Nearly every American will be exposed to a medical device during his or her life, and tens of millions of people will be treated with an implantable device. However, regulatory and public health systems in the United States and internationally have critical gaps. The U.S. Food and Drug Administration (FDA) has released a national medical device postmarket surveillance plan1, in which the agency identifies as a main priority the goal of promoting the development of national and international medical device registries for selected products. Another top priority of the FDA is the creation of a unique device identification (UDI) system for medical devices in response to a 2007 federal law2.
Orthopaedic devices are a good choice for demonstrating the importance of registries and UDI implementation worldwide because they are the most commonly used devices and important for public health3. The experience of DePuy Synthes (Warsaw, Indiana) with ASR (Articular Surface Replacement) implants as well as the recalling of metal-on-metal implants in general are changing our frameworks of evaluation4,5. Coverage in The New York Times6 and articles in high-impact medical journals have highlighted the changes in public perceptions of device safety, device regulation, and available evidence5,7. The public health importance is imminent given that more than 1,100,000 joint replacements were performed in 2011 in the United States alone3. Moreover, a dramatic increase in annual volume seems to match or even outperform projections of more than three million annual joint replacement surgeries by 20308. The costs are also expected to substantially increase8.
The Epicenter for Implementation and Advancement
The International Consortium of Orthopaedic Registries (ICOR) initiative was launched in 2011 to address the major gap in evidence and data related to implants. The inaugural conference was held in May 2011, at the headquarters of the FDA in Silver Spring, Maryland. More than seventy stakeholders and over thirty orthopaedic registries for total hip and knee replacement representing fourteen nations are currently part of the network9. Since September 2012, the ICOR has been working on the implementation of a worldwide surveillance system and meaningful use of UDI in orthopaedics through a contract with the FDA. ICOR is focused on two important goals: major demonstration projects of research and surveillance for hip and knee implants, and the harmonization of worldwide implant data through the creation of an implant library.
Registry-Based Demonstration Projects: Major Comparative Studies of Hip and Knee Implants
To demonstrate the potential of and to build a surveillance system, seven national and regional registries with relevant data participated in the investigation of hip and knee implants. These included the registries of Australia (Australian Orthopaedic Association National Joint Replacement Registry), the Catalan region of Spain (Catalan Arthroplasty Register), the Emilia-Romagna region of Italy (Register of the Orthopaedic Prosthetic Implants [RIPO]), Kaiser Permanente and HealthEast in the United States, Sweden (Swedish Knee Arthroplasty Register), and Norway (Norwegian Arthroplasty Register). The eighth registry, New Zealand National Joint Registry, participated by providing supplementary information that will be combined with other registry data in the near future.
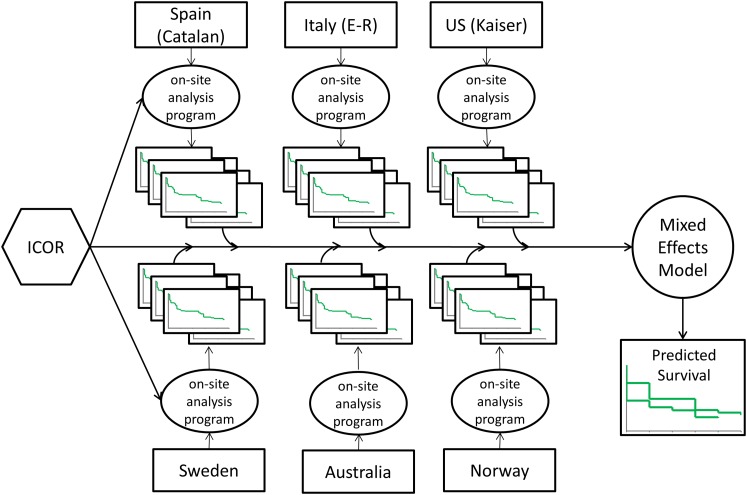
Investigators from the registries agreed that it is critical for scientists and clinicians to undertake analyses of the outcomes of specific implants and relate them to generic characteristics (attributes) of a group of devices. First, the ICOR established a distributed data system as outlined in the initial plans10. Standardized data extraction programs were created by the ICOR coordinating center and distributed to participating registries (Fig. 1). Each registry then completed the analyses of its own data, and completely de-identified data summaries were returned to the coordinating center. The data were then combined with use of multivariable hierarchical models in order to evaluate comparative outcomes of devices regarding the main patient-centered outcome—revision surgery after initial device implantation. The ICOR investigators agreed that all-cause revision after surgery adequately reflects patient experience, particularly in the first ten years after surgery, where revision indicates failure of the implant as well as the pain and other discomforts that necessitate a second arthroplasty, regardless of the implant components that failed. The details of this advanced innovative methodology are summarized in the paper in this series by Banerjee et al. (http://dx.doi.org/10.2106/JBJS.N.00642)11.
Fig. 1.
Example depiction of the ICOR distributed research network and analytics.
Multinational Investigations of Hip Bearings and Fixation
The ICOR began these studies by establishing priorities, inclusion and exclusion criteria, and a control group for all investigations of hip bearing surface. The expert consensus was to reduce the heterogeneity and increase the potential to determine the effects of bearing type by focusing on patients with osteoarthritis who had undergone uncemented stemmed hip replacement (no resurfacing or cemented implants) and who were less than sixty-five years of age. The control group was defined as the bearing that is commonly used, has little heterogeneity in terms of the effect of implant head size on outcomes, and represents that which is used in modern practice in the United States.
After extensive analytic investigations, the control for bearing studies was identified to be metal on highly cross-linked polyethylene (HXLPE). Allepuz and colleagues reported that there were no differences in outcomes of surgery when metal-on-HXLPE implants with larger or smaller femoral head size were used and that larger head diameter should not be considered advantageous or detrimental to device survival (http://dx.doi.org/10.2106/JBJS.N.00461)12. Metal on HXLPE was evaluated in all subsequent investigations of other bearing effects, including evaluations of head-size effects related to those bearings.
Paxton and colleagues compared metal-on-HXLPE with metal-on-conventional (non-cross-linked) polyethylene bearing surfaces and found that non-cross-linked polyethylene was not associated with significantly worse outcomes compared with metal on HXLPE (http://dx.doi.org/10.2106/JBJS.N.00460)13. While the estimate of the effect trended in the direction of higher revision occurrence with metal on non-cross-linked polyethylene, the difference was not significant (hazard ratio [HR], 1.20; 95% confidence interval [CI], 0.80 to 1.79).
One of the most important studies conducted by the ICOR investigators was the comparison by Furnes et al. of metal-on-HXLPE with metal-on-metal bearings (http://dx.doi.org/10.2106/JBJS.N.00459)14. The public health concern and the controversy related to metal-on-metal bearings are well known. Where the DePuy ASR device is a known outlier implant, the investigators excluded the ASR from the analyses from the outset. The study focused on substantiating the effect on revision risk of large-head-size, metal-on-metal implants; a prior ICOR investigation indicated the need for this study on the basis of individual registry reports15. Furnes et al. found an interaction of time and bearing type. The effect of large-head-size, metal-on-metal bearing on revision risk more than doubled over time when compared with that of metal-on-HXLPE, from no difference between bearings (HR, 0.95; 95% CI, 0.74 to 1.23) at zero to two years of follow-up to more than two-times higher risk of revision (HR, 2.15; 95% CI, 1.63 to 2.83) at six to seven years of follow-up14. Additionally, there was no difference between smaller-head-size, metal-on-metal and metal-on-HXLPE bearings, but the effect was confounded in some registries with the use of best-performing metal-on-metal bearings. The ICOR is in the process of a more comprehensive evaluation of smaller-head-size, metal-on-metal bearings.
The final ICOR investigation of hip bearings presents the results of an analysis of ceramic-on-ceramic compared with metal-on-HXLPE (http://dx.doi.org/10.2106/JBJS.N.00465)16. We found a 37% higher risk of revision associated with ceramic-on-ceramic implants with a smaller head size compared with ceramic-on-ceramic implants with larger head size and a similar, 36% higher risk of revision when they were compared with metal-on-HXLPE bearings, with both results significant. Overall, use of ceramic-on-ceramic devices with a larger head size was not substantially different from use of metal-on-HXLPE, but there was a small and insubstantial protective effect noted for the larger-size ceramic-on-ceramic devices in the first two years that dissipated over the long-term. This means that the selection of a large-size, ceramic-on-ceramic bearing would prevent fewer than one out of 500 patients from requiring a revision within two years after hip arthroplasty, with no difference in later time periods.
Regarding fixation, the major study undertaken by ICOR investigators was not limited in terms of age and fixation method but focused on osteoarthritis patients who had undergone stemmed hip replacement (no resurfacing arthroplasty procedures). In their comprehensive study, which included 239,442 patients, Stea and colleagues reported substantial variability in the choice of fixation globally (http://dx.doi.org/10.2106/JBJS.N.00463)17. While European countries often use cemented fixation (with a high prevalence of cementless as well), most of the practice in the United States and Australia is based on cementless or hybrid fixation (cemented stem and uncemented cup). In a recent limited study of Scandinavian registries, the authors reported that uncemented fixation is associated with worse outcomes among patients sixty-five years of age or older18. The ICOR study includes much more generalizable data collected worldwide and found that cementless fixation is associated with approximately 58% higher risk of revision surgery in patients seventy-five years of age or older when compared with a hybrid approach. The effect of cementless fixation was significant but not as strong in the intermediate age group (sixty-five to seventy-four years of age) and among patients forty-five to sixty-four years of age. Importantly, the ICOR investigation established that hybrid fixation is a safe and efficient choice for hip replacement.
Multinational Investigations of Knee Devices
The investigations of knee devices focused on mobile and fixed-bearing total knee replacements and also addressed the practice of posterior stabilization. Given the availability of various mobile-bearing devices within the context of both posterior stabilization and non-posterior stabilization (cruciate-retaining implants), posterior-stabilized and non-posterior-stabilized mobile-bearing implants were examined in separate studies.
Regarding non-posterior-stabilized implants, Namba and colleagues compared the effect of mobile and fixed bearing and found over 40% higher risk of revision surgery associated with mobile bearings, with no interaction of effect with time (http://dx.doi.org/10.2106/JBJS.N.00466)19. The study included 319,616 total knee replacements and has tremendous implications for worldwide practice, given that almost 20% of the knee arthroplasties used mobile bearings.
The second study of mobile bearings included 137,616 posterior-stabilized knee prostheses; 17.6% had a mobile bearing (http://dx.doi.org/10.2106/JBJS.N.00556)20. In this report, Graves and colleagues found a significant interaction of bearing with time: patients with mobile bearings had a >85% higher chance of revision surgery within the first year postoperatively (HR, 1.86; 95% CI, 1.28 to 2.7) compared with those with fixed bearings. For all other time intervals, the mobile-bearing devices had higher HR estimates, but these differences were not significant. There was also no evidence that bearing effects were modified by age, sex, or patella resurfacing.
In the final registry-based study of knee implants, by Comfort et al., fixed posterior-stabilized implants were compared with fixed non-posterior-stabilized implants (cruciate-retaining) (http://dx.doi.org/10.2106/JBJS.N.00462)21. The study included 371,527 knee implants and found that posterior-stabilized devices were associated with a much higher risk of revision than non-posterior-stabilized devices when the patella was not resurfaced. The difference was much greater in the first two years (year zero to one: HR, 2.15; 95% CI, 1.56 to 2.95; and year one to two: HR, 1.61; 95% CI, 1.48 to 1.75) than in later years. In the subgroup analyses of patella resurfacing, the estimates were less strong but still significant. Separately, the investigators found that patella resurfacing was protective against revision occurrence in the first ten years after surgery.
Other ICOR Studies of Devices and Outcomes
In a study of 1536 children and young adults thirty years of age or younger who were operated on in Australia between 1999 and 2012, we found that patients who underwent hip and knee replacement had very different diagnoses compared with adults, including a high prevalence of tumor (http://dx.doi.org/10.2106/JBJS.N.00541)22. The revision surgery rate was similar for this population compared with that of older patients. However, the sample size was not large, and the follow-up period was limited. It is important for registries to continue to collect data relevant to this cohort to better understand the safety and effectiveness of devices as well as the unmet health needs of these patients.
Keurentjes and colleagues performed a meta-analysis of cohort studies of hip devices (http://dx.doi.org/10.2106/JBJS.N.00397)23. Estimates of revision surgery were extracted from all relevant studies as well as registries (annual reports). Each study reported failure rates of devices, and investigators combined these data. The authors reviewed all-cause revision as well as aseptic loosening at ten years of follow-up. After reviewing 5513 papers related to thirty-four types of acetabular components and thirty-two types of femoral components, they found that eight types of acetabular cups and fifteen types of femoral stems performed better than the United Kingdom National Institute for Health and Care Excellence benchmark of a ten-year revision rate of ≤10%.
Two studies addressed two different, but interconnected, questions of organization of the data collection and understanding minimum clinically important differences in patient-reported outcome measures (PROMs). Romero et al. evaluated nineteen registry reports and 1052 articles and found that only one report and two studies mentioned the use of PROMs and minimum clinically important differences for revision rates after hip or knee replacement (http://dx.doi.org/10.2106/JBJS.N.00464)24. This demonstrates limited understanding of a minimum clinically important difference in its association with other patient-centered clinical outcomes. In the other study, Franklin and colleagues reported that U.S. joint replacement registries have limited success in implementing PROMs and related data collection (http://dx.doi.org/10.2106/JBJS.N.00328)25. They believe that it is important to measure both pain and function and reduce data-collection burden. The experience with the FORCE-TJR (Function and Outcomes Research for Comparative Effectiveness in Total Joint Replacement) registry26 shows that successful implementation of PROM data collection is possible if proper attention addresses the selection of the outcome measure, mode and timing of postoperative administration, and minimization of the data-collection burden.
Creating an Orthopaedic Implant Library: The Role of Registries
The creation of an implant library and relevant nomenclature for device attributes and characteristics is the critical link within the clinical and research community interested in devices from a postmarket surveillance and research perspective when using registries. Registries are critically important for uniquely identifying devices, as almost none of the nation’s electronic health records at this time can automatically uniquely identify a device and link it to the outcome data of individual patients27. Similarly, very limited national data exist that can be used to identify medical devices and link them to patient outcomes within claim systems27. Large U.S. and international registries might be very attractive in this context and potentially could meet the needs of the FDA and regulators worldwide. In orthopaedics, large registries or networks of registries capture device information on a very detailed level and are particularly important for active surveillance and postmarket evaluation. The registries can provide denominator data for adverse events related to specific implants and allow proper comparative effectiveness studies.
UDI Rule and ICOR Contribution
Under the FDA’s proposed UDI rule, manufacturers must label medical devices with a UDI code that identifies model and production characteristics. Additionally, manufacturers must provide the FDA with attributes of UDI-labeled devices to populate the Global Unique Device Identification Database (GUDID), a public hub of standardized UDI data intended to integrate with billing, inventory, and electronic health records systems.
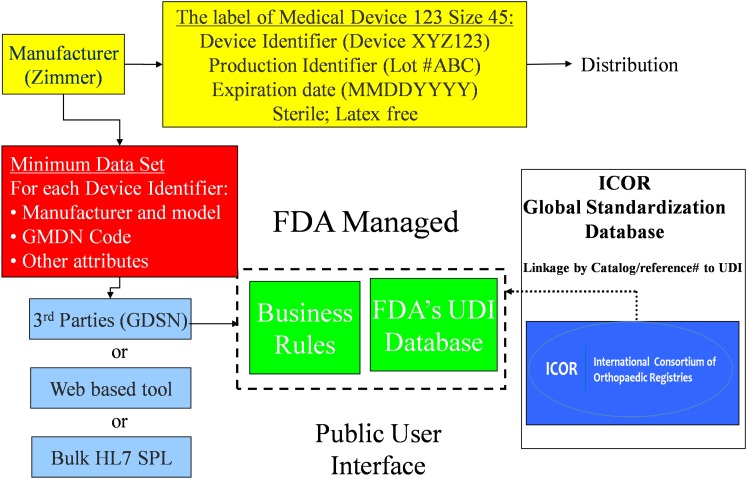
The ICOR contribution to this process is depicted in Figure 2; the ICOR database of clinical attributes and characteristics is shown as an adjunct database to GUDID.
Fig. 2.
Illustrated example of UDI database collection and distribution, including the contribution of ICOR. GMDN = global medical device nomenclature, GDSN = global data synchronization network, and HL7 SPL = Health Level-7 structured product labeling.
To monitor and evaluate total joint arthroplasty procedures, the specific devices must be accurately identified and classified. Registries have developed implant libraries to achieve this goal, but these have usually been developed in an ad hoc way by each registry, and there has been no standardization. The ICOR facilitated a standardized process that enabled the development of a universal implant library that all registries could use for consistency of reporting and enhanced inter-registry collaboration. In the absence of UDI, the ICOR process is based on the catalog number assigned by a company to an implant so that it is specifically identified. It can be numeric, alphabetic, or alphanumeric, and it will be specific to a particular implant size or configuration. Any change to the design of an implant necessitates a change in the catalog number. However, there has been no worldwide consensus on the encoding of part numbers, and, in some instances, different devices have been identified with the same catalog number, and different numbers have been used for the same implant, depending on where it was being sold. Nevertheless, the ICOR identified that the combination of manufacturer name and catalog number leads to unique identification of 99% of products. The ICOR is continuing to work with registries to reduce the burden they have in maintaining and updating their individual device databases on the basis of catalog numbers. As UDI is implemented, the registries will also link implant characteristics to the UDI database. We believe that this process enhances the value of GUDID in the eyes of the clinical and research community (Fig. 2).
In conclusion, ICOR achievements to date have important implications for medical device postmarket surveillance system development in the United States and worldwide. The ability to create an international, distributed research network for medical devices is unprecedented and opens new opportunities for the development of investigations of comparative effectiveness and device safety. ICOR experience in addressing hip and knee replacements showcases a scalable model for use for other implantable and surgical devices. Comparative studies of hip and knee devices illustrate the ability of the ICOR to evaluate global evidence for various classes of devices and help surgeons and patients make evidence-based choices.
Funding Disclosure
The ICOR initiative, including the coordinating center at Weill Cornell Medical College and Kaiser Permanente, is financed by contract HHSF223201110172C from the U.S. Food and Drug Administration (FDA), Silver Spring, Maryland; one of the authors (A.S.) was the principal investigator.
Footnotes
The studies in this supplement were presented at the joint meeting of the International Society of Arthroplasty Registries (ISAR) and the International Consortium of Orthopaedic Registries (ICOR) in Stratford-upon-Avon, United Kingdom, June 1-3, 2013, and at the ICOR meeting at the American Academy of Orthopaedic Surgeons (AAOS) Annual Meeting in New Orleans, Louisiana, March 10-11, 2014.
Disclosure: One or more of the authors received payments or services, either directly or indirectly (i.e., via his or her institution), from a third party in support of an aspect of this work. None of the authors, or their institution(s), have had any financial relationship, in the thirty-six months prior to submission of this work, with any entity in the biomedical arena that could be perceived to influence or have the potential to influence what is written in this work. Also, no author has had any other relationships, or has engaged in any other activities, that could be perceived to influence or have the potential to influence what is written in this work. The complete Disclosures of Potential Conflicts of Interest submitted by authors are always provided with the online version of the article.
References
- 1.Center for Devices and Radiological Health. U.S. Food and Drug Administration. Strengthening our national system for medical device postmarket surveillance: Update and next steps. 2013. http://www.fda.gov/downloads/MedicalDevices/Safety/CDRHPostmarketSurveillance/UCM348845.pdf. Accessed 2014 Jul 28.
- 2.Gross TP, Crowley J. Unique device identification in the service of public health. N Engl J Med. 2012October25;367(17):1583-5 Epub 2012 Sep 26. [DOI] [PubMed] [Google Scholar]
- 3.Pfuntner A, Wier LM, Stocks C. Agency for healthcare research and quality. most frequent procedures performed in U.S. hospitals, 2011. HCUP Statistical Brief #165. 2013 Oct. http://www.hcup-us.ahrq.gov/reports/statbriefs/sb165.pdf. Accessed 2014 Jul 8. [PubMed]
- 4.Cohen D. Out of joint: the story of the ASR. BMJ. 2011;342(13):d2905. Epub 2011 May 13. [DOI] [PubMed] [Google Scholar]
- 5.Sedrakyan A. Metal-on-metal failures—in science, regulation, and policy. Lancet. 2012March31;379(9822):1174-6 Epub 2012 Mar 13. [DOI] [PubMed] [Google Scholar]
- 6.Meier B. With warning, a hip device is withdrawn. NY Times. 2010March10 http://www.nytimes.com/2010/03/10/business/10device.html?pagewanted=all&_r=0. Accessed 2011 Sep 15.
- 7.Godlee F. The trouble with medical devices. BMJ. 2011;342(13):d3123. [Google Scholar]
- 8.Kurtz SM, Lau E, Ong K, Zhao K, Kelly M, Bozic KJ. Future young patient demand for primary and revision joint replacement: national projections from 2010 to 2030. Clin Orthop Relat Res. 2009October;467(10):2606-12 Epub 2009 Apr 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sedrakyan A, Paxton EW, Phillips C, Namba R, Funahashi T, Barber T, Sculco T, Padgett D, Wright T, Marinac-Dabic D. The International Consortium of Orthopaedic Registries: overview and summary. J Bone Joint Surg Am. 2011December21;93(Suppl 3):1-12. [DOI] [PubMed] [Google Scholar]
- 10.Sedrakyan A, Paxton EW, Marinac-Dabic D. Stages and tools for multinational collaboration: the perspective from the coordinating center of the International Consortium of Orthopaedic Registries (ICOR). J Bone Joint Surg Am. 2011December21;93(Suppl 3):76-80. [DOI] [PubMed] [Google Scholar]
- 11.Banerjee S, Cafri G, Isaacs AJ, Graves S, Paxton E, Marinac-Dabic D, Sedrakyan A. A distributed health data network analysis of survival outcomes: the international consortium of orthopaedic registries perspective. J Bone Joint Surg Am. 2014December17;96(Suppl 1):7-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Allepuz A, Havelin L, Barber T, Sedrakyan A, Graves S, Bordini B, Hoeffel D, Cafri G, Paxton E. Effect of femoral head size on metal-on-HXLPE Hip Arthroplasty Outcome in a Combined Analysis of Six National and Regional Registries. J Bone Joint Surg Am. 2014December17;96(Suppl 1):12-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Paxton E, Cafri G, Havelin L, Stea S, Pallisό F, Graves S, Hoeffel D, Sedrakyan A. Risk of revision following total hip arthroplasty: metal-on-conventional polyethylene compared with metal-on-highly cross-linked polyethylene bearing surfaces: international results from six registries. J Bone Joint Surg Am. 2014December17;96(Suppl 1):19-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Furnes O, Paxton E, Cafri G, Graves S, Bordini B, Comfort T, Coll Rivas M, Banerjee S, Sedrakyan A. Distributed analysis of hip implants using six national and regional registries: comparing metal-on-metal with metal-on-highly cross-linked polyethylene bearings in cementless total hip arthroplasty in young patients. J Bone Joint Surg Am. 2014December17;96(Suppl 1):25-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Graves SE, Rothwell A, Tucker K, Jacobs JJ, Sedrakyan A. A multinational assessment of metal-on-metal bearings in hip replacement. J Bone Joint Surg Am. 2011December21;93(Suppl 3):43-7. [DOI] [PubMed] [Google Scholar]
- 16.Sedrakyan A, Graves S, Bordini B, Pons M, Havelin L, Mehle S, Paxton E, Barber T, Cafri G. Comparative effectiveness of ceramic-on-ceramic implants in stemmed hip replacement: a multinational study of six national and regional registries. J Bone Joint Surg Am. 2014December17;96(Suppl 1):34-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stea S, Comfort T, Sedrakyan A, Havelin L, Marinelli M, Barber T, Paxton E, Banerjee S, Isaacs AJ, Graves S. Multinational comprehensive evaluation of the fixation method used in hip replacement: interaction with age in context. J Bone Joint Surg Am. 2014December17;96(Suppl 1):42-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mäkelä KT, Matilainen M, Pulkkinen P, Fenstad AM, Havelin L, Engesaeter L, Furnes O, Pedersen AB, Overgaard S, Kärrholm J, Malchau H, Garellick G, Ranstam J, Eskelinen A. Failure rate of cemented and uncemented total hip replacements: register study of combined Nordic database of four nations. BMJ. 2014;348(13):f7592. Epub 2014 Jan 13. [DOI] [PubMed] [Google Scholar]
- 19.Namba R, Graves S, Robertsson O, Furnes O, Stea S, Puig-Verdié L, Hoeffel D, Cafri G, Paxton E, Sedrakyan A. International comparative evaluation of knee replacement with fixed or mobile non-posterior-stabilized implants. J Bone Joint Surg Am. 2014December17;96(Suppl 1):52-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Graves S, Sedrakyan A, Baste V, Gioe TJ, Namba R, Martínez Cruz O, Stea S, Paxton E, Banerjee S, Isaacs AJ, Robertsson O. International comparative evaluation of knee replacement with fixed or mobile-bearing posterior-stabilized prostheses. J Bone Joint Surg Am. 2014December17;96(Suppl 1):59-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Comfort T, Baste V, Froufe MA, Namba R, Bordini B, Robertsson O, Cafri G, Paxton E, Sedrakyan A, Graves S. International comparative evaluation of fixed-bearing non-posterior-stabilized and posterior-stabilized total knee replacements. J Bone Joint Surg Am. 2014December17;96(Suppl 1):65-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sedrakyan A, Romero L, Graves S, Davidson D, de Steiger R, Lewis P, Solomon M, Vial R, Lorimer M. Survivorship of hip and knee implants in pediatric and young adult populations: analysis of registry and published data. J Bone Joint Surg Am. 2014December17;96(Suppl 1):73-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Keurentjes JC, Pijls BG, Val Tol FR, Mentink JF, Mes SD, Schoones JW, Fiocco M, Sedrakyan A, Nelissen RG. Which implant should we use for primary total hip replacement? a systematic review and meta-analysis. J Bone Joint Surg Am. 2014December17;96(Suppl 1):79-97. [DOI] [PubMed] [Google Scholar]
- 24.Romero L, Nieuwenhuijse M, Carr A, Sedrakyan A. Review of clinical outcomes-based anchors of minimum clinically important differences in hip and knee registry-based reports and publications. J Bone Joint Surg Am. 2014December17;96(Suppl 1):98-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Franklin PD, Lewallen D, Bozic K, Hallstrom B, Jiranek W, Ayers DC. Implementation of patient-reported outcome measures in U.S. total joint replacement registries: rationale, status, and plans. J Bone Joint Surg Am. 2014December17;96(Suppl 1):104-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Franklin PD, Allison JJ, Ayers DC. Beyond joint implant registries: a patient-centered research consortium for comparative effectiveness in total joint replacement. JAMA. 2012September26;308(12):1217-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Campion TR Jr, Johnson SB, Paxton EW, Mushlin AI, Sedrakyan A. Implementing unique device identification in electronic health record systems: organizational, workflow, and technological challenges. Med Care. 2014January;52(1):26-31. [DOI] [PubMed] [Google Scholar]