Abstract
An alkaline phosphatase immunochemically similar to placental alkaline phosphatase (EC 3.1.3.1) was purified from liver metastases of a giant-cell carcinoma of the lung. Some properties of its physical and chemical structure were determined and compared to those of purified placental alkaline phosphatase. The purified tumor phosphatase and the placental phosphatase were similar with regard to the following properties: (1) NH2-terminal sequence, (2) peptide map, (3) subunit molecular weight, and (4) isoelectric point. The physical properties and NH2-terminal sequence of alkaline phosphatase isoenzyme of liver differed from the placental and the tumor enzyme. The data from the present study strongly support the hypothesis that the tumor and the placental alkaline phosphatases are products of the same gene.
Keywords: NH2-terminal sequence, peptide map, tumor protein
Full text
PDF
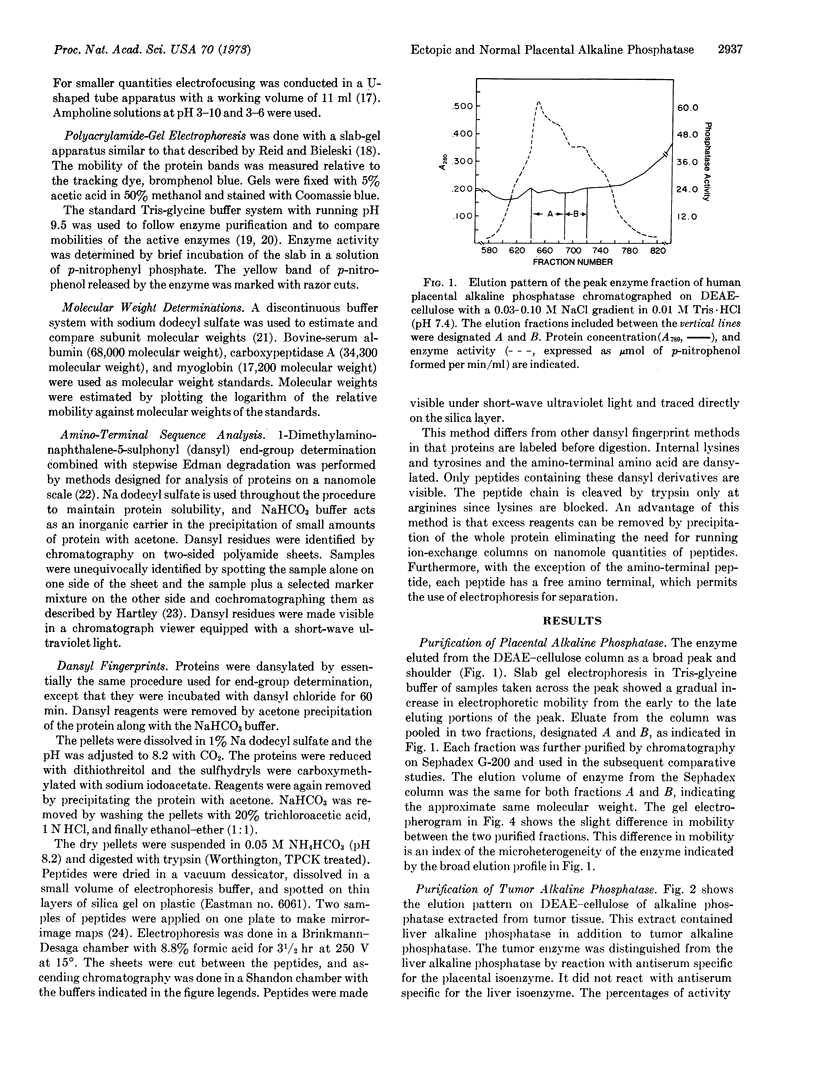
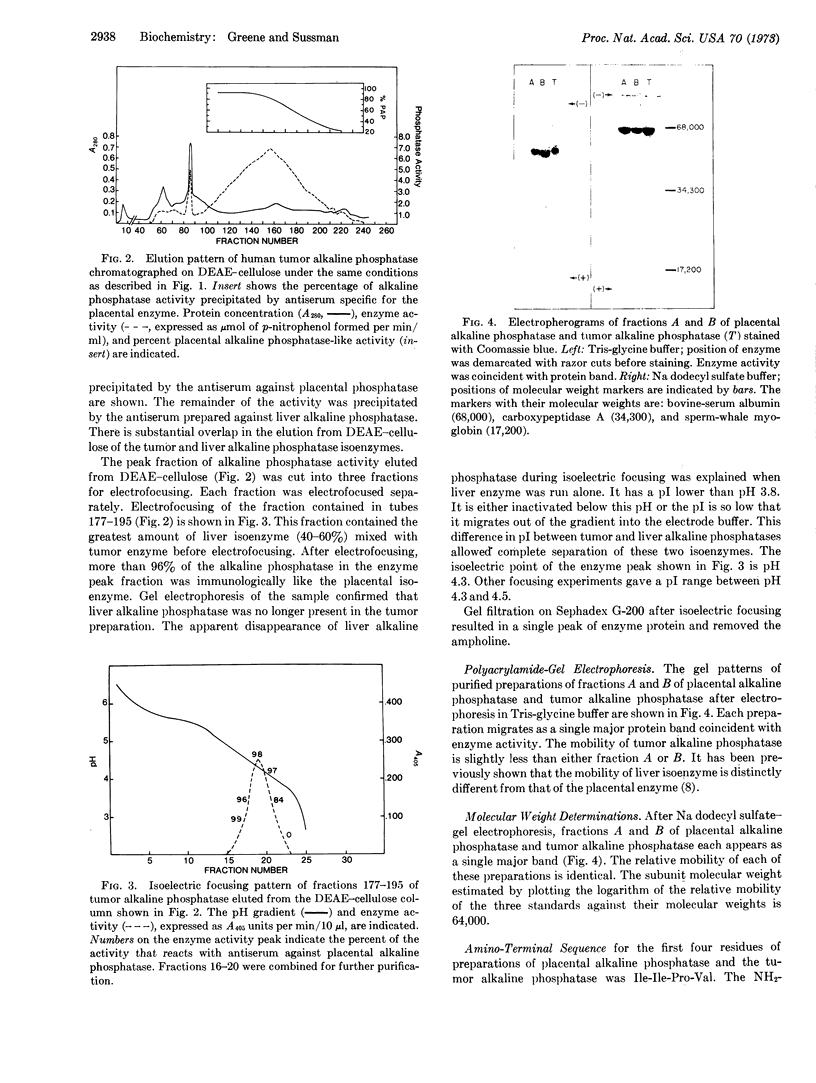


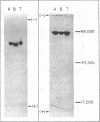
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alpert E., Drysdale J. W., Isselbacher K. J., Schur P. H. Human -fetoprotein. Isolation, characterization, and demonstration of microheterogeneity. J Biol Chem. 1972 Jun 25;247(12):3792–3798. [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Faiman C., Colwell J. A., Ryan R. J., Hershman J. M., Shields T. W. Gonadotropin secretion from a bronchogenic carcinoma. Demonstration by radioimmunoassay. N Engl J Med. 1967 Dec 28;277(26):1395–1399. doi: 10.1056/NEJM196712282772604. [DOI] [PubMed] [Google Scholar]
- George J. M., Capen C. C., Phillips A. S. Biosynthesis of vasopressin in vitro and ultrastructure of a bronchogenic carcinoma. Patient with the syndrome of inappropriate secretion of antidiuretic hormone. J Clin Invest. 1972 Jan;51(1):141–148. doi: 10.1172/JCI106784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh N. K. Purification and molecular properties of placental and intestinal alkaline phosphatases. Ann N Y Acad Sci. 1969 Oct 14;166(2):604–640. doi: 10.1111/j.1749-6632.1969.tb46423.x. [DOI] [PubMed] [Google Scholar]
- Gottlieb A. J., Sussman H. H. Human placental alkaline phosphatase: molecular weight and subunit structure. Biochim Biophys Acta. 1968 Jun 26;160(2):167–171. doi: 10.1016/0005-2795(68)90083-4. [DOI] [PubMed] [Google Scholar]
- Harkness D. R. Studies on human placental alkaline phosphatase. II. Kinetic properties and studies on the apoenzyme. Arch Biochem Biophys. 1968 Aug;126(2):513–523. doi: 10.1016/0003-9861(68)90436-0. [DOI] [PubMed] [Google Scholar]
- Hartley B. S. Strategy and tactics in protein chemistry. Biochem J. 1970 Oct;119(5):805–822. doi: 10.1042/bj1190805f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacoby B., Bagshawe K. D. Placental-type alkaline phosphatase from human tumour tissue. Clin Chim Acta. 1971 Dec;35(2):473–481. doi: 10.1016/0009-8981(71)90223-3. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lipsett M. B. Humoral syndromes associated with cancer. Cancer Res. 1965 Aug;25(7):1068–1073. [PubMed] [Google Scholar]
- NEALE F. C., CLUBB J. S., HOTCHKIS D., POSEN S. HEAT STABILITY OF HUMAN PLACENTAL ALKALINE PHOSPHATASE. J Clin Pathol. 1965 May;18:359–363. doi: 10.1136/jcp.18.3.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama T., Yoshida M., Kitamura M. L-leucine sensitive, heat-stable alkaline-phosphatase isoenzyme detected in a patient with pleuritis carcinomatosa. Clin Chim Acta. 1970 Nov;30(2):546–548. doi: 10.1016/0009-8981(70)90152-x. [DOI] [PubMed] [Google Scholar]
- Odell W. D., Ross G. T., Rayford P. L. Radioimmunoassay for human luteinizing hormone. Metabolism. 1966 Apr;15(4):287–289. doi: 10.1016/0026-0495(66)90142-9. [DOI] [PubMed] [Google Scholar]
- Rathnam P., Saxena B. B. Subunits of luteinizing hormone from human pituitary glands. J Biol Chem. 1971 Dec 10;246(23):7087–7094. [PubMed] [Google Scholar]
- Reid M. S., Bieleski R. L. A simple apparatus for vertical flat-sheet polyacrylamide gel electrophoresis. Anal Biochem. 1968 Mar;22(3):374–381. doi: 10.1016/0003-2697(68)90278-9. [DOI] [PubMed] [Google Scholar]
- Robson E. B., Harris H. Genetics of the alkaline phosphatase polymorphism of the human placenta. Nature. 1965 Sep 18;207(5003):1257–1259. doi: 10.1038/2071257a0. [DOI] [PubMed] [Google Scholar]
- Sargent J. R., Vadlamudi B. P. Electrophoresis of peptides on thin layers of silica gel. Anal Biochem. 1968 Oct 24;25(1):583–587. doi: 10.1016/0003-2697(68)90137-1. [DOI] [PubMed] [Google Scholar]
- Stolbach L. L., Krant M. J., Fishman W. H. Ectopic production of an alkaline phosphatase isoenzyme in patients with cancer. N Engl J Med. 1969 Oct 2;281(14):757–762. doi: 10.1056/NEJM196910022811403. [DOI] [PubMed] [Google Scholar]
- Sussman H. H., Small P. A., Jr, Cotlove E. Human alkaline phosphatase. Immunochemical identification of organ-specific isoenzymes. J Biol Chem. 1968 Jan 10;243(1):160–166. [PubMed] [Google Scholar]
- Vesterberg O., Svensson H. Isoelectric fractionation, analysis, and characterization of ampholytes in natural pH gradients. IV. Further studies on the resolving power in connection with separation of myoglobins. Acta Chem Scand. 1966;20(3):820–834. doi: 10.3891/acta.chem.scand.20-0820. [DOI] [PubMed] [Google Scholar]
- Weiner A. M., Platt T., Weber K. Amino-terminal sequence analysis of proteins purified on a nanomole scale by gel electrophoresis. J Biol Chem. 1972 May 25;247(10):3242–3251. [PubMed] [Google Scholar]
- Weintraub B. D., Rosen S. W. Ectopic production of human chorionic somatomammotropin by nontrophoblastic cancers. J Clin Endocrinol Metab. 1971 Jan;32(1):94–101. doi: 10.1210/jcem-32-1-94. [DOI] [PubMed] [Google Scholar]
- Weller D. L., Heaney A., Sjogren R. E. A simple apparatus and procedure for electrofocusing experiments: pL of lactate dehydrogenase and isocitrate lyase. Biochim Biophys Acta. 1968 Dec 3;168(3):576–579. doi: 10.1016/0005-2795(68)90194-3. [DOI] [PubMed] [Google Scholar]