were: 1) sufficient understanding of consent form and study procedures, 2) age 18 years or higher, 3) regular ST use, defined as using ST products daily and at least 1.5 tins per week for at least 1 year, and 4) ability to attend 5 separate laboratory sessions. Exclusion criteria were: 1) evident intoxication on any visit, 2) pregnancy, 3) current ST cessation or reduction efforts, and 4) significant ST or smoking-related disease by history. All exclusion criteria were self-reported by the participants. Participants were paid $35 per hour with a $100 bonus for completion of all visits. Data collection occurred between March and August 2011 at Battelle's Human Exposure Assessment Laboratory (HEAL) in Baltimore, MD.
Study products
Referent unflavored moist snuff, CRP2, was obtained from North Carolina State University (NCSU) tobacco support program. The unaltered product had a water content of 54.8% and contained a blend of dark fire-cured (25.9%), air-cured (7.9%) and burley (3.7%) tobaccos. The nicotine content was 1.2% (12 mg/gm). Aqueous suspensions of the product have a pH of 7.7. The CRP2 product was amended at Portland State University (PSU) by addition of wintergreen (methyl salicylate) flavoring and variants of pH. Methyl salicylate was added to CRP2 to flavor the product to a concentration of 2 mg/gm of moist snuff (w/w). The pH of CRP2 was adjusted to 5.4 (low pH) or 8.3 (high pH) with the addition of citric acid or sodium carbonate, respectively. According to the Henderson-Hasselbach equation, the % of unionized nicotine in the products was: low pH, <0.5%, high pH 66%; un-amended 32%. The pH of the products was reevaluated after the addition of flavorings. The products were packaged individually in coded and sealed vials. A group of three coded containers were bundled for each participant containing: un-amended CRP2 (unflavored; pH=7.7); wintergreen flavored with low pH; and wintergreen flavored with high pH. The containers were stored and shipped refrigerated until use.
Study design and procedures
The current research employed a double-blind, within-subjects study design. Each participant visited Battelle's HEAL for three experimental sessions separated by at least 24 hours, during which 2 grams of ST product was used by mouth in one of the following randomized conditions:
Condition 1: ST with altered low pH of 5.4 with wintergreen flavoring;
Condition 2: ST with altered high pH of 8.3 with wintergreen flavoring;
Condition 3: ST with unaltered pH of 7.7 with no wintergreen flavoring.
Participants were required to refrain from tobacco use at least 2 hours prior to the lab visits. The sessions were about 75 minutes long: 15 minute baseline period, 30 minutes with ST product in the mouth, and 30 minute period without ST product in the mouth. The presentations of the conditions were randomized.
At the first visit, participants were introduced to the study and an IRB-approved informed consent document was signed. At the first visit, each participant completed a smoking history questionnaire, a nicotine dependency test, and was familiarized with the visual analog questionnaires and other study procedures. Blood specimens to determine plasma nicotine levels were collected from a forearm vein 10 minutes before and at 5, 10, 15, 20, 30, 35, 45, and 60 minutes after the product was placed in the mouth. The product (2 gm) was retained in the mouth for 30 minutes.
Dependent measures
Plasma nicotine
Blood samples were centrifuged and the plasma was transferred into tubes and frozen at −20°C until they were shipped on dry ice overnight to LabStat international (Kitchener, Ontario, Canada) where gas chromatography/thermal specific ionic detection was used to determine nicotine levels (LOQ=1.0 ng/mL). Nicotine exposure was assessed with three outcomes: nicotine boost, total nicotine absorption, and speed of nicotine absorption. Nicotine boost was calculated as the difference between the baseline (BL) nicotine level and the highest nicotine level obtained. Total nicotine absorption was determined by the area under the nicotine plasma level by time curve (AUC) using the trapezoidal rule for unequal intervals [16]. Speed of nicotine absorption was determined from estimates of the slope of nicotine plasma levels between BL and the 30-minute time point (total of 4 points: BL, 5, 15, and 30 minutes). The best fit of the line and slope was estimated according to methods described by Tallarida and Murray [16].
Cardiovascular measures
Heart rate (HR) and blood pressure (BP) were measured with a DRE EZ Waveline Monitor (DRE Inc., Louisville, KY) before and at 10 minute intervals during the experimental sessions. Using the participant's systolic blood pressures (SBP) and diastolic blood pressures (DBP), the mean arterial pressure (MAP) was calculated at each interval using the following equation: MAP=[(2 x DBP) + SBP)]/3. MAP data values were then plotted on a curve of MAP over time and an AUC was calculated for data analyses.
Subjective measures
Nicotine dependence
ST nicotine dependence was assessed using the Fagerstrom Test for Nicotine Dependence – Smokeless Tobacco (FTND-ST) [17], which is a modified version of FTND questionnaire designed for combustible cigarettes by Heatherton et al. [18]. The FTNDST is comprised of 7 questions including: “How many tins/ pouches of smokeless tobacco do you typically use each week?”; “How often do you use smokeless tobacco?”; “Do you intentionally swallow tobacco juices?”; “Do you use smokeless tobacco when you are sick or have mouth sores?”; “How soon after awakening from your normal sleeping period do you use chewing tobacco or snuff?”; “Do you smoke cigarettes?”; and “Is it difficult for you not to use smokeless tobacco where its use is restricted or not allowed?”. The FTND-ST score can range from 0-9 [19].
Tobacco use history
Subjects answered a questionnaire regarding their tobacco history, including questions about ST use, cigarette use and alternative cigarette product use. Examples include, number of ST chews/cigarettes per day, their age of initiation, and number of years as a user for all products used
Subjective strength of the product
Product strength was measured using a single question as we used in previous research [7]. The question was answered on a 140 mm visual analog scale (VAS), anchored with the labels “Not at all” to “Extremely”. The subjects also answered VAS questions on “Strength”, “Liking”, ”Head Rush” , and “Alert”. Scale values were converted to a percentage where 0% is “Not at all” and 100% is “Extremely”. Subjective strength of the product was measured at the same time as blood draw with the exception of the baseline blood draw. The data was plotted on a curve of perceived product strength over time and an AUC was calculated for data analyses.
Product experience
A 7-tem questionnaire was used to evaluate the subjective experience of the product [7] and consisted of 140 mm VASbased questions, also anchored with the labels “not at all” to “extremely” and was converted to percentages. Questions were designed to assess the participant's perception of overall strength, amount swallowed, how well the product packed, increased salivation, burning sensations in the mouth, mouth tingling, and nausea [7,20]. The data were plotted on a curve of product ‘liking’ over time and an AUC was calculated to further analyze the data.
Statistical analyses
All statistical analyses were conducted with Excel (Microsoft Office 2007). Due to the small sample size descriptive statistics only were reported for most variables.
Results
Participants
Participant characteristics are presented in Table 1. The study was completed by 7 participants (all men, 4 Whites and 3 African Americans) who met eligibility criteria and attended all five visits. The average age was 45 ± 11 years (range: 28-62). Participants had used ST for an average of 15 ± 17 years (range: 1-42), and the number of ST tins used per day was 2 ± 3 (range: 0.3-10). About half of the participants (43%) smoked conventional cigarettes in addition to using ST products.
Table 1.
Participants’ characteristics
| N = 7 | |
|---|---|
| Sex Male | 100% |
| Race African American | 43% |
| Caucasian | 57% |
| Other | -- |
| Age Mean (SD) | 45 (12) |
| Concurrent cigarette smoker and ST user | 43% |
| ST tins or pouchesper day Mean (SD) | 2 (3) |
| Years of ST use Mean (SD) | 15 (17) |
| FTND-ST Score Mean (SD) | 4.3 (1.6) |
Mean (SD): Arithmetic mean with standard deviation
Dependent measures
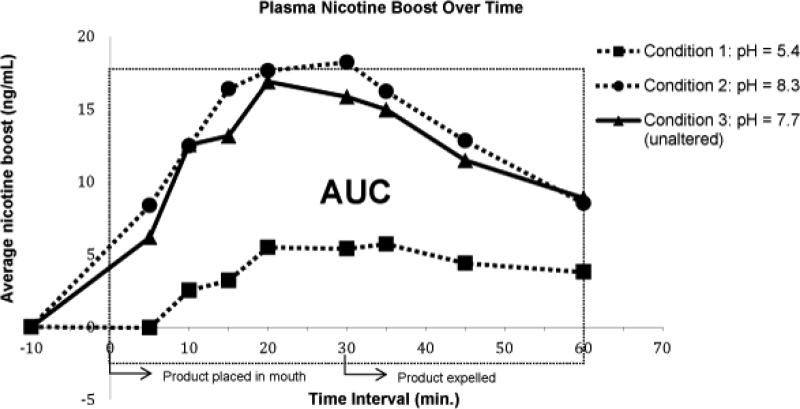
As shown in Figure 1, plasma levels of nicotine immediately and markedly increased in two referent conditions: the altered high pH with wintermint flavoring (Condition 2) and the unaltered pH without wintermint flavoring (Condition 3). Conversely, only a slight increase in plasma nicotine levels were seen when the condition was altered to low pH with wintergreen flavoring (Condition 1). At conditions 2 and 3, the average plasma nicotine levels and AUC were comparably high. At condition 2, the average plasma nicotine level was 20.9 ± 7.6 ng/mL (range: below LOD - 32.9), with an average AUC of 1251 ± 223 (range: 980 -1564), which was comparable to condition 3 with an average plasma nicotine level of 21.4 ± 12.3 ng/mL (range: below LOD −51.3) and an average AUC of 1195 ± 706 (range: 472-2450). Conversely, at condition 1, average plasma nicotine levels were relatively low at 12.5 ± 8.1 ng/mL (range: below LOD-33.1), with an average AUC of 736 ± 461 (range: 286-1590). The peak plasma nicotine levels, in all three conditions, occurred between 20 and 35 minutes from the initial time in which the product was placed into the participant's mouth. After adjusting for baseline nicotine concentrations, the maximal average (Cmax) nicotine boost was 6.6 ± 3.9 (range: 1.2 - 11.8) ng/mL in altered low pH condition with flavoring; 20.0 ± 4.2 (range: 14.1 - 25.8) ng/mL in the altered high pH with flavoring and 19.5 ± 6.5 (range: 9.1-28.7) ng/mL in unaltered pH without flavoring.
Figure 1.
Plasma nicotine boost over time.
Cardiovascular measures
Mean Arterial Pressure (MAP) was unchanged across all three conditions. Heart rate increased by 4 to 6 beats per minute after the high pH flavored and the un-amended product but did not change after the low pH flavored product.
Self-Report subjective measures
Nicotine dependence and tobacco use history
Participants were moderately dependent on nicotine with an average FTND-ST score of 4.3 ± 1.6 (range: 3 - 7). The average age of the participants when they first tried ST was 27 ± 15 (range: 12 - 47) and the average age when participants started to use ST regularly was 31 ± 15 (range: 13 - 48). Four participants had tried to quit using ST in the past. Three participants also smoke conventional cigarettes either some days or every day. The average number of cigarettes smoked per day in the past 30 days was 10 ± 9 (range: 5 - 20).
Subjective strength of the product
No significant differences were seen between perceived product strengths, all conditions had less than 15% difference.
Product experience
All difference in preference between products was less than 15%.
Discussion
The presented study clearly shows that ST use delivered large amounts of nicotine to the bloodstream. The unamended referent product and the high pH flavored product delivered significantly more nicotine than the flavored low pH product. The time course of nicotine delivery from the higher pH products was similar to that seen in a previous study [7] where nicotine levels quickly increased and remained at high levels for the time the product was retained in the mouth (30 min). After removal, the plasma levels decreased rapidly. Absorption of nicotine from moist snuff continued in some subjects even after the removal of the product from mouth. This could be due to the slow distribution of nicotine into the plasma from the mucosa. Absorption of swallowed nicotine in the gut could also play a role. The peak nicotine increase from the high pH products averaged19.6 ng/mL;a boost in plasma nicotine that is similar to that observed after smoking a single cigarette [21], a cigarillo [10], a little cigar [9], or in some instances after e- cigarette [11]. A primary feature of dependence producing drugs is the rapid increase in plasma levels - thus subjective reports of a “high”, where the head rush and increased preference are more associated with rapid delivery. For example de Wit reported that low availability of pentobarbital and fast delivery were distinguished by higher liking from the fast delivery even though overall total delivery was similar [22]. Similarly slow nicotine delivery from transdermal patches is not associated with liking and abuse potential whereas rapid delivery of nicotine from cigarette smoking is associated with a high risk of abuse [8]. The mechanism for the difference appears to be related to higher plasma and arterial levels of nicotine reaching the brain rapidly and causing dopamine release in the reward centers: nucleus accumbens and medial forebrain bundle [23].
In a previous study on ST products, a direct association between pH and nicotine absorption was reported [7]. A limitation of that study was that in addition to differences in pH, the ST products differed in flavorings, form (one was a pouch whereas others were loose), and nicotine content of the tobacco. Other differences between the products might have been present but were not systematically characterized including buffering capacity, particle size and surface area, wettability, and moisture content, which could also influence amount and rate of absorption. The present study used a single ST product that was amended to change pH and flavor, but no other characteristics were changed. Thus the differences in nicotine absorption are most likely related to the pH of the product. The addition of the small amount of wintergreen flavoring did not seem to increase nicotine absorption – comparing the high pH flavored product with the unflavored referent product. Chen et al. reported a wide range of mint flavor content in ST [15]. A follow up study would be necessary to measure nicotine absorption after parametrically altering the flavoring content through the range of commercially available concentrations while holding the pH constant. For example, the range of wintergreen (methyl salicylate) is quite large from 2 to 30 mg/g. And the range of menthol concentration ST was between 0.9 to 5.3 mg/g. It is possible that mint flavoring may influence nicotine absorption because it increases local blood flow, increases saliva production and increases permeability across membranes. All of these actions could enhance nicotine absorption but we saw no evidence of that in the present study.
The present study replicates and expands on early theoretical and experimental findings that indicate that pH is an important determinant of buccal absorption of nicotine from ST products. To our knowledge, the pH of vapor from electronic nicotine delivery systems (ENDS, e-cigarettes) has not been reported. The measure of mainstream smoke pH from combustible tobacco products has been addressed using distinct methodologies and approaches [24,25] and the interpretation of the results have been challenged [26]. Generally, the pH of mainstream cigar smoke is higher than the pH of cigarette smoke. Furthermore during cigar smoking the pH increases markedly whereas the pH of cigarette smoke decreases slightly [27].
Acknowledgements
The unamended ST products were obtained from North Carolina Agricultural Research Service; pH and flavor amendments were prepared at Portland State University (Dr James Pankow). We gratefully acknowledge the editorial assistance of Katherine Kim and Amanda Chan, pharmacy intern students from the Notre Dame of Maryland University, School of Pharmacy, Baltimore, MD.
Funding
This work was supported by the National Cancer Institute at the National Institutes of Health (grant number: 5R21CA141639).
Footnotes
DECLARATION OF INTERESTS
The authors report no competing interests.
References
- 1.Mackay J, Eriksen M, Shafey O. The Tobacco Atlas. 2ndedn American Cancer Society; USA.: 2006. [Google Scholar]
- 2.American Cancer Society Smokeless Tobacco. What is spit or smokeless tobacco? 2012 [Google Scholar]
- 3.Centers for Disease Control and Prevention (CDC) Tobacco product use among middle and high school students--United States, 2011 and 2012. MMWR Morb Mortal Wkly Rep. 2013;62:893–897. [PMC free article] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention (CDC) State-specific prevalence of cigarette smoking and smokeless tobacco use among adults --- United States, 2009. MMWR Morb Mortal Wkly Rep. 2010;59:1400–1406. [PubMed] [Google Scholar]
- 5.Piano MR, Benowitz NL, Fitzgerald GA, Corbridge S, Heath J, et al. Impact of smokeless tobacco products on cardiovascular disease: implications for policy, prevention, and treatment: a policy statement from the American Heart Association. Circulation. 2010;122:1520–1544. doi: 10.1161/CIR.0b013e3181f432c3. [DOI] [PubMed] [Google Scholar]
- 6.Tomar SL, Alpert HR, Connolly GN. Patterns of dual use of cigarettes and smokeless tobacco among US males: findings from national surveys. Tob Control. 2010;19:104–109. doi: 10.1136/tc.2009.031070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fant RV, Henningfield JE, Nelson RA, Pickworth WB. Pharmacokinetics and pharmacodynamics of moist snuff in humans. Tob Control. 1999;8:387–392. doi: 10.1136/tc.8.4.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.United States Department of Health and Human Services . The Health Consequences of Smoking: Nicotine Addiction. A Report of the Surgeon General. Centers for Disease Control and Prevention; 1988. [Google Scholar]
- 9.Koszowski B, Potts J, Viray L, Ressenberry R. Smoking patterns and effects among little cigar and cigarette dual users.. 2014 SRNT 20th Annual Meeting; Seattle, USA. 2009.2014. [Google Scholar]
- 10.Fabian LA, Canlas LL, Potts J, Pickworth WB. Ad lib smoking of Black & Mild cigarillos and cigarettes. Nicotine Tob Res. 2012;14:368–371. doi: 10.1093/ntr/ntr131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vansickel AR, Eissenberg T. Electronic cigarettes: effective nicotine delivery after acute administration. Nicotine Tob Res. 2013;15:267–270. doi: 10.1093/ntr/ntr316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fant RV, Pickworth WB, Henningfield JE. The addictive effects of nicotine are related to the speed of delivery. In: Opitz K, editor. Nicotine as a Therapeutic Agent, Immunity and the Environment. Gustav-Fisher; Stuttgart: 1997. pp. 53–61. [Google Scholar]
- 13.Hatsukami DK, Jensen J, Anderson A, Broadbent B, Allen S, et al. Oral tobacco products: preference and effects among smokers. Drug Alcohol Depend. 2011;118:230–236. doi: 10.1016/j.drugalcdep.2011.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Borgerding MF, Bodnar JA, Curtin GM, Swauger JE. The chemical composition of smokeless tobacco: a survey of products sold in the United States in 2006 and 2007. Regul Toxicol Pharmacol. 2012;64:367–387. doi: 10.1016/j.yrtph.2012.09.003. [DOI] [PubMed] [Google Scholar]
- 15.Chen C, Isabelle LM, Pickworth WB, Pankow JF. Levels of mint and wintergreen flavorants: smokeless tobacco products vs. confectionery products. Food Chem Toxicol. 2010;48:755–763. doi: 10.1016/j.fct.2009.12.015. [DOI] [PubMed] [Google Scholar]
- 16.Tallarida RJ, Murray RB. Manual of Pharmacologic Calculations with Computer Programs. Springer-Verlag; New York: 1981. Area under a Curve: Simpson's Rule and Trapezoidal Rule. pp. 47–49. [Google Scholar]
- 17.Ebbert JO, Patten CA, Schroeder DR. The Fagerström Test for Nicotine Dependence-Smokeless Tobacco (FTND-ST). Addict Behav. 2006;31:1716–1721. doi: 10.1016/j.addbeh.2005.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heatherton TF, Kozlowski LT, Frecker RC, Fagerström KO. The Fagerström Test for Nicotine Dependence: a revision of the Fagerström Tolerance Questionnaire. Br J Addict. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- 19.Ferketich AK, Wee AG, Shultz J, Wewers ME. A measure of nicotine dependence for smokeless tobacco users. Addict Behav. 2007;32:1970–1975. doi: 10.1016/j.addbeh.2007.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gray JN, Breland AB, Weaver M, Eissenberg T. Potential reduced exposure products (PREPs) for smokeless tobacco users: clinical evaluation methodology. Nicotine Tob Res. 2008;10:1441–1448. doi: 10.1080/14622200802323258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Benowitz NL, Hukkanen J, Jacob P., 3rd Nicotine chemistry, metabolism, kinetics and biomarkers. Handb Exp Pharmacol. 2009:29–60. doi: 10.1007/978-3-540-69248-5_2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de Wit H, Bodker B, Ambre J. Rate of increase of plasma drug level influences subjective response in humans. Psychopharmacology (Berl) 1992;107:352–358. doi: 10.1007/BF02245161. [DOI] [PubMed] [Google Scholar]
- 23.Benowitz NL. Nicotine addiction. N Engl J Med. 2010;362:2295–2303. doi: 10.1056/NEJMra0809890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pankow JF, Tavakoli AD, Luo W, Isabelle LM. Percent free base nicotine in the tobacco smoke particulate matter of selected commercial and reference cigarettes. Chem Res Toxicol. 2003;16:1014–1018. doi: 10.1021/tx0340596. [DOI] [PubMed] [Google Scholar]
- 25.Watson CH, Trommel JS, Ashley DL. Solid-phase microextraction-based approach to determine free-base nicotine in trapped mainstream cigarette smoke total particulate matter. J Agric Food Chem. 2004;52:7240–7245. doi: 10.1021/jf049455o. [DOI] [PubMed] [Google Scholar]
- 26.Seeman JI. Possible role of ammonia on the deposition, retention, and absorption of nicotine in humans while smoking. Chem Res Toxicol. 2007;20:326–343. doi: 10.1021/tx600290v. [DOI] [PubMed] [Google Scholar]
- 27.Brunnemann KD, Hoffmann D. The pH of tobacco smoke. Food Cosmet Toxicol. 1974;12:115–124. doi: 10.1016/0015-6264(74)90327-7. [DOI] [PubMed] [Google Scholar]