Abstract
The objectives of this study were to examine the seasonal changes in the risk of gastrointestinal (GI) illness of beachgoers in the tropics, to compare the association between GI illness and water quality using various indicator organisms, and to study other beach health hazards. A prospective cohort study during two seasonal periods (summer and autumn) was conducted in a beach surrounded by intensive residential development. Analyses demonstrated that although densities of indicators were well below water quality standards throughout the study, they were significantly higher during the autumn season. The incidence of GI illness among beachgoers was also higher during the rainy season. A higher incidence of GI illness was observed for bathers during the autumn season when compared to non-bathers, while a somewhat lower incidence was observed during the summer. This study showed that rainfall contributes to higher levels of microbial contaminants and GI risk to beachgoers. The association between GI illness and Enterococcus using culture counts showed the highest odds ratio among all indicator parameters including those using molecular methods. A much higher risk of GI illness among children under 5 years was observed among all beachgoers.
Keywords: Bacteroidales, cohort, Enterococcus, gastrointestinal illness, qPCR, tropical climate
INTRODUCTION
Many of the world's coasts are becoming increasingly urban. In Puerto Rico, the urbanization of coasts has brought with it demands for fresh water and sewage treatment, as well as damage to coastal ecosystems. As coastal communities grow, sewage can become a threat to local waterways. Studies have shown that populations living near the coast may increase the chance of water pollution with urban, industrial wastes and sewage effluents with increased levels of pathogenic agents (Corbett et al. 1993; Crowther et al. 2001). Also, regional domestic sewage outlets and urban runoff problems potentiate beach contamination (Turbow et al. 2003). Exposure to sewage-contaminated water can cause infections and transmit diseases, particularly among children under 5 years (Creel 2003).
Other factors that promote survival and dispersion of pathogens in the beach include tidal phenomena, the presence of animals, and bather load, among others (Pan American Health Organization (PAHO) 2003; WHO 2003).
Climatic events also are a major factor in assessing the level of risk to bathers of recreational water illness. Fecal indicator bacteria and pathogen concentrations are influenced, in part, by their flux from the land, which is exacerbated during rainfall (Walters et al. 2010). Levels of microorganisms in water can increase by several orders of magnitude after as little as 2 cm of rainfall (Schwab 2007).
Extensive research with the aim of establishing guidelines and standards for recreational water quality has been conducted all over the world (Elmanama et al. 2005). In Puerto Rico, the Environmental Quality Board (EQB), under the Beach Monitoring and Notification Program, has the responsibility of performing bacteriological monitoring on the beaches most used by bathers. This program was created by the United States (US) Federal Government through the Beaches Environmental Assessment and Coastal Health Act (BEACH Act), which was promulgated by US Congress in 2000 (United States Environmental Protection Agency (USEPA) 2007). Bacteriological monitoring consists of taking samples of marine water to look for fecal coliforms and Enterococcus densities, which are indicators of fecal contamination. The Water Quality Standards Regulation of the EQB establishes: (1) the fecal coliform geometric mean of a series of representative samples (at least five samples) of the waters taken sequentially shall not exceed 200 colony forming units (CFU)/100 mL; and (2) the enterococci density in terms of geometric mean of at least five representative samples taken sequentially shall not exceed 35 CFU/100 mL (Puerto Rico Environmental Quality Board (PREQB) 2010).
Exposure to microbiological contaminants in recreational sea waters of Puerto Rico is a public health concern representing actual and potential hazards to bathers and other users of the beach for sport and recreation. Epidemiologic investigations of illness in fresh and marine recreational bathers have addressed health effects from exposure to point sources of fecal contamination (Fleisher et al. 1998; McBride et al. 1998; Pruss 1998; Prieto et al. 2001; Wade et al. 2006, 2008, 2010), storm-drain runoff (Haile et al. 1999), and non-point sources (Colford et al. 2007; Fleisher et al. 2010; Marion et al. 2010; Abdelzaher et al. 2011). Few studies have been conducted in subtropical and tropical marine waters except for those performed by Fleisher et al. in Florida (2010), Abdelzaher and collaborators (2011), also in Florida, and the Boquerón Beach study in Puerto Rico by Wade et al. (USEPA 2009). We carried out an epidemiological study to obtain more information about the possible health hazards associated with bathing in Puerto Rico's tropical marine environment and to compare different recreational water quality indicators. Accordingly, in the cohort study presented here, the incidence of gastrointestinal (GI) illness among beach-goers visiting Costa Azul beach, located in Luquillo, Puerto Rico was determined, and comparisons of illness risks were made between the exposed (bathers) and non-exposed groups (non-bathers). We also considered the difference in illness risks between bathers’ age groups and between exposure seasons.
METHODS
We conducted a prospective cohort study of beachgoers during two seasonal periods (summer and autumn 2008) on Costa Azul beach, located in Luquillo, Puerto Rico. Luquillo is a municipality located in the northeast coast of Puerto Rico, an island in the northeastern Caribbean Sea.
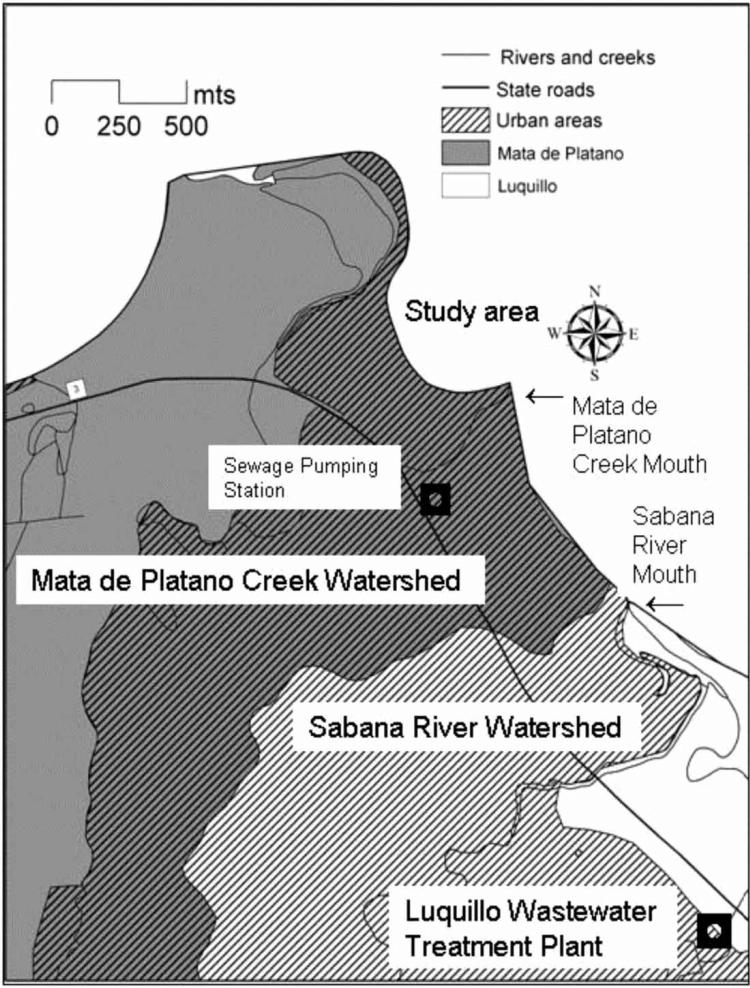
The study area was selected specifically because it was potentially affected by the discharge of both point and non-point sources (Figure 1). The Sabana River, which originates in the municipality of Luquillo, and measures approximately 12.6 km (Encyclopedia of Puerto Rico 2009), discharges into the north coast of Luquillo one mile east (up-stream) of the study site. This river receives the effluent of the Luquillo Wastewater Treatment Plant as well as effluent from overflow of malfunctioning septic tanks near the coast. These effluents accumulate in a small lagoon retained by a sand bank at the Sabana River mouth during low flow periods. During intense rainfall events, the discharge of this river breaks the sand barrier and carries storm water with very high levels of fecal organisms from this lagoon. The treatment plant served at the time nearly twelve thousand people (12,000), and discharged approximately one mile inland from the Sabana River mouth.
Figure 1.
Map showing urbanized portions of Mata de Platano Creek and Sabana River near study area.
The study transect 5 at Costa Azul beach receives the direct discharge of a storm water sewer which serves an adjacent densely populated residential and tourist condominium area, and receives the overflow of some malfunctioning septic tanks, animal droppings, and lixiviates from overflowed commercial garbage dumpsters during intense storms (Figure 2).
Figure 2.
Outflow from main culvert draining storm water from residential area into transect 5 of the study area.
The beach also receives the natural discharge of a nearby creek (Mata de Plátano), a few tens of meters east of the study area during intense storms (Figure 3). This creek drains a catchment area with malfunctioning septic tanks and receives bypass flows of raw wastewater from a sewage pumping station adjacent to the creek. It is estimated that the catchment area contains septic tanks that serve close to 8,000 people, since the Luquillo wastewater treatment plant serves 12,000 people and the estimated population of the municipality in 2010 was 20,068 (City Population.DE 2011). This number is consistent with statistics offered by the Puerto Rico Aqueduct and Sewers Authority, which estimates that 50% of Puerto Rico's population relies on septic tanks that offer poor treatment and therefore pollute ground water and surface water island wide (Díaz 2012). Most of the Luquillo residences lie within the catchment area of the Mata de Plátano creek.
Figure 3.
Mata de Plátano creek draining into Costa Azul beach after breaching sand bar and receiving raw wastewater discharges from pumping station bypass flows.
This pumping station has regular malfunctions and corresponding bypass flows into the creek, as documented by reports from the sewer utility to the Environmental Protection Agency (EPA) as required by law. This discharge causes accumulation of raw wastewater in the nearby creek, which acts as a pond during dry periods when retained by a coastal sand bank, probably causing the foul smell and decaying conditions observed in it. Extremely high fecal indicator counts were recorded in one sample taken of this pond during the study. This provokes sewage discharges into the sea during heavy rainfall events.
Costa Azul beach was also of particular interest since it is one of the beaches most visited by residents from different municipalities of Puerto Rico, as well as by itinerant occupants of adjacent condominiums who have short-term and long-term rentals. Usually, beachgoers bring their own food and drinks, and also cook on the beach. Even though this beach does not have bathroom or shower facilities, it is especially crowded on holidays and weekends. It is estimated that approximately 8,000 people visit Costa Azul beach during the summer season to engage in recreational activities.
All study instruments and protocols were reviewed and approved by the Committee for the Protection of Human Subjects at the University of Puerto Rico, Medical Sciences Campus, San Juan, Puerto Rico. All participants had to sign an informed consent form before enrollment in the study.
Health data collection
Data were collected using standard questionnaires from the EPA utilized for the National Epidemiological and Environmental Assessment of Recreational Water Study (USEPA 2004). Questionnaires were adapted and calibrated for the Puerto Rican population prior to its use. Spanish and English versions of the questionnaires were available.
Exclusion criteria included: (1) participation in the study within the last 28 day period; (2) unaccompanied minors (<18 years); and (3) inability to speak Spanish or English. In brief, interviewers approached all beachgoers and those who agreed to participate were recruited after signing consent. Participants answered questions for other household members who were at the beach. Information on swimming status was ascertained before leaving the beach. The survey was mostly conducted on Sundays from 14 June 2008 to 3 August 2008, and from 14 September 2008 to 19 October 2008, between 10:30 a.m. and 5:00 p.m.
During the interview at the beach, respondents were asked about the presence of underlying health conditions and that of their household members (acute and long-term chronic conditions), demographic information, bathing and other beach activities, use of protective clothing or equipment, food and drink consumption, use of insect repellant, and contact with unknown animals.
Follow-up telephone interviews were conducted 10–12 days after the beach interview to collect information about health symptoms developed subsequent to the beach visit. The survey included questions regarding other bathing or water-related activities, contact with unknown animals, and contact with ill people since the beach visit. The telephone interviewers were blinded with regard to water exposure of the participants and the results of the water samples.
Collection of water samples
Water samples were collected and analyzed on each study day. They were collected three times a day (10:00 a.m., 12:00 p.m. and 2:00 p.m.), along each of three transects perpendicular to the shoreline, one in shin-high water (0.3 m deep) and one in waist-high water (1 m deep). In addition, 100 mL were taken from each transect sample at shin and waist depth and a mixture (composite) was made. Transects were located ≥60 m apart to encompass the swimming area, maintaining the sampling points throughout the whole study in the areas with maximum density of swimmers (Haugland et al. 2005). Water samples were placed in coolers and maintained in ice at 1–4 °C during the time before analyses.
Water samples were analyzed within 6 hours of collection using membrane filtration or culture methods. Fecal indicator bacteria by culture methods, Enterococcus and fecal coliforms, were quantified in colony forming units (CFU/100 mL). Viable Enterococcus was enumerated by the USEPA Method 1600 2002) on membrane-Enterococcus indoxyl-β-D-glucoside agar (mEI) plates. Viable fecal coliforms were enumerated by the American Public Health Association (APHA) Method 9222D on membrane-fecal coliform agar (mFC) plates (APHA 1995).
We also filtered water samples for quantitative polymer-ase chain reaction (qPCR) analysis, within 6 hours of water sample collection, for the detection of Enterococcus species and Bacteroidales. We decided to include Bacteroidales in the analysis of water quality since it has been used in various studies in subtropical and tropical environments (Colford et al. 2007; USEPA 2009; Sinigalliano et al. 2010). This relatively new alternative indicator is abundant in the intestinal flora; is highly concentrated in human feces; is unlikely to persist or reproduce in aquatic environments; and results can be obtained in a matter of hours (Bernhard & Field 2000; Converse et al. 2009; Haugland et al. 2010). Results of the logistic regression analysis served to compare the qPCR parameters with culture method parameters as recreational water quality indicators. A detailed description of sample preparation and analysis has been described by Haugland et al. (2005). In brief, fecal indicator organisms in water samples were collected by polycarbonate membrane filters, total DNA was extracted, and polymerase chain reaction (PCR) amplifications of a genus specific DNA sequence of Enterococcus and Bacteroidales were carried out using the TaqMan PCR product detection system. The reactions were performed in a thermal cycling instrument (Smart Cycler System, Cepheid, Sunnyvale, CA) that automated the detection and quantitative measurement of the fluorescent signals produced by probe degradation during each cycle of amplification. Ratios of the target sequences in a test sample were compared with a calibrator sample using an arithmetic formula, referred to as the Comparative Cycle Threshold Method (Applied Biosystems 1997). If no threshold was achieved after 45 cycles, the sample was considered below the limit of detection (LOD). Results are reported in qPCR cell equivalents (CE) per 100 mL of original sample.
Health assessments
Although respiratory, ear, eye, and skin rash symptoms were also evaluated, we are presenting results only for GI illness. Data collected for respiratory, ear, eye, and skin rash symptoms are currently under analysis and will be reported in a future paper. GI illness was defined as any of the following: diarrhea (having more than three loose stools within a 24-hour period); episodes of vomiting; nausea and stomach ache symptoms; stomach ache that affected regular activity; nausea that affected regular activity; and stomach ache and fever symptoms. This definition was used to improve the sensitivity and identification of GI cases, to minimize losses due to inter-individual variations in the general perception of what constitutes a case of GI illness and to provide compatibility with the definition of this illness used in other studies (Fleisher et al. 1998, 2010; McBride et al. 1998; Colford et al. 2007; Wade et al. 2008, 2010). Consistent with several studies (McBride et al. 1998; Colford et al. 2007; Wade et al. 2008, 2010; Fleisher et al. 2010; Sinigalliano et al. 2010) GI illness was not restricted to individuals with fever, since infections such as norovirus and Escherichia coli 0157:H7 can produce GI illness without fever. Diarrhea was also considered as a stand-alone outcome because it is a commonly used definition of GI illness in population-based surveillance (Herikstad et al. 2002; Imhoff et al. 2004; Wade et al. 2010).
Data analysis
GI incidence rates were calculated for bathers and non-bathers. Bathers were defined as individuals who completely immersed their body and head in the water. Body immersion without head immersion was not considered for analysis since only 11% of respondents (158) reported immersing their bodies up to the waist and shoulders. The small number of participants in this exposure category (body immersion without head immersion) imposes caution in the interpretation of results (due to this small sample size, making conclusions about this specific group was difficult). Non-bathers were defined as those who spent the day at the beach during the survey date but did not have direct water contact (did not wade, play in the water, or swim). Logistic regression models were constructed to describe the strength of the association between types of water exposure and the incidence of illness among bathers and non-bathers, and to evaluate the association between risk of illness and water quality (as measured by the fecal indicator organisms).
The arithmetic mean of the log-transformed (base 10) values was utilized to summarize water quality at a given day and time. The analyses were focused primarily on the daily average of all samples. The daily average represented average water quality at the beach on a particular day. Robust estimates of variance were used to account for the correlation of observations within households to avoid an exaggeration of the statistical precision.
The adjusted odds ratio (AOR) of GI illness and associated 95% confidence interval (CI) were calculated with logistic regression models that compared bathers and non-bathers. For models assessing the association between water quality indicators among bathers, the AOR was expressed as the increase in the odds of illness per unit of increase in the water quality measure among all participants, with the associated 95% CI.
Covariates strongly associated with illness, water quality and water contact, or those considered to be potential confounding factors were considered and evaluated in the logistic regression models. Confounding was first examined using a backwards elimination approach starting with a full model that included all covariates. Covariates that resulted in <5% change, in one or more of the estimates for water quality exposure and illness relationship were then dropped from the model until a final reduced model was obtained. Potential confounding factors evaluated in initial models were age; sex; contact with unknown animals; contact with other people with GI illness; presence of chronic GI illness; sand exposure (collecting shells, making sand castles, burying body in sand, and getting sand into mouth); consumption of raw meat, fish or undercooked eggs; and swimming after the beach interview. The selection procedure reduced the number of covariates to age, sex, chronic GI illness, and sand exposure. We used Stata, version 10.1 (StataCorp. 2009, College Station, TX) for the data analysis.
RESULTS
A total of 828 households were offered enrollment. Of these, 26 households refused to participate or were ineligible. A total of 1,699 individuals from 802 households agreed to participate and were enrolled in the study. Data were available for a total of 718 households (1,531 individuals) who completed the follow-up telephone interview and were eligible for analysis. Of note, the household interviewing response rate (completed/attempted) through the completion of the telephone interview was 90%.
Note that in the following tables, deviations of the total from 1,531 were due to missing responses, except for tables for incident GI illness where respondents (bathers and non-bathers) with underlying GI illness (3 days before their beach visit) were also excluded.
Water quality
The mean daily average density (CFU/100 mL) of Enterococcus by culture method was significantly higher (p = 0.001) in the autumn season (log10 = 0.33) than in the summer season (log10 = 0.19). The current Puerto Rico recreational water standard for Enterococcus is 35 CFU/100 mL as geometric mean (log10 = 1.54), and was not exceeded on any sampling date. It was also observed that the mean daily average density of fecal coliforms was significantly higher (p = 0.001) in the autumn season (log10 = 0.82) than in the summer season (log10 = 0.64). The current Puerto Rico recreational water standard for fecal coliforms is 200 CFU/100 mL as geometric mean (log10 = 2.30), and was not exceeded either. The results of daily analyses of samples are shown in Table 1.
Table 1.
Summary statistics by season for log10 indicator organisms, measured by culture methods
| Indicator Season | Enterococcusa Summer | Autumn | Fecal coliformsa Summer | Autumn |
|---|---|---|---|---|
| No. of days | 8 | 5 | 7 | 5 |
| No. of samples | 144 | 90 | 126 | 90 |
| Log10 CFU/100 mL | ||||
| Mean of daily averages | 0.19 | 0.33 | 0.64 | 0.82 |
| Median of daily averages | 0.13 | 0.28 | 0.48 | 0.76 |
| Standard deviation | 0.19 | 0.41 | 0.40 | 0.69 |
| Minimum/maximum | 0.02/0.61 | –0.03/0.80 | 0.21/1.28 | 0.06/1.71 |
p-value = 0.001 for seasonal difference in mean of log10 averages (chi-squared test).
Enterococcus counts (log10 CFU/100 mL) by culture methods showed similar mean levels at shin depth (0.66) and waist depth (0.61). Collection time was not a significant factor in the variability of Enterococcus densities. Mean levels of log10 Enterococcus CFU/100 mL were 0.57, 0.66, and 0.67 at 10:00 a.m., 12:00 p.m., and 2:00 p.m., respectively. Mean levels of fecal coliforms log10 densities at shin depth (1.27) were also similar to waist depth densities (1.37). Collection time was not an important factor in the variability of fecal coliforms densities. Mean levels of log10 fecal coliforms CFU/100 mL were 1.52, 1.17, and 1.18 at 10:00 a.m., 12:00 p.m., and 2:00 p.m., respectively.
The average qPCR CE results for the measurements of indicator organisms are shown in Table 2. There were no results below the LOD for Bacteroidales or for Enterococcus, and levels were generally low, indicating clean waters. The mean of daily average qPCR CE for Bacteroidales was higher than for Enterococcus.
Table 2.
Summary statistics by season for log10 indicator organisms measured by qPCR
| Indicator Season | Enterococcus Summer | Autumn | Bacteroidales Summer | Autumn |
|---|---|---|---|---|
| No. of days | 8 | 5 | 8 | 5 |
| No. of composite samplesa | 48 | 30 | 48 | 30 |
| Log10 qPCR CEb/100 mL | ||||
| Mean of daily averages | 1.14 | 1.07 | 2.70 | 2.40 |
| Median of daily averages | 1.15 | 0.93 | 2.72 | 2.12 |
| Standard deviation | 0.13 | 0.45 | 0.24 | 0.63 |
| Minimum/maximum | 0.90/1.30 | 0.65/1.84 | 2.34/3.09 | 2.08/3.51 |
The composite samples were a mixture of three individual samples, each representing one transect location.
CE = cell equivalents.
Densities of (log10) Bacteroidales showed similar mean levels at shin depth (2.62) and waist depth (2.62). Collection time was not a significant factor in the variability of Bacteroi-dales qPCR CE. Mean levels of log10 Bacteroidales qPCR CE/100 mL were 2.46, 2.47, and 2.82 at 10:00 a.m., 12:00 p.m., and 2:00 p.m., respectively. Mean levels of log10 densities of Enterococcus qPCR CE at shin depth (1.14) were also similar to waist depth densities (1.14). Collection time was not an important factor in the variability of Enterococcus qPCR CE. Mean levels of log10 Enterococcus qPCR CE/100 mL were 1.15, 1.12, and 1.16 at 10:00 a.m., 12:00 p.m., and 2:00 p.m., respectively.
Incidence of GI illness by beach activities and seasonal period
Overall, bathers were younger (median age = 26; ±16.4) than non-bathers (median age = 37; ±17.9); more likely to eat or drink beverages at the beach, and more likely to experience sand exposure (collecting shells, making sand castles, burying body in sand, getting sand into mouth, and digging in beach sand). There were more female (53%) than male beachgoers. Among non-bathers, 61% were female. Sex distribution was equal among bathers. Non-bathers were more likely to have had prior chronic GI illness (6.6%) than bathers (3.9%). Beachgoer and activity data are shown in Table 3.
Table 3.
General characteristics of beachgoers on Costa Azul beach, Luquillo, Puerto Rico
| Bathers |
Non-bathers |
Total |
||||
|---|---|---|---|---|---|---|
| Characteristics | No. | (%) | No. | (%) | No. | (%) |
| Age (years) | ||||||
| <5 | 75 | (6.82) | 13 | (3.35) | 88 | (5.91) |
| 5–14 | 250 | (22.75) | 10 | (2.58) | 260 | (17.47) |
| 15–24 | 239 | (21.75) | 85 | (21.65) | 324 | (21.77) |
| 25–34 | 190 | (17.29) | 83 | (21.39) | 273 | (18.35) |
| 35–44 | 158 | (14.38) | 61 | (15.72) | 219 | (14.72) |
| 45–54 | 126 | (11.46) | 68 | (17.53) | 194 | (13.04) |
| >54 | 61 | (5.55) | 69 | (17.78) | 130 | (8.74) |
| Mean age (±SDa) | 26 (±16.4) | 37 (±17.9) | 29 (±17.5) | |||
| Sex | ||||||
| Females | 567 | (50) | 243 | (61) | 810 | (53) |
| Males | 567 | (50) | 154 | (39) | 721 | (47) |
| Food and beverages consumption at the beach | 977 | (86.23) | 319 | (80.30) | 1,296 | (84.71) |
| Sand exposure | 349 | (30.78) | 36 | (9.09) | 385 | (25.15) |
| Chronic GI illness | 44 | (3.88) | 26 | (6.57) | 70 | (4.58) |
| Lost to follow-up | 105 | (8.48) | 57 | (12.58) | 162 | (10) |
SD = standard deviation of mean.
Data were collected and analyzed across seasons (summer and autumn), which are characterized by rainfall differences. The first period was from 14 June to 3 August, and the second one was from 14 September to 19 October 2008. The average 72-hour total rainfall before each survey date was 9.6 mm (0.38 inches) during the first period and 17.57 mm (0.69 inches) during the second period.
The incidence of GI illness among bathers and non-bathers is shown in Table 4. The incidence of GI illness among beachgoers was 5.08 per 100. When beach activities were considered, the overall incidence of GI illness among those who had no direct contact with water (non-bathers) was 6.35 per 100, while the incidence of GI illness among those who had direct contact with water (bathers) was 4.56 per 100. However, when stratified by season, the percent incidence of GI illness was, during summer, bathers 3.87 and non-bathers 5.93, and during autumn (rainy season), bathers 7.60 and non-bathers 7.41. The overall incidence of GI illness during the autumn period was 7.53 per 100 compared to 4.41 per 100 during the summer period.
Table 4.
Incidence of GI illness by water contact and season (excluding those with pre-existing GI illness)
| Number of participants (% of total) | GI illness incidence per 100 overall | GI illness incidence per 100 summer | GI illness incidence per 100 autumn | |
|---|---|---|---|---|
| Bathers | 921 (71) | 4.56 | 3.87 | 7.60 |
| Non-bathers | 378 (29) | 6.35 | 5.93 | 7.41 |
| Total | 1,299 (100) | 5.08 | 4.41 | 7.9 |
Adjusted odds ratios of GI illness by bathing activities and seasonal period
The AORs estimated from logistic regression models included the following covariates: (1) age; (2) sex; (3) chronic GI illness; and (4) sand exposure. The AORs for bathing activity and season, estimated from logistic regression models, are shown in Table 5. The overall AOR of GI illness associated with the autumn period was 1.77 (95% CI: 0.93–3.38) compared to the summer period.
Table 5.
Adjusted odds ratio (AOR) of GI illness among beachgoers by season and water contact (excluding those with pre-existing GI Illness)a
| Season | Number of participants (% of total) | Multivariate AOR overall | (95% CI)b | Multivariate AORsa Bathers | (95% CI)b For bathing activity |
|---|---|---|---|---|---|
| Autumn | 315 (22) | 1.77 | (0.93–3.38)c | 1.35 | (0.39–4.63) |
| Summer | 1,142 (78) | 1.00 | Reference | 0.73 | (0.36–1.45) |
| Total | 1,457 (100) | 0.88 | (0.47–1.63) |
The multivariate logistic regression model was used to adjust for age, sex, chronic GI illness, and sand exposure.
CI = confidence interval.
p-value = 0.088.
When stratified by season of exposure, an AOR of 1.35 (95% CI: 0.39–4.63) for GI illness was observed for bathers during the autumn season, while an AOR of 0.73 (95% CI: 0.36–1.45) was observed during the summer when compared to non-bathers. Overall AOR of GI illness among bathers with respect to non-bathers was 0.88 (95% CI: 0.47–1.63). These findings were not statistically significant.
Water quality and GI illness
Overall risks of GI illness associated with daily averages of Enterococcus and fecal coliform densities (measured by culture method) are shown in Table 6. A log10 increase in the daily average density of Enterococcus was associated with a 3.59 (95% CI: 0.63–20.57) increase in the odds of GI illness for bathers. The AOR observed for those having no contact with water was 2.30 (95% CI: 0.31–17.21).
Table 6.
AORs of GI illness associated with each log10 increase in indicator densities by culture methods
|
Enterococcus
a
|
Fecal coliformsa |
|||
|---|---|---|---|---|
| Indicator | Multivariate AORsb | (95% CI)c | Multivariate AORsb | (95% CI)c |
| Bathers | 3.59 | (0.63–20.57) | 0.70 | (0.28–1.76) |
| Non-bathers | 2.30 | (0.31–17.21) | 0.89 | (0.30–2.64) |
Based on daily averages of log10 indicator parameter.
The multivariate logistic regression model was used to adjust for age, sex, chronic GI illness, and sand exposure among all beachgoers.
CI = confidence interval.
No association was observed between overall daily average of fecal coliform densities (measured by culture method) and the odds of GI illness for any type of water contact.
For parameters measured by the qPCR technique, the AOR of GI illness associated with the overall daily average of Enterococcus and Bacteroidales are shown in Table 7. No associations were observed between overall daily average of Enterococcus and Bacteroidales CE and the odds of GI illness for bathers.
Table 7.
AORs of GI illness associated with each log10 increase in indicator densities by qPCR
|
Enterococcus
a
|
Bacteroidales
a
|
|||
|---|---|---|---|---|
| Indicator | Multivariate AORsb | (95% CI)c | Multivariate AORsb | (95% CI)c |
| Bathers | 0.39 | (0.05–2.81) | 0.57 | (0.18–1.74) |
| Non-bathers | 0.53 | (0.05–5.23) | 0.54 | (0.15–1.91) |
Based on daily averages of log10 indicator parameter measured by qPCR.
The multivariate logistic regression model was used to adjust for age, sex, chronic GI illness, and sand exposure among all beachgoers.
CI = confidence interval.
Adjusted multivariate GI illness predictive models were constructed and stratified by season using Enterococcus measured by culture as a water quality covariate. Results showed that among summer participants, a one log10 increase in the daily Enterococcus average density resulted in a 6.73 increase in the adjusted odds of GI illness (95% CI: 0.44–103.92). During autumn, this AOR was 1.38 (95% CI: 0.32–5.89). Women reported more GI illness than men, with a 1.04 times higher risk during the summer (95% CI: 0.57–1.89) and 2.59 times higher risk during autumn (95% CI: 1.02–6.58). This elevated risk for women during the rainy season was found to be statistically significant (p <0.05). The odds among children younger than 5 years was 12.35 times higher than those aged 5–14 years during the summer (95% CI: 2.76–57.26). This difference was statistically signifi-cant (p < 0.05). During autumn, the odds among children younger than 5 years was 6.51 higher than the reference group (95% CI: 0.77–55.02). Data from these multivariate predictive models are shown in Table 8.
Table 8.
AORs of GI illness based on multivariate model using the Enterococcus (log10) culture method indicator excluding participants with pre-existing and chronic GI illness
| Outcome Season | Gastrointestinal (GI) illness |
|
|---|---|---|
| Summer AORsa (95% CI)b | Autumn AORsa (95% CI)b | |
| Type of water contact | ||
| Bathers | 0.69 (0.34–1.38) | 1.33 (0.38–4.61) |
| Non-bathers | 1.00 (Reference) | 1.00 (Reference) |
| Sex | ||
| Female | 1.04 (0.57–1.89) | 2.59 (1.02–6.58)c |
| Male | 1.00 (Reference) | 1.00 (Reference) |
| Age (years) | ||
| <5 | 12.35 (2.76–55.26)c | 6.51 (0.77–55.02) |
| 5–14 | 1.00 (Reference) | 1.00 (Reference) |
| 15–24 | 3.87 (0.83–18.13) | 1.35 (0.17–10.59) |
| 25–34 | 3.68 (0.76–17.90) | 2.20 (0.37–13.97) |
| 35–44 | 7.09 (1.49–33.64)c | 0.42 (0.03–5.15) |
| 45–54 | 6.51 (1.43–29.65)c | 2.68 (0.31–23.18) |
| >54 | 3.20 (0.56–18.21) | 1.14 (0.07–19.58) |
| Sand exposure | ||
| Yes | 1.35 (0.57–3.23) | 0.92 (0.27–3.14) |
| No | 1.00 (Reference) | 1.00 (Reference) |
| Water quality | ||
| Daily average of log10 indicator parameter | 6.73 (0.44–103.92) | 1.38 (0.32–5.89) |
Adjusted for water contact, sex, age, sand exposure, and water quality.
CI = confidence interval.
p-value <0.05.
DISCUSSION
In this prospective cohort study we assessed differences in the risk of GI illness between seasons, between participants with and without water contact, and as a function of fecal indicator densities. We also studied beachgoer characteristics as predictors of GI illness. Findings suggest that precipitation contributes to higher densities of Enterococcus and fecal coliforms by culture methods during the rainy season (p < 0.001) as compared to the summer season (Table 1). Even though microbial densities were well below regulated water quality standards, a borderline significant increase in the odds of GI illness (p = 0.088) was observed during the rainy season as compared to the summer season (Table 5). We can infer that increased rainfall runoff during the autumn is carrying a load of pathogens of fecal origin to the Costa Azul beach and thus increasing the risk of GI illness. It is known that nearly half the population of Puerto Rico uses septic tanks as means of domestic wastewater disposal and that a large proportion of those facilities are of inadequate design and operation. Also, increased flow through the nearby Mata de Plátano creek during storm flow conditions can create a breach in the sandbar that holds the effluent of bypass flows from a nearby raw wastewater pumping station, therefore permitting the access of large amounts of fecal organisms to the Costa Azul beach.
According to the results of this study, microbial contaminants affecting water quality originate mostly from non-point sources, and their densities in recreational water increase with rainfall. In prior studies, high bacterial loads have been shown to increase in water bodies after rain events (Crowther et al. 2001; Lipp et al. 2001; Abdelzaher et al. 2011). More investigation is needed focusing on the fecal indicators response to significant rainfall events and timing of antecedent rainfall events.
Many of the previous studies have identified beaches affected mainly by sewage outflows from wastewater treatment plants, in other words point sources of water contamination (Fleisher et al. 1998; Pruss 1998; Prieto et al. 2001; Wade et al. 2006. 2008, 2010). Our study site is mostly affected by non-point sources such as septic tanks, since higher levels of bacterial counts were found in coastal recreational water during the rainy season.
Notwithstanding the occasional loads of organisms reaching our study beach, the lack of a statistically signifi-cant difference in GI illness risk between bathers and non-bathers in this study is probably due to overall clean water conditions observed during the study. As observed in Table 1, this study measured very low bacteria indicator levels when compared with the Puerto Rico recreational water quality standard and the EPA recommended bacterial water-quality criteria for recreational coastal waters. The clean water conditions at this study site suggest that discharges from the Luquillo sewage treatment plant into the nearby Sabana River were actually not impacting on the beach as suspected, despite the proximity of the Sabana River's mouth (one mile east upstream). The Sabana River, carrying the effluent from the Luquillo sewage treatment plant, empties into the Atlantic Ocean, where strong wave action probably disperses its contaminants into the open ocean and away from Costa Azul beach. If the study beach were affected by the discharge of the Luquillo wastewater treatment plant, a continuous input of bacteria affecting the water at the beach would be expected. Results of this study are comparable to those found by Wade and collaborators at Boquerón Beach in Puerto Rico (2009) where low levels of indicator bacteria were found despite the presence of two nearby wastewater treatment plants.
Although not statistically significant, a somewhat elevated overall risk of GI illness in non-bathers compared to bathers was found in this study. However, when we stratified the data among seasons, an expected pattern was observed of a slightly elevated GI illness risk among bathers during the rainy season. This is the season in which water quality is worse due to rainfall runoff. On the other hand, during the summer non-bathers showed a higher incidence of GI illness than bathers. This occurred under very clean water conditions and during the absence of extreme storm flow events.
A higher incidence of GI illness in bathers has been demonstrated in most published studies (Wade et al. 2006, 2008). This is probably due to the fact that most other studies found higher levels of microbial contaminants in water than our study. Also, the variability observed across beaches in other studies could indicate that the risks may be site-specific. Risks may depend on differences in the populations such as susceptibility to the etiologic agents, cultural differences and uses of recreational waters and beach areas.
Under the clean water conditions of this study, especially during the summer, pathways of exposure to pathogens of fecal origin other than head immersion in the water may gain relevance.
We theorize that on Costa Azul beach, microbial contaminants in storm water may present a risk of GI illness among beachgoers due to their longer survival in beach sand than in water. The storm water can reach the sand due to the direct discharge of the main culvert serving the Costa Azul residential area and to the waves and tides carrying water with bacterial loads from the contaminated creek into the sand. Sand filters bacteria out of the water and sustains their growth, especially after it dries (Mohammed et al. 2012). Thus sand can harbor pathogens even when recreational water has had time to clean after rainfalls. This condition exists during the summer in Costa Azul beach. This could represent an elevated risk to beachgoers spending more time exposed to sand or lower risk to bathers who wash their bodies through immersion in clean marine water during the summer.
Several studies have shown that beach sand can harbor significant levels of bacteria, which are often present in higher concentrations than in the water column (Ghinsberg et al. 1994; Oshiro & Fujioka 1995; Obiri & Jones 1999). Bacteria harbored in the sand may persist longer than in the water because they can adhere to sand particles. Beach sand may also act as a physical barrier protecting pathogens from solar and UV radiation that has been shown to influence cell inactivation (Noble et al. 2003). Beach sand contact has been associated with GI illness in prospective cohort studies (Bonilla et al. 2007; Heany et al. 2009). The study in South Florida (Bonilla et al. 2007) found indicator densities in dry sand 100 times that of adjacent recreational water, under conditions similar to the tropical Costa Azul beach during the summer. This could explain why clean water conditions can be found in Costa Azul beach waters during the summer when few rainfall events occur, while the sand could be harboring and allowing the reproduction of bacteria of fecal origin.
It was beyond the scope of this study to sample beach sand. However, our findings suggest that beach sand may play a role in supporting a higher density of fecal organisms than water, which can increase the risk of marine water-related illnesses. The extent of beachgoers’ exposure to sand with higher densities of fecal indicator bacteria than water at Costa Azul beach during the summer should be explored. This is especially important for children under 5 years, who showed a much higher risk of GI illness than other age groups during the summer.
The association between Enterococcus densities in water (measured by culture methods) and GI illness showed the highest odds ratio (OR) among water quality indicator parameters. This association was not statistically significant when the multivariate model was constructed. The lack of a consistent association between enterococci densities in water and bather health has been observed in previous studies of subtropical marine beaches impacted by non-point sources (Fleisher et al. 2010; Abdelzaher et al. 2011).
Fecal coliform densities were not associated with GI illness in this study. It has been demonstrated that these microbes do not survive long in sea water, and that their presence in marine waters is considered an indicator of recent fecal contamination. Fecal coliforms as a group have been determined to be a poor indicator of the risk of digestive system illness (USEPA 2008).
In this study, CE densities of Enterococcus and Bacteroidales using the qPCR method were low, and no association was found between GI illness and these indicators. QPCR parameters showed low densities in water throughout the study and less seasonal variability than culture parameters. They showed no significant statistical association with GI illness in this study site. It is possible that we saw little relationship of qPCR parameters and GI illness because the water was of high quality. Wade et al. (2008) recognized that the relationships observed between Enterococcus densities measured by qPCR CE and GI illness could not be extrapolated to marine beaches, or to recreational waters affected by different sources of fecal contamination.
Addressing which indicators the Puerto Rico EQB should keep measuring will require additional research to understand how the bacterial indicators relate to the presence of pathogens that directly impact public health. It should be taken into consideration that measuring fecal coliform densities is probably not cost-effective and if budgets are limited, the effort expended in monitoring it should be reconsidered. A better approach would be to sample more beach sites or sample at more frequent intervals to protect public health.
General limitations
This study used a prospective cohort design which has been used by numerous others to evaluate the risks associated with recreational water exposures (Corbett et al. 1993; Haile et al. 1999; Prieto et al. 2001; Colford et al. 2007; Wade et al. 2008, 2010). In spite of the fact that this type of study design is affected by participant losses in the follow-up, the 10% lost to follow-up was equally distributed among bathers and non-bathers.
Bathers and non-bathers differed with respect to bathing, demographic characteristics, and pre-existing GI illness. Non-bathers were older (mean ≥37 years old) than bathers (mean ≥26 years old). More non-bathers were female (61%) than male (39%). A higher proportion of non-bathers reported pre-existing GI illness. Differences in the study population may have been responsible for the higher overall risk in GI illness among non-bathers compared with bathers.
Water quality at Luquillo beach was good. This fact could have affected the association between fecal indicators densities and incidence of GI illness. Perhaps with more polluted waters, this result might have differed importantly. An additional issue was the low proportion of non-bathers (29%). Seventy-one percent of respondents reported bathing exposure. This fact might have reduced the ability to accurately describe differences between bathers and non-bathers. The Boquerón Beach study done by Wade and collaborators (2009) showed a similar proportion of swimmers compared to non-swimmers.
Sand monitoring was not done in this study, so the validity of the sand contamination contention is supported only by results of other studies in subtropical beaches, and because it is the most plausible explanation of the apparent protective effect of swimming during the higher water quality period in the summer at Costa Azul beach. Plans are being made to include sand monitoring in upcoming studies of marine recreational waters in the near future.
CONCLUSIONS
Additional research is recommended on recreational marine sites in tropical environments impacted by non-point pollution, especially after rain events, before extrapolating results to other beaches. Beach sand samples should be collected and analyzed not only for fecal indicator bacteria, but also for common pathogens of fecal origin. Additional studies should also focus on identifying the local sources of contamination and a determination of whether the indicator microbes are of human, animal or environmental origin. As stated by Cheung et al. (1990), it is important that individual countries can conduct their own epidemiological studies, so that health-related bathing water quality standards which suit their particular conditions could be developed.
The use of fecal coliforms in Puerto Rico to measure water quality should be further evaluated to determine if this fecal indicator is valid from a regulatory perspective. Also, actions should be taken by the Puerto Rico EQB to advice potential beachgoers of increased risks of bathing during the rainy season. These advisories should make specific reference to the much higher risk faced by small children exposed to beach water after recent rainfall events.
ACKNOWLEDGEMENTS
This work was supported by a fellowship from US EPANCER grant MA-91685001–1.
QPCR analysis was carried out by Idennys Magly under the generous guidance of Dr Richard Haugland at EPA-NERL. We thank Dr Timothy Wade, Dr Alfred Dufour, and Elizabeth Sams of EPA-NHEERL for their valuable advice and Dr Pablo Méndez for map construction.
Contributor Information
Lyzbeth Cordero, Environmental Health Department, Graduate School of Public Health, Medical Sciences Campus, University of Puerto Rico, PO Box 365067 San Juan, PR 00936-5067, Puerto Rico.
Jose Norat, Environmental Health Department, Graduate School of Public Health, Medical Sciences Campus, University of Puerto Rico, PO Box 365067 San Juan, PR 00936-5067, Puerto Rico.
Hernando Mattei, Social Sciences Department, Graduate School of Public Health, Medical Sciences Campus, University of Puerto Rico, PO Box 365067 San Juan, PR 00936-5067, Puerto Rico.
Cruz Nazario, Biostatistics and Epidemiology Department, Graduate School of Public Health, Medical Sciences Campus, University of Puerto Rico, PO Box 365067 San Juan, PR 00936-5067, Puerto Rico.
REFERENCES
- Abdelzaher AM, Wright ME, Ortega C, Rasem Hasan A, Shibata T, Solo-Gabriele HM, Kish J, Withum K, He G, Elmir SM, Bonilla A, Bonilla TD, Palmer CJ, Scott TM, Lukasik J, Harwood VJ, McQuaig S, Sinigalliano CD, Gidley ML, Wanless D, Plano LRW, Garza AC, Zhu X, Stewart JR, Dickerson JW, Jr., Yampara-Iquise H, Carson C, Fleisher JM, Fleming LE. Daily measures of microbes and human health at a non-point source marine beach. J. Water Health. 2011;9:443–457. doi: 10.2166/wh.2011.146. [DOI] [PubMed] [Google Scholar]
- American Public Health Association (APHA), American Water Works Association and Water Environment Foundation . Standard Methods for the Examination of Water and Wastewater. 19th edition. American Public Health Association; Washington, DC.: 1995. [Google Scholar]
- Applied Biosystems . ABI PRISM 7700 Sequence Detection System. Bulletin #2 of the Applied Biosystems Corporation; Foster City, CA.: 1997. [Google Scholar]
- Bernhard AE, Field KG. A PCR assay to discriminate human and ruminant feces on the basis of host differences in Bacteroides-Prevotella genes encoding 16S rRNA. Appl. Environ. Microbiol. 2000;66:4571–4574. doi: 10.1128/aem.66.10.4571-4574.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonilla TD, Nowosielski K, Cuvelier M, Hartz A, Green M, Esiobu N, McCorquodale DS, Fleisher JM, Rogerson A. Prevalence and distribution of fecal indicator organisms in South Florida beach sand and preliminary assessment of health effects associated with beach sand exposure. Mar. Pollut. Bull. 2007;54:1472–1482. doi: 10.1016/j.marpolbul.2007.04.016. [DOI] [PubMed] [Google Scholar]
- Cheung WH, Chang KC, Hung RP, Kleevens JW. Health effects of beach water pollution in Hong Kong. Epidemiol. Infect. 1990;105:139–162. doi: 10.1017/s0950268800047737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- City Population.DE [July 5, 2012];Luquillo. 2011 Available from: http://www.citypopulation.de/php/puertorico.php?adm2id=72089.
- Colford JM, Jr., Wade TJ, Schiff KC, Wright CC, Griffith JF, Sandhu SK, Burns S, Lovelace G, Weisberg SB. Water quality indicators and the risk of illness at beaches with nonpoint sources of fecal contamination. Epidemiology. 2007;18:27–35. doi: 10.1097/01.ede.0000249425.32990.b9. [DOI] [PubMed] [Google Scholar]
- Converse RR, Blackwood AD, Kirs M, Griffith JF, Noble RT. Rapid QPCR-based assay for fecal Bacteroides spp. as a tool for assessing fecal contamination in recreational waters. Water Res. 2009;43:4828–4837. doi: 10.1016/j.watres.2009.06.036. [DOI] [PubMed] [Google Scholar]
- Corbett SJ, Rubin GL, Curry GK, Kleinbaum DG. The health effects of swimming at Sydney beaches. The Sydney Beach Users Study Advisory Group. Am. J. Public Health. 1993;83:1701–1706. doi: 10.2105/ajph.83.12.1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creel L. Population Reference Bureau. Ripple effects: population and Coastal Regions. [April 12, 2009];Available from. 2003 http://www.prb.org/pdf/RippleEffects_Eng.pdf.
- Crowther J, Kay D, Wyer MD. Relationships between microbial water quality and environmental conditions in coastal recreational waters: The Fylde Coast, UK. Water Res. 2001;35:4029–4038. doi: 10.1016/s0043-1354(01)00123-3. [DOI] [PubMed] [Google Scholar]
- Díaz A. Prasa, Soderberg tackle septic-tank proliferation. [July 5, 2012];Caribbean Business 40 Available from. 2012 http://www.caribbeanbusiness.pr/prnt_ed/print_detail.php?nw_id=6900&ct_id=0.
- Elmanama AA, Fahd MI, Afifi S, Abdallah S, Bahr S. Microbiological beach sand quality in Gaza Strip in comparison to seawater quality. Environ. Res. 2005;99:1–10. doi: 10.1016/j.envres.2004.12.014. [DOI] [PubMed] [Google Scholar]
- Encyclopedia of Puerto Rico [October 19, 2009];Luquillo. 2009 Available from: http://www.enciclopediapr.org/ing/article.cfm?ref=09032603.
- Fleisher JM, Fleming LE, Solo-Gabriele HM, Kish JK, Sinigalliano CD, Plano LRW, Elmir SM, Wang JD, Withum K, Shibata T, Gidley ML, Abdelzaher A, He G, Ortega C, Zhu X, Wright M, Hollenbeck J, Backer LC. The BEACHES Study: Health effects and exposures from non-point source microbial contaminants in subtropical recreational marine waters. Int. J. Epidemiol. 2010;39:1291–1298. doi: 10.1093/ije/dyq084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleisher JM, Kay D, Wyer MD, Godfree AF. Estimates of the severity of illnesses associated with bathing in marine recreational waters contaminated with domestic sewage. Int. J. Epidemiol. 1998;27:722–726. doi: 10.1093/ije/27.4.722. [DOI] [PubMed] [Google Scholar]
- Ghinsberg RC, Bar-Dov L, Rogol M, Sheinberg Y, Nitzan Y. Monitoring of selected bacteria and fungi in sand and sea water along Tel Aviv coast. Microbios. 1994;77:29–40. [PubMed] [Google Scholar]
- Haile RW, Witte JS, Gold M, Cressey R, McGee C, Millikan RC, Glasser A, Harawa N, Ervin C, Harmon P, Harper J, Dermand J, Alamillo J, Barrett K, Nides M, Wang G. The health effects of swimming in ocean water contaminated with storm drain runoff. Epidemiology. 1999;10:355–363. [PubMed] [Google Scholar]
- Haugland RA, Siefring SC, Wymer LJ, Brenner KP, Dufour AP. Comparison of Enterococcus measurements in freshwater at two recreational beaches by quantitative polymerase chain reaction and membrane filter culture analysis. Water Res. 2005;39:559–568. doi: 10.1016/j.watres.2004.11.011. [DOI] [PubMed] [Google Scholar]
- Haugland RA, Varma M, Sivaganesan M, Kelty C, Peed L, Shanks OC. Evaluation of genetic markers from the 16S rRNA gene V2 region for use in quantitative detection of selected Bacteroidales species and human fecal waste by qPCR. Syst. Appl. Microbiol. 2010;33:348–357. doi: 10.1016/j.syapm.2010.06.001. [DOI] [PubMed] [Google Scholar]
- Heany CD, Sams E, Wing S, Marshall S, Brenner K, Dufour AP, Wade TJ. Contact with beach sand among beachgoers and risk of illness. Am. J. Epidemiol. 2009;170:164–172. doi: 10.1093/aje/kwp152. [DOI] [PubMed] [Google Scholar]
- Herikstad H, Yang S, Van Gilder TJ, Vugia D, Hadler J, Blake P, Deneen V, Shiferaw B, Angulo FJ. A population-based estimate of the burden of diarrheal illness in the United States: FoodNet, 1996–7. Epidemiol. Infect. 2002;129:9–17. doi: 10.1017/s0950268801006628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imhoff B, Morse D, Shiferaw B, Hawkins M, Vugia D, Lance-Parker S, Hadler J, Medus C, Kennedy M, Moore MR, Van Gilder T. Burden of self-reported acute diarrheal illness in FoodNet surveillance areas, 1998–1999. Clin. Infect. Dis. 2004;38:S219–226. doi: 10.1086/381590. [DOI] [PubMed] [Google Scholar]
- Lipp EK, Kurz R, Vincent R, Rodriguez-Palacios C, Farrah SR, Rose JB. The effects of seasonal variability and weather on microbial fecal pollution and enteric pathogens in a subtropical estuary. Estuaries. 2001;24:266–276. [Google Scholar]
- Marion JW, Lee J, Lemeshow S, Buckley TJ. Association of gastrointestinal illness and recreational water exposure at an inland US beach. Water Res. 1998;44:4796–4804. doi: 10.1016/j.watres.2010.07.065. [DOI] [PubMed] [Google Scholar]
- Mcbride GB, Salmond CE, Bandaranayake DR, Turner SJ, Lewis GD, Till DG. Health effects of marine bathing in New Zealand. Int. J. Environ. Health Res. 1998;8:173–189. [Google Scholar]
- Mohammed RL, Echeverry A, Stinson CM, Green M, Bonilla TD, Hartz A, McCorquodale DS, Rogerson A, Esiobu N. Survival trends of Staphylococcus aureus, Pseudomonas aeruginosa, and Clostridium perfringens in a sandy South Florida beach. Mar. Pollut. Bull. 2012;64:1201–1209. doi: 10.1016/j.marpolbul.2012.03.010. [DOI] [PubMed] [Google Scholar]
- Noble RT, Mooreb DF, Leecasterc MK, McGeed CD, Weisberge SB. Comparison of total coliform, fecal coliform, and Enterococcus bacterial indicator response for ocean recreational water quality testing. Water Res. 2003;37:1637–1643. doi: 10.1016/S0043-1354(02)00496-7. [DOI] [PubMed] [Google Scholar]
- Obiri-Danso K, Jones K. Distribution and seasonability of microbial indicators and thermophilic campylobacters in two freshwater bathing sites on the River Lune in northwest England. J. Appl. Microbiol. 1999;87:822–832. doi: 10.1046/j.1365-2672.1999.00924.x. [DOI] [PubMed] [Google Scholar]
- Oshiro R, Fujioka R. Sand, soil, and pigeon droppings: Sources of indicator bacteria in the waters of Hanauma Bay, Oahu, Hawaii. Water Sci. Technol. 1995;31:251–254. [Google Scholar]
- PAHO Promoting the healthy, safe use of recreational waters. Pan Am. J. Public Health. 2003;14:364–369. [PubMed] [Google Scholar]
- PREQB [April 10, 2010];Water Quality Standards Regulation of Puerto Rico. 2010 Available from: http://www.gobierno.pr/NR/rdonlyres/5A9F2F2E-94AE-4C69-8453-CA08D616ED7D/0/Reg_Estádares_Calidad_Agua_2010.pdf.
- Prieto MD, López B, Juanes JA, Revilla JA, Llorca M, Delgado-Rodríguez M. Recreation in coastal waters: health risks associated with bathing in sea water. J. Epidemiol. Community Health. 2001;55:442–447. doi: 10.1136/jech.55.6.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruss A. Review of epidemiological studies on health effects from exposure to recreational water. Int. J. Epidemiol. 1998;27:1–9. doi: 10.1093/ije/27.1.1. [DOI] [PubMed] [Google Scholar]
- Schwab KJ. Are existing bacterial indicators adequate for determining recreational water illness in waters impacted by non point pollution? Epidemiology. 2007;18:21–22. doi: 10.1097/01.ede.0000249537.24445.c8. [DOI] [PubMed] [Google Scholar]
- Sinigalliano CD, Fleisher JM, Gidley ML, Solo-Gabriele HM, Shibata T, Plano LRW, Elmir SM, Wanless D, Bartkowiak J, Boiteau R, Withum K, Abdelzaher AM, He G, Ortega C, Zhu X, Wright ME, Kish J, Hollenbeck J, Scott T, Backer LC, Fleming LE. Traditional and molecular analyses for fecal indicator bacteria in non-point source subtropical recreational marine waters. Water Res. 2010;44:3763–3772. doi: 10.1016/j.watres.2010.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- StataCorp . Stata SE, Version 10.1. StataCorp; College Station, TX: 2009. [Google Scholar]
- Turbow DJ, Osgood ND, Jiang SC. Evaluation of recreational health risk in coastal waters based on Enterococcus densities and bathing patterns. Environ. Health Perspect. 2003;111:598–603. doi: 10.1289/ehp.5563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- USEPA [September 16, 2007];Method 1600: Enterococci in Water by Membrane Filtration Using membrane-Enterococcus Indoxyl-β-DGlucoside Agar (mEI) 2002 EPA Publication 821-R-02-022. Available from: http://www.epa.gov/nerlcwww/1600sp02.pdf.
- USEPA Questionnaires for the NEEAR study (provided by A. Dufour) 2004 [Google Scholar]
- USEPA [September 16, 2007];Beach Act. 2007 Available from: http://www.epa.gov/waterscience/beaches/rules/act.html.
- USEPA [April 1, 2008];Fecal bacteria. 2009 Available from: http://www.epa.gov/volunteer/stream/vms511.html.
- USEPA [January 24, 2012];Report on 2009 National Epidemiologic and Environmental Assessment of Recreational Water Epidemiology Studies. Tech Rep EPA/600/R-10/168. 2009 Available from: http://www.epa.gov/neear/files/Report2009v5_508comp.pdf.
- Wade TJ, Calderon RL, Brenner KP, Sams E, Beach M, Haugland R, Wymer L, Dufour AP. High sensitivity of children to swimming-associated gastrointestinal illness: results using a rapid assay of recreational water quality. Epidemiology. 2008;19:375–383. doi: 10.1097/EDE.0b013e318169cc87. [DOI] [PubMed] [Google Scholar]
- Wade TJ, Calderon RL, Sams E, Beach M, Brenner KP, Williams AH, Dufour AP. Rapidly measured indicators of recreational water quality are predictive of swimming-associated gastrointestinal illness. Environ. Health Perspect. 2006;114:24–28. doi: 10.1289/ehp.8273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade TJ, Sams E, Beach M, Brenner KP, Haugland R, Chern E, Beach M, Wymer L, Clifford CC, Love D, Li Q, Noble R, Dufour AP. Rapidly measured indicators of recreational water quality and swimming-associated illness at marine beaches: a prospective cohort study. Environ. Health. 2010;9:66–79. doi: 10.1186/1476-069X-9-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walters SP, Thebo AL, Boehm AB. Impact of urbanization and agriculture on the occurrence of bacterial pathogens and stx genes in coastal waterbodies of central California. Water Res. 2010;45:1752–1762. doi: 10.1016/j.watres.2010.11.032. [DOI] [PubMed] [Google Scholar]
- WHO [October 16, 2006];Guidelines for Safe Recreational Waters Environments. Volume 1. Coastal and Fresh Waters. 2003 Available from: http://www.who.int/water_sanitation_health/bathing/en/