Left untreated, pediatric obstructive sleep apnea (OSA) can lead to significant morbidity and several adverse effects on growth, behaviour and cardiopulmonary function. Children with craniofacial abnormalities present from birth or as a result of surgical interventions are particularly at increased risk for OSA. Despite its adverse effects, however, little is known about the incidence of OSA in this high-risk population. This prospective study used a validated tool to predict the incidence of moderate to severe OSA in a group of consecutive patients at a pediatric tertiary care centre in the United States.
Keywords: Cleft lip/palate, Obstructive sleep apnea, Pediatric Sleep Questionnaire
Abstract
OBJECTIVE:
To determine the incidence of obstructive sleep apnea (OSA) in children with isolated cleft lip and/or palate (CL/P).
METHODS:
The present prospective study was performed at a pediatric tertiary care centre. Consecutive patients evaluated at the cleft clinic from January 2011 to August 2013 were identified. Patients’ families prospectively completed the Pediatric Sleep Questionnaire (PSQ), a validated tool used to predict moderate to severe OSA. Patients with CL/P and an underlying syndrome or other craniofacial diagnosis were excluded. A positive OSA screen was recorded if the ratio of positive to total responses was >0.33. Risk factors associated with a positive screen were identified using the Student’s t or ANOVA test.
RESULTS:
A total of 867 patients completed the PSQ, 489 of whom with isolated CL/P met inclusion criteria. The mean age was 8.4 years. The overall incidence of positive screening was 14.7%. The most commonly reported symptoms among positive screeners were ‘fidgets with hands or feet’ (73.6%), ‘interrupts others’ (69.4%) and ‘mouth breather during the day’ (69.4%). The most sensitive items were ‘stops breathing during the night’ and ‘trouble breathing during sleep’, with positive predictive values of 0.78 and 0.67, respectively. Sex, body mass index, ancestry and cleft type were not significantly associated with increased risk for positive screening.
CONCLUSION:
One in seven children with isolated CL/P screened positively for OSA according to the PSQ. This finding highlights the potential importance of routine screening in this at-risk group.
Abstract
OBJECTIF :
Déterminer l’incidence d’apnée obstructive du sommeil (AOS) chez les enfants ayant une fente labiale ou palatine isolée (F/LP).
MÉTHODOLOGIE :
La présente étude prospective a été effectuée dans un centre de soins tertiaires. Des patients consécutifs évalués à la clinique de fente labiale ou palatine entre janvier 2011 et août 2013 ont été repérés. Les familles des patients ont rempli prospectivement un questionnaire sur le sommeil pédiatrique (PSQ), un outil validé utilisé pour prédire l’AOS modérée à grave. Les patients ayant une F/LP et un syndrome sous-jacent ou un autre diagnostic crâniofacial étaient exclus. Le dépistage d’AOS était positif si le ratio entre la réponse positive et les réponses totales était supérieur à 0,33. Les facteurs de risque de dépistage positif ont été déterminés à l’aide du test t de Student ou du test ANOVA.
RÉSULTATS :
Au total, 867 patients ont rempli le PSQ, dont 489 avaient une F/LP qui respectait les critères d’inclusion. Ils avaient un âge moyen de 8,4 ans. L’incidence globale de dépistage positif s’élevait à 14,7 %. Les symptômes les plus déclarés chez les personnes dépistées comme positives étaient « agite les mains ou les pieds » (73,6 %), « interrompt » (69,4 %) et « respire par la bouche pendant la journée » (69,4 %). Les faits les plus inquiétants étaient « arrête de respirer pendant la nuit et « a de la difficulté à respirer pendant qu’il dort », dont les valeurs prédictives positives étaient respectivement de 0,78 et de 0,67. Le sexe, l’indice de masse corporelle, l’ascendance et le type de fente ne s’associaient pas de manière significative à un risque accru de dépistage positif.
CONCLUSION :
Un enfant sur sept ayant une F/LP isolée était positif à l’AOS selon le PSQ. Cette observation fait ressortir l’importance potentielle du dépistage systématique dans ce groupe à haut risque.
Recent prospective studies evaluating the prevalence of obstructive sleep apnea (OSA) in the general pediatric population using polysomnography (PSG) have estimated the prevalence to range from 1.2% to 5.8% (1–3). Pediatric OSA is an important cause of morbidity and, if untreated, can lead to adverse effects on growth, behaviour, cognition and cardiopulmonary function (4). Children with craniofacial deformities, including cleft lip and palate (CL/P), are at increased risk for OSA due to abnormalities in the naso- and oropharyngeal anatomy present from birth or acquired as a result of surgical intervention (5,6). Children with CL/P commonly exhibit midface hypoplasia and a retrognathic maxilla, which persist after surgical intervention (7,8). Moreover, surgical treatments for oral rehabilitation include palato- and pharyngoplasty, which have been shown to increase OSA risk (6–8). However, despite its adverse effects, little is known about the incidence of OSA in this high-risk population.
Despite the paucity of literature, the American Academy of Pediatrics acknowledges an increased risk for OSA in children with CL/P (9). Previous studies involving children with CL/P are limited by highly heterogeneous study populations, small patient cohorts and specific surgical interventions. From an epidemiological perspective, children with isolated, nonsyndromic CL/P constitute the majority of patients seen at most cleft clinics (10). Thus, it makes empirical sense to characterize the risk for OSA in this specific population. Furthermore, although PSG remains the gold standard for OSA diagnosis, issues of cost and access to care commonly limit the clinical utility of this tool. Thus, it has been suggested that the majority of children in the United States are treated for OSA based purely on clinical assessment without a PSG diagnosis (11).
In the present study, we used a validated screening tool to assess the incidence of OSA symptoms in children with isolated, nonsyndromic CL/P treated at a large urban cleft centre. We hypothesized that this patient population is at high risk for positive OSA screening. Secondary objectives aimed to characterize clinical and demographic variables that increase risk for positive OSA screening.
METHODS
An Institutional Review Board-approved retrospective chart review was performed on consecutive patients evaluated at the Cleft Clinic at the Children’s Hospital of Philadelphia (Pennsylvania, USA) between January 2011 and August 2013. As part of standard clinical care, the Sleep-Related Breathing Disorder subscale of the Pediatric Sleep Questionnaire (PSQ) was prospectively administered to all patients cared for at the Cleft Clinic during the study period. This validated tool is a parent-completed survey that predicts moderate to severe OSA with a sensitivity of 83% and a specificity of 87% in otherwise healthy children (12). The PSQ consists of 22 ‘yes/no’ questions regarding symptoms of sleep-disordered breathing, daytime sleepiness and behavioural issues. Questionnaires were scored by the number of positive responses; a ratio of positive to total responses >0.33 was considered to be a positive OSA screen. Unanswered questions were eliminated from the survey so that ratios were generated only from the number of answered questions. In the event that an answer other than ‘yes’ or ‘no’ was recorded, the answer was considered to be a positive response unless clear evidence argued to the contrary. For example, a written answer response of ‘sometimes’ was considered a positive response equivalent to ‘yes’.
A chart review was performed on all patients who completed the PSQ to obtain demographic information, clinical diagnoses and surgical history. Demographic variables included age at time of survey, sex, body mass index (BMI) and ancestry. For purposes of comparison, BMI was normalized to age-matched control data from the Centers for Disease Control & Prevention (Georgia, USA) and reported as a percentile. Measured weight was used if it was collected within six months of PSQ administration. Race was reported as Caucasian, African American, Hispanic or Asian. Only nonsyndromic children with isolated CL/P who were otherwise healthy were included in the present study. Patients with underlying chromosomal abnormalities or other craniofacial disorders were excluded. The diagnosis of CL/P was classified as isolated cleft lip (CL), submucosal cleft palate, and CL/P according to the Veau classification: cleft of the soft palate only (Veau I), cleft of the soft and hard palate (Veau II), unilateral complete CL/P (Veau III), and bilateral complete CL/P (Veau IV). Surgical status was documented based on the timing of surgical procedure relative to administration of the PSQ. Surgical procedures for primary cleft closure included Furlow double-reversing Z-palatoplasty and Veau-Wardill-Kilner V-Y pushback. Revision procedures included posterior pharyngeal flap (PPF) and sphincter pharyngoplasty (SPP). For purposes of comparison according to surgical status or clinical diagnosis, the Student’s t test or ANOVA test with Tukey’s method was used where appropriate. All statistical calculation was performed using STATA version 13 (StataCorp, USA); P<0.05 was considered to be statistically significant.
RESULTS
A total of 867 patients seen in the clinic were administered the PSQ during the study period and 489 with isolated CL/P met inclusion criteria. The mean (± SD) age at time of PSQ administration was 8.4±4.3 years (range two to 18 years). The cohort descriptors are shown in Table 1. The study population was predominately male (59.5%) and Caucasian (60.7%). One-quarter of the cohort was Asian (25.9%). Nearly one-half of the subjects had a Veau class III cleft (49.7%) and nearly one-quarter had a Veau class IV cleft (23.2%).
TABLE 1.
Patient demographics at time of administration of the Pediatric Sleep Questionnaire (n=489)
| Patient characteristic | |
|---|---|
| Age, years | |
| 2–4 | 130 (26.6) |
| 5–9 | 196 (40.1) |
| 10–13 | 90 (18.4) |
| 14–18 | 73 (14.9) |
| Sex, male/female, n/n (%/%) | 291/198 (59.5/40.5) |
| Body mass index, percentile* | |
| 0–20 | 89 (18.7) |
| 21–40 | 78 (16.4) |
| 41–60 | 88 (18.5) |
| 61–80 | 110 (23.2) |
| 81–100 | 110 (23.2) |
| Ancestry | |
| Caucasian | 298 (60.7) |
| African American | 36 (7.5) |
| Hispanic | 29 (5.9) |
| Asian | 126 (25.9) |
| Cleft type | |
| Cleft lip | 23 (4.7) |
| Submucous cleft palate | 14 (2.9) |
| †Veau I | 33 (6.7) |
| †Veau II | 63 (12.8) |
| †Veau III | 243 (49.7) |
| †Veau IV | 113 (23.2) |
| Surgical status | |
| Posterior pharyngoplasty | 26 (5.3) |
| Sphincter pharyngoplasty | 9 (1.8) |
| Tonsillectomy | 9 (1.8) |
| Adenoidectomy | 10 (2.0) |
| Combined tonsillectomy + adenoidectomy | 14 (2.9) |
Data presented as n (%) unless otherwise indicated.
14 participants did not have available body mass index data;
Veau classifications: Veau I – cleft of the soft palate only; Veau II – cleft of the soft and hard palate; Veau III – unilateral complete cleft lip and palate; Veau IV – bilateral complete celft lip and palate
Of children with nonsyndromic CL/P, 14.7% had a positive screen for OSA. Table 2 summarizes the prevalence of positive OSA screening according to age and sex. There was no conferred risk for positive screening according to either sex or age group in the cohort (Figure 1 and Table 2). Table 3 summarizes the prevalence of positive OSA screening according to BMI percentile while Table 4 summarizes according to ancestry. Neither higher BMI percentile nor ancestry were associated with increased positive OSA screening. Moreover, there were no significant differences between cleft type and positive OSA screening (Table 5).
TABLE 2.
Prevalence of positive obstructive sleep apnea (OSA+) screening according to sex and age group
| Age, years | Male/female, n/n | Patients with OSA+ screen, n (%) | OSA+ screen | P | |
|---|---|---|---|---|---|
|
| |||||
| Male | Female | ||||
| 2–4 | 68/61 | 13 (10.1) | 9 | 4 | 0.21 |
| 5–9 | 124/74 | 25 (12.6) | 17 | 8 | 0.60 |
| 10–13 | 51/38 | 13 (14.6) | 7 | 6 | 0.79 |
| 14–18 | 48/25 | 3 (4.1) | 1 | 2 | 0.24 |
| Total | 489 | 54 (11.0) | 34 | 20 | 0.58 |
Figure 1).
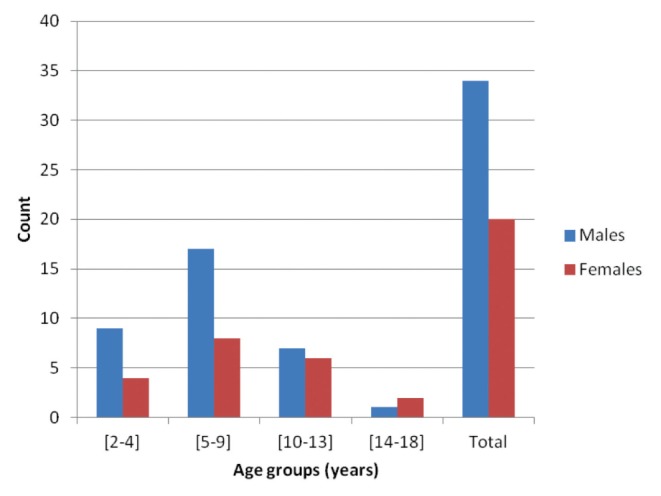
Distribution of positive obstructive sleep apnea screening according to sex and age group. No significant differences between age, sex and positive obstructive sleep apnea screen (all P>0.21)
TABLE 3.
Prevalence of pediatric positive obstructive sleep apnea (OSA+) screening according to body mass index percentile
| Body mass index percentile | n | OSA+ screen, n (%) |
|---|---|---|
| 0–20 | 89 | 8 (9.0) |
| 21–40 | 78 | 7 (9.0) |
| 41–60 | 88 | 11 (12.5) |
| 61–80 | 110 | 13 (11.8) |
| 81–100 | 110 | 15 (13.6) |
TABLE 4.
Prevalence of Pediatric Sleep Questionnaire-diagnosed obstructive sleep apnea (OSA) according to ancestry
| Ancestry | n | Patients with OSA+ screen, n (%) |
|---|---|---|
| Caucasian | 298 | 31 (10.4) |
| African American | 36 | 6 (16.7) |
| Hispanic | 29 | 1 (3.4) |
| Asian | 126 | 17 (13.5) |
+ Positive
TABLE 5.
Patient presentation of positive obstructive sleep apnea (OSA+) screening according to age and cleft type
| Cleft type | OSA+ screen, n | Age at Pediatric Sleep Questionnaire, years, n (%) | |||
|---|---|---|---|---|---|
|
| |||||
| 2–4 | 5–9 | 10–13 | 14–18 | ||
| Submucous cleft palate | 0 | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Cleft lip | 4 | 1 (25.0) | 1 (25.0) | 2 (50.0) | 0 (0) |
| *Veau I | 4 | 1 (25.0) | 2 (50.0) | 1 (25.0) | 0 (0) |
| *Veau II | 5 | 1 (20.0) | 3 (60.0) | 1 (20.0) | 0 (0) |
| *Veau III | 28 | 7 (25.0) | 12 (42.9) | 7 (25.0) | 2 (7.1) |
| *Veau IV | 13 | 3 (23.1) | 7 (53.8) | 2 (15.4) | 1 (7.7) |
There were no significant differences between type of cleft and OSA+ screening.
Veau classifications: Veau I – cleft of the soft palate only; Veau II – cleft of the soft and hard palate; Veau III – unilateral complete cleft lip and palate; Veau IV – bilateral complete celft lip and palate
The frequency of individual OSA symptoms was evaluated (Table 6). The most commonly reported symptoms in the general population were ‘mouth breather during the day’ (29.5%), ‘interrupts others’ (27.2%) and ‘fidgets with hands or feet’ (25.6%). In children with a positive screen, the most common symptoms were ‘fidgets with hands or feet’ (73.6%), ‘interrupts others’ (69.4%), ‘mouth breather during the day’ (69.4%) and ‘interrupts others’ (69.4%). Questions with the highest positive predictive values were ‘stops breathing during the night’ and ‘trouble breathing during sleep’, with values of 0.78 and 0.67, respectively.
TABLE 6.
Frequency of individual obstructive sleep apnea symptoms in patients with cleft lip and palate
| Symptom | Frequency of symptoms, % | ||
|---|---|---|---|
|
| |||
| All patients | Positive screeners | Negative screeners | |
| Snores more than one-half the time | 21.9 | 61.1 | 13.7 |
| Always snores | 9.7 | 29.2 | 5.3 |
| Snores loudly | 13.1 | 45.8 | 6.2 |
| ‘Heavy’ or loud breathing during sleep | 23.5 | 59.7 | 15.6 |
| Trouble breathing during sleep | 4.1 | 16.7 | 1.4 |
| Stops breathing during the night | 3.7 | 19.4 | 1.0 |
| Mouth breather during the day | 29.5 | 69.4 | 21.3 |
| Wakes in the morning with a dry mouth | 22.1 | 58.3 | 14.1 |
| Occasionally wets the bed | 20.0 | 33.3 | 17.0 |
| Wakes up feeling unrefreshed | 14.7 | 38.9 | 9.1 |
| Problem with sleepiness during the day | 9.7 | 27.8 | 6.5 |
| Others comment on child appearing sleepy | 2.5 | 9.7 | 1.2 |
| Hard to wake in the morning | 15.4 | 38.9 | 10.3 |
| Wakes in the morning with a headache | 4.1 | 16.7 | 1.7 |
| Ever stopped growing at a normal rate | 4.4 | 15.3 | 2.2 |
| Overweight | 3.2 | 6.9 | 2.4 |
| Does not seem to listen when spoken to directly | 16.6 | 59.7 | 8.6 |
| Difficulty organizing tasks | 12.2 | 47.2 | 5.5 |
| Easily distracted | 22.8 | 69.4 | 14.6 |
| Fidgets with hands or feet | 25.6 | 73.6 | 17.3 |
| ‘On the go’ or ‘driven by a motor’ | 24.7 | 62.5 | 17.3 |
| Interrupts others | 27.2 | 69.4 | 19.4 |
In contrast, children with a negative OSA screen most commonly reported ‘mouth breather during the day’ (21.3%), ‘interrupts others’ (19.4%), ‘fidgets with hands or feet’ (17.3%), ‘on the go or driven by a motor’ (17.3%) and ‘occasionally wets the bed’ (17.0%). Questions least predictive of a positive PSQ screen were ‘fidgets with hands and feet’ and ‘easily distracted’, with negative predictive values of 0.95. Additionally, questions most commonly blank were ‘mouth breather during the day’ (3.5%), ‘wakes in the morning with a dry mouth’ (3.7%) and ‘occasionally wets the bed’ (3.3%).
Thirty-two patients underwent PPF surgery before taking the PSQ (6.5%), and 12 underwent dynamic SPP. The mean time from surgery to completion of the PSQ was 5.83 and 5.56 years, respectively; 1.8% of patients underwent tonsillectomy before OSA screening and 2.0% underwent adenoidectomy. The mean time from surgery to completion of the PSQ was 4.82 and 4.61 years, respectively; 2.9% of patients underwent combined adenotonsillectomy. None of the interventions were associated with increased risk for positive OSA screening (P>0.05) (Table 7).
TABLE 7.
Surgical procedures affecting the upper airway according to age at time of surgery
| Age, years | Tonsillectomy + adenoidectomy | Adenoidectomy | Tonsillectomy | PPF | SPP |
|---|---|---|---|---|---|
| 2–4 | 9 | 4 | 5 | 9 | 3 |
| 5–9 | 5 | 5 | 2 | 14 | 6 |
| 10–13 | 0 | 1 | 1 | 8 | 2 |
| 14–18 | 0 | 0 | 1 | 1 | 1 |
| Total | 14 | 10 | 9 | 32 | 12 |
Data presented as n. PPF Posterior pharyngoplasty; SPP Sphincter pharyngoplasty
DISCUSSION
The present study used prospective administration of an OSA screening tool with retrospective chart review to report the prevalence of positive OSA screening in a large cohort of children with nonsyndromic CL/P. The prevalence of children with a positive screen was 14.7%. Thus, relative to their unaffected peers, children with CL/P may be seven times more likely to develop OSA symptoms (1–3). Our findings are consistent with the well-documented under-recognition of OSA in children with clefts (13,14). Because pediatric OSA is linked to delays in growth (4), neurodevelopment (15) and adverse metabolic effects (16), it is plausible that delayed recognition of OSA is responsible for increased morbidity in children with CL/P. The majority of studies investigating OSA in children with cleft are limited through the use of heterogeneous cohorts of craniofacial patients and patients undergoing specific surgical interventions (17–19). The present study is the first to report the prevalence of positive OSA screening in a large cohort of children with isolated, nonsyndromic CL/P.
A PSG can distinguish between OSAs and central sleep apneas, and recognize periodic limb movements and other sleep abnormalities that may mimic OSA symptoms. As such, it remains the gold standard in the diagnosis of OSA; however, in cases in which OSA is well documented clinically, PSG may not always be absolutely indicated (4). In addition to its cost, inconvenience and lack of availability, PSG has numerous limitations that undermine its utility. Although PSG can be used to detect sleep abnormalities related to airway obstruction, gas exchange and disturbances in sleep architecture, it has never been validated to predict adverse clinical sequelae or response to medical treatment (20). Thus, even in the absence of PSG confirmation, symptoms of sleep-disordered breathing commonly result in adverse neurocognitive and behavioural consequences. Furthermore, the lack of consistent acquisition between sleep laboratories and standard criteria for abnormal sleep studies further limits the utility of PSG as a diagnostic tool (21).
Thus, an important area of research is the development of effective OSA screening tools, thereby obviating the need for PSG clinically. While initially developed for research, the Chervin Pediatric Sleep Questionnaire (PSQ) is among the best screening tools for pediatric OSA (22) and, as such, is often used in the clinic (23). The PSQ is a 22-question survey that asks questions related to snoring and troubled breathing, daytime sleepiness, inattentiveness and other common symptoms of pediatric OSA. This instrument has not been adequately studied in children with orofacial clefts and our study is the first to report PSQ findings in a large cohort of children with nonsyndromic CL/P. While unique in this homogenous population, several studies have reported PSQ findings from other craniofacial settings. MacLean et al (19) reported an incidence of positive OSA screening in 31.4% in a cohort of children with Pierre Robin sequence and underlying chromosomal abnormalities.
There were many items on the PSQ that were frequently under-reported in the study cohort despite the high prevalence of positive OSA screening. There are several reasons to explain these differences and it must be emphasized that the PSQ was designed and validated for healthy children without CL/P. Children with these disorders have different etiologies for upper airway obstruction that may manifest as differences in ‘normal’ breathing. These differences can significantly alter the PSQ because symptoms of day- and night-time breathing account for approximately one-third of all questions. Additionally, families of children with CL/P may have an altered sensitivity to OSA symptoms as they are exposed to a lifetime of medical and surgical interventions that may alter their expectations. Furthermore, children with CL/P commonly demonstrate increased risk for impairments in neurocognitive function (24,25), which may be less likely related to OSA than in the general pediatric population. Similarly, factors in the general pediatric population commonly associated with increased risk for OSA failed to demonstrate a statistical correlation in our cohort, such as obesity, a well-established and increasingly common risk factor for OSA (26). Additionally, some studies have shown increased prevalence and severity of OSA among African American children relative to their Caucasian peers (27,28). Finally, pediatric OSA has classically been described to exhibit male predominance (28); however, several large studies have found no significant difference in OSA prevalence according to sex (29,30). Again, differences in our results may be due to complex underlying medical conditions unique to this patient population.
OSA is a well-described complication of surgical interventions for the treatment of velopharyngeal insufficiency (17,31,32). Despite possessing normal mandibular architecture, children with CL/P are at greater risk for upper airway obstruction. A deviated nasal septum is common in children with unilateral CL, and often results after surgical intervention (6). Furlow palatoplasty can both lengthen and thicken the palate, and has a tendency to decrease airway space. Additionally, acute airway obstruction is well documented in patients undergoing PPF and SPP. This chronic obstruction can compromise the nasal airway and manifest as OSA. Interestingly, our study did not find an association between these interventions and increased risk for positive OSA screening. This could be secondary to the relatively small cohort of children with nonsyndromic cleft requiring these complex procedures or length of follow-up.
Combined adenotonsillectomy is the most common surgical procedure to address pediatric OSA (33), with more than a half-million surgeries performed annually in the United States alone (34). For children with CL/P, a partial instead of full adenoidectomy is frequently performed to prevent future velopharyngeal insufficiency. While common in the pediatric population, only 2.9% of children in our cohort underwent this procedure. Despite demonstrated benefits of a recent randomized control trial (35), we did not find a protective effect of adenotonsillectomy from positive OSA screening.
There were some limitations to the present study and, thus, future areas for investigation. The first is the nature of the screening tool we used to determine the prevalence of positive OSA screening. The PSQ validated by Chervin et al (12) had a reported sensitivity and specificity of 0.83 and 0.87, respectively, for the diagnosis of moderate to severe OSA in children two to 18 years of age. To validate the PSQ, overnight PSG was used. The American Academy of Pediatrics recognizes that, despite a lack of consensus, PSG is currently the best available tool for the diagnosis of OSA (9). Until better tools are developed, PSG and screening questionnaires based on its reliability must be used in the diagnosis and treatment of OSA. Second, parents commonly omitted questions from the PSQ that did not pertain to their child. For example, the symptom of ‘occasionally wets the bed’ has limited applicability to a child still wearing diapers. This may compromise the utility of the PSQ and is a limitation of paper administration of the PSQ. Additionally, the present study was limited in the fact that a control group was not included for comparison and, as such, current published data were used. A control group would have increased the strength of our study by providing an objective benchmark for questionnaire results specific to our practice. With any retrospective study, our analysis was susceptible to inherent biases in patients presenting to clinic, transfer of care issues and different follow-up periods. Thus, we cannot draw strong conclusions regarding PSQ correlations with clinical variables.
CONCLUSIONS
One of seven children with isolated CL/P screened positively for OSA using PSQ. This is out of proportion to the general pediatric population, and highlights the potential importance of routine screening in this high-risk group. Future work will correlate these findings with diagnostic tests for OSA to determine true prevalence of the disease.
REFERENCES
- 1.Bonuck KA, Chervin RD, Cole TJ, et al. Prevalence and persistence of sleep disordered breathing symptoms in young children: A 6-year population-based cohort study. Sleep. 2011;34:875–84. doi: 10.5665/SLEEP.1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bixler EO, Vgontzas AN, Lin HM, et al. Sleep disordered breathing in children in a general population sample: Prevalence and risk factors. Sleep. 2009;32:731–6. doi: 10.1093/sleep/32.6.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li AM, So HK, Au CT, et al. Epidemiology of obstructive sleep apnoea syndrome in Chinese children: A two-phase community study. Thorax. 2010;65:991–7. doi: 10.1136/thx.2010.134858. [DOI] [PubMed] [Google Scholar]
- 4.Marcus CL, Brooks LJ, Draper KA, et al. Diagnosis and management of childhood obstructive sleep apnea syndrome. Pediatrics. 2012;130:e714–55. doi: 10.1542/peds.2012-1672. [DOI] [PubMed] [Google Scholar]
- 5.Ettinger RE, Oppenheimer AJ, Lau D, et al. Obstructive sleep apnea after dynamic sphincter pharyngoplasty. J Craniofac Surg. 2012;23(7 Suppl 1):1974–6. doi: 10.1097/SCS.0b013e31825b3ba9. [DOI] [PubMed] [Google Scholar]
- 6.Scott AR, Moldan MM, Tibesar RJ, Lander TA, Sidman JD. A theoretical cause of nasal obstruction in patients with repaired cleft palate. Am J Rhinol Allergy. 2011;25:58–60. doi: 10.2500/ajra.2011.25.3544. [DOI] [PubMed] [Google Scholar]
- 7.Hermann NV, Kreiborg S, Darvann TA, Jensen BL, Dahl E, Bolund S. Craniofacial morphology and growth comparisons in children with Robin Sequence, isolated cleft palate, and unilateral complete cleft lip and palate. Cleft Palate Craniofac J. 2003;40:373–96. doi: 10.1597/1545-1569_2003_040_0373_cmagci_2.0.co_2. [DOI] [PubMed] [Google Scholar]
- 8.Rose E, Thissen U, Otten JE, Jonas I. Cephalometric assessment of the posterior airway space in patients with cleft palate after palatoplasty. Cleft Palate Craniofac J. 2003;40:498–503. doi: 10.1597/1545-1569_2003_040_0498_caotpa_2.0.co_2. [DOI] [PubMed] [Google Scholar]
- 9.Section on Pediatric Pulmonology, Subcommittee on Obstructive Sleep Apnea Syndrome American Academy of Pediatrics. Clinical practice guideline: Diagnosis and management of childhood obstructive sleep apnea syndrome. Pediatrics. 2002;109:704–12. doi: 10.1542/peds.109.4.704. [DOI] [PubMed] [Google Scholar]
- 10.Calzolari E, Pierini A, Astolfi G, Bianchi F, Neville AJ, Rivieri F. Associated anomalies in multi-malformed infants with cleft lip and palate: An epidemiologic study of nearly 6 million births in 23 EUROCAT registries. Am J Med Genet A. 2007;143:528–37. doi: 10.1002/ajmg.a.31447. [DOI] [PubMed] [Google Scholar]
- 11.Mitchell RB, Pereira KD, Friedman NR. Sleep-disordered breathing in children: Survey of current practice. Laryngoscope. 2006;116:956–8. doi: 10.1097/01.MLG.0000216413.22408.FD. [DOI] [PubMed] [Google Scholar]
- 12.Chervin RD, Hedger K, Dillon JE, Pituch KJ. Pediatric sleep questionnaire (PSQ): Validity and reliability of scales for sleep-disordered breathing, snoring, sleepiness, and behavioral problems. Sleep Med. 2000;1:21–32. doi: 10.1016/s1389-9457(99)00009-x. [DOI] [PubMed] [Google Scholar]
- 13.MacLean JE, Fitzsimons D, Hayward P, Waters KA, Fitzgerald DA. The identification of children with cleft palate and sleep disordered breathing using a referral system. Pediatr Pulmonol. 2008;43:245–50. doi: 10.1002/ppul.20763. [DOI] [PubMed] [Google Scholar]
- 14.Muntz H, Wilson M, Park A, Smith M, Grimmer JF. Sleep disordered breathing and obstructive sleep apnea in the cleft population. Laryngoscope. 2008;118:348–53. doi: 10.1097/MLG.0b013e318158195e. [DOI] [PubMed] [Google Scholar]
- 15.Gozal D, Kheirandish-Gozal L. Neurocognitive and behavioral morbidity in children with sleep disorders. Curr Opin Pulm Med. 2007;13:505–9. doi: 10.1097/MCP.0b013e3282ef6880. [DOI] [PubMed] [Google Scholar]
- 16.Waters KA, Sitha S, O’brien LM, et al. Follow-up on metabolic markers in children treated for obstructive sleep apnea. Am J Respir Crit Care Med. 2006;174:455–60. doi: 10.1164/rccm.200401-110OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saint Raymond C, Bettega G, Deschaux C, et al. Sphincter pharyngoplasty as a treatment of velopharyngeal incompetence in young people: A prospective evaluation of effects on sleep structure and sleep respiratory disturbances. Chest. 2004;125:864–71. doi: 10.1378/chest.125.3.864. [DOI] [PubMed] [Google Scholar]
- 18.Oosterkamp BC, Remmelink HJ, Pruim GJ, Hoekema A, Dijkstra PU. Craniofacial, craniocervical, and pharyngeal morphology in bilateral cleft lip and palate and obstructive sleep apnea patients. Cleft Palate Craniofac J. 2007;44:1–7. doi: 10.1597/05-175. [DOI] [PubMed] [Google Scholar]
- 19.Maclean JE, Waters K, Fitzsimons D, Hayward P, Fitzgerald DA. Screening for obstructive sleep apnea in preschool children with cleft palate. Cleft Palate Craniofac J. 2009;46:117–23. doi: 10.1597/07-215.1. [DOI] [PubMed] [Google Scholar]
- 20.Jambhekar S, Carroll JL. Diagnosis of pediatric obstructive sleep disordered breathing: Beyond the gold standard. Expert Rev Respir Med. 2008;2:791–809. doi: 10.1586/17476348.2.6.791. [DOI] [PubMed] [Google Scholar]
- 21.Friedman NR. Polysomnography should not be required both before and after adenotonsillectomy for childhood sleep disordered breathing. J Clin Sleep Med. 2007;3:678–80. [PMC free article] [PubMed] [Google Scholar]
- 22.Spruyt K, Gozal D. Pediatric sleep questionnaires as diagnostic or epidemiological tools: A review of currently available instruments. Sleep Med Rev. 2011;15:19–32. doi: 10.1016/j.smrv.2010.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peña-Zarza JA, Osona-Rodriguez de Torres B, Gil-Sanchez JA, Figuerola-Mulet J. Utility of the pediatric sleep questionnaire and pulse oximetry as screening tools in pediatric patients with suspected obstructive sleep apnea syndrome. Sleep Disord. 2012;2012:819035. doi: 10.1155/2012/819035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jocelyn LJ, Penko MA, Rode HL. Cognition, communication, and hearing in young children with cleft lip and palate and in control children: A longitudinal study. Pediatrics. 1996;97:529–34. [PubMed] [Google Scholar]
- 25.Broen PA, Devers MC, Doyle SS, Prouty JM, Moller KT. Acquisition of linguistic and cognitive skills by children with cleft palate. J Speech Lang Hear Res. 1998;41:676–87. doi: 10.1044/jslhr.4103.676. [DOI] [PubMed] [Google Scholar]
- 26.Nevin MA. Pediatric obesity, metabolic syndrome, and obstructive sleep apnea syndrome. Pediatr Ann. 2013;42:205–10. doi: 10.3928/00904481-20130924-11. [DOI] [PubMed] [Google Scholar]
- 27.Stepanski E, Zayyad A, Nigro C, Lopata M, Basner R. Sleep-disordered breathing in a predominantly African-American pediatric population. J Sleep Res. 1999;8:65–70. doi: 10.1046/j.1365-2869.1999.00136.x. [DOI] [PubMed] [Google Scholar]
- 28.Lumeng JC, Chervin RD. Epidemiology of pediatric obstructive sleep apnea. Proc Am Thorac Soc. 2008;5:242–52. doi: 10.1513/pats.200708-135MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wing YK, Hui SH, Pak WM, et al. A controlled study of sleep related disordered breathing in obese children. Arch Dis Child. 2003;88:1043–7. doi: 10.1136/adc.88.12.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sánchez-Armengol A, Fuentes-Pradera MA, Capote-Gil F, et al. Sleep-related breathing disorders in adolescents aged 12 to 16 years: Clinical and polygraphic findings. Chest. 2001;119:1393–400. doi: 10.1378/chest.119.5.1393. [DOI] [PubMed] [Google Scholar]
- 31.Liao YF, Chuang ML, Chen PK, Chen NH, Yun C, Huang CS. Incidence and severity of obstructive sleep apnea following pharyngeal flap surgery in patients with cleft palate. Cleft Palate Craniofac J. 2002;39:312–6. doi: 10.1597/1545-1569_2002_039_0312_iasoos_2.0.co_2. [DOI] [PubMed] [Google Scholar]
- 32.Orr WC, Levine NS, Buchanan RT. Effect of cleft palate repair and pharyngeal flap surgery on upper airway obstruction during sleep. Plast Reconstr Surg. 1987;80:226–32. doi: 10.1097/00006534-198708000-00010. [DOI] [PubMed] [Google Scholar]
- 33.Brietzke SE, Gallagher D. The effectiveness of tonsillectomy and adenoidectomy in the treatment of pediatric obstructive sleep apnea/hypopnea syndrome: A meta-analysis. Otolaryngol Head Neck Surg. 2006;134:979–84. doi: 10.1016/j.otohns.2006.02.033. [DOI] [PubMed] [Google Scholar]
- 34.Bhattacharyya N, Lin HW. Changes and consistencies in the epidemiology of pediatric adenotonsillar surgery, 1996–2006. Otolaryngol Head Neck Surg. 2010;143:680–4. doi: 10.1016/j.otohns.2010.06.918. [DOI] [PubMed] [Google Scholar]
- 35.Marcus CL, Moore RH, Rosen CL, et al. A randomized trial of adenotonsillectomy for childhood sleep apnea. N Engl J Med. 2013;368:2366–76. doi: 10.1056/NEJMoa1215881. [DOI] [PMC free article] [PubMed] [Google Scholar]


