Abstract
Background
Co-infection with S. mansoni and Human Immunodeficiency Virus-1 (HIV-1) has been described in sub-Saharan Africa. However, few community-based studies have been conducted to assess the association between the two diseases. The present study examined whether the infection with HIV-1 is associated with an altered susceptibility to S. mansoni infection by comparing the prevalence and intensity of S. mansoni infection among those infected and not infected with HIV-1. Any influence of HIV-1 associated immunodeficiency on the intensity of S. mansoni infection was also investigated.
Methods
A cross-sectional study was conducted among 1,785 randomly selected adults (aged 21–55 years) in fishing villages of north-western Tanzania. Single stool samples were obtained and examined for S. mansoni eggs using the Kato Katz technique. Finger prick and venous blood samples were collected for HIV-1 screening and CD4+ cell quantification. Demographic information was collected by questionnaire.
Results
Of the 1,785 individuals from whom complete data were obtained, 854 (47.85%, 95% CI; 40.46 – 56.57) were infected with S. mansoni and had a mean intensity of 183.21(95% CI; 165.61-202.70) eggs per gram of faeces (epg). A total of 125 individuals (6.29%, 95% CI 3.59-11.04) were infected with HIV-1 and only 40% (n=50) of them were co-infected with S. mansoni. No differences in prevalence of S. mansoni infection or intensities of infection, as estimated by egg count (epg), were observed between HIV-1 sero-positive individuals and HIV-1 negative individuals. In generalized regression models (adjusted for sex, age, occupation, residence and level of education), being infected with HIV-1 did not increase the risk (APR=1.01, 95%; 0.83-1.21, P=0.93) or intensity (AOR = 0.84, 95% CI; 0.56-1.25, P = 0.33) of S. mansoni infection. Among individuals co-infected with HIV-1 and S. mansoni infection, the intensity of infection (epg) was not associated (P = 0.21) or correlated (P = 0.13) with CD4+ cell counts.
Conclusion
Our findings suggest that HIV-1 infection may not have a major effect on S. mansoni infection or on the excretion of eggs from the co-infected individuals. However, further studies are needed to understand the biological interaction between HIV-1 and S. mansoni in a large cohort of co-infected individuals.
Keywords: S. mansoni, HIV-1, Co-infection, Fishing villages, Risk factors, Tanzania
Background
In the past two decades, epidemiological studies have reported an overlap of Human Immunodeficiency (HIV-1) and S. mansoni infections in sub-Saharan Africa [1-3], leading to co-infection in highly endemic regions [1-4]. Schistosoma mansoni is focally distributed in the region, its distribution being largely influenced by the distribution of its intermediate snail host and human–water contact behaviours [5,6]. In addition, the development of its related morbidities is influenced by genetic factors of the affected population [7], immune responses against the parasites or eggs [8] and changes in body physiology with increased age [9]. These factors may influence the prevalence and intensity of infection patterns in endemic populations. In contrast, HIV-1 is widely distributed and its transmission involves a combination of multiple factors including demographic factors and socio-economic status, epidemiological settings (rural versus urban) and sexual behaviours [10-12].
In areas where S. mansoni is highly endemic, such as in fishing villages along the Lake Victoria shores in east Africa [13], the migratory behaviour of fishermen and women [14], multiple sexual partner networks [11], living in clusters and isolated localities away from basic health services, a high prevalence of sexual transmitted diseases [15] and social cultural behaviours [11,12,15] increase the risk of HIV-1 transmission. The overlap of multiple risk factors associated with the two diseases in the same geographical setting or the biological interaction between HIV-1 and S. mansoni [2,10-12,14] have been proposed to increase the risk of individuals to be co-infected with both HIV-1 and S. mansoni [2,11,13,15]. In co-infected individuals, immunological studies have described a number of biological mechanisms through which chronic HIV-1 infection could affect S. mansoni related morbidities [16]. These mechanisms could result in differences in the prevalence and intensity of S. mansoni infection [17,18]; the efficiency of parasite egg excretion [4,17,18]; morbidity patterns [19] and the response to anthelmintic treatment among HIV-1 infected and non infected people [20,21]. However, despite the possible interaction between the two diseases, few community based studies have been conducted to explore the possible interaction between HIV-1 and S.mansoni infections [4,17,18]. The current study examined whether infection with HIV-1 is associated with an altered susceptibility to S. mansoni infection by comparing the prevalence and intensity of S. mansoni infection among those infected and not infected with HIV-1. As HIV-1 infection is associated with reduced CD4+ cell counts, and as the human immunological status is known to influence schistosome-human reactions, we also tested if there was an association or correlation between the intensity of S.mansoni infection and the immunological status, as measured by CD4+ cell counts, in people co-infected with HIV-1 and S. mansoni.
Methods
Study population and study area
The study was conducted at Ilemela district, Mwanza region, located at 32-34°E and 2 - 4°S, on the southern shores of the Lake Victoria, north-western Tanzania. The area experiences a temperature range from 18°C to 28°C and the mean annual rainfall of 1068 mm. Four villages Sangabuye, Kayenze, Igalagala and Igombe, were chosen for the present study due to their close proximity to the lake. The majority of the population are of Sukuma tribe. Other migrant tribes are Kerewe, Jita, and Kara. The majority of the villagers in the four villages depend on the lake for domestic and economic activities such as washing, bathing, cooking, drinking and recreation. The main economic activities in the four villages are fishing and farming. For high water contact levels, the residents have a high risk of being infected with S. mansoni [22,23] and high occupational exposure maintains high intensities of S. mansoni infection into adulthood [24]. Annual mass drug administration (MDA) using praziquantel and albendazole against helminth infections in these villages is organised within the school environment and focuses on school-going children, not the adult population.
The high rate of sexual mixing within the fishing villages increases the risk of HIV-1 transmission in the adult population [11]. In 2003, the prevalence of HIV-1 infection in individuals aged 15–60 years living in Kayenze and Sangabuye villages was estimated to be 10% [22] and the National HIV-1 seroprevalence results of 2012 have indicated that the prevalence of HIV-1 is 6% in the age group 15–60 years [25].
Study design, inclusion and exclusion criteria
This cross-sectional study was conducted between September 2012 and December 2012. All people who had lived in the study villages for more than two years, and were aged 21 to 55 years, were eligible for enrolment. Individuals with a history of treatment for schistosomiasis (praziquantel) in the past six months and those who were on anti-retroviral treatment were excluded from the study.
To determine an adequate sample size, we assumed that among people not infected with HIV-1, 50% were infected with S. mansoni. In order to determine a difference of 20% among people with HIV-1 who were infected with S. mansoni, at a power of 80% and 95% confidence interval, we required at least 103 people infected with HIV-1. This sample size was calculated using Stata 12 using the command sampsi 0.5 0.7, power (.80). To achieve this number of HIV infected we needed to screen 1,760 people assuming an HIV-1 prevalence of 6% [25] and refusal rate of 10%.
A two-step sampling procedure was used to select households and household members to participate in the study. At first, random sampling was used to select 1,785 households from a list of 4586 households. Then, from every selected household, random sampling was used to select one individual from all eligible persons in the household. If the selected individual was not present on the day of screening, the house was revisited three times on different days and if the selected subject declined to participate or was not available after multiple attempts, a new member of the household was chosen. Similarly, if the selected household remained vacant after multiple visits, the household was substituted by a neighbouring one.
Data collection
-
(i)
Human Immunodeficiency Virus screening and CD4+analysis
Human Immunodeficiency Virus-1 testing was conducted according to the Tanzanian National HIV algorithms which recommend the use of a rapid test qualitative immunoassay [26]. Study participants were counselled before and after HIV testing as per requirement of the country algorithms. The study used Determine® (Alere Determine, Chiba, Japan) followed by UNI-GOLD® (Trinity Biotech PLC, Bray, Ireland) for the participants who had a positive result with Determine [26]. The test was conducted according to the standards provided by the manufacturers. For individuals who had a Determine positive test but a negative UNIGOLD test, a dry blood spot was obtained for Enzyme Immunosobent Assay (ELISA) testing. Within 24-hours of blood sample collection, the quantification of CD4+ cells was carried out using a FACSCalibur machine (Becton Dickinson-BD Biosciences, San Jose, CA, USA) following standard procedures [27].
-
(ii)
Parasitological screening forSchistosoma mansoni
A single stool sample was collected from all study participants. Four Kato Katz thick smears were prepared from different parts of the single stool sample using a template of 41.7 mg (Vestergaard Frandsen, Lausanne, Switzerland), following a standard protocol [28-30]. After 24 hours, the smears were independently examined for S. mansoni eggs by two experienced laboratory technicians of the National Institute for Medical Research (NIMR) laboratory. For quality assurance, a random sample of 10% of the negative and positive Kato Katz thick smears were re-examined by a third technician.
In addition to stool and blood examination, demographic information on sex, age, occupation, marital status, village of residence, number of years lived in current residence and level of education were collected by questionnaire.
Ethical considerations
Ethical approval was obtained from the Higher Degrees Research and Ethics Committee of the School of Public Health, Makerere University (Institutional Review Board (IRB) -00005856/2011) and from the Bugando University College of Health Sciences and Allied Sciences-Institutional Review Board, (BREC/001/32/2011). Ethical clearance was granted by the National Ethical Review Committee, National Institute for Medical Research, Tanzania and the study was registered in the clinical trial network, Clinical Trial (Number:- NCT-01541631). The study received further clearance from the regional and district administrative authorities of Mwanza region and Ilemela district. Swahili translated informed assent and consent forms were used to obtain children and adult participants’ consent respectively. For illiterate individuals, a thumb print was used to sign the assent and consent forms after a clear description of the study objective and acceptance to participate.
All study participants who were infected with S. mansoni were treated with praziquantel (40 mg/kg) according to World Health Organization (WHO) guidelines, irrespective of their HIV-1 sero-status. All HIV-1 infected individuals found to have CD4+ < 350 cells/μL were referred to the Care and Treatment Clinic (CTC) for assessment of their eligibility for antiretroviral therapy (ART).
Statistical analysis
The data were double entered using CSPro and the final data set was stored in a MySQL database. Data analysis was performed using Stata version 12 (Stata Corp, College station, Texas, USA). The prevalence of S. mansoni 95% confidence interval (95% CI) were obtained by binomial logistic regression taking into account clustering by villages. Comparisons of prevalence by demographic factors for S. mansoni infection were tested for significance using the chi-square test (χ2) or the Fisher exact test for categorical variables before running regression models. The arithmetic mean of S. mansoni egg counts for each participant was calculated from the counts of four Kato Katz thick smears and multiplied by 24 to obtain individual eggs per gram of faeces. S. mansoni egg counts were over dispersed so were logarithmically transformed and used to calculate the geometric mean egg per gram of feaces (GM-epg) that was obtained as the antilog of the mean of the transformed egg counts. Geometric mean egg counts for S. mansoni between various demographic factors were compared using t-test or ANOVA. Intensity of infection was categorized according to WHO criteria as : 1–99 epg, 100–399 epg, ≥400 epg defined as low, moderate and heavy intensities of infection respectively [29,30]. To test for association between intensity of S. mansoni infection and degree of immunosupression with HIV-1 as measured by CD4+ cell counts, linear regression analyses were done to calculate Pearson correlation coefficient for individuals co-infected with S. mansoni and HIV-1 adjusting for sex and age.
We also assessed whether using cut-offs of 350 and 200 CD4+ cells/μL, which are used for treatment referral and as a definition of Acquired Immunodeficiency Syndromes (AIDS) [31] respectively, influence susceptibility with S.mansoni. To control for the confounding effects of age, sex, occupation, marital status, level of education, village of residences and duration of residence on the association between HIV-1 and S. mansoni infections binomial logistic and generalized regressions models were done for all people whether infected with S. mansoni or not. Stepwise backward procedures were used to determine whether these variables were independent factors of S. mansoni infection by use of adjusted prevalence ratio (APR) for logistic regression. To control for confounding regarding intensity of infection, linear regression models were run for people who were infected with S .mansoni. Stepwise backward procedures were used to determine whether these variables were independent factors of intensity of S. mansoni infection by using adjusted odds ratios (AOR) for linear models and the 95% confidence interval (CI).
Results
-
(i)
Demographic characteristics of study participants round decimals to one point
A total of 1,785 individuals who participated in this study, 52.9% (945/1785) were female and 47.0% (n = 840) were male. The mean age of the study participants was 35.6 ± 9.74 years (with no significant difference in mean age between males and females, P = 0.4). The majority of the study participants had received a primary school education n = 1078, (60.39%), n = 76 (4.3%) had attained secondary/college education, n = 631, (35.4%) were illiterate. The majority were married (n = 1,257 (70.9%), 234 (13.1%) reported being in a long-term, stable relationship and 291 (16.4%) were either separated or divorced/widowed. There were 1,256 (70.36%) farmers, 255 (14.3%) fishermen and 223 (15.4%) small-scale business men and women. Overall, 97% of the study participants performed their economic activities within their villages.
-
(ii)
Prevalence ofSchistosoma mansoniinfection and association with HIV-1
The overall prevalence of S. mansoni was 854/1,785 (47.84%, 95%CI, 40.46-56.57), which was higher among males than females (472/840, 56.19% versus 382/941, 40.59%, P < 0.0001) (Table 1). There was a relationship between age and S. mansoni infection, the older age had lower prevalence compared to young age groups (P < 0.00001). Fishing-related occupations were associated with a higher prevalence of infection compared to other economic activities (P < 0.0001). Similarly, the prevalence varied significantly by villages of residence (P < 0.0001), with inhabitants of Kayenze and Igalagala having the highest prevalence. However, S. mansoni infection did not vary by duration of residence (Table 1). In relation to HIV-1 serostatus, 50/125 (7.00%) were co-infected with HIV-1 and S. mansoni infection. HIV-1 seronegative individuals had a higher prevalence of S. mansoni infection compared to the HIV-1 seropositive individuals but this difference was not statistically significant (P = 0.06) (Table 1). In generalised regression models (Table 1) adjusted for sex, age, marital status, education levels, occupation, village of residence, and duration of stay, HIV-1 was not an independent risk factor for infection with S. mansoni (APR = 1.01,95% CI; 0.84 - 1.21, P = 0.93). The main independent risk factors of S. mansoni infection were being of the male gender (APR = 1.27, 95% CI;1.14-1.42, P < 0.001), involvement in fishing activities (APR = 1.28,95% CI;1.07–1.53, P < 0.01), living in Igalagala (APR = 1.31,95% CI;1.12-1.52, P < 0.001) and Kayenze (APR = 1.32,95% CI;1.15-1.51, P < 0.001) villages.
-
(iii)
Intensity ofS.mansoniinfection and its association with HIV-1
Table 1.
Prevalence and risk factors of Schistosoma mansoni among residence of fishing villages of north-western Tanzania
| Variable | No. infected | Prevalence of S. mansoni (95% CI) | Crude prevalence ratio (95% CI) | Adjusted prevalence ratio (95% CI) |
|---|---|---|---|---|
| Overall | 854 | 47.84 (40.41 – 56.60) | ----- | ----- |
| Sex | ||||
| Female | 382 | 40.29 (32.43 – 50.00) | 1 | 1 |
| Male | 472 | 56.14 (49.19 – 64.06)* | 1.39 (1.26 – 1.54)** | 1.27 (1.14 – 1.42)*** |
| Age (in years) | ||||
| 21 – 30 | 365 | 53.18 (47.48 – 59.55)* | 1.22 (1.99 – 1.48) | 1.19 (0.98 – 1.45) |
| 31 – 40 | 292 | 47.66 (38.38 – 59.19) | 1.09 (0.99 – 1.19) | 1.12 (0.92 – 1.37) |
| 41 – 50 | 125 | 38.96 (29.80 – 50.93) | 0.89 (0.76 – 1.04) | 0.91 (0.72 – 1.13) |
| 51 – 55 | 72 | 43.71 (35.49 – 53.83) | 1 | 1 |
| Marital status | ||||
| Married | 591 | 47.20 (36.04 – 61.83) | 1 | 1 |
| Single | 263 | 48.63 (29.60 – 79.87) | 1.03 (0.82 – 1.29) | 1.05 (0.95 – 1.16) |
| Education level | ||||
| Literate | 564 | 49.04 (43.27 – 55.59) | 1 | 1 |
| Illiterate | 290 | 45.44 (34.83 – 59.28) | 0.93 (0.91 – 0.94) | 0.98 (0.88 – 1.08) |
| Occupation | ||||
| SME | 129 | 44.14 (36.14 – 53.91) | 1 | 1 |
| Peasants | 561 | 45.11 (37.98 – 53.58 | 1.02 (0.89 – 1.16) | 1.06 (0.86 – 1.24) |
| Fishing | 164 | 66.28 (55.77 – 78.78)* | 1.52 (1.42 – 1.59)** | 1.27 (1.06 – 1.53)*** |
| Village of residences | ||||
| Igombe | 176 | 40.42 (29.82 – 54.78) | 1 | 1 |
| Igalagala | 141 | 55.47 (39.08 – 78.72)* | 1.37 (1.31 – 1.44)** | 1.31 (1.12 – 1.52)*** |
| Kayenze | 351 | 54.21 (42.96 – 68.38)* | 1.34 (1.25 – 1.44)** | 1.32 (1.15 – 1.51)*** |
| Sangabuye | 186 | 41.24 (26.34 – 64.58) | 1.02 (0.88 – 1.78) | 1.06 (0.89 – 1.25) |
| Duration of residences (in years) | ||||
| 3 – 5 | 152 | 50.16 (43.42 – 57.94) | 1 | 1 |
| 6 – 10 | 169 | 50.21 (38.90 – 64.79) | 1.01 (0.80 – 1.25) | 1.01 (0.86 – 1.18) |
| 11 – 20 | 187 | 49.76 (38.92 – 63.61) | 0.98 (0.85 – 1.16) | 1.01 (0.88 – 1.16) |
| ≥21 | 346 | 48.69 (43.06 – 55.06) | 0.97 (0.92 – 0.99) | 0.96 (0.85 – 1.09) |
| CD4 + cell counts/μL | ||||
| <350 | 28 | 38.36 (28.63 – 51.38) | 1 | ----- |
| ≥350 | 22 | 44.89 (28.96 – 69.61) | 1.17 (0.91 – 1.51) | ----- |
| HIV-1 sero-status | ||||
| Negative | 804 | 48.12 (41.04 – 56.43) | 1 | 1 |
| Positive | 50 | 39.52 (27.64 – 56.49) | 0.92 (0.78 – 1.09) | 1.01 (0.84 – 1.21) |
*P < 0.001 from χ2; **P < 0.001 and ***P < 0.001 from binomial family model.
The overall geometrical mean egg per gram of faeces (GM-epg) for study participants with detectable S. mansoni eggs was 182.85epg (95% CI: 165.27 – 202.31). Males had higher intensities of infection (238.31epg, 95% CI: 206.63 – 274.84) than females (132.07epg, 95% CI: 115.29 – 151.28, P < 0.0001) (Table 2). The youngest age group (21–30 years) had the highest intensities of infection than older age groups (Table 2). Also, those involved in fishing activities had the highest intensities of infection compared to other economic activities (P < 0.0012). In addition, illiterate individuals had the highest intensity of infection compared to literate individuals (P < 0.034). Inhabitants living in Kayenze and Igalagala villages had the highest intensities of infection compared to those who were living in the other two villages (P < 0.002) (Table 2). Of the 854 individuals with detectable S. mansoni eggs, 352/854 (41.21%), 232/854 (27.16%) and 270/854 (31.61%) had light, moderate and heavy infections respectively, as defined by WHO [29,30].
Table 2.
Factors associated with infection intensity among people infected with S.mansoni residing in fishing villages of north-western Tanzania
| Variable | No. infected | GM-epg (95% CI) | F -ratio ( P -level) | COR (95% CI) | AOR (95% CI) |
|---|---|---|---|---|---|
| Overall | 854 | 183.21 (165.61 – 202.70) | ------ | ------- | ------- |
| Sex | |||||
| Female | 382 | 132.02 (115.33 – 151.14) | − 4.7386* (P < 0.0001) | 1 | 1 |
| Male | 472 | 238.86 (207.14 – 275.44) | 1.61 (1.48 – 2.21) | 1.65 (1.32 – 2.08) | |
| Age (in years) | |||||
| 21 – 30 | 368 | 220.20 (188.16 – 257.71) | 2.03** (P = 0.11) | 1.61 (0.65 – 1.54) | 1.68 (1.14 – 2.48) |
| 31 – 40 | 286 | 177.55 (149.10 – 211.41) | 1.30 (0.89 – 1.91) | 1.32 (0.89 – 1.97) | |
| 41 – 50 | 127 | 136.85 (109.18 – 171.54) | 1.004 (0.65 – 1.91) | 1.08 (0.69 – 1.67) | |
| 51 – 55 | 73 | 136.85 (109.18 – 171.54) | 1 | 1 | |
| Marital status | |||||
| Married | 591 | 173.06 (153.51 – 195.09) | − 0.8094* (P = 0.42) | 1 | 1 |
| Single* | 263 | 204.46 (168.53 – 248.04) | 1.02 (0.93 – 1.04 | 0.99 (0.94 – 1.05) | |
| Occupation | |||||
| SSB | 129 | 141.93 (108.59 – 185.21) | 6.75** P < 0.0012 | 1 | 1 |
| Peasants | 561 | 161.72 (142.87 – 183.06) | 1.14 (0.82 – 1.57) | 1.12 (0.81 – 1.54) | |
| Fishing | 164 | 308.84 (246.20 – 387.41 | 2.18 1.50 – 3.15) | 1.62 (1.10 – 2.41) | |
| Education level | |||||
| Literate | 564 | 173.54 (153.35 – 196.38) | −2.1185* (P < 0.0344) | 1 | 1 |
| Illiterate | 290 | 197.38 (164.80 – 236.40) | 1.14 (0.91 – 1.41) | 1.33 (1.07 – 1.66) | |
| Village of residence | |||||
| Igombe | 176 | 144.14 (116.73 – 178.00) | 3.26** (P < 0.0211) | 1 | 1 |
| Igalagala | 141 | 224.83 (175.65 – 287.77) | 1.56 (1.12 – 2.17) | 1.71 (1.23 – 2.40) | |
| Kayenze | 351 | 202.39 (173.12 – 236.61) | 1.40 (1.07 – 1.84) | 1.62 (1.23 – 2.13) | |
| Sangabuye | 186 | 163.07 (129.35 – 205.58) | 1.13 (0.83 – 1.54) | 1.38 (1.001 – 1.89) | |
| Duration of residence (in years) | |||||
| 3 − 5 | 152 | 230.94 (180.85 – 294.91) | 1.89** (P = 0.13) | 1 | 1 |
| 6 – 10 | 169 | 160.53 (122.74 – 209.95) | 0.69 0.48 – 1.01 | 0.71 (0.50 – 1.01) | |
| 11 – 20 | 187 | 188.01 (154.38 – 228.95) | 0.81 (0.59 – 1.11) | 0.86 (0.64 – 1.17) | |
| ≥21 | 346 | 165.24 (141.06 – 193.58) | 0.71 (0.54 – 0.95) | 0.73 (0.55 – 1.04) | |
| CD4 + cell counts/μL | |||||
| <350 | 28 | 154.27 (85.16 – 279.48) | 1.5410* (P = 0.13) | 1 | |
| ≥350 | 22 | 93.26 (60.42 – 143.95) | 1.09 (0.54 – 2.08) | ------ | |
| HIV-1 serostatus | |||||
| Negative | 804 | 186.83 (168.15 – 207.59) | 0.882* (P = 0.38) | 1 | 1 |
| Positive | 50 | 126.78 (86.59 – 185.61) | 0.76 (0.51 – 1.13) | 0.84 (0.56 – 1.25) | |
● Determined by based on t-tests **Determined by Anova.
● COR = Crude Odd Ratio.
● AOR = Adjusted Odd Ratio.
● Single* - male of female individuals living alone/not married.
HIV-1 seronegative individuals had higher intensities of infection (186.98epg, 95% CI; 168.39-207.61) than HIV-1 seropositive individuals (126.78epg, 95% CI; 86.59 – 185.61) but the observed difference did not reach significance (P = 0.38). In a multiple linear regression model adjusted for age, sex, occupation, marital status, education level, residence and duration of stay, the intensity of S. mansoni infection was not associated with HIV-1 infection (AOR = 0.84,95% CI; 0.56 – 1.25) (Table 2). The intensity of infection was associated with being of male gender (AOR = 1.65,95% CI; 1.32-2.08, P < 0.0001), young ages (21–30 years; AOR = 1.68,95% CI; 1.14-2.48, P < 0.01), involvement in fishing activities (AOR = 1.62,95% CI; 1.10-2.41, P < 0.01), being illiterate (AOR = 1.33,95% CI; 1.07 – 1.66, P < 0.014) and living in the study villages.
-
(iv)
Association between immune status andS. mansoniinfection
Of the 125 individuals infected with HIV-1, 50/125, (40.00%, 95% CI; 31.58 - 48.42) had detectable S. mansoni eggs in their stool samples. The overall prevalence of co-infection for the entire cohort was 50/1,785 (2.80%). Of the co-infected individuals, the proportions of lightly, moderately and heavily co-infected individuals were 25/50, (50.00%, 14/50 (28.00%) and 11/50 (22.00%) as defined by WHO [29,30]. The prevalence of S. mansoni in HIV-1 infected individuals with CD4+ counts <350 cells/μL was 28/75 (37.33%) compared to 22/50 (44.00%) in those who had CD4+ counts ≥350 cells/μL (P = 0.47). Co-infected individuals with CD4+ counts <350 cells/μl had a higher GM-epg of S. mansoni infection (154.27epg, 95% CI; 85.16-279.48) compared to those who had CD4+ counts ≥350 cells/μl (93.26 GM-epg, 95% CI: 60.42- 143.95), but the difference was not significant (P = 0.13). Among those with CD4+ counts <200 cells/μL, 24.44% (n = 11) were co-infected with HIV-1 and S. mansoni, while among those with CD4+ cell counts ≥ 200 cells/μL, 50.65% (n = 39) were co-infected. Considering the intensities of S. mansoni infection, those who had CD4+ counts of <200 cells/μL had higher GM-epg of S. mansoni infection (184.83epg, 95% CI: 54.85-622.82), compared to those who had CD4+ counts of ≥200 cells/μL (110.376epg, 95% CI; 75.63 – 161.07), but the difference between the two groups did not reach significance (P = 0.13).
For individuals who were mono-infected with HIV-1, 45/75 (60.00%) had CD4+ counts <350 cells/μL and 30/75 (40.00%) had CD4+ counts ≥ 350 cells/μL. The median CD4+ counts for individuals who were infected with HIV-1 only was 231.5(range: 132–512.5) cells/μL and those who were co-infected with S. mansoni and HIV-1 was 308.5 (range: 202–586) cells/μL (P = 0.14).
For individuals who were co-infected, the intensity of infection was not associated with CD4+ count at both univariate (P = 0.11) and multivariate level (P = 0.21) (correcting for age, sex, village of occupation, residence, education level) (R2 = 0.3027, adjusted R2 = 0.1458, F = 0.075). No correlation was observed between intensity of S. mansoni infection (epg) and CD4+ cell counts among the co-infected individuals (r = −0.37, P = 0.13) (Figure 1).
Figure 1.
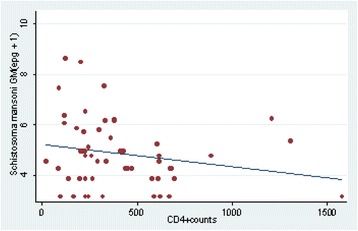
Scatter plot of correlation of S. mansoni egg counts and CD4 + counts in individuals co-infected with HIV-1 and S. mansoni . No correlation was observed between egg excretions and CD4+ counts levels (r = −0.37, P = 0.13).
Discussion
The present study villages of North-western Tanzania are endemic to S. mansoni and HIV-1 infections. Co-infection with HIV-1 and S. mansoni in a single human host does also occur in the study population. In the present study area, no differences in prevalence of S. mansoni infection or egg counts were observed between HIV-1 sero-positive participants and HIV-1 sero-negative individuals. After adjusting for other factors in the statistical models, no association was observed between the HIV-1 serostatus and the prevalence as well as the intensity of S.mansoni infection. Furthermore, no correlation was observed between CD4+ cell counts and S. mansoni egg counts.
Our observations were consistent with the study of Kallestrup et al. [18], in rural Zimbabwe; although that study was limited by low intensities of S. mansoni infection, no difference in egg counts or prevalence was observed between HIV-1 seropositive or HIV-1 sero-negative participants infected with S. mansoni [18]. These observations are inconsistent with reports from previous similar studies in Ethiopia and Western Kenya [4,17], where individuals co-infected with HIV-1 and S. mansoni infections excreted fewer egg counts per gram of faeces than HIV-1 negative individuals infected with S. mansoni despite the two groups having similar worm burdens [4], as determined by Circulating Cathodic Antigen (CCA) levels (a S. mansoni gut antigen which is regurgitated by juvenile and adult stages as a by-product of host red blood cell digestion) [32]. Complementary to these observations in human hosts, a lower egg output in immunodeficient murine models has also been described [33-38]. In these models, the maturation of the worm is delayed; the fecundity of the female worm affected and transposition of the eggs to the lumen of the intestine is reduced. These combine to significantly lower the level of S.mansoni egg output compared with immunocompetent mice, despite having a comparable number of adult worms [33-35,37,38].
In the present study cohort, co-infected individuals with lower CD4+ cell counts levels categorised either at <200 cells/μL or <350 cells/μL had higher GM-epg of S. mansoni infection compared to those who had higher CD4+ cell count levels. However the difference was not statistically significant. Previous similar studies have reported lower S. mansoni egg counts in individuals with lower CD4+ cell counts [4,18]. It is important to note that, HIV-1 seropositive individuals included in the present study had higher median CD4+ cell counts as compared to similar cohort included in other similar study in rural Zimbabwe, which had very low CD4+ cell counts [18]. It is possible that the previous studies, which observed lower S. mansoni egg counts in HIV-1 seropositive individuals with lower CD4+ cell counts, were focused on cohorts suffering more advanced stages of HIV-1 associated disease [4,18]. It is worthwhile noting that decreased CD4+ cell counts have also been reported in HIV-1 seronegative individuals infected with S. mansoni, indicating an association between CD4+ lymphocytopenia and S. mansoni infection [18,39].
In the present study, no direct association or correlation was observed between the geometrical mean S. mansoni epg and CD4+ cell counts among individuals co-infected with HIV-1 and S.mansoni. Our observations were consistent to a similar study in rural Zimbabwe [18]. The results of the present study and those of other authors [18] indicate that even HIV-1 co-infected individuals, regardless of the immune status as measured by CD4+ count levels, could have egg counts close to HIV-1 negative individuals infected with S. mansoni [18]. In contrast, in co-infected human hosts in western Kenya, lower CD4+ cell counts correlated positively with lower egg excretion ratio/CCA ratio [22,32]. The CD4+ T-helper cells lymphocyte responses are thought to play a central role in excretion efficiencies of S. mansoni eggs, with decreasing CD4+ cell counts correlating with reduced excretion efficiencies of S. mansoni eggs in murine models [33-38]. It is worth to note that the degree of induced immunonosuppression observed in murine models studies, which resulted in lower S. mansoni egg output and correlated positively with CD4+ cells, was very severe and cannot be compared to moderately CD4+ depleted individuals observed in the present study. Together, the low numbers of co-infected individuals observed in the present study, combined with their relatively modest CD4+ depletion, could have reduced the likelihood of observing a significant correlation between CD4+ cell count and S. mansoni egg output.
Our study was subject to some limitations. The cross-sectional nature of the study design may have contributed to the lack of temporal association between HIV-1, S. mansoni and some of the study variables. Thus, even though this study was a community-based study with a large number of study participants, some caution must be taken with the interpretation of these findings.
Conclusion
In conclusion, no differences in prevalence of S. mansoni infection or egg count were observed between HIV-1 sero-positive individuals and HIV-1 sero-negative individuals. In addition, no association was observed between HIV-1 serostatus and prevalence or intensity of infection with S.mansoni. Furthermore, no correlation was observed between CD4+ cell count levels and S. mansoni egg count levels. Our findings suggest that HIV-1 infection may not affect S. mansoni disease development and excretion of eggs from the co-infected individuals. Further research is needed to test biological interaction using more sensitive methods of diagnosing S. mansoni, such as quantitative CCA, and with a much bigger sample size of co-infected individuals with S. mansoni and HIV-1.
Acknowledgements
This work was supported by Training Health Researchers into Vocational Excellence in East Africa (THRiVE), grant number 087540 funded by the Wellcome Trust. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the supporting offices. DWD and HDM received additional funds from Cambridge- Africa Alborada grant, we acknowledge their support. HDM received additional funding support from Dan Davis Prize Scholarship, Tel Aviv University, Israel. The investigation received financial support from TDR, the Special Programme for Research and Training in Tropical Diseases, co-sponsored by UNICEF, UNDP, the World Bank and WHO”, we acknowledge their support.
We acknowledge the technical support from the main laboratory staff and the management team of National Institute for Medical Research (NIMR) Mwanza centre for accepting to house all the logistics of this work and financial management of the project, Catholic University of Health and Allied Sciences and Bugando Medical Centre, Mwanza. We thank all the study participants and the local government for participation in this study.
Footnotes
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
HDM, DWD, SMK, DM, FN designed the study. AP, SW, FMJ and FN participated in data analysis. HDM drafted the first version of the manuscript and all authors read, contributed to its contents and approved the manuscript.
Contributor Information
Humphrey D Mazigo, Email: humphreymazigo@gmail.com.
David W Dunne, Email: dwd10@cam.ac.uk.
Shona Wilson, Email: sw320@cam.ac.uk.
Safari M Kinung’hi, Email: kinunghi_csm@hotmail.com.
Angela Pinot de Moira, Email: acp44@cam.ac.uk.
Frances M Jones, Email: fmj20@cam.ac.uk.
Domenica Morona, Email: dmorona@gmail.com.
Fred Nuwaha, Email: nuwahaf@yahoo.co.uk.
References
- 1.Brown M, Mawa PA, Kaleebu P, Elliott AM. Helminths and HIV infection: epidemiological observations on immunological hypotheses. Parasite Immunol. 2006;28(11):613–623. doi: 10.1111/j.1365-3024.2006.00904.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mazigo HD, Nuwaha F, Wilson S, Kinung’hi SM, Morona D, Waihenya R, Heukelbach J, Dunne DW. Epidemiology and interactions of human immunodeficiency virus - 1 and Schistosoma mansoni in sub-Saharan Africa. Infect Dis Poverty. 2013;2(1):2. doi: 10.1186/2049-9957-2-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Secor WE. Interactions between schistosomiasis and infection with HIV-1. Parasite Immunol. 2006;28:597–603. doi: 10.1111/j.1365-3024.2006.00887.x. [DOI] [PubMed] [Google Scholar]
- 4.Karanja DM, Colley DG, Nahlen BL, Ouma JH, Secor WE. Studies on schistosomiasis in western Kenya: I. Evidence for immune-facilitated excretion of schistosome eggs from patients with Schistosoma mansoni and human immunodeficiency virus coinfections. Am J Trop Med Hyg. 1997;56(5):515–521. doi: 10.4269/ajtmh.1997.56.515. [DOI] [PubMed] [Google Scholar]
- 5.Steinmann P, Keiser J, Bos R, Tanner M, Utzinger J. Schistosomiasis and water resources development: systematic review, meta-analysis, and estimates of people at risk. Lancet Infect Dis. 2006;6(7):411–425. doi: 10.1016/S1473-3099(06)70521-7. [DOI] [PubMed] [Google Scholar]
- 6.Gryseels B, Polman K, Clerinx J, Kestens L. Human schistosomiasis. Lancet. 2006;368(9541):1106–1118. doi: 10.1016/S0140-6736(06)69440-3. [DOI] [PubMed] [Google Scholar]
- 7.Mohamed-Ali Q, Elwali NE, Abdelhameed AA, Mergani A, Rahoud S, Elagib KE, Saeed OK, Abel L, Magzoub MM, Dessein AJ. Susceptibility to periportal (Symmers) fibrosis in human Schistosoma mansoni infections: evidence that intensity and duration of infection, gender, and inherited factors are critical in disease progression. J Infect Dis. 1999;180(4):1298–1306. doi: 10.1086/314999. [DOI] [PubMed] [Google Scholar]
- 8.Butterworth AE, Curry AJ, Dunne DW, Fulford AJ, Kimani G, Kariuki HC, Klumpp R, Koech D, Mbugua G, Ouma JH. Immunity and morbidity in human schistosomiasis mansoni. Trop Geogr Med. 1994;46(4 Spec No):197–208. [PubMed] [Google Scholar]
- 9.Fulford AJ, Webster M, Ouma JH, Kimani G, Dunne DW. Puberty and age-related changes in susceptibility to schistosome infection. Parasit Today. 1998;14(1):23–26. doi: 10.1016/S0169-4758(97)01168-X. [DOI] [PubMed] [Google Scholar]
- 10.Johnson L, Budlender D. HIV risk factors: a review of the demographic, socio-economic, biomedical and behavioral determinants of HIV prevalence in South Africa. Centre Actuar Res. 2002;2002:5–6. [Google Scholar]
- 11.Pickering H, Okongo M, Bwanika K, Nnalusiba B, Whitworth J. Sexual behaviour in a fishing community on Lake Victoria, Uganda. Health Trans Rev. 1997;7(1):13–20. [PubMed] [Google Scholar]
- 12.Kissling E, Allison EH, Seeley JA, Russell S, Bachmann M, Musgrave SD, Heck S. Fisherfolk are among groups most at risk of HIV: cross-country analysis of prevalence and numbers infected. AIDS. 2005;19(17):1939–1946. doi: 10.1097/01.aids.0000191925.54679.94. [DOI] [PubMed] [Google Scholar]
- 13.Muok EMO, Onguru DO, Karanja DM, Mwinzi PNM, Nganga ZW, Ofula AV. Participation of fishing communities in neglected tropical disease/HIV Co-infection cohort studies in Western Kenya. J Trop Dis. 2013;1:1. [Google Scholar]
- 14.Kwena ZA, Camlin CS, Shisanya CA, Mwanzo I, Bukusi EA. Short-term mobility and the risk of HIV infection among married couples in the fishing communities along Lake Victoria, Kenya. PLoS One. 2013;8(1):e54523. doi: 10.1371/journal.pone.0054523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Asiki G, Mpendo J, Abaasa A, Agaba C, Nanvubya A, Nielsen L, Seeley J, Kaleebu P, Grosskurth H, Kamali A. HIV and syphilis prevalence and associated risk factors among fishing communities of Lake Victoria, Uganda. Sex Trans Infec. 2011;87(6):511–515. doi: 10.1136/sti.2010.046805. [DOI] [PubMed] [Google Scholar]
- 16.Secor WE. The effects of schistosomiasis on HIV/AIDS infection, progression and transmission. Curr Opin HIV AIDS. 2012;7(3):254–259. doi: 10.1097/COH.0b013e328351b9e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fontanet AL, Woldemichael T, Sahlu T, van Dam GJ, Messele T, Rinke De Wit T, Masho W, Yeneneh H, Coutinho RA, van Lieshout L. Epidemiology of HIV and Schistosoma mansoni infections among sugar-estate residents in Ethiopia. Ann Trop Med Parasit. 2000;94(2):145–155. doi: 10.1080/00034980057482. [DOI] [PubMed] [Google Scholar]
- 18.Kallestrup P, Zinyama R, Gomo E, Butterworth AE, van Dam GJ, Erikstrup C, Ullum H. Schistosomiasis and HIV-1 infection in rural Zimbabwe: implications of coinfection for excretion of eggs. J Infect Dis. 2005;191(8):1311–1320. doi: 10.1086/428907. [DOI] [PubMed] [Google Scholar]
- 19.Mwinzi PN, Karanja DM, Kareko I, Magak PW, Orago AS, Colley DG, Secor WE. Short report: evaluation of hepatic fibrosis in persons co-infected with Schistosoma mansoni and human immunodeficiency virus 1. Am J Trop Med Hyg. 2004;71(6):783–786. [PubMed] [Google Scholar]
- 20.Kallestrup P, Zinyama R, Gomo E, Butterworth AE, van Dam GJ, Gerstoft J, Erikstrup C, Ullum H. Schistosomiasis and HIV in rural Zimbabwe: efficacy of treatment of schistosomiasis in individuals with HIV coinfection. Clin Infect Dis. 2006;42(12):1781–1789. doi: 10.1086/504380. [DOI] [PubMed] [Google Scholar]
- 21.Karanja DM, Boyer AE, Strand M, Colley DG, Nahlen BL, Ouma JH, Secor WE. Studies on schistosomiasis in western Kenya: II. Efficacy of praziquantel for treatment of schistosomiasis in persons coinfected with human immunodeficiency virus-1. Am J Trop Med Hyg. 1998;59(2):307–311. doi: 10.4269/ajtmh.1998.59.307. [DOI] [PubMed] [Google Scholar]
- 22.Malenganisho WLM: The Role of HIV, Micronutrient Status and Treatment in Schistosoma Mansoni Infection and Morbidity: A Cohort Study among Adult of Ukerewe and Mwanza Districts, Tanzania. In PhD thesis. Faculty of Science, University of Copenhagen and DBL- Institute for Health Research and Development, Denmark: 2005.
- 23.Malenganisho WL, Magnussen P, Friis H, Siza J, Kaatano G, Temu M, Vennervald BJ. Schistosoma mansoni morbidity among adults in two villages along Lake Victoria shores in Mwanza District, Tanzania. Trans Roy Soc Trop Med Hyg. 2008;102(6):532–541. doi: 10.1016/j.trstmh.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 24.Kardorff R, Gabone RM, Mugashe C, Obiga D, Ramarokoto CE, Mahlert C, Spannbrucker N, Lang A, Gunzler V, Gryseels B. Schistosoma mansoni-related morbidity on Ukerewe Island, Tanzania: clinical, ultrasonographical and biochemical parameters. Trop Med Intern Health. 1997;2(3):230–239. doi: 10.1046/j.1365-3156.1997.d01-269.x. [DOI] [PubMed] [Google Scholar]
- 25.Tanzania government HIV-1/Malaria survey report: Tanzania HIV/AIDS and Malaria Indicator Survey, 2011–12. Tanzania Commission for AIDS, Dar Es Salaam, Tanzania, Tanzania Government print. 1-235
- 26.Lyamuya EF, Aboud S, Urassa WK, Sufi J, Mbwana J, Ndugulile F, Massambu C. Evaluation of simple rapid HIV assays and development of national rapid HIV test algorithms in Dar es Salaam, Tanzania. BMC Infect Dis. 2009;9:19. doi: 10.1186/1471-2334-9-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Borato DCK, Carraro E, Ribas SRW, Kalva-Filho CA, Vellosa JCR. Comparison of Two methodologies for CD4+ T lymphocytes relative counting on immune monitoring of patients with human immunodeficiency virus. Sci World J. 2012;ᅟ:1–5. doi: 10.1100/2012/906873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Katz N, Chaves A, Pellegrino J. A simple device for quantitative stool thick-smear technique in Schistosomiasis mansoni. Rev Inst Med Trop Sao Paulo. 1972;14(6):397–400. [PubMed] [Google Scholar]
- 29.WHO: Basic laboratory methods in medical parasitology. World Health Organization, Geneva, ISBN 9241544104, 1991, 1-111.
- 30.WHO: Prevention and control of schistosomiasis and soil-transmitted helminthiasis. World Health Org Tech Rep Ser 2002, 912:i. [PubMed]
- 31.WHO: WHO case definitions of hiv for surveillance and revised clinical staging and immunological classification of hiv-related disease in adults and children. World Health Org 2007, ISBN: 978 92 4 159562 9, World Health Organization, 2007; 4-40.
- 32.Van Dam G, Wichers J, Ferreira TF, Ghati D, Van Amerongen A, Deelder A. Diagnosis of schistosomiasis by reagent strip test for detection of circulating cathodic antigen. J Clin Microb. 2004;42(12):5458–5461. doi: 10.1128/JCM.42.12.5458-5461.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Doenhoff MJ, Bain J. The immune-dependence of schistosomicidal chemotherapy: relative lack of efficacy of an antimonial in Schistosoma mansoni-infected mice deprived of their T-cells and the demonstration of drug-antiserum synergy. Clin Exper Immunol. 1978;33(2):232–238. [PMC free article] [PubMed] [Google Scholar]
- 34.Doenhoff MJ, Pearson S, Dunne DW, Bickle Q, Lucas S, Bain J, Musallam R, Hassounah O. Immunological control of hepatotoxicity and parasite egg excretion in Schistosoma mansoni infections: stage specificity of the reactivity of immune serum in T-cell deprived mice. Trans Roy Soc Trop Med Hyg. 1981;75(1):41–53. doi: 10.1016/0035-9203(81)90012-2. [DOI] [PubMed] [Google Scholar]
- 35.Doenhoff MJ, Hassounah O, Murare H, Bain J, Lucas S. The schistosome egg granuloma: immunopathology in the cause of host protection or parasite survival? Trans Roy Soc Trop Med Hyg. 1986;80(4):503–514. doi: 10.1016/0035-9203(86)90126-4. [DOI] [PubMed] [Google Scholar]
- 36.Davies SJ, Grogan JL, Blank RB, Lim KC, Locksley RM, McKerrow JH. Modulation of blood fluke development in the liver by hepatic CD4+ lymphocytes. Science. 2001;294(5545):1358–1361. doi: 10.1126/science.1064462. [DOI] [PubMed] [Google Scholar]
- 37.Dunne DW, Hassounah O, Musallam R, Lucas S, Pepys MB, Baltz M, Doenhoff M. Mechanisms of Schistosoma mansoni egg excretion: parasitological observations in immunosuppressed mice reconstituted with immune serum. Parasite Immunol. 1983;5(1):47–60. doi: 10.1111/j.1365-3024.1983.tb00722.x. [DOI] [PubMed] [Google Scholar]
- 38.Harrison R, Doenhoff M. Retarded development of Schistosoma mansoni in immunosuppressed mice. Parasitology. 1983;86(03):429–438. doi: 10.1017/S0031182000050629. [DOI] [PubMed] [Google Scholar]
- 39.Kalinkovich A, Weisman Z, Greenberg Z, Nahmias J, Eitan S, Stein M, Bentwich Z. Decreased CD4 and increased CD8 counts with T cell activation is associated with chronic helminth infection. Clin Exp Immunol. 1998;114(3):414–421. doi: 10.1046/j.1365-2249.1998.00736.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


