Abstract
Background
EGFR mutation might be a predictive factor for applying EGFR-tyrosine kinase inhibitors (EGFR-TKIs, including gefitinib, erlotinib and afatinib) in non-small-cell lung cancer (NSCLS) patients. Thus, it is necessary to pool previous trials to compare the effect of EGFR-TKIs versus cytotoxic chemotherapy in EGFR mutation positive (mut+) and negative (mut−) patients.
Material/Methods
This study identified 8 first-line and 9 second-line phase III trials in databases. Hazard ratio (HR) was pooled to assess the risk of progression-free survival (PFS), and overall survival (OS), while odds ratio (OR) was pooled to assess objective response, disease control, and toxicity of EGFR-TKIs verses chemotherapy.
Results
In EGFR mut+ patients, EGFR-TKIs were associated with significantly lower risk of disease progression in the first-line setting, but this trend was only observed in the gefitinib group, not in the erlotinib group in the second-line setting. In EGFR mut− patients, gefitinib and erlotinib had significantly higher risk of disease progression in first-line and second-line setting, respectively. Compared with chemotherapy, the effects of EGFR-TKIs on OS in both first-line and second-line settings were not evident. Regarding toxicity, EGFR-TKIs had significantly higher risk of rash and lower hematological toxicity compared with chemotherapy.
Conclusions
All of the 3 EGFR-TKIs and gefitinib alone regimens had better effects in prolonging PFS in EGFR mut+ patients in first-line and second-line setting, respectively, but chemotherapy seemed more effective in EGFR mut− patients than EGFR-TKIs. Therefore, accurate identification of EGFR mutation status is useful to decide on an appropriate regimen for treatment of NSCLC patients.
MeSH Keywords: Carcinoma, Non-Small-Cell Lung; Genes, erbB-1; Meta-Analysis
Background
The EGFR signaling pathway plays a critical role in regulating the development and progression of non-small-cell lung cancer (NSCLC) [1,2]. Activation of this signal pathway might stimulate cancer cell proliferation and invasion and inhibit tumor cell apoptosis [2,3]. Gefitinib and erlotinib are the first generation of small and reversible molecular EGFR-tyrosine kinase inhibitors (TKIs) inhibiting the EGFR/ERBB1 receptor [4], while afatinib is the second generation of TKI inhibiting the EGFR/HER-1/ERBB1 and HER-2/ERBB2 receptors [5]. These three agents have been extensively used in patients with NSCLC [5].
Accumulating evidence shows that NSCLC patients with somatic mutations in certain genes have varied risks of lung cancer [6]. Somatic mutation in the region of EGFR genes encoding tyrosine kinase had higher response rate to EGFR-TKIs [7,8]. Therefore, EGFR-TKIs-based regimens, which were originally limited to use in patients who failed in previous platinum-based chemotherapy, have been gradually applied as first-line strategy for selective NSCLC patients who might have greater response to EGFR-TKIs. About 30–35% of NSCLC patients in Asia [9] and 10–15% of NSCLC patients in Europe [10] have EGFR activate mutation, in which the deletions in exon 19 and substitution of leucine-858 with arginine (L858R) in exon 21 of the EGFR kinase domain were the most common types [10,11].
Several phase III randomized controlled trials (RCTs) have been conducted to compare EGFR-TKIs (gefitinib, erlotinib, or afatinib) versus the standard doublet chemotherapy (platinum plus a new third-generation agent) as first-line treatment or EGFR-TKIs (gefitinib or erlotinib) versus a single new third-generation chemotherapy agent in second-line treatment for patients with advanced NSCLC. Most of the first-line treatments reported that EGFR-TKIs were associated with higher response rate and better progression-free survival (PFS) versus cytotoxic chemotherapy. In second-line treatment, the effect of EGFR-TKIs was not significant or was even associated with worse effect compared with cytotoxic chemotherapy. However, the small size of individual studies might not have sufficient statistical power to estimate the possible difference in experimental and control arms. Therefore, this study aimed to assess the efficacy of EGFR-TKIs versus chemotherapy in progression-free survival (PFS) and/or overall survival (OS) among patients with or without EGFR mutation.
Material and Methods
Search strategy
Trials were searched in MEDLINE, the Cochrane Central Register of Controlled Trials (CENTRAL), and EMBASE databases by using the following search terms and strategy: (“lung neoplasms” OR “non-small-cell lung cancer” OR “NSCLC”) AND (“gefitinib” OR “erlotinib” OR “afatinib” OR “tyrosine kinase inhibitor”) AND (“randomized” OR “trial” OR “RCT”) AND (“epidermal growth factor receptor” OR “EGFR”). The time range of the search was restricted to January 2000 to April 2014. No language restriction was applied for searching. Reference lists of included trials and other relevant reviews or meta-analyses were manually searched to avoid missing eligible trials.
Criteria for inclusion and exclusion
RCTs meeting the following criteria simultaneously were included for this meta-analysis: (1) phase III RCTs; (2) patients >18 years; and (3) studies compared EGFR-TKIs monotherapy vs. any chemotherapy in first-line or second-line trials for NSCLC patients. Trials were included regardless of publication status, date of publication, and language. Trials with a combination of chemotherapy and EGFR-TKIs in the experiment arm or merely with placebo in control arm were excluded.
Data extraction
Data extraction from original trials was independently performed by 2 authors (WQZ and TL). Disagreement was resolved by referring to original studies with a third author (HL) through group discussion. Data extracted include first author, year of publication, country/region in which the trials were conducted, regimen design in experiment and control group, and clinicopathological data including EGFR mutation, progression-free survival (PFS), overall response, disease control rate, and overall survival (OS). In addition, severe drug toxicities (grade III or above adverse effects), including rash, fatigue/asthenia, diarrhea, vomiting/nausea, anemia, neutropenia, thrombocytopenia, and leukocytopenia were extracted for pooled analysis.
Statistical analyses
Cochrane Review Manager (version 5.2, Cochrane Collaboration, Copenhagen, Denmark) was used for statistical analysis. Hazard ratios (HRs) and the associated 95% confidence intervals (CIs) for PFS and OS and odds ratio (OR) and associated 95% CIs for objective response, disease control, and toxicity in original trials were extracted to compared the efficacy of EGFR-TKIs versus chemotherapy in first-line and second-line setting. In addition, subgroup analysis was performed by stratifying EGFR-TKIs within EGFR mut+ and EGFR mut− subgroups. If results of the trials were updated, the most recent OS data was used for analysis. The HR results were pooled by using inverse variance weighted method. A fixed-effects model was applied firstly to test heterogeneity (I2 and p values of χ2 Cochran Q test were used to detect heterogeneity across the different studies and between subgroups). If I2 >50% or p<0.1, a random-effects model would be applied. P<0.05 was considered as significant in Z test of pooled results.
Results
Search results
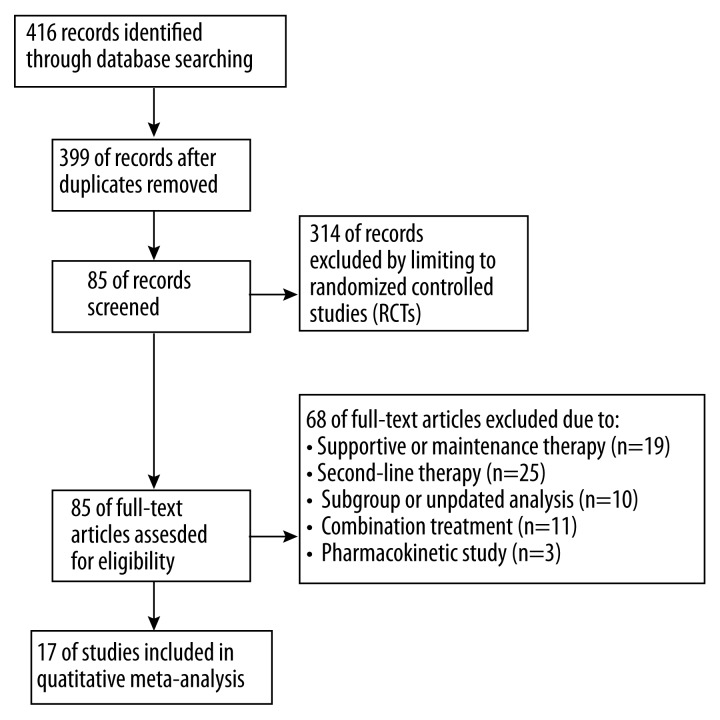
The literature search identified 17 qualified phase III clinical trials. Among them, 8 studies compared gefitinib, erlotinib, or afatinib versus chemotherapy in first-line treatment and 9 compared gefitinib or erlotinib with chemotherapy in second-line treatment in patients with NSCLC. The search and screening process of qualified trials are described in Figure 1. The 8 first-line trials include IPASS [12], WJTOG3405 [13], NEJ002 [14] and First-SIGNAL [15] which compared gefitinib with chemotherapy, OPTIMAL [16,17] and EURTAC [18] which compared erlotinib with chemotherapy and LUX-lung 3 [19] and LUX-lung 6 [20], which compared afatinib with chemotherapy. The 9 second-line trials include V-15-32 [21], KCSG-LU08-01 [22], ISTANA [23] and Interest [24] that compared gefitinib with chemotherapy and TITAN [25], TAILOR [26], PROSE [27], HORG [28] and Delta [29] that compared erlotinib with chemotherapy. The key information of the 8 first-line and 9 second-line trials are summarized in Tables 1 and Table 2, respectively. Among the 8 first-line trials, 6 only included patients with EGFR mutation [13,14,16–20]. In second-line trials, EGFR mutation status varied significantly. One study included only patients without mutation [26], 1 study did not report EGFR mutation status [23], while the other 6 had mixed patients with mutation, without mutation, or with unknown mutation status. Table 1 and 2 show the available HR data for PFS, OS, and OR data for objective response and disease control pooled. In the first-line setting, EGFR-TKIs were associated with better effect in prolonging PFS (HR 0.45, 95% CI 0.30–0.67, p<0.0001) and had a high ratio of objective response (OR 4.09, 95% CI 2.35–7.15, p<0.0001) and disease control (OR 1.86, 95% CI 1.01–3.41, p=0.05). But the effect in OS was similar to chemotherapy (HR 0.95, 95% CI 0.86–1.04, p=0.24) (Table 1). In second-line setting, EGFR-TKIs had similar effects in PFS (HR 1.01, 95% CI 0.87–1.17, p=0.92), objective response (OR 1.13, 95% CI 0.89–1.43, p=0.33), and OS (HR 1.01, 95% CI 0.93–1.08, p=0.86) as chemotherapy, but the effect in disease control was significantly worse than chemotherapy (OR 0.76, 95% CI 0.59–0.98, p=0.03) (Table 2).
Figure 1.
The search and screening process.
Table 1.
Characteristics of first-line trials included.
| Study | Country/region | Treatment | Total no. pts | No. of EGFR+ pts (%) | No. of EGFR− pts (%) | No. of EGFR unknown pts (%) | Progression-free survival | Objective response | Disease control | Overall survival |
|---|---|---|---|---|---|---|---|---|---|---|
| First-line | HR (95% CI) | OR (95% CI) | OR (95% CI) | HR (95% CI) | ||||||
| WJTOG3405 (2010) | Japan | Gefitinib vs. Cis+D | 172 | 172 (100) | 0 | 0 | 0.49 (0.34–0.71) | 3.4 (1.6–7.4) | 3.8 (1.2–12.5) | 1.64 (0.75–3.59) |
| NEJ002 (2010) | Japan | Gefitinib vs. Car+Pa | 228 | 228 (100) | 0 | 0 | 0.32 (0.24–0.43) | 6.3 (3.6–11.2) | 2.1 (1.0–4.6) | 0.89 (0.63–1.26) |
| IPASS (2009) | East Asian | Gefitinib vs. Car+Pa | 1217 | 261 (21) | 176 (15) | 780 (64) | 0.74 (0.65–0.84) | 1.59 (1.25–2.01) | 0.70 (0.54, 0.92) | 0.90 (0.79–1.03) |
| First-SIGNAL (2012) | Korean | Gefitinib vs. Cis+G | 309 | 43 (14) | 54 (17) | 212 (69) | 1.20 (0.94–1.53) | 1.46 (0.93–2.28) | 0.56 (0.34, 0.94) | 0.93 (0.72–1.20) |
| Optimal (2011) | China | Erlotinib vs. Car+G | 154 | 154 (100) | 0 | 0 | 0.16 (0.10–0.26) | 8.6 (4.1–18.2) | 5.8 (1.6–21.3) | 1.07 (0.79–1.45) |
| EURTAC (2012) | Europe | Erlotinib vs. platinum+G/ or platinum+D | 173 | 173 (100) | 0 | 0 | 0.37 (0.25–0.55) | 7.9 (3.8–16.4) | 2.0 (1.0–3.9) | 1.04 (0.65–1.66) |
| LUX-Lung 3 (2012) | Global | Afatinib vs. Cis+Pe | 354 | 354 (100) | 0 | 0 | 0.58 (0.43–0.78) | 4.4 (2.6–7.3) | 2.1 (1.1–4.0) | 1.12 (0.73–1.72) |
| LUX-Lung 6 (2013) | Asian | Afatinib vs. Cis+G | 364 | 364 (100) | 0 | 0 | 0.28(0.20–0.39) | 6.8 (4.1–11.2) | 3.9 (2.1–7.3) | 0.95 (0.68–1.33) |
| Pooled HR (95% CI), p value | Pooled OR (95% CI), p value | Pooled OR (95% CI), p value | Pooled HR (95% CI), p value | |||||||
| 0.45 (0.30, 0.67), p<0.0001 | 4.09 (2.35, 7.15), p<0.0001 | 1.86 (1.01, 3.41), p=0.05 | 0.95 (0.86, 1.04), p=0.24 |
EGFR+ – EGFR mutation positive; EGFR− – EGFR mutation negative; Car – carboplatin; Cis – cisplatin; D – docetaxel; Pa – paclitaxel; Pe – pemetrexed; G – gemcitabine; N.A. – not available; OR – odd ratio; HR – hazard ratio; CI – confidential interval; Pts – patients.
Table 2.
Characteristics of second-line trials included.
| Study | Country/region | Treatment | Total no. pts | No. of EGFR+ pts (%) | No. of EGFR− pts (%) | No. of EGFR unknown pts (%) | Progression-free survival | Response | Disease control | Overall survival |
|---|---|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | OR (95% CI) | OR (95% CI) | HR (95% CI) | |||||||
| V-15-32 (2008) | Japan | Gefitinib vs. D | 489 | 31 (6) | 26 (6) | 432 (88) | 0.90 (0.72–1.13) | 2.14 (1.21–3.78) | 1.08 (0.69–1.68) | 1.12 (0.89–1.41) |
| KCSG-LU08-01 (2012) | Korean | Gefitinib vs. Pe | 135 | 33 (24) | 38 (28) | 64 (48) | 0.54 (0.37–0.79) | 1.10 (0.55–2.20) | 1.02 (0.44–2.34) | 0.80 (0.50–1.28) |
| ISTANA (2010) | Korean | Gefitinib vs. D | 161 | N.A. | N.A. | N.A. | 0.73 (0.53–1.01) | 0.85 (0.44–1.61) | 0.98 (0.48–2.00) | 0.87 (0.61–1.24) |
| Interest (2008) | Global | Gefitinib vs. D | 1466 | 44 (3) | 253 (17) | 1169 (80) | 1.04 (0.93–1.16) | 1·22 (0·82–1.84) | N.A. | 1.02 (0.91–1.14) |
| TITAN (2012) | Global | Erlotinib vs. Pe or D | 424 | 11 (3) | 149 (35) | 264 (62) | 1.19 (0.97–1.46) | 1.27 (0.60–2.66) | 0.70 (0.47–1.03) | 0.96 (0.78–1.18) |
| TAILOR (2012) | Italy | Erlotinib vs. D | 219 | 0 | 219 (100) | 0 | 1.45 (1.08–1.95) | N.A. | N.A. | N.A. |
| PROSE (2014) | Italy | Erlotinib vs. Pe | 263 | 14(5) | 163 (62) | 86 (33) | 1.27 (0.99–1.63) | 0.72 (0.30–1.70) | 0.47 (0.28–0.77) | 1.14 (0.88–1.48) |
| HORG (2013) | Greece | Erlotinib vs. Pe | 332 | 11(3) | 112(34) | 209(63) | 0.88 (0.73–1.06) | 0.77 (0.38–1.57) | 0.67 (0.42–1.07) | 0.99 (0.80–1.23) |
| Delta (2013) | Japan | Erlotinib vs. D | 301 | 56(19) | 199(66) | 46(15) | 1.22 (0.97–1.53) | 0.96 (0.53–1.76) | N.A. | 0.91 (0.68–1.22) |
| Pooled HR (95% CI), p value | Pooled OR (95% CI), p value | Pooled OR (95% CI), p value | Pooled HR (95% CI), p value | |||||||
| 1.01 (0.87, 1.17), p=0.92 | 1.13 (0.89, 1.43), p=0.33 | 0.76 (0.59, 0.98), p=0.03 | 1.01 (0.93, 1.08), p=0.86 |
EGFR+ – EGFR mutation positive; EGFR− – EGFR mutation negative; Car – carboplatin; Cis – cisplatin; D – docetaxel; Pa – paclitaxel; Pe – pemetrexed; G – gemcitabine; N.A. – not available; OR – odd ratio; HR – hazard ratio; CI – confidential interval; Pts – patients.
The effect of EGFR-TKIs vs. chemotherapy on PFS
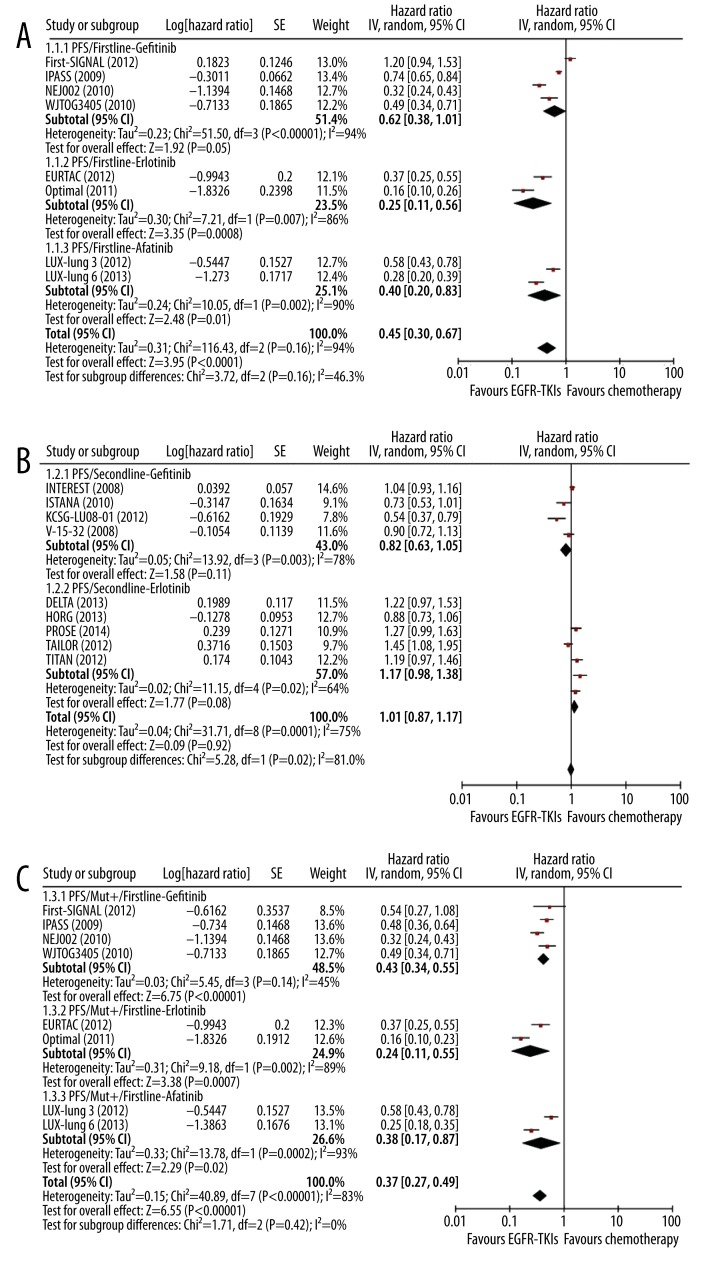
Eight studies evaluated EGFR-TKIs as first-line agents (4 gefitinib, 2 erlotinib, and 2 afatinib studies) and 9 studies assessed EGFR-TKIs as second-line agent in comparison to chemotherapy. The treatment effect in different settings is given in Figure 2. Generally, EGFR-TKIs were associated with significantly better PFS compared with chemotherapy in first-line setting (HR 0.45, 95% CI 0.30–0.67, p<0.0001) (Figure 2A). No significant heterogeneity was observed in subgroup difference (I2=46.3%). The trend of better PFS was consistent in the 3 EGFR-TKI subgroups: gefitinib (HR 0.62, 95% CI 0.38–1.01, p=0.05), erlotinib (HR 0.25, 95% CI 0.11–0.56, p=0.0008), and afatinib (HR 0.40, 95% CI 0.20–0.83, p=0.01) (Figure 2A). However, this trend was not observed in second-line setting, in which both gefitinib and erlotinib were associated with similar PFS as chemotherapy (gefitinib vs. chemotherapy, HR 0.82, 95% CI: 0.63–1.05, p=0.11; erlotinib vs. chemotherapy, HR 1.17, 95% CI 0.98–1.38, p=0.08) (Figure 2B).
Figure 2A–C.
Meta-analysis of the effect of EGFR-TKIs on PFS in first-line and second-line settings. Comparison of the effect on PFS between EGFR-TKIs and chemotherapy in first-line setting (A) and second-line settings (B); Comparison of the effect on PFS in mut+ patients in first-line setting (C) and in second-line setting (D); Comparison of the effect on PFS in mut− patients in first-line setting (E) and in second-line setting (F).
In patients with EGFR mutations, EGFR-TKIs treatment was associated with significantly lower risk of disease progression as first-line setting (gefitinib: HR 0.43, 95% CI 0.34–0.55, P<0.00001; erlotinib: HR 0.24, 95% CI 0.11–0.55, p=0.0007; and afatinib: HR 0.38, 95% CI 0.17–0.87, p=0.02) (Figure 2C). However, in second-line setting, significantly reduced risk was observed in the gefitinib group (HR 0.32, 95% CI 0.15–0.69, P=0.004) but not in the erlotinib group (HR 0.93, 95% CI 0.51–1.67, p=0.80) (Figure 2D).
Figure 2D–F.
Meta-analysis of the effect of EGFR-TKIs on PFS in first-line and second-line settings. Comparison of the effect on PFS between EGFR-TKIs and chemotherapy in first-line setting (A) and second-line settings (B); Comparison of the effect on PFS in mut+ patients in first-line setting (C) and in second-line setting (D); Comparison of the effect on PFS in mut− patients in first-line setting (E) and in second-line setting (F).
In patients without EGFR mutations, only 2 studies compared the effect of gefitinib versus chemotherapy in first-line setting. Pooled results showed that gefitinib had significantly higher risk of disease progression compared with chemotherapy (HR 2.09, 95% CI 1.06–4.11, p=0.03) (Figure 2E). In second-line setting, gefitinib was associated with similar risk as chemotherapy (HR 0.91 95% CI 0.54–1.54, p=0.72) (Figure 2F). Three studies compared erlotinib vs. chemotherapy and the pooled data showed significantly higher risk in the erlotinib arm (HR 1.40, 95% CI 1.17–1.66, p=0.0002) (Figure 2F).
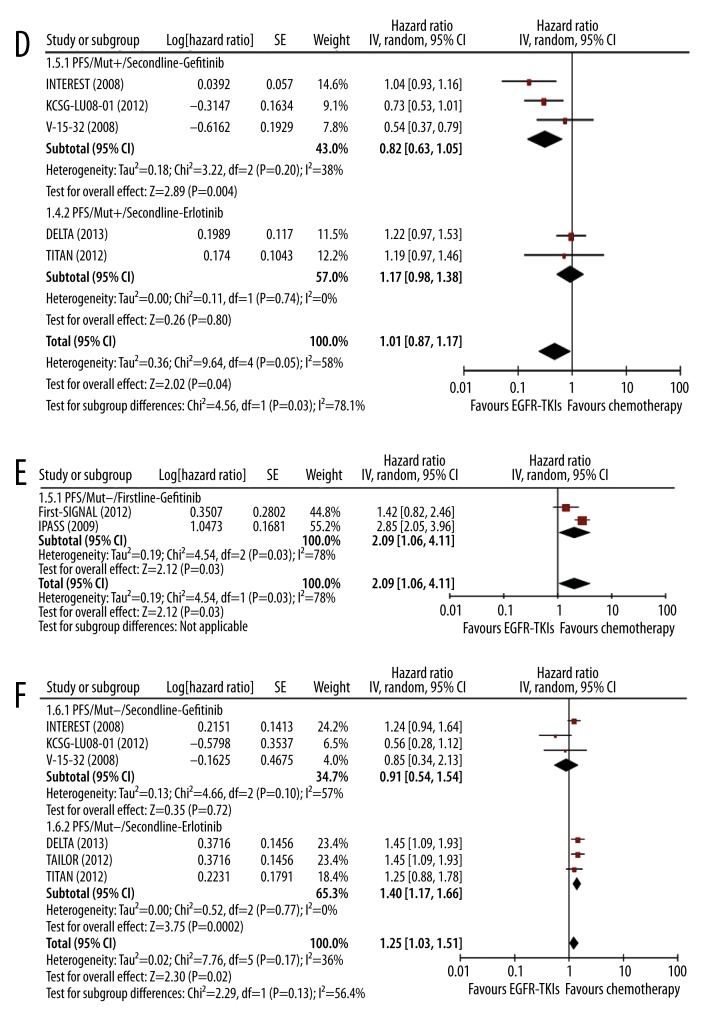
The effect of EGFR-TKIs vs. chemotherapy on OS
Generally, EGFR-TKIs had no significant benefits in OS compared with chemotherapy in both first-line (HR 0.95, 95% CI 0.86–1.04, p=0.24) (Figure 3A) and second-line setting (HR 1.01, 95% CI 0.93–1.08, p=0.86) (Figure 3B). No significant heterogeneity was observed in subgroup difference in both first-line and second-line settings (I2=0%). Sub-group analysis showed that all 3 agents had no obvious effects on OS compared with chemotherapy: gefitinib (HR 0.91, 95% CI 0.82–1.02, p=0.11), erlotinib (HR 1.06, 95% CI 0.82–1.37, p=0.65), and afatinib (HR 1.01, 95% CI 0.78–1.32, p=0.93) (Figure 3A). In second-line setting, subgroup analysis also showed gefitinib and erlotinib had similar effects as chemotherapy (gefitinib vs. chemotherapy, HR 1.01, 95% CI 0.92–1.12, p=0.77; erlotinib vs. chemotherapy, HR 1.00, 95% CI 0.88–1.12, p=0.94) (Figure 3B).
Figure 3A–C.
Meta-analysis of the effect of EGFR-TKIs on OS in first-line and second-line settings. Comparison of the effect on OS between EGFR-TKIs and chemotherapy in first-line setting (A) and in second-line setting (B); Comparison of the effect on OS in mut+ patients in first-line setting (C) and in second-line setting (D); Comparison of the effect on OS in mut− patients in first-line setting (E) and in second-line setting (F).
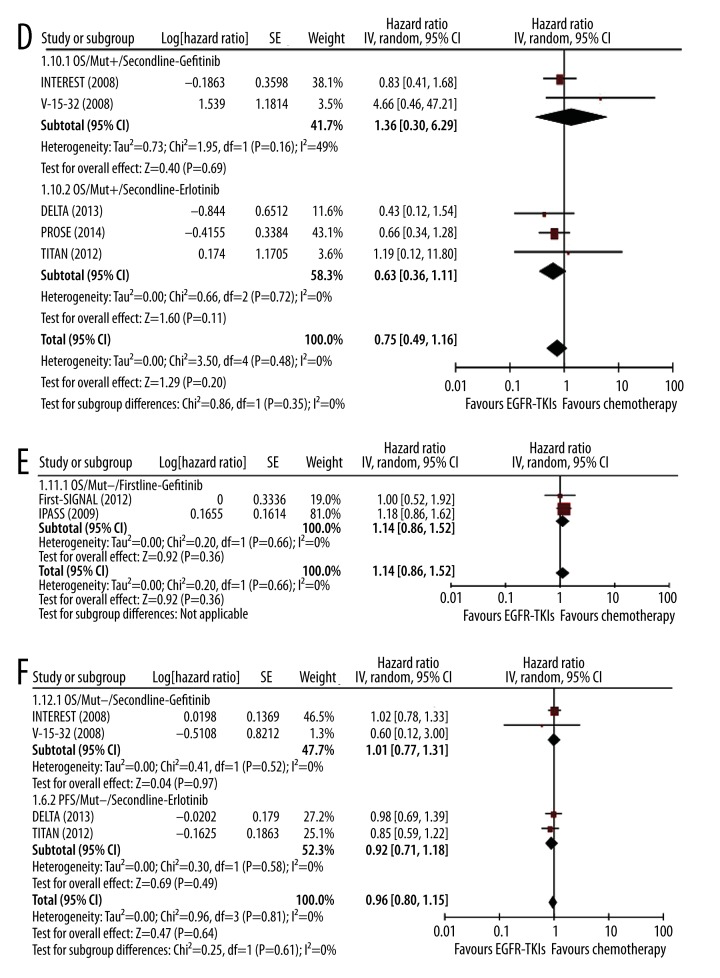
In patients with EGFR mutations, EGFR-TKIs, including gefitinib, erlotinib, and afatinib, had no significant difference in OS compared with chemotherapy in first-line setting (gefitinib: HR 1.00, 95% CI 0.82–1.22, p=0.97; erlotinib: HR 1.06, 95% CI 0.82–1.37, p=0.65; and afatinib: HR 1.01, 95% CI 0.78–1.32, p=0.93) (Figure 3C). In second-line setting, the difference was also not significant (gefitinib: HR 1.36, 95% CI 0.30–6.29, p=0.69; erlotinib: HR 0.63, 95% CI 0.36–1.11, p=0.11) (Figure 3D).
Figure 3D–F.
Meta-analysis of the effect of EGFR-TKIs on OS in first-line and second-line settings. Comparison of the effect on OS between EGFR-TKIs and chemotherapy in first-line setting (A) and in second-line setting (B); Comparison of the effect on OS in mut+ patients in first-line setting (C) and in second-line setting (D); Comparison of the effect on OS in mut− patients in first-line setting (E) and in second-line setting (F).
In patients without EGFR mutations, similar findings were observed as in patients with EGFR mutation. Compared with chemotherapy, gefitinib had no significant effect in OS in first-line setting (HR 1.14, 95% CI 0.86–1.52, p=0.36) (Figure 3E) or second-line setting (HR 1.01, 95% CI 0.77–1.31, p=0.97) (Figure 3F). The effect of erlotinib on OS was also not significant in second-line setting (HR 0.92, 95% CI 0.71–1.18, p=0.49) (Figure 3F).
Drug toxicity of EGFR-TKIs vs. chemotherapy
Severe drug toxicities (grade III or above adverse effects) were extracted for pooled analysis. Compared with chemotherapy, the most common severe adverse effects of EGFR-TKIs were rash in both first-line (OR 24.54, 95% CI 6.81–88.47, p<0.00001) and second-line (OR 7.72, 95% CI 3.70–16.11, p<0.00001) setting (Table 3). However, EGFR-TKIs had significantly lower hematological toxicity compared with chemotherapy. The risks of anemia, neutropenia, thrombocytopenia, and leukocytopenia were all significantly lower than in the chemotherapy group in both first-line and second-line settings (Table 3).
Table 3.
Meta-analysis of adverse effects, EGFR-TKIs vs. chemotherapy.
| Grade 3/4 adverse effects | Number of studies | Pooled OR (95%CI) | I2 | P-H | P |
|---|---|---|---|---|---|
| Firstline setting | |||||
| Rash | 5 | 24.54 [6.81, 88.47] | 0% | 0.65 | <0.00001 |
| Fatigue/Asthenia | 5 | 0.23 [0.11, 0.45] | 11% | 0.34 | <0.0001 |
| Diarrhea | 5 | 13.96 [3.81, 51.14] | 0% | 0.65 | <0.0001 |
| Vomiting/Nausea | 4 | 0.18 [0.03, 1.28] | 80% | 0.02 | 0.09 |
| Aneamia | 5 | 0.07 [0.03, 0.19] | 33% | 0.2 | <0.00001 |
| Neutropenia | 5 | 0.01 [0.00, 0.03] | 0% | 0.55 | <0.00001 |
| Thrombocytopenia | 4 | 0.02 [0.01, 0.09] | 0% | 0.68 | <0.00001 |
| Leucocytopenia | 3 | 0.02 [0.01, 0.06] | 0% | 0.45 | <0.00001 |
| Secondline setting | |||||
| Rash | 7 | 7.72 [3.70, 16.11] | 3% | 0.41 | <0.00001 |
| Fatigue/Asthenia | 6 | 0.42 [0.17, 1.04] | 56% | 0.04 | 0.06 |
| Diarrhea | 6 | 0.98 [0.57, 1.67] | 0% | 0.73 | 0.94 |
| Vomiting/Nausea | 8 | 0.71 [0.43, 1.18] | 0% | 0.43 | 0.19 |
| Aneamia | 4 | 0.54 [0.30, 0.96] | 0% | 0.66 | 0.04 |
| Neutropenia | 7 | 0.03 [0.01, 0.06] | 63% | 0.01 | <0.00001 |
| Thrombocytopenia | 3 | 0.10 [0.01, 0.78] | 0% | 0.77 | 0.03 |
| Leucocytopenia | 2 | 0.02 [0.00, 0.78] | 92% | 0.0006 | 0.04 |
CI – confidence interval; OR – odd ratio; P – p value; P-H – P value of Q for heterogeneity test; I2 – 0–25%, no heterogeneity; 25–50%, modest heterogeneity; 50% or above, high heterogeneity; fixed effects model was used when I2 <50%; random effects model was used when I2 ≥50%.
Discussion
Platinum-based doublet chemotherapy has been considered as the standard first-line treatment for advanced NSCLC cancer patients. However, the efficacy of this therapy is poor in certain groups of patients [30]. Many previous studies have tried to identify predictors of NSCLC development and how they can be used for better disease management [8,31]. The major strengths of this study include comprehensive review of the most recent phase III trials and stratified trials into gefitinib, erlotinib, and afatinib subgroups, rather than assuming these 3 agents have the same efficacy and to pool them into 1 group. Therefore, the exact effects of the 3 agents in EGFR mut+ and mut− patients in both first-line and second-line settings are clearly demonstrated.
This study pooled the most recent clinical results based on 8 first-line phase III trials and 9 second-line phase III trials involving 6761 patients, and addressed the most clinically important molecular factor (EGFR mutation) relevant to the treatment. For EGFR mut+ patients, this meta-analysis found that all of the 3 EGFR-TKIs had a considerable advantage in PFS over chemotherapy in first-line setting. In second-line setting, previous studies only compared gefitinib or erlotinib versus chemotherapy, and subgroup analysis showed that gefitinib was superior to chemotherapy. In EGFR mut− patients, a superior effect of EGFR-TKIs over chemotherapy was not observed. In first-line setting, although only 2 studies [12,15] examined the effect of EGFR-TKI, typically, gefitinib in EGFR mut− NSCLC patients, their findings were consistent and confirmed better efficacy of chemotherapy over gefitinib. In second-line setting for EGFR mut− patients, gefitinib did not present better effect, while erlotinib had worse effect than chemotherapy. Currently, available therapy for patients who failed in first-line treatment includes targeted therapy or further chemotherapy. Traditionally, examination of EGFR mutation status is considered unnecessary and time-consuming. However, this meta-analysis demonstrates that in second-line setting, gefitinib had better PFS in EGFR mut+ patients than chemotherapy, while erlotinib had worse PFS in EGFR mut− patients. Even when used as first-line agents, EGFR-TKIs should be recommended to patients with EGFR mutation. Therefore, identifying EGFR mutation status is quite helpful to guide therapeutic regimen development. Based on these findings, it can be concluded that EGFR mutation is a biomarker to predict the benefit of EGFR-TKIs in both first-line and second-line settings.
Even with over 2791 and 3790 patient cases in first-line and second-line settings, respectively, this study failed to demonstrate the OS advantage of EGFR-TKIs over chemotherapy. The most likely reason is the high crossover rate after progression. In both first-line and second-line settings, none of the trials prohibited patients from crossing over to the other treatment arm. For example, in the first-line trial NEJ002, most of the patients randomly assigned to either gefitinib or chemotherapy had subsequent treatment. In the chemotherapy group, 94.6% of patients had second-line gefitinib therapy to control disease progression [32]. In the second-line INTEREST trial, 31% of patients in the gefitinib arm had docetaxel as subsequent therapy, while in the docetaxel arm, 37% of patients had subsequent EGFR-TKI therapy [24]. In addition, in the V-15-32 trial, 36% of patients in the gefitinib arm had docetaxel as subsequent therapy, while 53% of patients in the docetaxel arm had subsequent gefitinib therapy [33]. One recent review indicated that PFS advantage is unlikely to be linked with OS advantage due to the impact of subsequent salvage therapy. Prolongation of survival due to salvage therapy after disease progression limits the use of OS as an indicator of therapeutic efficiency [34].
Regarding severe drug toxicity, this study showed that although EGFR-TKIs produced more rashes, their risks of hematological adverse effects were significantly lower compared with chemotherapy. Rash can be properly managed through supportive care and protocol-defined dose reductions. The rarity of hematological events EGFR-TKIs contributes to substantial improvements in overall health status and quality of life of the patients compared with chemotherapy.
This study had several limitations. Firstly, EGFR mutation status was not fully examined in original studies. In second-line trials, 2431 out of 3790 (64%) patients had EGFR mutation status unidentified. Therefore, treatment efficacy in mut+ and mut− patients was only estimated based on a relatively small proportion (36%) of total patients. Secondly, the methods used to detect EGFR mutation were not consistent in the trials involved. Direct sequencing, PCR clamp, and amplification refractory mutation system were used in different trials. Since different methods have different sensitivities in detecting mutations, the possible bias associated with detection methods may directly influence the accuracy of pooled results.
Conclusions
All of the 3 EGFR-TKIs and gefitinib alone regimens had better effect in prolonging progression-free survival in EGFR mut+ patients in first-line and second-line settings, respectively, but chemotherapy seemed more effective in EGFR mut− patients than EGFR-TKIs. Therefore, accurate identification of EGFR mutation status is useful in deciding on an appropriate regimen for NSCLC patients.
Footnotes
Source of support: Self financing
References
- 1.Sun S, Schiller JH, Gazdar AF. Lung cancer in never smokers – a different disease. Nat Rev Cancer. 2007;7:778–90. doi: 10.1038/nrc2190. [DOI] [PubMed] [Google Scholar]
- 2.Sato M, Shames DS, Gazdar AF, et al. A translational view of the molecular pathogenesis of lung cancer. J Thorac Oncol. 2007;2:327–43. doi: 10.1097/01.JTO.0000263718.69320.4c. [DOI] [PubMed] [Google Scholar]
- 3.Bozcuk H, Gumus A, Ozbilim G, et al. Cluster analysis of p-glycoprotein, c-erb-B2 and P53 in relation to tumor histology strongly indicates prognosis in patients with operable non-small cell lung cancer. Med Sci Monit. 2005;11(6):HY11–20. [PubMed] [Google Scholar]
- 4.Suda K, Mizuuchi H, Maehara Y, et al. Acquired resistance mechanisms to tyrosine kinase inhibitors in lung cancer with activating epidermal growth factor receptor mutation--diversity, ductility, and destiny. Cancer Metastasis Rev. 2012;31:807–14. doi: 10.1007/s10555-012-9391-7. [DOI] [PubMed] [Google Scholar]
- 5.Hynes NE, Lane HA. ERBB receptors and cancer: the complexity of targeted inhibitors. Nat Rev Cancer. 2005;5:341–54. doi: 10.1038/nrc1609. [DOI] [PubMed] [Google Scholar]
- 6.Flego V, Ristic S, Devic Pavlic S, et al. Tumor necrosis factor-alpha gene promoter -308 and -238 polymorphisms in patients with lung cancer as a second primary tumor. Med Sci Monit. 2013;19:846–51. doi: 10.12659/MSM.889554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shepherd FA, Rodrigues Pereira J, Ciuleanu T, et al. Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med. 2005;353:123–32. doi: 10.1056/NEJMoa050753. [DOI] [PubMed] [Google Scholar]
- 8.Dahabreh IJ, Linardou H, Siannis F, et al. Somatic EGFR mutation and gene copy gain as predictive biomarkers for response to tyrosine kinase inhibitors in non-small cell lung cancer. Clin Cancer Res. 2010;16:291–303. doi: 10.1158/1078-0432.CCR-09-1660. [DOI] [PubMed] [Google Scholar]
- 9.Yoshida K, Yatabe Y, Park JY, et al. Prospective validation for prediction of gefitinib sensitivity by epidermal growth factor receptor gene mutation in patients with non-small cell lung cancer. J Thorac Oncol. 2007;2:22–28. [PubMed] [Google Scholar]
- 10.Rosell R, Moran T, Queralt C, et al. Screening for epidermal growth factor receptor mutations in lung cancer. N Engl J Med. 2009;361:958–67. doi: 10.1056/NEJMoa0904554. [DOI] [PubMed] [Google Scholar]
- 11.Paez JG, Janne PA, Lee JC, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science. 2004;304:1497–500. doi: 10.1126/science.1099314. [DOI] [PubMed] [Google Scholar]
- 12.Fukuoka M, Wu YL, Thongprasert S, et al. Biomarker analyses and final overall survival results from a phase III, randomized, open-label, first-line study of gefitinib versus carboplatin/paclitaxel in clinically selected patients with advanced non-small-cell lung cancer in Asia (IPASS) J Clin Oncol. 2011;29:2866–74. doi: 10.1200/JCO.2010.33.4235. [DOI] [PubMed] [Google Scholar]
- 13.Mitsudomi T, Morita S, Yatabe Y, et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol. 2010;11:121–28. doi: 10.1016/S1470-2045(09)70364-X. [DOI] [PubMed] [Google Scholar]
- 14.Inoue A, Kobayashi K, Maemondo M, et al. Updated overall survival results from a randomized phase III trial comparing gefitinib with carboplatin-paclitaxel for chemo-naive non-small cell lung cancer with sensitive EGFR gene mutations (NEJ002) Ann Oncol. 2013;24:54–59. doi: 10.1093/annonc/mds214. [DOI] [PubMed] [Google Scholar]
- 15.Han JY, Park K, Kim SW, et al. First-SIGNAL: first-line single-agent iressa versus gemcitabine and cisplatin trial in never-smokers with adenocarcinoma of the lung. J Clin Oncol. 2012;30:1122–28. doi: 10.1200/JCO.2011.36.8456. [DOI] [PubMed] [Google Scholar]
- 16.Zhou C, Wu YL, Chen G, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 2011;12:735–42. doi: 10.1016/S1470-2045(11)70184-X. [DOI] [PubMed] [Google Scholar]
- 17.Zhou C WY, Liu X, et al. Overall survival (OS) results from OPTIMAL (CTONG0802), a phase III trial of erlotinib (E) versus carboplatin plus gemcitabine (GC) as first-line treatment for Chinese patients with EGFR mutation-positive advanced non-small cell lung cancer (NSCLC) J Clin Oncol (Meeting Abstracts) 2012;30(Suppl) [Google Scholar]
- 18.Rosell R, Carcereny E, Gervais R, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012;13:239–46. doi: 10.1016/S1470-2045(11)70393-X. [DOI] [PubMed] [Google Scholar]
- 19.Sequist LV, Yang JC, Yamamoto N, et al. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol. 2013;31:3327–34. doi: 10.1200/JCO.2012.44.2806. [DOI] [PubMed] [Google Scholar]
- 20.Wu YL ZC, Hu CP, et al. LUX-Lung 6: A randomized, openlabel, phase III study of afatinib (A) versus gemcitabine/cisplatin (GC) as first-line treatment for Asian patients (pts) with EGFR mutationpositive (EGFR M+) advanced adenocarcinoma of the lung. J Clin Oncol (Meeting Abstracts) 2013:31. [Google Scholar]
- 21.Sekine I, Ichinose Y, Nishiwaki Y, et al. Quality of life and disease-related symptoms in previously treated Japanese patients with non-small-cell lung cancer: results of a randomized phase III study (V-15-32) of gefitinib versus docetaxel. Ann Oncol. 2009;20:1483–88. doi: 10.1093/annonc/mdp031. [DOI] [PubMed] [Google Scholar]
- 22.Sun JM, Lee KH, Kim SW, et al. Gefitinib versus pemetrexed as second-line treatment in patients with nonsmall cell lung cancer previously treated with platinum-based chemotherapy (KCSG-LU08-01): an open-label, phase 3 trial. Cancer. 2012;118:6234–42. doi: 10.1002/cncr.27630. [DOI] [PubMed] [Google Scholar]
- 23.Lee DH, Park K, Kim JH, et al. Randomized Phase III trial of gefitinib versus docetaxel in non-small cell lung cancer patients who have previously received platinum-based chemotherapy. Clin Cancer Res. 2010;16:1307–14. doi: 10.1158/1078-0432.CCR-09-1903. [DOI] [PubMed] [Google Scholar]
- 24.Kim ES, Hirsh V, Mok T, et al. Gefitinib versus docetaxel in previously treated non-small-cell lung cancer (INTEREST): a randomised phase III trial. Lancet. 2008;372:1809–18. doi: 10.1016/S0140-6736(08)61758-4. [DOI] [PubMed] [Google Scholar]
- 25.Ciuleanu T, Stelmakh L, Cicenas S, et al. Efficacy and safety of erlotinib versus chemotherapy in second-line treatment of patients with advanced, non-small-cell lung cancer with poor prognosis (TITAN): a randomised multicentre, open-label, phase 3 study. Lancet Oncol. 2012;13:300–8. doi: 10.1016/S1470-2045(11)70385-0. [DOI] [PubMed] [Google Scholar]
- 26.Garassino MC, Bettini A, Floriani I, et al. TAILOR: A phase III trial comparing erlotinib with docetaxel as the second-line treatment of NSCLC patients with wild-type (wt) EGFR. J Clin Oncol (Meeting Abstracts) 2012:30. [Google Scholar]
- 27.Gregorc V, Novello S, Lazzari C, et al. Predictive value of a proteomic signature in patients with non-small-cell lung cancer treated with second-line erlotinib or chemotherapy (PROSE): a biomarker-stratified, randomised phase 3 trial. Lancet Oncol. 2014;15:713–21. doi: 10.1016/S1470-2045(14)70162-7. [DOI] [PubMed] [Google Scholar]
- 28.Karampeazis A, Voutsina A, Souglakos J, et al. Pemetrexed versus erlotinib in pretreated patients with advanced non-small cell lung cancer: a Hellenic Oncology Research Group (HORG) randomized phase 3 study. Cancer. 2013;119:2754–64. doi: 10.1002/cncr.28132. [DOI] [PubMed] [Google Scholar]
- 29.Kawaguchi T, Ando M, Asami K, et al. Randomized Phase III Trial of Erlotinib Versus Docetaxel As Second- or Third-Line Therapy in Patients With Advanced Non-Small-Cell Lung Cancer: Docetaxel and Erlotinib Lung Cancer Trial (DELTA) J Clin Oncol. 2014;32:1902–8. doi: 10.1200/JCO.2013.52.4694. [DOI] [PubMed] [Google Scholar]
- 30.Schiller JH, Harrington D, Belani CP, et al. Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med. 2002;346:92–98. doi: 10.1056/NEJMoa011954. [DOI] [PubMed] [Google Scholar]
- 31.Bir F, Aksoy Altinboga A, et al. Potential utility of p63 expression in differential diagnosis of non-small-cell lung carcinoma and its effect on prognosis of the disease. Med Sci Monit. 2014;20:219–26. doi: 10.12659/MSM.890394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maemondo M, Inoue A, Kobayashi K, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. New Engl J Med. 2010;362:2380–88. doi: 10.1056/NEJMoa0909530. [DOI] [PubMed] [Google Scholar]
- 33.Maruyama R, Nishiwaki Y, Tamura T, et al. Phase III study, V-15-32, of gefitinib versus docetaxel in previously treated Japanese patients with non-small-cell lung cancer. J Clin Oncol. 2008;26:4244–52. doi: 10.1200/JCO.2007.15.0185. [DOI] [PubMed] [Google Scholar]
- 34.Hotta K, Kiura K, Fujiwara Y, et al. Role of survival post-progression in phase III trials of systemic chemotherapy in advanced non-small-cell lung cancer: a systematic review. PloS One. 2011;6:e26646. doi: 10.1371/journal.pone.0026646. [DOI] [PMC free article] [PubMed] [Google Scholar]