Abstract
Background
Human parathyroid hormone (PTH) (1-34) or teriparatide (TPTD) is an anabolic agent for osteoporosis. This recombinant protein stimulates positive bone formation balance and bone remodeling. However, when concomitantly used with antiresorptive (AR) agents, previous studies reported conflicting results in their potential additive and synergistic effects on bone metabolism and bone mineral density (BMD). This study aimed to integrate previous evidence to assess the effect of TPTD monotherapy and the additive effect of TPTD on AR agents in postmenopausal women with osteoporosis.
Material/Methods
This meta-analysis identified 9 RCTs from databases. To assess the therapeutic effect on osteoporosis, the weighted mean differences (WMDs) were used to pool the percentage change of BMD along with the 95% confidence intervals (CIs). BMD of 3 skeletal sites, including lumbar spine, total hip, and femoral neck were assessed.
Results
TPTD alone could significantly improve BMD of all 3 skeletal sites compared with placebo, although the effect on the femoral neck was less conclusive. The additive effect of TPTD over hormone replacement therapy (HRT) and denosumab (DEN) agents is evident in all 3 skeletal sites. But TPTD plus Alendronate (ALN) did not demonstrate additive effect in total hip and femoral neck.
Conclusions
TPTD alone could significantly improve BMD of lumbar spine, total hip, and femoral neck. BMD outcomes of concomitant use of TPTD and AR agents are site-dependent and vary depending on the specific AR agent used and the timing of AR therapy initiation.
MeSH Keywords: Bone Density, Meta-Analysis, Osteoporosis, Teriparatide
Background
Osteoporosis is characterized as microarchitectural deterioration of bone tissue and decreased bone mass [1]. Postmenopausal woman are more vulnerable to osteoporosis, partly due to estrogen deficiency and rapid loss of calcium [2,3]. Estrogen deficiency can directly result in excessive bone resorption and inadequate bone formation [4,5]. Therefore, postmenopausal women are a population with high risk of fracture. Although the option for osteoporosis treatment has expanded greatly due to the emerging agents during the past decade, none of them could restore skeletal integrity in most patients with established osteoporosis [6,7]. Development of effective therapy is thus quite meaningful for this group of patients.
Currently, the medications approved for postmenopausal osteoporosis mainly include 2 categories. The first category is the antiresorptive (AR) agents, including nitrogen-containing bisphosphonates (such as Alendronate (ALN)), hormone replacement therapy (HRT), denosumab (DEN), raloxifene (RAL), and calcitonin. Bisphosphonates are established as the first-line treatment options for postmenopausal osteoporosis [4]. Although these agents differ in cellular and molecular mechanisms, they have the same ability to inhibit osteoclastic bone resorption. The second category is the anabolic agents such as human parathyroid hormone (PTH) (1-84) and teriparatide [(PTH) 1-34 (TPTD)]. These recombinant proteins stimulate positive bone formation balance and subsequent bone remodeling [8]. An animal study and subsequent randomized controlled studies (RCTs) generally found that daily injection of TPTD contributed to restoration of bone mass and strength [9–11]. Previous attempts to combine 2 or more antiresorptive agents failed to demonstrate significant additive effects [12]. However, the various mechanisms of antiresorptive and anabolic agents suggest that their combination might lead to additive bone formation and bone resorption inhibition effect, which is better than the use of either alone [6]. However, previous studies concerning this combination reported conflicting results in their potential additive and synergistic effects on bone metabolism and bone mineral density (BMD) [6]. Therefore, effects of this combination therapy are still not quite clear. This study aimed to integrate previous evidence to assess the effect of TPTD monotherapy and the additive effect of TPTD on antiresorptive agents in postmenopausal women with osteoporosis.
Material and Methods
Search strategy
Relevant trials were searched in the electronic databases, including PubMed, Medline, the Cochrane Central Register of Controlled Trials (CENTRAL), and ClinicalTrial.gov from January 1990 to June 2014. The following keywords and strategy were used for searching: (“osteoporosis”) AND (“parathyroid hormone” OR “teriparatide” OR “PTH”) AND (“random” OR “randomized” OR “randomly”). After a preliminary search, reference lists of qualified trials were searched manually to ensure all relevant and qualified studies were included. If updated or duplicated studies were found, only the studies include all participants and with the latest outcomes were included. No language restriction was set during searching. Qualified trials were included regardless of publication status. Therefore, both published and unpublished studies were included as long as they met the inclusion criteria.
Inclusion criteria
The following criteria were set for screening eligible studies for this meta-analysis: (1) randomized controlled trials (RCTs); (2) participants were postmenopausal woman with primary osteoporosis; (3) participants were randomly assigned to daily subcutaneous injection of TPTD or a combination of daily TPTD with antiresorptive agents in experimental arm, with placebo or antiresorptive agent alone in control arm; (4) treatment lasted at least 6 months; (5) detailed data of percentage change of BMD from baseline to the end of follow-up of the experiment and control group could be extracted. Studies with TPTD plus antiresorptive agent in the experiment arm, but only with TPTD alone in the control arm were excluded. Two authors independently performed the search process and screened the trials according to the criteria. Disagreements were resolved by group discussion.
Quality assessment of the included trials was based on the methodological quality item of RCT according to the Cochrane Handbook for Systematic Reviews of Interventions. The 6 quality items include random sequence generation, allocation concealment, blinding of patient and personnel, blinding of outcome assessment, incomplete outcome data, and selective reporting risk. Publication bias was assessed by visual inspection of funnel plots. The risk of publication bias is revealed if the plots are asymmetrical about the pooled WMD of percentage change of BMD.
Data extraction and analysis
Two authors (JS and ZJ) independently performed data extraction. Crosschecking was performed by a third author (FC) to ensure accuracy of the data. Original data extracted included first author, year of publication, place in which the study was conducted, number of participants and medication strategies in experiment and control arm, duration of study, BMD sites measured, and methods to measure BMD at the end of follow-up. The primary outcome assessed was the percentage change of BMD in 3 skeletal sites, including lumbar spine, total hip, and femoral neck. All data synthesis and analysis in this study were performed using RevMan 5.2 (Cochrane Collaboration). The weighted mean differences (WMDs) were used to pool the percentage change of BMD along with the 95% confidence intervals (CIs). If a study had more than 1 TPTD dosage group, the different dose group was treated as a different experiment arm. To assess the additive effect of combined TPTD and AR agents and to minimize possible heterogeneity, subgroup analysis was performed by stratifying AR agents.
Between-studies heterogeneity was assessed with Chi square-based Q test and I2. P<0.1 or I2>50% was considered as significant heterogeneity. A primary analysis was performed with a fixed-effects model. If I2≤50% and p≥0.1, a fixed-effects model with Mantel-Haenszel method was used to make estimates. However, if significant heterogeneity was observed, the sources of heterogeneity were further analyzed. If there was no significant clinical heterogeneity, a random-effects model was used. The significance of pooled estimates was assessed with Z test and p<0.05 was considered as significant. Sensitivity analyses were then conducted to test the robustness of the results by excluding the trial with the largest weight that might dominant the finding.
Results
Searching and screening of eligible trials
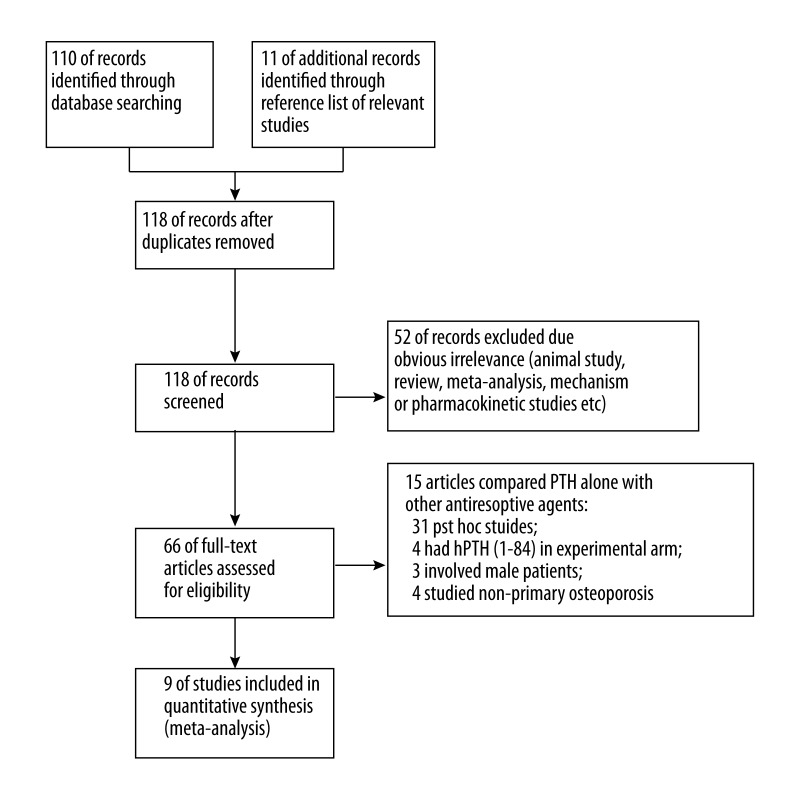
A total of 121 studies were obtained from the primary search in the databases and the reference list of relevant studies. The detailed screening process is given in Figure 1. Finally, 9 RCTs were included in this meta-analysis [10,11,13–19].
Figure 1.
The general search process.
Characteristics and quality of trials included
These 9 RCTs involved a total of 2447 participants, with 1536 in the experimental group and 911 in the control group. Four studies compared TPTD alone vs. placebo [10,11,13,14] and 5 studies compared TPTD plus AR agents vs. AR agents alone [15–19] (Table 1). Particularly, 2 studies had multi-intervention arms [10,11]. In the following forest plot figures, Neer 2001a and 2001b represented 20 and 40 μg hPTH (1-34)/d group, while Miyauchi 2008a, 2008b, and 2008c represented 10, 20, and 40 μg hPTH (1-34)/d group, respectively. Among the 5 trials with TPTD plus AR agents as experiment arm, 2 assessed TPTD plus ALN [17,18], 2 assessed TPTD plus HRT [15,16], and 1 assessed TPTD plus DEN [19]. The dose of TPTD ranged from 10 to 40 μg/d in the 9 trials. Duration varied from 6 months to 24 months. Lumbar spine, total hip, and femoral neck were the most common sites with BMD measured. In all studies involved, BMD was measured by dual-energy x-ray absorptiometry (DXA) (Table 1). The quality of the included studies is summarized in Table 2.
Table 1.
Characteristics of trials included.
| Study | No patients | Delivery | Intervention | Control | Concomitant therapy | Duration | Skeletal site of BMD measured | BMD assessment | |
|---|---|---|---|---|---|---|---|---|---|
| E | C | ||||||||
| Neer 2001 | 541/552 | 544 | SI | 20/40 μg hPTH (1-34)/d | Placebo | Calcium: 1000 mg/d; Vitamin D: 400–1200 IU/d | 21 m | LS, total hip, femoral neck, trochanter, intertrochanter, distal radius, radius shaft, TB | DXA |
| Sethi 2008 | 41 | 41 | SI | 20 μg hPTH (1-34)/d | Placebo | Calcium: 1000 mg/d; Vitamin D: 500 IU/d | 6 m | LS, femoral neck | DXA |
| Miyauchi 2008 | 39/39/38 | 38 | SI | 10/20/40 μg hPTH (1-34)/d | Placebo | Calcium: 610 mg/d; Vitamin D: 400 IU/d | 6 m | LS, total hip, femoral neck | DXA |
| Cosman 2010 | 33 | 33 | SI | 20 μg hPTH (1-34)/d | Placebo | Calcium: 500 mg/d; Vitamin D: 1000 IU/d | 24 m | LS, total hip, femoral neck, distal forearm | DXA |
| Cosman 2001 | 27 | 25 | SI | 25 μg hPTH (1-34)/d + HRT | HRT | Calcium: 1200–1500 mg/d; Vitamin D: 400 IU/d | 36 m | LS, total hip, TB | DXA |
| Ste-Marie 2006 | 122 | 125 | SI | 20 μg hPTH (1-34)/d + HRT | HRT | Calcium: 1000 mg/d; Vitamin D: 400–1200 IU/d | 13.8 m | LS, total hip, femoral neck, distal radius, TB | DXA |
| Cosman 2005 | 43 | 43 | SI | 25 μg hPTH (1-34)/d +ALN | ALN | Calcium: 1200–1500 mg/d; Vitamin D: maintain at least 20 ng/ml at serum level | 15 m | LS, total hip | DXA |
| Finkelstein 2010 | 31 | 31 | SI | 40 μg hPTH (1-34)/d +ALN | ALN/TPTD | Calcium: 1000–1200 mg/d; Vitamin D: 400 IU/d | 6 m | LS, total hip, femoral neck, radius shaft, TB, spinal trabecular | DXA |
| Tsai 2013 | 30 | 31 | SI | 25 μg hPTH (1-34)/d + DEN | DEN/TPTD | Calcium: 1200 mg/d; Vitamin D: maintain at least 50 nmol/L at serum level | 12 m | LS, total hip, femoral neck, radius shaft | DXA |
HRT – hormone replacement therapy; ALN – alendronate; BMD – bone mineral density; SI – subcutaneous injection; LS – lumbar spine; TB – total body; hPTH – human parathyroid hormone; DEN – Denosumab; DXA – dual-energy x-ray absorptiometry.
Table 2.
Quality assessments of RCTs included.
| Study/ quality components | Adequate random sequence generation (selection bias) | Adequate method of allocation concealment (selection bias) | Blinding of participants and personnel (performance bias) | Blinding of outcome assessment (detection bias) | Incomplete outcome data (attrition bias) | Selective reporting (reporting bias) |
|---|---|---|---|---|---|---|
| Neer 2001 | ? | ? | ? | ? | ? | Y |
| Sethi 2008 | ? | ? | N | ? | Y | Y |
| Miyauchi 2008 | ? | ? | Y | ? | ? | Y |
| Cosman 2010 | Y | ? | ? | Y | ? | Y |
| Cosman 2001 | Y | ? | ? | Y | ? | Y |
| Ste-Marie 2006 | ? | ? | Y | ? | ? | Y |
| Cosman 2005 | Y | ? | N | Y | Y | Y |
| Finkelstein 2010 | Y | ? | N | Y | ? | Y |
| Tsai 2013 | Y | Y | ? | ? | Y | Y |
‘Y’ – indicating low risk of bias; ‘N’ – indicating high risk of bias; ‘?’ – indicating insufficient data for judgment.
The effect of TPTD on BMD of lumbar spine
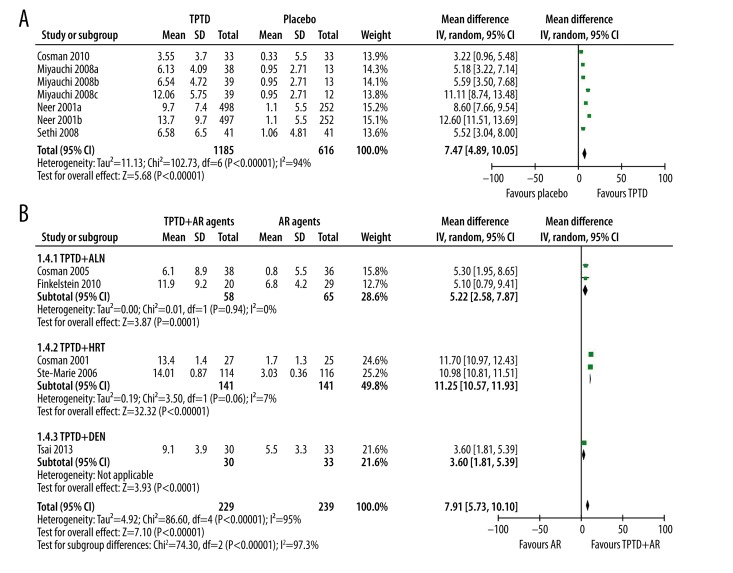
Four studies reported the effect of TPTD alone vs. placebo and 5 studies reported TPTD+AR agents vs. AR agents on BMD of the lumbar spine. Generally, TPTD alone could significantly improve BMD of the lumbar spine (WMD: 7.47%, 95% CI 4.89% to 10.05%, p<0.00001). Significant between-study heterogeneity was observed (p<0.00001, I2=94%) (Figure 2A). However, these studies reported consistent findings of the benefits of TPTD over placebo. The major cause of the heterogeneity is the various dose-related therapeutic effects. Sensitivity analysis showed that exclusion of the Neer et al. study and the 40 μg/d group of Miyauchi et al. (Miyauchi 2008c) could significantly reduce heterogeneity, while the overall benefits of TPTD did not change (WMD: 4.90%, 95% CI 3.82% to 5.99%, p<0.00001, I2=0%).
Figure 2.
The effect of single and combined use of TPTD on BMD of lumbar spine. (A) Single of use TPTD; (B) Combined use of TPTD with antiresorptive agents.
When TPTD was combined with AR agents, although the general analysis showed that the combination contributed to better BMD improvement than AR agents alone (WMD: 7.91%, 95% CI 5.73% to 10.10%, p<0.00001), significant between-study heterogeneity (I2=95%, P<0.00001) and subgroup difference (I2=97.3%, P<0.000001) was observed. Subgroup analysis showed that the additive effect was significant in all 3 subgroups: TPTD+ALN group (WMD: 5.22%, 95% CI 2.58% to 7.87%, p=0.0001, I2=0%), TPTD+HRT group (WMD: 11.25%, 95% CI 10.57% to 11.93%, p<0.00001, I2=71%), and TPTD+DEN group (WMD: 3.60%, 95% CI 1.81% to 5.39%, p<0.0001) (Figure 2B).
The effect of TPTD on BMD of total hip
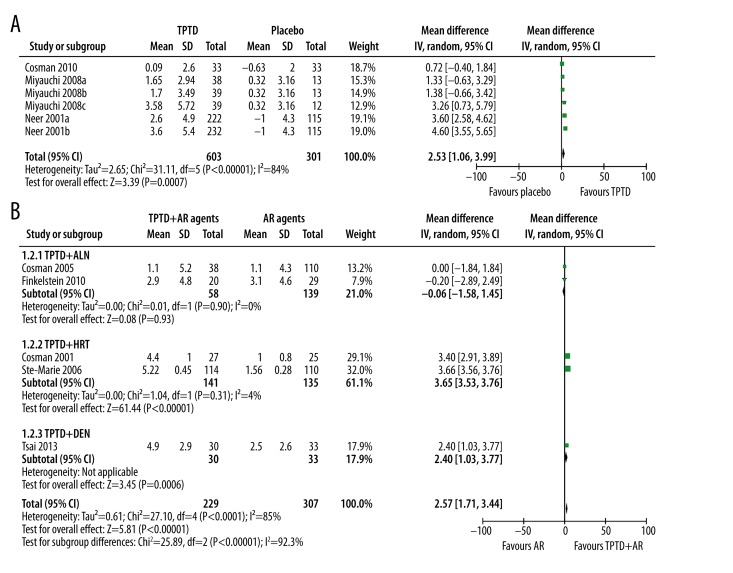
Three studies reported the effect of TPTD alone vs. placebo and 5 studies reported TPTD+AR agents vs. AR agents on BMD of total hip. Generally, TPTD alone could significantly improve BMD of total hip (WMD: 2.53%, 95% CI 1.06% to 3.99%, p=0.0007). However, significant heterogeneity was observed (p<0.00001, I2=84%) (Figure 3A). Subsequent sensitivity analysis showed that exclusion of Neer et al. study, which had the largest sample size that might dominate the pooled result, did not change TPTD’s benefits, but significantly reduced heterogeneity (WMD: 1.26%, 95% CI 0.36% to 2.15%, p=0.006, I2=9%).
Figure 3.
The effect of single and combined use of TPTD on BMD of total hip. (A) Single of use TPTD; (B) Combined use of TPTD with antiresorptive agents.
When TPTD was combined with AR agents, although the general analysis showed that the combination contributed to better BMD improvement than AR agents alone (WMD: 2.57%, 95% CI 1.71% to 3.44%, p<0.00001), significant between-study heterogeneity (I2=85%, P<0.0001) and subgroup difference (I2=92.3%, P<0.00001) was observed. Subgroup analysis showed that the additive effect were significant in TPTD+HRT (WMD: 3.65%, 95% CI 3.53% to 3.76%, p<0.00001, I2=4%) and TPTD+DEN group (WMD: 2.40%, 95% CI 1.03% to 3.77%, p<0.0006), but not in TPTD+ALN group (WMD: −0.06%, 95% CI −1.58% to 1.45%, p=0.93, I2=0%) (Figure 3B).
The effect of TPTD on BMD of the femoral neck
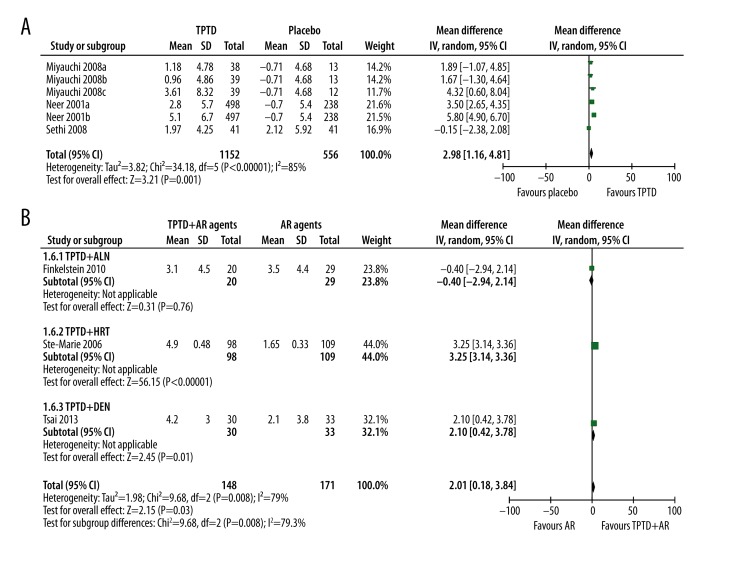
Three studies reported the effect of TPTD alone vs. placebo and 3 studies reported TPTD+AR agents vs. AR agents on BMD of the femoral neck. Generally, TPTD alone could significantly improve BMD of the femoral neck (WMD: 2.98%, 95% CI 1.16% to 4.81%, p=0.001). Significant between-study heterogeneity was observed (p<0.00001, I2=85%) (Figure 4A). Subsequent sensitivity analysis showed that exclusion of Neer et al. study (with the largest weight) significantly reduced heterogeneity, but the overall benefits of TPTD also changed to a non-significant level (WMD: 1.55%, 95% CI −0.19% to 3.29%, p=0.08, I2=31%).
Figure 4.
The effect of single and combined use of TPTD on BMD of femoral neck. (A) Single of use TPTD; (B) Combined use of TPTD with antiresorptive agents.
When TPTD was combined with AR agents, although the general analysis showed that the combination contributed to better BMD improvement than AR agents alone (WMD: 2.01%, 95% CI 0.18% to 3.84%, p=0.03), significant heterogeneity in subgroup difference (I2=79%, p=0.008) was observed. Subgroup analysis showed that the additive effects were significant in the TPTD+HRT (WMD: 3.25%, 95% CI 3.14% to 3.36%, p<0.00001) and TPTD+DEN group (WMD: 2.10%, 95% CI 0.42% to 3.78%, p=0.01), but not in the TPTD+ALN group (WMD: −0.40%, 95% CI −2.94% to 2.14%, p=0.76) (Figure 4B).
Publication bias
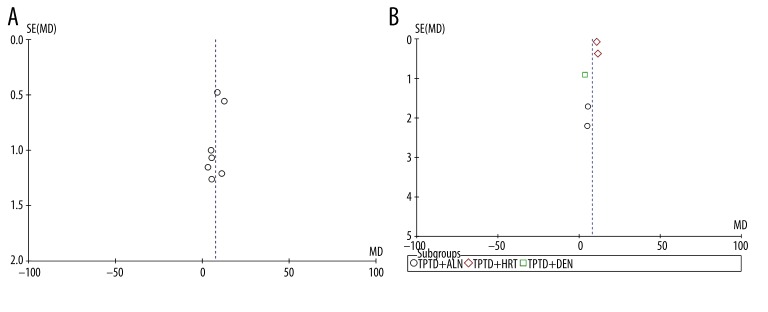
Funnel plots for studies reporting the BMD change of the lumbar spine in TPTD vs. placebo (Figure 5A) and TPTD+AR agents vs. AR agents alone (Figure 5B) were used to assess publication bias. The 2 plots demonstrate mild asymmetry, suggesting potential publication bias. However, due to the small number of studies involved in this meta-analysis, it is difficult to estimate the publication bias accurately.
Figure 5.
Funnel plot analysis of publication bias. (A) BMD change of the lumbar spine in TPTD group vs. placebo group. (B) MD change of the lumbar spine in TPTD+AR agents group vs. AR agents alone group.
Discussion
This study generally confirmed that TPTD alone could significantly improve BMD at the 3 skeletal sites compared with placebo for postmenopausal women with osteoporosis, although the effect on the femoral neck was less conclusive. The additive effect of TPTD over AR agents was clearly evident in most of the skeletal sites, except TPTD plus ALN in the site of total hip and femoral neck. This finding is consistent with previous studies that showed PTH treatment alone could improve BMD significantly [20,21]. Although Schwarz et al. observed an age-dependent therapeutic effect of PTH on BMD of spine [20], according to Boonen et al. large RCT, this treatment-by-age interaction might be caused by increase in BMD in the control group. In addition, there is no treatment-by-age interaction in hip and femoral neck [20,21], suggesting the therapeutic effect of TPTD was consistent in the older and younger women.
When used as monotherapy, TPTD showed consistent effects over placebo in all 3 skeletal sites, including lumbar spine, total hip, and femoral neck. In all treatment groups, bone formation marker, and serum procollagen type I N-terminal propeptide (P1NP) levels increased significantly, suggesting the positive effects of TPTD on bone formation. When TPTD is concomitantly used with AR agents, the additive effects were significantly affected by the specific AR agents used and the timing of introduction of AR agents. Muschitz’s recent study showed that addition of ALN 9 months after TPTD treatment could significantly improve BMD of both lumbar spine and hip region compared with TPTD monotherapy [22]. However, trials by Finkelstein and Cosman included in this meta-analysis all had ALN administration long before (6 months and 1 year respectively) TPTD therapy. The distinct BMD results of these trials could be partly explained by the different time point of initiation of ALN treatment, which might affect the balance between bone formation and resorption. In Finkelstein’s study, although TPTD plus ALN contributed to slightly increased bone formation marker P1NP, the increase was significantly smaller than that of TPTD monotherapy. At 1-year measurement after initiation of TPTD monotherapy or concomitant use of TPTD and ALN, P1NP level in the TPTD plus ALN group was only approximately 1/3 that of the TPTD alone group. However, Muschitz observed that at the end of combination therapy (9 months), P1NP level of the TPTD plus ALN group was still more than half that of the TPTD alone group. Therefore, adding ALN after long-term TPTD administration seemed have less influence on P1NP level compared with adding TPTD after long-term ALN administration. In other studies that combined TPTD and bisphosphonates for postmenopausal women with osteoporosis, the results were also far from satisfying. Cosman et al. studied the effect of adding TPTD to ongoing RAL therapy compared with switching to TPTD monotherapy for 18 months and did not find any significant difference in BMD between these groups [23]. In another study exploring combined intravenous zoledronic acid and TPTD as the initial treatment for bisphosphonate-naive patients over 1 year, the BMD increase in the combination group and in the zoledronic acid monotherapy group had no significant difference [24]. These results suggest that bisphosphonates might negatively affect the balance between bone formation and resorption of anabolic treatment [18], but its effect might be significantly related to the time point of treatment initiation. TPTD plus ALN might not be beneficial when applied after long-term treatment with ALN alone. However, this combination might generate some additive effects when used after anabolic therapies through facilitating mineralization of the newly formed bone.
For postmenopausal women with osteoporosis, hormone deficiency is definitely a cause of osteoporosis [7]. With the long-term HRT, the addition of TPTD helped to generate excessive increases in BMD. Findings of this study showed consistent additive effects of TPTD over HRT. One early study, but with accurate data unavailable, also showed that the combination of PTH (1-34) with HRT was beneficial to improve BMD of vertebral and total-body bone [25]. The combination of TPTD with DEN has little influence on TPTD-induced modeling-based bone. Thus, this combination also has an additive effect over monotherapy [26]. Currently, the pharmacological mechanism for the lack of additive effects in the combination of TPTD and selective AR agents remain largely unknown. Thus, more pharmacological studies are required to explore the underlying mechanisms.
This study also has several limitations. First, significant heterogeneity was observed when pooling BMD in the monotherapy and combined therapy groups. However, with subgroup and sensitivity analysis, the sources of heterogeneity was identified and solved. The heterogeneity was mainly related to specific agents and the dose used. Second, although heterogeneity was significantly reduced through subgroup analysis, the number of trials in each subgroup is relatively small. This might lead to risk of bias in reporting results. Thus, more large RCTs are required to confirm findings of this study. Third, although BMD is an important indicator of osteoporosis, it cannot directly reflect the risk of fracture. Only 2 trials in a monotherapy group and 1 study in a combined therapy group reported fracture results. Thus, it was impossible to make a pooled analysis to assess the risk of fracture in the current study.
Conclusions
TPTD alone could significantly improve BMD of the lumbar spine, total hip, and femoral neck. BMD outcomes of concomitant use of TPTD and AR agents are site-dependent and vary depending on the specific AR agent used and the timing of AR therapy initiation.
Footnotes
Conflicts of interest
The authors had no conflicts of interest.
Source of support: Departmental sources
References
- 1.Nih Consensus Development Panel on Osteoporosis Prevention D, Therapy. Osteoporosis prevention, diagnosis, and therapy. Jama. 2001;285(6):785–95. doi: 10.1001/jama.285.6.785. [DOI] [PubMed] [Google Scholar]
- 2.Riggs BL, Khosla S, Melton LJ., III A unitary model for involutional osteoporosis: estrogen deficiency causes both type I and type II osteoporosis in postmenopausal women and contributes to bone loss in aging men. J Bone Miner Res. 1998;13(5):763–73. doi: 10.1359/jbmr.1998.13.5.763. [DOI] [PubMed] [Google Scholar]
- 3.Godfrin-Valnet M, Khan KA, Guillot X, et al. Sirtuin 1 activity in peripheral blood mononuclear cells of patients with osteoporosis. Med Sci Monit Basic Res. 2014;20:142–45. doi: 10.12659/MSMBR.891372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Epstein S. Update of current therapeutic options for the treatment of postmenopausal osteoporosis. Clin Ther. 2006;28(2):151–73. doi: 10.1016/j.clinthera.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 5.Heiss C, Govindarajan P, Schlewitz G, et al. Induction of osteoporosis with its influence on osteoporotic determinants and their interrelationships in rats by DEXA. Med Sci Monit. 2012;18(6):BR199–207. doi: 10.12659/MSM.882895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cosman F. Combination therapy for osteoporosis: a reappraisal. Bonekey Rep. 2014;3:518. doi: 10.1038/bonekey.2014.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Govindarajan P, Schlewitz G, Schliefke N, et al. Implications of combined ovariectomy/multi-deficiency diet on rat bone with age-related variation in bone parameters and bone loss at multiple skeletal sites by DEXA. Med Sci Monit Basic Res. 2013;19:76–86. doi: 10.12659/MSMBR.883815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dobnig H, Sipos A, Jiang Y, et al. Early changes in biochemical markers of bone formation correlate with improvements in bone structure during teriparatide therapy. J Clin Endocrinol Metab. 2005;90(7):3970–77. doi: 10.1210/jc.2003-1703. [DOI] [PubMed] [Google Scholar]
- 9.Kimmel DB, Bozzato RP, Kronis KA, et al. The effect of recombinant human (1-84) or synthetic human (1-34) parathyroid hormone on the skeleton of adult osteopenic ovariectomized rats. Endocrinology. 1993;132(4):1577–84. doi: 10.1210/endo.132.4.8462456. [DOI] [PubMed] [Google Scholar]
- 10.Neer RM, Arnaud CD, Zanchetta JR, et al. Effect of parathyroid hormone (1-34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N Engl J Med. 2001;344(19):1434–41. doi: 10.1056/NEJM200105103441904. [DOI] [PubMed] [Google Scholar]
- 11.Miyauchi A, Matsumoto T, Shigeta H, et al. Effect of teriparatide on bone mineral density and biochemical markers in Japanese women with postmenopausal osteoporosis: a 6-month dose-response study. J Bone Mineral Metab. 2008;26(6):624–34. doi: 10.1007/s00774-008-0871-3. [DOI] [PubMed] [Google Scholar]
- 12.Compston J. The use of combination therapy in the treatment of postmenopausal osteoporosis. Endocrine. 2012;41(1):11–18. doi: 10.1007/s12020-011-9554-2. [DOI] [PubMed] [Google Scholar]
- 13.Sethi BK, Chadha M, Modi KD, et al. Efficacy of teriparatide in increasing bone mineral density in postmenopausal women with osteoporosis – an Indian experience. J Assoc Physicians India. 2008;56:418–24. [PubMed] [Google Scholar]
- 14.Cosman F, Lane NE, Bolognese MA, et al. Effect of transdermal teriparatide administration on bone mineral density in postmenopausal women. J Clin Endocrinol Metab. 2010;95(1):151–58. doi: 10.1210/jc.2009-0358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cosman F, Nieves J, Woelfert L, et al. Parathyroid hormone added to established hormone therapy: effects on vertebral fracture and maintenance of bone mass after parathyroid hormone withdrawal. J Bone Miner Res. 2001;16(5):925–31. doi: 10.1359/jbmr.2001.16.5.925. [DOI] [PubMed] [Google Scholar]
- 16.Ste-Marie LG, Schwartz SL, Hossain A, et al. Effect of teriparatide [rhPTH(1-34)] on BMD when given to postmenopausal women receiving hormone replacement therapy. J Bone Miner Res. 2006;21(2):283–91. doi: 10.1359/JBMR.051020. [DOI] [PubMed] [Google Scholar]
- 17.Cosman F, Nieves J, Zion M, et al. Daily and cyclic parathyroid hormone in women receiving alendronate. N Engl J Med. 2005;353(6):566–75. doi: 10.1056/NEJMoa050157. [DOI] [PubMed] [Google Scholar]
- 18.Finkelstein JS, Wyland JJ, Lee H, Neer RM. Effects of teriparatide, alendronate, or both in women with postmenopausal osteoporosis. J Clin Endocrinol Metab. 2010;95(4):1838–45. doi: 10.1210/jc.2009-1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tsai JN, Uihlein AV, Lee H, et al. Teriparatide and denosumab, alone or combined, in women with postmenopausal osteoporosis: the DATA study randomised trial. Lancet. 2013;382(9886):50–56. doi: 10.1016/S0140-6736(13)60856-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schwarz P, Jorgensen NR, Mosekilde L, Vestergaard P. Effects of increasing age, dosage, and duration of PTH treatment on BMD increase – a meta-analysis. Calcif Tissue Int. 2012;90(3):165–73. doi: 10.1007/s00223-011-9564-3. [DOI] [PubMed] [Google Scholar]
- 21.Boonen S, Marin F, Mellstrom D, et al. Safety and efficacy of teriparatide in elderly women with established osteoporosis: bone anabolic therapy from a geriatric perspective. J Am Geriatr Soc. 2006;54(5):782–89. doi: 10.1111/j.1532-5415.2006.00695.x. [DOI] [PubMed] [Google Scholar]
- 22.Muschitz C, Kocijan R, Fahrleitner-Pammer A, et al. Antiresorptives overlapping ongoing teriparatide treatment result in additional increases in bone mineral density. J Bone Miner Res. 2013;28(1):196–205. doi: 10.1002/jbmr.1716. [DOI] [PubMed] [Google Scholar]
- 23.Cosman F, Wermers RA, Recknor C, et al. Effects of teriparatide in postmenopausal women with osteoporosis on prior alendronate or raloxifene: differences between stopping and continuing the antiresorptive agent. J Clin Endocrinol Metab. 2009;94(10):3772–80. doi: 10.1210/jc.2008-2719. [DOI] [PubMed] [Google Scholar]
- 24.Cosman F, Eriksen EF, Recknor C, et al. Effects of intravenous zoledronic acid plus subcutaneous teriparatide [rhPTH(1-34)] in postmenopausal osteoporosis. J Bone Miner Res. 2011;26(3):503–11. doi: 10.1002/jbmr.238. [DOI] [PubMed] [Google Scholar]
- 25.Lindsay R, Nieves J, Formica C, et al. Randomised controlled study of effect of parathyroid hormone on vertebral-bone mass and fracture incidence among postmenopausal women on oestrogen with osteoporosis. Lancet. 1997;350(9077):550–55. doi: 10.1016/S0140-6736(97)02342-8. [DOI] [PubMed] [Google Scholar]
- 26.Miller PD, Delmas PD, Lindsay R, et al. Early responsiveness of women with osteoporosis to teriparatide after therapy with alendronate or risedronate. J Clin Endocrinol Metab. 2008;93(10):3785–93. doi: 10.1210/jc.2008-0353. [DOI] [PMC free article] [PubMed] [Google Scholar]