Abstract
An important distinction in research on the neural mechanisms of emotion regulation involves the relatively limited duration of emotional states vs. emotional traits which are defined as the stable tendency to experience particular emotions in daily life. Neuroimaging investigations of the regulation of anger states point to involvement of reciprocal changes in prefrontal cortex and amygdala activity, but the neural substrate of trait anger has received less attention. We used resting-state functional magnetic resonance imaging (rsfMRI) to determine whether variation in the strength of functional connectivity between amygdala and orbitofrontal cortex is associated with trait anger. Sixteen healthy male subjects completed the Speilberger State-Trait Anger Expression Inventory. Correlational analysis for resting-state functional connectivity (RSFC) was conducted with left and right amygdala as separate seed regions. Anger measures were correlated to RSFC involving right and left amygdala on a voxel-by-voxel basis across all subjects. We found that Trait Anger was inversely associated with the strength of RSFC between amygdala and contralateral middle orbitofrontal cortex. The association was stronger for the right amygdala-left orbitofrontal connection. Anger Control, the tendency to try to control expressions of anger, showed the opposite pattern of being positively correlated with amygdala-orbitofrontal connectivity. The present study provides evidence that RSFC in a cortico-limbic circuit might subserve stable differences in anger regulation. Our findings also suggest that RSFC may prove valuable as a trait marker for disorders characterized by emotional dysregulation such as depression, anxiety and personality disorders.
Keywords: functional magnetic resonance imaging, negative emotion, emotion regulation, prefrontal cortex
Introduction
Emotional traits are stable and reliable individual differences in the experience of emotions and are central features of personality. Negative emotional traits (anger, anxiety, depression) are believed to be a core mechanism underlying disorders of mood, anxiety and aggression [1], and are independent risk factors for a variety of stress-related chronic illnesses and premature mortality [2]. Trait anger has been defined as the general tendency to experience anger or to respond with anger when one feels unfairly criticized or treated.
Studies of emotion regulation using neuroimaging methods have primarily focused on state-dependent activation in response to presentation of emotional stimuli. For example, the presentation of threatening facial expressions (fear or anger) reliably elicits activation of the amygdala. [3] Paradigms that involve cognitive or linguistic processing of emotional stimuli, or attempts to modulate emotional response, have implicated a cortico-limbic interplay involving reciprocal connections between the amygdala and areas of prefrontal cortex in emotion regulation. [4] Thus, suppression of negative emotions elicits activation of orbitofrontal, medial, dorsolateral, and ventrolateral regions of prefrontal cortex, as well as dorsal anterior cingulate cortex, coupled with suppression of activity in the amygdala and insula. This functional coupling is disrupted in psychiatric disorders characterized by emotional dysregulation. [5]
Relatively less is known about the neural correlates of stable individual differences in emotion regulation. Recent evidence suggests that intersubject variability in amygdala response to threatening facial expressions is stable over time [6], and that trait differences in personality are associated with this trait-like variability in fMRI responses to threat-related stimuli. [7] Resting-state functional connectivity (RSFC), with its ability to quantify stable functional connections between brain regions within specific neural circuitries, may help to elucidate the role of the neuronal circuitry in emotional traits. In the present study we have utilized rsfMRI to investigate the relationship between functional connectivity within a key neural circuit underpinning emotion and variation in a negative emotional trait. Specifically, we hypothesized that trait anger would be associated with individual variation in RSFC strength in the amygdala-orbitofrontal circuit.
Methods
Participants
Sixteen healthy right-handed males (mean 34 years, S.D. 14.42) provided informed consent. Exclusion criteria included taking psychotropic medications or drugs of abuse, and traumatic brain injury. The study was approved by the Institutional Review Board of the University of Massachusetts Medical School.
Psychometric measures
Subjects completed the Trait Anger and Anger Control subscales of the State-Trait Anger Expression Inventory-2 (STAXI-2, [8]) prior to being scanned. Trait Anger measures the general tendency to experience anger, and Anger Control measures how often an individual attempts to control angry feelings by calming down or control the outward expression of anger. Participants endorse statements on a 4-point scale from 1 (not at all/almost never) to 4 (very much so/almost always). The instrument has good psychometric properties and in the current sample of healthy men STAXI-2 scores fell within the normative range (not shown), which is based on a sample of over 600 healthy men [8].
MRI acquisition
RSFC was assessed by instructing subjects to remain relaxed with eyes closed as fMRI images were continuously collected for 5 min in a 3T scanner (Philips Achieva). 3D high-resolution structural T1-weighted images were first obtained to provide anatomical details with parameters: field of view (FOV) = 256×256×192 mm3, 256×256×192 matrix size, inversion time (TI) = 400 ms, repetition time (TR) = 2460 ms, echo time (TE) = 3.4 ms, spatial resolution = 1×1×1 mm3), followed by 100 volumes of T2*-weighted images acquired at the resting state using the echo-planner imaging sequence with the following parameters: FOV = 256×256 mm2, 128×128 in-plane matrix size, slice number = 29, slice thickness = 3 mm, TR = 3000 ms, TE = 35 ms, in-plane spatial resolution = 2×2 mm2.
Data Acquisition and Analysis
Imaging data was preprocessed using Statistical Parametric Mapping (SPM8) software (Wellcome Department of Cognitive Neurology, London, UK) running under the MATLAB environment (Mathworks, Inc., Sherborn, MA). The data were initially corrected for motion (threshold of 2 mm), slice scan time correction, spatial smoothing using a 3D Gaussian filter (4-mm FWHM), and voxel-wise linear detrending and 0.01–0.08Hz band-pass filtering. Structural and functional data of each participant were then transformed to a standard stereotaxic space (MNI space) [9] to facilitate group analysis.
Functional connectivity maps were generated using correlational analysis on a voxel-by voxel basis. Left and right amygdala were selected as separate seed regions. All ROI definitions were based on Automated Anatomical Labeling (AAL) [10] built in the MarsBaR toolbox of SPM8. RSFC maps for each seed of individual subjects were calculated using Resting-State fMRI Data Analysis Toolkit (REST, http://restfmri.net/forum/?q=rest). Briefly, a regionally averaged time course from all voxels inside each seed region was used as a reference time course. Pearson cross-correlation coefficients between reference time courses and the time course of each individual voxel were calculated. This correlational analysis was carried out for each subject. Since correlation coefficients are not normally distributed, it is not appropriate to average them. Therefore, in order to obtain group-averaged RSFC maps, correlation coefficients were transformed using Fisher’s z transformation to make the r values normally distributed, and the resultant z values were then averaged across subjects. Subsequently, the averaged z values were transformed back to r values, yielding a mean correlation map for each seed. Significant functional connectivity was thresholded at mean r value > 0.35 (equivalent to p < 0.001, uncorrected). [11]
To examine relationships between anger traits and amygdala-prefrontal RSFC, we quantified the strength of RSFC (using r values) within the amygdala-prefrontal circuit for individual subjects and correlated it to the subscale of anger trait on a voxel-by-voxel basis. Specifically, for each voxel, a correlation between the connectional strength to left/right amygdala and trait anger was calculated. All voxels with a correlation coefficient R > 0.6 and cluster size > 30 voxels (equivalent to p = 0.001, uncorrected [11]) were considered to comprise the circuitry associated with anger trait regulation.
Results
Behavior measures were not correlated with age or years of education. Trait measures for anger showed adequate variance for examining individual differences (Trait Anger Mean 17.8 (S.D. 6.67), Range 11–30; Anger Control-Out Mean 25.4 (S.D. 6.30) Range 14–32, and agreed with published norms.
The RSFC maps of unilateral amygdala are shown in Figure 1. Strong connections were seen between amygdala and cortical regions including orbital-frontal cortex, cingulate cortex, temporal cortex, precuneus/cuneus, parahippocampal gyrus and paracentral lobule, and subcortical regions including thalamus, caudate and putamen.
Figure 1. Resting-state functional connectivity maps from unilateral amygdala.
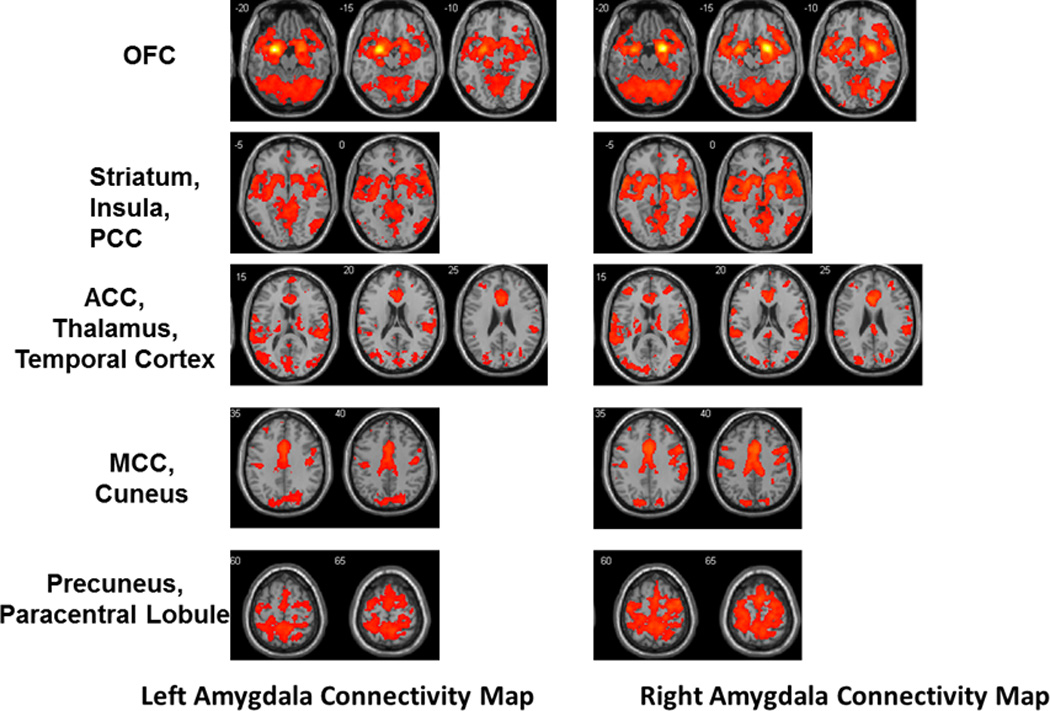
Abbreviations: OFC, orbitofrontal cortex; PCC, posterior cingulate cortex; ACC, anterior cingulate cortex; MCC, middle cingulate cortex.
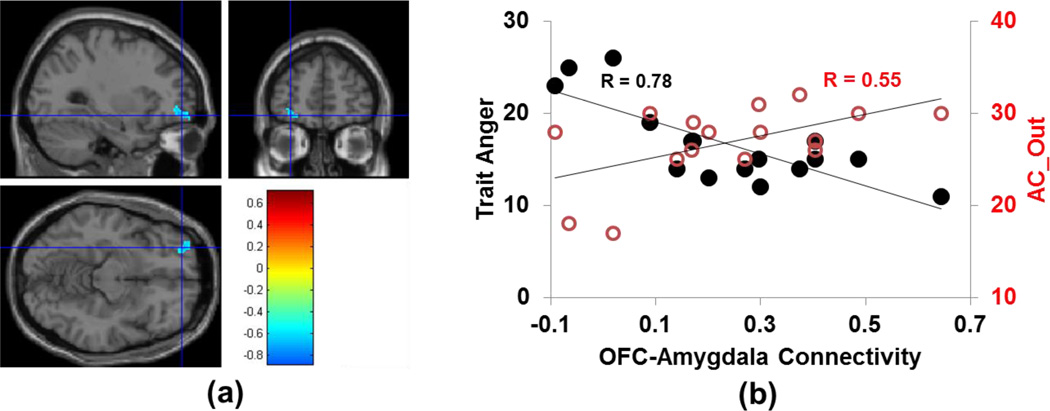
As predicted, trait anger measures showed strong inverse association with RSFC between amygdala and orbital-frontal cortex. This inverse correlation was strongest for right amygdala and contralateral middle orbital-frontal cortex (Fig. 2a). A weaker inverse correlation was also observed for left amygdala and contralateral orbital-frontal cortex when the threshold of clustering size was lowered to 5 voxels. The covariation between functional connectivity strength between right amygdala-left orbital-frontal cortex is depicted graphically in Figure 2b. In contrast to Trait Anger, Anger Control subscales (Anger Control-In and Anger Control-Out) showed the opposite relationship: a strong positive correlation to the strength of RSFC between right amygdala and contralateral middle orbital-frontal cortex (Figure 2b).
Figure 2. Co-variation of Functional Connectivity with Trait Anger.
(a) Trait Anger showed strong inverse association with the strength of RSFC between right amygdala and contralateral middle orbital-frontal cortex. All voxels with a correlation coefficient R > 0.6 and cluster size > 30 voxels (equivalent to p = 0.001, uncorrected) [11] were considered to be significantly associated with the circuitry responsible for anger trait regulation. (b) Trait Anger showed strong inverse association with the functional connectivity strength between right amygdala and left middle orbital-frontal cortex (closed circles), and Anger Control-Out (AC_Out, open circles) showed strong positive correlation (in red) to the strength of RSFC between right amygdala and contralateral middle orbital-frontal cortex. The correlation coefficients depicted in (b) were calculated from the cluster in the orbital-frontal cortex shown in (a).
Discussion
In the present study we have provided evidence that RSFC of the amygdala- orbital-frontal cortex circuit is strongly correlated with trait measures of anger. The central roles of the amygdala and prefrontal cortex in processing emotional responses have been well established in humans and animals.[12] Our finding regarding the association of the amygdala-orbitofrontal circuit with anger regulation complements task-based neuroimaging studies of emotion regulation in humans. [13–14] These studies have shown that the magnitude of fMRI signal decrease in amygdala predicts increased signal in ventromedial prefrontal cortex when subjects are instructed to regulate their emotional response to negative stimuli [14], and that the strength of functional coupling between areas of prefrontal cortex and the amygdala predicts the ability to suppress negative emotion in a cognitive reappraisal task [15]. The results of our resting state analysis presented here provide additional information about a stable pattern of emotional regulation and its relationship with the strength of resting state functional connectivity in the amygdala-prefrontal circuit.
The intrinsic activity within various brain networks, as demonstrated by resting state analysis, has been shown to predict task-induced activation patterns. [16] In addition, the RSFC of brain networks has been shown in several studies to have remarkable consistency, and recent research has demonstrated moderate to high test-test reliability over periods of months to a year as well. [17] We have shown here that a trait measure of anger, reflecting a stable pattern of emotional reactivity, is associated with RSFC in the amygdala-orbitofrontal network. Therefore, we speculate that the intrinsic activity in this network will be predictive of interindividual variation in state-dependent expression and regulation of anger.
Interestingly, the strongest association between anger measures and amygdala- orbital-frontal cortex connectivity in our study is for right amygdala with the contralateral middle orbitofrontal region of prefrontal cortex. This finding is consistent with anatomical studies in nonhuman primates [18] as well as functional connectivity studies in humans [19] demonstrating robust contralateral connectivity. Another interesting observation is the remarkable asymmetry between functional connectivity networks involving left and right amygdala, suggesting that right amygdala may play a more prominent role in anger regulation, consistent with previous evidence for right/left differences in the amygdala’s role in fear conditioning and depression. [20] This spatial pattern of amygdala functional connectivity observed in this study is consistent with structural and functional studies of the amygdala. [21]
The findings presented here may have relevance for the pathophysiology of clinical disorders characterized by problems with anger and aggression. For example, functional coupling between orbitofrontal cortex and amygdala is disrupted in depressed patients with anger attacks [22] and patients with Personality Disorder [23]. In schizophrenia, amygdala- orbital-frontal cortex RSFC is disrupted and is inversely correlated with aggression ratings. [24] According to the cognitive control model of trait anger proposed by Wilkowski and Robinson [25], individuals low in trait anger exhibit greater tendency to utilize cognitive processes when confronted with hostile situational input, resulting in lesser tendencies toward reactive anger and aggression. The association described in the present study of low trait anger with greater functional connectivity between cortex and amygdala may suggest a neural substrate for this model that links cognitive processing with modulation of limbic reactivity. Taken together, our findings in healthy subjects and research on clinical disorders characterized by anger and aggression converge to suggest that the functional integrity of amygdala- orbital-frontal cortex connectivity may represent a continuum from relatively weaker connectivity associated with individual differences in trait anger to more severe disruptions associated with clinical problems with aggression.
A limitation of this study is the modest sample size and the inclusion of only male subjects. Future studies will be needed to demonstrate that the findings reported here generalize to women and can be replicated in a larger sample, and to address the possibility that latent variables mediate the correlation between trait anger and functional connectivity.
In conclusion, our findings support the hypothesis that anger traits correlate with individual differences in connectivity strength between amygdala and orbitofrontal cortex. Additional studies are warranted to further investigate the relationship between cortico-limbic resting state connectivity and state-dependent activation during anger-related tasks, and the applications of these findings to disorders characterized by emotional dysregulation such as depression, anxiety and personality disorders.
Acknowledgments
We thank Rasheed Hardaway, Athene K. Lee, and Dr. Wei Huang for technical assistance, and Drs David Gansler, Matthew Jerram and David Kennedy for expert advice and discussions on the manuscript.
This publication was made possible by the NIH Grant numbers 1RO1 MH067096-02 and 5R01DA021846-02 from the National Institute of Health (JK) and a UMass Department of Psychiatry Fellowship Award (CF). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Footnotes
The authors declare no conflicts of interest.
References
- 1.Campbell-Sills L, Barlow D. Incorporating emotion regulationinto conceptualizations and treatments of anxiety and mood disorders. In: G JJ, editor. Handbook of Emotion Regulation. New York: Guilford; 2007. pp. 542–559. [Google Scholar]
- 2.Everson-Rose SA, Lewis TT. Psychosocial factors and cardiovascular diseases. Annu Rev Public Health. 2005;26:469–500. doi: 10.1146/annurev.publhealth.26.021304.144542. [DOI] [PubMed] [Google Scholar]
- 3.Phan KL, Wager T, Taylor SF, Liberzon I. Functional neuroanatomy of emotion: a meta-analysis of emotion activation studies in PET and fMRI. Neuroimage. 2002;16:331–348. doi: 10.1006/nimg.2002.1087. [DOI] [PubMed] [Google Scholar]
- 4.Quirk GJ, Beer JS. Prefrontal involvement in the regulation of emotion: convergence of rat and human studies. Curr Opin Neurobiol. 2006;16:723–727. doi: 10.1016/j.conb.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 5.New AS, Hazlett EA, Newmark RE, Zhang J, Triebwasser J, Meyerson D, et al. Laboratory induced aggression: a positron emission tomography study of aggressive individuals with borderline personality disorder. Biol Psychiatry. 2009;66:1107–1114. doi: 10.1016/j.biopsych.2009.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Manuck SB, Brown SM, Forbes EE, Hariri AR. Temporal stability of individual differences in amygdala reactivity. Am J Psychiatry. 2007;164:1613–1614. doi: 10.1176/appi.ajp.2007.07040609. [DOI] [PubMed] [Google Scholar]
- 7.Gianaros PJ, Hariri AR, Sheu LK, Muldoon MF, Sutton-Tyrrell K, Manuck SB. Preclinical atherosclerosis covaries with individual differences in reactivity and functional connectivity of the amygdala. Biol Psychiatry. 2009;65:943–950. doi: 10.1016/j.biopsych.2008.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Spielberger C. State-Trait Anger Expression Inventory-2: Professional Manual. Psychological Assessment Resources, Inc.; 1999. [Google Scholar]
- 9.Brett M, Johnsrude IS, Owen AM. The problem of functional localization in the human brain. Nat Rev Neurosci. 2002;3:243–249. doi: 10.1038/nrn756. [DOI] [PubMed] [Google Scholar]
- 10.Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- 11.Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster-size threshold. Magn Reson Med. 1995;33:636–647. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- 12.Phelps EA, LeDoux JE. Contributions of the amygdala to emotion processing: from animal models to human behavior. Neuron. 2005;48:175–187. doi: 10.1016/j.neuron.2005.09.025. [DOI] [PubMed] [Google Scholar]
- 13.Ochsner KN, Ray RD, Cooper JC, Robertson ER, Chopra S, Gabrieli JD, et al. For better or for worse: neural systems supporting the cognitive down- and up-regulation of negative emotion. Neuroimage. 2004;23:483–499. doi: 10.1016/j.neuroimage.2004.06.030. [DOI] [PubMed] [Google Scholar]
- 14.Urry HL, van Reekum CM, Johnstone T, Kalin NH, Thurow ME, Schaefer HS, et al. Amygdala and ventromedial prefrontal cortex are inversely coupled during regulation of negative affect and predict the diurnal pattern of cortisol secretion among older adults. Journal of Neuroscience. 2006;26:4415–4425. doi: 10.1523/JNEUROSCI.3215-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Banks SJ, Eddy KT, Angstadt M, Nathan PJ, Phan KL. Amygdala-frontal connectivity during emotion regulation. Soc Cogn Affect Neurosci. 2007;2:303–312. doi: 10.1093/scan/nsm029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mennes M, Kelly C, Zuo XN, Di Martino A, Biswal BB, Castellanos FX, et al. Inter-individual differences in resting-state functional connectivity predict task-induced BOLD activity. Neuroimage. 2010;50:1690–1701. doi: 10.1016/j.neuroimage.2010.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zuo XN, Kelly C, Adelstein JS, Klein DF, Castellanos FX, Milham MP. Reliable intrinsic connectivity networks: test-retest evaluation using ICA and dual regression approach. Neuroimage. 2010;49:2163–2177. doi: 10.1016/j.neuroimage.2009.10.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McDonald AJ. Cortical pathways to the mammalian amygdala. Prog Neurobiol. 1998;55:257–332. doi: 10.1016/s0301-0082(98)00003-3. [DOI] [PubMed] [Google Scholar]
- 19.Robinson JL, Laird AR, Glahn DC, Lovallo WR, Fox PT. Metaanalytic connectivity modeling: delineating the functional connectivity of the human amygdala. Hum Brain Mapp. 2010;31:173–184. doi: 10.1002/hbm.20854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.LaBar KS, Gatenby JC, Gore JC, LeDoux JE, Phelps EA. Human amygdala activation during conditioned fear acquisition and extinction: a mixed-trial fMRI study. Neuron. 1998;20:937–945. doi: 10.1016/s0896-6273(00)80475-4. [DOI] [PubMed] [Google Scholar]
- 21.Aggleton JP. The amygdala : a functional analysis. Oxford, OX ; New York: Oxford University Press; 2000. [Google Scholar]
- 22.Dougherty DD, Rauch SL, Deckersbach T, Marci C, Loh R, Shin LM, et al. Ventromedial prefrontal cortex and amygdala dysfunction during an anger induction positron emission tomography study in patients with major depressive disorder with anger attacks. Arch Gen Psychiatry. 2004;61:795–804. doi: 10.1001/archpsyc.61.8.795. [DOI] [PubMed] [Google Scholar]
- 23.New AS, Hazlett EA, Buchsbaum MS, Goodman M, Mitelman SA, Newmark R, et al. Amygdala-prefrontal disconnection in borderline personality disorder. Neuropsychopharmacol. 2007;32:1629–1640. doi: 10.1038/sj.npp.1301283. [DOI] [PubMed] [Google Scholar]
- 24.Hoptman MJ, D'Angelo D, Catalano D, Mauro CJ, Shehzad ZE, Kelly AM, et al. Amygdalofrontal functional disconnectivity and aggression in schizophrenia. Schizophr Bull. 2010;36:1020–1028. doi: 10.1093/schbul/sbp012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wilkowski BM, Robinson MD. The anatomy of anger: an integrative cognitive model of trait anger and reactive aggression. J Pers. 2010;78:9–38. doi: 10.1111/j.1467-6494.2009.00607.x. [DOI] [PubMed] [Google Scholar]