Abstract
Extracellular shed vesicles (ESVs) facilitate a unique mode of cell cell communication wherein vesicle uptake can induce a change in the recipient cell’s state. Despite the intensity of ESV research, currently reported data represent bulk characterization of concentrated vesicle samples with little attention paid to heterogeneity. ESV populations likely represent diversity in mechanisms of formation, cargo, and size. To better understand ESV subpopulations and the signaling cascades implicated in their formation, we characterize ESV size distributions to identify subpopulations in normal and cancerous epithelial cells. We discovered that cancer cells exhibit bimodal ESV distributions, one small-diameter and another large-diameter population, suggesting that two mechanisms may govern ESV formation, an exosome population and a cancer-specific microvesicle population. Altered glutamine metabolism in cancer is thought to fuel cancer growth but may also support metastatic niche formation through microvesicle production. We describe the role of a glutaminase inhibitor, compound 968, in ESV production. We discovered that inhibiting glutamine metabolism significantly impairs large-diameter microvesicle production in cancer cells.
Keywords: Extracellular Shed Vesicle, Microvesicle, Exosome, Cellular Communication, Biomarker, Cancer, Pancreatic Cancer, KRAS, Microenvironment, Oncosome
1. Introduction
Cells shed heterogeneous vesicular structures into their local environment and throughout the body (1-5). These vesicles facilitate a unique mode of cell cell communication, akin to paracrine signaling, wherein cargo-laden packages are submitted from the originating, or parent, cell to the recipient cell. The uptake of extracellular shed vesicles (ESVs) can induce a change in the recipients’ state and thus its behavior and function (6-9). The changes induced by ESV uptake are as diverse as the family of messengers. ESVs, first described in the literature as exosomes in the 1970s and 1980s (10-13), have been identified and interrogated throughout the years (1, 4, 5, 14-18). ESVs have been described with multiple names (2) including exosomes (19, 20), microvesicles (21, 22), microparticles (23, 24), and oncosomes (25, 26), among others; the nomenclature is not currently standardized (4). The given names, to some degree, indicate provenance, function, or properties. The term exosome typically refers to intraendosomal vesicles released by the cell (12), whereas the term microvesicle typically refers to structures that bud directly from cancer cell surfaces (1, 2, 22). Tumor-released exosomes have been implicated in cancer immunity (15), whereas tumor-derived microvesicles are implicated in the development of the metastatic niche (1, 9, 18, 22, 27-30). Importantly, microvesicles are different from apoptotic bodies (31), as their contents do not merely represent a random sampling of cell constituents, but rather specifically packaged cargo (1, 31). Many assays have been executed to characterize exosome and microvesicle content (32-34), mechanisms of formation (2, 18), and biological activity (7, 19). Although ESV interrogation (2, 35-39) and clinical and commercial application (ExoQuick™; Exo-Flow™; ExoELISA™) (40-42) represent areas of intense research and activity, little has been done to characterize ESV subpopulations emanating from a single cell source (22, 43). Limitations in processing techniques are presently responsible for the sparseness of subpopulation analysis to date.
Currently reported data represent bulk characterization of concentrated ESV samples with little or no attention paid to heterogeneity. ESV populations, even those that emanate from a single cell type, likely represent a diverse population with unique cargo and mechanisms of formation. To better describe ESV populations, an understanding of constituent subpopulations and the signaling cascades implicated in their formation and shedding is necessary (22). Of particular interest is the dissemination of cancer cell-derived microvesicles and their role in priming the metastatic niche. We are unaware of any thorough characterization of cancer cell-derived ESV size distributions. In this work, we characterize ESV size distributions of species sourced from model cancer cell lines to identify distinct subpopulations with a goal of informing subsequent interrogation. We compare these results, in the case of pancreatic cancer, to ESV signatures from a model normal epithelial pancreas cell line.
Among cancers, pancreatic cancer is the fourth-leading cause of cancer death in the United States (44) and the most lethal malignancy, with pancreatic ductal adenocarcinoma (PDAC) being the most common (45). The overall survival rate of pancreatic cancer is less than 5% (46). These abysmal outcomes result, in part, from a typically asymptomatic progression until late-stage cancer has developed. Clinically recommended means of early detection do not exist, even though early dissemination of tumor cells has been implicated in the low survival rates and rapid progression of pancreatic cancer (47, 48). In murine xenograft models, the interaction between cancer cells and normal pancreas cells promotes pancreatic cancer progression (49, 50); and microvesicles harvested from cancer cells interact with and change the state of stromal cells (1, 51, 52). These results indicate that, in pancreatic cancer, cancer cell-derived microvesicles can transform normal cells and prime the tumor microenvironment.
Pancreatic cancer often results from mutations in the RAS family of genes, typically KRAS (47, 53-55). In cells, the binding of guanosine triphosphate (GTP) to KRAS results in its activation (54, 56) and ability to initiate signaling cascades that promote cell proliferation, migration, and differentiation (54, 56). Mutations that cause KRAS to be in a persistently active, GTP-bound state send excessive signals that stimulate cell growth, thus contributing to tumor formation (54, 57, 58). Recently, modification of glutamine metabolism by oncogenic KRAS has been identified as a primary player in maintaining tumor growth and survival (59-64). Consequently, the ability to metabolize glutamine is markedly increased as the cell relies more heavily upon anabolic processes (60, 65, 66). These alterations in cellular metabolism are thought to provide the fuel necessary not only for cancer cell growth but also for microvesicle (MV) production. Given that altered glutamine metabolism results from the ubiquitous KRAS mutations, among other causes (67, 68), in pancreatic cancer, we investigated the effect that treating model pancreatic cancer cell lines with glutaminase inhibitor, compound 968, would have on ESV production. We discovered that inhibiting glutamine metabolism blocked the ability of pancreatic cancer cell lines to generate MVs. These findings underscore the functional connections between the altered metabolic state of cancer cells and their ability to generate ESVs.
2. Methods
2.1. Cells and Culture
U87 MG (U87, HTB-14™, glioblastoma), MDAMB231 (HTB-26™, metastatic mammary gland adenocarcinoma), PANC-1 (CRL-1469™, pancreatic ductal carcinoma), BxPC-3 (CRL-1687™, pancreatic adenocarcinoma), and hTERT-HPNE (CRL-4023™, hTERT-immortalized normal pancreatic duct) model cell lines were obtained from the American Type Culture Collection (ATCC®, Manassas, Virginia). U87 cells were transfected so as to stably express epidermal growth factor receptor variant III (EGFRvIII), see Section 2.2, which is associated with increased proliferation in glioma cells (1, 69, 70); this cell line will subsequently be referred to as U87+EGFRvIII.
2.1.1. Cell Culture
MDAMB231, U87, U87+EGFRvIII, and BxPC-3 cell lines were grown in Roswell Park Memorial Institute (RPMI-1640; Lonza, Walkersville, MD) cell medium containing 10% fetal bovine serum (FBS; Gemini BioProducts, West Sacramento, CA). The PANC-1 cell line was grown in Dulbecco’s Modified Eagle Medium (DMEM; Mediatech, Manassas, VA) containing 10% FBS. The hTERT-HPNE cells were grown in a medium consisting of 75% DMEM (Sigma-Aldrich, St. Louis, MO) and 25% M3™Base (Incell, San Antonio, TX) containing 5% FBS. All lines were maintained at 37 °C in a humidified, 5% carbon dioxide environment. The medium in each flask was exchanged every 2-3 days and rinsed with phosphate buffered saline (PBS) according to standard sterile techniques. All cell cultures are maintained in 25 cm2 rectangular cell culture flasks.
2.2. Generation of Stable Cell Lines
The pcDNA3 construct encoding human EGFRvIII was transfected into U87 cells using Lipofectamine (Invitrogen, Carlsbad, CA). Clones of U87 cells stably expressing EGFRvIII were selected by culturing the cells in RPMI-1640 containing 10% FBS and 1 μg mL−1 puromycin. Once individual clones expressing EGFRvIII were obtained, the cells were then maintained in the same growth medium supplemented with 0.3 μg mL−1 puromycin.
2.3. ESV Harvesting Protocol
Prior to obtaining the ESVs, nearly confluent culture flasks were rinsed with PBS and then subjected to serum-free medium culture conditions for 12 hours. The resultant conditioned media, each from approximately 2.5 × 106 serum-starved cells, was collected for analysis. Conditioned media was centrifuged in two stages, 300×g for 10 minutes and 12000×g for 20 minutes, to pellet intact cells and cell debris, respectively. 500 μL aliquots of the supernatant were extracted for measurement.
2.3.1. Cell Treatment with a Glutaminase Inhibitor
A glutaminase inhibitor, compound 968 (968; EMD Millipore, Billerica, MA), was prepared by dissolving 968 in dimethyl sulfoxide (DMSO; Sigma-Aldrich, St. Louis, MO) at 30 mM. 36 hours prior to ESV harvesting, cells were treated with 968 at 10 μM (71, 72); a total of 1.67 μL of 968 in DMSO was added to 5 mL of culture medium (0.03% by volume). Following the protocol described in Section 2.3, cells were treated with 10 μM 968 under serum-starved conditions for 12 hours. Subsequent harvesting and centrifugation steps are identical to those previously described.
2.4. Immunoblot Analysis
Cultures of cells treated as indicated were rinsed with PBS and then lysed with cell lysis buffer (25 mM Tris, 100 mM NaCl, 1% Triton X-100, 1 mM EDTA, 1 mM DTT, 1 mM NaVO4, 1 mM β-glycerol phosphate, 1 μg mL−1 aprotinin, 1 μg mL−1 leupeptin). To generate ESV lysates, the partially clarified conditioned medium (medium cleared of intact cells and cell debris), see Section 2.3, was filtered using a Steri-Flip PVDF filter with a pore size of 0.2 μM (Millipore Corporation, Billerica, MA). The ESVs retained by the filter were washed thoroughly with PBS and then lysed with 300 μL cell lysis buffer. An equal number of cells from each cell line (5e3 cells) and a corresponding equal ratio of ESVs generated by the cell lines were resolved by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and the proteins were transferred to PVDF membranes. The membranes were blocked in 5% dry milk diluted in TBST (20 mM Tris, 135 mM NaCl, and 0.02% Tween 20), and then were incubated with Flottilin-2 (Cell Signaling, Inc., Danvers, MA), RhoC (Cell Signaling, Inc.), or actin (Sigma-Aldrich) antibodies prepared in TBST. Horseradish-peroxidase conjugated secondary antibodies were used to detect the primary antibodies, followed by extensive washing with TBST and then exposure to enhanced chemiluminescence (ECL) reagent.
2.5. Dynamic Light Scattering
ESV preparations were characterized at 25 °C using dynamic light scattering (DLS; He-Ne laser, 633 nm; 173°backscattered light detection) on a Nano Series Zetasizer (zetasizer, Malvern Instruments, Southborough, MA). 500 μL samples were loaded into a microcuvette (ZEN0118, Malvern Instruments) for measurement. Each measurement represents 3 unique preparations with 3 runs per preparation with at least 12 unique measurements per run. All data reported represent 108 total measurements.
2.6. Data Analysis
All data were processed using an author-scripted MATLAB® routine. This routine employs a nonlinear least-squares regression on multiple 4-parameter skew-normal distributions to fit each data set. The relative scattering intensity [a.u.] of each size distribution figure represents the volume distribution, as reported by the zetasizer which converts intensity distribution data using Mie theory; the magnitude of the figures are scaled by an arbitrary factor to enable facile comparisons among data sets. The peak amplitudes correspond to the total volume represented by the associated particle size. In all figures, unless stated otherwise, error bars represent standard error of the mean for 9 samples.
3. Results
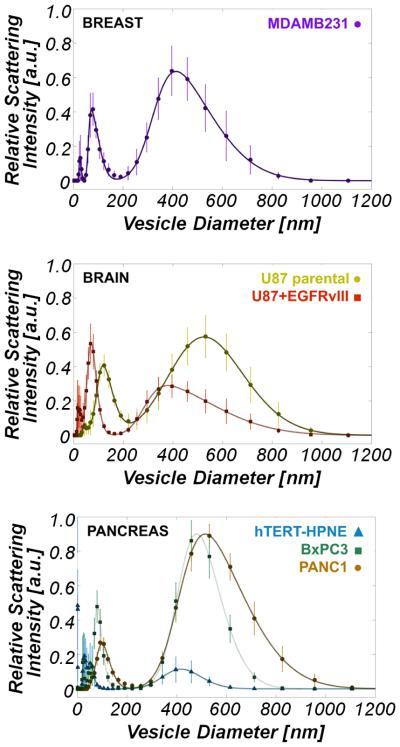
The purpose of these experiments is to identify and establish distinct ESV subpopulations shed from normal and cancerous epithelial cells (MDAMB231; U87, U87+EGFRvIII; PANC-1, BxPC-3, and hTERT-HPNE, Figure 1) by means of dynamic light scattering (DLS). DLS determines the size distribution of particles in solution by measuring their Brownian motion over time and measures relative sample concentrations through total recorded backscattered light. As particles move, light impinging on the particle is scattered; the time-associated scattered light readings are used to generate an autocorrelation curve. From this data, a diffusion coefficient is extracted, and thus sizes can be inferred from the Stokes-Einstein relation. A hallmark feature revealed by this investigation is the characteristic bimodal distribution of ESVs, derived from three distinct types of cancer (breast, brain, and pancreatic cancer), see Figure 1. Across all of the cancer cell lines, the small-diameter population exhibits a peak position of 88 ± 19 nm and an α, skewness, of 3.11 ± 1.17; the large-diameter population exhibits an average peak position of 462 ± 58 nm and an α of 2.85 ± 1.18. The skewness parameters indicate that both ESV subpopulations are dominated by the presence of the relatively larger diameter vesicles. Upon comparing the normal epithelial cell line, hTERT-HPNE, large-diameter peak position (417 ± 11 nm) and magnitude to those of the cancer cell lines, there is no statistically significant difference between the large-diameter peak position of hTERT-HPNE vesicles as compared to those of cancer cells, but approximately an order-of-magnitude difference between the peak amplitudes.
Figure 1.
Dynamic light scattering measurements reveal a bimodal vesicle population among cancer cell types examined. (a) ESV size distribution in MDAMB231 (□) cell lines. MDAMB231 peaks are located at 73 ± 1 nm and 413 ± 4 nm. (b) ESV size distribution in U87 (⚫) and U87+EGFRvIII (■) cell lines. U87 peaks are located at 120 ± 1 nm and 525 ± 5 nm. U87+EGFRvIII peaks are located at 70 ± 3 nm and 378 ± 2 nm. (c) ESV size distribution in PANC-1 (⚫) , BxPC-3 (◾), and hTERT-HPNE (▲) cell lines. The PANC-1 peaks are located at 98 ± 3 nm and 515 ± 3 nm. The BxPC-3 peaks are located at 80 ± 1 nm and 480 ± 2 nm. The hTERT-HPNE peaks are located at 31 ± 1 nm and 51 ± 1 nm, and 417 ± 11 nm. Of particular interest is the striking difference in ESV signatures between the normal pancreas cell line, hTERT-HPNE, and those of the two pancreatic cancer lines, BxPC-3 and PANC-1. Peaks at approximately 30 nm for the brain, breast, and BxPC-3 (pancreas) lines are an artifact of the culture medium (RPMI-1640). All deviations from the peak locations represent those values falling within the 95% confidence interval predicted by nonlinear least squares regression.
Western blots, as shown in Figure 2, were carried-out on the cells and the MVs that the cells shed into the medium to verify that the particles examined with DLS are ESVs. Flotillin-2 is a protein associated with membrane transport and fusion (8, 73) and should be present in ESVs (1, 17, 17, 70). Actin, a major component of the cytoskeleton, is abundantly expressed in both whole cell lysates and ESV lysates (17, 20, 74). In the case of large-diameter ESVs that bud directly from the cell surface, it is possible that actin is essential in the maturation of budding vesicles (17). RhoC, which is involved in extracellular matrix assembly, cytoskeletal reorganization, and cell migration (75, 76), is expressed in the cytosol but is not involved in ESV formation (18). As can be seen in Figure 1 and 2, the immortalized normal epithelial cells (hTERT-HPNE) make almost undetectable levels of MVs as determined by DLS and flotillin-2 and actin staining. Furthermore, ESV preparations are devoid of cytosolic contamination as determined by the fact that RhoC is exclusively present in whole cell lysates (WCLs) and not in ESV lysates (ESVLs).
Figure 2.
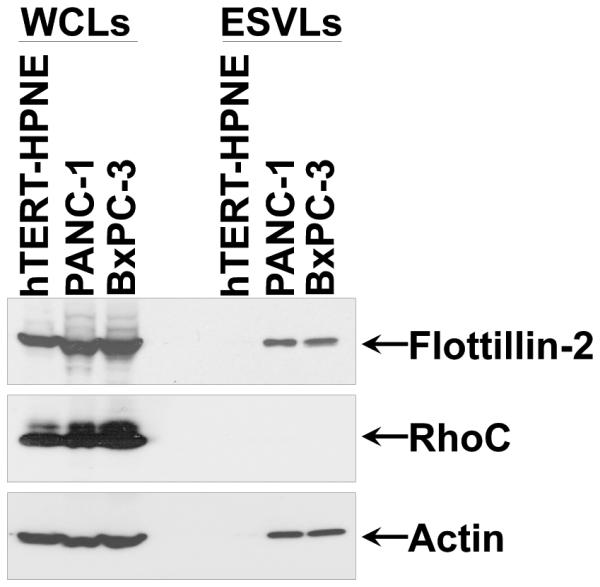
Immunoblot Assay. Serum-starved hTERT-HPNE, PANC-1, and BxPC-3 cells were lysed, and the ESVs shed into the medium by the cells were isolated and lysed as well. The whole cell lysates (WCLs) and the ESV lysates (ESVLs) were subjected to western blot analysis with antibodies against the ESV marker flotillin-2, the cytosolic-specific marker RhoC, and the loading control actin. Two blank channels separate WCLs and ESVLs.
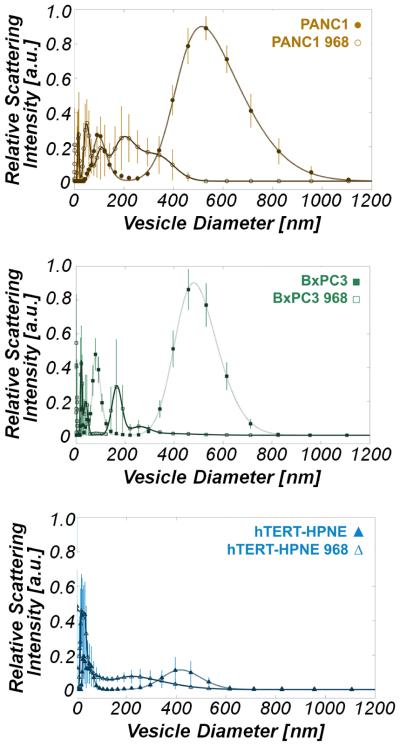
To explore the conjecture that MVs can be related to oncogenic processes, we asked whether glutamine metabolism, which is significantly upregulated in cancer, is important for the ability of cancer cells to generate MVs. Both cancerous epithelial cell lines, PANC-1 and BxPC-3, which rely heavily on glutamine for survival (67, 77), produced significantly more ESVs than the normal epithelial line, hTERT-HPNE. PANC-1 cells produced approximately twice as many ESVs as BxPC-3 cells. Compound 968, a glutaminase inhibitor, was added to the model pancreas lines and its impact upon ESV production was determined by DLS. We found that treating cells with compound 968 drastically altered ESV size distributions, as seen in Figure 3, and diminished microvesicle production, as seen in Figure 3 and Figure 4. The total calculated ESV volume for cancer cells was significantly reduced upon exposure to compound 968, but ESV volume in normal pancreas cells was not significantly affected. Relative to the non-treated cases, there was a 96.00 ± 30.97 % reduction in total PANC-1 vesicle volume, a 97.61 ± 28.49 % reduction in total BxPC-3 vesicle volume, and no statistically significant change in total hTERT-HPNE vesicle volume following treatment with compound 968, see Figure 4.
Figure 3.
Dynamic light scattering measurements demonstrate that treatment of cancer cells with compound 968 substantially reduces large-diameter microvesicle production. (a) ESV size distribution in untreated PANC-1 (⚫) and 968-treated PANC-1 (○) cells. (b) ESV size distribution in BxPC-3 (◾) and 968-treated BxPC-3 (□) cells. (c) ESV size distribution in hTERT-HPNE (▴) and 968-treated hTERT-HPNE (△) cells.
Figure 4.
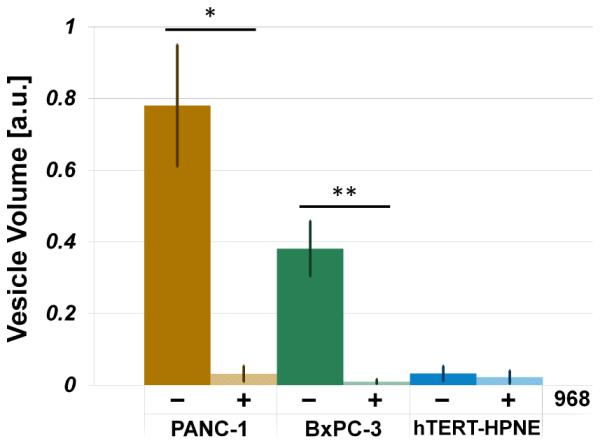
Total vesicle volume analysis demonstrates that treatment of cancer cells with compound 968 dramatically reduces vesicle production in cancer cells (PANC-1, *p = 0.0006; BxPC-3, **p = 0.0002) and has no statistically significant effect on vesicle production in normal epithelial cells (hTERT-HPNE, p = 0.7).
4. Discussion
We used dynamic light scattering to identify distinct cancer cell-derived ESV subpopulations. DLS has the benefit of extracting particle size distributions in a repeatable fashion and is an established, effective tool for characterizing particle sizes, including exosome populations in blood (36, 43, 78). Compared with other measurement approaches, DLS measures ESVs without requiring sample pelleting or dehydration, which could damage ESVs and alter their geometric parameters (73, 79), but does not directly report absolute particle concentration, number, or volume (38). A relative measure of total ESV volume can be calculated by integrating the product of volume percent backscattered light and ESV volume. This calculated total volume is proportional to the total vesicle volume (cargo) that could be delivered to recipient cells. Changes in total vesicle volume resulting from cell treatment or modification, such as exposure to compound 968, provides insight into changes in total ESV production resulting from treatment.
We have demonstrated the presence of two distinct subpopulations within cancer cell-derived vesicles. As suggested in other reports (1, 5, 34, 38, 80), vesicles shed by cancer cells exhibit a size range from approximately 20 nm to over 1 μm; our findings, for breast, brain, and pancreas cells, as shown in Figure 1, are consistent with this reported range. For each cancer cell line examined herein, the peak locations, skewness, and areas of the bimodal ESV distributions were quantified by fitting multiple 4-parameter skew-normal distributions by nonlinear least squares regression. Given the narrow range of peak positions and magnitudes for each cancer cell-derived ESV subpopulation, these data suggest that the processes governing ESV production for both small- and large-diameter vesicles may be tightly regulated and conserved across cancer types (22, 73). Despite similarity in ESV size distributions among cancer types, PANC-1 cells, which express oncogenic KRAS, produce substantially more ESVs than do BxPC-3 cells, which express wild-type KRAS (81). The presence of small- and large-diameter populations in cancerous epithelial cell lines suggests that two unique mechanisms may govern the biogenesis of each unique subpopulation (2, 22). It is possible, although yet to be determined, that the small-diameter population is representative of a normal cell exosome signature and that the large-diameter vesicles represent an aberrant, cancer-related microvesicle signature. Evidence in a small cohort of stomach and liver cancer patients (82, 83) further bolsters this claim.
We determined that in the cell lines considered large-diameter ESVs (microvesicles) are a cancer-specific signature associated with dysregulated glutamine metabolism. Cancer activates, upregulates, modifies, or creates specific signaling cascades that are dysregulated versions of host pathways not expressed under normal conditions (18, 63, 84, 85). Given the role of glutamine metabolism in pancreatic cancer (54, 58-60, 67, 68), we explored the impact of a glutaminase inhibitor, compound 968, on microvesicle production. Compound 968 has been shown to inhibit cancer growth but has no impact on the proliferation of normal cells (71, 86, 87). We found that the results of treating cells with this glutaminase inhibitor included a preferential reduction in the large-diameter vesicle population, see Figure 3, and a drastic reduction in total vesicle production, as shown in Figure 3 and Figure 4. This outcome is consistent with the hypothesis that large-diameter ESVs (microvesicles) are cancer-specific, as the introduction of a glutaminase inhibitor significantly disrupted their production in cancer cells but only had a limited effect on normal epithelial cells, see Figure 3. These data also suggest that treating cancer cells with glutaminase inhibitors may serve as a means not only to preferentially starve, and perhaps eliminate, cancer cells (71, 88), but also as a way to restrict their MV production, which could reduce their ability to prepare the metastatic niche (7, 28, 78, 89). More work is necessary to appropriately interrogate and describe the role of glutaminase inhibitors in ESV production in cancer.
5. Conclusion
Understanding the mechanisms of ESV generation, as well as their cargo and properties, is essential. This is particularly crucial, as ESVs putatively induce changes of state in recipient cells and prime local environments for primary and metastatic tumor establishment and growth. As metastases are responsible for 90% of cancer-related death (7, 90), unlocking the processes by which ESVs are prepared provides insight into cancer treatment and inhibition of its progression. We discovered that inhibition of glutamine metabolism in model cancer cell lines significantly impairs large-diameter microvesicle production. This result suggests that vesicle formation in cancer cells can be reduced by applying a metabolic inhibitor and indicates that the large-diameter population may be representative of cancer cell-derived microvesicles. We have also characterized the size distributions and total relative volumes of ESVs from multiple model cell lines of primary tumor and metastatic origin, as well as a normal epithelial cell line. We discovered that each cancer cell population exhibits a bimodal distribution of vesicles, including one small population of particles with diameters less than approximately 200 nm and another large population bearing a diameter range from approximately 200 nm to 1.10 μm. The presence of a significant large-diameter ESV population appears only in cancer cells and not in normal epithelial cells. This feature of ESV subpopulations bolsters the argument that there may be two mechanisms governing ESV formation, a small-diameter, exosome, population and a large-diameter, cancer-specific, microvesicle population. Further investigations are required to definitively establish the specific signaling cascades that govern ESV formation processes, as this could elucidate both mechanisms of vesicle formation and vesicle roles in priming the metastatic niche.
Acknowledgements
This work was supported by the National Cancer Institutes under Award Number U54CA143876, the National Science Foundation GK-12 Program under Award Number 0841291, and the Alfred P. Sloan Foundation. hTERT-HPNE cells were provided by National Institutes of Health Physical Sciences in Oncology Network Bioresource Core Facility.
Abbreviations list
- ESVs
Extracellular shed vesicles
- MVs
Microvesicles
- GTP
Guanosine triphosphate
- PDAC
pancreatic ductal adenocarcinoma
- EGFRvIII
Epidermal Growth Factor Receptor variant III
- DLS
Dynamic light scattering
- ESVLs
Extracellular shed vesicles lysates
- WCLs
Whole cell lysates
References
- [1].Antonyak Marc A, Bo Li K Lindsey, Johnson Jared L, Druso Joseph E, Bryant Kirsten L, Holowka David A, Cerione Richard A., Boroughs Lindsey K. Cancer cell-derived microvesicles induce transformation by transferring tissue transglutaminase and fibronectin to recipient cells. Proceedings of the National Academy of Sciences. 2011 Sep;108(42):17569–17569. doi: 10.1073/pnas.1017667108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Lee Tae Hoon, D’Asti Esterina, Magnus Nathalie, Al-Nedawi Khalid, Meehan Brian, Rak Janusz. Microvesicles as mediators of intercellular communication in cancer– the emerging science of cellular ’debris’. Seminars in immunopathology. 2011 Sep;33(5):455–67. doi: 10.1007/s00281-011-0250-3. [DOI] [PubMed] [Google Scholar]
- [3].Li Xiao-Bo, Zhang Zhi-Ren, Schluesener Hermann J., Xu Shun-Qing. Role of exosomes in immune regulation. Journal of Cellular and Molecular Medicine. 2006 Apr;10(2):364–375. doi: 10.1111/j.1582-4934.2006.tb00405.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Bobrie Angélique, Colombo Marina, Raposo Graça, Théry Clotilde. Exosome secretion: molecular mechanisms and roles in immune responses. Traffic (Copenhagen, Denmark) 2011 Dec;12(12):1659–68. doi: 10.1111/j.1600-0854.2011.01225.x. [DOI] [PubMed] [Google Scholar]
- [5].Muralidharan-Chari Vandhana, Clancy James W, Sedgwick Alanna, D’Souza-Schorey Crislyn. Microvesicles: mediators of extracellular communication during cancer progression. Journal of cell science. 2010 May;123:1603–11. doi: 10.1242/jcs.064386. Pt 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Keller Sascha, Sanderson Michael P, Stoeck Alexander, Altevogt Peter. Exosomes: From biogenesis and secretion to biological function. Immunology Letters. 2006;107:102–108. doi: 10.1016/j.imlet.2006.09.005. [DOI] [PubMed] [Google Scholar]
- [7].Peinado Héctor, Lavotshkin Simon, Lyden David. The secreted factors responsible for pre-metastatic niche formation: old sayings and new thoughts. Seminars in cancer biology. 2011 Apr;21(2):139–46. doi: 10.1016/j.semcancer.2011.01.002. [DOI] [PubMed] [Google Scholar]
- [8].van Niel Guillaume, Porto-Carreiro Isabel, Simoes Sabrina, Raposo Graça. Exosomes: a common pathway for a specialized function. Journal of biochemistry. 2006 Jul;140(1):13–21. doi: 10.1093/jb/mvj128. [DOI] [PubMed] [Google Scholar]
- [9].Guo Feng, French Jarrod B, Li Peng, Zhao Hong, Yu Chan Chung, Fick James R, Benkovic Stephen J, Jun Huang Tony. Probing cell-cell communication with microfluidic devices. Lab on a chip. 2013 Aug;13(16):3152–62. doi: 10.1039/c3lc90067c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Harding C, Heuser J, Stahl P. Endocytosis and intracellular processing of transferrin and colloidal gold-transferrin in rat reticulocytes: demonstration of a pathway for receptor shedding. European journal of cell biology. 1984 Nov;35(2):256–63. [PubMed] [Google Scholar]
- [11].Pan BT. Electron microscopic evidence for externalization of the transferrin receptor in vesicular form in sheep reticulocytes. The Journal of Cell Biology. 1985 Sep;101(3):942–948. doi: 10.1083/jcb.101.3.942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Johnstone RM, Adam M, Hammond JR, Orr L, Turbide C. Vesicle formation during reticulocyte maturation. Association of plasma membrane activities with released vesicles (exosomes) J. Biol. Chem. 1987 Jul;262(19):9412–9420. [PubMed] [Google Scholar]
- [13].Friend C, Marovitz W, Henle G, Henle W, Tsuei D, Hirschhorn K, Holland JG, Cuttner J. Observations on Cell Lines Derived from a Patient with Hodgkin’s Disease. Cancer Res. 1978 Aug;38(8):2581–2591. [PubMed] [Google Scholar]
- [14].Johnstone Rose M. Exosomes biological significance: a concise review. Blood Cells, Molecules, and Diseases. 2006 Apr;36(2):315–21. doi: 10.1016/j.bcmd.2005.12.001. [DOI] [PubMed] [Google Scholar]
- [15].Iero M, Valenti R, Huber V, Filipazzi P, Parmiani G, Fais S, Rivoltini L. Tumour-released exosomes and their implications in cancer immunity. Cell death and differentiation. 2008 Jan;15(1):80–8. doi: 10.1038/sj.cdd.4402237. [DOI] [PubMed] [Google Scholar]
- [16].Van Doormaal FF, Kleinjan A, Di Nisio M, Büller HR, Nieuwland R. Cell-derived microvesicles and cancer. Neth J Med. 2009;67(7):266–273. [PubMed] [Google Scholar]
- [17].Li B, Antonyak MA, Zhang J, Cerione Richard A. RhoA triggers a specific signaling pathway that generates transforming microvesicles in cancer cells. Oncogene. 2012 Nov;31(45):4740–9. doi: 10.1038/onc.2011.636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Antonyak Marc A, Wilson Kristin F, Cerione Richard A. R(h)oads to microvesicles. Small GTPases. 2012;3(4):219–24. doi: 10.4161/sgtp.20755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Schorey Jeffrey S, Bhatnagar Sanchita. Exosome function: from tumor immunology to pathogen biology. Traffic. 2008;9:871–881. doi: 10.1111/j.1600-0854.2008.00734.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Simpson Richard J, Lim Justin We E, Moritz Robert L, Mathivanan Suresh. Exosomes: proteomic insights and diagnostic potential. Expert review of proteomics. 2009 Jun;6(3):267–83. doi: 10.1586/epr.09.17. [DOI] [PubMed] [Google Scholar]
- [21].Castellana Donatello, Toti Florence, Freyssinet Jean-Marie. Membrane microvesicles: Macromessengers in cancer disease and progression. Thrombosis Research. 2010;125:S84–S88. doi: 10.1016/S0049-3848(10)70021-9. Suppl. [DOI] [PubMed] [Google Scholar]
- [22].D’Souza-Schorey Crislyn, Clancy James W. Tumor-derived microvesicles: shedding light on novel microenvironment modulators and prospective cancer biomarkers. Genes & development. 2012 Jun;26(12):1287–99. doi: 10.1101/gad.192351.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Taylor Douglas D, Gercel-Taylor Cicek. Exosomes/microvesicles: mediators of cancer-associated immunosuppressive microenvironments. Seminars in immunopathology. 2011 Sep;33(5):441–54. doi: 10.1007/s00281-010-0234-8. [DOI] [PubMed] [Google Scholar]
- [24].Tushuizen Maarten E, Diamant Michaela, Sturk Augueste, Nieuwland Rienk. Cell-derived microparticles in the pathogenesis of cardiovascular disease: friend or foe? Arteriosclerosis, thrombosis, and vascular biology. 2011 Jan;31(1):4–9. doi: 10.1161/ATVBAHA.109.200998. [DOI] [PubMed] [Google Scholar]
- [25].Al-Nedawi Khalid, Meehan Brian, Micallef Johann, Lhotak Vladimir, May Linda, Guha Abhijit, Rak Janusz. Intercellular transfer of the oncogenic receptor EGFRvIII by microvesicles derived from tumour cells. Nature cell biology. 2008 May;10(5):619–24. doi: 10.1038/ncb1725. [DOI] [PubMed] [Google Scholar]
- [26].Morello Matteo, Minciacchi Valentina R, de Candia Paola, Yang Julie, Posadas Edwin, Kim Hyung, Griffiths Duncan, Bhowmick Neil, Chung Leland WK, Gandellini Paolo, Freeman Michael R, Demichelis Francesca, Di Vizio Dolores. Large oncosomes mediate intercellular transfer of functional microRNA. Cell Cycle. 2013 Nov;12(22):59–69. doi: 10.4161/cc.26539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Ariztia Edgardo V., Lee Catherine J., Gogoi Radhika, Fishman David A. The Tumor Microenvironment: Key to Early Detection. Critical reviews in clinical laboratory sciences. 2008 Oct;43(5-6):393–425. doi: 10.1080/10408360600778836. [DOI] [PubMed] [Google Scholar]
- [28].Park Jung Eun, Tan Hon Sen, Datta Arnab, Lai Ruenn Chai, Zhang Huoming, Meng Wei, Lim Sai Kiang, Sze Siu Kwan. Hypoxic tumor cell modulates its microenvironment to enhance angiogenic and metastatic potential by secretion of proteins and exosomes. Molecular & cellular proteomics : MCP. 2010 Jun;9(6):1085–99. doi: 10.1074/mcp.M900381-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].lPsaila Bethan, Lyden David. The metastatic niche: adapting the foreign soil. Nature reviews. Cancer. 2009 Apr;9(4):285–93. doi: 10.1038/nrc2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Im Hyungsoon, Shao Huilin, Park Yong Il, Peterson Vanessa M, Castro Cesar M, Weissleder Ralph, Lee Hakho. Label-free detection and molecular profiling of exosomes with a nano-plasmonic sensor. Nature biotechnology. 2014 May;32(5):490–5. doi: 10.1038/nbt.2886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Journal of immunology (Baltimore, Md. : 1950) 2001 Jun;166(12):7309–18. doi: 10.4049/jimmunol.166.12.7309. [DOI] [PubMed] [Google Scholar]
- [32].Mathivanan Suresh, Simpson Richard J. ExoCarta: A compendium of exosomal proteins and RNA. Proteomics. 2009 Nov;9(21):4997–5000. doi: 10.1002/pmic.200900351. [DOI] [PubMed] [Google Scholar]
- [33].Valadi Hadi, Ekström Karin, Bossios Apostolos, Sjöstrand Margareta, Lee James J, Lötvall Jan O. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nature cell biology. 2007 Jun;9(6):654–9. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- [34].Choi Dong-Sic, Lee Jae-Min, Park Gun Wook, Lim Hyeon-Woo, Bang Joo Young, Kim Yoon-Keun, Kwon Kyung-Hoon, Kwon Ho Jeong, Kim Kwang Pyo, Gho Yong Song. Proteomic analysis of microvesicles derived from human colorectal cancer cells. Journal of proteome research. 2007 Dec;6(12):4646–55. doi: 10.1021/pr070192y. [DOI] [PubMed] [Google Scholar]
- [35].Yuana Y, Oosterkamp TH, Bahatyrova S, Ashcroft B, Garcia Rodriguez P, Bertina RM, Osanto S. Atomic force microscopy: a novel approach to the detection of nanosized blood microparticles. Journal of thrombosis and haemostasis : JTH. 2010 Feb;8(2):315–23. doi: 10.1111/j.1538-7836.2009.03654.x. [DOI] [PubMed] [Google Scholar]
- [36].Lawrie AS, Albanyan A, Cardigan RA, Mackie IJ, Harrison P. Microparticle sizing by dynamic light scattering in fresh-frozen plasma. Vox sanguinis. 2009 Apr;96(3):206–12. doi: 10.1111/j.1423-0410.2008.01151.x. [DOI] [PubMed] [Google Scholar]
- [37].Jorgensen Malene, Baek Rikke, Pedersen Shona, Sondergaard Evo K.L., Kristensen Soren R., Varming Kim. Extracellular Vesicle (EV) Array: microarray capturing of exosomes and other extracellular vesicles for multiplexed phenotyping. Journal of Extracellular Vesicles. 2013;2 doi: 10.3402/jev.v2i0.20920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].van der Pol E, Hoekstra AG, Sturk A, Otto C, van Leeuwen TG, Nieuwland R. Optical and non-optical methods for detection and characterization of microparticles and exosomes. Journal of thrombosis and haemostasis : JTH. 2010 Dec;8(12):2596–607. doi: 10.1111/j.1538-7836.2010.04074.x. [DOI] [PubMed] [Google Scholar]
- [39].Ratajczak J, Wysoczynski M, Hayek F, Janowska-Wieczorek A, Ratajczak MZ. Membrane-derived microvesicles: important and underappreciated mediators of cell-to-cell communication. Leukemia. 2006 Sep;20(9):1487–1495. doi: 10.1038/sj.leu.2404296. [DOI] [PubMed] [Google Scholar]
- [40].Dai Shengming, Wei Dong, Wu Zhen, Zhou Xiangyang, Wei Xiaomou, Huang Haixin, Li Guisheng. Phase I clinical trial of autologous ascites-derived exosomes combined with GM-CSF for colorectal cancer. Molecular therapy : the journal of the American Society of Gene Therapy. 2008 Apr;16(4):782–90. doi: 10.1038/mt.2008.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Escudier Bernard, Dorval Thierry, Chaput Nathalie, André Fabrice, Caby Marie-Pierre, Novault Sophie, Flament Caroline, Leboulaire Christophe, Borg Christophe, Amigorena Sebastian, Boccaccio Catherine, Bonnerot Christian, Dhellin Olivier, Movassagh Mojgan, Piperno Sophie, Robert Caroline, Serra Vincent, Valente Nancy, Le Pecq Jean-Bernard, Spatz Alain, Lantz Olivier, Tursz Thomas, Angevin Eric, Zitvogel Laurence. Vaccination of metastatic melanoma patients with autologous dendritic cell (DC) derived-exosomes: results of thefirst phase I clinical trial. Journal of translational medicine. 2005 Mar;3(1):10. doi: 10.1186/1479-5876-3-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Viaud Sophie, Théry Clotilde, Ploix Stéphanie, Tursz Thomas, Lapierre Valérie, Lantz Olivier, Zitvogel Laurence, Chaput Nathalie. Dendritic cell-derived exosomes for cancer immunotherapy: what’s next? Cancer research. 2010 Feb;70(4):1281–5. doi: 10.1158/0008-5472.CAN-09-3276. [DOI] [PubMed] [Google Scholar]
- [43].György Bence, Módos Károly, Pállinger Eva, Pálóczi Krisztina, Pásztói Mária, Misják Petra, Deli Mária A, Sipos Aron, Szalai Anikó, Voszka István, Polgár Anna, Tóth Kálmán, Csete Mária, Nagy György, Gay Steffen, Falus András, Kittel Agnes, Buzás Edit I. Detection and isolation of cell-derived microparticles are compromised by protein complexes resulting from shared biophysical parameters. Blood. 2011 Jan;117(4):e39–48. doi: 10.1182/blood-2010-09-307595. [DOI] [PubMed] [Google Scholar]
- [44].Siegel Rebecca, Naishadham Deepa, Jemal Ahmedin. Cancer statistics. CA: a cancer journal for clinicians. 2012;2012;62(1):10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- [45].Tuveson DavidA., Neoptolemos JohnP. Understanding Metastasis in Pancreatic Cancer: A Call for New Clinical Approaches. Cell. 2012;148(1):21–23. doi: 10.1016/j.cell.2011.12.021. [DOI] [PubMed] [Google Scholar]
- [46].Eser Stefan, Messer Marlena, Eser Philipp, von Werder Alexander, Seidler Barbara, Bajbouj Monther, Vogelmann Roger, Meining Alexander, von Burstin Johannes, Algül Hana, Pagel Philipp, Schnieke Angelika E, Esposito Irene, Schmid Roland M, Schneider Günter, Saur Dieter. In vivo diagnosis of murine pancreatic intraepithelial neoplasia and early-stage pancreatic cancer by molecular imaging. Proceedings of the National Academy of Sciences of the United States of America. 2011 Jun;108(24):9945–50. doi: 10.1073/pnas.1100890108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Rhim Andrew D, Mirek Emily T, Aiello Nicole M, Maitra Anirban, Bailey Jennifer M, McAllister Florencia, Reichert Maximilian, Beatty Gregory L, Rustgi Anil K, Vonderheide Robert H, Leach Steven D, Stanger Ben Z. EMT and dissemination precede pancreatic tumor formation. Cell. 2012 Jan;148(1-2):349–61. doi: 10.1016/j.cell.2011.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Rhim Andrew D., Thege Fredrik I., Santana Steven M., Lannin Timothy B., Saha Trisha N., Tsai Shannon, Maggs Lara R., Kochman Michael L., Ginsberg Gregory G., Lieb John G., Chandrasekhara Vinay, Drebin Jeffrey A., Ahmad Nuzhat, Yang Yu-Xiao, Kirby Brian J., Stanger Ben Z. Detection of Circulating Pancreas Epithelial Cells in Patients with Pancreatic Cystic Lesions. Gastroenterology. 2013 doi: 10.1053/j.gastro.2013.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Schneiderhan Wilhelm, Diaz Fredy, Fundel Martin, Zhou Shaoxia, Siech Marco, Hasel Cornelia, Möller Peter, Gschwend Jürgen E, Seufferlein Thomas, Gress Thomas, Adler Guido, Bachem Max G. Pancreatic stellate cells are an important source of MMP-2 in human pancreatic cancer and accelerate tumor progression in a murine xenograft model and CAM assay. Journal of cell science. 2007 Feb;120:512–9. doi: 10.1242/jcs.03347. Pt 3. [DOI] [PubMed] [Google Scholar]
- [50].Bachem Max G, Zhou Shaoxia, Buck Karin, Schneiderhan Wilhelm, Siech Marco. Pancreatic stellate cells–role in pancreas cancer. Langenbeck’s archives of surgery / Deutsche Gesellschaft für Chirurgie. 2008 Nov;393(6):891–900. doi: 10.1007/s00423-008-0279-5. [DOI] [PubMed] [Google Scholar]
- [51].Camussi Giovanni, Deregibus Maria C, Bruno Stefania, Cantaluppi Vincenzo, Biancone Luigi. Exosomes/microvesicles as a mechanism of cell-to-cell communication. Kidney international. 2010 Nov;78(9):838–48. doi: 10.1038/ki.2010.278. [DOI] [PubMed] [Google Scholar]
- [52].Pucci Ferdinando, Pittet Mikael J. Molecular pathways: tumor-derived microvesicles and their interactions with immune cells in vivo. Clinical cancer research : an official journal of the American Association for Cancer Research. 2013 May;19(10):2598–604. doi: 10.1158/1078-0432.CCR-12-0962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Hezel Aram F, Kimmelman Alec C, Stanger Ben Z, Bardeesy Nabeel, Depinho Ronald A. Genetics and biology of pancreatic ductal adenocarcinoma. Genes & development. 2006 May;20(10):1218–49. doi: 10.1101/gad.1415606. [DOI] [PubMed] [Google Scholar]
- [54].di Magliano Marina Pasca, Logsdon Craig D. Roles for KRAS in Pancreatic Tumor Development and Progression. Gastroenterology. 2013;144(6):1220–1229. doi: 10.1053/j.gastro.2013.01.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Fernández-Medarde Alberto, Santos Eugenio. Ras in cancer and developmental diseases. Genes & cancer. 2011 Mar;2(3):344–58. doi: 10.1177/1947601911411084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Spaargaren M, Bischoff JR, McCormick F. Signal transduction by Ras-like GTPases: a potential target for anticancer drugs. Gene expression. 1995 Jan;4(6):345–56. [PMC free article] [PubMed] [Google Scholar]
- [57].Biankin Andrew V, Waddell Nicola, Kassahn Karin S, Gingras Marie-Claude, Muthuswamy Lakshmi B, Johns Amber L, Miller David K, Wilson Peter J, Patch Ann-Marie, Wu Jianmin, Chang David K, Cowley Mark J, Gardiner Brooke B, Song Sarah, Harliwong Ivon, Idrisoglu Senel, Nourse Craig, Nourbakhsh Ehsan, Manning Suzanne, Wani Shivangi, Gongora Milena, Pajic Marina, Scarlett Christopher J, Gill Anthony J, Pinho Andreia V, Rooman Ilse, Anderson Matthew, Holmes Oliver, Leonard Conrad, Taylor Darrin, Wood Scott, Xu Qinying, Nones Katia, Fink J Lynn, Christ Angelika, Bruxner Tim, Cloonan Nicole, Kolle Gabriel, Newell Felicity, Pinese Mark, Mead R Scott, Humphris Jeremy L, Kaplan Warren, Jones Marc D, Colvin Emily K, Nagrial Adnan M, Humphrey Emily S, Chou Angela, Chin Venessa T, Chantrill Lorraine A, Mawson Amanda, Samra Jaswinder S, Kench James G, Lovell Jessica A, Daly Roger J, Merrett Neil D, Toon Christopher, Epari Krishna, Nguyen Nam Q, Barbour Andrew, Zeps Nikolajs, Kakkar Nipun, Zhao Fengmei, Wu Yuan Qing, Wang Min, Muzny Donna M, Fisher William E, Charles Brunicardi F, Hodges Sally E, Reid Jeffrey G, Drummond Jennifer, Chang Kyle, Han Yi, Lewis Lora R, Dinh Huyen, Buhay Christian J, Beck Timothy, Timms Lee, Sam Michelle, Begley Kimberly, Brown Andrew, Pai Deepa, Panchal Ami, Buchner Nicholas, De Borja Richard, Denroche Robert E, Yung Christina K, Serra Stefano, Onetto Nicole, Mukhopadhyay Debabrata, Tsao Ming-Sound, Shaw Patricia A, Petersen Gloria M, Gallinger Steven, Hruban Ralph H, Maitra Anirban, Iacobuzio-Donahue Christine A, Schulick Richard D, Wolfgang Christopher L, Morgan Richard A, Lawlor Rita T, Capelli Paola, Corbo Vincenzo, Scardoni Maria, Tortora Giampaolo, Tempero Margaret A, Mann Karen M, Jenkins Nancy A, Perez-Mancera Pedro A, Adams David J, Largaespada David A, Wessels Lodewyk F A, Rust Alistair G, Stein Lincoln D, Tuveson David A, Copeland Neal G, Musgrove Elizabeth A, Scarpa Aldo, Eshleman James R, Hudson Thomas J, Sutherland Robert L, Wheeler David A, Pearson John V, McPherson John D, Gibbs Richard A, Grimmond Sean M. Pancreatic cancer genomes reveal aberrations in axon guidance pathway genes. Nature. 2012 Nov;491(7424):399–405. doi: 10.1038/nature11547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Jones Siân, Zhang Xiaosong, Parsons D Williams, Lin Jimmy Cheng-Ho, Leary Rebecca J, Angenendt Philipp, Mankoo Parminder, Carter Hannah, Kamiyama Hirohiko, Jimeno Antonio, Hong Seung-Mo, Fu Baojin, Lin Ming-Tseh, Calhoun Eric S, Kamiyama Mihoko, Walter Kimberly, Nikolskaya Tatiana, Nikolsky Yuri, Hartigan James, Smith Douglas R, Hidalgo Manuel, Leach Steven D, Klein Alison P, Jaffee Elizabeth M, Goggins Michael, Maitra Anirban, Iacobuzio-Donahue Christine, Eshleman James R, Kern Scott E, Hruban Ralph H, Karchin Rachel, Papadopoulos Nickolas, Parmigiani Giovanni, Vogelstein Bert, Velculescu Victor E, Kinzler Kenneth W. Core signaling pathways in human pancreatic cancers revealed by global genomic analyses. Science (New York, N.Y.) 2008 Sep;321(5897):1801–6. doi: 10.1126/science.1164368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Son Jaekyoung, Lyssiotis Costas A, Ying Haoqiang, Wang Xiaoxu, Hua Sujun, Ligorio Matteo, Perera Rushika M, Ferrone Cristina R, Mullarky Edouard, Shyh-Chang Ng, Kang Ya’an, Fleming Jason B, Bardeesy Nabeel, Asara John M, Haigis Marcia C, DePinho Ronald A, Cantley Lewis C, Kimmelman Alec C. Glutamine supports pancreatic cancer growth through a KRAS-regulated metabolic pathway. Nature. 2013 Apr;496(7443):101–5. doi: 10.1038/nature12040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Ying Haoqiang, Kimmelman AlecC., Lyssiotis CostasA., Hua Sujun, Chu GeraldC., Fletcher-Sananikone Eliot, Locasale JasonW., Son Jaekyoung, Zhang Hailei, Coloff JonathanL., Yan Haiyan, Wang Wei, Chen Shujuan, Viale Andrea, Zheng Hongwu, Paik Ji-hye, Lim Carol, Guimaraes AlexanderR., Martin EricS., Chang Jeffery, Hezel AramF., Perry SamuelR., Hu Jian, Gan Boyi, Xiao Yonghong, Asara JohnM., Weissleder Ralph, Wang Y.Alan, Chin Lynda, Cantley LewisC., DePinho RonaldA. Oncogenic Kras Maintains Pancreatic Tumors through Regulation of Anabolic Glucose Metabolism. Cell. 2012;149(3):656–670. doi: 10.1016/j.cell.2012.01.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Lyssiotis Costas A, Son Jaekyoung, Cantley Lewis C, Kimmelman Alec C. Pancreatic cancers rely on a novel glutamine metabolism pathway to maintain redox balance. Cell cycle (Georgetown, Tex.) 2013 Jul;12(13):1987–8. doi: 10.4161/cc.25307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Ward PatrickS., Thompson CraigB. Metabolic Reprogramming: A Cancer Hallmark Even Warburg Did Not Anticipate. Cancer Cell. 2012;21(3):297–308. doi: 10.1016/j.ccr.2012.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Hsu Peggy P., Sabatini David M. Cancer Cell Metabolism: Warburg and Beyond. Cell. 2008 Sep;134(5):703–7. doi: 10.1016/j.cell.2008.08.021. [DOI] [PubMed] [Google Scholar]
- [64].Vander Heiden Matthew G, Cantley Lewis C, Thompson Craig B. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science (New York, N.Y.) 2009 May;324(5930):1029–33. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Wise David R., Thompson Craig B. Glutamine addiction: a new therapeutic target in cancer. Trends in Biochemical Sciences. 2010;35(8):427–433. doi: 10.1016/j.tibs.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Cantor Jason R, Sabatini David M. Cancer cell metabolism: one hallmark, many faces. Cancer discovery. 2012 Oct;2(10):881–98. doi: 10.1158/2159-8290.CD-12-0345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Kaadige Mohan R, Looper Ryan E, Kamalanaadhan Sadhaasivam, Ayer Donald E. Glutamine-dependent anapleurosis dictates glucose uptake and cell growth by regulating MondoA transcriptional activity. Proceedings of the National Academy of Sciences of the United States of America. 2009 Sep;106(35):14878–83. doi: 10.1073/pnas.0901221106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Sloan Elizabeth J, Ayer Donald E. Myc, mondo, and metabolism. Genes & cancer. 2010 Jun;1(6):587–96. doi: 10.1177/1947601910377489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Montano Nicola, Cenci Tonia, Martini Maurizio, Giorgio D’Alessandris Quintino, Pelacchi Federica, Ricci-Vitiani Lucia, Maira Giulio, De Maria Ruggero, Maria Larocca Luigi, Pallini Roberto. Expression of EGFRvIII in glioblastoma: prognostic significance revisited. Neoplasia (New York, N.Y.) 2011 Dec;13(12):1113–21. doi: 10.1593/neo.111338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Shao Huilin, Chung Jaehoon, Balaj Leonora, Charest Alain, Bigner Darell D, Carter Bob S, Hochberg Fred H, Breakefield Xandra O, Weissleder Ralph, Lee Hakho. Protein typing of circulating microvesicles allows real-time monitoring of glioblastoma therapy. Nature medicine. 2012 Dec;18(12):1835–40. doi: 10.1038/nm.2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Wang Jian-Bin, Erickson Jon W., Fuji Reina, Ramachandran Sekar, Gao Ping, Dinavahi Ramani, Wilson Kristin F., Ambrosio Andre L.B., Dias Sandra M.G., Dang Chi V., Cerione Richard A. Targeting Mitochondrial Glutaminase Activity Inhibits Oncogenic Transformation. Cancer Cell. 2010;18(3):207–219. doi: 10.1016/j.ccr.2010.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Katt William P, Ramachandran Sekar, Erickson Jon W, Cerione Richard A. Dibenzophenanthridines as inhibitors of glutaminase C and cancer cell proliferation. Molecular cancer therapeutics. 2012 Jun;11(6):1269–78. doi: 10.1158/1535-7163.MCT-11-0942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].van der Pol Edwin, Böing Anita N, Harrison Paul, Sturk Augueste, Nieuwland Rienk. Classification, functions, and clinical relevance of extracellular vesicles. Pharmacological reviews. 2012 Jul;64(3):676–705. doi: 10.1124/pr.112.005983. [DOI] [PubMed] [Google Scholar]
- [74].Coren Lori V, Shatzer Teresa, Ott David E. CD45 immunoaffinity depletion of vesicles from Jurkat T cells demonstrates that exosomes contain CD45: no evidence for a distinct exosome/HIV-1 budding pathway. Retrovirology. 2008 Jan;5(1):64. doi: 10.1186/1742-4690-5-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Clark EA, Golub TR, Lander ES, Hynes RO. Genomic analysis of metastasis reveals an essential role for RhoC. Nature. 2000 Aug;406(6795):532–5. doi: 10.1038/35020106. [DOI] [PubMed] [Google Scholar]
- [76].Kutay Ulrike, Stournaras Christos, Vega Francisco M., Ridley Anne J. Rho GTPases in cancer cell biology. FEBS Letters. 2008;582(14):2093–2101. doi: 10.1016/j.febslet.2008.04.039. [DOI] [PubMed] [Google Scholar]
- [77].Dang Chi V. MYC, microRNAs and glutamine addiction in cancers. Cell cycle (Georgetown, Tex.) 2009 Oct;8(20):3243–5. doi: 10.4161/cc.8.20.9522. [DOI] [PubMed] [Google Scholar]
- [78].Grange Cristina, Tapparo Marta, Collino Federica, Vitillo Loriana, Damasco Christian, Deregibus Maria Chiara, Tetta Ciro, Bussolati Benedetta, Camussi Giovanni. Microvesicles released from human renal cancer stem cells stimulate angiogenesis and formation of lung premetastatic niche. Cancer research. 2011 Aug;71(15):5346–56. doi: 10.1158/0008-5472.CAN-11-0241. [DOI] [PubMed] [Google Scholar]
- [79].György Bence, Szabó Tamás G, Pásztói Mária, Pál Zsuzsanna, Misják Petra, Aradi Borbála, László Valéria, Pállinger Eva, Pap Erna, Kittel Agnes, Nagy György, Falus András, Buzás Edit I. Membrane vesicles, current state-of-the-art: emerging role of extracellular vesicles. Cellular and molecular life sciences : CMLS. 2011 Aug;68(16):2667–88. doi: 10.1007/s00018-011-0689-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Cocucci Emanuele, Racchetti Gabriella, Meldolesi Jacopo. Shedding microvesicles: artefacts no more. Trends in cell biology. 2009 Feb;19(2):43–51. doi: 10.1016/j.tcb.2008.11.003. [DOI] [PubMed] [Google Scholar]
- [81].Deer Emily L, González-Hernández Jessica, Coursen Jill D, Shea Jill E, Ngatia Josephat, Scaife Courtney L, Firpo Matthew A, Mulvihill Sean J. Phenotype and genotype of pancreatic cancer cell lines. Pancreas. 2010 May;39(4):425–35. doi: 10.1097/MPA.0b013e3181c15963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Baran Jaroslaw, Baj-Krzyworzeka Monika, Weglarczyk Kazimierz, Szatanek Rafal, Zembala Maria, Barbasz Jakub, Czupryna Antoni, Szczepanik Antoni, Zembala Marek. Circulating tumour-derived microvesicles in plasma of gastric cancer patients. Cancer immunology, immunotherapy : CII. 2010 Jun;59(6):841–50. doi: 10.1007/s00262-009-0808-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Wang Wenjie, Li Huiyu, Yan Zhou, Shenghua Jie. Cancer Biomarkers. 5. Vol. 13. IOS Press; 2013. Peripheral blood microvesicles are potential biomarkers for hepatocellular carcinoma; pp. 351–357. Cancer Biomarkers - Volume 13, Number 5 / 2013. [DOI] [PubMed] [Google Scholar]
- [84].Davies PCW, lLineweaver CH. Cancer tumors as Metazoa 1.0: tapping genes of ancient ancestors. Physical biology. 2011 Feb;8(1):015001. doi: 10.1088/1478-3975/8/1/015001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Cairns Rob A, Harris Isaac S, Mak Tak W. Regulation of cancer cell metabolism. Nature reviews. Cancer. 2011 Feb;11(2):85–95. doi: 10.1038/nrc2981. [DOI] [PubMed] [Google Scholar]
- [86].Lu Weiqin, Pelicano Helene, Huang Peng. Cancer metabolism: is glutamine sweeter than glucose? Cancer cell. 2010 Sep;18(3):199–200. doi: 10.1016/j.ccr.2010.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Wilson Kristin F., Erickson Jon W., Antonyak Marc A., Cerione Richard A. Rho GTPases and their roles in cancer metabolism. Trends in Molecular Medicine. 2013;19(2):74–82. doi: 10.1016/j.molmed.2012.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Lukey Michael J, Wilson Kristin F, Cerione Richard A. Therapeutic strategies impacting cancer cell glutamine metabolism. Future medicinal chemistry. 2013 Oct;5(14):1685–700. doi: 10.4155/fmc.13.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Skog Johan, Würdinger Tom, van Rijn Sjoerd, Meijer Dimphna H, Gainche Laura, Sena-Esteves Miguel, Curry William T, Carter Bob S, Krichevsky Anna M, Breakefield Xandra O. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nature cell biology. 2008 Dec;10(12):1470–6. doi: 10.1038/ncb1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Chaffer Christine L, Weinberg Robert A. A perspective on cancer cell metastasis. Science (New York, N.Y.) 2011 Mar;331(6024):1559–64. doi: 10.1126/science.1203543. [DOI] [PubMed] [Google Scholar]