Abstract
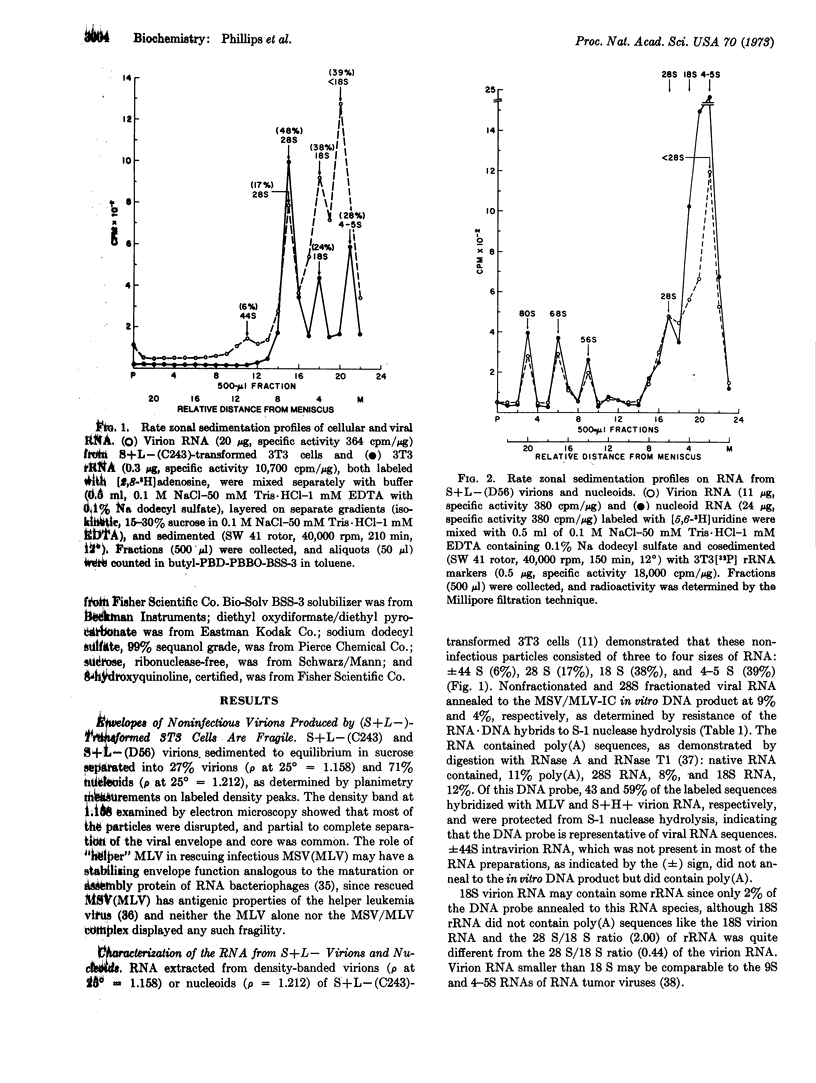
RNA from noninfectious virions produced by two established clonal lines of sarcoma positive-leukemia negative (S+L-)-transformed 3T3 cells has been characterized. RNA from virions or nucleoids of S+L--(C243) cells consisted of three to four sizes: ±44 S (6%), 28 S (17%), 18 S (38%), and <18 S (39%). 28S virion RNA contained some virus-specific information demonstrable by RNA·DNA hybridization with a DNA probe derived from the RNA-directed DNA polymerase product of murine sarcoma-leukemia virus, while parallel studies indicated that 28S ribosomal RNA from ribosomal subunits of transformed and nontransformed 3T3 cells did not contain virus-specific information. In contrast to the S+L-(C243) virions, RNA from virions or nucleoids of S+L-(D56) cells consisted of five sizes: 80 S (6%), 68 S (8%), 56 S (5%), 28 S (28%), and <28 S (53%). Thermal dissociation studies suggested that this S+L- genome is comprised of 28S RNA subunits. From these studies we postulate that the 28S viral RNA is most probably the monomeric genome of S+L- virions.
Keywords: density gradient sedimentation, poly(A), polysomes, rate zonal sedimentation, ribosomes, RNA·DNA hybridization
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ando T. A nuclease specific for heat-denatured DNA in isolated from a product of Aspergillus oryzae. Biochim Biophys Acta. 1966 Jan 18;114(1):158–168. doi: 10.1016/0005-2787(66)90263-2. [DOI] [PubMed] [Google Scholar]
- Bader J. P., Steck T. L. Analysis of the ribonucleic acid of murine leukemia virus. J Virol. 1969 Oct;4(4):454–459. doi: 10.1128/jvi.4.4.454-459.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassin R. H., Phillips L. A., Kramer M. J., Haapala D. K., Peebles P. T., Nomura S., Fischinger P. J. Transformation of mouse 3T3 cells by murine sarcoma virus: release of virus-like particles in the absence of replicating murine leukemia helper virus. Proc Natl Acad Sci U S A. 1971 Jul;68(7):1520–1524. doi: 10.1073/pnas.68.7.1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassin R. H., Tuttle N., Fischinger P. J. Isolation of murine sarcoma virus-transformed mouse cells which are negative for leukemia virus from agar suspension cultures. Int J Cancer. 1970 Jul 15;6(1):95–107. doi: 10.1002/ijc.2910060114. [DOI] [PubMed] [Google Scholar]
- Bassin R. H., Tuttle N., Fischinger P. J. Rapid cell culture assay technic for murine leukaemia viruses. Nature. 1971 Feb 19;229(5286):564–566. doi: 10.1038/229564b0. [DOI] [PubMed] [Google Scholar]
- Biswal N., McCain B., Maccain B., Benyeshh-Melnick M. The DNA of murine sarcoma-leukemia virus. Virology. 1971 Sep;45(3):697–706. doi: 10.1016/0042-6822(71)90183-8. [DOI] [PubMed] [Google Scholar]
- Cheung K. S., Smith R. E., Stone M. P., Joklik W. K. Comparison of immature (rapid harvest) and mature Rous sarcoma virus particles. Virology. 1972 Dec;50(3):851–864. doi: 10.1016/0042-6822(72)90439-4. [DOI] [PubMed] [Google Scholar]
- Coffin J. M., Temin H. M. Hybridization of Rous sarcoma virus deoxyribonucleic acid polymerase product and ribonucleic acids from chicken and rat cells infected with Rous sarcoma virus. J Virol. 1972 May;9(5):766–775. doi: 10.1128/jvi.9.5.766-775.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duesberg P. H. Physical properties of Rous Sarcoma Virus RNA. Proc Natl Acad Sci U S A. 1968 Aug;60(4):1511–1518. doi: 10.1073/pnas.60.4.1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duesberg P. H., Vogt P. K. Differences between the ribonucleic acids of transforming and nontransforming avian tumor viruses. Proc Natl Acad Sci U S A. 1970 Dec;67(4):1673–1680. doi: 10.1073/pnas.67.4.1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erikson R. L. Studies on the RNA from avian myeloblastosis virus. Virology. 1969 Jan;37(1):124–131. doi: 10.1016/0042-6822(69)90313-4. [DOI] [PubMed] [Google Scholar]
- Fischinger P. J., Moore C. O., O'Connor T. E. Isolation and identification of a helper virus found in the Moloney sarcoma-leukemia virus complex. J Natl Cancer Inst. 1969 Apr;42(4):605–622. [PubMed] [Google Scholar]
- Gazdar A. F., Phillips L. A., Sarma P. S., Peebles P. T., Chopra H. C. Presence of sarcoma genome in a "non-infectious" mammalian virus. Nat New Biol. 1971 Nov 17;234(46):69–72. doi: 10.1038/newbio234069a0. [DOI] [PubMed] [Google Scholar]
- Gazdar A. F., Sarma P. S., Bassin R. H. Properties of a murine sarcoma virus isolated from a tumor arising in an NZW-NZB F 1 hybrid mouse. II. Physical and biological characteristics. Int J Cancer. 1972 Jan 15;9(1):234–241. doi: 10.1002/ijc.2910090125. [DOI] [PubMed] [Google Scholar]
- Green M., Cartas M. The genome of RNA tumor viruses contains polyadenylic acid sequences. Proc Natl Acad Sci U S A. 1972 Apr;69(4):791–794. doi: 10.1073/pnas.69.4.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HANAFUSA H., HANAFUSA T., RUBIN H. ANALYSIS OF THE DEFECTIVENESS OF ROUS SARCOMA VIRUS, II. SPECIFICATION OF RSV ANTIGENICITY BY HELPER VIRUS. Proc Natl Acad Sci U S A. 1964 Jan;51:41–48. doi: 10.1073/pnas.51.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haapala D. K., Fischinger P. J. Molecular relatedness of mammalian RNA tumor viruses as determined by DNA hybridization. Science. 1973 Jun 1;180(4089):972–974. doi: 10.1126/science.180.4089.972. [DOI] [PubMed] [Google Scholar]
- Hanafusa H., Baltimore D., Smoler D., Watson K. F., Yaniv A., Spiegelman S. Absence of polymerase protein in virions of alpha-type rous sarcoma virus. Science. 1972 Sep 29;177(4055):1188–1191. doi: 10.1126/science.177.4055.1188. [DOI] [PubMed] [Google Scholar]
- Hartley J. W., Rowe W. P. Production of altered cell foci in tissue culture by defective Moloney sarcoma virus particles. Proc Natl Acad Sci U S A. 1966 Apr;55(4):780–786. doi: 10.1073/pnas.55.4.780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iglewski W. J., Franklin R. M. Denaturation and renaturation of viral ribonucleic acid. I. Annealing R17 ribonucleic acid with denatured replicative form or with denatured replicative intermediate. J Virol. 1967 Aug;1(4):793–803. doi: 10.1128/jvi.1.4.793-803.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarrett O., Pitts J. D., Whalley J. M., Clason A. E., Hay J. Isolation of the nucleic acid of feline leukemia virus. Virology. 1971 Jan;43(1):317–320. doi: 10.1016/0042-6822(71)90252-2. [DOI] [PubMed] [Google Scholar]
- Leong J. A., Garapin A. C., Jackson N., Fanshier L., Levinson W., Bishop J. M. Virus-specific ribonucleic acid in cells producing rous sarcoma virus: detection and characterization. J Virol. 1972 Jun;9(6):891–902. doi: 10.1128/jvi.9.6.891-902.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manly K. F., Smoler D. F., Bromfeld E., Baltimore D. Forms of deoxyribonucleic acid produced by virions of the ribonucleic acid tumor viruses. J Virol. 1971 Jan;7(1):106–111. doi: 10.1128/jvi.7.1.106-111.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen C. R. Tables for estimating sedimentation through linear concentration gradients of sucrose solution. Anal Biochem. 1967 Jul;20(1):114–149. doi: 10.1016/0003-2697(67)90271-0. [DOI] [PubMed] [Google Scholar]
- Obara T., Bolognesi D. P., Bauer H. Ribosomal RNA in avian leukosis virus particles. Int J Cancer. 1971 May 15;7(3):535–546. doi: 10.1002/ijc.2910070320. [DOI] [PubMed] [Google Scholar]
- Peebles P. T., Bassin R. H., Haapala D. K., Phillips L. A., Nomura S., Fischinger P. J. Rescue of murine sarcoma virus from a sarcoma-positive leukemia-negative cell line: requirement for replicating leukemia virus. J Virol. 1971 Nov;8(5):690–694. doi: 10.1128/jvi.8.5.690-694.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peebles P. T., Haapala D. K., Gazdar A. F. Deficiency of viral ribonucleic acid-dependent deoxyribonucleic acid polymerase in noninfectious virus-like particles released from murine sarcoma virus-transformed hamster cells. J Virol. 1972 Mar;9(3):488–493. doi: 10.1128/jvi.9.3.488-493.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips L. A., Franklin R. M. The in vivo distribution of bacterial polysomes, ribosomes, and ribosomal subunits. Cold Spring Harb Symp Quant Biol. 1969;34:243–253. doi: 10.1101/sqb.1969.034.01.030. [DOI] [PubMed] [Google Scholar]
- Phillips L. A., Hotham-Iglewski B., Franklin R. M. Polyribosomes of Escherichia coli. II. Experiments to determine the in vivo distribution of polysomes, ribosomes and ribosomal subunits. J Mol Biol. 1969 Oct 14;45(1):23–38. doi: 10.1016/0022-2836(69)90207-1. [DOI] [PubMed] [Google Scholar]
- Reitz M., Gillespie D., Saxinger W. C., Robert M., Gallo R. C. Poly (rA) tracts of tumor virus 70S RNA are not transcribed in endogenous or reconstituted reactions of viral reverse transcriptase. Biochem Biophys Res Commun. 1972 Dec 4;49(5):1216–1224. doi: 10.1016/0006-291x(72)90598-0. [DOI] [PubMed] [Google Scholar]
- Roberts G. C., Dennis E. A., Meadows D. H., Cohen J. S., Jardetzky O. The mechanism of action of ribonuclease. Proc Natl Acad Sci U S A. 1969 Apr;62(4):1151–1158. doi: 10.1073/pnas.62.4.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson W. S., Robinson H. L. DNA polymerase in defective Rous sarcoma virus. Virology. 1971 May;44(2):457–462. doi: 10.1016/0042-6822(71)90278-9. [DOI] [PubMed] [Google Scholar]
- Sarma P. S., Log T., Huebner R. J. Trans-species rescue of defective genomes of murine sarcoma virus from hamster tumor cells with helper feline leukemia virus. Proc Natl Acad Sci U S A. 1970 Jan;65(1):81–87. doi: 10.1073/pnas.65.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scales B. A new scintillator for liquid scintillation counting. Int J Appl Radiat Isot. 1967 Jan;18(1):1–6. doi: 10.1016/0020-708x(67)90165-2. [DOI] [PubMed] [Google Scholar]
- Scheele C. M., Hanafusa H. Electrophoretic analysis of the RNA of avian tumor viruses. Virology. 1972 Dec;50(3):753–764. doi: 10.1016/0042-6822(72)90429-1. [DOI] [PubMed] [Google Scholar]
- Scherrer K., Marcaud L., Zajdela F., Breckenridge B., Gros F. Etude des RNA nucléaires et cytoplasmiques à marquage rapide dans les cellules érythropoiétiques aviaires différenciées. Bull Soc Chim Biol (Paris) 1966;48(10):1037–1075. [PubMed] [Google Scholar]
- Spiegelman S., Pace N. R., Mills D. R., Levisohn R., Eikhom T. S., Taylor M. M., Peterson R. L., Bishop D. H. The mechanism of RNA replication. Cold Spring Harb Symp Quant Biol. 1968;33:101–124. doi: 10.1101/sqb.1968.033.01.015. [DOI] [PubMed] [Google Scholar]
- Steitz J. A. Identification of the A protein as a structural component of bacteriophage R17. J Mol Biol. 1968 May 14;33(3):923–936. doi: 10.1016/0022-2836(68)90328-8. [DOI] [PubMed] [Google Scholar]
- Stephenson M. L., Wirthlin L. S., Scott J. F., Zamecnik P. C. The 3'-terminal nucleosides of the high molecular weight RNA of avian myeloblastosis virus. Proc Natl Acad Sci U S A. 1972 May;69(5):1176–1180. doi: 10.1073/pnas.69.5.1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temin H. M., Mizutani S. RNA-dependent DNA polymerase in virions of Rous sarcoma virus. Nature. 1970 Jun 27;226(5252):1211–1213. doi: 10.1038/2261211a0. [DOI] [PubMed] [Google Scholar]
- VINOGRAD J., HEARST J. E. Equilibrium sedimentation of macromolecules and viruses in a density gradient. Fortschr Chem Org Naturst. 1962;20:373–422. [PubMed] [Google Scholar]
- WARNER J. R., KNOPF P. M., RICH A. A multiple ribosomal structure in protein synthesis. Proc Natl Acad Sci U S A. 1963 Jan 15;49:122–129. doi: 10.1073/pnas.49.1.122. [DOI] [PMC free article] [PubMed] [Google Scholar]



