Abstract
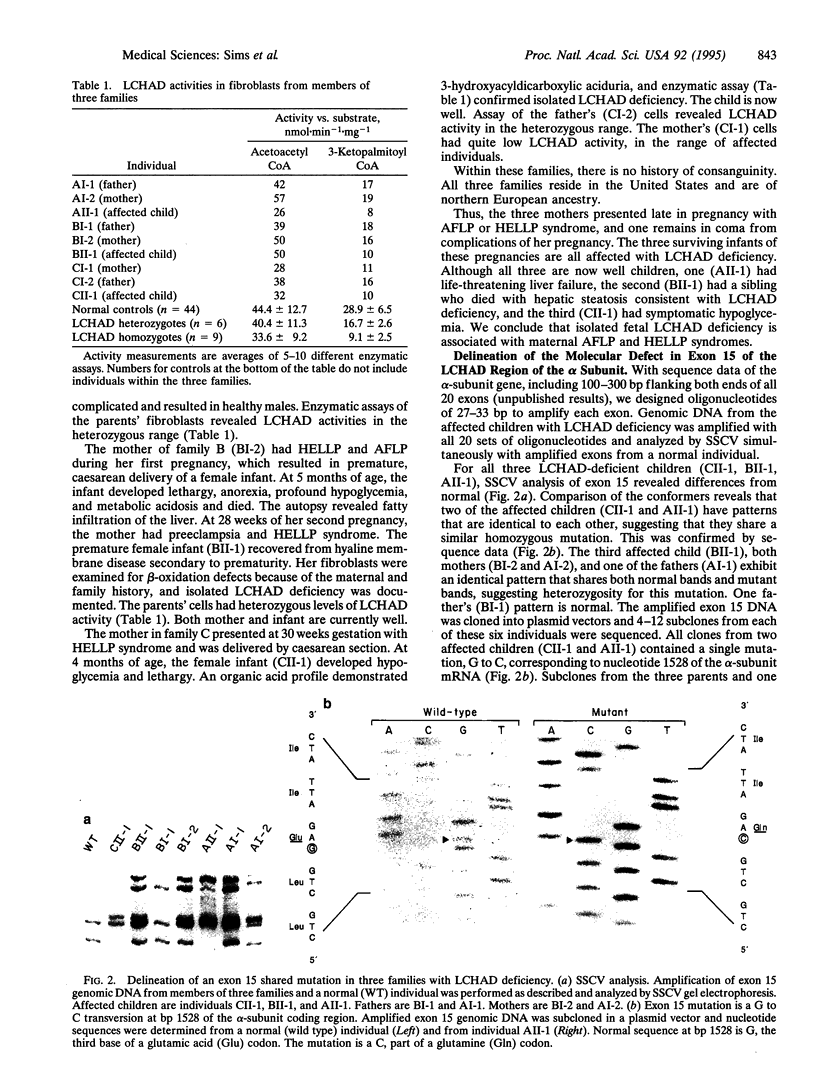
Mitochondrial long chain fatty acid beta-oxidation provides the major source of energy in the heart. Deficiencies of human beta-oxidation enzymes produce sudden, unexplained death in childhood, acute hepatic encephalopathy, skeletal myopathy, or cardiomyopathy. Long chain 3-hydroxyacyl-CoA dehydrogenase [LCHAD; long-chain-(S)-3-hydroxyacyl-CoA:NAD+ oxidoreductase, EC 1.1.1.211] catalyzes the third step in beta-oxidation, and this activity is present on the C-terminal portion of the alpha subunit of mitochondrial trifunctional protein. We used single-stranded conformation variance analysis of the exons of the human LCHAD (alpha subunit) gene to determine the molecular basis of LCHAD deficiency in three families with children presenting with sudden unexplained death or hypoglycemia and abnormal liver enzymes (Reye-like syndrome). In all families, the mothers had acute fatty liver and associated sever complications during pregnancies with the affected infants. The analysis in two affected children revealed a G to C mutation at position 1528 (G1528C) of the alpha subunit of the trifunctional protein on both alleles. This is in the LCHAD domain and substitutes glutamine for glutamic acid at position 474 of mature alpha subunit. The third child had this G1528C mutation on one allele and a different mutation (C1132T) creating a premature termination codon (residue 342) on the second allele. Our results demonstrate that mutations in the LCHAD domain of the trifunctional protein alpha subunit in affected offspring are associated with maternal acute fatty liver of pregnancy. This is the initial delineation of the molecular basis of isolated LCHAD deficiency.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Billadello J. J., Kelly D. P., Roman D. G., Strauss A. W. The complete nucleotide sequence of canine brain B creatine kinase mRNA: homology in the coding and 3' noncoding regions among species. Biochem Biophys Res Commun. 1986 Jul 16;138(1):392–398. doi: 10.1016/0006-291x(86)90294-9. [DOI] [PubMed] [Google Scholar]
- Birktoft J. J., Holden H. M., Hamlin R., Xuong N. H., Banaszak L. J. Structure of L-3-hydroxyacyl-coenzyme A dehydrogenase: preliminary chain tracing at 2.8-A resolution. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8262–8266. doi: 10.1073/pnas.84.23.8262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitar K. G., Perez-Aranda A., Bradshaw R. A. Amino acid sequence of L-3-hydroxyacyl CoA dehydrogenase from pig heart muscle. FEBS Lett. 1980 Jul 28;116(2):196–198. doi: 10.1016/0014-5793(80)80642-9. [DOI] [PubMed] [Google Scholar]
- Gottlieb E. The 3' untranslated region of localized maternal messages contains a conserved motif involved in mRNA localization. Proc Natl Acad Sci U S A. 1992 Aug 1;89(15):7164–7168. doi: 10.1073/pnas.89.15.7164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hale D. E., Bennett M. J. Fatty acid oxidation disorders: a new class of metabolic diseases. J Pediatr. 1992 Jul;121(1):1–11. doi: 10.1016/s0022-3476(05)82532-6. [DOI] [PubMed] [Google Scholar]
- Hill K. E., Lloyd R. S., Burk R. F. Conserved nucleotide sequences in the open reading frame and 3' untranslated region of selenoprotein P mRNA. Proc Natl Acad Sci U S A. 1993 Jan 15;90(2):537–541. doi: 10.1073/pnas.90.2.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson S., Kler R. S., Bartlett K., Briggs H., Bindoff L. A., Pourfarzam M., Gardner-Medwin D., Turnbull D. M. Combined enzyme defect of mitochondrial fatty acid oxidation. J Clin Invest. 1992 Oct;90(4):1219–1225. doi: 10.1172/JCI115983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamijo T., Aoyama T., Komiyama A., Hashimoto T. Structural analysis of cDNAs for subunits of human mitochondrial fatty acid beta-oxidation trifunctional protein. Biochem Biophys Res Commun. 1994 Mar 15;199(2):818–825. doi: 10.1006/bbrc.1994.1302. [DOI] [PubMed] [Google Scholar]
- Kamijo T., Aoyama T., Miyazaki J., Hashimoto T. Molecular cloning of the cDNAs for the subunits of rat mitochondrial fatty acid beta-oxidation multienzyme complex. Structural and functional relationships to other mitochondrial and peroxisomal beta-oxidation enzymes. J Biol Chem. 1993 Dec 15;268(35):26452–26460. [PubMed] [Google Scholar]
- Kamijo T., Wanders R. J., Saudubray J. M., Aoyama T., Komiyama A., Hashimoto T. Mitochondrial trifunctional protein deficiency. Catalytic heterogeneity of the mutant enzyme in two patients. J Clin Invest. 1994 Apr;93(4):1740–1747. doi: 10.1172/JCI117158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly D. P., Strauss A. W. Inherited cardiomyopathies. N Engl J Med. 1994 Mar 31;330(13):913–919. doi: 10.1056/NEJM199403313301308. [DOI] [PubMed] [Google Scholar]
- Matsubara Y., Indo Y., Naito E., Ozasa H., Glassberg R., Vockley J., Ikeda Y., Kraus J., Tanaka K. Molecular cloning and nucleotide sequence of cDNAs encoding the precursors of rat long chain acyl-coenzyme A, short chain acyl-coenzyme A, and isovaleryl-coenzyme A dehydrogenases. Sequence homology of four enzymes of the acyl-CoA dehydrogenase family. J Biol Chem. 1989 Sep 25;264(27):16321–16331. [PubMed] [Google Scholar]
- Orita M., Iwahana H., Kanazawa H., Hayashi K., Sekiya T. Detection of polymorphisms of human DNA by gel electrophoresis as single-strand conformation polymorphisms. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2766–2770. doi: 10.1073/pnas.86.8.2766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne R. M., Haas R. C., Strauss A. W. Structural characterization and tissue-specific expression of the mRNAs encoding isoenzymes from two rat mitochondrial creatine kinase genes. Biochim Biophys Acta. 1991 Jul 23;1089(3):352–361. doi: 10.1016/0167-4781(91)90176-m. [DOI] [PubMed] [Google Scholar]
- Sternberg N. Bacteriophage P1 cloning system for the isolation, amplification, and recovery of DNA fragments as large as 100 kilobase pairs. Proc Natl Acad Sci U S A. 1990 Jan;87(1):103–107. doi: 10.1073/pnas.87.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treem W. R., Rinaldo P., Hale D. E., Stanley C. A., Millington D. S., Hyams J. S., Jackson S., Turnbull D. M. Acute fatty liver of pregnancy and long-chain 3-hydroxyacyl-coenzyme A dehydrogenase deficiency. Hepatology. 1994 Feb;19(2):339–345. [PubMed] [Google Scholar]
- Treem W. R., Witzleben C. A., Piccoli D. A., Stanley C. A., Hale D. E., Coates P. M., Watkins J. B. Medium-chain and long-chain acyl CoA dehydrogenase deficiency: clinical, pathologic and ultrastructural differentiation from Reye's syndrome. Hepatology. 1986 Nov-Dec;6(6):1270–1278. doi: 10.1002/hep.1840060608. [DOI] [PubMed] [Google Scholar]
- Uchida Y., Izai K., Orii T., Hashimoto T. Novel fatty acid beta-oxidation enzymes in rat liver mitochondria. II. Purification and properties of enoyl-coenzyme A (CoA) hydratase/3-hydroxyacyl-CoA dehydrogenase/3-ketoacyl-CoA thiolase trifunctional protein. J Biol Chem. 1992 Jan 15;267(2):1034–1041. [PubMed] [Google Scholar]
- Venizelos N., Ijlst L., Wanders R. J., Hagenfeldt L. beta-Oxidation enzymes in fibroblasts from patients with 3-hydroxydicarboxylic aciduria. Pediatr Res. 1994 Jul;36(1 Pt 1):111–114. doi: 10.1203/00006450-199407001-00020. [DOI] [PubMed] [Google Scholar]
- Wilcken B., Leung K. C., Hammond J., Kamath R., Leonard J. V. Pregnancy and fetal long-chain 3-hydroxyacyl coenzyme A dehydrogenase deficiency. Lancet. 1993 Feb 13;341(8842):407–408. doi: 10.1016/0140-6736(93)92993-4. [DOI] [PubMed] [Google Scholar]
- Yaffe D., Nudel U., Mayer Y., Neuman S. Highly conserved sequences in the 3' untranslated region of mRNAs coding for homologous proteins in distantly related species. Nucleic Acids Res. 1985 May 24;13(10):3723–3737. doi: 10.1093/nar/13.10.3723. [DOI] [PMC free article] [PubMed] [Google Scholar]