Abstract
Background
The purpose of this study was to address the affects of mood modifying drugs on the transcriptome, in a tissue culture model, using qPCR arrays as a cost effective approach to identifying regulatory networks and pathways that might coordinate the cell response to a specific drug.
Methods
We addressed the gene expression profile of 90 plus genes associated with human mood disorders using the StellARray™ qPCR gene expression system in the human derived SH-SY5Y neuroblastoma cell line.
Results
Global Pattern Recognition (GPR) analysis identified a total of 9 genes (DRD3⁎, FOS†, JUN⁎, GAD1⁎†, NRG1⁎, PAFAH1B3⁎, PER3⁎, RELN⁎ and RGS4⁎) to be significantly regulated in response to cellular challenge with the mood stabilisers sodium valproate (⁎) and lithium (†). Modulation of FOS and JUN highlights the importance of the activator protein 1 (AP-1) transcription factor pathway in the cell response. Enrichment analysis of transcriptional networks relating to this gene set also identified the transcription factor neuron restrictive silencing factor (NRSF) and the oestrogen receptor as an important regulatory mechanism.
Limitations
Cell line models offer a window of what might happen in vivo but have the benefit of being human derived and homogenous with regard to cell type.
Conclusions
This data highlights transcription factor pathways, acting synergistically or separately, in the modulation of specific neuronal gene networks in response to mood stabilising drugs. This model can be utilised in the comparison of the action of multiple drug regimes or for initial screening purposes to inform optimal drug design.
Abbreviations: AP-1, activator protein 1; ENCODE, Encyclopaedia of DNA Elements; ERK, extracellular-signal-regulated kinase; GPR, Global Pattern Recognition; G×E, Gene×Environment; NRSF, neuron restrictive silencing factor; REST, repressor element-1 silencing transcription factor
Keywords: Global Pattern Recognition, Mood disorders, Mood-modifying drugs, Neuronal signalling, NRSF, Pathway analysis
1. Introduction
Mental health is in part dependent upon transcriptional responses to cues which can be environmental, chemical, physiological and psychological; this is termed the Gene×Environment (G×E) component. These changes not only affect our health in the short term, but can have medium to long term impact via epigenetic modulation of gene expression, altering our response to environmental challenges. Genetic polymorphism can modulate the G×E response and offer insight into the mechanisms underpinning such pathways (Quinn et al., 2013). Earlier genetic studies targeted association of one genetic variant to a specific disorder; this had limited success and focused predominantly on candidate genes such as those in the monoaminergic pathways. These correlations are now being readdressed by analysing multiple variants in such pathways or by stratification of the cohorts based on environmental factors. Our recent work on a promoter polymorphism of the monoamine oxidase A gene and maternal parameters affecting infant behaviour is an example of the latter (Hill et al., 2013). It is difficult to address the signal cascade in response to specific challenges in vivo due to the heterogeneity of cells involved in processing the environmental signals mediating a cellular response. However, in vitro cell line models offer an opportunity to address in fine detail the signal pathways modulated in response to a specific challenge. In this study we analysed the response to distinct drugs in the human neuroblastoma cell line SH-SY5Y targeting a commercially available compilation of mood disorder genes to address whether they leave a molecular signature of transcriptional change to the challenge. The drugs chosen for comparison included two psychostimulant challenges, amphetamine and cocaine, and two mood stabilisers, sodium valproate and lithium. All of these drugs have been shown previously to modulate signal pathways in SH-SY5Y cells at the transcriptional and/or post-transcriptional level (Asghari et al., 1998, Di Daniel et al., 2005, Kantor et al., 2002, Lew, 1992, Pan et al., 2005, Warburton et al., 2014). Our analysis identified similarities and differences in the networks modified by the drug challenge which suggested an overlap in the pathways of the mood stabilisers. These changes reflect one window for the spectrum of changes that could occur in vivo, but nonetheless outline the potential for a concerted cellular response to drug exposure.
2. Materials and methods
2.1. Cell culture and drug treatment
Human derived SH-SY5Y neuroblastoma cells (American Type Culture Collection) were maintained in Earle׳s modified Eagle׳s medium (EMEM) (Sigma) and HAM׳s F12 (Sigma) at a ratio of 1:1, supplemented with 10% foetal calf serum (FCS) (Sigma), 1% 200 mM l-glutamine, 1% 100 mM sodium pyruvate and 100 U/ml penicillin/100 ug/ml streptomycin at 37 °C and 5% CO2. Amphetamine, cocaine hydrochloride, lithium chloride and valproic acid sodium salt were purchased from Sigma and stock solutions made using sterile filtered dH2O. Drug regimes were 1 h treatment with either: vehicle control (sterile filtered dH2O), 10 µM amphetamine (Jones and Kauer, 1999, Shyu et al., 2004), 10 µM cocaine (Warburton et al., 2014), 1 mM lithium (Hing et al., 2012, Roberts et al., 2007) or 5 mM sodium valproate (Pan et al., 2005, Phiel et al., 2001, Zhang et al., 2003). For each drug treatment, n=4. Basal (untreated) cells were also included.
2.2. RNA extraction and quantitative polymerase chain reaction (qPCR) analysis
Total RNA was extracted using Trizol reagent (Invitrogen) and the resulting RNA pellets resuspended in RNase-free water. 500 ng RNA was reverse transcribed into cDNA using the GoScript™ RT system (Promega). qPCR analysis was performed on an iQ5 real-time PCR system (Bio-Rad) using 1 µl of cDNA per reaction and GoTaq® qPCR Master Mix (Promega) with the addition of Fluorescene Calibration Dye (Bio-Rad) at a final concentration of 10 nM. Changes in gene expression were analysed on the Lonza Web site (http://array.lonza.com/gpr), using the Global Pattern Recognition™ (GPR) analysis software designed by Bar Harbor Biotechnology (https://www.bhbio.com/BHB/dw/home.html). This algorithm internally normalised the real-time qPCR data set of each gene with respect to all genes within the experiment and generated a list of genes that are ranked on the basis of the difference between the test and control expression levels and the consistency of the data between the biological replicates. This proprietary software calculated both the fold-change data and the respective p-values. The results are displayed as change with respect to the genes that showed minimal changes, which were defined on Ct values obtained using the Global Pattern Recognition analysis software (Akilesh et al., 2003).
A list of genes on the mood array is presented in Table 1.
Table 1.
Gene name and description for the Human Mood Disorder 96-well qPCR StellARray™.
| Gene name | Entrez gene | Description |
|---|---|---|
| ACE | 1636 | Angiotensin I converting enzyme (peptidyl-dipeptidase A) 1 |
| ADCYAP1 | 116 | Adenylate cyclase activating polypeptide 1 (pituitary) |
| ADRBK2 | 157 | Adrenergic, beta, receptor kinase 2 |
| ARNTL | 406 | Aryl hydrocarbon receptor nuclear translocator-like |
| ATP2A2 | 488 | ATPase, Ca++ transporting, cardiac muscle, slow twitch 2 |
| BCR | 613 | Breakpoint cluster region |
| BDNF | 627 | Brain-derived neurotrophic factor |
| CASP8 | 841 | Caspase 8, apoptosis-related cysteine peptidase |
| CCND2 | 894 | Cyclin D2 |
| CHRNA7 | 1139 | Cholinergic receptor, nicotinic, alpha 7 |
| CIT | 11113 | Citron rho-interacting serine/threonine kinase |
| CLOCK | 9575 | Clock circadian regulator |
| COMT | 1312 | Catechol-O-methyltransferase |
| CREB1 | 1385 | CAMP responsive element binding protein 1 |
| CRH | 1392 | Corticotropin releasing hormone |
| CRHBP | 1393 | Corticotropin releasing hormone binding protein |
| DAO | 1610 | d-amino-acid oxidase |
| DISC1 | 27185 | Disrupted in schizophrenia 1 |
| DLX1 | 1745 | Distal-less homeobox 1 |
| DRD1 | 1812 | Dopamine receptor D1 |
| DRD3 | 1814 | Dopamine receptor D3 |
| DRD4 | 1815 | Dopamine receptor D4 |
| DTNBP1 | 84062 | Dystrobrevin binding protein 1 |
| ERBB3 | 2065 | V-erb-b2 erythroblastic leukemia viral oncogene homolog 3 |
| FAT1 | 2195 | FAT atypical cadherin 1 |
| FKBP5 | 2289 | FK506 binding protein 5 |
| FOS | 2353 | FBJ murine osteosarcoma viral oncogene homolog |
| GABRA5 | 2558 | Gamma-aminobutyric acid (GABA) A receptor, alpha 5 |
| GAD1 | 2571 | Glutamate decarboxylase 1 (brain, 67 kDa) |
| GCH1 | 2643 | GTP cyclohydrolase 1 |
| GPR50 | 9248 | G protein-coupled receptor 50 |
| GRIK3 | 2899 | Glutamate receptor, ionotropic, kainate 3 |
| GRIK4 | 2900 | Glutamate receptor, ionotropic, kainate 4 |
| GRIN2B | 2904 | Glutamate receptor, ionotropic, N-methyl d-aspartate 2B |
| GRM3 | 2913 | Glutamate receptor, metabotropic 3 |
| GRM4 | 2914 | Glutamate receptor, metabotropic 4 |
| GSK3B | 2932 | Glycogen synthase kinase 3 beta |
| Hs18s | – | Human 18S ribosomal RNA |
| HS Genomic | – | Human genomic DNA control |
| HSP90B1 | 7184 | Heat shock protein 90 kDa beta (Grp94), member 1 |
| HSPA5 | 3309 | Heat shock 70 kDa protein 5 (glucose-regulated protein, 78 kDa) |
| HTR1B | 3351 | 5-hydroxytryptamine (serotonin) receptor 1B |
| HTR2A | 3356 | 5-hydroxytryptamine (serotonin) receptor 2A |
| IL1RN | 3557 | Interleukin 1 receptor antagonist |
| IMPA1 | 3612 | Inositol(myo)-1(or 4)-monophosphatase 1 |
| IMPA2 | 3613 | Inositol(myo)-1(or 4)-monophosphatase 2 |
| INPP1 | 3628 | Inositol polyphosphate-1-phosphatase |
| ISYNA1 | 51477 | Myo-inositol 1-phosphate synthase A1 |
| JUN | 3725 | Jun oncogene |
| KCNN3 | 3782 | Potassium intermediate/small conductance calcium-activated channel, subfamily N, member 3 |
| MAG | 27307 | Malignancy-associated gene |
| MAL | 4118 | Mal, T-cell differentiation protein |
| MAOA | 4128 | Monoamine oxidase A |
| MLC1 | 23209 | Megalencephalic leukoencephalopathy with subcortical cysts 1 |
| MOBP | 4336 | Myelin-associated oligodendrocyte basic protein |
| MOG | 4340 | Myelin oligodendrocyte glycoprotein |
| MTHFR | 4524 | 5,10-methylenetetrahydrofolate reductase (NADPH) |
| NAPG | 8774 | N-ethylmaleimide-sensitive factor attachment protein, gamma |
| NCAM1 | 4684 | Neural cell adhesion molecule 1 |
| ND4 | 4538 | Mitochondrially encoded NADH dehydrogenase 4 |
| NDUFV1 | 4723 | NADH dehydrogenase (ubiquinone) flavoprotein 1, 51 kDa |
| NDUFV2 | 4729 | NADH dehydrogenase (ubiquinone) flavoprotein 2, 24 kDa |
| NOS1AP | 9722 | Nitric oxide synthase 1 (neuronal) adaptor protein |
| NR1D1 | 9572 | Nuclear receptor subfamily 1, group D, member 1 |
| NR3C1 | 2908 | Nuclear receptor subfamily 3, group C, member 1 (glucocorticoid receptor) |
| NRG1 | 3084 | Neuregulin 1 |
| NTRK2 | 4915 | Neurotrophic tyrosine kinase, receptor, type 2 |
| OLIG2 | 10215 | Oligodendrocyte lineage transcription factor 2 |
| P2RX7 | 5027 | Purinergic receptor P2X, ligand-gated ion channel, 7 |
| PAFAH1B1 | 5048 | Platelet-activating factor acetylhydrolase, isoform Ib, alpha subunit 45 kDa |
| PAFAH1B3 | 5050 | Platelet-activating factor acetylhydrolase, isoform Ib, gamma subunit 29 kDa |
| PCNT | 5116 | Pericentrin |
| PDLIM5 | 10611 | PDZ and LIM domain 5 |
| PER3 | 8863 | Period circadian clock 3 |
| PIP4K2A | 5305 | Phosphatidylinositol-5-phosphate 4-kinase, type II, alpha |
| PLA2G1B | 5319 | Phospholipase A2, group IB (pancreas) |
| PLA2G4A | 5321 | Phospholipase A2, group IVA (cytosolic, calcium-dependent) |
| PLCG1 | 5335 | Phospholipase C, gamma 1 |
| PLP1 | 5354 | Proteolipid protein 1 |
| POLG | 5428 | Polymerase (DNA directed), gamma |
| PTGS2 | 5743 | Prostaglandin-endoperoxide synthase 2 (prostaglandin G/H synthase and cyclooxygenase) |
| RELN | 5649 | Reelin |
| RFX4 | 5992 | Regulatory factor X, 4 (influences HLA class II expression) |
| RGS4 | 5999 | Regulator of G-protein signaling 4 |
| SLC12A6 | 9990 | Solute carrier family 12 (potassium/chloride transporters), member 6 |
| SLC6A2 | 6530 | Solute carrier family 6 (neurotransmitter transporter, noradrenalin), member 2 |
| SLC6A3 | 6531 | Solute carrier family 6 (neurotransmitter transporter, dopamine), member 3 |
| SLC6A4 | 6532 | Solute carrier family 6 (neurotransmitter transporter, serotonin), member 4 |
| SULT1A1 | 6817 | Sulfotransferase family, cytosolic, 1A, phenol-preferring, member 1 |
| SYNGR1 | 9145 | Synaptogyrin 1 |
| TAAR6 | 319100 | Trace amine associated receptor 6 |
| TF | 7018 | Transferrin |
| TIMELESS | 8914 | Timeless circadian clock |
| TPH1 | 7166 | Tryptophan hydroxylase 1 (tryptophan 5-monooxygenase) |
| TPH2 | 121278 | Tryptophan hydroxylase 2 |
| XBP1 | 7494 | X-box binding protein 1 |
2.3. Bioinformatic analysis
Gene expression data generated from GPR analysis was uploaded into the online biological pathway analysis software MetaCore™, version 6.15 build 62452. Functional enrichment of the experimental dataset was performed using: 1) the Pathway Map analysis tool to identify significantly associated pathways based on p-value and GPR Fold-change and 2) Build Network for Your Experimental Data feature using the Transcription Factor Targets Modelling algorithm with default settings under Analyse Networks (Transcription Factors) to generate sub-networks based on the presence of transcription factors and/or receptor targets within the original input file. Such genes/proteins uploaded from experimental datasets and from which pathways were built upon were termed ‘seed nodes’.
In silico analysis of NRSF binding sites over the significantly altered genes across the different treatment conditions from the qPCR array data were identified using Transcription Factor ChIP-seq from ENCODE (Encyclopaedia of DNA Elements), version 4, available on the UCSC Genome Browser (http://genome.ucsc.edu/index.html). Upstream and downstream flank sequences (10 Kb) were included and the position of NRSF binding sites calculated. For genes with multiple transcripts, the locus for the largest isoform was used. The full list of NRSF binding sites is detailed in Table 3.
Table 3.
Predicted NRSF regulation of genes affecting mood.
| Gene | Locus | Strand | NRSF site | Size (Bp) | Position |
|---|---|---|---|---|---|
| ACE | chr17:61554422–61575741 | + | chr17:61553914–61554174 | 260 | −508 |
| ACE | chr17:61554422–61575741 | + | chr17:61554504–61554774 | 270 | 82 |
| ACE | chr17:61554422–61575741 | + | chr17:61556270–61556594 | 324 | 1848 |
| ACE | chr17:61554422–61575741 | + | chr17:61557174–61557444 | 270 | 2752 |
| ACE | chr17:61554422–61575741 | + | chr17:61558309–61558579 | 270 | 3887 |
| ADRBK2 | chr22:25960861–26125258 | + | chr22:25961290–25961560 | 270 | 429 |
| ADRBK2 | chr22:25960861–26125258 | + | chr22:26052841–26053085 | 244 | 91980 |
| ADRBK2 | chr22:25960861–26125258 | + | chr22:26097050–26097320 | 270 | 136189 |
| ARNTL | chr11:13277734–13387266 | + | chr11:13283216–13283586 | 370 | 5482 |
| ARNTL | chr11:13277734–13387266 | + | chr11:13298458–13299341 | 883 | 20724 |
| ARNTL | chr11:13277734–13387266 | + | chr11:13310624–13311040 | 416 | 32890 |
| ARNTL | chr11:13277734–13387266 | + | chr11:13312905–13313275 | 370 | 35171 |
| ARNTL | chr11:13277734–13387266 | + | chr11:13351630–13351900 | 270 | 73896 |
| ARNTL | chr11:13277734–13387266 | + | chr11:13361071–13361575 | 504 | 83337 |
| ARNTL | chr11:13277734–13387266 | + | chr11:13364729–13364973 | 244 | 86995 |
| ARNTL | chr11:13277734–13387266 | + | chr11:13365612–13366116 | 504 | 87878 |
| BCR | chr22:23522552–23660224 | + | chr22:23525622–23525892 | 270 | 3070 |
| BCR | chr22:23522552–23660224 | + | chr22:23546679–23546949 | 270 | 24127 |
| BCR | chr22:23522552–23660224 | + | chr22:23562075–23562399 | 324 | 39523 |
| BCR | chr22:23522552–23660224 | + | chr22:23566052–23566322 | 270 | 43500 |
| BCR | chr22:23522552–23660224 | + | chr22:23591914–23592184 | 270 | 69362 |
| BCR | chr22:23522552–23660224 | + | chr22:23624008–23624332 | 324 | 101456 |
| BCR | chr22:23522552–23660224 | + | chr22:23647903–23648174 | 271 | 125351 |
| BCR | chr22:23522552–23660224 | + | chr22:23651156–23651400 | 244 | 128604 |
| BDNF | chr11:27676442–27743605 | − | chr11:27667673–27667943 | 270 | −8499 |
| BDNF | chr11:27676442–27743605 | − | chr11:27671454–27671716 | 262 | −4726 |
| BDNF | chr11:27676442–27743605 | − | chr11:27680076–27680346 | 270 | 63259 |
| BDNF | chr11:27676442–27743605 | − | chr11:27721240–27721484 | 244 | 22121 |
| BDNF | chr11:27676442–27743605 | − | chr11:27723005–27723329 | 324 | 20276 |
| BDNF | chr11:27676442–27743605 | − | chr11:27739843–27740167 | 324 | 3438 |
| BDNF | chr11:27676442–27743605 | − | chr11:27740692–27741122 | 430 | 2483 |
| BDNF | chr11:27676442–27743605 | − | chr11:27741795–27742502 | 707 | 1103 |
| BDNF | chr11:27676442–27743605 | − | chr11:27742701–27743071 | 370 | 534 |
| BDNF | chr11:27676442–27743605 | − | chr11:27743607–27744258 | 651 | +2 |
| BDNF | chr11:27676442–27743605 | − | chr11:27744566–27744890 | 324 | +961 |
| CASP8 | chr2:202098166–202152434 | + | chr2:202096900–202097280 | 380 | −1266 |
| CASP8 | chr2:202098166–202152434 | + | chr2:202098061–202098441 | 380 | −105 |
| CASP8 | chr2:202098166–202152434 | + | chr2:202122713–202123093 | 380 | 24547 |
| CRH | chr8:67088612–67090846 | − | chr8:67089099–67090281 | 1182 | 565 |
| CRH | chr8:67088612–67090846 | − | chr8:67090287–67090659 | 372 | 187 |
| CRH | chr8:67088612–67090846 | − | chr8:67090956–67091280 | 324 | +110 |
| CRH | chr8:67088612–67090846 | − | chr8:67091915–67092285 | 370 | +1069 |
| CRH | chr8:67088612–67090846 | − | chr8:67098519–67098889 | 370 | +7673 |
| DISC1 | chr1:231762561–232177019 | + | chr1:231795960–231796330 | 370 | 33399 |
| DISC1 | chr1:231762561–232177019 | + | chr1:231814930–231815200 | 270 | 52369 |
| DISC1 | chr1:231762561–232177019 | + | chr1:231925791–231926295 | 504 | 163230 |
| DISC1 | chr1:231762561–232177019 | + | chr1:231963016–231963520 | 504 | 200455 |
| DISC1 | chr1:231762561–232177019 | + | chr1:231964053–231964309 | 256 | 201492 |
| DISC1 | chr1:231762561–232177019 | + | chr1:232067746–232067990 | 244 | 305185 |
| DISC1 | chr1:231762561–232177019 | + | chr1:232148522–232148892 | 370 | 385961 |
| DRD3 | chr3:113847557–113918254 | − | chr3:113871366–113871690 | 324 | 46564 |
| DRD3 | chr3:113847557–113918254 | − | chr3:113874262–113874642 | 380 | 43612 |
| DRD3 | chr3:113847557–113918254 | − | chr3:113897607–113898013 | 406 | 20241 |
| DRD3 | chr3:113847557–113918254 | − | chr3:113898443–113898813 | 370 | 19441 |
| DRD4 | chr11:637305–640705 | + | chr11:640330–640654 | 324 | 3025 |
| DTNBP1 | chr6:15523032–15663289 | − | chr6:15552018–15552288 | 270 | 111001 |
| DTNBP1 | chr6:15523032–15663289 | − | chr6:15621994–15622224 | 230 | 41065 |
| DTNBP1 | chr6:15523032–15663289 | − | chr6:15662506–15662830 | 324 | 459 |
| FKBP5 | chr6:35541362–35696397 | − | chr6:35656504–35656848 | 344 | 39549 |
| FKBP5 | chr6:35541362–35696397 | − | chr6:35687515–35687759 | 244 | 8638 |
| FKBP5 | chr6:35541362–35696397 | − | chr6:35695292–35695562 | 270 | 835 |
| FKBP5 | chr6:35541362–35696397 | − | chr6:35695873–35696103 | 230 | 294 |
| FKBP5 | chr6:35541362–35696397 | − | chr6:35699743–35700105 | 362 | −3346 |
| FOS | chr14:75745481–75748937 | + | chr14:75743830–75744074 | 244 | −1651 |
| FOS | chr14:75745481–75748937 | + | chr14:75745296–75745800 | 504 | −185 |
| GABRA5 | chr15:27111866–27194357 | + | chr15:27110041–27110545 | 504 | −1825 |
| GABRA5 | chr15:27111866–27194357 | + | chr15:27111625–27112129 | 504 | −241 |
| GAD1 | chr2:171673200–171717659 | + | chr2:171670663–171671101 | 438 | −2537 |
| GAD1 | chr2:171673200–171717659 | + | chr2:171671290–171671546 | 256 | −1910 |
| GAD1 | chr2:171673200–171717659 | + | chr2:171672190–171672567 | 377 | −1010 |
| GAD1 | chr2:171673200–171717659 | + | chr2:171679546–171679776 | 230 | 6346 |
| GAD1 | chr2:171673200–171717659 | + | chr2:171701873–171702253 | 380 | 28673 |
| GRIK3 | chr1:37261128–37499844 | − | chr1:37269486–37269856 | 370 | 229988 |
| GRIK3 | chr1:37261128–37499844 | − | chr1:37301874–37302144 | 270 | 197700 |
| GRIK3 | chr1:37261128–37499844 | − | chr1:37329834–37330078 | 244 | 169766 |
| GRIK3 | chr1:37261128–37499844 | − | chr1:37331752–37332256 | 504 | 167588 |
| GRIK3 | chr1:37261128–37499844 | − | chr1:37332540–37332784 | 244 | 167060 |
| GRIK3 | chr1:37261128–37499844 | − | chr1:37388506–37388750 | 244 | 111094 |
| GRIK3 | chr1:37261128–37499844 | − | chr1:37389788–37390253 | 465 | 109591 |
| GRIK3 | chr1:37261128–37499844 | − | chr1:37411488–37411732 | 244 | 88112 |
| GRIK3 | chr1:37261128–37499844 | − | chr1:37431706–37432281 | 575 | 67563 |
| GRIK3 | chr1:37261128–37499844 | − | chr1:37486267–37486654 | 387 | 13190 |
| GRIK3 | chr1:37261128–37499844 | − | chr1:37494616–37494860 | 244 | 4984 |
| GRIK3 | chr1:37261128–37499844 | − | chr1:37504779–37505043 | 264 | −4935 |
| GRM3 | chr7:86273230–86494192 | + | chr7:86290343–86290599 | 256 | 17113 |
| GRM3 | chr7:86273230–86494192 | + | chr7:86322086–86322456 | 370 | 48856 |
| GRM3 | chr7:86273230–86494192 | + | chr7:86476174–86476554 | 380 | 202944 |
| GRM3 | chr7:86273230–86494192 | + | chr7:86497476–86497720 | 244 | +3284 |
| JUN | chr1:59246463–59249785 | − | chr1:59249472–59249885 | 413 | −100 |
| MAG | chr19:35782989–35820133 | + | chr19:35796870–35797100 | 230 | 13881 |
| MAG | chr19:35782989–35820133 | + | chr19:35809956–35810280 | 324 | 26967 |
| MAOA | chrX:43515409–43606068 | + | – | – | – |
| MLC1 | chr22:50497820–50523781 | − | – | – | – |
| MOBP | chr3:39543557–39567857 | + | chr3:39540121–39540386 | 265 | −3436 |
| MOBP | chr3:39543557–39567857 | + | chr3:39558349–39558719 | 370 | 14792 |
| MOBP | chr3:39543557–39567857 | + | chr3:39574318–39574698 | 380 | +6461 |
| MTHFR | chr1:11845787–11866160 | − | chr1:11845214–11845454 | 240 | +573 |
| MTHFR | chr1:11845787–11866160 | − | chr1:11850982–11851306 | 324 | 14854 |
| MTHFR | chr1:11845787–11866160 | − | chr1:11856563–11856793 | 230 | 9367 |
| MTHFR | chr1:11845787–11866160 | − | chr1:11857775–11857960 | 185 | 8200 |
| MTHFR | chr1:11845787–11866160 | − | chr1:11858618–11858699 | 81 | 7461 |
| MTHFR | chr1:11845787–11866160 | − | chr1:11863764–11864034 | 270 | 2126 |
| MTHFR | chr1:11845787–11866160 | − | chr1:11865502–11865882 | 380 | 278 |
| MTHFR | chr1:11845787–11866160 | − | chr1:11866038–11866425 | 387 | −265 |
| NAPG | chr18:10525873–10552766 | + | chr18:10525815–10526242 | 427 | −58 |
| NCAM1 | chr11:112831969–113092626 | + | chr11:112831909–112832179 | 270 | −60 |
| NCAM1 | chr11:112831969–113092626 | + | chr11:112977293–112977549 | 256 | 145324 |
| NCAM1 | chr11:112831969–113092626 | + | chr11:113008930–113009200 | 270 | 176961 |
| NCAM1 | chr11:112831969–113092626 | + | chr11:113011853–113012123 | 270 | 179884 |
| NCAM1 | chr11:112831969–113092626 | + | chr11:113023160–113023664 | 504 | 191191 |
| NCAM1 | chr11:112831969–113092626 | + | chr11:113074175–113074445 | 270 | 242206 |
| NR1D1 | chr17:38249037–38256973 | − | chr17:38244467–38244847 | 380 | +4570 |
| NR1D1 | chr17:38249037–38256973 | − | chr17:38254215–38254595 | 380 | 2378 |
| NR1D1 | chr17:38249037–38256973 | − | chr17:38255228–38255666 | 438 | 1307 |
| NR1D1 | chr17:38249037–38256973 | − | chr17:38256685–38257094 | 409 | −121 |
| NR1D1 | chr17:38249037–38256973 | − | chr17:38257324–38257828 | 504 | −351 |
| NR1D1 | chr17:38249037–38256973 | − | chr17:38264445–38264769 | 324 | −7472 |
| NR3C1 | chr5:142657496–142783254 | − | chr5:142784785–142785394 | 609 | −2140 |
| NRG1 | chr8:31496911–32622558 | + | chr8:31499444–31499814 | 370 | 2533 |
| NRG1 | chr8:31496911–32622558 | + | chr8:31612484–31612740 | 256 | 115573 |
| NRG1 | chr8:31496911–32622558 | + | chr8:31629195–31629565 | 370 | 132284 |
| NRG1 | chr8:31496911–32622558 | + | chr8:31652781–31653242 | 461 | 155870 |
| NRG1 | chr8:31496911–32622558 | + | chr8:31691004–31691508 | 504 | 194093 |
| NRG1 | chr8:31496911–32622558 | + | chr8:31817830–31818086 | 256 | 320919 |
| NRG1 | chr8:31496911–32622558 | + | chr8:31896212–31896582 | 370 | 399301 |
| NRG1 | chr8:31496911–32622558 | + | chr8:32084240–32084744 | 504 | 587329 |
| NRG1 | chr8:31496911–32622558 | + | chr8:32122327–32122831 | 504 | 625416 |
| NRG1 | chr8:31496911–32622558 | + | chr8:32189091–32189595 | 504 | 692180 |
| NRG1 | chr8:31496911–32622558 | + | chr8:32191794–32192298 | 504 | 694883 |
| NRG1 | chr8:31496911–32622558 | + | chr8:32200953–32201685 | 732 | 704042 |
| NRG1 | chr8:31496911–32622558 | + | chr8:32245491–32245735 | 244 | 748580 |
| NRG1 | chr8:31496911–32622558 | + | chr8:32276508–32276752 | 244 | 779597 |
| NRG1 | chr8:31496911–32622558 | + | chr8:32284202–32284706 | 504 | 787291 |
| NRG1 | chr8:31496911–32622558 | + | chr8:32392615–32392985 | 370 | 895704 |
| NRG1 | chr8:31496911–32622558 | + | chr8:32405958–32406282 | 324 | 909047 |
| NRG1 | chr8:31496911–32622558 | + | chr8:32406492–32406892 | 400 | 909581 |
| NRG1 | chr8:31496911–32622558 | + | chr8:32411341–32411845 | 504 | 914430 |
| NRG1 | chr8:31496911–32622558 | + | chr8:32487206–32487506 | 300 | 990295 |
| NRG1 | chr8:31496911–32622558 | + | chr8:32488853–32489109 | 256 | 991942 |
| NRG1 | chr8:31496911–32622558 | + | chr8:32503654–32504024 | 370 | 1006743 |
| NRG1 | chr8:31496911–32622558 | + | chr8:32546371–32546746 | 375 | 1049460 |
| NRG1 | chr8:31496911–32622558 | + | chr8:32572641–32573145 | 504 | 1075730 |
| NRG1 | chr8:31496911–32622558 | + | chr8:32581201–32581705 | 504 | 1084290 |
| NRG1 | chr8:31496911–32622558 | + | chr8:32582687–32583047 | 360 | 1085776 |
| PAFAH1B3 | chr19:42801185–42806952 | − | chr19:42806435–42806939 | 504 | −13 |
| PER3 | chr1:7844714–7905237 | + | ~14 Kb upstream of 5׳UTR | – | – |
| PDLIM5 | chr4:95373038–95509370 | + | chr4:95372903–95373283 | 380 | −135 |
| PDLIM5 | chr4:95373038–95509370 | + | chr4:95406777–95407007 | 230 | 33739 |
| PDLIM5 | chr4:95373038–95509370 | + | chr4:95418920–95419164 | 244 | 45882 |
| PDLIM5 | chr4:95373038–95509370 | + | chr4:95455973–95456203 | 230 | 82935 |
| PDLIM5 | chr4:95373038–95509370 | + | chr4:95456267–95456511 | 244 | 83229 |
| PDLIM5 | chr4:95373038–95509370 | + | chr4:95471601–95471831 | 230 | 98563 |
| PDLIM5 | chr4:95373038–95509370 | + | chr4:95499407–95499663 | 256 | 126369 |
| RELN | chr7:103112231–103629963 | − | chr7:103127865–103128245 | 380 | 501718 |
| RELN | chr7:103112231–103629963 | − | chr7:103276613–103276992 | 379 | 352971 |
| RELN | chr7:103112231–103629963 | − | chr7:103297949–103298179 | 230 | 331784 |
| RELN | chr7:103112231–103629963 | − | chr7:103301028–103301258 | 230 | 328705 |
| RELN | chr7:103112231–103629963 | − | chr7:103354935–103355205 | 270 | 274758 |
| RELN | chr7:103112231–103629963 | − | chr7:103438111–103438481 | 370 | 191482 |
| RELN | chr7:103112231–103629963 | − | chr7:103451010–103451107 | 97 | 178856 |
| RELN | chr7:103112231–103629963 | − | chr7:103484281–103484449 | 168 | 145514 |
| RELN | chr7:103112231–103629963 | − | chr7:103491745–103492249 | 504 | 137714 |
| RELN | chr7:103112231–103629963 | − | chr7:103559848–103560078 | 230 | 69885 |
| RELN | chr7:103112231–103629963 | − | chr7:103580845–103581215 | 370 | 48748 |
| RELN | chr7:103112231–103629963 | − | chr7:103636658–103636861 | 203 | −6898 |
| RFX4 | chr12:106976685–107156582 | + | chr12:106975282–106975646 | 364 | −1403 |
| RFX4 | chr12:106976685–107156582 | + | chr12:106975776–106976119 | 343 | −909 |
| RFX4 | chr12:106976685–107156582 | + | chr12:107147300–107147544 | 244 | 170615 |
| RGS4 | chr1:163038396–163046592 | + | chr1:163039054–163039341 | 287 | 658 |
| SLC12A6 | chr15:34522197–34630265 | − | chr15:34516950–34517512 | 562 | +5247 |
| SLC12A6 | chr15:34522197–34630265 | − | chr15:34610582–34611086 | 504 | 19179 |
| SLC12A6 | chr15:34522197–34630265 | − | chr15:34630069–34630393 | 324 | −128 |
| SLC12A6 | chr15:34522197–34630265 | − | chr15:34634991–34635543 | 552 | −4726 |
| SLC6A2 | chr16:55689542–55737700 | + | chr16:55686047–55686317 | 270 | −3495 |
| SLC6A2 | chr16:55689542–55737700 | + | chr16:55689638–55689908 | 270 | 96 |
| SLC6A2 | chr16:55689542–55737700 | + | chr16:55690575–55690845 | 270 | 1033 |
| SLC6A2 | chr16:55689542–55737700 | + | chr16:55693927–55694197 | 270 | 4385 |
| SLC6A2 | chr16:55689542–55737700 | + | chr16:55695818–55696088 | 270 | 6276 |
| SLC6A2 | chr16:55689542–55737700 | + | chr16:55696686–55696956 | 270 | 7144 |
| SLC6A2 | chr16:55689542–55737700 | + | chr16:55744402–55744761 | 359 | +7061 |
| SLC6A2 | chr16:55689542–55737700 | + | chr16:55746277–55746521 | 244 | +8821 |
| SLC6A4 | chr17:28523378–28562954 | − | – | – | – |
| SULT1A1 | chr16:28616908–28634907 | − | chr16:28621167–28621407 | 240 | 13500 |
| TF | chr3:133419211–133497850 | + | chr3:133461483–133461863 | 380 | 42272 |
| TF | chr3:133419211–133497850 | + | chr3:133465027–133465407 | 380 | 45816 |
| TF | chr3:133419211–133497850 | + | chr3:133472690–133472920 | 230 | 53479 |
| TIMELESS | chr12:56810157–56843200 | − | chr12:56811537–56811907 | 370 | 31293 |
| TIMELESS | chr12:56810157–56843200 | − | chr12:56842752–56843263 | 511 | −63 |
| TPH2 | chr12:72332626–72426221 | + | chr12:72332400–72332889 | 489 | −226 |
| TPH2 | chr12:72332626–72426221 | + | chr12:72374868–72375372 | 504 | 42242 |
| TPH2 | chr12:72332626–72426221 | + | chr12:72410895–72411165 | 270 | 78269 |
| XBP1 | chr22:29190548–29196560 | − | chr22:29196394–29196960 | 566 | −400 |
| XBP1 | chr22:29190548–29196560 | − | chr22:29198252–29198482 | 230 | −1922 |
NRSF binding sites over top 10 affected genes across all drug treatments from Transcription Factor ChIP-seq from ENCODE version 4. Bold font indicates genes significantly affected by drug challenge. Negative and positive values under Position represent the location of the NRSF site upstream of the gene transcriptional start site and downstream of the 3׳UTR, respectively. Values not assigned +/− represent binding sites within the gene sequence. For genes with multiple transcripts, binding site positions are with respect to the largest isoform.
3. Results
3.1. Gene expression profiling of human SH-SY5Y cells in response to mood-modifying drugs using Global Pattern Recognition analysis
To investigate the effects of mood modifying drugs on the expression of a panel of genes associated with mood disorders (Human Mood Disorder 96 StellARray™), SH-SY5Y neuroblastoma cells after treatment for 1 h under one of the specified conditions were analysed using the proprietary Global Pattern Recognition (GPR) algorithm which compares the change in expression of a gene normalised to the expression of every other gene in the array (Akilesh et al., 2003). This software calculates both the fold-change data and the respective p-values with respect to genes that showed minimal changes. We and others have recently demonstrated that drugs used in the treatment of mood disorders can differentially affect the expression stability of traditionally used housekeeping genes, impacting upon their usefulness as normalising factors (D’Souza et al., 2013, Powell et al., 2013, Sugden et al., 2010). Unfortunately, these large changes in gene expression may mask small but biologically important changes in gene expression, such as master regulator genes (e.g., transcription factors). The data in Table 2 therefore represents a more appropriate display of the genes most changed within the experiment by comparing all genes against themselves. As the array contains validated mood genes we addressed the top 10 genes which significantly changed in response to each drug to define pathways and networks within the larger gene list.
Table 2.
Gene expression profiling of SH-SY5Y cells following exposure to drugs affecting mood.
| Lithium |
Sodium valproate |
||||||
|---|---|---|---|---|---|---|---|
| Gene | Description | p | Fold change | Gene | Description | p | Fold change |
| FOS | FBJ murine osteosarcoma viral oncogene homolog | 0.012 | −2.57 | DRD3 | Dopamine receptor D3 | 0.001 | −7.98 |
| GAD1 | Glutamate decarboxylase 1 | 0.023 | −3.48 | RGS4 | Regulator of G-protein signaling 4 | 0.007 | −2.08 |
| RGS4 | Regulator of G-protein signaling 4 | 0.063 | −1.51 | JUN | Jun oncogene | 0.008 | 2.49 |
| PER3 | Period circadian clock 3 | 0.067 | −1.38 | RELN | Reelin | 0.012 | −1.78 |
| NRG1 | Neuregulin 1 | 0.068 | −1.43 | PER3 | Period circadian clock 3 | 0.026 | −1.48 |
| NR1D1 | Nuclear receptor subfamily 1, group D, member 1 | 0.069 | −1.48 | PAFAH1B3 | Platelet-activating factor acetylhydrolase, isoform Ib, gamma subunit 29 kDa | 0.034 | 1.61 |
| RELN | Reelin | 0.078 | −1.94 | GAD1 | Glutamate decarboxylase 1 | 0.035 | −7.45 |
| ACE | Angiotensin I converting enzyme (peptidyl-dipeptidase A) 1 | 0.099 | 1.31 | NRG1 | Neuregulin 1 | 0.044 | −1.34 |
| Hs18s | Human 18S ribosomal RNA | 0.105 | 1.64 | MTHFR | Methylenetetrahydrofolate reductase (NADPH) | 0.083 | 1.53 |
| BDNF | Brain-derived neurotrophic factor | 0.106 | −1.39 | RFX4 | Regulatory factor X, 4 (influences HLA class II expression) | 0.092 | −1.49 |
| Cocaine | Amphetamine | ||||||
| Gene | Description | p | Fold change | Gene | Description | p | Fold change |
| SULT1A1 | Sulfotransferase family, cytosolic, 1A, phenol-preferring, member 1 | 0.088 | 1.68 | MOBP | Myelin-associated oligodendrocyte basic protein | 0.080 | 2.08 |
| DRD3 | Dopamine receptor D3 | 0.110 | −2.08 | XBP1 | X-box binding protein 1 | 0.093 | 1.34 |
| FOS | FBJ murine osteosarcoma viral oncogene homolog | 0.142 | −1.45 | NR1D1 | Nuclear receptor subfamily 1, group D, member 1 | 0.109 | −1.35 |
| MOBP | Myelin-associated oligodendrocyte basic protein | 0.161 | 1.85 | MAG | Malignancy-associated gene | 0.138 | 2.81 |
| SLC6A2 | Solute carrier family 6 (neurotransmitter transporter, noradrenalin), member 2 | 0.176 | −1.28 | PAFAH1B3 | Platelet-activating factor acetylhydrolase, isoform Ib, gamma subunit 29 kDa | 0.141 | 1.33 |
| GRIK3 | Glutamate receptor, ionotropic, kainate 3 | 0.194 | −1.67 | FKBP5 | FK506 binding protein 5 | 0.159 | −1.34 |
| TIMELESS | Timeless circadian clock | 0.200 | −1.20 | RELN | Reelin | 0.198 | −1.30 |
| NCAM1 | Neural cell adhesion molecule 1 | 0.206 | −1.20 | BCR | Breakpoint cluster region | 0.207 | 1.22 |
| ND4 | Mitochondrially encoded NADH dehydrogenase 4 | 0.232 | 1.15 | MLC1 | Megalencephalic leukoencephalopathy with subcortical cysts 1 | 0.208 | 2.52 |
| NR1D1 | Nuclear receptor subfamily 1, group D, member 1 | 0.233 | −1.28 | GABRA5 | Gamma-aminobutyric acid (GABA) A receptor, alpha 5 | 0.213 | −1.78 |
Top 10 changes in gene expression levels between treated (10 µM amphetamine, 10 µM cocaine, 1 mM lithium and 5 mM sodium valproate) and untreated conditions measured using qPCR arrays (Human Mood Disorder 96 StellARrayTM) and Global Pattern Recognition (GPR) statistical analysis. Fold change values are represented as treated conditions normalised to the drug vehicle. Bold font indicates significant changes in gene expression, p<0.05.
Following treatment with the mood stabiliser sodium valproate, 8 genes were significantly (p<0.05) up- or down-regulated compared to the vehicle control; 2 up-regulated (JUN and PAFAH1B3) and 6 down-regulated (DRD3, GAD1, NRG1, PER3, RELN and RGS4). When compared to the results obtained after treatment with another common mood stabiliser, lithium, similarities in the gene expression profile with respect to the top 10 altered genes were observed; namely down-regulation of GAD1, NRG1, PER3, RELN and RGS4, but, only GAD1 reached statistical significance at this time point for lithium treatment. In addition, FOS was significantly down-regulated in response to lithium. Treatment with the two psychomotor stimulants cocaine and amphetamine demonstrated no statistically significant changes in gene expression following 1 h treatment. Furthermore the genes with the lowest p-values were distinct between the psychostimulants apart from MOBP (Table 2) demonstrating that these drugs might be preferentially targeting distinct pathways for their action. However due to the low p-values obtained under these experimental conditions we did not pursue their analysis further.
3.2. Network analysis of genes significantly modulated in response to mood stabilisers
To further explore potential gene networks important in the response to drug challenge, we analysed only the genes whose expression was most affected by lithium and sodium valproate using the Analyse Networks (Transcription Factors) algorithm from MetaCore™. This generates sub-networks through relative enrichment of the uploaded dataset based on the presence of transcription factors and/or receptor targets within the original input file. The gene set used was composed of GAD1, NRG1, PER3, RELN, RGS4, PAFAH1B3, DRD3, FOS and JUN, the first five of which were observed for both lithium and sodium valproate and the remaining were those significantly modified in response to either exposure.
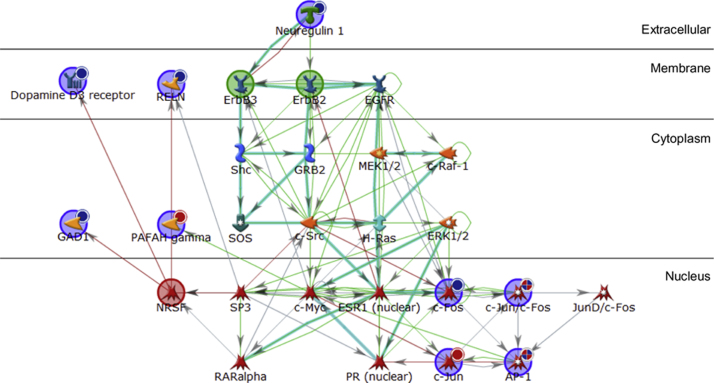
A network containing NRSF, ErbB2 and ErbB3 as seed nodes was the highest ranked using this approach, and was defined as genes/proteins uploaded from experimental datasets or genes/proteins directly linked to uploaded gene lists from which networks are built (Fig. 1). It included 7 of our 9 input genes (DRD3, FOS, GAD1, JUN, NRG1, PAFAH1B3 and RELN) and had a p-value of 5.24×10−29 based on hypergeometric distribution which calculated the probability of a particular pathway map arising by chance given the number of genes across all gene pathways, within a particular pathway or sub-network and within the present experimental dataset. The transcription factors identified as being important regulators of this network were c-Fos and c-Jun (collectively AP-1), c-Myc, ESR1, NRSF, PR, RAR-alpha and SP3.
Fig. 1.
Network analysis of genes significantly modulated in response to mood stabilisers. Genes shown to be significantly up or down regulated in human SH-SY5Y cells in response to 1 h treatment with the mood stabilisers sodium valproate and lithium were uploaded into MetaCore™ for network analysis. The gene list was analysed under the Build Network feature using the Transcription Factor Targets Modelling algorithm. Seed nodes from which the network was built upon are encompassed by a large circle; blue circles represent genes from the experimental data, green circles represent molecules from which the pathway is expanded from and red circles represent molecules on which the pathway terminates. Genes uploaded from the experimental data are also marked with a smaller circle in their top right hand corner; red circles represent genes that were significantly up-regulated, whereas blue circles represent genes significantly down-regulated. Connecting arrows indicate interactions; green arrows represent activation, red arrows represent inhibition and blue arrows are unspecified. Overlaid cyan lines represent canonical pathways. Gene names/symbols within the network from top to bottom, left to right: Neuregulin 1, Dopamine D3 receptor, RELN, ErbB3, ErbB2, EGFR, Shc, GRB2, MEK1/2, c-Raf-1, GAD1 PAFAH gamma, SOS, c-Src, H-Ras, ERK1/2, NRSF, SP3, c-Myc, ESR1 (nuclear), c-Fos, c-Jun/c-Fos, JunD/c-Fos, RARalpha, PR (nuclear) c-Jun, and AP-1. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
As our gene expression data showed that 7/9 of the significantly modulated genes were down-regulated (Table 2) and NRSF which predominantly functions as a transcriptional repressor was identified as an important regulator of our gene set, we addressed predicted NRSF binding sites using ENCODE data from the Transcription Factor ChIP-seq track (The ENCODE Project Consortium, 2011; Rosenbloom et al., 2013) on the UCSC Genome Browser. This identified NRSF binding at the promoter regions (within 5 Kb of the transcriptional start site) of DRD3 (transcript variant a, e and g), FOS, GAD1, JUN, NRG1 (transcript variant HRG-gamma1/2/3, HRG-beta1/d-, 2- and 3b, ndf43/b/c, HRG-alpha and SMDF), PAFAH1B3 and RGS4 (transcript variant 2/3) which, with the exception of JUN and PAFAH1B3, were all down-regulated in response to 1 h treatment with sodium valproate (or lithium with respect to FOS).
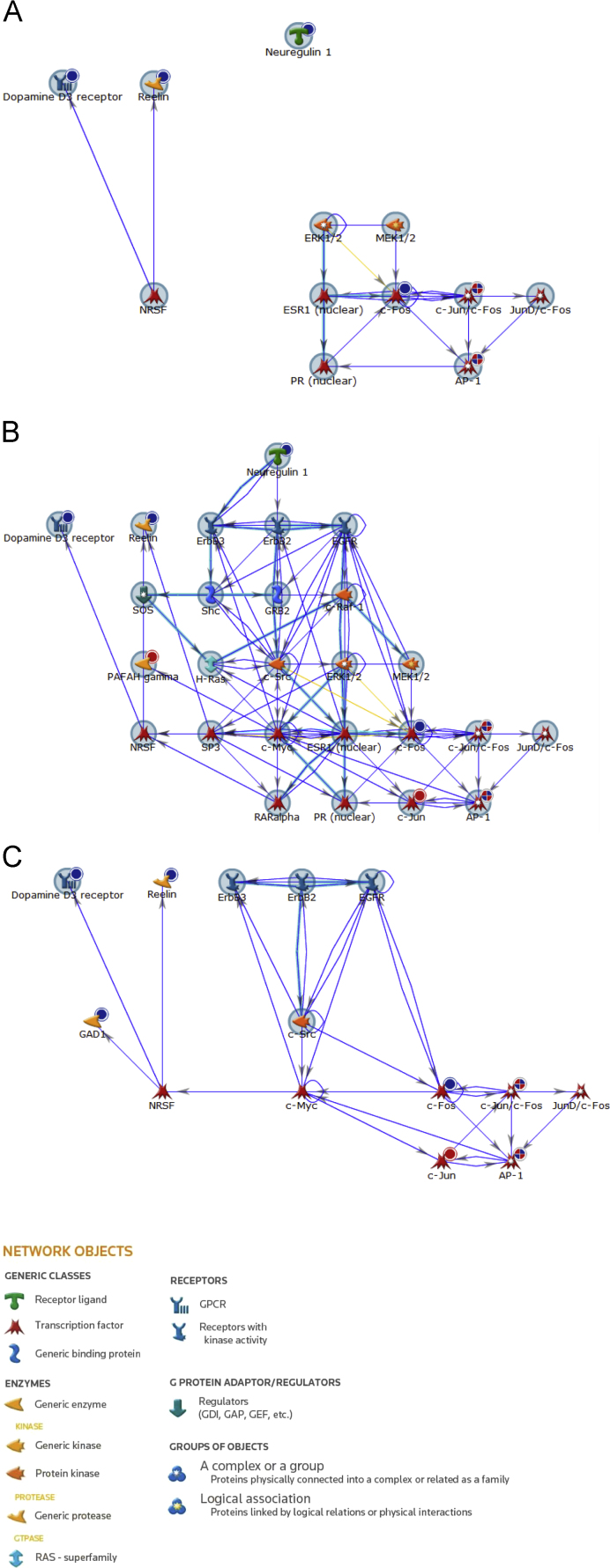
To determine how these regulatory pathways were most relevant for mood disorders, we filtered our dataset using the MetaCore™ ‘Filter by Disease’ feature which traces all of the known associated interactions for a particular disease process. This assigned 46.15% of our network, not unexpectedly to disease processes relating to mood (Fig. 2A). Furthermore, it identified NRSF and ERK1/2 signalling along the oestrogen receptor pathway as important regulators of processes relevant to mood disorders involving this subset of genes. In addition to disorders of the CNS, filtering of our dataset by disease showed there to be significant associations (96.15%) with breast, skin and gastrointestinal neoplasia; GAD1 being the only gene not to be involved in these cancer-related pathologies (Fig. 2B). To further assess which signalling pathways may be operating in response to challenge with these mood stabilisers, we also filtered our experimental network for Drug Responses under the Gene Ontology (GO) Processes filter. This identified the fibroblast growth factor, ERBB and neurotrophin TRK receptor signalling pathways as important cellular responses, with the dopamine D3 receptor, EGFR, ErbB2, ErbB3 and c-Src highlighted as therapeutic targets (Fig. 2C).
Fig. 2.
Network analysis filters for disease and gene ontology processes. The network generated in relation to genes significantly regulated in response to SH-SY5Y cell treatment with sodium valproate and lithium (Fig. 1) was filtered to show the relevant disease pathways (A and B) and gene ontology processes (C). (A and B) Disease processes relevant to mood disorders (A), represents 46.15% of the gene network; and breast, skin and gastrointestinal neoplasms (B), represents 96.15% of the gene network. (C) Gene ontology processes relevant to drug response. Seed nodes from which the network was built upon are encompassed by a large blue circle. Genes uploaded from the experimental data are also marked with a smaller circle in their top right hand corner; red circles represent genes that were significantly up-regulated, whereas blue circles represent genes significantly down-regulated. Connecting blue arrows indicate direct interactions, yellow arrows indicate interactions that are in the base but do not form part of the network and overlaid cyan lines represent canonical pathways. Gene names/symbols within network A, from top to bottom, left to right: Neuregulin 1, Dopamine D3 receptor, Reelin, ERK1/2, MEK1/2, NRSF, ESR1 (nuclear), c-Fos, c-Jun/c-Fos, JunD/c-Fos, PR (nuclear) and AP-1; B, from top to bottom, left to right: Neuregulin 1, Dopamine D3 receptor, Reelin, ErbB3, ErbB2, EGFR, SOS, Shc, GRB2, c-Raf-1, PAFAH gamma, H-Ras, c-Src, ERK1/2, MEK1/2, NRSF, SP3, c-Myc, ESR1 (nuclear), c-Fos, c-Jun/c-Fos, JunD/c-Fos, RARalpha, PR (nuclear), c-Jun and AP-1; and C, from top to bottom, left to right: Dopamine D3 receptor, Reelin, ErbB3, ErbB2, EGFR, GAD1, c-Src, NRSF, c-Myc, c-Fos, c-Jun/c-Fos, JunD/c-Fos, c-Jun and AP-1. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
4. Discussion
Understanding the mechanism of action for a drug to alter the cell phenotype, in addition to the initial cellular targets recognised by the drug, is important for both clinical application and pharmaceutical development. Transcriptome profiling allows for global scale interrogation of potential regulatory mechanisms involved in modulating cellular responses to a particular drug through the use of pathway analysis tools. The aim of this study was to address the effects of mood modifying drugs on the expression profile of a commercially available panel of genes associated with mood disorders by network analysis to compare and contrast their mode of action.
We used two mood stabilisers (lithium and sodium valproate) and two mood stimulants (cocaine and amphetamine). Only the mood stabilisers reached statistical significance and interestingly they shared 5 genes in their top 9 most modified genes, Table 2; we therefore focused on this set of genes for further analysis. Valproate significantly modified 8 genes, lithium only two, GAD1 and FOS, with GAD1 being significantly down-regulated for both drugs. GAD1 encodes one of several forms of glutamic acid decarboxylase which is a key enzyme for the synthesis of the inhibitory neurotransmitter GABA. GAD1 is implicated from both genetic and functional analysis as a modulator of mood (Domschke et al., 2013, Hettema et al., 2006, Karolewicz et al., 2010, Lundorf et al., 2005, Thompson et al., 2009, Weber et al., 2012). FOS and JUN proteins constitute the AP-1 transcription factor complex which was a target for modulation. These factors represent a family of proteins that heterodimerise to regulate the AP-1 DNA site (Quinn, 1991, Quinn et al., 1989a, Quinn et al., 1989b, Takimoto et al., 1989). Lithium and sodium valproate have both been demonstrated to modulate the AP-1 complex (Chen et al., 2008, Ozaki and Chuang, 2002). The genes shared in common by the mood stabilisers sodium valproate and lithium were GAD1, NRG1, PER3, RELN and RGS4. The remainder, DRD3, JUN and PAFAH1B3 were specific for sodium valproate. Although some of these genes were modified with cocaine and amphetamine, the statistical significance was low, certainly lower than all the genes in the 9 most differentially expressed genes in Table 2. We have previously used cocaine and amphetamine in SH-SY5Y and found that we can observe significant changes in genes involved in mental health. For example, recently in the approximate same passage number of cells as used in this experiment, we have demonstrated that cocaine altered the expression of the schizophrenia candidate gene MIR137 (Warburton et al., 2014). However under the current experimental conditions this gene set targeting mood disorders is not responding as robustly to cocaine and amphetamine as lithium and sodium valproate. We therefore attempted to determine whether the significant mood stabiliser gene set defined a specific pathway or network of genes to explain their concerted response to drug exposure.
Pathway analysis using both the Analyse Networks (Transcription Factors) and Filter by Disease algorithms available on the online pathway analysis software MetaCore™ identified the transcription factor NRSF, also termed REST (repressor element-1 silencing transcription factor), to be strongly associated with the pathways supporting these networks of genes. NRSF has a direct association with DRD3, GAD1 and RELN genes based on the network analysis, Fig. 1. Bioinformatic analysis of predicted NRSF binding sites using ENCODE (Encyclopaedia of DNA Elements) data from the Transcription Factor ChIP-seq track (The ENCODE Project Consortium, 2011; Rosenbloom et al., 2013) on the UCSC Genome Browser identified NRSF binding at the promoter regions (within 5 Kb of the transcriptional start site) of the FOS, NRG1 and RGS4 genes, Table 3. This ENCODE analysis also demonstrated NRSF binding sites in similar genomic locations on DRD3, GAD1, JUN, PAFAH1B3 and RELN. Aberrant signalling of NRSF and its target genes has been shown to be involved in the pathophysiology of several CNS disorders including schizophrenia (Loe-Mie et al., 2010), major depressive disorder (Otsuki et al., 2010) and alcoholism and depression (Ukai et al., 2009), with genetic variants influencing age-related cognitive function (Miyajima et al., 2008). More recently it has been highlighted as a major player in Alzheimer׳s disease (Lu et al., 2014). NRSF has the properties to modulate epigenetic factors in its target genes due to its association with a plethora of co-activators, such as members of the SWI/SNF family, which can modify histones by post-translational modifications (Loe-Mie et al., 2010). These epigenetic modifications could result in medium to long term changes in gene expression that underlie drug exposure in addition to the immediate modulation of the transcriptome. Our data suggest that lithium and sodium valproate, with different initial cellular targets, may modulate related signalling pathways leading to overlapping cellular responses mediated in part by the NRSF pathway. It should be noted that we performed this experiment at 1 h postexposure to capture an early response of the cell to the drug. As in any stimulus induction modification of gene expression many of these changes will be transient, especially in the short term for transcription factors such as AP-1 and NRSF. This is in keeping with the transient response of AP-1 and NRSF in stimulus inducible gene expression models we have previously observed at 1 h postexposure (Gillies et al., 2009, Howard et al., 2008, Quinn, 1991, Spencer et al., 2006). A more extensive timescale would perhaps have demonstrated a different or related set of genes, nevertheless, our strategy allowed the observation of the differential gene set acting as a signature for the mood stabilisers and allows for future optimisation.
Filtering our dataset by disease also identified ERK1/2 signalling along with the oestrogen receptor pathway as a potentially important regulatory network for this gene set (Fig. 2). Oestrogen receptor signalling has been well documented in the modulation of behaviours relating to aggression (Nomura et al., 2002), anxiety and depression (Furuta et al., 2013). The action of sex hormones may in part explain why in conditions such as panic disorder these phenotypes are more prevalent among females. Our data would be consistent with GAD1 SNP variation being tentatively associated for the higher susceptibility of females to panic disorder (Weber et al., 2012) via modulation by oestrogen. This oestrogen pathway could overlap with other transcription factor pathways identified in our analysis, for example synergistic action of the oestrogen and AP-1 pathways on gene expression (Fujimoto and Kitamura, 2004). The extended networks identified in this study (AP-1, oestrogen and NRSF) may also work synergistically, for example NRSF activity is important for E2 stimulation of the cell cycle (Bronson et al., 2010) and oestrogen receptor B is enriched at NRSF binding sites (Le et al., 2013). Such interactions between these three pathways can be further modified by the glucocorticoid receptor, so linking these pathways to a major driver of mood (Abramovitz et al., 2008, Karmakar et al., 2013). Glucocorticoid sensitivity is strongly associated with several mood related disorders (Spijker and van Rossum, 2012) and anti-glucocorticoid drugs have been used in the treatment of such conditions (Gallagher et al., 2008, Wolkowitz and Reus, 1999, Wolkowitz et al., 1999). Mood disorder susceptibility has also been linked to glucocorticoid signalling through its modulation of the stress response along the hypothalamic–pituitary–adrenal (HPA) axis (Lupien et al., 2009, Spijker and van Rossum, 2012).
Our data points to a cost effective and rapid assessment of expression changes in selected genes using GPR analysis, which can help delineate the pathways targeted by drugs to modify mood. In particular, we have identified dopamine and glutamine pathways as being important; perhaps not unexpectedly as the gene set is enriched for known genes involved in mood disorders. Alteration in the regulation of these pathways would be expected to modulate mood and is reflected in the range of drugs currently used in targeting these pathways. However the modulation of the AP-1 pathway and the involvement of factors such as NRSF and ERK1/2 highlight a more general modulation of neurotransmitter pathways in response to mood modifying drugs. Our model can therefore be used to determine mechanisms associated with off target and long term affects of particular drugs and can be extrapolated to predict in vivo responses, utilised in the comparison of multiple drug regimes or used as an initial screening process to inform optimal drug design.
Role of funding source
Warburton, Peeney, Bubb and Quinn are funded by the Biotechnology and Biological Sciences Research Council (BBSRC) (BB/F016905/1), Myers and Quinn are funded by the Wellcome Trust (grant no. WT091483/Z) and Savage was funded by the University of Liverpool (UoL). BBSRC, Wellcome Trust and UoL had no role in the experimental design; acquisition, analysis and interpretation of data; writing of the manuscript and decision to submit the paper for publication.
Conflicts of interest
The authors report no conflicts of interest
Contribution of authors
Warburton was involved in experimental design, data acquisition, analysis of data and manuscript preparation. Savage and Myers were involved in experimental design and data acquisition. Peeney was involved in analysis of data. Quinn and Bubb were involved in experimental design, analysis of data and manuscript preparation. All authors have approved the final manuscript.
Acknowledgements
The authors wish to thank Kate Haddley for laboratory assistance and the Biotechnology and Biological Sciences Research Council (BBSRC), Wellcome Trust and University of Liverpool for funding.
References
- The ENCODE Project Consortium, 2011. A user׳s guide to the encyclopedia of DNA elements (ENCODE) PLoS Biol. 9, e1001046. [DOI] [PMC free article] [PubMed]
- Abramovitz L., Shapira T., Ben-Dror I., Dror V., Granot L., Rousso T., Landoy E., Blau L., Thiel G., Vardimon L. Dual role of NRSF/REST in activation and repression of the glucocorticoid response. J. Biol. Chem. 2008;283:110–119. doi: 10.1074/jbc.M707366200. [DOI] [PubMed] [Google Scholar]
- Akilesh S., Shaffer D.J., Roopenian D. Customized molecular phenotyping by quantitative gene expression and pattern recognition analysis. Genome Res. 2003;13:1719–1727. doi: 10.1101/gr.533003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asghari V., Wang J.F., Reiach J.S., Young L.T. Differential effects of mood stabilizers on Fos/Jun proteins and AP-1 DNA binding activity in human neuroblastoma SH-SY5Y cells. Mol. Brain Res. 1998;58:95–102. doi: 10.1016/s0169-328x(98)00107-7. [DOI] [PubMed] [Google Scholar]
- Bronson M.W., Hillenmeyer S., Park R.W., Brodsky A.S. Estrogen coordinates translation and transcription, revealing a role for NRSF in human breast cancer cells. Mol. Endocrinol. 2010;24:1120–1135. doi: 10.1210/me.2009-0436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G., Huang L.-D., Jiang Y.-M., Manji H.K. The mood-stabilizing agent valproate inhibits the activity of glycogen synthase kinase-3. J. Neurochem. 2008;72:1327–1330. doi: 10.1046/j.1471-4159.2000.0721327.x. [DOI] [PubMed] [Google Scholar]
- D’Souza U.M., Powell-Smith G., Haddley K., Powell T.R., Bubb V.J., Price T., McGuffin P., Quinn J.P., Farmer A.E. Allele-specific expression of the serotonin transporter and its transcription factors following lamotrigine treatment in vitro. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2013;162B:474–483. doi: 10.1002/ajmg.b.32178. [DOI] [PubMed] [Google Scholar]
- Di Daniel E., Mudge A.W., Maycox P.R. Comparative analysis of the effects of four mood stabilizers in SH-SY5Y cells and in primary neurons. Bipolar Disord. 2005;7:33–41. doi: 10.1111/j.1399-5618.2004.00164.x. [DOI] [PubMed] [Google Scholar]
- Domschke K., Tidow N., Schrempf M., Schwarte K., Klauke B., Reif A., Kersting A., Arolt V., Zwanzger P., Deckert J. Epigenetic signature of panic disorder: a role of glutamate decarboxylase 1 (GAD1) DNA hypomethylation? Prog. Neuropsychopharmacol. Biol. Psychiatry. 2013;46:189–196. doi: 10.1016/j.pnpbp.2013.07.014. [DOI] [PubMed] [Google Scholar]
- Fujimoto N., Kitamura S. Effects of environmental estrogenic chemicals on AP1 mediated transcription with estrogen receptors alpha and beta. J. Steroid Biochem. Mol. Biol. 2004;88:53–59. doi: 10.1016/j.jsbmb.2003.10.006. [DOI] [PubMed] [Google Scholar]
- Furuta M., Numakawa T., Chiba S., Ninomiya M., Kajiyama Y., Adachi N., Akema T., Kunugi H. Estrogen, predominantly via estrogen receptor alpha, attenuates postpartum-induced anxiety- and depression-like behaviors in female rats. Endocrinology. 2013;154(10):3807–3816. doi: 10.1210/en.2012-2136. Epub 2013 Aug 2. [DOI] [PubMed] [Google Scholar]
- Gallagher P., Malik N., Newham J., Young A.H., Ferrier I.N., Mackin P. Antiglucocorticoid treatments for mood disorders. Cochrane Database of Syst. Rev. 2008 doi: 10.1002/14651858.CD005168.pub2. Issue 1. Art. no.: CD005168. [DOI] [PubMed] [Google Scholar]
- Gillies S., Haddley K., Vasiliou S., Bubb V.J., Quinn J.P. The human neurokinin B gene, TAC3, and its promoter are regulated by Neuron Restrictive Silencing Factor (NRSF) transcription factor family. Neuropeptides. 2009;43:333–340. doi: 10.1016/j.npep.2009.05.004. [DOI] [PubMed] [Google Scholar]
- Hettema J.M., An S.S., Neale M.C., Bukszar J., van den Oord E.J., Kendler K.S., Chen X. Association between glutamic acid decarboxylase genes and anxiety disorders, major depression, and neuroticism. Mol. Psychiatry. 2006;11:752–762. doi: 10.1038/sj.mp.4001845. [DOI] [PubMed] [Google Scholar]
- Hill J., Breen G., Quinn J., Tibu F., Sharp H., Pickles A. Evidence for interplay between genes and maternal stress in utero: monoamine oxidase A polymorphism moderates effects of life events during pregnancy on infant negative emotionality at 5 weeks. Genes Brain Behav. 2013;12:388–396. doi: 10.1111/gbb.12033. [DOI] [PubMed] [Google Scholar]
- Hing B., Davidson S., Lear M., Breen G., Quinn J., McGuffin P., MacKenzie A. A polymorphism associated with depressive disorders differentially regulates brain derived neurotrophic factor promoter IV activity. Biol. Psychiatry. 2012;71:618–626. doi: 10.1016/j.biopsych.2011.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard M.R., Millward-Sadler S.J., Vasilliou A.S., Salter D.M., Quinn J.P. Mechanical stimulation induces preprotachykinin gene expression in osteoarthritic chondrocytes which is correlated with modulation of the transcription factor neuron restrictive silence factor. Neuropeptides. 2008;42:681–686. doi: 10.1016/j.npep.2008.09.004. [DOI] [PubMed] [Google Scholar]
- Jones S., Kauer J.A. Amphetamine depresses excitatory synaptic transmission via serotonin receptors in the ventral tegmental area. J. Neurosci. 1999;19:9780–9787. doi: 10.1523/JNEUROSCI.19-22-09780.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantor L., Park Y.H., Wang K.K., Gnegy M. Enhanced amphetamine-mediated dopamine release develops in PC12 cells after repeated amphetamine treatment. Eur. J. Pharmacol. 2002;451:27–35. doi: 10.1016/s0014-2999(02)02190-8. [DOI] [PubMed] [Google Scholar]
- Karmakar S., Jin Y., Nagaich A.K. Interaction of glucocorticoid receptor (GR) with estrogen receptor (ER) alpha and activator protein 1 (AP1) in dexamethasone-mediated interference of ERalpha activity. J. Biol. Chem. 2013;288:24020–24034. doi: 10.1074/jbc.M113.473819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karolewicz B., Maciag D., O’Dwyer G., Stockmeier C.A., Feyissa A.M., Rajkowska G. Reduced level of glutamic acid decarboxylase-67 kDa in the prefrontal cortex in major depression. Int. J. Neuropsychopharmacol. 2010;13:411–420. doi: 10.1017/S1461145709990587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le T.P., Sun M., Luo X., Kraus W.L., Greene G.L. Mapping ERbeta genomic binding sites reveals unique genomic features and identifies EBF1 as an ERbeta interactor. PLoS One. 2013;8:e71355. doi: 10.1371/journal.pone.0071355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lew G.M. Microtubular tau protein after cocaine in cultured SH-SY5Y human neuroblastoma. Gen. Pharmacol. 1992;23:1111–1113. doi: 10.1016/0306-3623(92)90295-u. [DOI] [PubMed] [Google Scholar]
- Loe-Mie Y., Lepagnol-Bestel A.M., Maussion G., Doron-Faigenboim A., Imbeaud S., Delacroix H., Aggerbeck L., Pupko T., Gorwood P., Simonneau M., Moalic J.M. SMARCA2 and other genome-wide supported schizophrenia-associated genes: regulation by REST/NRSF, network organization and primate-specific evolution. Hum. Mol. Genet. 2010;19:2841–2857. doi: 10.1093/hmg/ddq184. [DOI] [PubMed] [Google Scholar]
- Lu T., Aron L., Zullo J., Pan Y., Kim H., Chen Y., Yang T.H., Kim H.M., Drake D., Liu X.S., Bennett D.A., Colaiacovo M.P., Yankner B.A. REST and stress resistance in ageing and Alzheimer׳s disease. Nature. 2014;507:448–454. doi: 10.1038/nature13163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundorf M.D., Buttenschon H.N., Foldager L., Blackwood D.H., Muir W.J., Murray V., Pelosi A.J., Kruse T.A., Ewald H., Mors O. Mutational screening and association study of glutamate decarboxylase 1 as a candidate susceptibility gene for bipolar affective disorder and schizophrenia. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2005;135B:94–101. doi: 10.1002/ajmg.b.30137. [DOI] [PubMed] [Google Scholar]
- Lupien S.J., McEwen B.S., Gunnar M.R., Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat. Rev. Neurosci. 2009;10:434–445. doi: 10.1038/nrn2639. [DOI] [PubMed] [Google Scholar]
- Miyajima F., Quinn J.P., Horan M., Pickles A., Ollier W.E., Pendleton N., Payton A. Additive effect of BDNF and REST polymorphisms is associated with improved general cognitive ability. Genes Brain Behav. 2008;7:714–719. doi: 10.1111/j.1601-183X.2008.00409.x. [DOI] [PubMed] [Google Scholar]
- Nomura M., Durbak L., Chan J., Smithies O., Gustafsson J.A., Korach K.S., Pfaff D.W., Ogawa S. Genotype/age interactions on aggressive behavior in gonadally intact estrogen receptor beta knockout (betaERKO) male mice. Horm. Behav. 2002;41:288–296. doi: 10.1006/hbeh.2002.1773. [DOI] [PubMed] [Google Scholar]
- Otsuki K., Uchida S., Wakabayashi Y., Matsubara T., Hobara T., Funato H., Watanabe Y. Aberrant REST-mediated transcriptional regulation in major depressive disorder. J. Psychiatr. Res. 2010;44:378–384. doi: 10.1016/j.jpsychires.2009.09.009. [DOI] [PubMed] [Google Scholar]
- Ozaki N., Chuang D.-M. Lithium increases transcription factor binding to AP-1 and cyclic AMP-responsive element in cultured neurons and rat brain. J. Neurochem. 2002;69:2336–2344. doi: 10.1046/j.1471-4159.1997.69062336.x. [DOI] [PubMed] [Google Scholar]
- Pan T., Li X., Xie W., Jankovic J., Le W. Valproic acid-mediated Hsp70 induction and anti-apoptotic neuroprotection in SH-SY5Y cells. FEBS Lett. 2005;579:6716–6720. doi: 10.1016/j.febslet.2005.10.067. [DOI] [PubMed] [Google Scholar]
- Phiel C.J., Zhang F., Huang E.Y., Guenther M.G., Lazar M.A., Klein P.S. Histone deacetylase is a direct target of valproic acid, a potent anticonvulsant, mood stabilizer, and teratogen. J. Biol. Chem. 2001;276:36734–36741. doi: 10.1074/jbc.M101287200. [DOI] [PubMed] [Google Scholar]
- Powell T.R., Schalkwyk L.C., Heffernan A.L., Breen G., Lawrence T., Price T., Farmer A.E., Aitchison K.J., Craig I.W., Danese A., Lewis C., McGuffin P., Uher R., Tansey K.E., D’Souza U.M. Tumor necrosis factor and its targets in the inflammatory cytokine pathway are identified as putative transcriptomic biomarkers for escitalopram response. Eur. Neuropsychopharmacol. 2013;23:1105–1114. doi: 10.1016/j.euroneuro.2012.09.009. [DOI] [PubMed] [Google Scholar]
- Quinn J.P. Variation in the composition of the AP1 complex in PC12 cells following induction by NGF and TPA. Mol. Cell. Neurosci. 1991;2:253–258. doi: 10.1016/1044-7431(91)90052-p. [DOI] [PubMed] [Google Scholar]
- Quinn J.P., Farina A.R., Gardner K., Krutzsch H., Levens D. Multiple components are required for sequence recognition of the AP1 site in the gibbon ape leukemia virus enhancer. Mol. Cell. Biol. 1989;9:4713–4721. doi: 10.1128/mcb.9.11.4713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn J.P., Takimoto M., Iadarola M., Holbrook N., Levens D. Distinct factors bind the AP-1 consensus sites in gibbon ape leukemia virus and simian virus 40 enhancers. J. Virol. 1989;63:1737–1742. doi: 10.1128/jvi.63.4.1737-1742.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn J.P., Warburton A., Myers P., Savage A.L., Bubb V.J. Polymorphic variation as a driver of differential neuropeptide gene expression. Neuropeptides. 2013;47:395–400. doi: 10.1016/j.npep.2013.10.003. [DOI] [PubMed] [Google Scholar]
- Roberts J., Scott A.C., Howard M.R., Breen G., Bubb V.J., Klenova E., Quinn J.P. Differential regulation of the serotonin transporter gene by lithium is mediated by transcription factors, CCCTC binding protein and Y-box binding protein 1, through the polymorphic intron 2 variable number tandem repeat. J. Neurosci. 2007;27:2793–2801. doi: 10.1523/JNEUROSCI.0892-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbloom K.R., Sloan C.A., Malladi V.S., Dreszer T.R., Learned K., Kirkup V.M., Wong M.C., Maddren M., Fang R., Heitner S.G., Lee B.T., Barber G.P., Harte R.A., Diekhans M., Long J.C., Wilder S.P., Zweig A.S., Karolchik D., Kuhn R.M., Haussler D., Kent W.J. ENCODE data in the UCSC Genome Browser: year 5 update. Nucleic Acids Res. 2013;41:D56–D63. doi: 10.1093/nar/gks1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shyu K.G., Wang B.W., Yang Y.H., Tsai S.C., Lin S., Lee C.C. Amphetamine activates connexin43 gene expression in cultured neonatal rat cardiomyocytes through JNK and AP-1 pathway. Cardiovasc. Res. 2004;63:98–108. doi: 10.1016/j.cardiores.2004.02.018. [DOI] [PubMed] [Google Scholar]
- Spencer E.M., Chandler K.E., Haddley K., Howard M.R., Hughes D., Belyaev N.D., Coulson J.M., Stewart J.P., Buckley N.J., Kipar A., Walker M.C., Quinn J.P. Regulation and role of REST and REST4 variants in modulation of gene expression in in vivo and in vitro in epilepsy models. Neurobiol. Dis. 2006;24:41–52. doi: 10.1016/j.nbd.2006.04.020. [DOI] [PubMed] [Google Scholar]
- Spijker A.T., van Rossum E.F. Glucocorticoid sensitivity in mood disorders. Neuroendocrinology. 2012;95:179–186. doi: 10.1159/000329846. [DOI] [PubMed] [Google Scholar]
- Sugden K., Pariante C.M., McGuffin P., Aitchison K.J., D’Souza U.M. Housekeeping gene expression is affected by antidepressant treatment in a mouse fibroblast cell line. J. Psychopharmacol. 2010;24:1253–1259. doi: 10.1177/0269881108099690. [DOI] [PubMed] [Google Scholar]
- Takimoto M., Quinn J.P., Farina A.R., Staudt L.M., Levens D. fos/jun and octamer-binding protein interact with a common site in a negative element of the human c-myc gene. J. Biol. Chem. 1989;264:8992–8999. [PubMed] [Google Scholar]
- Thompson M., Weickert C.S., Wyatt E., Webster M.J. Decreased glutamic acid decarboxylase(67) mRNA expression in multiple brain areas of patients with schizophrenia and mood disorders. J. Psychiatr. Res. 2009;43:970–977. doi: 10.1016/j.jpsychires.2009.02.005. [DOI] [PubMed] [Google Scholar]
- Ukai W., Ishii T., Hashimoto E., Tateno M., Yoshinaga T., Ono T., Watanabe K., Watanabe I., Shirasaka T., Saito T. The common aspects of pathophysiolgy of alcoholism and depression. Nihon Arukoru Yakubutsu Igakkai Zasshi. 2009;44:704–711. [PubMed] [Google Scholar]
- Warburton A., Breen G., Rujescu D., Bubb V.J., Quinn J.P. Characterization of a REST-regulated internal promoter in the schizophrenia genome-wide associated gene MIR137. Schizophr. Bull. 2014 doi: 10.1093/schbul/sbu117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber H., Scholz C.J., Domschke K., Baumann C., Klauke B., Jacob C.P., Maier W., Fritze J., Bandelow B., Zwanzger P.M., Lang T., Fehm L., Strohle A., Hamm A., Gerlach A.L., Alpers G.W., Kircher T., Wittchen H.U., Arolt V., Pauli P., Deckert J., Reif A. Gender differences in associations of glutamate decarboxylase 1 gene (GAD1) variants with panic disorder. PLoS One. 2012;7:e37651. doi: 10.1371/journal.pone.0037651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolkowitz O.M., Reus V.I. Treatment of depression with antiglucocorticoid drugs. Psychosom. Med. 1999;61:698–711. doi: 10.1097/00006842-199909000-00011. [DOI] [PubMed] [Google Scholar]
- Wolkowitz O.M., Reus V.I., Chan T., Manfredi F., Raum W., Johnson R., Canick J. Antiglucocorticoid treatment of depression: double-blind ketoconazole. Biol. Psychiatry. 1999;45:1070–1074. doi: 10.1016/s0006-3223(98)00267-4. [DOI] [PubMed] [Google Scholar]
- Zhang M.M., Xiao C., Yu K., Ruan D.Y. Effects of sodium valproate on synaptic plasticity in the CA1 region of rat hippocampus. Food Chem. Toxicol. 2003;41:1617–1623. doi: 10.1016/s0278-6915(03)00195-9. [DOI] [PubMed] [Google Scholar]