Abstract
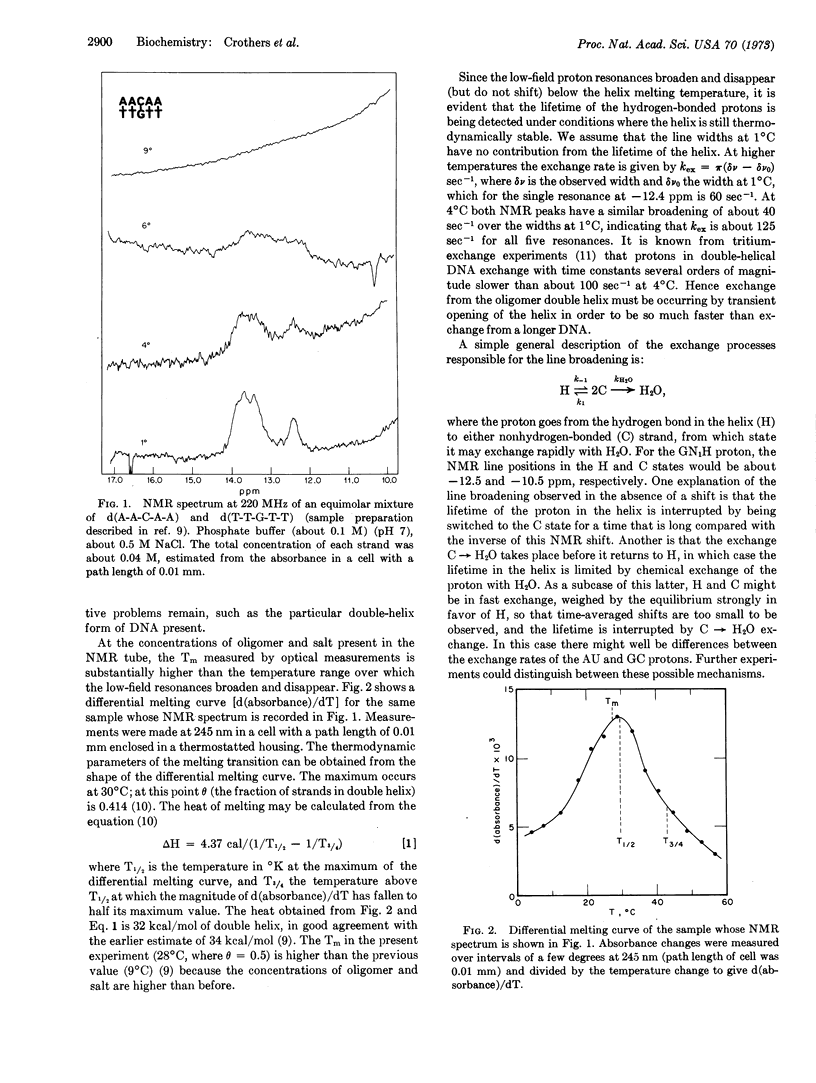
We have studied the low-field proton magnetic resonance spectrum of the double-helical complex of d(A-A-C-A-A) with d(T-T-G-T-T). Well-resolved resonances for the thymidine N3 and the guanosine N1 protons are seen at 1°C, which broaden by 4°C and disappear above 9°C. The actual Tm of the complex was found to be 28°C by measurement of UV-absorbance change. There are several factors that could limit the observed exchange broadening of the resonance lines; the model that best fits the data is one in which the rate of double-helix dissociation limits the rate of exchange of the H-bonding protons with water.
Keywords: proton exchange, oligonucleotide melting
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Craig M. E., Crothers D. M., Doty P. Relaxation kinetics of dimer formation by self complementary oligonucleotides. J Mol Biol. 1971 Dec 14;62(2):383–401. doi: 10.1016/0022-2836(71)90434-7. [DOI] [PubMed] [Google Scholar]
- Cross A. D., Crothers D. M. A proton magnetic resonance study of single-stranded and double-helical deoxyribooligonucleotides. Biochemistry. 1971 Oct 26;10(22):4015–4023. doi: 10.1021/bi00798a002. [DOI] [PubMed] [Google Scholar]
- Giessner-Prettre C., Pullman B. Intermolecular nuclear shielding values for protons of purines and flavins. J Theor Biol. 1970 Apr;27(1):87–95. doi: 10.1016/0022-5193(70)90130-x. [DOI] [PubMed] [Google Scholar]
- Gralla J., Crothers D. M. Free energy of imperfect nucleic acid helices. 3. Small internal loops resulting from mismatches. J Mol Biol. 1973 Aug 5;78(2):301–319. doi: 10.1016/0022-2836(73)90118-6. [DOI] [PubMed] [Google Scholar]
- Katz L., Penman S. Association by hydrogen bonding of free nucleosides in non-aqueous solution. J Mol Biol. 1966 Jan;15(1):220–231. doi: 10.1016/s0022-2836(66)80222-x. [DOI] [PubMed] [Google Scholar]
- Kearns D. R., Patel D. J., Shulman R. G. High resolution nuclear magnetic resonance studies of hydrogen bonded protons of tRNA in water. Nature. 1971 Jan 29;229(5283):338–339. doi: 10.1038/229338a0. [DOI] [PubMed] [Google Scholar]
- McConnell B., von Hippel P. H. Hydrogen exchange as a probe of the dynamic structure of DNA. I. General acid-base catalysis. J Mol Biol. 1970 Jun 14;50(2):297–316. doi: 10.1016/0022-2836(70)90194-4. [DOI] [PubMed] [Google Scholar]
- Pörschke D., Eigen M. Co-operative non-enzymic base recognition. 3. Kinetics of the helix-coil transition of the oligoribouridylic--oligoriboadenylic acid system and of oligoriboadenylic acid alone at acidic pH. J Mol Biol. 1971 Dec 14;62(2):361–381. doi: 10.1016/0022-2836(71)90433-5. [DOI] [PubMed] [Google Scholar]
- Sheard B., Yamane T., Shulman R. G. Nuclear magnetic resonance study of cyanoferrimyoglobin; identification of pseudocontact shifts. J Mol Biol. 1970 Oct 14;53(1):35–48. doi: 10.1016/0022-2836(70)90044-6. [DOI] [PubMed] [Google Scholar]
- Shoup R. R., Miles H. T., Becker E. D. NMR evidence of specific base-pairing between purines and pyrimidines. Biochem Biophys Res Commun. 1966 Apr 19;23(2):194–201. doi: 10.1016/0006-291x(66)90527-4. [DOI] [PubMed] [Google Scholar]
- Wong Y. P., Kearns D. R., Reid B. R., Shulman R. G. Investigation of exchangeable protons and the extent of base pairings in yeast phenylalanine transfer RNA by high resolution nuclear magnetic resonance. J Mol Biol. 1972 Dec 30;72(3):725–740. doi: 10.1016/0022-2836(72)90187-8. [DOI] [PubMed] [Google Scholar]
- Yang S. K., Crothers D. M. Conformational changes of transfer ribonucleic acid. Comparison of the early melting transition of two tyrosine-specific transfer ribonucleic acids. Biochemistry. 1972 Nov 7;11(23):4375–4381. doi: 10.1021/bi00773a026. [DOI] [PubMed] [Google Scholar]



