Abstract
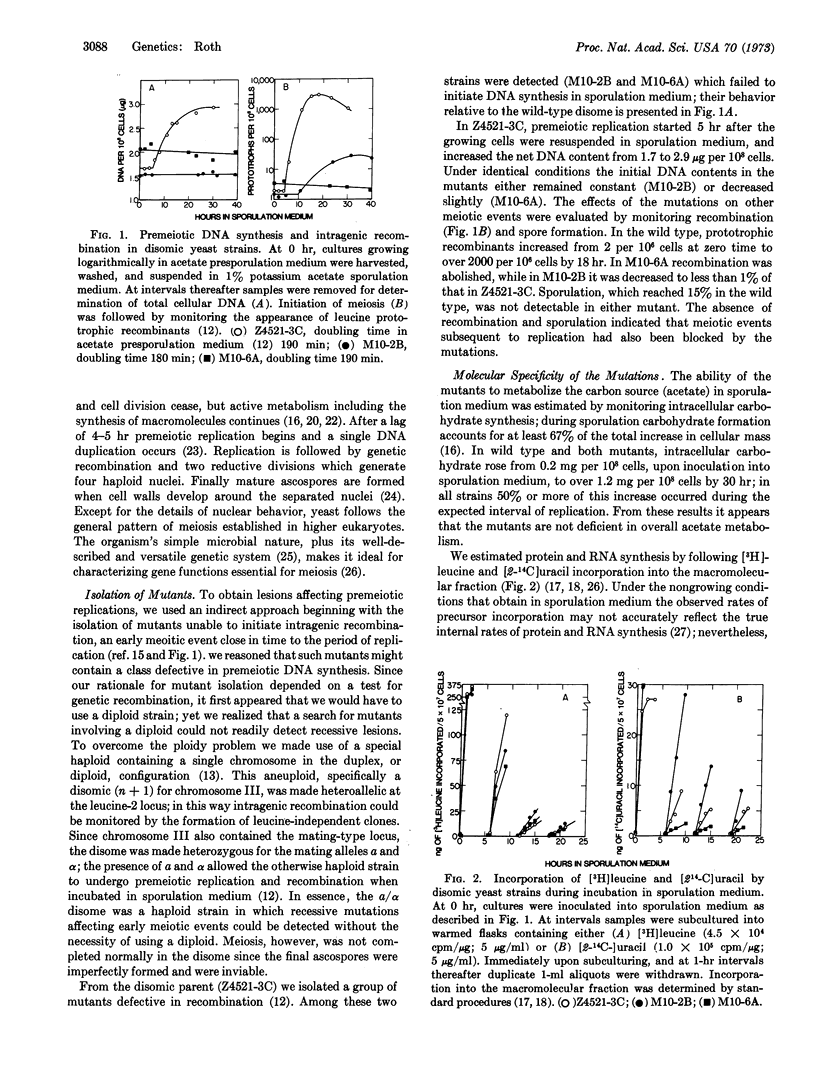
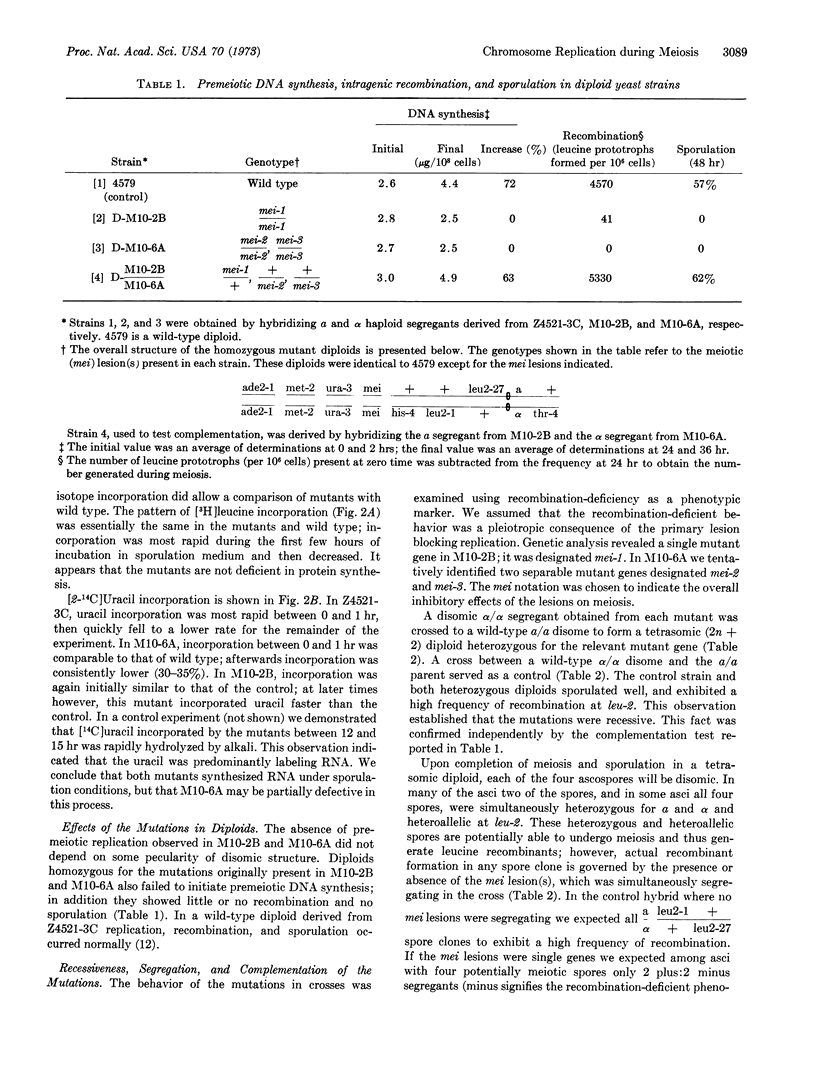
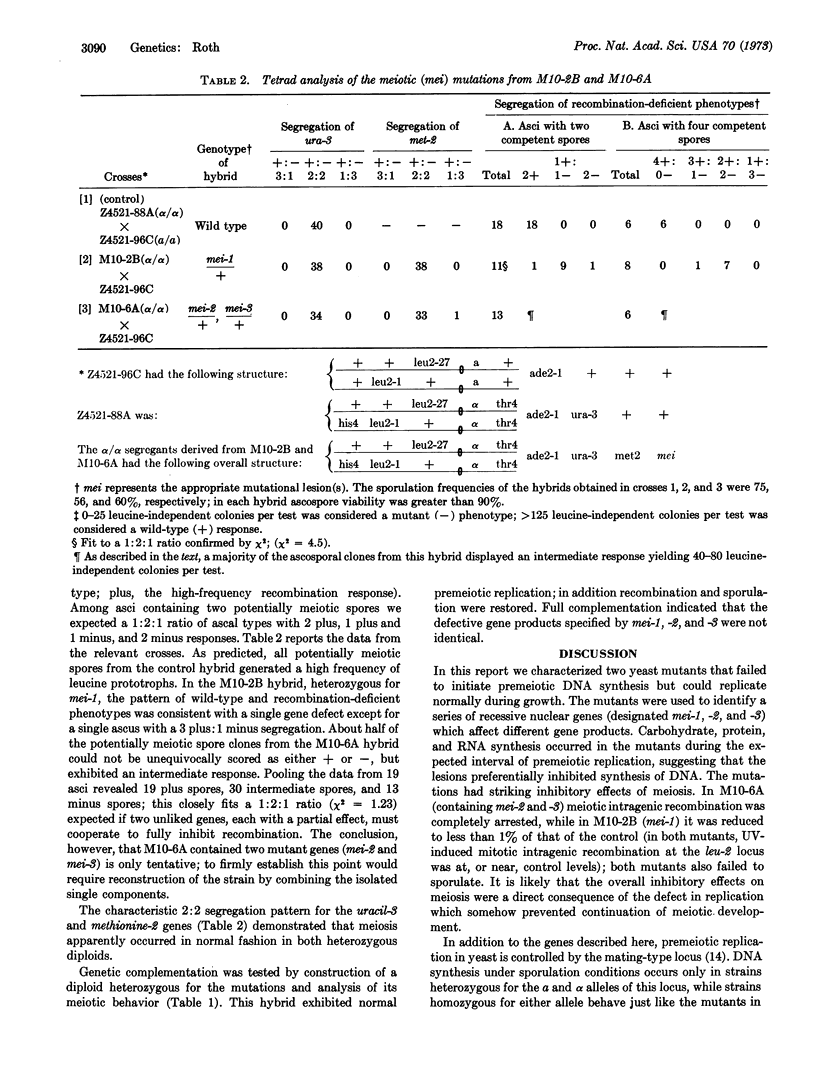
Recent comparisons of chromosome replication in meiotic and mitotic cells have revealed significant differences in both the rate and pattern of DNA synthesis during the final duplication preceding meiosis. These differences suggested that unique gene functions might be required for premeiotic replication that were not necessary for replication during growth. To provide evidence for such functions, we isolated stage-specific mutants in the yeast Saccharomyces cerevisiae which permitted vegetative replication but blocked the round of replication before meiosis. The mutants synthesized carbohydrate, protein, and RNA during the expected interval of premeiotic replication, suggesting that their lesions preferentially affected synthesis of DNA. The mutations blocked meiosis, as judged by a coincident inhibition of intragenic recombination and ascospore formation. The lesions were characterized as recessive nuclear genes, and were designated mei-1, mei-2, and mei-3; complementation indicated that the relevant gene products were not identical.
Keywords: yeast
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Callan H. G. Replication of DNA in the chromosomes of eukaryotes. Proc R Soc Lond B Biol Sci. 1972 Apr 18;181(1062):19–41. doi: 10.1098/rspb.1972.0039. [DOI] [PubMed] [Google Scholar]
- Callan H. G., Taylor J. H. A radioautographic study of the time course of male meiosis in the newt Triturus vulgaris. J Cell Sci. 1968 Dec;3(4):615–626. doi: 10.1242/jcs.3.4.615. [DOI] [PubMed] [Google Scholar]
- Croes A. F. Duplication of DNA during meiosis in baker's yeast. Exp Cell Res. 1966 Feb;41(2):452–454. doi: 10.1016/s0014-4827(66)80151-9. [DOI] [PubMed] [Google Scholar]
- Esposito M. S., Esposito R. E., Arnaud M., Halvorson H. O. Acetate utilization and macromolecular synthesis during sporulation of yeast. J Bacteriol. 1969 Oct;100(1):180–186. doi: 10.1128/jb.100.1.180-186.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposito M. S., Esposito R. E., Arnaud M., Halvorson H. O. Conditional mutants of meiosis in yeast. J Bacteriol. 1970 Oct;104(1):202–210. doi: 10.1128/jb.104.1.202-210.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartwell L. H. Genetic control of the cell division cycle in yeast. II. Genes controlling DNA replication and its initiation. J Mol Biol. 1971 Jul 14;59(1):183–194. doi: 10.1016/0022-2836(71)90420-7. [DOI] [PubMed] [Google Scholar]
- Hartwell L. H. Macromolecule synthesis in temperature-sensitive mutants of yeast. J Bacteriol. 1967 May;93(5):1662–1670. doi: 10.1128/jb.93.5.1662-1670.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotta Y., Stern H. Analysis of DNA synthesis during meiotic prophase in Lilium. J Mol Biol. 1971 Feb 14;55(3):337–355. doi: 10.1016/0022-2836(71)90322-6. [DOI] [PubMed] [Google Scholar]
- Kofman-Alfaro S., Chandley A. C. Meiosis in the male mouse. An autoradiographic investigation. Chromosoma. 1970;31(4):404–420. doi: 10.1007/BF00285832. [DOI] [PubMed] [Google Scholar]
- Mills D. Effect of pH on adenine and amino acid uptake during sporulation in Saccharomyces cerevisiae. J Bacteriol. 1972 Oct;112(1):519–526. doi: 10.1128/jb.112.1.519-526.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moens P. B., Rapport E. Spindles, spindle plaques, and meiosis in the yeast Saccharomyces cerevisiae (Hansen). J Cell Biol. 1971 Aug;50(2):344–361. doi: 10.1083/jcb.50.2.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roman H., Sands S. M. Heterogeneity of Clones of Saccharomyces Derived from Haploid Ascospores. Proc Natl Acad Sci U S A. 1953 Mar;39(3):171–179. doi: 10.1073/pnas.39.3.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth R. Carbohydrate accumulation during the sporulation of yeast. J Bacteriol. 1970 Jan;101(1):53–57. doi: 10.1128/jb.101.1.53-57.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth R., Fogel S. A system selective for yeast mutants deficient in meiotic recombination. Mol Gen Genet. 1971;112(4):295–305. doi: 10.1007/BF00334431. [DOI] [PubMed] [Google Scholar]
- Roth R., Halvorson H. O. Sporulation of yeast harvested during logarithmic growth. J Bacteriol. 1969 May;98(2):831–832. doi: 10.1128/jb.98.2.831-832.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth R., Lusnak K. DNA synthesis during yeast sporulation: genetic control of an early developmental event. Science. 1970 Apr 24;168(3930):493–494. doi: 10.1126/science.168.3930.493. [DOI] [PubMed] [Google Scholar]
- SHERMAN F., ROMAN H. Evidence for two types of allelic recombination in yeast. Genetics. 1963 Feb;48:255–261. doi: 10.1093/genetics/48.2.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SWIFT H. H. The desoxyribose nucleic acid content of animal nuclei. Physiol Zool. 1950 Jul;23(3):169–198. doi: 10.1086/physzool.23.3.30152074. [DOI] [PubMed] [Google Scholar]
- SWIFT H. The constancy of desoxyribose nucleic acid in plant nuclei. Proc Natl Acad Sci U S A. 1950 Nov;36(11):643–654. doi: 10.1073/pnas.36.11.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaffer B., Brearley I., Littlewood R., Fink G. R. A stable aneuploid of Saccharomyces cerevisiae. Genetics. 1971 Apr;67(4):483–495. doi: 10.1093/genetics/67.4.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simchen G., Piñon R., Salts Y. Sporulation in Saccharomyces cerevisiae: premeiotic DNA synthesis, readiness and commitment. Exp Cell Res. 1972 Nov;75(1):207–218. doi: 10.1016/0014-4827(72)90538-1. [DOI] [PubMed] [Google Scholar]
- Stern H., Hotta Y. DNA synthesis in relation to chromosome pairing and chiasma formation. Genetics. 1969;61(1 Suppl):27–39. [PubMed] [Google Scholar]
- TAYLOR J. H., McMASTER R. D. Autoradiographic and microphotometric studies of desoxyribose nucleic acid during microgametogenesis in Lilium longiflorum. Chromosoma. 1954;6(6-7):489–521. doi: 10.1007/BF01259951. [DOI] [PubMed] [Google Scholar]
- Taylor J. H., Woods P. S., Hughes W. L. THE ORGANIZATION AND DUPLICATION OF CHROMOSOMES AS REVEALED BY AUTORADIOGRAPHIC STUDIES USING TRITIUM-LABELED THYMIDINEE. Proc Natl Acad Sci U S A. 1957 Jan 15;43(1):122–128. doi: 10.1073/pnas.43.1.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WIMBER D. E., PRENSKY W. AUTORADIOGRAPHY WITH MEIOTIC CHROMOSOMES OF THE MALE NEWT (TRITURUS VIRIDESCENS) USING H3-THYMIDINE. Genetics. 1963 Dec;48:1731–1738. doi: 10.1093/genetics/48.12.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]



