Abstract
Hepatic capsular retraction is an imaging feature that deserves the attention of the radiologist. Hepatic capsular retraction is associated with a number of hepatic lesions, benign or malignant, treated or untreated. The purpose of this pictorial review is to discuss the most common benign and malignant hepatic lesions associated with this feature with an emphasis on magnetic resonance imaging (MRI).
Keywords: Capsular retraction, hepatic tumors, fibrous stroma, magnetic resonance imaging (MRI)
Introduction
Hepatic capsular retraction is defined as a focal irregularity, flattening, or concavity of the normally convex border of liver capsule, facing a circumscribed lesion or not. Prior to any treatment, this feature is a rare finding on cross-sectional imaging and its prevalence is approximately 2% (1,2). Initial reports have regarded hepatic capsular retraction as a specific sign of malignancy with a positive and negative predictive value of 100% and 21.8%, respectively (2). Actually, this sign can be seen in malignant or benign, treated or non-treated, hepatic and biliary tumors (either primary or secondary). Non-tumoral etiologies have also been described to be associated with capsular retraction (3). In tumors, the mechanism of this retraction is explained mostly by three reasons: sub-capsular location of the lesion, fibrous stroma of the underlying lesion (which is observed in 70% of malignant lesions prior any treatment (4)), and necrosis and desmoplastic reaction induced by the treatment. With the development of imaging techniques for the liver, this imaging feature has gained more attention from radiologists, hepatologists, and oncologists, and can be used, in association with clinical context, biology, and other imaging features of the lesion, to give clues for specific tumor characterization. From a technical point of view, the most efficient sequence for the detection of hepatic capsular retraction in magnetic resonance imaging (MRI) is a T2-weighted (T2W) spin-echo sequence preferably without fat saturation to benefit a better contrast between intra-abdominal fat and hepatic parenchyma. T1-weighted (T1W) sequences before and after gadolinium injection play a key role in the etiological diagnosis of the capsular retraction and particularly for the characterization of tumoral lesions.
Throughout this pictorial review, our goal is to fulfill four learning objectives:
to differentiate true hepatic capsular retraction from pseudocapsular retraction;
to describe the benign and malignant etiologies associated with hepatic capsular retraction;
to understand the diagnostic value of this sign in patients who did not receive any treatment;
to understand the main mechanisms of capsular retraction such as fibrous component, sub-capsular location of lesions and the role of treatment in malignant liver tumors.
Variants and pitfalls
It is important to distinguish true hepatic capsular retraction from other contour abnormalities. Pseudo-retraction of the liver capsule may be caused by accessory hepatic fissures and invagination of the liver by diaphragm or ribs (Fig. 1). Accessory fissures are incomplete fissures formed by indentations of the diaphragm. They are most common in the right liver dome, near the diaphragm, and usually cause shallow indentation. Their frequency increases with age. Normal liver parenchyma between two exophytic masses and perihepatic disease such as peritoneal carcinomatosis (Fig. 2) and pseudomyxoma peritonei can also mimic capsular retraction. Pericapsular implants are commonly bi-convex while pseudomyxoma is responsible for scalloping of the liver parenchyma.
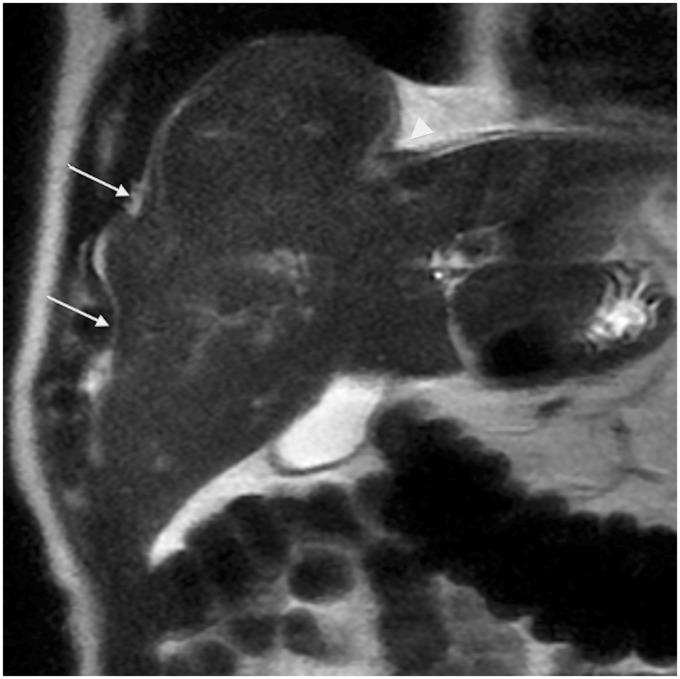
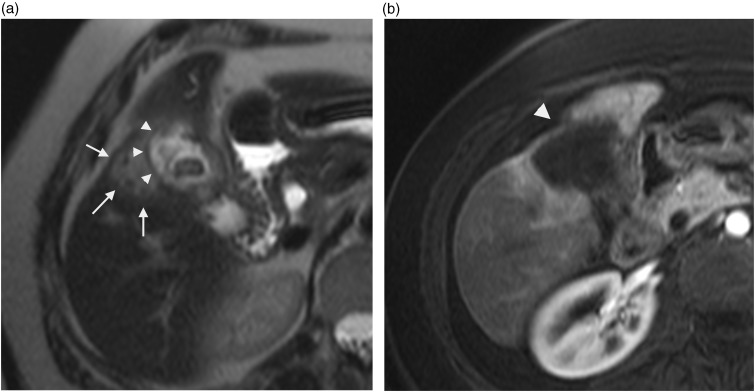
Fig. 1.
A 75-year-old man with pseudo liver capsular retraction. Coronal T2W TSE not fat-suppressed MRI in this patient followed for colonic adenocarcinoma shows pseudo retraction adjacent to ribs (arrow) and diaphragm (arrowhead).
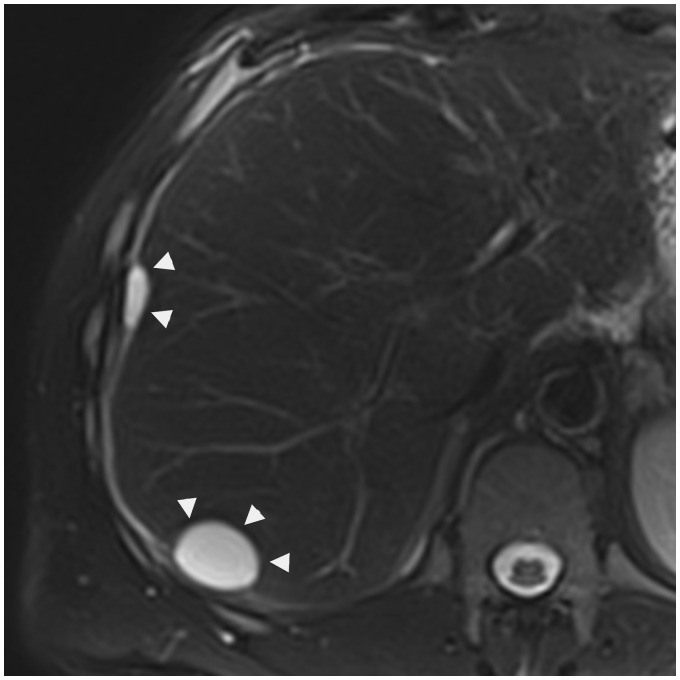
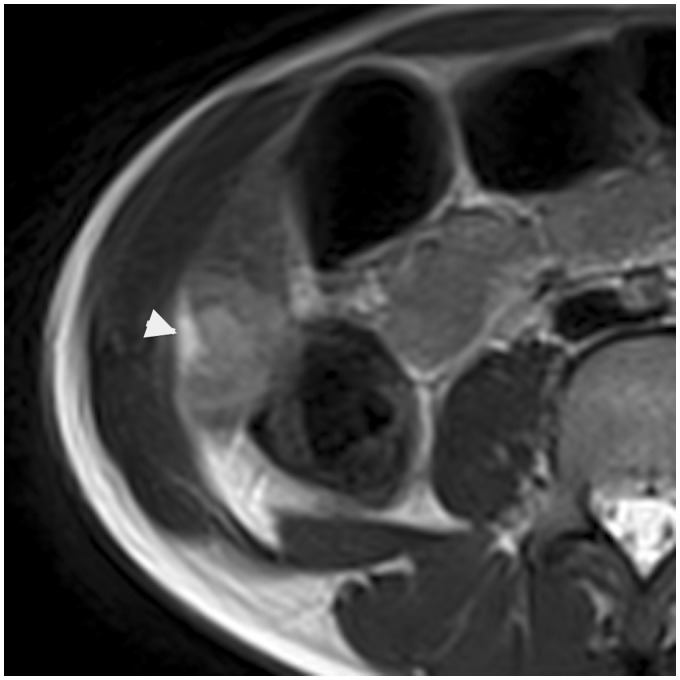
Fig. 2.
A 49-year-old man presenting a pancreatic neoplasia with peritoneal carcinomatosis. Axial T2W TSE fat-suppressed MRI shows two hepatic pericapsular implants of peritoneal carcinomatosis (arrowheads), biconvex, in high signal iontensity.
Benign primary liver tumors
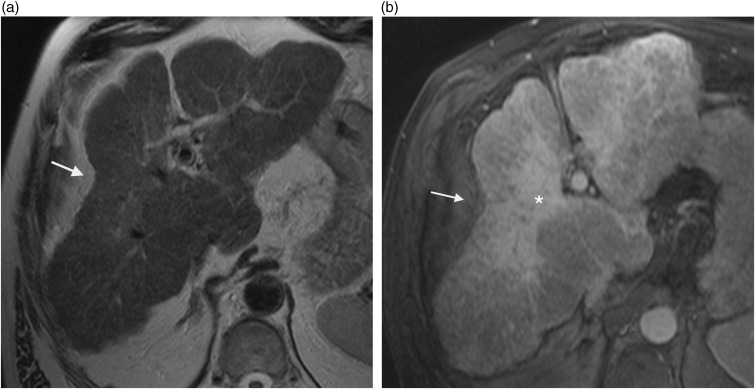
Hepatic hemangioma represents the most common hepatic benign lesion. Most hemangiomas have a typical appearance on cross-sectional imaging. On computed tomography (CT), they usually are well-defined, hypo-attenuated lesion on unenhanced CT scans and show peripheral, progressive, and nodular centripetal enhancement after contrast medium administration. The key findings on MR are a similar lesion enhancement and strong signal intensity on T2W images. Typically, capsular retraction is not seen in hepatic hemangiomas. However, case reports have described such association in three circumstances: (i) hemangiomas that undergo central thrombosis and fibrous replacement of large vascular channels also called sclerosed, thrombosed, or hyalinized hemangiomas (5,6); (ii) giant hemangiomas; and (iii) hemangiomas occurring in cirrhotic patients (4–7). It seems that internal changes such as major fibrous transformation or presence of chronic liver disease which may induce progressive reduction in size of hemangioma could favor capsular retraction (Fig. 3). Hepatic giant hemangiomas are also associated with capsular retraction (8).
Fig. 3.
A 59-year-old man with sclerosed hemangioma developed on alcoholic cirrhosis. T2W TSE not fat-supressed MRI shows hyperintense wedge-shaped lesion (arrow) with underlying capsular retraction.
To the best of our knowledge, hepatic hemangioma is the only benign tumor described with capsular retraction in the literature.
Malignant primary liver tumors
Capsular hepatic retraction is observed in 20% of cholangiocarcinoma in the literature (9,10). This malignant tumor is the second most common primary liver cancer. It may develop either in the extrahepatic or intrahepatic bile ducts and can be classified into three types according to tumor location: common bile duct, hilar, and peripheral. Moreover, intrahepatic cholangiocarcinoma can be further divided into mass-forming, periductal infiltrating, and intraductal types, the mass-forming type being the most frequent by far. The common risk factor for this tumor is chronic biliary inflammation although most cholangiocarcinomas develop in normal livers. Cross-sectional imaging is often suggestive showing a large, non-encapsulated mass, with irregular contours and satellite nodules (Fig. 4), which presents on MRI variable signal intensity on T2W sequence, low signal intensity on T1W sequence, and gradual centripetal enhancement after injection of gadolinium. Central delayed enhancement of the fibrous stroma can be more evident on MRI. Biliary dilatation and lobar atrophy are more often found in hilar cholangiocarcinomas than peripheral ones (9,11,12). Capsular hepatic retraction is thought to be the result of prominent tumoral fibrous stroma whereas segmental hepatic parenchymal atrophy is due to both chronic bile duct obstruction and portal invasion (9).
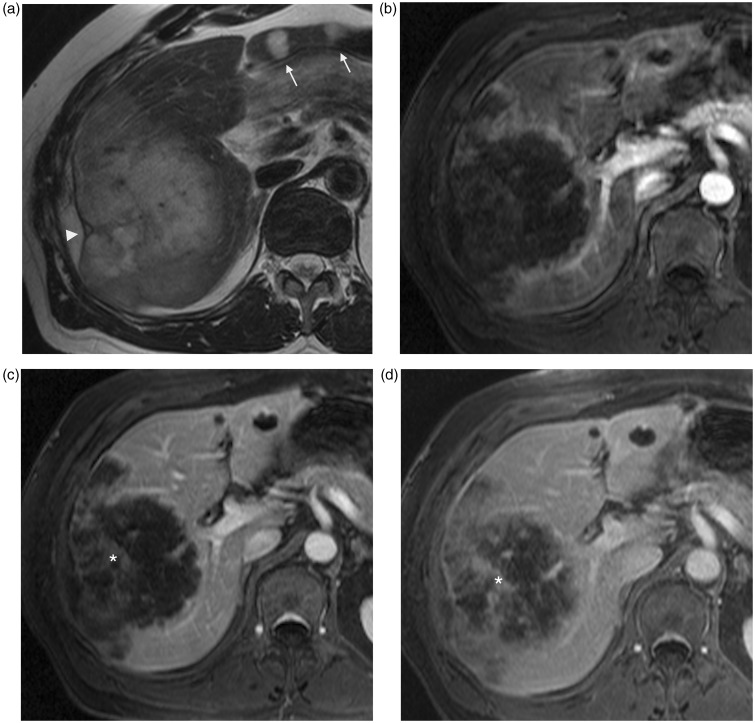
Fig. 4.
A 76-year-old man with intrahepatic cholangiocarcinoma. Axial T2W TSE not fat-supressed MRI (a) shows high intensity mass of right hepatic lobe with ill-defined margins, capsular retraction, and smaller diffuse nodules (arrows). On contrast-enhanced T1W GE MRI, the principal lesion shows peripheral enhancement on arterial phase (b, arrowheads), with progressive centripetal enhancement (*) on portal (c) and late (d) phases.
Hepatic capsular retraction may also be seen in gallbladder carcinoma invading liver parenchyma (Fig. 5).
Fig. 5.
A 58-year-old man with gallbladder adenocarcinoma. Axial T2W TSE not fat-supressed MRI (a) shows high intensity infiltrative mass (arrows) with poor-defined interface with gallbladder (arrowheads). Mild progressive peripheral centripetal enhancement was noted on arterial (b), portal and late contrast-enhanced T1W GE images with adjacent hepatic capsular retraction (arrowhead).
Capsular retraction is rare and unusual in classical hepatocellular carcinoma (HCC), probably due to the lack of stromal fibrous component. Consequently, some authors have used this sign to exclude the diagnosis of HCC (13,14). However two types of HCC may occasionally have capsular retraction due to their stromal fibrous component: fibrolamellar HCC and hepato-cholangiocarcinoma.
Fibrolamellar HCC is characteristically a large hepatic mass that appears in adolescent or young adult patients, with no underlying liver disease or risk factors for HCC, and with serum hepatocellular tumor markers that are typically normal. On imaging, this tumor appears as a large lobulated heterogeneously arterial-enhancing mass with often a central fibrous scar, in a normal underlying liver. Capsular retraction was seen in less than 10% of the Armed Forces Institute of Pathology cases (15). Besides, hepatic capsular retraction, tumor heterogeneity, central calcification seen in most cases, and enlarged lymphadenopathy are striking findings for establishing the diagnosis of fibrolamellar HCC and differentiating this tumor from focal nodular hyperplasia (FNH) (15).
Combined hepatocellular-cholangiocarcinoma constitutes 3–5% of primary liver cancer and is classified into three types: two separate masses of HCC and cholangiocarcinoma in the same liver, contiguous but independent masses of both, and a mass of mixed components of both. Theoretically, the latter shows features of HCC and cholangiocarcinoma on imaging are as demonstrating hypervascularity on arterial phase and wash-out on portal venous phases, while others having persistent enhancement on delayed phase. However, the cholangiocarcinoma portion is often predominant, explaining that capsular retraction has been reported in 27% of cases (16).
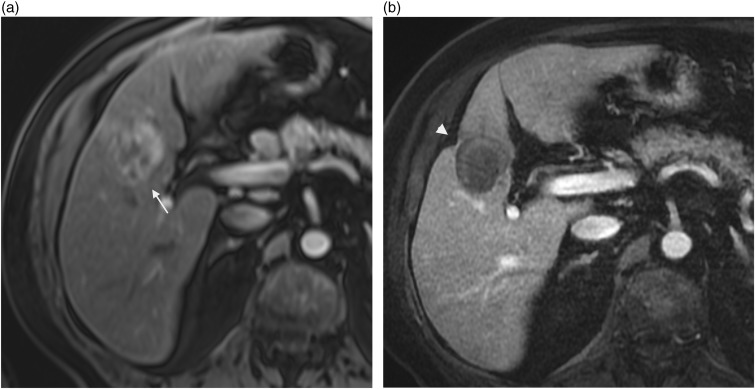
Hepatic epithelioid hemangioendothelioma is a rare primary hepatic tumor of vascular origin, with intermediate malignant potential. Most of the cases have been reported in young adults with a female predominance. It may be found incidentally or presenting with non-specific symptoms but typical CT and MR features can facilitate an accurate diagnosis. Two different patterns have been described (17). The early stage is characterized by a nodular type with multiple peripheral lesions showing target pattern. On contrast-enhanced CT scan the lesions are characterized by a hypo-attenuating central zone corresponding to a sclerotic area, a hypervascular peripheral enhancement corresponding to a hypercellular zone, and a hypo-attenuating rim corresponding to edematous connective tissue. On MRI the nodules appear hypointense on T1W images with a dynamic enhancement pattern similar to that seen on CT images. There may be heterogeneous signal intensity on T2W images, with a more pronounced hyperintensity of the sclerotic zone. In the advanced phase or diffuse type, the lesions coalesce and form extensive mass that infiltrates the surrounding liver parenchyma and the hepatic vessels. High fibrous myxoid stroma and subcapsular location explain the classical association with capsular retraction (Fig. 6) seen in about 10–25% of the lesions (17) but in a great majority of the patients (69%) according to Miller et al. (18). Capsular retraction is one of the key findings. It has been reported in the range of 25–69% (17,18). A stronger association is seen in advanced hepatic epithelioid hemangioendotheliomas.
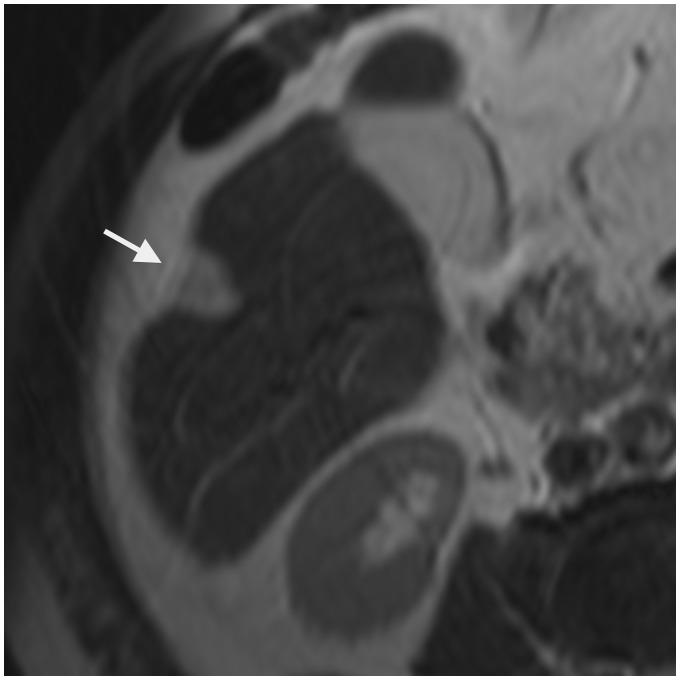
Fig. 6.
An 11-year-old boy presenting with epithelioid hemangioendothelioma of the liver. On T2W TSE not fat-supressed image, capsular retraction is depicted next to the subcapsular hyperintense lesion in the segment 6 (arrowhead).
Hepatic metastases
A small percentage of untreated liver metastases are associated with capsular retraction. The primary tumors are generally fibrous or associated with a desmoplastic reaction and frequently correspond to carcinoid tumors, colon, pancreatic, and gallbladder carcinoma (1,2,4,19). Metastases can have several imaging aspects and should be suspected in the proper context when associated to capsular retraction, particularly in case of multiple lesions. Lesion behavior on arterial phase CT or MR can be variable but most lesions show delayed uptake of the contrast agent which is related to fibrous stroma.
Very occasionally, untreated liver metastases originating from breast cancer may mimic cirrhosis with lobulated contours and morphologic changes of the liver. This pattern has been called “hepar lobatum”.
Treated hepatic tumors
A part of hepatic capsular retraction is usually observed during or after non-surgical treatment of hepatic tumor, especially with metastases (4). Capsular retraction is mostly seen in patients with liver lesion receiving chemotherapy (Fig. 7), radiation therapy, transcatheter chemoembolization, and radiofrequency (RF) ablation (Figs. 8 and 9). In this particular context, capsular retraction is due to post-treatment changes including necrosis and fibrosis of the lesions, with surrounding parenchymal atrophy (1,2,19). A pattern of pseudo-cirrhosis with capsular retraction (Fig. 10) has also been described with treated breast cancer liver metastasis due to the hepatotoxic effects of chemotherapy and/or tumoral hepatic infiltration (20,21). Appearance of capsular retraction in treated hepatic tumor is often associated with a decrease in size of the lesion and major histological response.
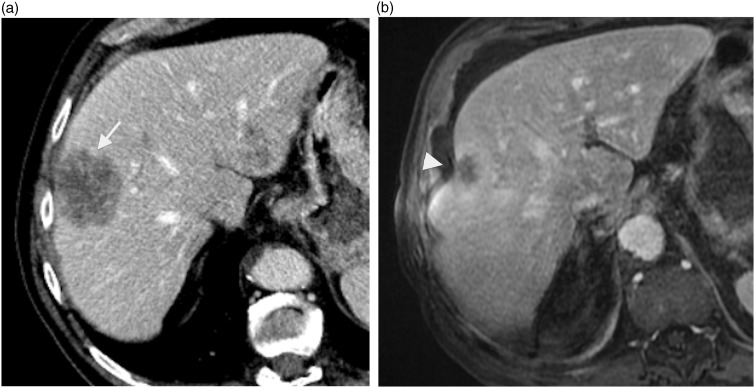
Fig. 7.
A 78-year-old-man with rectum adenocarcinoma metastatic to liver and lungs. Contrast-enhanced CT image (a) shows subcapsular metastatic mass of segment VIII (arrow) prior to treatment. Contrast-enhanced T1W GE image (b) shows shrinking of this mass with underlying capsular retraction (arrowhead) after systemic chemotherapy.
Fig. 8.
A 76-year-old-man with hepatocellular carcinoma on cirrhotic liver. (a) axial contrast-enhanced T1W GE image showing lesion with marked enhancement (arrow) prior to any treatment. (b) same lesion after RF ablation showing no enhancement with adjacent capsular retraction (arrowhead).
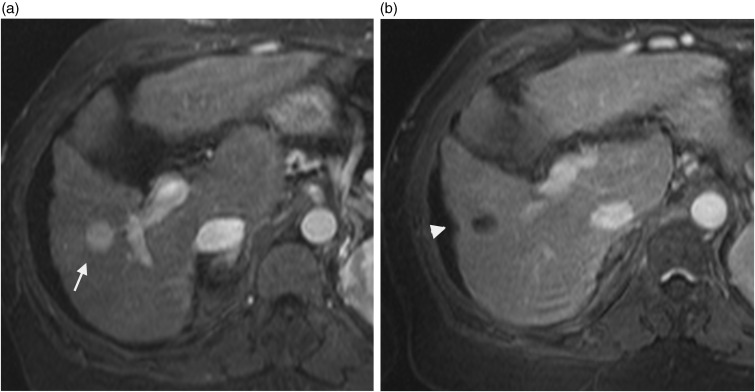
Fig. 9.
A 78-year-old-man with hepatocellular carcinoma. Contrast-enhanced T1W GE (a) image showing an enhanced nodule at the arterial phase in segment 4 prior to treatment (arrow). This lesion was treated with transcatheter chemoembolization and RF ablation; contrast-enhanced T1W GE image after treatment (b) showing associated capsular retraction (arrowhead) and no abnormal enhancement.
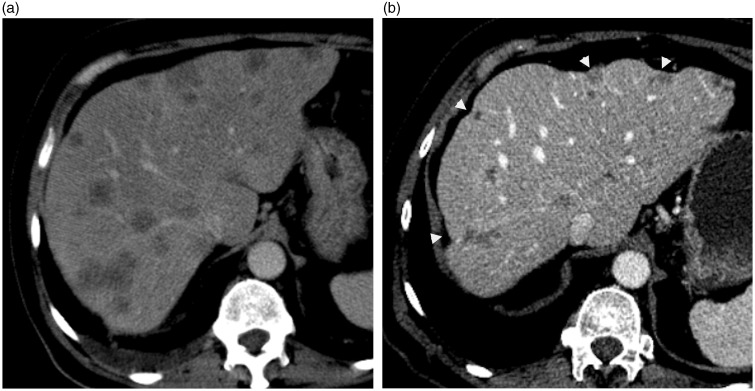
Fig. 10.
A 65-year-old man with metastatic colon carcinoma. (a) axial-enhanced CT scan during the portal phase demonstrates multiple hypoattenuating hepatic metastases before chemotherapy. (b) axial-enhanced CT scan of same patient few months after chemotherapy shows pseudocirrhosis with a markedly deformed liver and capsular retraction (arrowheads).
Non-tumoral etiologies
Capsular retraction is commonly encountered in patients with cirrhosis and frequently corresponds to focal confluent hepatic fibrosis (Fig. 11). This is a mass-like atrophic region described in 14% of patients with alcoholic or viral cirrhosis (22,23). It typically affects the anterior portion of the right lobe and the segment 4. Lesions are frequently wedge-shaped and radiated from the porta hepatis, more rarely band-like. Associated volume loss is seen as retraction of the overlying hepatic capsule or total shrinkage of the area of involvement in 88% of cases (22,23). Retraction index can increase over time (24). Focal confluent fibrosis is usually hypo-attenuating on unenhanced and arterial phase CT images, iso-attenuating on portal venous phase images and enhance on delayed phase. On MRI, areas of fibrosis show the same enhancement pattern with hyperintensity on T2W images. Rarely, early arterial phase enhancement may occur corresponding to hyperarterialized confluent fibrosis indicating immature fibrosis simulating an infiltrative tumor process. Recognizing this particular pattern is important to avoid misdiagnosis, especially HCC. Unlike intrahepatic cholangiocarcinoma, no segmental intrahepatic biliary duct dilatation is associated. Trapped vessels can be found in 15% of cases (24).
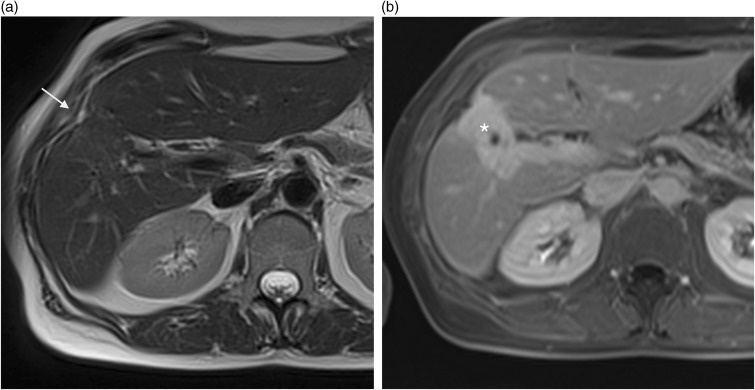
Fig. 11.
A 64-year-old man with cirrhosis and confluent hepatic fibrosis. On axial T2W TSE not fat-supressed (a) and contrast-enhanced T1W GE in the delayed phase (b) MRI images show a wedge-shaped peripheral area involving right anterior segments with marked hepatic capsular retraction (arrow). Hyperintensity of the lesion (*) on delayed contrast-enhanced image is due to retention of contrast within the fibrous stroma.
Portal branch occlusion and segmental cholestasis, by different mechanisms, are two others etiologies of focal parenchyma fibrosis and atrophy with liver capsular retraction associated.
Post-traumatic subcapsular hepatic lesions or pseudolesions can also be associated to overlying capsular retraction (3), due to scarring and fibrosis which is part of the healing process of hepatic injuries (Fig. 12). Iatrogenic injuries to hepatic capsule with capsular retraction association can be seen after liver biopsy and percutaneous biliary or hepatic abscess drainage (3). A previous history of abdominal trauma or hepatic procedure is the key to diagnosis. The capsular retraction is usually of small size, peripheral, and located next to the area of the liver injury.
Fig. 12.
A 48-year-old woman with non-specific hepatic lesion and history of abdominal trauma. (a) axial T2W TSE not fat-supressed MRI showing capsular retraction (arrow) with no evidence of underlying lesion. Axial T1W GE on portal enhancement phase (b) MRI showing hypointense wedge-shaped lesion that strongly enhances on late phases (*) which correlate with a fibrous scar.
Hepatic abscess, if it is near to the capsule, can cause by regressing a small capsular retraction, always in relation to scarring and fibrosis of the hepatic parenchyma, even in the absence of percutaneous drainage.
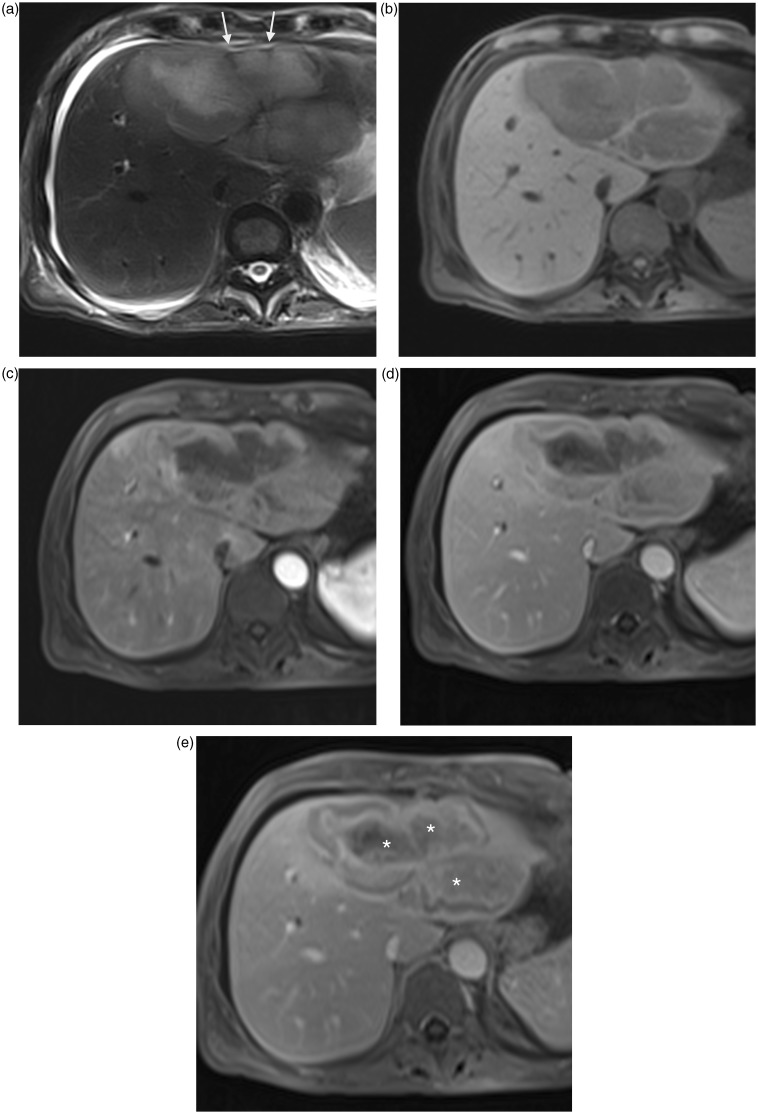
Hepatic inflammatory pseudotumors (IPT) of liver (Fig. 13) have also been more recently described as a rare cause of hepatic capsular retraction on a case report (25). They are rare lesions whose etiology and pathogenesis remain unknown (infectious agents, genetic abnormalities, autoimmune disease) (26). Treatment is based on antibiotic and/or corticosteroid therapy in most cases, the decrease or even resolution of IPT (27). The radiological diagnosis of these benign lesions is often difficult and they can mimic hepatic malignant tumors particularly because of their very variable presentation. Some authors distinguish two types of IPT (28), based on anatomo-pathological and radiological analysis:
active – with a dense fibroblastic IPT which is large (average 5 cm), vascularized, and heterogeneous at imaging associated to inflammatory syndrome;
non-active type – with a necrotic lesion which is smaller, hypovascularized, homogeneous, and without inflammatory syndrome.
Fig. 13.
A 64-year-old man with inflammatory pseudotumor of liver. Large liver lesion in segments II and IV with capsular retraction (arrows) in high signal intensity on T2W TSE fat-suppressed image (a), low signal intensity on T1W GE image (b). Contrast-enhanced T1W GE images during arterial (c), portal (d), and delayed (e) phases show a fibrous central component with progressive enhancement (*) on portal and late phase although a perilesional halo is enhanced as soon the arterial phase.
In the literature, in MRI these lesions have low signal intensity at T1W images, iso or high signal intensity at T2W images (27), and variable presentation on contrast-enhanced T1W images. As we mentioned before, differential diagnosis with malignant tumor can be very difficult and a histological verification is essential. In this context, percutaneous lesion biopsy can help to make the diagnosis and avoid a major hepatic surgery although the role of percutaneous biopsy in the investigation and management of solitary hepatic masses potentially malignant is questionable.
Conclusion
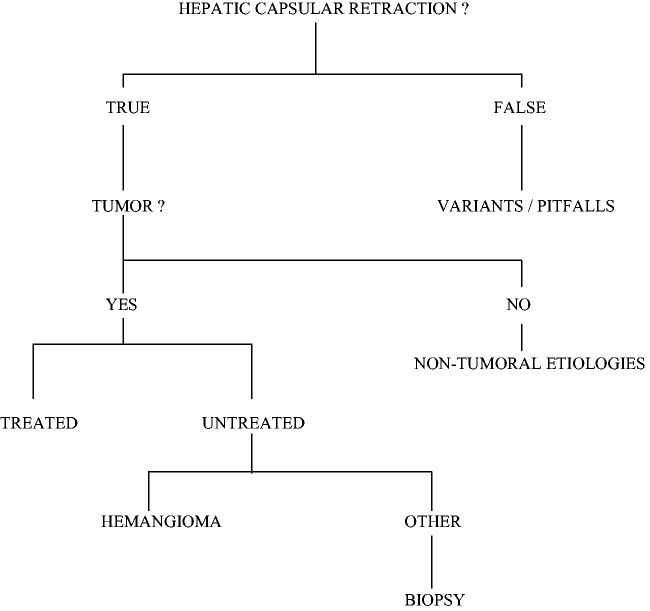
Hepatic capsular retraction is not a specific sign of malignancy but the presence of this sign adjacent to a circumscribed tumor, while non-specific, is suspicious (Fig. 14). The subcapsular location and the fibrous component of the tumor seem to be determining in the development of capsular retraction.
Fig. 14.
Diagnosis algorithm.
From etiological point of view, few benign tumors may be associated with hepatic capsular retraction, essentially sclerosing liver hemangiomas. For malignant lesions, metastases constitute the most common cause while intrahepatic cholangiocarcinoma and epithelioid hemangioendothelioma of the liver are the main primary tumors. “Classical” HCC and untreated HCC do not cause hepatic capsular retraction except for fibrolamellar HCC and combined hepatocellular-cholangiocarcinoma.
However, it is necessary to keep in mind that much of hepatic capsular retraction is iatrogenic, secondary to non-surgical treatments of hepatic tumors as chemotherapy, RF ablation, radiotherapy, or chemoembolization.
Conflict of interest
None declared.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- 1.Soyer P, Bluemke DA, Vissuzaine C, et al. CT of hepatic tumors: prevalence and specificity of retraction of the adjacent liver capsule. AJR Am J Roentgenol 1994; 162: 1119–1122. [DOI] [PubMed] [Google Scholar]
- 2.Sans N, Fajadet P, Galy-Fourcade D, et al. Is capsular retraction a specific CT sign of malignant liver tumor? Eur Radiol 1999; 9: 1543–1545. [DOI] [PubMed] [Google Scholar]
- 3.Blachar A, Federle MP, Brancatelli G. Hepatic capsular retraction: spectrum of benign and malignant etiologies. Abdom Imaging 2002; 27: 690–699. [DOI] [PubMed] [Google Scholar]
- 4.Da Ines D, Petitcolin V, Lannareix V, et al. [Liver capsule retraction adjacent to a circumscribed liver lesion: review of 26 cases with histological confirmation]. J Radiol 2009; 90: 1067–1074. [DOI] [PubMed] [Google Scholar]
- 5.Doyle DJ, Khalili K, Guindi M, et al. Imaging features of sclerosed hemangioma. AJR Am J Roentgenol 2007; 189: 67–72. [DOI] [PubMed] [Google Scholar]
- 6.Vilgrain V, Boulos L, Vullierme MP, et al. Imaging of atypical hemangiomas of the liver with pathologic correlation. Radiographics 2000; 20: 379–397. [DOI] [PubMed] [Google Scholar]
- 7.Brancatelli G, Federle MP, Blachar A, et al. Hemangioma in the cirrhotic liver: diagnosis and natural history. Radiology 2001; 219: 69–74. [DOI] [PubMed] [Google Scholar]
- 8.Yang DM, Yoon MH, Kim HS, et al. Capsular retraction in hepatic giant hemangioma: CT and MR features. Abdom Imaging 2001; 26: 36–38. [DOI] [PubMed] [Google Scholar]
- 9.Vilgrain V, Van Beers BE, Flejou JF, et al. Intrahepatic cholangiocarcinoma: MRI and pathologic correlation in 14 patients. J Comput Assist Tomogr 1997; 21: 59–65. [DOI] [PubMed] [Google Scholar]
- 10.Soyer P. Capsular retraction of the liver in malignant tumor of the biliary tract MRI findings. Clin Imaging 1994; 18: 255–257. [DOI] [PubMed] [Google Scholar]
- 11.Chung YE, Kim M-J, Park YN, et al. Varying appearances of cholangiocarcinoma: radiologic-pathologic correlation. Radiographics 2009; 29: 683–700. [DOI] [PubMed] [Google Scholar]
- 12.Lim JH. Cholangiocarcinoma: morphologic classification according to growth pattern and imaging findings. AJR Am J Roentgenol 2003; 181: 819–827. [DOI] [PubMed] [Google Scholar]
- 13.Vilgrain V. [Retraction of the liver capsule: a useful imaging finding]. J Radiol 2009; 90: 1019–1020. [DOI] [PubMed] [Google Scholar]
- 14.Baron RL, Peterson MS. From the RSNA refresher courses: screening the cirrhotic liver for hepatocellular carcinoma with CT and MR imaging: opportunities and pitfalls. Radiographics 2001; 21: S117–S132. [DOI] [PubMed] [Google Scholar]
- 15.McLarney JK, Rucker PT, Bender GN, et al. Fibrolamellar carcinoma of the liver: radiologic-pathologic correlation. Radiographics 1999; 19: 453–471. [DOI] [PubMed] [Google Scholar]
- 16.Ebied O, Federle MP, Blachar A, et al. Hepatocellular-cholangiocarcinoma: helical computed tomography findings in 30 patients. J Comput Assist Tomogr 2003; 27: 117–124. [DOI] [PubMed] [Google Scholar]
- 17.Läuffer JM, Zimmermann A, Krähenbühl L, et al. Epithelioid hemangioendothelioma of the liver. A rare hepatic tumor. Cancer 1996; 78: 2318–2327. [DOI] [PubMed] [Google Scholar]
- 18.Miller WJ, Dodd GD, 3rd, Federle MP, et al. Epithelioid hemangioendothelioma of the liver: imaging findings with pathologic correlation. AJR Am J Roentgenol 1992; 159: 53–57. [DOI] [PubMed] [Google Scholar]
- 19.Yang DM, Kim HS, Cho SW, et al. Pictorial review: various causes of hepatic capsular retraction: CT and MR findings. Br J Radiol 2002; 75: 994–1002. [DOI] [PubMed] [Google Scholar]
- 20.Nascimento AB, Mitchell DG, Rubin R, et al. Diffuse desmoplastic breast carcinoma metastases to the liver simulating cirrhosis at MR imaging: report of two cases. Radiology 2001; 221: 117–121. [DOI] [PubMed] [Google Scholar]
- 21.Robinson PJA. The effects of cancer chemotherapy on liver imaging. Eur Radiol 2009; 19: 1752–1762. [DOI] [PubMed] [Google Scholar]
- 22.Ohtomo K, Baron RL, Dodd GD, 3rd, et al. Confluent hepatic fibrosis in advanced cirrhosis: appearance at CT. Radiology 1993; 188: 31–35. [DOI] [PubMed] [Google Scholar]
- 23.Ohtomo K, Baron RL, Dodd GD, 3rd, et al. Confluent hepatic fibrosis in advanced cirrhosis: evaluation with MR imaging. Radiology 1993; 189: 871–874. [DOI] [PubMed] [Google Scholar]
- 24.Brancatelli G, Baron RL, Federle MP, et al. Focal confluent fibrosis in cirrhotic liver: natural history studied with serial CT. AJR Am J Roentgenol 2009; 192: 1341–1347. [DOI] [PubMed] [Google Scholar]
- 25.Ganesan K, Viamonte B, Peterson M, et al. Capsular retraction: an uncommon imaging finding in hepatic inflammatory pseudotumour. Br J Radiol 2009; 82: e256–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ntinas A, Kardassis D, Miliaras D, et al. Inflammatory pseudotumor of the liver: a case report and review of the literature. J Med Case Reports 2011; 5: 196–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Park JY, Choi MS, Lim Y-S, et al. Clinical features, image findings, and prognosis of inflammatory pseudotumor of the liver: a multicenter experience of 45 cases. Gut Liver 2014; 8: 58–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Abbey-Toby A, Cazals-Hatem D, Colombat M, et al. [Inflammatory pseudo-tumor of the liver: is pre-operative diagnosis possible?]. Gastroentérologie Clin Biol 2003; 27: 883–90. [PubMed] [Google Scholar]