Abstract
Increasing evidence link nanomaterials with adverse biological outcomes and due to the variety of applications and potential human exposures to nanoparticles it is thus important to evaluate their toxicity for the risk assessment of workers and consumers. It is crucial to understand the underlying mechanisms of their toxicity as observation of similar effects after different nanomaterial exposures does not reflect similar intracellular processing and organelle interactions. A thorough understanding of mechanisms is not only needed for accurate prediction of potential toxicological impacts but also for the development of safer nanoapplications by modulating the physico-chemical characteristics. Furthermore biomedical applications may also take advantage of an in depth knowledge about the mode of action of nanotoxicity to design new nanoparticle-derived drugs.
In the present manuscript we discuss the similarities and differences in molecular pathways of toxicity after carbon black and TiO2 nanoparticle exposures and identify the main toxicity mechanisms induced by these two nanoparticles which may also be indicative for the mode of action of other insoluble nanomaterials. We address the translocation, cell death induction, genotoxicity and inflammation induced by titanium dioxide and carbon black nanoparticles which depend on their internalisation, ROS production capacities and/or protein interactions. We summarise their distinct cellular mechanisms of toxicity and the crucial steps which may be targeted to avoid adverse effects or to induce them for nanomedical purposes. Several physico-chemical characteristics could influence these general toxicity pathways depicted here and the identification of common toxicity pathways could support the grouping of nanomaterials in terms of toxicity.
Keywords: Nanotoxicology, mechanisms, carbon black, titanium dioxide TiO2, nanoparticle and nanomaterial, adverse effects, signaling, apoptosis, inflammation, protein interactions, translocation, grouping, safer by design
Introduction
Nanomaterial induced biological processes are among the most studied topics in the recent literature. However, molecular mechanisms leading to these processes are not fully understood and are hot topics for further explorations. Increasing evidence link nanomaterials with adverse biological outcomes and a thorough understanding of underlying mechanisms is not only needed for accurate prediction of potential toxicological impacts but also for the development of safer nanotechnology applications or to conceive new biomedical applications.
Titanium dioxide (TiO2) nanoparticles are increasingly used for various industrial and consumer product applications including paints, waste water treatment, sterilization, cosmetics, food additive, biomedical ceramic and implant biomaterials. Current estimates indicate that 3800–7800 tons of nano-TiO2 are produced annually in the US1. Woodrow Wilson Center database indicate that approximately 9% of the consumer products currently listed to contain nanomaterials contain TiO2 nanoparticles2. These products include but are not limited to personal care products such as sunscreens, cosmetics, and food products. Nano TiO2 is also used in biomedical applications like scaffolds, coatings and dental implants. Both nano and micro TiO2 are useful tools in tissue engineering to repair, replace or enhance tissue function3. Due to the wide variety of applications, TiO2 nanoparticles present substantial potential for human exposures. Human exposures can occur during manufacturing and utilization of these nanoparticles. Limited data are available on human exposure to TiO2 nanoparticles through inhalation (most relevant route for toxicological outcomes). Based on rodent chronic inhalation studies, NIOSH has recommended airborne exposure limits of 0.3 mg. m−3 (10 h/day, 40 h/week) for workplace exposure and 1.2mg/m3 by Japanese NEDO (8 h/day, 40 h/week)4. Other major routes for TiO2 exposures are dermal (through application of cosmetics and sunscreens) and gastrointestinal (through food (E171 food additive etc)). However, minimal toxic responses are observed after exposure through both of these routes.
Carbon Black (CB) nanoparticles are produced in huge quantities and current worldwide production is about 8.1 million metric tons (http://www.carbon-black.org/files/carbonblackuserguide.pdf). Major applications of CB nanoparticles are in rubber (90% of the production), and pigment industry (9%). It is important to note that engineered CB particles differ significantly from soot particles which are unwanted carbonaceous by-products of incomplete combustion of hydrocarbon-containing materials. These differences include purity (higher in CB which is nearly 97% carbon) surface uniformity of nodules (higher in CB), agglomeration (higher in CB) and polycyclic aromatic hydrocarbon content (higher in soot). In working environments, daily doses of CB can reach up to 0.12 mg/kg (assuming a threshold limiting value (TLV) for respirable CB of 2.5 mg/m3, 60% deposition, an average weight of a person of 70 kg, an 8 hour work day, and a respiratory volume of 0.7 m3 pr. hour)5.
Due to the variety of applications and potential human exposures to CB and TiO2 nanoparticles it is thus important to evaluate their toxicity for the risk assessment of workers and consumers. It is crucial to understand the underlying mechanisms of their toxicity as observation of similar effects after different nanomaterial exposures does not reflect similar intracellular processing and organelle interactions. Modern approaches of toxicology focus on the understanding of molecular and cellular pathways rather than just investigating the final outcome as this may allow the identification of possible early stage effects or responses to low doses. Thus, it is imperative to meticulously elaborate molecular pathways after nanomaterial exposure to make well informed decisions about nano-safety and for the development of safer nanomedical applications.
Nanomaterial induced toxicity cannot be explained by any single factor and a combination of physical (physicochemical characteristics of particles) and biological (cell type and nano-bio interactions) factors determines the final outcome. These factors also dictate what type of intracellular signaling cascades will be activated after nanomaterial exposure. CB and TiO2 are both low-solubility nanoparticles but differ in many physicochemical characteristics including crystal structure, photo activation, Reactive oxygen species generation abilities and surface reactivity. These may not only influence the final outcome of exposure but also explain differences in cellular mechanisms. Unfortunately very few studies directly compared the effect of these two nanomaterials and thus this review aim to elucidate their respective toxicity mechanisms. We illustrate that CB and TiO2 nanoparticles induce similar effects (apopotosis, genotoxicity, inflammation etc) but through very distinct mechanisms. In the present manuscript we discuss the similarities and differences in molecular pathways of toxicity after CB and TiO2 nanoparticle exposures and identify the main toxicity mechanisms induced by these two nanoparticles which may also be indicative for the mode of action of other low-solubility nanomaterials. Several physico-chemical characteristics could influence these general toxicity pathways depicted in this review and the identification of common toxicity pathways could support the grouping of nanomaterials in terms of toxicity.
Biodistribution of TiO2 and carbon black nanoparticles
The deposition of nanoparticles after inhalation is more dependent on the aerodynamic or thermodynamic diameter of the nanoparticle aggregates than on their chemical nature6. However, the alveolar clearance through nanoparticle uptake by macrophages could be influenced by the composition and interaction of nanoparticles with the lung lining fluids will also depend on surface characteristics such as charge and hydrophobicity which differ between TiO2 and carbonaceous nanoparticles. Translocation of nanoparticles to the systemic circulation has been observed after inhalation but the fraction of nanomaterials reaching the blood circulation is generally very low4, 6, 7. This translocation towards blood and secondary organs is influenced by the chemical composition of the nanomaterial. Indeed, the biodistribution of iridium nanoparticles is much higher (10%) than those of CB and TiO2 nanoparticles which demonstrated similar translocation (3% and 2% respectively7).
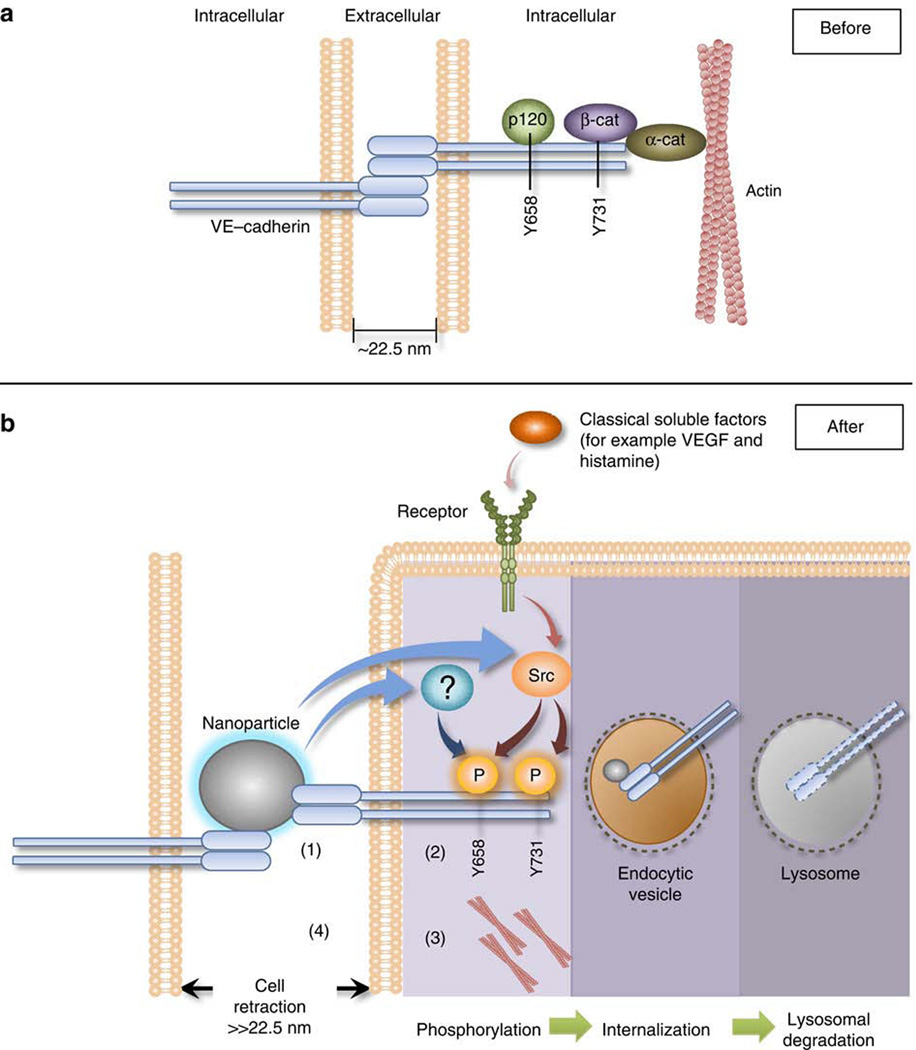
Translocation of nanomaterials through biological barriers depends on the physico-chemical characteristics such as size, shape and surface charge, especially if the nanomaterial crosses the epithelial or endothelial barrier by transcytosis involving endocytic mechanisms. It has for instance been shown that neutral or positively charged TiO2 nanoparticles are taken up by airway epithelial cells in contrast to negatively charged TiO28. However, the paracellular passage of the endothelium may be a less specific process as nicely illustrated by a mechanistic study showing that nanoparticles could enter the intercellular space and disrupt adherens junctions (Figure 1). This disruption has been observed with several nanomaterials including TiO2 nanoparticles9 and was attributed to the small size (less than 22.5 nm) of the particles allowing their penetration into the intercellular space where the nanoparticles could get in contact with the junctional protein Vascular Endothelial (VE)-cadherin disrupting their interactions and inducing the phosphorylation of VE-cadherin and subsequent cellular signal transduction pathways leading to cell retraction through actin remodeling. This direct physical interaction with VE-cadherin led thus to endothelial leakiness9 which has been confirmed in vivo after subcutaneous and intravenous injections of TiO2. Epithelial and blood-brain barriers present however also tight junctions to prevent passage of molecules and which may be less easily disrupted by nanomaterials.
Figure 1. Proposed mechanism of Nano-Endothelial Leakiness.
a) The anatomy of an adherens junction. Intact monolayer of connected endothelial cells is maintained by stable VE–cadherin homophilic interactions with neighbouring cells. VE–cadherin forms a trans-homophillic interaction at the EC domains with another cis-paired VE–cadherin complex. β-catenin, p120 and VE–cadherin form a complex. Formation of this ternary complex stabilizes the adherens junction. Distance of adherens junction is at least 22.5 nm.
b) TiO2–NM are small enough to migrate into the adherens junction; they bind and disrupt VE–cadherin homophilic interaction (1). This disruption induces the phosphorylation of Y658 of VE-cadherin via a currently unknown kinase pathway, while the Y731 residue is phosphorylated by Src kinase. The phosphorylation at the two residues induces the loss of interaction between VE–cadherin and β-catenin and with p120 (2). The loss of interaction of the VE–cadherin–β-catenin–p120 complex destabilizes actin and lead to actin remodelling (3). As a result the cell retracts and leakiness occurs (4). After the binding of TiO2-NM to VE–cadherin, VE–cadherin might be internalized and further degraded by lysosomes. Fate of VE–cadherin: phosphorylation of VE–cadherin due to NanoEL may result in internalization and lysosomal degradation. This minimizes the overall amounts of VE–cadherin near the vicinity of the cell membrane. TiO2–NM might be internalized alongside VE–cadherin as it remained bound to VE–cadherin but the final fate of the TiO2–NM is uncertain. From Setyawati et al. 20139.
Reproduced by permission of Nature Publishing Group.
Cell death mechanisms and genotoxicity induced by TiO2 and carbon black nanomaterials
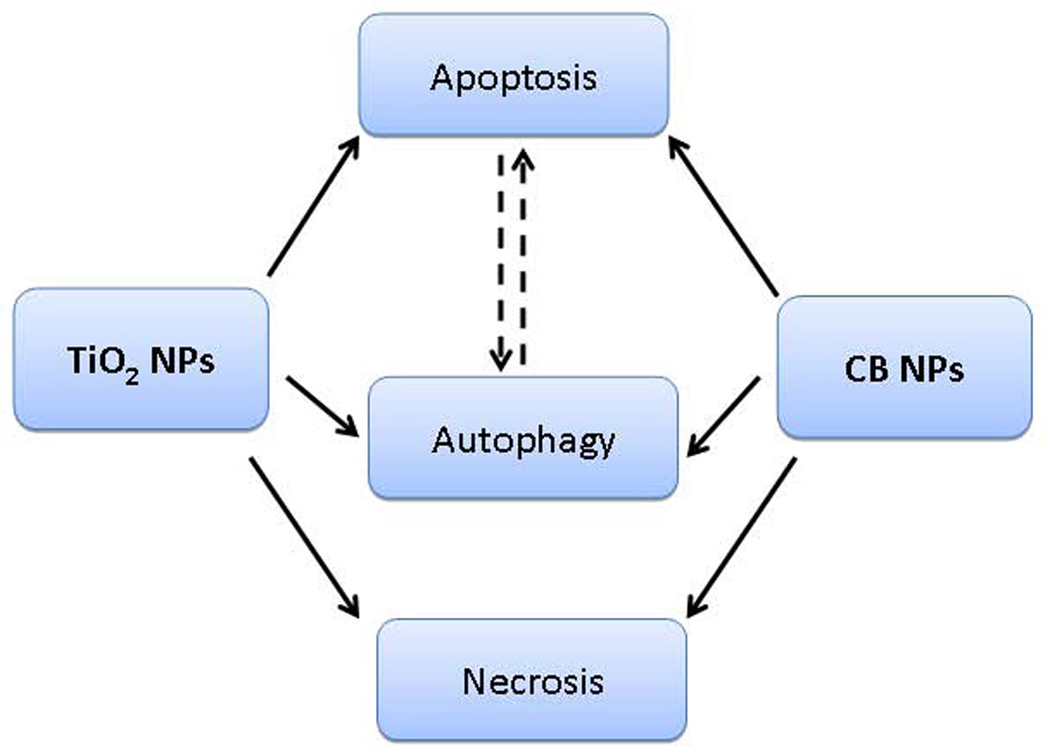
While induction of cell death may be beneficial in some cases (e.g. killing of tumor cells during therapy), induction of exuberant cytotoxicity in healthy tissue usually ensue deleterious consequences. TiO2 and CB nanoparticle exposures result in induction of a variety of cell death modalities (Figure 2), and thus lead to distinct pathophysiological consequences. Uncontrolled cell death by necrosis will lead to inflammatory outcomes whereas controlled cell death, such as apoptosis induction, will avoid inflammation. On the other hand cells could also die through an inflammatory cell death called pyroptosis or cell death could be prevented by autophagy, a process which allows to eliminate damaged cell structures or organelles (for review10). These different types of cell deaths (necrosis, apotosis and pyroptosis) could be activated by nanomaterials through specific molecular and cellular signaling pathways (for review11). Physiologically, apoptosis of for instance airway epithelial cells functions as 1) mechanism to reduce the hyperplastic cell numbers due to allergen or chemical induced hyperplasia 2) eliminate damaged cells 3) control inflammation and thereby support barrier function12. However, an exuberant apoptotic response is noted in various airway pathologies e.g chronic obstructive pulmonary disease (COPD), asthma, cystic fibrosis and interstitial lung disease. Viral/bacterial infections could lead to apoptosis to protect the organism against these invading pathogens and environmental particle exposures have also been shown to induce apoptosis.
Figure 2. Types of cell death induced by CB and TiO2 nanoparticles.
Various modalities of cell death could be induced by CB and TiO2 NPs in human cells.
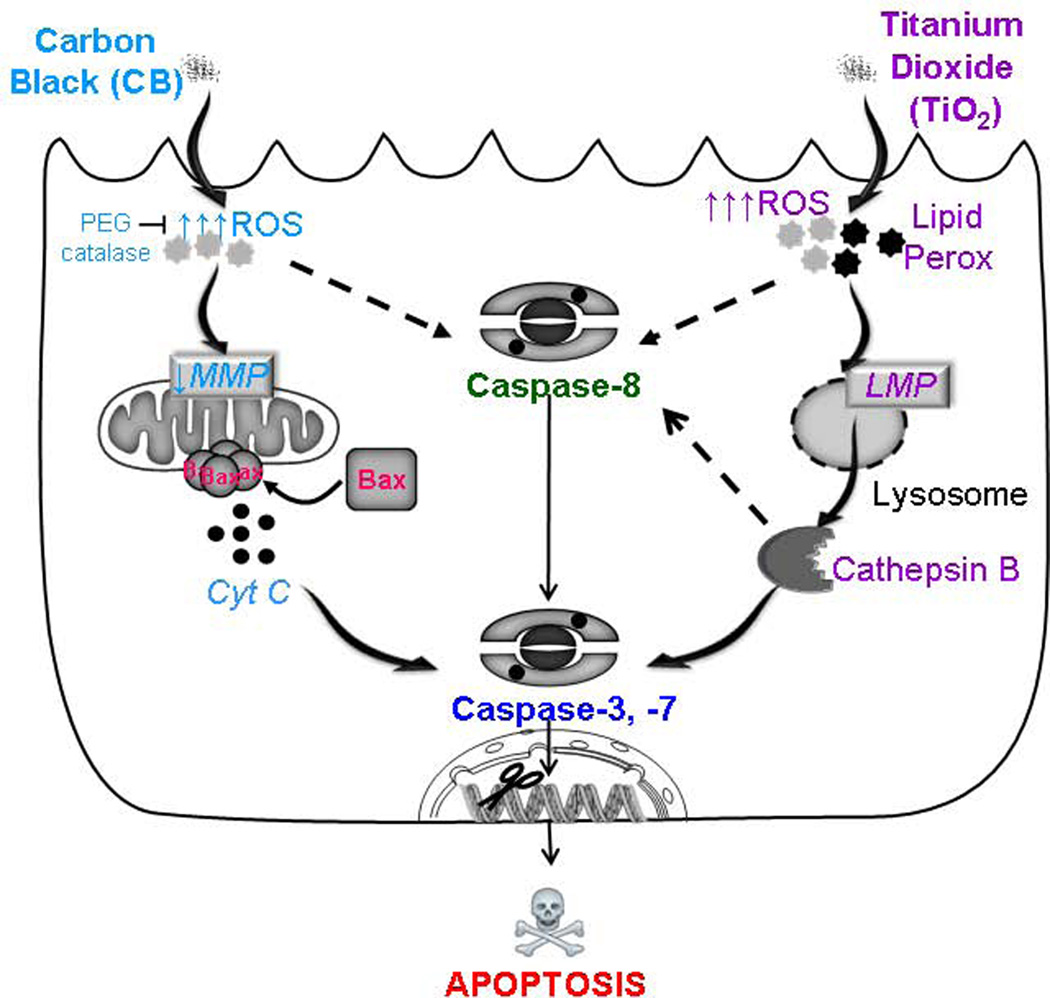
Various in vitro and in vivo studies have demonstrated the ability of CB or TiO2 nanoparticles to induce toxicity towards the respiratory system but most of them did not address the molecular pathways of toxicity. A detailed comparative analysis of CB and TiO2 nanoparticle induced toxic mechanisms in human bronchial epithelial cells (transformed and primary cells) was performed13 and an overview of the resulting biological mechanisms is presented in figure 3. CB and TiO2 nanoparticle exposure for at least 4 hours was sufficient to induce significant toxicity in bronchial epithelial cells. Both types of nanoparticles induced cell death through apoptosis induction at same concentrations (20 µg. cm−2) but through distinct pathways. CB nanoparticle exposure induced mitochondrial membrane potential loss (↓∆Ψm), activation of the pro-apoptotic protein BAX and release of cytochrome c from the damaged mitochondria. In contrast, TiO2 nanoparticle exposure led to lipid peroxidation, lysosomal damage, and release of cathepsin B.
Figure 3. Schema of the hypothetic pathways of cell death induction by CB and TiO2 NPs in bronchial epithelial cells.
CB NP induce apoptosis by a ROS dependent mitochondrial pathway involving loss of the mitochondrial membrane potential, activation of bax and release of cytochrome c resulting in activation of caspases and subsequent DNA fragmentation. TiO2NPs induce cell death through lipid peroxidation and lysosomal membrane destabilization leading to cathepsin B release and subsequent activation of caspases and final apoptotic events. Modulation of oxidative stress by PEG catalase prevents cell death by blocking downstream events only in case of CB NPs. (Image drawn in part using Servier medical art)(From Hussain et al. 201013).
Differences in signaling cascades for apoptosis induction by CB and TiO2 nanoparticles can be explained partly by their inherent capacity to generate reactive oxygen species (ROS) under acellular conditions14. CB nanoparticles demonstrated particle size/surface area dependent oxidation of DTT (dithiothreitol) while TiO2 nanoparticles were minimally reactive. These cellular assays may however give false negative findings due to similar redox potentials between the test substance (nanomaterials) and detection agents (DTT, ascorbate) but inside the cells multiple redox partners are present with varying redox potentials which could interact with the nanomaterials. Apoptotic effects of CB nanoparticles were completely inhibited by a specific hydrogen peroxide (H2O2) scavenger (pegylated catalase) while there was no significant effect on TiO2 nanoparticle induced toxicity. This further confirmed that CB induced H2O2 mediated apoptosis (partly produced extracellular by surface reactions) while TiO2 induced different types of ROS as the cellular effects were not inhibited by this H2O2 scavenger. ROS production by CB nanoparticles has also been shown to be responsible for the induction of apoptosis through activation of epidermal growth factor receptor (EGFR) and downstream signaling15. Another alternative cell death induction mechanism induced by nanomaterials is cell death receptor activation and TiO2 nanoparticle exposure has been shown to activate FAS receptor4.
It is interesting to note that these distinct signaling cascades of TiO2 and CB nanoparticles depicted in figure 3 were not due to differential localization or uptake kinetics of these nanomaterials16. However, TiO2 nanoparticles formed larger sized intracellular aggregates with sharp edges which potentially explain the selective lysosomal damage induced by these nanomaterials leading to the release of lysosomal proteins as well as TiO2 NPs into the cytoplasm which are thus no longer membrane bound. This is in line with TEM images showing that CB nanoparticles are mostly present in membrane bound vesicles in contrast to TiO2 nanoparticle aggregates which could be observed in membrane bound vesicles as well as free in the cytoplasm14.
Several studies have confirmed the induction of lysosomal perturbations by TiO2 in different cell types4 and membrane damages, either physically or through ROS production by TiO2 nanoparticles, resulted in dose dependent hemolysis of erythrocytes17. Attachment of nanoparticles induced erythrocyte surface changes leading to hemagglutination and abnormal sedimentation17. Both nano and micro sized particles of TiO2 were also shown to induce hemolysis in human erythrocytes18. However, it is unlikely that TiO2 exposures could cause intravascular hemolysis as plasma was shown to effectively abolish this response.
Interestingly, some reports describe that TiO2 nanoparticles induce mitochondria mediated apoptosis in other cell types and mitochondrial dysfunction in vivo4,19,20. The mitochondrial damage by nanoparticles could however be induced indirectly after lysosomal rupture through intracellular ROS production or lysosomal hydrolase release10. Discrepancies between studies may also be due to cell type, concentrations, endocytic mechanisms and nanomaterial used. Indeed, crystal structures (anatase or rutile) or size are shown to determine OHo radical generation by TiO2 nanoparticles21 and these parameters influence accordingly the apoptosis induction as anatase TiO2 nanoparticles had greater effects in neuronal cells than rutile nanoparticles and nanometer sized TiO2 was more cytotoxic than micrometer TiO220.
TiO2 and CB nanoparticles have also been shown to induce genotoxicity in vitro and in vivo demonstrating their clastogenicity, mutagenicity and carcinogenicity4,22. Even though some investigations have shown negative results, several studies have evidenced the induction of micronuclei, gene mutations, DNA strand breaks, chromosomal alterations and cell transformations mostly through ROS dependent but also some ROS independent mechanisms. For instance, TiO2 nanoparticles induced ROS independent phosphorylation of H2A histone family member X (H2AX), a sensitive marker of DNA strand breaks, in an internalization dependent manner23. Interestingly, surface coating of nanoparticles with albumin attenuates phosphorylation of histone H2AX. The reduction of TiO2 nanoparticle toxicity by coating was also observed in other studies using PEGylation of TiO2 or silica coating (for review4). Genotoxic effects of nanoparticles are also observed in vivo but are often reported to be due to the induction of inflammation leading to oxidative stress. The role of inflammation in TiO2 nanoparticle genotoxicity has been evidenced in the lung but also in spleen, kidney and liver (for review4). However, DNA damage in the liver has also been shown to be due to direct interaction of TiO2 nanoparticles with oxygen or nitrogen atoms of the nucleotides forming P-O(N)-TiO bonds24.
Toxicity mechanisms leading to inflammation
Many in vitro studies have shown pro-inflammatory responses after treatment with these two nanoparticles involving mitogen-activated protein kinases (MAPK), nuclear factor-kappaB or receptor activation to induce cytokine production (for review4,25). Some comparative studies report however different inflammogenic potencies of TiO2 and CB nanoparticles. For instance, exposure of lung fibroblasts to TiO2 nanoparticles induced IL-1beta secretion and subsequent MMP1 induction in contrast to CB nanoparticles which had no effect26. This could be due to the activation of the inflammasome by TiO2 nanoparticles which allows the maturation of pro-IL1beta by caspase-1 activation. Cathepsin B could activate the inflammasome and it has indeed been shown that Il-1beta release in response to rutile and anatase TiO2 nanoparticles is dependent on active cathepsin B and caspase-127. The release of cathepsin B from lysosomes has been demonstrated after TiO2 nanoparticle treatment due to lysosomal rupture as reported above13. Thus, apoptosis induction and pro-inflammatory responses in response to TiO2 nanoparticle exposure could be induced by the same mechanism of inflammasome activation through lysosomal desatbilisation. Contradictory results were however obtained by Reisetter et al28 who compared the toxicity mechanisms of CB and TiO2 nanoparticles in alveolar macrophages showing that TiO2 nanoparticles did not elicit toxicity at the tested concentrations which may however be due to different types of TiO2 nanoparticles used. Furthermore, at equal mass the CB nanomaterial used in this study had a much greater surface area compared to the TiO2 nanoparticles which may also explain the greater effect of the CB samples. Interestingly, they demonstrated that CB nanoparticles induced an inflammasome dependent pyroptosis, a specific form of inflammatory cell death that requires caspase-1 activity. This activation could however be due to ROS production which are also able to activate caspase-129 and further studies are necessary to confirm these novel results and to understand the underlying mechanisms.
Inflammation after in vivo subchronic inhalation of TiO2 and CB nanoparticles has been illustrated30 but was often attributed to lung overload. TiO2 and CB nanoparticles induced similar inflammatory potency after 24h at same surface area doses31 but interestingly comparative time course studies have however shown a more persistent inflammation for TiO2 nanoparticles compared to CB nanoparticles. Short term inhalation confirmed the induction of inflammation by TiO2 but not CB nanoparticles30.
Nanoparticles can also have immunomodulatory effects32 which have been demonstrated for TiO2 as well as carbonaceous nanomaterials. Low doses of TiO2 nanoparticles, which did not induce inflammation in healthy animals, induced epithelial damage and aggravated inflammatory and asthmatic responses in a mouse model of occupational asthma33 and a similar effect was observed for CB nanoparticles in ovalbumin sensitized mice34. In another study it was demonstrated that nano TiO2 significantly increase the dermal sensitization potency of a known skin sensitizer by skewing the immune response towards a Th2 phenotype35. It is worth mentioning that the micro-scale form of TiO2 failed to elicit similar effects. These findings demonstrate the ability of TiO2 as well as CB nanoparticles to induce toxicity in compromised subjects with pre-existing conditions and modulate the immune responses. The underlying molecular and cellular mechanisms are however still unclear. This immunomodulatory effect could be due to activation of dendritic cells as several in vitro studies have shown maturation of dendritic cells by TiO2 and CB36,37. However, for the moment the findings are still contradictory as some studies report maturation towards Th1-biased responses via activation of the inflammasome complex36 and a protective effect of TiO2 in asthmatic animals38 while others demonstrate induction of Th2 type37,33,34,35 responses (or Th1 as well as Th2 responses39). More immunotoxicological studies are thus needed to clearly establish the immunomodulatory effect of these NPs and to understand the differences in these studies which could be due to different concentrations, treatment times or different nanoparticles used.
The cardiovascular effects induced by nanomaterials may either be due to direct interactions of nanomaterial reaching the bloodstream or to indirect consequences of inflammation induced in target organs. For instance, acute and chronic inflammation could induce the so called acute phase response which is strongly linked to increased risk of cardiovascular disease. Saber et al40 have shown that pulmonary inflammation after nanomaterial exposure, including TiO2 and CB nanoparticles, correlated strongly with acute phase protein expression in the lung but interestingly not in the liver. The authors state that this pulmonary acute phase response may constitute a direct link between particle inhalation and risk of cardiovascular disease and may be a predictive biomarker. Indeed, the acute phase protein serum amyloid A which was increased by TiO2 and CB nanoparticles is known to promote atherosclerotic plaque progression and impairment of endothelial dysfunction which have been reported after exposure to nanoparticles including TiO2 and CB4, 41.
Consequences of the interaction of nanomaterials with proteins
Another probable mechanism through which CB and TiO2 nanomaterial can induce toxicity include their propensity to interact with proteins. The adsorption of proteins on the surface of nanomaterials in physiological environments leads to the formation of the so called protein corona which has been extensively studied42. This corona could modulate the effect of nanomaterials by influencing the aggregation state, biodistribution, cellular uptake and reactivity of the nanomaterials. To predict the protein affinity and selectivity in a corona formation process, a biological surface adsorption index (BSAI) was developed and specific nanodescriptors representing different adsorption forces were defined43. This approach allowed to cluster successfully metal oxide nanomaterial such as TiO2 nanoparticles as weak and carbonaceous nanomaterial as high adsorption materials.
On the other hand, this interaction of nanomaterials with proteins could impact the activity of the protein and thus have important physiological consequences. Interaction of nanomaterials with proteins essential for cellular functions could inactivate them either by conformational changes and/or denaturation. Both CB and TiO2 nanoparticles were shown to inhibit enzyme activity and induce conformational changes in Arylamine N-Acetyletransferase-144,45, a xenobiotic metabolizing enzyme involved in activation/detoxification of carcinogens. Although in this case both nanomaterials induce similar effects, the magnitude of the response varies hugely. Interestingly, the interaction of nanomaterials with proteins could also lead to gain of function42 as shown for fibrinogen unfolding and subsequent receptor activation after binding to gold nanoparticles. A variety of other proteins are also known to bind with either CB or TiO2 nanomaterials including cytokines. A comparative study has shown that both, CB and TiO2 nanoparticles, adsorbed cytokines but the binding was dependent on the nature of cytokine as well as on the nanoparticles46. Furthermore, binding of nanoparticles to proteins of the complement system could induce the activation of this innate immune system32.
Interactions of several nanomaterials (including TiO2) with the cytoskeleton have also been reported, leading to retardation of cell migration due to destabilization of microtubule network47. Impairment of the cytoskeleton could, beside other cellular perturbations, disturb lysosomal trafficking leading to autophagy dysfunctions which are commonly observed after nanomaterial exposures (for review10). As discussed above, nanoparticles able to enter the intercellular space have been shown to bind to junctional proteins inducing adherence junction rupture and induction of intracellular signal transduction pathways leading to cell retraction and endothelial leakiness9. The interaction of nanomaterials with ion channels or cellular receptors, either directly or through the protein corona, could also induce cell signaling. It has for instance been shown that TiO2 nanoparticles could activate membrane L-type calcium channels and Toll Like Receptors (TLR) and respective downstream signaling4 which however needs to be confirmed. Cell death receptors could also be induced by nanomaterials as discussed above. CB nanoparticles induce EGFR signaling leading to disruption of gap junctional intercellular communication4,48, cell proliferation and apoptosis15. CB nanoparticles may however activate EGFR indirectly through ROS production and activation of lipid raft signaling49.
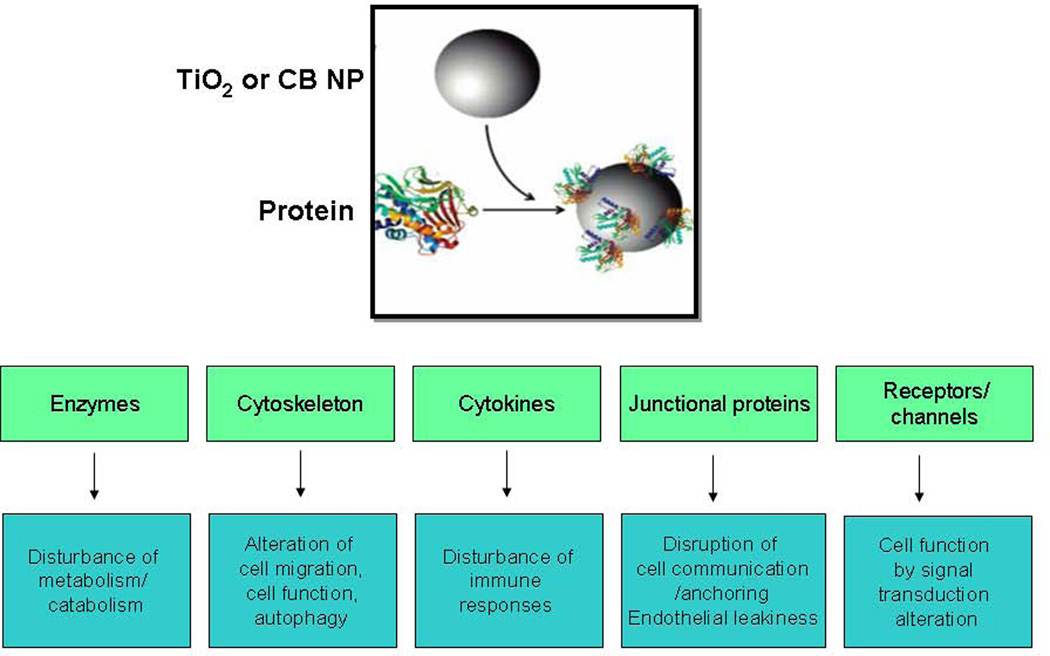
The binding/adsorption of TiO2 and CB nanoparticles to proteins can thus lead to unpredicted consequences. On the one hand the protein corona which will form around the nanomaterials in physiological environments will influence the aggregation state, biodistribution, cellular uptake and reactivity of the nanomaterials. On the other hand the interaction of nanomaterials with different types of proteins could lead to activation/inhibition of surface or cellular proteins which could induce a variety of changes in normal cell function (depicted in Figure 4). The interaction with enzymes or cytokines could lead respectively to disturbance of the metabolism and immune responses while the modification of structural proteins such as cytoskeleton or cell junctional proteins could lead to important changes in cell functioning (autophagy, cell migration/anchoring, cell communication or barrier function). The normal cell function could also be disrupted by an interaction of nanomaterials with proteins involved in signal transduction pathways such as receptors or ion channels, leading to the activation or inhibition of cell signaling. Unfortunately, there are not enough current research efforts for these adverse scenarios and there is urgent need to explore these processes as these different protein-nanomaterial interactions could induce important perturbations of cell function which may ultimately modulate or induce diseases ranging from immune, neurodegenerative or metabolic disorders to cancer.
Figure 4. Consequences of protein-nanomaterial interactions.
Nanomaterials can interact with different types of proteins which could lead to a variety of changes in normal cell function.
Conclusions
TiO2 and CB nanoparticles could induce a large variety of cellular mechanisms leading to toxicity, some of which are similar between these two nanomaterials and represent maybe general pathways of nanotoxicity, whereas others are specific to each particle due to their different physico-chemical characteristics. Understanding the underlying mechanisms which drive the cellular effects is not only important to predict the toxicity of nanomaterials but also to allow their grouping in terms of hazard characterisation. A thorough mechanistic comprehension is also necessary to produce nanomaterials safer by design by modulating the physico-chemical characteristics responsible for the induction of signalling pathways leading to adverse effects, especially in case of utilization of nanomaterials in nanomedicine. On the other hand biomedical applications may also take advantage of an in depth knowledge about the mode of action of nanomaterial toxicity to design new nanoparticle-derived drugs.
Table 1 and figure 5 summarise the distinct cellular mechanisms of toxicity induced by these two nanomaterials and the crucial steps which may be targeted to avoid adverse effects or in contrast to induce them for nanomedical purposes. Other nanomaterials could share these mechanisms induced by TiO2 and/or CB nanoparticle and deciphering their distinct mode of action or similarities may thus allow depicting some common nanotoxicological pathways and physico-chemical characteristics responsible for their toxicity.
Table 1.
Summery of the cellular effects and pathways induced by CB and TiO2 nanoparticles (the most relevant references discussed in this review are indicated by their respective reference number).
| Protein interactions | Junctional proteins (TiO29) |
| Metabolizing enzymes (CB44,45, TiO245) | |
| Cytoskeleton (TiO247) | |
| Membrane proteins (CB48,15, TiO24) | |
| Cytokines (CB46, TiO246) | |
| Apoptosis | Mitochondrial pathway (CB13, TiO24,20) |
| Lysosomal pathway (TiO24,13) | |
| Cell death receptors (TiO24) | |
| Pyroptosis (CB28) | |
| Autophagy | Induction or disturbance (CB10, TiO210) |
| Genotoxicity | Clastogenic, mutagenic, carcinogenic (CB22, TiO222,23,24) |
| Immune response | NF-κB pathway activation (CB25, TiO24) |
| Inflammasome activation (CB28, TiO227) | |
| Immunomodulatory effects (CB34,37,39, TiO233,35,36,38) | |
| Cardiovascular effects | Endothelial dysfunction (CB41, TiO24) |
| Acute phase response (CB40, TiO240) |
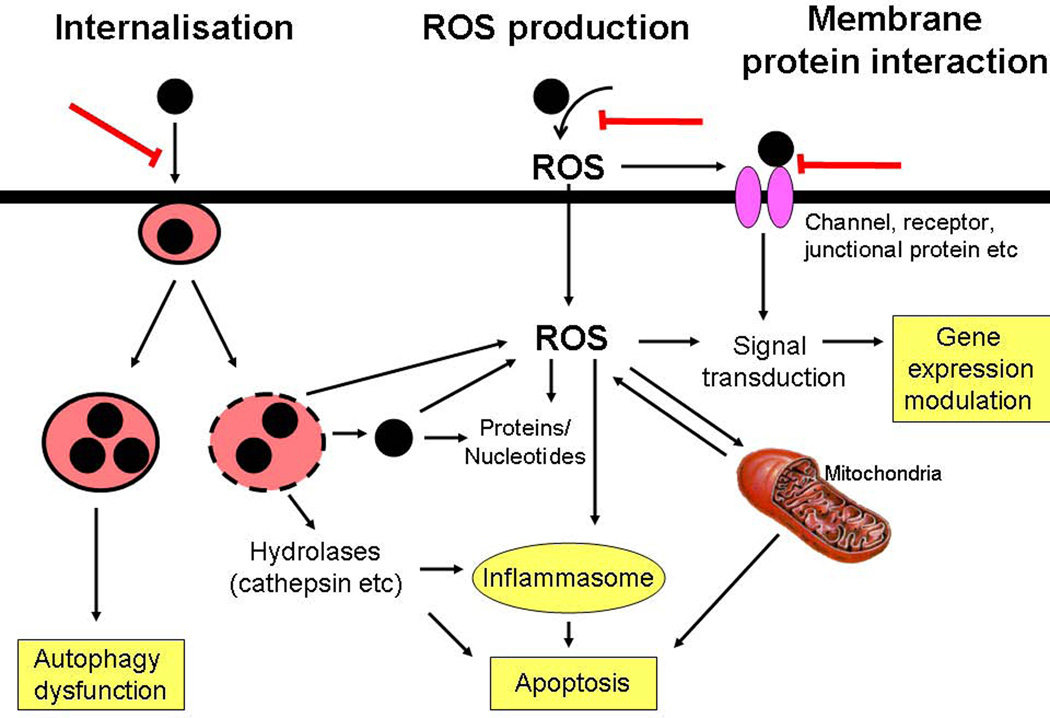
Figure 5. Main toxicity pathways induced by TiO2 and CB nanoparticles.
Three main pathways could be identified which are induced by TiO2 and/or CB nanoparticles and which could be shared by other nanomaterials. The internalization of nanomaterial could lead to lysosomal destabilization or accumulation. Nanomaterials could produce reactive oxygen species through surface reactions. The interaction of nanomaterial with membrane proteins could lead to activation or inhibition of cell signaling pathways. These different pathways could induce autophagy, inflammasome activation, apoptosis or gene expressions and crucial steps may be targeted to avoid these cellular effects (red bars).
The internalisation of TiO2 nanoparticles is a crucial step for their toxicity as the accumulation in lysosomes leads to their rupture and release of hydrolases such as cathepsins and intracellular ROS production. Furthermore, nanomaterials will also be released into the cytoplasm allowing their access to essential biomolecules or oxidative stress induction. Predictive toxicology or strategies to prevent TiO2 toxicity may thus focus on the endocytosis of TiO2 or other nanomaterials able to destabilise lysosomes. For instance, negative surface charge or coating of TiO2 nanoparticles successfully prevented uptake and toxicity. In addition, accumulation of nanomaterial within intact lysosomes may also lead to perturbation of normal cellular functioning such as autophagy dysfunction. Conversely, CB nanoparticles induce toxic mechanisms mainly through ROS production which already occurs extracellularly. Inhibiting the internalisation of CB nanoparticles may thus not be sufficient to avoid toxicity and reduction of surface reactivity could instead be aimed to reduce toxicity. It is important to decipher the chronology of the entire cellular mechanism as for instance the destabilisation of lysosomes could induce the production of ROS which in turn can activate signalling pathways or alter mitochondria leading to apoptosis. CB, TiO2 nanoparticles as well as many other nanoparticles could interact directly with membrane proteins (channels, receptors or junctional proteins etc) responsible for the induction of signal transduction pathways leading to altered gene expressions etc. Studying and predicting the adsorption capacities of nanomaterials and protein interactions is thus not only important for determining the protein corona in physiological environments, but also to understand the possible activation/inhibition of surface or cellular proteins.
In conclusion, three main modes of toxicity mechanisms could be identified: internalisation with subsequent lysosomal destabilization or accumulation, ROS production capacities and protein interactions. Many of the toxic effects observed after nanomaterial treatment could be explained by these three modes of actions described here, yet other mechanisms could also be induced by nanomaterials, especially if they are soluble. Cross activations and retroactions of these pathways exist such as activation of membrane proteins by ROS etc and this schematic illustration of nanomaterial toxicity mechanisms maybe incomplete and not represent the whole complexity of cellular mechanisms. The toxicity mechanisms triggered by nanomaterials will also depend on the cell-type used (depending on their phagocytic capacities, anti-oxidant defence mechanisms etc), the dose and coating of the nanomaterial by bio-molecules present in body fluids. Further studies are needed to confirm the importance of these mechanisms and to verify whether these are representative of other low-solubility nanomaterials. More comparative mechanistic studies are needed which should not only compare different physicochemical characteristics of the same nanomaterial but also evaluate other types of nanomaterials with similar properties in parallel. This identification of common toxicity pathways will support the grouping of nanomaterials in terms of toxicity and could help to identify relevant endpoints for qualitative structure-toxicity relationship modelling approaches. Several physico-chemical characteristics will indeed influence these three general toxicity pathways depicted here and represent promising tools to design safer or more reactive nanomaterials for biomedical applications.
Acknowledgement
This work was supported (in part) by the Intramural Research Program of the NIH, National Institute of Environmental Health Sciences (NIEHS) and by the French National Research Agency (ANR) and the German Federal Ministry of Education and Research (BMBF), under the frame of SIINN, the ERA-NET for a Safe Implementation of Innovative Nanoscience and Nanotechnology.
Abbreviations
- ROS
Reactive oxygen species
- CB
Carbon black
- EGFR
Epidermal growth factor receptor
- VE-cadherin
Vascular endothelial-cadherin
- TiO2
Titanium dioxide
- H2AX
H2A histone family member X
Footnotes
Conflict of interest
All authors disclose any potential sources of conflict of interest.
FURTHER READING/RESOURCES
Books:
Systems Toxicology : from omics technology to nanotechnology » Eds: Casciano DA. and Sahu SC, John Wiley and Sons Ltd. (2011)
Encyclopedia of nanotechnology, Ed: Bharat Bhushan, Springer. (2011)
Nanoethics and Nanotoxicology, Eds: Houdy P., Lahmani M., Marano F., Springer (2011)
Nanomaterials: A Danger or a Promise? Eds : Brayner R. Fievet F., Cordani T., Springer (2013)
Nanotoxicology (second edition): Progress Towards Nanomedicine, Eds: N.A. Monteiro-Riviere and C. L. Tran, CRC press (2014)
Nanomaterial: Impacts on Cell Biology and Medicine, Eds: Y Chen and D. Capco, Springer (2014)
References
- 1.Hendren CO, Mesnard X, Droge J, Wiesner MR. Estimating production data for five engineered nanomaterials as a basis for exposure assessment. Environ Sci Technol. 2011;45(7):2562–2569. doi: 10.1021/es103300g. [DOI] [PubMed] [Google Scholar]
- 2.PEN. The project on emerging nanotechnologies. Washington, DC, USA: The Woodrow Wilson International Center for Scholars; 2014. [Google Scholar]
- 3.Schanen BC, Karakoti AS, Seal S, Drake DR, Warren WL, Self WT. Exposure to titanium dioxide nanomaterials provokes inflammation of an in vitro human immune construct. ACS Nano. 2009;3(9):2523–2532. doi: 10.1021/nn900403h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shi H, Magaye R, Castranova V, Zhao J. Titanium dioxide nanoparticles: a review of current toxicological data. Part Fibre Toxicol. 2013;10:15. doi: 10.1186/1743-8977-10-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vesterdal LK, Folkmann JK, Jacobsen NR, Sheykhzade M, Wallin H, Loft S, Moller P. Pulmonary exposure to carbon black nanoparticles and vascular effects. Part Fibre Toxicol. 2010;7:33. doi: 10.1186/1743-8977-7-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Geiser M, Kreyling WG. Deposition and biokinetics of inhaled nanoparticles. Part Fibre Toxicol. 2010;7:2. doi: 10.1186/1743-8977-7-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kreyling WG, Semmler-Behnke M, Takenaka S, Möller W. Differences in the Biokinetics of Inhaled Nano- versus Micrometer-Sized Particles. Acc Chem Res. 2012 doi: 10.1021/ar300043r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vranic S, Boggetto N, Contremoulins V, Mornet S, Reinhardt N, Marano F, Baeza-Squiban A, Boland S. Deciphering the mechanisms of cellular uptake of engineered nanoparticles by accurate evaluation of internalization using imaging flow cytometry. Part Fibre Toxicol. 2013;10:2. doi: 10.1186/1743-8977-10-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Setyawati MI, Tay CY, Chia SL, Goh SL, Fang W, Neo MJ, Chong HC, Tan SM, Loo SC, Ng KW, et al. Titanium dioxide nanomaterials cause endothelial cell leakiness by disrupting the homophilic interaction of VE-cadherin. Nat Commun. 2013;4:1673. doi: 10.1038/ncomms2655. [DOI] [PubMed] [Google Scholar]
- 10.Stern ST, Adiseshaiah PP, Crist RM. Autophagy and lysosomal dysfunction as emerging mechanisms of nanomaterial toxicity. Part Fibre Toxicol. 2012;9:20. doi: 10.1186/1743-8977-9-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.De Stefano D, Carnuccio R, Maiuri MC. Nanomaterials toxicity and cell death modalities. J Drug Deliv. 2012;2012:167896. doi: 10.1155/2012/167896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tesfaigzi Y. Roles of apoptosis in airway epithelia. Am J Respir Cell Mol Biol. 2006;34(5):537–547. doi: 10.1165/rcmb.2006-0014OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hussain S, Thomassen LC, Ferecatu I, Borot MC, Andreau K, Martens JA, Fleury J, Baeza-Squiban A, Marano F, Boland S. Carbon black and titanium dioxide nanoparticles elicit distinct apoptotic pathways in bronchial epithelial cells. Part Fibre Toxicol. 2010;7:10. doi: 10.1186/1743-8977-7-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hussain S, Boland S, Baeza-Squiban A, Hamel R, Thomassen LC, Martens JA, Billon-Galland MA, Fleury-Feith J, Moisan F, Pairon JC, et al. Oxidative stress and proinflammatory effects of carbon black and titanium dioxide nanoparticles: role of particle surface area and internalized amount. Toxicology. 2009;260(1–3):142–149. doi: 10.1016/j.tox.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 15.Sydlik U, Bierhals K, Soufi M, Abel J, Schins RP, Unfried K. Ultrafine carbon particles induce apoptosis and proliferation in rat lung epithelial cells via specific signaling pathways both using EGF-R. Am J Physiol Lung Cell Mol Physiol. 2006;291(4):L725–L733. doi: 10.1152/ajplung.00131.2006. [DOI] [PubMed] [Google Scholar]
- 16.Belade E, Armand L, Martinon L, Kheuang L, Fleury-Feith J, Baeza-Squiban A, Lanone S, Billon-Galland MA, Pairon JC, Boczkowski J. A comparative transmission electron microscopy study of titanium dioxide and carbon black nanoparticles uptake in human lung epithelial and fibroblast cell lines. Toxicol In Vitro. 2012;26(1):57–66. doi: 10.1016/j.tiv.2011.10.010. [DOI] [PubMed] [Google Scholar]
- 17.Li SQ, Zhu RR, Zhu H, Xue M, Sun XY, Yao SD, Wang SL. Nanotoxicity of TiO(2) nanoparticles to erythrocyte in vitro. Food Chem Toxicol. 2008;46(12):3626–3631. doi: 10.1016/j.fct.2008.09.012. [DOI] [PubMed] [Google Scholar]
- 18.Aisaka Y, Kawaguchi R, Watanabe S, Ikeda M, Igisu H. Hemolysis caused by titanium dioxide particles. Inhal Toxicol. 2008;20(9):891–893. doi: 10.1080/08958370802304123. [DOI] [PubMed] [Google Scholar]
- 19.Hu R, Zheng L, Zhang T, Gao G, Cui Y, Cheng Z, Cheng J, Hong M, Tang M, Hong F. Molecular mechanism of hippocampal apoptosis of mice following exposure to titanium dioxide nanoparticles. J Hazard Mater. 2011;191(1–3):32–40. doi: 10.1016/j.jhazmat.2011.04.027. [DOI] [PubMed] [Google Scholar]
- 20.Wu J, Sun J, Xue Y. Involvement of JNK and P53 activation in G2/M cell cycle arrest and apoptosis induced by titanium dioxide nanoparticles in neuron cells. Toxicol Lett. 2010;199(3):269–276. doi: 10.1016/j.toxlet.2010.09.009. [DOI] [PubMed] [Google Scholar]
- 21.Li M, Yin JJ, Wamer WG, Lo YM. Mechanistic characterization of titanium dioxide nanoparticle-induced toxicity using electron spin resonance. J Food Drug Anal. 2014;22(1):76–85. doi: 10.1016/j.jfda.2014.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roller M. Carcinogenicity of inhaled nanoparticles. Inhal Toxicol. 2009;21(Suppl 1):144–157. doi: 10.1080/08958370902942541. [DOI] [PubMed] [Google Scholar]
- 23.Toyooka T, Amano T, Ibuki Y. Titanium dioxide particles phosphorylate histone H2AX independent of ROS production. Mutat Res. 2012;742(1–2):84–91. doi: 10.1016/j.mrgentox.2011.12.015. [DOI] [PubMed] [Google Scholar]
- 24.Li N, Ma L, Wang J, Zheng L, Liu J, Duan Y, Liu H, Zhao X, Wang S, Wang H, et al. Interaction Between Nano-Anatase TiO(2) and Liver DNA from Mice In Vivo. Nanoscale Res Lett. 2009;5(1):108–115. doi: 10.1007/s11671-009-9451-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Donaldson K, Tran L, Jimenez LA, Duffin R, Newby DE, Mills N, MacNee W, Stone V. Combustion-derived nanoparticles: a review of their toxicology following inhalation exposure. Part Fibre Toxicol. 2005;2:10. doi: 10.1186/1743-8977-2-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Armand L, Dagouassat M, Belade E, Simon-Deckers A, Le Gouvello S, Tharabat C, Duprez C, Andujar P, Pairon JC, Boczkowski J, et al. Titanium dioxide nanoparticles induce matrix metalloprotease 1 in human pulmonary fibroblasts partly via an interleukin-1β-dependent mechanism. Am J Respir Cell Mol Biol. 2013;48(3):354–363. doi: 10.1165/rcmb.2012-0099OC. [DOI] [PubMed] [Google Scholar]
- 27.Morishige T, Yoshioka Y, Tanabe A, Yao X, Tsunoda S, Tsutsumi Y, Mukai Y, Okada N, Nakagawa S. Titanium dioxide induces different levels of IL-1beta production dependent on its particle characteristics through caspase-1 activation mediated by reactive oxygen species and cathepsin B. Biochem Biophys Res Commun. 2010;392(2):160–165. doi: 10.1016/j.bbrc.2009.12.178. [DOI] [PubMed] [Google Scholar]
- 28.Reisetter AC, Stebounova LV, Baltrusaitis J, Powers L, Gupta A, Grassian VH, Monick MM. Induction of inflammasome-dependent pyroptosis by carbon black nanoparticles. J Biol Chem. 2011;286(24):21844–21852. doi: 10.1074/jbc.M111.238519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alfonso-Loeches S, Urena-Peralta JR, Morillo-Bargues MJ, De La Cruz JO, Guerri C. Role of mitochondria ROS generation in ethanol-induced NLRP3 inflammasome activation and cell death in astroglial cells. Front. Cell. Neurosci. 2014 doi: 10.3389/fncel.2014.00216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Klein CL, Wiench K, Wiemann M, Ma-Hock L, van Ravenzwaay B, Landsiedel R. Hazard identification of inhaled nanomaterials: making use of short-term inhalation studies. Arch Toxicol. 2012;86(7):1137–1151. doi: 10.1007/s00204-012-0834-2. [DOI] [PubMed] [Google Scholar]
- 31.Sager TM, Castranova V. Surface area of particle administered versus mass in determining the pulmonary toxicity of ultrafine and fine carbon black: comparison to ultrafine titanium dioxide. Part Fibre Toxicol. 2009;6:15. doi: 10.1186/1743-8977-6-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hussain S, Vanoirbeek JA, Hoet PH. Interactions of nanomaterials with the immune system. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2012;4(2):169–183. doi: 10.1002/wnan.166. [DOI] [PubMed] [Google Scholar]
- 33.Hussain S, Vanoirbeek JA, Luyts K, De Vooght V, Verbeken E, Thomassen LC, Martens JA, Dinsdale D, Boland S, Marano F, et al. Lung exposure to nanoparticles modulates an asthmatic response in a mouse model. Eur Respir J. 2011;37(2):299–309. doi: 10.1183/09031936.00168509. [DOI] [PubMed] [Google Scholar]
- 34.Alessandrini F, Beck-Speier I, Krappmann D, Weichenmeier I, Takenaka S, Karg E, Kloo B, Schulz H, Jakob T, Mempel M, et al. Role of oxidative stress in ultrafine particle-induced exacerbation of allergic lung inflammation. Am J Respir Crit Care Med. 2009;179(11):984–991. doi: 10.1164/rccm.200807-1061OC. [DOI] [PubMed] [Google Scholar]
- 35.Hussain S, Smulders S, De Vooght V, Ectors B, Boland S, Marano F, Van Landuyt KL, Nemery B, Hoet PH, Vanoirbeek JA. Nano-titanium dioxide modulates the dermal sensitization potency of DNCB. Part Fibre Toxicol. 2012;9:15. doi: 10.1186/1743-8977-9-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schanen BC, Das S, Reilly CM, Warren WL, Self WT, Seal S, Drake DR. Immunomodulation and T helper TH1/TH2 response polarization by CeO2 and TiO2 nanoparticles. PLoS One. 2013;8(5):e62816. doi: 10.1371/journal.pone.0062816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Koike E, Takano H, Inoue K, Yanagisawa R, Kobayashi T. Carbon black nanoparticles promote the maturation and function of mouse bone marrow-derived dendritic cells. Chemosphere. 2008;73(3):371–376. doi: 10.1016/j.chemosphere.2008.05.054. [DOI] [PubMed] [Google Scholar]
- 38.Scarino A, Noël A, Renzi PM, Cloutier Y, Vincent R, Truchon G, Tardif R, Charbonneau M. Impact of emerging pollutants on pulmonary inflammation in asthmatic rats: ethanol vapors and agglomerated TiO2 nanoparticles. Inhal Toxicol. 2012;24(8):528–538. doi: 10.3109/08958378.2012.696741. [DOI] [PubMed] [Google Scholar]
- 39.van Zijverden M, van der Pijl A, Bol M, van Pinxteren FA, de Haar C, Penninks AH, van Loveren H, Pieters R. Diesel exhaust, carbon black, and silica particles display distinct Th1/Th2 modulating activity. Toxicol Appl Pharmacol. 2000;168(2):131–139. doi: 10.1006/taap.2000.9013. [DOI] [PubMed] [Google Scholar]
- 37.Saber AT, Lamson JS, Jacobsen NR, Ravn-Haren G, Hougaard KS, Nyendi AN, Wahlberg P, Madsen AM, Jackson P, Wallin H, et al. Particle-induced pulmonary acute phase response correlates with neutrophil influx linking inhaled particles and cardiovascular risk. PLoS One. 2013;8(7):e69020. doi: 10.1371/journal.pone.0069020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Møller P, Mikkelsen L, Vesterdal LK, Folkmann JK, Forchhammer L, Roursgaard M, Danielsen PH, Loft S. Hazard identification of particulate matter on vasomotor dysfunction and progression of atherosclerosis. Crit Rev Toxicol. 2011;41(4):339–368. doi: 10.3109/10408444.2010.533152. [DOI] [PubMed] [Google Scholar]
- 39.Walkey CD, Chan WC. Understanding and controlling the interaction of nanomaterials with proteins in a physiological environment. Chem Soc Rev. 2012;41(7):2780–2799. doi: 10.1039/c1cs15233e. [DOI] [PubMed] [Google Scholar]
- 40.Xia XR, Monteiro-Riviere NA, Mathur S, Song X, Xiao L, Oldenberg SJ, Fadeel B, Riviere JE. Mapping the surface adsorption forces of nanomaterials in biological systems. ACS Nano. 2011;5(11):9074–9081. doi: 10.1021/nn203303c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sanfins E, Dairou J, Hussain S, Busi F, Chaffotte AF, Rodrigues-Lima F, Dupret JM. Carbon black nanoparticles impair acetylation of aromatic amine carcinogens through inactivation of arylamine N-acetyltransferase enzymes. ACS Nano. 2011;5(6):4504–4511. doi: 10.1021/nn103534d. [DOI] [PubMed] [Google Scholar]
- 42.Deng ZJ, Butcher NJ, Mortimer GM, Jia Z, Monteiro MJ, Martin DJ, Minchin RF. Interaction of human arylamine N-acetyltransferase 1 with different nanomaterials. Drug Metab Dispos. 2014;42(3):377–383. doi: 10.1124/dmd.113.055988. [DOI] [PubMed] [Google Scholar]
- 43.Val S, Hussain S, Boland S, Hamel R, Baeza-Squiban A, Marano F. Carbon black and titanium dioxide nanoparticles induce pro-inflammatory responses in bronchial epithelial cells: need for multiparametric evaluation due to adsorption artifacts. Inhal Toxicol. 2009;21(Suppl 1):115–122. doi: 10.1080/08958370902942533. [DOI] [PubMed] [Google Scholar]
- 44.Tay CY, Cai P, Setyawati MI, Fang W, Tan LP, Hong CH, Chen X, Leong DT. Nanoparticles strengthen intracellular tension and retard cellular migration. Nano Lett. 2014;14(1):83–88. doi: 10.1021/nl4032549. [DOI] [PubMed] [Google Scholar]
- 45.Ale-Agha N, Albrecht C, Klotz LO. Loss of gap junctional intercellular communication in rat lung epithelial cells exposed to carbon or silica-based nanoparticles. Biol Chem. 2010;391(11):1333–1339. doi: 10.1515/BC.2010.133. [DOI] [PubMed] [Google Scholar]
- 46.Peuschel H, Sydlik U, Grether-Beck S, Felsner I, Stöckmann D, Jakob S, Kroker M, Haendeler J, Gotić M, Bieschke C, et al. Carbon nanoparticles induce ceramide- and lipid raft-dependent signalling in lung epithelial cells: a target for a preventive strategy against environmentally-induced lung inflammation. Part Fibre Toxicol. 2012;9:48. doi: 10.1186/1743-8977-9-48. [DOI] [PMC free article] [PubMed] [Google Scholar]