Abstract
We have investigated the effects of hypoxia and myocardial ischemia/reperfusion on the structure and function of cytochrome c oxidase (CcO). Hypoxia (0.1% O2 for 10 h) and cAMP-mediated inhibition of CcO activity were accompanied by hyperphosphorylation of subunits I, IVi1, and Vb and markedly increased reactive O2 species production by the enzyme complex in an in vitro system that uses reduced cytochrome c as an electron donor. Both subunit phosphorylation and enzyme activity were effectively reversed by 50 nm H89 or 50 nm myristoylated peptide inhibitor (MPI), specific inhibitors of protein kinase A, but not by inhibitors of protein kinase C. In rabbit hearts subjected to global and focal ischemia, CcO activity was inhibited in a time-dependent manner and was accompanied by hyperphosphorylation as in hypoxia. Additionally, CcO activity and subunit phosphorylation in the ischemic heart were nearly completely reversed by H89 or MPI added to the perfusion medium. Hyperphosphorylation of subunits I, IVi1, and Vb was accompanied by reduced subunit contents of the immunoprecipitated CcO complex. Most interestingly, both H89 and MPI added to the perfusion medium dramatically reduced the ischemia/reperfusion injury to the myocardial tissue. Our results pointed to an exciting possibility of using CcO activity modulators for controlling myocardial injury associated with ischemia and oxidative stress conditions.
Cytochrome c oxidase (CcO)3 is the terminal oxidase of the mitochondrial electron transport chain, whose activity is modulated in response to O2 tension and the work load of the tissue (1-6). This rate-limiting enzyme is an important site of regulation of mitochondrial respiration and oxidative phosphorylation (7). In the yeast, altered CcO activity in response to aerobic and anaerobic conditions is associated with the differential expression of the two isologs of the CcO Vb gene (8), although the precise mechanism by which the mammalian CcO modulates its activity remains unknown. Mitochondrial electron transport chain complexes are major sources of cellular ROS under both normoxic and hypoxic conditions (9, 10). Hypoxia-tolerant and hypoxia-sensitive human glioma cells exhibit distinct patterns of mitochondrial function in response to hypoxia (9, 11). Submitochondrial particles exposed to hypoxic conditions in vitro show reduced CcO activity (1, 10, 12). Some studies also suggest that the myocardial ischemia/reperfusion injury is anifested through altered CcO activity and reduced mitochondrial oxidative phosphorylation (13, 14).
Protein kinases have been suggested to play a role in the modulation of myocardial ischemia/reperfusion injury (15), although the roles of different cellular components in mediating this injury remain unclear. The presence of PKA and PKC activities in the mitochondrial inner membrane-matrix compartment and the role of PKC-mediated phosphorylation in the regulation of pyruvate dehydrogenase activity are well established (16). An 18-kDa subunit of the NADH dehydrogenase (complex I) (17) and subunits I, II, and Vb of CcO (complex IV) are phosphorylated in vitro when incubated with PKA and [γ-32P]ATP (18). Furthermore, in vitro phosphorylation of bovine heart and rat heart CcO results in 40–70% reduced activity (18, 19). Nevertheless, phosphorylation of CcO subunits in vivo in whole cells or tissues and its effect on enzyme activity have not yet been investigated.
In addition to possible regulation by protein phosphorylation, CcO activity in mammalian tissues is also modulated by lipid composition and reactive oxygen species (ROS). For example, a decline in CcO activity during ischemia and reperfusion (8, 9) or hypoxia (1, 12) has been attributed, at least in part, to a decreased cardiolipin content or its modification by peroxy radicals. Indeed cardiolipin content plays an important role in CcO activity and reconstitution of the detergent-solubilized enzyme complex (17). Additionally, studies by Darley-Usmar and co-workers (20) have shown that mitochondrially generated NO during various pathophysiological conditions modulates CcO activity by direct binding to the heme and altering the activity of the enzyme complex.
In the present study we have used murine macrophage cells in culture and in vitro perfused rabbit heart system to investigate the effects of hypoxia and ischemia, respectively, on the phosphorylation status and activity of CcO enzyme. Our results show that both experimental hypoxia and ischemia cause increased mitochondrial PKA activity and increased phosphorylation of CcO subunits. These conditions resulted in significantly lower CcO activity and vastly increased ROS production by the CcO complex. More important, specific inhibitors of PKA but not PKC rendered marked protection against ischemia/reperfusion injury in the perfused rabbit heart system.
MATERIALS AND METHODS
Cell Culture and Hypoxic Conditions
RAW 264.7 mouse monocyte macrophages were cultured in Dulbecco’s modified Eagle’s medium supplemented with 10% (v/v) heat-inactivated fetal calf serum as described before (6). Cells at 80–90% confluency were grown under hypoxic conditions (0.1% O2) for 10 h or normoxic conditions (21% O2) for 10 h as described before (6).
Ischemia and Perfusion Protocol
All animal procedures were carried out in conformance with the Guidelines for the Care and Use of Laboratory Animals from the National Institutes of Health. Hearts from New Zealand male white rabbits (2–2.5 kg) were perfused using a Langendorff perfusion apparatus as described before (21). The aorta was cannulated and the heart suspended on a Langendorff perfusion apparatus in which the coronary arteries were perfused from a reservoir with oxygenated temperature-controlled Tyrode’s solution at a constant pressure of 80 mm Hg. The heart was surrounded by a temperaturecontrolled 50-ml chamber with an overflow so that the heart was submerged in its effluence. Coronary flow was measured by a timed dripcount from the overflow. Two orthogonal bipolar electrocardiograms (X and Y leads) were recorded by two pairs of 1 cm in diameter goldplated electrodes that were affixed to the walls of the chamber. In order to monitor contractility, a water-filled latex balloon connected to a pressure gauge was inserted into the left ventricular cavity. The preload was adjusted to ~5–10 mm Hg. The pulse pressure was measured to the nearest 0.5 mm Hg. All other instrumental settings and measurements were done as described previously (21).
We were able to induce global ischemia without complete disruption of coronary circulation by reducing aortic perfusion to 2.5 ml/min. This level of perfusion is below the critical level for producing ischemic changes but still provides a coronary flow in order to ensure continued delivery of agents. Focal ischemia was produced by ligating the anterior division of the left coronary artery near its origin by using a snare made with 2-0-gauge silk suture passed under the artery and sheathed in a 20-gauge polyethylene tube. For experimental groups involving inhibitors, either PKA-specific inhibitor H89 or MPI (50 nm each) or PKC-specific inhibitor Go6850 (10 nm) was added to the perfusion medium before the onset of the experiment.
Evaluation of the Ischemic Damage
Ischemia/reperfusion damage caused by anterior coronary artery occlusion was evaluated by delineating the nonperfused volume of myocardium at risk during the occlusion and the volume of myocardial necrosis after 2 h of reperfusion. After reperfusion (2 h) the snare was reapplied, and the heart was perfused with india ink to delineate the nonperfused volume at risk. The heart was removed from the apparatus, sectioned transversely from apex to base in 3-mm thick sections, and incubated for 20 min at 36 °C in 1% triphenyltetrazolium chloride solution to delineate the necrotic zone. The area of the necrotic region and the area at risk were measured from enlarged photographs of the sections using computer-based planimetry. The volumes of the necrotic regions and the risk regions were calculated using the thickness of the slices.
Isolation of Mitochondria and Assay of CcO Activity
Mitochondria were isolated from cells and myocardial tissue by differential centrifugation of cell and tissue homogenates in mitochondria isolation buffer (MIB: 70 mM sucrose, 220 mM mannitol, 2.5 mM HEPES, pH 7.4, and 2 mM EDTA) as described before (22). Mitochondria were washed three times with MIB and in some experiments were treated with digitonin (75μg/mg protein) as described before (22). Submitochondrial particles (SMP) were prepared as described (6). Briefly, mitochondria were sonicated for 120 s with a 10-s on/off cycle using ultrasonic processor model GE130PB and probe CU188-3548 and centrifuged at 12,000 × g for 10 min. The supernatant was centrifuged again at 150,000 × g for 45 min using Sorvall RC M120EX ultracentrifuge. The pellet was suspended and used as SMP. CcO activity was assayed by two different approaches. In the first approach, CcO activity was measured in permeabilized cells as described before (23) under conditions that inflict minimal membrane perturbation. Briefly, cells were permeabilized by the addition of 0.01% digitonin for 15 min with agitation. The assay medium consisted of 4 μm 3,3′-diaminobenzidine tetrachloride (DAB), 100 μm reduced cytochrome c, 2 μg/ml catalase in 0.01 M sodium phosphate buffer, pH 7.0. An increase in oxidized DAB, which is directly proportional to the CcO activity, was measured at 450 nm. In the second approach, CcO was solubilized from SMP with 1.5% sodium cholate and reconstituted into asolectin-cardiolipin vesicles as described (24, 25). Orientation of CcO in the vesicles was determined by spectral analysis following sequential reduction with ascorbate and dithionate (26). The CcO activity of proteoliposomes was assayed as described (26, 27) by measuring the rate of oxidation of ferrocytochrome c (0.2–0.8 mm) at 550 nm in a final volume of 1 ml using a Cary-1E spectrophotometer (Varian Instruments, Walnut Creek, CA).
Assay for PKA and PKC Activity
The PKA activity was measured using a kit from Calbiochem, which combines affinity binding and ultrafiltration and uses a 32P-labeled biotinylated peptide substrate. Assays were carried out as per the manufacturer’s recommended protocol. The PKC activity (specific for Ca2+-dependent isoforms) was measured following partial purification on DEAE-Sephacel microcolumns using a kit from Invitrogen. The assay system employs a PKC-specific peptide substrate and a pseudosubstrate inhibitor in 50-μl volumes using 1 μCi of [γ-32P]ATP.
Electrophoresis of Proteins and Immunoblot Analysis
Proteins were subjected to electrophoresis on 12–18% SDS-polyacrylamide gels (28). The conditions for immunoblot analysis of proteins were similar to those described before (12). The immunoblots were developed using the Super Signal ULTRA chemiluminescent substrate kit from Pierce. The blots were imaged and quantified in a Bio-Rad Fluor-S imaging system.
Developing Holoenzyme Antibody to CcO
CcO enzyme from beef heart mitochondria was purified using a combination of (NH4)2SO4 fractionation and DEAE chromatography (29). New Zealand female rabbit (2.0 kg) was immunized by subcutaneous injection with 200 μg of CcO in complete Freund’s adjuvant using standard procedures. The total serum proteins were fractionated with (NH4)2SO4 for enriching the IgG fraction (30). The polyclonal antibody interacted with at least 11 of the 13 subunits and immunoprecipitated intact CcO containing all 13 subunits (results not shown).
Assay of ROS Production
Mitochondrial oxidative stress was evaluated by superoxide production using two different methods for detection of ROS. The lucigenin-enhanced chemiluminescence method was used as described by Li et al. (31), using a Turner Designs TD-20/20 luminometer. Briefly, freshly isolated mitoplasts or SMP (100–200 μg of protein) were used in a 200-μl assay system containing 0.5 mM NAD(P)H with or without added 0.1 mM reduced ferrocytochrome c as electron donor, and inhibitors of respiratory complexes, rotenone (2.5 μm), antimycin A (10 μm), and myxothiazol (10 μm), for the assay of ROS. Chemiluminescence was initiated by adding 5 μm lucigenin, and light emission was recorded every 60 s for 5–10 min and was expressed as mean arbitrary light unit per min per mg of protein as described before (32). It was shown previously that at the substrate level of 5 μm used, the nonspecific signal due to redox recycling of lucigenin is insignificant (31).
ROS generation was also measured by the dichlorofluorescein fluorescence method modified from LeBel et al. (33). Stable nonfluorescent DCF-DA (1 mM in methanol) was used in a 1-ml assay mixture containing reconstituted membrane vesicles as described above. In whole cells, DCF-DA is hydrolyzed by cellular esterases to a nonfluorescent DCF. DCF is then rapidly oxidized by peroxy radicals to highly fluorescent dichlorofluorescein. Because mitochondria or reconstituted membrane vesicles may have limited esterase activity to convert DCF-DA to DCF, we have used a partially purified enzyme fraction from rat brain cytosol (10 μg of protein/ml of reaction). The cytosolic enzyme was purified by (NH4)2SO4 fractionation (45–80% saturation) followed by dialysis for 16 h. Appropriate controls were used to normalize the effect of cytosol alone on ROS generation. In some cases, the ROS were also measured in the presence of SOD (30 units/ml) or catalase (10 units/ml), both from Sigma. The fluorescence was recorded by using a spectrofluorometer LPS-220B from Photon Technology International at an excitation at 488 nm and emission at 525 nm for 20 min.
Assay of Other Respiratory Complexes
Respiratory complex I (NADH:ubiquinone oxidoreductase), complex II (succinate:ubiquinone oxidoreductase), and complex III (ubiquinol:ferrocytochrome c oxidoreductase) activities were measured in the freshly prepared mitochondrial membrane fraction from macrophages or rabbit heart essentially as described by Birch-Machin and Turnbull (34).
Statistical Analysis
Data from the cultured macrophages and isolated heart studies were presented as means ± S.D. Differences between paired variables were determined using two-tailed Student’s t tests for paired data. Differences among nonpaired variables were evaluated using a single factor analysis of variance followed by individual comparisons using two-tailed Student’s t test for nonpaired data. p values <0.05 were considered statistically significant.
RESULTS
Effects of cAMP and PKA Inhibitors on CcO Activity in Cultured Macrophages
Ours and a number of other studies reported altered CcO activity in cells subjected to hypoxia (6, 11). In this study we evaluated the possible role of protein phosphorylation in altered enzyme activity in cultured macrophages. Initially, CcO activity was assayed by the DAB substrate method using digitonin-permeabilized cells. This system allowed the assaying of CcO activity under membrane lipid conditions that closely mimic in vivo conditions. A low concentration of digitonin (23) used for cell permeabilization inflicts minimal damage to the inner membrane-associated enzyme complex and the lipid environment. It is seen from Fig. 1A that treatment with H89 (50 nm) and MPI (50 nm), both specific inhibitors of PKA or Go6850 (10 nm), which inhibits the α, β, δ, and ζ forms of PKC, had no significant effect on the CcO activity in cells grown under normoxic conditions. Under identical assay conditions, cells grown under hypoxia for 10 h showed about 55% inhibition of CcO activity, which was nearly completely reversed by added H89 or MPI (50 nm each). Go6850 (10 nm), however, had no detectable effect on the hypoxia-mediated inhibition of CcO activity.
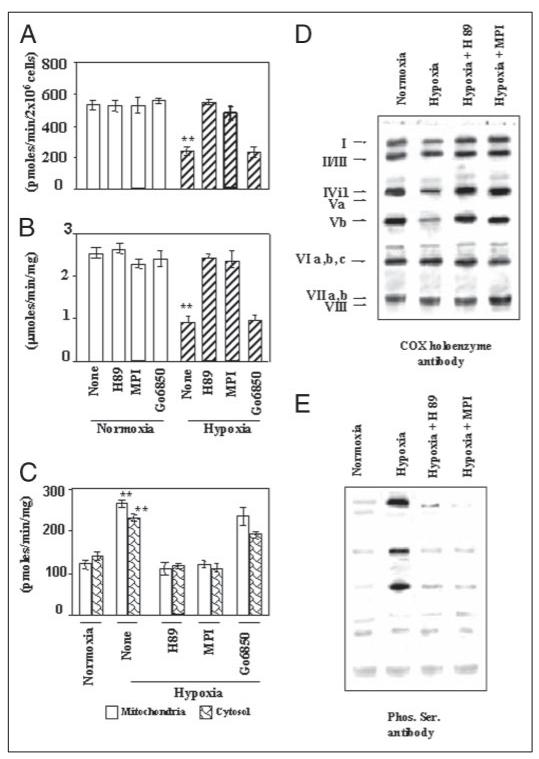
Figure 1. Inhibition of CcO activity in macrophages during hypoxia and reversal by PKA inhibitors H89 and MPI.
RAW 264.7 mouse monocyte macrophages were grown under normoxia or hypoxia (0.1% O2 for 10 h) with or without added H89 or MPI (50 nm each) or 10 nm Go6850 and used for assays. A, CcO activity was measured in permeabilized macrophages grown under normoxia or hypoxia using the DAB method. B, CcO activity was measured spectrophotometrically following the oxidation of cytochrome c in enzyme reconstituted in asolectin-cardiolipin vesicles as described under “Materials and Methods.” C, PKA activity was measured in mitochondria and cytosol from macrophages grown under different conditions. D and E, CcO enzyme from cells grown under different conditions was solubilized in 1.5% sodium cholate and immunoprecipitated with the holoenzyme antibody. Equal amounts of immunoprecipitates were resolved on two companion gels, and the proteins were probed with polyclonal antibody against CcO (D) and antibody to Ser phosphate (E). The conditions for isolation of mitochondria, immunoprecipitation, and immunoblotting were as described under “Materials and Methods.” A–C, the average ± S.D. were calculated from 3 to 4 separate assays. Asterisks indicate significant differences (**, p =<0.01).
A significant change in cardiolipin content of mitochondrial inner membrane during ischemia (35) has been implicated as an important cause of altered CcO activity during ischemia/reperfusion. With a view to clearly resolve the contribution of altered cardiolipin content on enzyme activity from those associated with protein modification, we used the liposome-reconstituted system (27). This system allows activity measurements of enzyme reconstituted in liposomes with defined cardiolipin composition. Fig. 1B shows that H89 or MPI (50 nm each) had no inhibitory effect on reconstituted enzyme from normoxic cells, whereas Go6850 had a marginal inhibitory effect. Enzyme from hypoxia-grown cells, however, showed nearly 60% inhibition of activity, which was nearly completely reversed by added H89 or MPI. Go6850, on the other hand, did not significantly reverse the hypoxia-mediated inhibition. Consistent with the H89- and MPI-mediated reversal of CcO activity, the PKA activity was induced 2–3-fold in both the cytosolic and mitochondrial fractions of cells subjected to hypoxia (Fig. 1C). The results also showed that H89 or MPI included in the culture medium markedly reduced the PKA activity, whereas Go6850 had only a marginal effect. These results showed that mitochondrial CcO activity is markedly inhibited during hypoxia, and that at least part of this inhibitory effect was probably because of direct changes in subunit composition or modification.
The possible subunit modification during hypoxia was investigated by immunoprecipitation of the CcO complex with holoenzyme antibody, which immunoprecipitates the intact or nearly intact complex. The immunoprecipitated complex was further analyzed for subunit phosphorylation. In Fig. 1, D and E, the immunoprecipitated CcO complexes from normoxic or hypoxic cells were subjected to SDS-PAGE, and two identical blots were probed either with CcO holoenzyme antibody (Fig. 1D) or antibody to Ser phosphate (Fig. 1E). As shown in Fig. 1E, 1st lane, the enzyme complex from normoxic cells resolved into multiple components. In the gel system used, subunits II and III, subunits VIa, -b, and -c, and subunits VIIa and -b and VIII comigrated forming complex bands. Additionally, the antibody interacted poorly with subunit Va such that a minor band was visible only when the gels were overloaded. Results also showed that the complex immunoprecipitated from hypoxic cells showed notable differences, including significantly lower contents of subunits I, IVi1, and Vb. Furthermore, the hypoxia-mediated reduction in these subunits was nearly completely reversed by H89 or MPI (50 nm each), suggesting a possible role for PKA-mediated phosphorylation. The immunoblot in Fig. 1E, probed with antibody to Ser phosphate, shows no detectable phosphorylation of CcO subunits from normoxic cells, whereas subunits I, IVi1, and Vb were found hyperphosphorylated in CcO from cells exposed to hypoxia. Furthermore, H89 and MPI (50 nm each) markedly reduced the level of phosphorylation of these subunits, confirming the role of PKA. These results suggested that the reduced CcO activity during hypoxia is related to reversible phosphorylation of subunits I, IVi1, and Vb and possible changes in subunit stoichiometry.
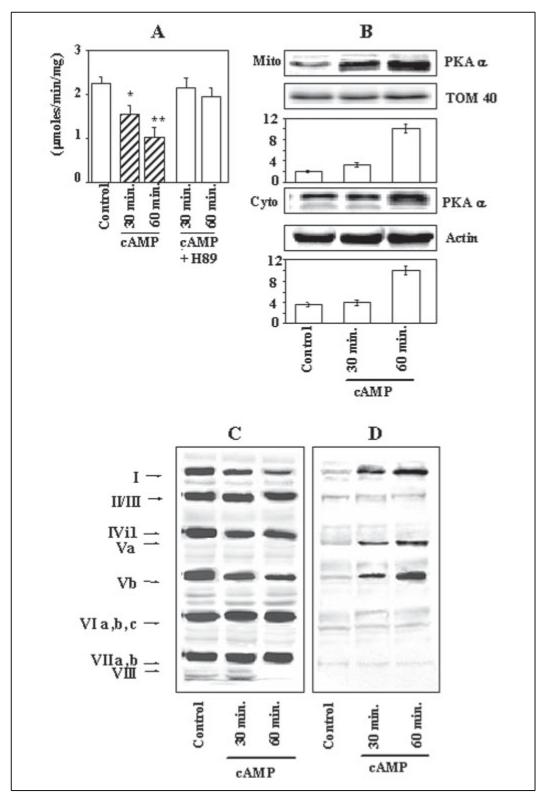
The role of PKA-mediated phosphorylation on CcO activity was further evaluated by increasing the cellular cAMP level in cultured macrophage cells. As shown in Fig. 2A, the CcO activity was progressively inhibited by dibutyryl cAMP (100 μm) by about 38 and 60%, respectively, at 30 and 60 min of exposure. Cultures supplemented with 50 nm H89, on the other hand, showed only marginal inhibition of 5 and 15%, respectively. Fig. 2B shows that under these growth conditions both the mitochondrial and cytosolic levels of PKAα, the catalytic subunit, were increased in a time-dependent manner, with a nearly 5-fold increase at the 60-min time period. Results of immunoprecipitation of the CcO complex with holoenzyme antibody showed that similar to what was shown in hypoxia-grown cells, addition of dibutyryl cAMP caused a selective subunit reduction and subunit phosphorylation. Fig. 2C shows that the levels of subunits I and Vb were reduced markedly in a timedependent manner, whereas subunit IVi1 was reduced marginally. The immunoblot with antibody to Ser phosphate (Fig. 2D) shows that subunits I, IVi1, and Vb were hyperphosphorylated, and the level of phosphorylation increased with increasing time of treatment with dibutyryl cAMP. These results confirmed that increased PKA levels that cause hyperphosphorylation of CcO subunits lead to inhibition of enzyme activity.
Figure 2. cAMP-mediated inhibition of CcO activity and reversal by H89 in murine macrophages.
Mitochondria (Mito) and cytosol from macrophages grown in presence or absence of 100 mM dibutyryl cAMP for 30 and 60 min were assayed for CcO activity and used for immunoprecipitation studies. A, CcO activity in reconstituted vesicles was measured as described in Fig. 1. B, the level of PKAα subunit was measured by immunoblot analysis of mitochondrial and cytosolic proteins (30 μg of protein each), and the blots were quantified by imaging through a Bio-Rad VersaDoc imaging system. The band intensities are presented as arbitrary absorbance units. C and D, CcO solubilized from mitochondria in 1.5% sodium cholate was immunoprecipitated and divided into two equal parts. Each part was resolved on two companion gels and subjected to immunoblot analysis with holoenzyme antibody (C) and Ser phosphate antibody (D). A and B, the values represent average ± S.D. of 3-4 independent estimates. Asterisks indicate significant differences (*, p < 0.05; **, p =<0.01).
Inhibition of CcO Activity during Ischemic Injury through a PKA-mediated Process
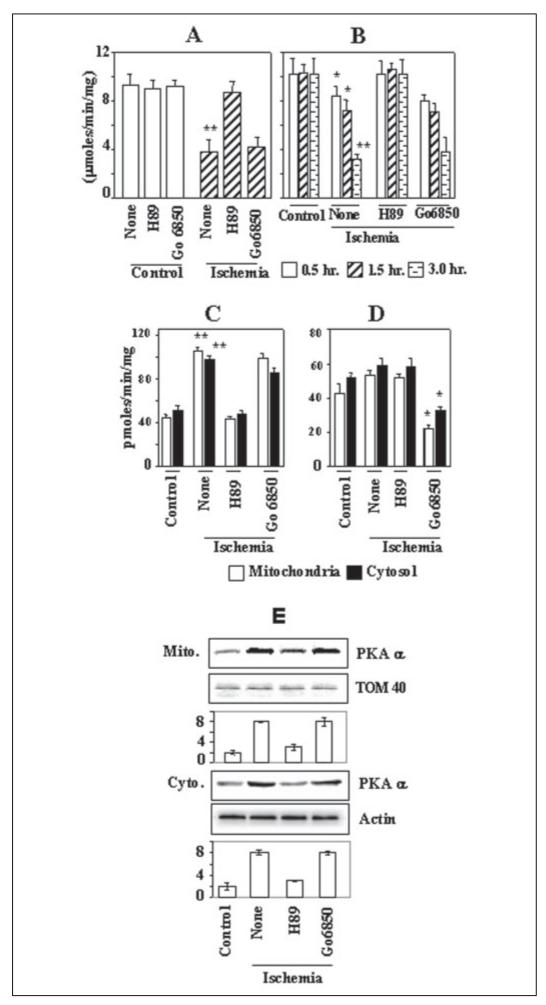
The effects of ischemia in the myocardial tissue on CcO activity were studied using in vitro perfused rabbit hearts subjected to focal ischemia by anterior coronary artery occlusion or global ischemia as described under “Materials and Methods” (21). The region of damage was identified with the snare in place by the lack of staining with india ink and was excised for enzyme activity. The sham-control hearts showed no indication of ischemic damage after 3 h of perfusion (data not shown). Fig. 3A shows that in rabbit hearts subjected to global ischemia for 20 min, the CcO activity was inhibited by about 60% of sham controls. Most interestingly, inclusion of 50 nm H89, a PKA inhibitor, markedly reversed the CcO activity, although the PKC inhibitor Go6850 had no significant effect. A similar protective effect of H89 on CcO activity during focal ischemia was observed (Fig. 3B). Hearts subjected to focal ischemia showed a 15–70% inhibition of CcO activity at 0.5–3.0 h of ischemia. 50 nm H89 added to the perfusion medium rendered complete protection, whereas the PKC inhibitor Go6850 had no effect. Although not shown, the PKA inhibitor MPI also yielded a protective effect similar to that observed with H89. Additionally, similar to that observed in macrophages grown under hypoxia, both the mitochondrial and cytosolic PKA activities in the heart tissue were increased nearly 2-fold during ischemia (Fig. 3C). As expected, in hearts pre-perfused with H89-containing buffer, the PKA activity was significantly lower. Results also showed that only a marginal increase of both cytosolic and mitochondrial PKC activity occurred in hearts subjected to ischemia (Fig. 3D), which was effectively inhibited by Go6850. Consistent with the PKA activities, the levels of catalytic subunit, PKAα, in both the mitochondrial and cytosolic compartments were increased by 4-fold in hearts subjected to focal ischemia (Fig. 3E). Most interestingly, inclusion of H89 in the perfusion medium prevented this increase. The reason for the observed action of H89 in modulating the PKAα subunit level remains unclear. These results demonstrate that CcO activity is altered in the myocardial tissue during ischemia probably through a PKA-dependent mechanism.
Figure 3. Inhibition of CcO activity in rabbit hearts subjected to focal or global ischemia.
Isolated rabbit hearts were subjected to global ischemia for 20 min (A) or focal ischemia for 0.5-3 h. In some cases 50 nm H89 or 10 nm Go6850 was added in the perfusion medium. At the end of in vitro perfusion and ischemia, tissue was excised, and subcellular fractions were isolated. CcO solubilized from SMP was assayed in reconstituted proteoliposomes as described under “Materials and Methods.” A, CcO activity during 20 min of global ischemia by reduced perfusion flow. B, focal ischemia by coronary occlusion and reperfusion for 0.5, 1.5, and 3.0 h. A and B, CcO activity was measured spectrophotometrically by following oxidation of reduced cytochrome c using enzyme reconstituted in lipid vesicles. C, PKA activity in mitochondria and cytosol; D, PKC activity in mitochondria and cytosol. E, the levels of PKAα subunit in the mitochondrial and cytosolic fractions of heart tissue. Immunoblot in E was carried out with 30 μg of protein in each case. The blots were reprobed with TOM40 antibody or actin antibody as loading controls for the mitochondrial and cytoplasmic proteins, respectively. A–D, the average values ± S.D. were calculated from 4 to 6 independent estimates. Asterisks indicate significant difference (*, p < 0.05; **, p < 0.01). Mito, mitochondria.
Phosphorylation State of CcO Subunits Under Hypoxia and Ischemia
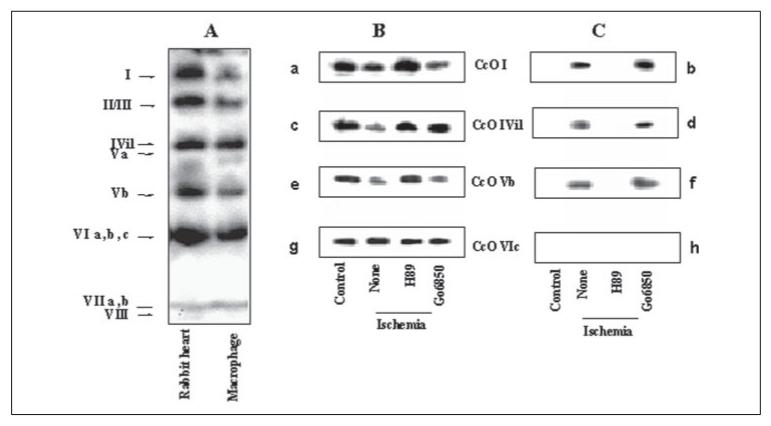
In Fig. 4A, cholate-solubilized CcO from rabbit heart and macrophages was immunoprecipitated with the holoenzyme antibody; the precipitated proteins were resolved on gradient polyacrylamide gel and analyzed by immunoblot by probing with CcO holoenzyme antibody. It is seen that the holoenzyme antibody immunoprecipitated intact or nearly intact complex from both macrophages and rabbit heart and cross-reacted with at least about 11 of the CcO subunits. In Fig. 4, B and C, immunoprecipitated complexes from control rabbit hearts or from those subjected to global ischemia were analyzed for subunit phosphorylation using subunit-specific antibody or Ser phosphate antibodies. Two parallel blots were probed with subunit-specific antibodies (Fig. 4, B and C, panels a, c, e, and g) or Ser phosphate antibody (panels b, d, f, and h). It is seen that in heart tissue subjected to ischemia, the levels of subunits I, IVi1, and Vb are significantly reduced in the complex immunoprecipitated with the holoenzyme antibody. The levels were restored to near control levels in heart tissue pretreated with H89. The PKA inhibitor MPI also yielded a similar protective effect (results not shown). The level of subunit VIc on the other hand was unaffected. Results also show that all four subunits exhibited no detectable phosphorylation in control tissues. Phosphorylation of subunits I, IVi1, and Vb was either fully or partly inhibited by H89 but not by PKC inhibitor Go6850. These results showed that similar to that observed with hypoxia-grown macrophages, at least three subunits, namely I, IVi1, and Vb, are phosphorylated under in vivo conditions and that PKA plays a critical role in the hypoxia- and ischemia-mediated phosphorylation of CcO.
Figure 4. Phosphorylation status of CcO subunits from rabbit hearts subjected to global ischemia.
A, sodium cholate (1.5%)-solubilized CcO from control macrophages and rabbit hearts was immunoprecipitated with antibody to holoenzyme, and the immunoprecipitates were probed with the same holoenzyme antibody. B, CcO from hearts subjected to 20 min of global ischemia under different conditions was immunoprecipitated as in A, and equal amounts of the immunoprecipitates were probed with subunitspecific antibodies against CcO subunits I, IVi1, Vb and VIc (panels a, c, e, and g) in B, or with antibody against Ser phosphate (panels b, d, f, and h) in C. In each case, heart mitochondrial membranes solubilized with 1.5% sodium cholate (500 μg of protein each) were used for immunoprecipitation as described under “Materials and Methods.”
Increased ROS Production by CcO Enzyme during Hypoxia or Ischemia
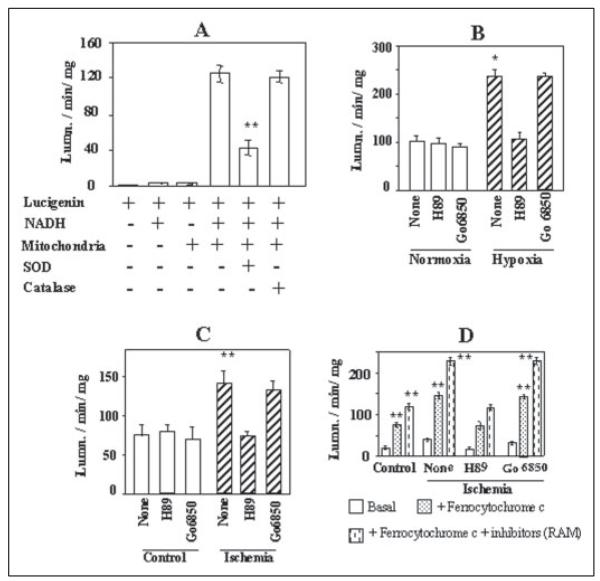
We determined the level of ROS production in swollen mitoplasts and liposome-reconstituted mitochondrial membranes by using the lucigenin method, which measures the level of O2. (superoxide radicals). We used 5 mM lucigenin substrate, which is known to produce minimal nonspecific signal due to redox recycling (31). Additionally, we used appropriate controls to ensure the specificity of the assay system. As shown in Fig. 5A, under our assay conditions lucigenin alone or with added NADH yielded very low signals. Also, the activity with swollen mitoplasts was dependent on added NADH. Furthermore, the activity was inhibited by added SOD but not by catalase, confirming that the system mainly detects radicals. Fig. 5B shows that mitochondria from cells grown under hypoxic conditions generated nearly 2.5-fold higher ROS, as compared with cells grown under normoxic conditions. Inhibitors of PKA and PKC had no significant effect on ROS production by control cell mitochondria. Most interestingly, H89 reversed the rate of mitochondrial ROS production in hypoxia-grown cells to near control cell levels, whereas Go6850 had only a marginal effect. Mitochondria from hearts subjected to global ischemia also generated a nearly 2-fold higher ROS as compared with sham-treated hearts (Fig. 5C). Perfusion with H89-containing buffer reduced the ROS production by about 50%, whereas PKC inhibitor Go6850 had no significant effect. Fig. 5D shows the rate of radical production by liposome-reconstituted mitochondrial membrane. It is seen that reduced cytochrome c added as an electron donor was able to induce a 2–4-fold higher ROS production in liposome-reconstituted CcO enzyme. Additionally, blocking the possible electron backflow from CcO to other complexes by adding a mixture of rotenone, antimycin, and myxothiazol further increased the ROS production by 30–60% in the reconstituted system. The reconstituted enzyme from the ischemic heart generated a 2-fold higher ROS as compared with enzyme from sham-treated controls (Fig. 5D). Most interestingly, increased ROS production by CcO from heart tissues subjected to hypoxic and ischemic injury can be effectively attenuated by PKA inhibitor H89 or MPI (latter results not shown). PKC inhibitor Go6850, on the other hand, exhibited no detectable reversal of ROS production. These results not only confirmed higher ROS production in mitochondria during hypoxia and ischemia but also provided new evidence for increased ROS production by CcO complex under these pathophysiological conditions.
Figure 5. Rate of production of mitochondrial ROS during hypoxia and ischemia.
A and B, digitonin-treated mitochondria from macrophages grown under normoxia or hypoxia were swollen by suspension in 50 mM KH2PO4 buffer and used for the assay.C, swollen mitoplasts from control heart and those subjected to 20 min of global ischemia were used for the assay. D, CcO from hearts subjected to ischemia and reconstituted in asolectin-cardiolipin vesicles were used for assay. ROS production (mostly O.2 radicals)in response to added NADH was measured by the lucigenin method. Ferrocytochrome c (100 μm), RAM inhibitors (2.5–10 μm), NADH (0.5 mM), SOD (30 units/ml), and catalase (10 units/ml) were added at the beginning of reaction before adding 5 mM lucigenin. Assays were run in 200-ml volumes and contained mitoplasts (100–200 μg) or reconstituted lipid vesicles (50–100 μg of protein) as enzyme source. Asterisks indicate significant differences (*, p < 0.05; **, p =<0.01).
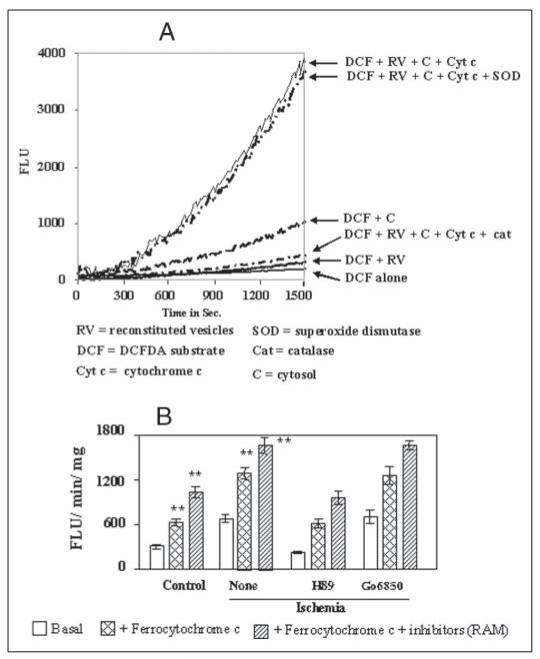
Because ROS production by CcO in different cell types is a controversial issue, we confirmed this possibility by using a more reliable DCF-DA method, which was modified to suit our in vitro assay method. DCF-DA is a stable, nonfluorescent molecule, which is required to be de-esterified by the intracellular esterases for reaction with H2O2 and other peroxy radicals to yield the fluorescence signal. We used an esterase-rich fraction from rat brain cytosol, which was partially purified by (NH4)2SO4 fractionation. This fraction also contains SOD activity for the conversion of radicals to H2O2· The control experiments in Fig. 6A show that DCF-DA alone or in the presence of added liposome-reconstituted CcO or the cytosolic fraction emitted very low fluorescence, indicating very low levels of H2O2 in the assay system. The liposome-reconstituted enzyme with added cytosol and reduced cytochrome c yielded a markedly higher rate of ROS formation. As expected, addition of SOD did not affect the activity of the complete system, whereas addition of catalase reduced the activity to the basal level. Fig. 6B shows the activity of reconstituted CcO from control and ischemic hearts under different experimental conditions. It is seen in Fig. 6B that the basal activity (DCF-DA + cytosol + reconstituted CcO) for ROS production was increased 2–3-fold in ischemic hearts as compared with control. The activities were further increased 2–3-fold by adding reduced cytochrome c as electron donor and the inhibitors that prevent backflow of electrons. Most interestingly, H89 added to the perfusion medium during ischemia attenuated ROS production below the control tissue level, whereas Go6850 had no effect. These results indeed confirmed that the CcO complex, hyperphosphorylated during hypoxia and ischemia, produces higher levels of ROS, which is attenuated by H89.
Figure 6. Measurement of ROS production by CcO using modified DCF-DA method.
A shows the characteristics of the modified DCF-DA method for in vitro assay of peroxy radicals generated by liposome-reconstituted CcO enzyme. The rate of fluorescence emission by reactions with added DCF-DA alone (1 μm), and those with added reconstituted CcO (RV) from control heart mitochondria (100 μg), reduced cytochrome c (100 mM), partially purified cytosolic protein fraction rich in esterase activity (10 μg/ml), catalase (10 units/ml), and SOD (30 units/ml) are shown. B, the rate of fluorescence at the linear range (between 600 and 1400 s from A) was integrated to obtain the fluorescence units/min/mg protein. CcO from control and ischemic hearts reconstituted in lipid vesicles as described under “Materials and Methods” and Fig. 1 were used in the assay. Results in B represent average ± S.D. of four independent assays. Asterisks indicate significant difference (**, p < 0.01).
Activities of Other Respiratory Complexes during Hypoxia and Ischemia
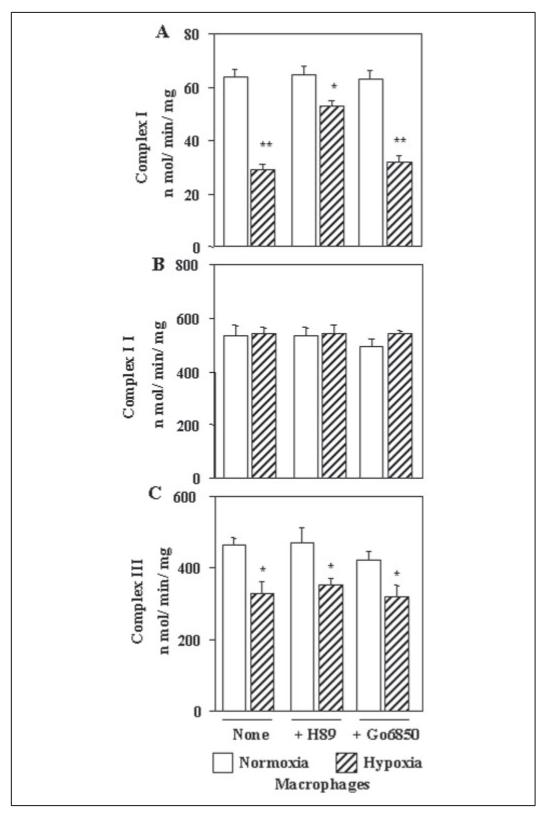
Fig. 7A shows that the NADH:ubiquinone oxidoreductase activity (complex I) is inhibited by about 50% in macrophages during hypoxia, which was restored to about 85% of control activity by H89. Go6850, on the other hand, had no significant protective effect. The succinate:ubiquinone oxidoreductase (complex II) was not affected during hypoxia (Fig. 7B). Both H89 and Go6850 added during hypoxia had no effect on the activity. The ubiquinol:ferrocytochrome c oxidoreductase activity (complex III) was inhibited by 25–30% during hypoxia that was not protected significantly either by H89 or Go6850 (Fig. 7C). These results showed that in addition to CcO, both complex I and complex III activities are inhibited during hypoxia and that H89 had a protective effect only on complex I.
Figure 7. Activities of other electron transport enzyme complexes in macrophages grown under hypoxic conditions.
Mitochondria from cells grown under normoxia or hypoxia were used for assays as described under “Materials and Methods.” A, NADH: ubiquinone oxidoreductase activity (complex I); B, succinate:ubiquinone oxidoreductase activity (complex II); and C, ubiquinol:ferrocytochrome c oxidoreductase activity (complex III). Values represent average ± S.D. of four independent assays. Asterisks indicate significant differences (*, p < 0.05; **, p =<0.01).
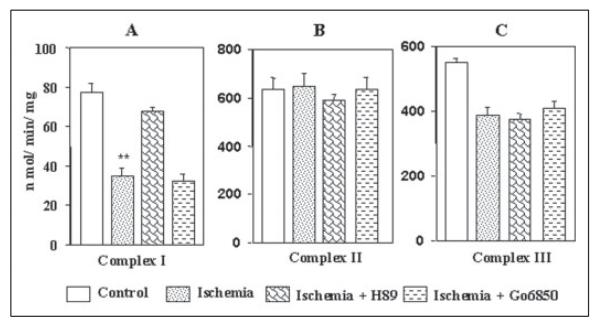
Fig. 8 shows that in rabbit hearts subjected to ischemia, the complex I activity was inhibited by about 60%, similar to that observed during hypoxia; H89 and MPI (latter results not shown) added to the perfusion medium, but not Go6850, provided a significant protective effect (Fig. 8A). The complex II activity was not affected significantly during global ischemia for 20 min. Results also showed that H89 added to the perfusion medium marginally inhibited complex II activity of hearts subjected to ischemia (Fig. 8B), but Go6850 had no effect. The reasons for the observed inhibitory effect of H89 on complex II remains unclear. Fig. 8C shows that the complex III activity is inhibited during ischemia by about 25–28%, which was not protected either by H89 or Go6850. Thus during both hypoxia and ischemia, mitochondrial complex I activity is affected that could be reversed by H89. Additionally, complex III activity is inhibited by about 25% in both experimental systems, which were not restored either by H89 or Go6850.
Figure 8. Activities of other electron transport enzyme complexes in hearts subjected to global ischemia.
Mitochondria from control hearts of those subjected to global ischemia for 20 min were used for assays as described in Fig. 7. Complex I activity (A), complex II activity (B), and complex III activity (C) values represent average ± S.D. of four independent assays. Asterisks indicate significant differences (**, p =<0.01).
The Role of PKA Inhibition on Heart Function and Necrosis during Ischemia/Reperfusion
Table 1 shows the base-line values and effects of 30 min of coronary occlusion on coronary flow, pulse pressure, and the electrocardiographic S-T segment. These values were derived from the analysis of 10 control hearts, 10 hearts pre-perfused with PKA inhibitor (H89 and MPI), and 6 hearts pre-perfused with Go6850 as listed in Table 2. Table 1 shows that as expected these parameters were changed by coronary occlusion. At 30 min of occlusion, coronary flow in the control group was significantly less at 83.3% of base-line level. Both H89/MPI and Go6850 groups showed a statistically significant decrease in flow (p = 0.006) ranging from 78.7 to 79.5 compared with their respective base-line values. Both the pulse pressure and S-T segment changes reflected the ischemic injury. The pulse pressure was reduced to 78.5–84.2% of base line across the groups. The ischemic S-T segment changes ranged from 0.38 to 0.52 mV. The difference in the S-T values was most significant in the PKA inhibitor-treated group (p = 0.002). Overall, the changes in these parameters indicated that the severity of the ischemia produced was comparable among the experimental groups. The decreases in coronary flow and the pulse pressure as well as the ischemic S-T segment changes during occlusion showed no differences among the groups by analysis of variance. This is also seen in Table 2 in that the volumes at risk were nearly similar for each group.
TABLE 1.
Coronary flow, pulse pressure, and S-T segment before and after 30 min of coronary occlusion
| Base line |
Coronary occlusion |
|||||
|---|---|---|---|---|---|---|
| Coronary flow | Pulse pressure | S-T segment | Coronary flow | Pulse pressure | S-T segment | |
| ml/min | mm Hg | μV | % base line | % base line | μ.V change | |
| Control | ||||||
| Mean | 37.8 | 63.7 | 0.21 | 83.3 | 84.2 | 0.38 |
| S.D. | 9.2 | 17.5 | 0.09 | 7.5 | 17.3 | 0.36 |
| p valuea | 0.0003 | 0.017 | 0.010 | |||
|
| ||||||
| +H89/MPIb | ||||||
| Mean | 40.2 | 73.2 | 0.16 | 79.5 | 78.5 | 0.52 |
| S.D. | 6.6 | 18.1 | 0.08 | 18.2 | 17.1 | 0.39 |
| p value | 0.006 | 0.012 | 0.002 | |||
|
| ||||||
| +Go6850 | ||||||
| Mean | 45.5 | 73.7 | 0.20 | 78.7 | 87.1 | 0.45 |
| S.D. | 7.4 | 17.5 | 0.16 | 10.2 | 8.9 | 0.30 |
| p value | 0.006 | 0.016 | 0.013 | |||
| p valuec | NSd | NS | NS | NS | NS | NS |
p value = paired t test versus base line.
MPI = four hearts perfused with myristoylated peptide inhibitor of PKA.
p value = analysis of variance across experimental groups (control, H89/MPI, and Go6850).
NS = no significant difference.
TABLE 2.
Myocardial ventricular volumes (cm3)
| Total | Risk | Necrotic | Necrotic/risk ratio |
|
|---|---|---|---|---|
| Control | ||||
| 1 | 10.946 | 1.107 | 0.683 | 0.618 |
| 2 | 9.743 | 1.336 | 0.660 | 0.494 |
| 3 | 13.488 | 2.805 | 1.253 | 0.447 |
| 4 | 9.191 | 0.943 | 0.517 | 0.549 |
| 5 | 9.870 | 1.061 | 0.636 | 0.599 |
| 6 | 9.003 | 1.401 | 0.885 | 0.632 |
| 7 | 8.255 | 0.418 | 0.325 | 0.778 |
| 8 | 9.114 | 1.365 | 0.894 | 0.655 |
| 9 | 5.735 | 0.356 | 0.288 | 0.809 |
| 10 | 10.136 | 1.281 | 1.115 | 0.871 |
| Mean | 9.548 | 1.207 | 0.726 | 0.645 |
| S.D. | 1.966 | 0.674 | 0.315 | 0.137 |
|
| ||||
| H89 or MPIa | ||||
| 1 | 10.884 | 0.805 | 0.412 | 0.512 |
| 2 | 7.757 | 0.285 | 0.122 | 0.426 |
| 3 | 8.661 | 1.684 | 0.400 | 0.237 |
| 4 | 9.716 | 1.173 | 0.486 | 0.399 |
| 5 | 7.881 | 0.580 | 0.162 | 0.280 |
| 6 | 10.866 | 0.550 | 0.249 | 0.452 |
| 7a | 7.072 | 2.188 | 0.535 | 0.244 |
| 8a | 8.014 | 1.329 | 0.689 | 0.518 |
| 9a | 7.100 | 0.263 | 0.121 | 0.461 |
| 10a | 10.337 | 4.398 | 1.662 | 0.378 |
| Mean | 8.829 | 1.326 | 0.484 | 0.391 |
| S.D. | 1.500 | 1.246 | 0.455 | 0.105 |
| p b | 0.370 | 0.795 | 0.184 | 0.0002 |
|
| ||||
| Go6850 | ||||
| 1 | 9.680 | 1.264 | 1.074 | 0.850 |
| 2 | 9.378 | 0.775 | 0.532 | 0.687 |
| 3 | 10.928 | 0.943 | 0.500 | 0.531 |
| 4 | 9.300 | 1.256 | 0.631 | 0.502 |
| 5 | 9.207 | 1.642 | 1.027 | 0.625 |
| 6 | 9.858 | 0.451 | 0.317 | 0.702 |
| Mean | 9.725 | 1.055 | 0.680 | 0.650 |
| S.D. | 0.638 | 0.421 | 0.305 | 0.127 |
| P | 0.836 | 0.629 | 0.782 | 0.951 |
| P c | 0.466 | 0.847 | 0.338 | 0.0001 |
MPI = hearts perfused with myristoylated peptide inhibitor of PKA.
p = value by nonpaired t test versus the control group.
p = value by analysis of variance across groups.
Although each group received the same degree of ischemia, the anatomical data of Table 2 confirm that prevention of phosphorylation of CcO by the PKA inhibitor H89 or MPI is protective. The ratio of the mean necrotic volume to volume at risk was significantly reduced by 40% compared with control (p = 0.0002). Less myocardial tissue became necrotic following reperfusion. This was not true for the hearts given the PKC blocker Go6850 (Table 2), where the values were similar to control values.
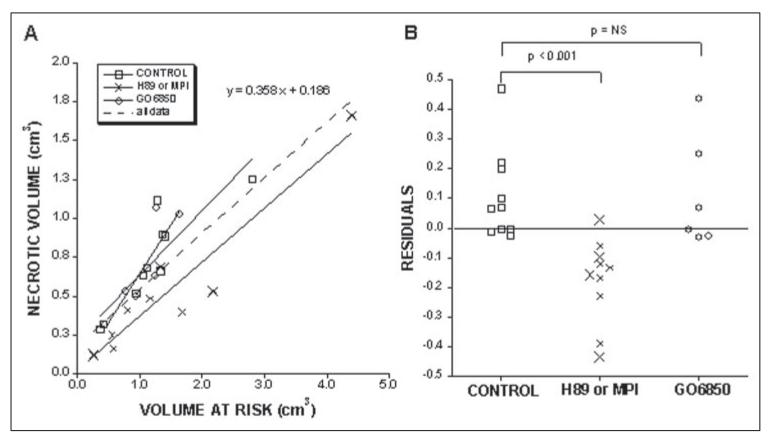
Because the size of the necrotic volume varies with the size of the risk volume (30), we applied another statistical test to the data. Linear regression was performed on the pooled data from the experimental groups relating the necrotic volume to the volume at risk (Fig. 9A). The residuals or the vertical distances of the individual data points from the pooled regression line were calculated for each group (Fig. 9B). An analysis of variance showed the residuals for the experimental groups to be significantly different with a p value of 0.001. The residuals were significantly different (less necrotic volume) between the H89/MPI group and the control group (p =<0.001 by nonpaired t test). There was no significant difference between the Go6850 group and the control group. Thus, blockage of PKA-mediated phosphorylation that is involved in the regulation of CcO activity provides protection against occlusionreperfusion injury.
Figure 9. The relationship of the necrotic volume to the volume at risk for the three experimental groups (A), and the residuals of the data points from the linear regression line of the pooled data (B).
The second experimental group (H89 or MPI) includes six hearts perfused with H89 (indicated by small ×) and four perfused with a myristoylated peptide inhibitor of PKA (indicated by the large ×). A, the solid lines are the regression lines for the individual groups. The dashed line is the regression line for the pooled data of all the groups. At the top is the equation for this line. B shows the residuals of the data points from this pooled line for each group. They were significantly different by analysis of variance (p < 0.001). The p values in B show the differences between individual groups using a nonpaired t test.
DISCUSSION
Mitochondrial dysfunction is an important contributing factor in myocardial injury during ischemia and reperfusion (13, 14). Progressive damage to the electron transport chain because of increased ROS production, Ca2+ overload, and selective depletion of cardiolipin are thought to be some of the causative factors. The initial phase of ischemia has been shown to accompany impaired activities of complex I and the ADP/ATP translocator. Prolonged ischemia and reperfusion injury are associated with dysfunction of other complexes, including CcO, and also vastly reduced oxidative phosphorylation, activation of permeability transition pore, and apoptotic cell death (36, 37). A number of studies have reported impaired CcO function during prolonged ischemia/reperfusion and hypoxia (6, 13, 14, 36). However, the precise mechanism of functional impairment of CcO under these pathophysiological conditions remains unclear. Results of this study show that PKA-mediated phosphorylation of subunits I, IVi1, and Vb during hypoxia and ischemia/reperfusion is the major cause of altered catalytic activity of the CcO enzyme. Notably, the results also show an increase in ROS production by phosphorylated CcO as a possible factor, which may contribute to irreversible myocardial injury.
Nearly 90% of respired O2 in the mammalian organisms is metabolized by CcO, and in most aerobic organisms the response to changes in O2 tension either through altered gene expression or altered energy metabolism is crucial to survival. In the yeast, CcO activity readily shifts in response to aerobic and anaerobic conditions by expression of CcO Va and Vb isologs, which are orthologs of the mammalian CcO IVi1 subunit (8). There is increasing evidence that as in the yeast system, the catalytic properties of mammalian CcO undergo subtle changes in response to altered O2 concentration as an adoptive response. Some studies also showed (6, 37) that under both in vitro (isolated enzyme) and in vivo (intact cells) conditions, CcO activity is reversibly decreased during exposure to prolonged or moderate hypoxia. Furthermore, the CcO activity (turnover number and Km for cytochrome c binding) in different tissues and compartments of the heart vary depending on the O2 content of tissues (12). In previous studies we observed reduced levels of subunits I, IVi1, and Vb in different rat tissues with varying O2 contents and experimental hypoxia induced by growth in low O2 levels or treatment with CoCl2 (19). In support of these observations, our present results show a time-dependent reduction of these same subunits under both hypoxia and ischemia. Although the precise mechanisms remain to be determined, it is likely that selective subunit phosphorylation may somehow be linked to reduction of subunits I, IVi1, and Vb under these pathophysiological conditions. We propose that subunit phosphorylation is an adoptive mechanism in the vertebrate system by which CcO activity is modulated in response to changing O2 tension.
The present study provides direct evidence for subunit modification by PKA-mediated phosphorylation as the basis for altered CcO activity in response to hypoxia and ischemia/reperfusion. First, in both macrophages exposed to hypoxia and rabbit hearts subjected to ischemia, mitochondrial PKA activity was increased in a time-dependent manner. In keeping with increased PKA activity, the levels of phosphorylation of subunits I, IVi1, and Vb increased under hypoxia and ischemia. Although the precise sites of phosphorylation under these conditions remain unclear, our results for the first time show changing phosphorylation levels under in vivo conditions. Increased PKA activity and subunit phosphorylation are accompanied by reduced CcO activity. Additionally, the PKA inhibitors, H89 and MPI, which reversed the phosphorylation status of subunits, also reversed the kinetic properties of CcO. An important observation of this study is that inclusion of PKA inhibitor in the perfusion media rendered protection against ischemic heart injury as seen by reduced necrotic volume (Table 2 and Fig. 9) and necrotic volume/risk ratio.
In both macrophages exposed to hypoxia and rabbit hearts subjected to ischemia, mitochondrial PKA activity was increased in a time-dependent manner at which time the levels of phosphorylation of CcO subunits I, IVi1, and Vb were also increased with concomitantly decreased CcO activity. Although externally added cAMP in the macrophage cell system caused increased mitochondrial PKA activity and increased phosphorylation of CcO subunits, it is not clear if the cAMP pathway is involved in the phosphorylation of CcO during ischemic myocardial injury. It is known that mitochondrial nitric-oxide synthase levls increase during hypoxia and ischemia/reperfusion, with increased production of NO (38, 39). An alternative and more likely possibility is that a NO-regulated soluble cGMP pathway is involved (20, 40). Recent studies indicate that the cAMP and cGMP can cross-activate PKG and PKA enzymes by an unknown mechanism (41, 42). Notably, in both macrophages exposed to hypoxia (Fig. 2B) and rabbit hearts subjected to ischemia (Fig. 3E), there is a marked increase in the cellular PKAα levels. It is therefore likely that mitochondrial NO or ROS may play a direct role in both the overexpression of PKA and its activation.
A previous study on the perfused dog heart system (43) showed that cardioprotective effect of the phosphodiesterase III inhibitor, olprinone, probably involved the action of PKA and p38 mitogen-activated protein kinase, although PKA by itself had no cardioprotective effect. In this study (43), the drug was administered for 30 min and removed before commencing ischemia by coronary occlusion, a condition quite different from the continuous perfusion with medium containing the inhibitors we have used. As indicated in our study, the mitochondrial PKA activity increased during ischemia/reperfusion, and it is therefore likely that the presence of the drug during this critical period is necessary for an effective pharmacological outcome.
In various species, including humans, brief ischemic episodes also render protection to the heart against subsequent ischemic damage through a process termed ischemic preconditioning (44, 45). There is evidence that activation and translocation of PKC and the subsequent activation of mitochondrial KATP channels play an important role in early phase ischemic preconditioning (46, 47). Our finding that inhibition of PKC does not alter the degree of ischemia/reperfusion damage suggests that the protection afforded by PKA blockade may be at least to some degree independent of the classic ischemic preconditioning pathway. A previous study (48) reported that after 30 min of reperfusion with the PKC inhibitor chelerythrine or Go6850, relaxation in rabbit heart was significantly impaired as indicated by increased diastolic pressure and reduced –dp/dt. Treatment with PKC inhibitor did not affect coronary flow or myocardial O2 consumption in post-ischemic heart and had no significant impact on myocardial infarct size. Our results essentially support these observations.
ROS play important roles in various physiological and pathological processes. It is believed that increased ROS production during hypoxia and ischemia is a contributory factor in both reversible and irreversible myocardial injury (49). Although various mitochondrial electron transport complexes are known to be important sites of ROS production, the role of CcO in this process has been controversial for some time (50-52). Therefore, in this study we wanted to address this question using a reconstituted system specific for CcO. A number of studies have shown that liposome-reconstituted CcO can accept electrons only from reduced cytochrome c or ascorbate (25). We reasoned that use of cytochrome c as an electron donor in liposome-reconstituted CcO could provide a definitive answer on the role of this electron transport chain complex in generating ROS. Results show that reconstituted CcO indeed generates ROS in a reduced cytochrome c-dependent manner. Notably, CcO from cells subjected to hypoxia and heart tissue subjected to ischemia showed nearly 2-fold higher levels of ROS formation. Although not shown, CcO from macrophages grown in the presence of dibutyryl cAMP also generated increased ROS. Furthermore, ROS production under these experimental conditions was reversed to control levels by pretreatment with the PKA inhibitor H89. These results are consistent with the possibility that hyperphosphorylated CcO with lower enzyme activity generates increased levels of ROS. Experiments with mitochondrial preparations using specific inhibitors suggest (results not shown) that CcO contributes to about 30–35% of total mitochondrial ROS production during hypoxic and ischemic injury. Our results not only show that CcO is an important site of ROS production but also that the rate of ROS production at this site is modulated by protein phosphorylation.
Because PKA is known to have many molecular targets in the myocardial tissue, it is likely that the observed protective effect of H89 and MPI may involve altered function/activity of many enzymes and proteins, including CcO, and complex I. However, the use of this system has enabled us to conclusively demonstrate the molecular basis for altered CcO activity during ischemia and its possible contribution to ischemic injury. These results may also help design more selective inhibitors of mitochondrial PKA or selective targeting to the mitochondrial compartment as possible therapeutic strategies for treating or minimizing ischemic heart injury.
Acknowledgments
We thank Kenneth Fitzgerald and Nick Galati for expert technical help. We also thank the members of the Avadhani laboratory for valuable discussions and suggestions.
Footnotes
The abbreviations used are: used: CcO, cytochrome c oxidase; ROS, reactive O2 species; PKA, protein kinase A; PKC, protein kinase C; MPI, myristoylated peptide inhibitor;SMP, submitochondrial particles; DCF-DA, 2[H11032],7[H11032]-dichlorofluorescein diacetate; DAB, 3,3[H11032]-diaminobenzidine-tetrachloride; SOD, superoxide dismutase.
This work was supported in part by National Institutes of Health Grant GM-49683 (to N. G. A.) and an American Heart Association grant (to J. F. S.). The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Chandel N, Budinger GR, Kemp RA, Schumacker PT. Am. J. Physiol. 1995;268:L918–L925. doi: 10.1152/ajplung.1995.268.6.L918. [DOI] [PubMed] [Google Scholar]
- 2.Chandel NS, Schumacker PT. J. Appl. Physiol. 2000;88:1880–1889. doi: 10.1152/jappl.2000.88.5.1880. [DOI] [PubMed] [Google Scholar]
- 3.Dagsgaard C, Taylor LE, O’Brien KM, Poyton RO. J. Biol. Chem. 2001;276:7593–7601. doi: 10.1074/jbc.M009180200. [DOI] [PubMed] [Google Scholar]
- 4.Merle P, Kadenbach B. Eur. J. Biochem. 1982;125:239–244. doi: 10.1111/j.1432-1033.1982.tb06674.x. [DOI] [PubMed] [Google Scholar]
- 5.Poyton RO, Dagsgaard CJ. Adv. Exp. Med. Biol. 2000;475:177–184. doi: 10.1007/0-306-46825-5_17. [DOI] [PubMed] [Google Scholar]
- 6.Vijayasarathy C, Damle S, Prabu SK, Otto CM, Avadhani NG. Eur. J. Biochem. 2003;270:871–879. doi: 10.1046/j.1432-1033.2003.03447.x. [DOI] [PubMed] [Google Scholar]
- 7.Capaldi RA. Annu. Rev. Biochem. 1990;59:569–596. doi: 10.1146/annurev.bi.59.070190.003033. [DOI] [PubMed] [Google Scholar]
- 8.Burke PV, Poyton RO. J. Exp. Biol. 1998;201:1163–1175. doi: 10.1242/jeb.201.8.1163. [DOI] [PubMed] [Google Scholar]
- 9.Turcotte ML, Parliament M, Franko A, Allalunis-Turner J. Br. J. Cancer. 2002;86:619–624. doi: 10.1038/sj.bjc.6600087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chandel NS, Budinger GR, Choe SH, Schumacker PT. J. Biol. Chem. 1997;272:18808–18816. doi: 10.1074/jbc.272.30.18808. [DOI] [PubMed] [Google Scholar]
- 11.Giordano FJ. J. Clin. Investig. 2005;115:500–508. doi: 10.1172/JCI200524408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vijayasarathy C, Biunno I, Lenka N, Yang M, Basu A, Hall IP, Avadhani NG. Biochim. Biophys. Acta. 1998;1371:71–82. doi: 10.1016/s0005-2736(97)00278-2. [DOI] [PubMed] [Google Scholar]
- 13.Lesnefsky EJ, Tandler B, Ye J, Slabe TJ, Turkaly J, Hoppel CL. Am. J. Physiol. 1997;273:H1544–H1554. doi: 10.1152/ajpheart.1997.273.3.H1544. [DOI] [PubMed] [Google Scholar]
- 14.Lesnefsky EJ, Hoppel CL. Arch. Biochem. Biophys. 2003;420:287–297. doi: 10.1016/j.abb.2003.09.046. [DOI] [PubMed] [Google Scholar]
- 15.Lasley RD, Noble MA, Mentzer RM., Jr. J. Mol. Cell. Cardiol. 1997;29:3345–3356. doi: 10.1006/jmcc.1997.0559. [DOI] [PubMed] [Google Scholar]
- 16.Sardanelli AM, Technikova-Dobrova Z, Speranza F, Mazzocca A, Scacco S, Papa S. FEBS Lett. 1996;396:276–278. doi: 10.1016/0014-5793(96)01112-x. [DOI] [PubMed] [Google Scholar]
- 17.Scacco S, Vergari R, Scarpulla RC, Technikova-Dobrova Z, Sardanelli A, Lambo R, Lorusso V, Papa S. J. Biol. Chem. 2000;275:17578–17582. doi: 10.1074/jbc.M001174200. [DOI] [PubMed] [Google Scholar]
- 18.Bender E, Kadenbach B. FEBS Lett. 2000;466:130–134. doi: 10.1016/s0014-5793(99)01773-1. [DOI] [PubMed] [Google Scholar]
- 19.Vijayasarathy C, Damle S, Lenka N, Avadhani NG. Eur. J. Biochem. 1999;266:191–200. doi: 10.1046/j.1432-1327.1999.00843.x. [DOI] [PubMed] [Google Scholar]
- 20.Shiva S, Oh JY, Landar AL, Ulasova E, Venkatraman A, Bailey SM, Darley-Usmar VM. Free Radic. Biol. Med. 2005;38:297–306. doi: 10.1016/j.freeradbiomed.2004.10.037. [DOI] [PubMed] [Google Scholar]
- 21.Spear JF, Moore EN. J. Cardiovasc. Pharmacol. 2002;39:761–776. doi: 10.1097/00005344-200205000-00018. [DOI] [PubMed] [Google Scholar]
- 22.Bhat NK, Niranjan BG, Avadhani NG. Biochemistry. 1982;21:2452–2460. doi: 10.1021/bi00539a026. [DOI] [PubMed] [Google Scholar]
- 23.Chrzanowska-Lightowers ZMA, Turnbull DM, Nightowlers RN. Anal. Biochem. 1993;214:45–49. doi: 10.1006/abio.1993.1454. [DOI] [PubMed] [Google Scholar]
- 24.Arnold S, Kadenbach B. FEBS Lett. 1999;443:105–108. doi: 10.1016/s0014-5793(98)01694-9. [DOI] [PubMed] [Google Scholar]
- 25.Buge U, Kadenbach B. Eur. J. Biochem. 1986;161:383–390. doi: 10.1111/j.1432-1033.1986.tb10457.x. [DOI] [PubMed] [Google Scholar]
- 26.Casey RP, Ariano BH, Azzi A. Eur. J. Biochem. 1982;122:313–318. doi: 10.1111/j.1432-1033.1982.tb05882.x. [DOI] [PubMed] [Google Scholar]
- 27.Napiwotzki J, Kadenbach B. Biol. Chem. 1998;379:335–339. doi: 10.1515/bchm.1998.379.3.335. [DOI] [PubMed] [Google Scholar]
- 28.Laemmli UK. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 29.Fuller SD, Capaldi RA, Henderson R. Biochemistry. 1982;21:2525–2529. doi: 10.1021/bi00539a036. [DOI] [PubMed] [Google Scholar]
- 30.Boopathi E, Lenka N, Prabu SK, Fang JK, Wilkinson F, Atchison M, Giallongo A, Avadhani NG. J. Biol. Chem. 2004;279:35242–35254. doi: 10.1074/jbc.M403160200. [DOI] [PubMed] [Google Scholar]
- 31.Li Y, Zhu H, Kuppusamy P, Roubaud V, Zweier JL, Trush MA. J. Biol. Chem. 1998;273:2015–2023. doi: 10.1074/jbc.273.4.2015. [DOI] [PubMed] [Google Scholar]
- 32.Raza H, Prabu SK, Robin MA, Avadhani NG. Diabetes. 2004;53:185–194. doi: 10.2337/diabetes.53.1.185. [DOI] [PubMed] [Google Scholar]
- 33.LeBel CP, Ali SF, McKee M, Bondy SC. Toxicol. Appl. Pharmacol. 1990;104:17–24. doi: 10.1016/0041-008x(90)90278-3. [DOI] [PubMed] [Google Scholar]
- 34.Birch-Machin MA, Turnbull DM. Methods Cell Biol. 2001;65:97–117. doi: 10.1016/s0091-679x(01)65006-4. [DOI] [PubMed] [Google Scholar]
- 35.Paradies G, Petrosillo G, Pistolese M, Ruggiero FM. FEBS Lett. 2000;466:323–326. doi: 10.1016/s0014-5793(00)01082-6. [DOI] [PubMed] [Google Scholar]
- 36.Lesnefsky EJ, Chen Q, Moghaddas S, Hassan MO, Tandler B, Hoppel CL. J. Biol. Chem. 2004;279:47961–47967. doi: 10.1074/jbc.M409720200. [DOI] [PubMed] [Google Scholar]
- 37.Chandel NS, Budinger GR, Schumacker PT. J. Biol. Chem. 1996;271:18672–18677. doi: 10.1074/jbc.271.31.18672. [DOI] [PubMed] [Google Scholar]
- 38.Maejima Y, Adachi S, Ito H, Nobori K, Tamamori-Adachi M, Isobe M. Cardiovasc. Res. 2003;59:268–270. doi: 10.1016/s0008-6363(03)00425-5. [DOI] [PubMed] [Google Scholar]
- 39.Borutaite V, Moncada S, Brown GC. Shock. 2005;23:319–323. doi: 10.1097/01.shk.0000156672.36439.2d. [DOI] [PubMed] [Google Scholar]
- 40.Nisoli E, Clementi E, Paolucci C, Cozzi V, Tonello C, Sciorati C, Bracale R, Valerio A, Francolini M, Moncada S, Carruba MO. Science. 2003;299:896–899. doi: 10.1126/science.1079368. [DOI] [PubMed] [Google Scholar]
- 41.Cornwell TL, Arnold E, Boerth NJ, Lincoln TM. Am. J. Physiol. 1994;267:C1405–C1413. doi: 10.1152/ajpcell.1994.267.5.C1405. [DOI] [PubMed] [Google Scholar]
- 42.Barman SA, Zhu S, Han G, White RE. Am. J. Physiol. 2003;284:L1004–L1011. doi: 10.1152/ajplung.00295.2002. [DOI] [PubMed] [Google Scholar]
- 43.Sanada S, Kitakaze M, Papst PJ, Asanuma H, Node K, Takashima S, Asakura M, Ogita H, Liao Y, Sakata Y, Ogai A, Fukushima T, Yamada J, Shinozaki Y, Kuzuya T, Mori H, Terada N, Hori M. Circulation. 2001;104:705–710. doi: 10.1161/hc3201.092216. [DOI] [PubMed] [Google Scholar]
- 44.Murry CE, Jennings RB, Reimer KA. Circulation. 1986;74:1124–1136. doi: 10.1161/01.cir.74.5.1124. [DOI] [PubMed] [Google Scholar]
- 45.Tomai F, Crea F, Chiariello L, Gioffre PA. Circulation. 1999;100:559–563. doi: 10.1161/01.cir.100.5.559. [DOI] [PubMed] [Google Scholar]
- 46.Hu K, Duan D, Li GR, Nattel S. Circ. Res. 1996;78:492–498. doi: 10.1161/01.res.78.3.492. [DOI] [PubMed] [Google Scholar]
- 47.Meldrum DR, Cleveland JC, Jr., Mitchell MB, Sheridan BC, Gamboni-Robertson F, Harken AH, Banerjee A. Am. J. Physiol. 1996;271:R718–R726. doi: 10.1152/ajpregu.1996.271.3.R718. [DOI] [PubMed] [Google Scholar]
- 48.Stamm C, Friehs I, Cowan DB, Cao-Danh H, Noria S, Munakata M, McGowan FX, Jr., del Nido PJ. Cardiovasc. Res. 2001;51:108–121. doi: 10.1016/s0008-6363(01)00249-8. [DOI] [PubMed] [Google Scholar]
- 49.Vanden Hoek TL, Li C, Shao Z, Schumacker PT, Becker LB. J. Mol. Cell. Cardiol. 1997;29:2571–2583. doi: 10.1006/jmcc.1997.0497. [DOI] [PubMed] [Google Scholar]
- 50.Chen Q, Vazquez EJ, Moghaddas S, Hoppel CL, Lesnefsky EJ. J. Biol. Chem. 2003;278:36027–36031. doi: 10.1074/jbc.M304854200. [DOI] [PubMed] [Google Scholar]
- 51.Lee I, Bender E, Kadenbach B. Mol. Cell. Biochem. 2002;234:63–70. [PubMed] [Google Scholar]
- 52.Turrens JF. J. Physiol. (Lond.) 2003;552:335–344. doi: 10.1113/jphysiol.2003.049478. [DOI] [PMC free article] [PubMed] [Google Scholar]