Abstract
Background
Cell types are defined at the molecular level during embryogenesis by a process called pattern formation and created by the selective utilization of combinations of sequence specific transcription factors. Developmental programs define the sets of genes that are available to each particular cell type, and real-time biochemical signaling interactions define the extent to which these sets are used at any given time and place. Gene expression is regulated through the integrated action of many cis-regulatory elements, including core promoters, enhancers, silencers, and insulators. The chromatin state in developing body parts provides a code to cellular populations that direct their cell fates. Chromatin profiling has been a method of choice for mapping regulatory sequences in cells that go through developmental transitions.
Results
We used antibodies against histone H3 lysine 4 trimethylations (H3K4me3) a modification associated with promoters and open/active chromatin, histone H3 lysine 27 trimethylations (H3K27me3) associated with Polycomb-repressed regions and RNA polymerase II (Pol2) associated with transcriptional initiation to identify the chromatin state signature of the mouse forelimb during mid-gestation, at embryonic day 12 (E12). The families of genes marked included those related to transcriptional regulation and embryogenesis. One third of the marked genes were transcriptionally active while only a small fraction were bivalent marked. Sequence specific transcription factors that were activated were involved in cell specification including bone and muscle formation.
Conclusion
Our results demonstrate that embryonic limb cells do not exhibit the plasticity of the ES cells but are rather programmed for a finer tuning for cell lineage specification.
Keywords: mouse genome, chromatin, forelimb, sequence-specific transcription factors
Introduction
One of the challenges in biology is understanding how one genome can generate an organism composed of hundreds of distinct cell types. The gene expression programs that specify and maintain cell states in mammals are controlled by thousands of transcription factors, cofactors and chromatin modifiers. Mis-regulation of these gene expression programs can lead to cellular transformation, organ malfunction, and disease. Identifying the cell state (type) based on the specific combinatorial gene regulatory networks can allow for cell reprogramming when a cell fails to follow the correct route or has lost its molecular memory over time. Embryonic development is the process that normally creates “cell types.” In mammals, the process of specifying “cell types” occurs during pattern-formation events that require highly complex spatial and temporal gene regulatory networks. A cell can execute the process of staying in, or exiting the cell cycle, and entering the post-mitotic differentiation state by using specific cis-regulatory modules (CRMs). CRMs are several hundred base pairs long, can exert their influence over distances as long as 100 kb, are comprised of multiple sequence-specific transcription factor (SSTF) binding sites and can direct the expression of developmental SSTFs and signaling molecules.1 CRMs act as switches to determine “availability” of associated loci for expression.2,3 SSTFs initiate lineage-specific gene expression programs, and epigenetic regulation contributes to stabilization of expression patterns.
The combination of chromatin immunoprecipitation (ChIP) and massive parallel sequencing (ChIP-seq) allows the thorough detection of protein-DNA interactions and enables the identification of regulatory events central to biological processes and disease states.4–7 Histone modifications modulate transcriptional initiation, elongation and enhancer activity or repression.8 Commonly studied interactions utilizing ChIP-seq include RNA polymerases, SSTFs, and histone marks.9 The advantage of this system is its high-throughput nature and ability to identify genome-wide de novo molecular interactions. ChIP-seq is one of the primary techniques used in the various Encyclopedia of DNA Elements (ENCODE) projects, with the goal of identifying functional elements in the genomes of humans, with similar consortiums focusing on model organisms.10
Open chromatin is characterized by the presence of histone modifications, such as Histone H3 lysine 4 trimethylation (H3K4me3), Histone H3 lysine 9 acetylation (H3K9ac) and Histone H4 acetylation (H4ac), while closed chromatin is characterized by the presence of Histone H3 lysine 9 trimethylation (H3K9me3) and Histone H3 lysine 27 trimethylation (H3K27me3). The presence of H3K4me3 on a promoter is a hallmark of active genes, open chromatin, and is highly correlated with the presence of RNA polymerase II (Pol2).11 Conversely, H3K27me3 is a marker of repressed genes, which negatively regulates transcription by promoting heterochromatin formation.12 The methylation of H3K4 and H3K27 is catalyzed by the highly conserved Trithorax (TrxG) and Polycomb (PcG) groups of proteins, respectively, and act as opposing forces to regulate transcription.12,13 However, H3K4me3 and H3K27me3 are not mutually exclusive and can bivalently mark chromatin. Bivalent genes are poised for either activation or repression, are often expressed at a low level, and tend to lose H3K27me3 marks as they differentiate from a stem cell state to a committed state.14 Broad H3K27me3 marks on bivalent genes were associated with a general repression of expression, and more “peak like” marks on the promoter region were often correlated with active gene expression.15 Thus, bivalently marked regions are of particular interest when studying developmental networks.
Methods
Mice
All research was conducted in accordance with Oregon State University Institutional Animal Care and Use Committee (IACUC) approval, under ACUP# 4227. ICR mice from Harlan Laboratories were bred and females were checked for the presence of a vaginal plug (E0.5). Multiple litters, each containing 6–15 embryos, were isolated at E12.5 and dissected for forelimbs in ice cold 1x phosphate-buffered saline (PBS). Biopsies were pooled with at least 10 embryos per 1.5 ml tube and immediately fixed.
ChIP and ChIP-seq
Biopsies from embryos were cross-linked for 20 min in freshly made fixation buffer (1% formaldehyde 100mM NaCl, 0.5mM EGTA, 50mM HEPES, pH 8.0) and quenched by the addition of glycine (125mM). Tissue was washed twice with ice cold PBS and snap-frozen in liquid nitrogen prior to sonication (14 cycles of 10 sec at 20% output with 60 sec on ice between cycles, Branson 450 sonicator) in lysis buffer (1% SDS, 10mM EDTA pH 8.0, 50mM Tris-HCl pH 8.0, protease inhibitors) to achieve an average sheared fragment size of 300bp, with the primary smear between 200–450bp. A portion of sheared chromatin from each preparation was reserved as the input control. Protein G magnetic beads (NEB S1430S) were prepared by washing 3x in PBS with 0.05% bovine serum albumin (BSA), bound overnight to rabbit anti-H3K4me3 (Abcam, ab1342), rabbit anti-H3K27me3 (Millipore, 07-449), or mouse anti-Pol2 (Millipore, 17-672) at 2 µg/20 µl beads, and washed 3x in PBS-BSA. Material for immunoprecipitation (IP) as diluted 1:10 in dilution buffer (1% Triton X-100, 150mM NaCl, 2mM EDTA pH 8.0, 20mM Tris-HCl pH 8.0, protease inhibitors), and 20 µl of Protein G magnetic beads were added to each ml of sheared chromatin (about 200 µg each) and allowed to rotate overnight at 4° C. The following day, samples were washed 1x in low-salt wash (1% Triton X-100, 0.1% SDS, 150mM NaCl, 2 mM EDTA pH 8.0, 10mM Tris HCl pH 8.0, protease inhibitors), 2x in high-salt wash (1% Triton X-100, 0.1% SDS, 500mM NaCl, 2mM EDTA pH 8.0, 10mM Tris HCl pH 8.0, protease inhibitors), 2x in lithium wash (100 mM Tris HCl pH 9.0, 500mM LiCl, 1% NP40, 1% deoxycholic acid), 3x in TE (10mM Tris-HCl pH 8.0, 1mM EDTA), and eluted in 100 µl of elution buffer containing RNaseA and Proteinase K (1% SDS, 100 mM NaHCO3, 10 mg/ml RNaseA, 5 M NaCl, 0.2 mg/ml Proteinase K). Elutants and respective input controls were de-crosslinked overnight, at 65° C, purified on Qiagen Miniprep columns and eluted in ddH2O. One set from each condition was reserved for qPCR validation and the remaining ChIP DNA were speed-vac dried. DNA was resuspended in 15 µl of ddH2O, quantified with the Qubit fluorometer (Invitrogen) or the NanoDrop spectrophotometer (Thermo Scientific), until at least 10 ng were collected for each sample. Single-end libraries were generated using the Illumina TruSeq ChIP seq library prep kit using 18 cycles for PCR sample enrichment, with the only protocol modification being size selection after fragment amplification. The library size selected was 200–300 bp in length and, after gel purification, size was confirmed with the Bioanalyzer (Agilent). Libraries were then sequenced on a 51 bp single-end run on the Illumina HiSeq 2000.
ChIP-seq Data Analysis
Primary Illumina data image analysis, base calling, and filtering was preformed by the Casava pipline (version 1.8.2, Illumina), and low quality filtered reads were removed from the data set prior to alignment. Filtered reads from the ChIP and input control libraries were aligned to the mouse genome (NCBI37/mm9) reference assembly using BWA (version 0.6.2). MACS (version 2.0.10) was used to identify genomic regions significantly enriched (q ≤ 0.05) in ChIP-seq samples over input controls, and the software “broad peaks” option was used to delineate the mapped extent of histone marks.16 The UCSC Genome Browser was used to visualize the mapping data and peak calls. Homer Tools were utilized for gene ontology annotation and comparisons of found peaks.17 DAVID Bioinformatics Tools was used for gene ontology clustering.18,19 Chip-seq data from this study is accessible under accession number GSE49010 from NCBI Gene Expression Omnibus (http://www.ncbi.nlm.nih.gov/geo).
Results
Genome Mapping of Mouse Embryonic Forelimb
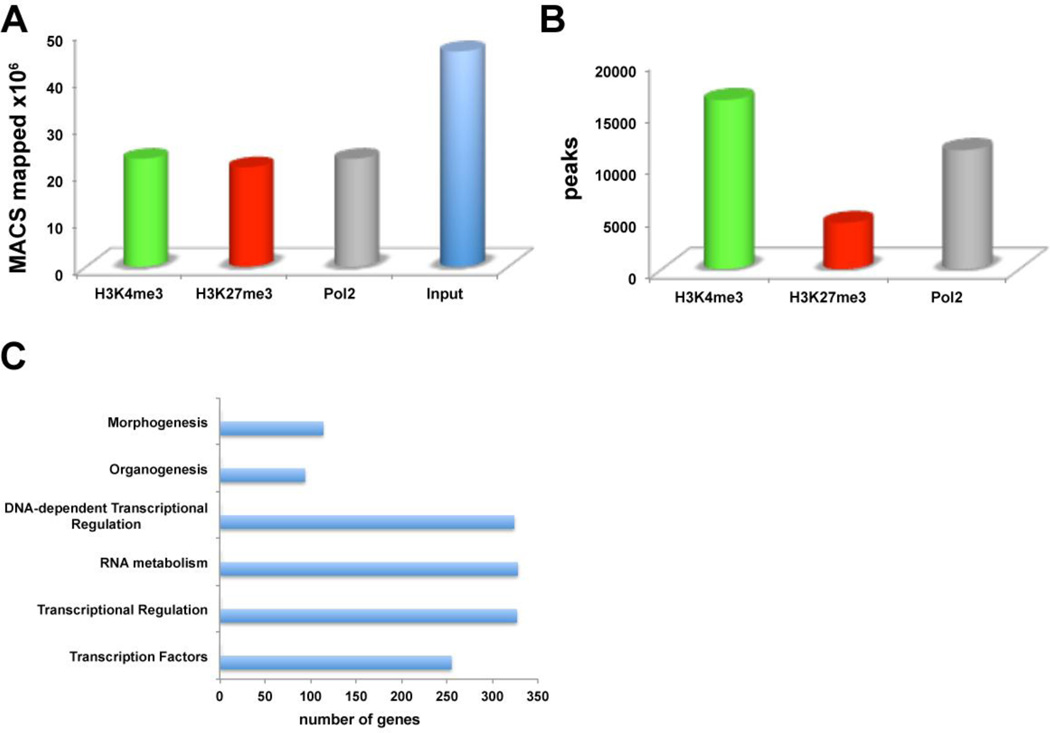
Multiple litters of ICR-mouse forelimbs at E12.5 were collected and processed for ChIP-seq. High-throughput sequencing produced between 37 and 47 million reads for each of the three (H3K4me3, H3K27me3, Pol2) ChIP samples. Each input control sample had 74 and 90 million reads. After initial quality filtering, about 96% of these reads were mapped to the mouse mm9 reference assembly, using BWA,20 that resulted in 22 million uniquely mapped reads for ChIP samples, and 46 uniquely mapped reads for input samples (Fig 1A). On average, 64% of the total raw reads were used for analysis. To identify peaks or regions that were enriched in each data set, Model-based Analysis of ChIP-Seq (MACS) was utilized with the input as the control to account for local sequencing bias of the genome.16 Histone marks are known to appear over broad regions, compared to transcription factors, and thus the broad-peak calling option was turned on for analysis that resulted in 16234, 4442, and 11425 broad peaks for H3K4me3, H3K27me3, and Pol2, respectively (Fig 1B). The greater number, 16234 of total peaks representing the 50%, in H3K4me3 was expected, as this trend can be seen on the publicly accessible datasets on the UCSC Genome Browser for various cell and tissue types. Genes that exhibited H3K4me3 and Pol2 presence represented the 31% of total peaks, while genes with both H3K4me3 and H3K27me3 presence (bivalent) represented the 7% and genes with H3K4me3, H3K27me3 and Pol2 presence (trivalent) represented the 2% of total peaks. These data suggest that the most developmental regulators are no longer bivalent as in ES cells, which represent the 22% of genes7, and by E12.5 cells lost their plasticity and cell stages have been determined.
Figure 1. Mapping the Mouse Embryonic Forelimb.
(A) Model-based Analysis of ChIP-Seq (MACS) for H3K4me3, H3K27me3, Pol2 and input ChIP samples. (B) Broad-peak analysis for H3K4me3, H3K27me3, and Pol2. (C) DAVID Bioinformatics Clustering tools identified enriched gene families in transcription and embryogenesis.
Chromatin State of Mouse Embryonic Forelimb
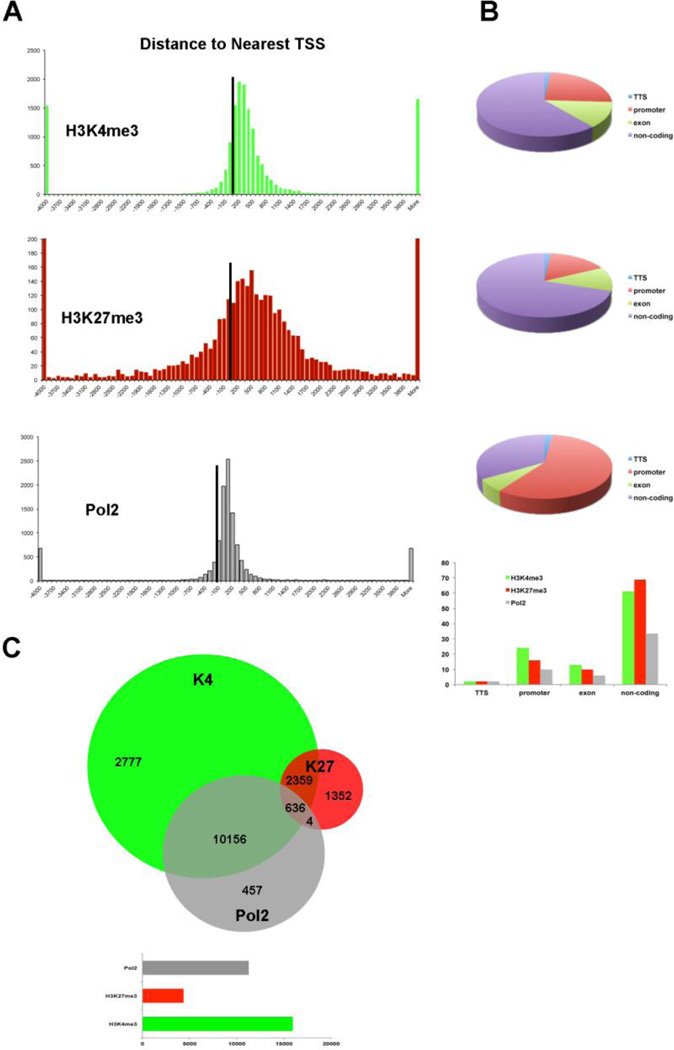
Homer tools were used to characterize the found peaks, the distance to the nearest transcriptional start site (TSS), defined as the start of a RefSeq gene, and the region in which the peak was present (Fig 2A).17 The centers of peaks for all three marks were detected at or just downstream (+200 for Pol2 and +500 for H3K4me3 and H3K27me3) of the TSS. The Pol2 marks were more tightly distributed near the TSS, while H3K27me3 marks often covered a much broader region over a gene. About 60% of the H3K4me3 and H3K27me3 peaks occurred over the body of a gene (intron, exon, 3´ UTR, 5´ UTR), and 60% of the Pol2 peaks occurred on the promoter regions. Homer tools were also used to characterize the occurrence of co-bound peaks, where one or more mark(s) overlapped (Fig 2B), with approximately 2,400 bivalent peaks. Bivalent domains are defined by the coexistence of the permissive (H3K4me3) and the repressive (H3K27me3) histone mark and are important in pluripotency and embryogenesis by keeping transcriptional regulators and developmental genes in a silenced state poised for activation upon differentiation21 DAVID Bioinformatics Clustering tools was applied to categorize and identify the gene families with bivalent chromatin marks 19. To filter out potential false positives, we identified the bivalent marks, within the 20kb of a transcriptional start of a gene. The enriched gene families included genes involved primarily in transcription and transcriptional regulation (80%) and in embryogenesis (15%), (Fig 1C). The presence of H3K4me3 was primarily noted in the promoter regions within the genes that are marked with it (Fig 2B), while the H3K27me3 was highly present in the intron of the genes that was detected (Fig 2B). Half of the genes exhibited H3K4me3 marks, 36% of them Pol2 marks and only 14% of them H3K27me3 marks, at this developmental stage. The presence of Pol2 on specific genes is often correlated with active transcription, which requires open and accessible chromatin. Over 95% of all Pol2 marks occurred in association with the H3K4me3 (Fig 1B). There were only four occurrences where a Pol2 mark occurred in association with only one H3K27me3 mark. However, the presence of Pol2 does not conclude that the gene is transcriptionally engaged, since additional assays can be included, such as RNA-seq to verify the actively transcribed genes.
Figure 2. Characterization of H3K4me3, H3K27me3, and Pol2 marks with respect to location on the genome and nearest gene.
(A) Red bars represent the relative location of the transcriptional start site (TSS), which is defined as the UCSC RefSeq start position of genes. H3K4me3 peaks occur mostly in close proximity to known genes, with a slight bias downstream from the TSS indicating marks occur along the gene body. H3K27me3 peaks occur with a bias to the downstream region of the TSS similar to that of H3K4me3 marks. Regions marked by H3K27me3 are broader and many are within the intergenic regions. RNA Pol2 binding occurs in a sharper peak. These figures should only be considered a general representation that can be compared only with each other. (B) Distribution of peak location within the genome. (C) Venn diagram illustrating co-occurrence of marked sites within limb biopsies. Bars indicate the marked genes within the mouse genome. Most notable is the large overlap of Pol2 marks with H3K4me3 marks. Pol2 requires genes to be accessible, and the presence of H3K4me3 is an indicator of accessible regions. Very few Pol2 peaks were found to be associated with H3K27me3, as it is associated with inactive regions of the genome. Figures were made using Homer tools merge Peaks.pl, and thus it is possible for two peaks in one sample to overlap with a single peak in a second sample and, therefore, be combined and considered one overlap instead of two.
Signaling Molecules in Mouse Embryonic Forelimb
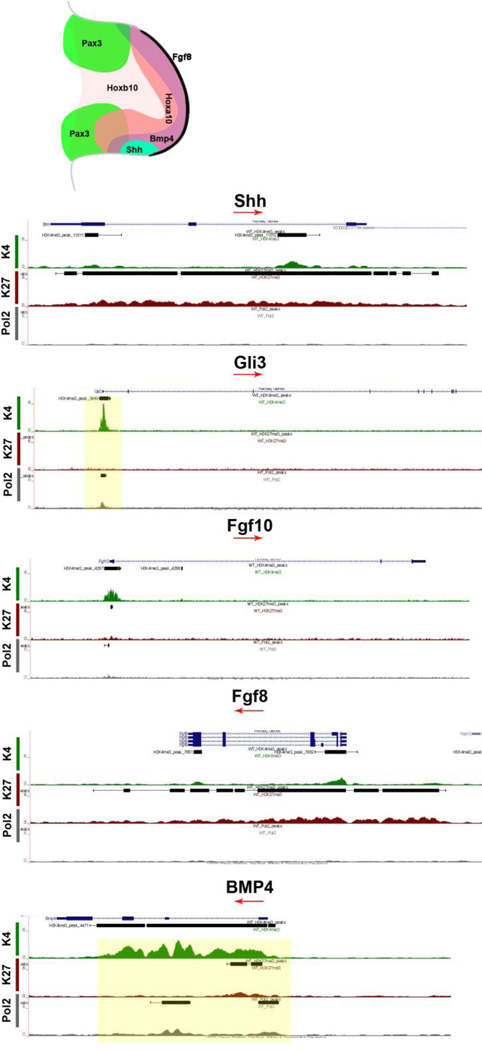
The mouse forelimb has a self-regulatory signaling system that involves several networks to regulate cell patterning, commitment and differentiation. FGFs that are expressed in the apical ectodermal ridge (AER) induce limb bud formation 22,23 and pattern the distal forelimb.24 Shh from the zone of polarizing area (ZPA) specifies the pattern formation via a concentration gradient.25 BMP signals from the distal part of the forelimb antagonize the effect of FGF signaling from the AER.26 By E12.5, the patterning events have been finalized and the signaling molecules should be poised for deactivation. Different network kernels that will guide the cell types toward their next developmental state are activated. Data from our analysis indicated that Shh and Fgf8 were not significantly marked by any of the histone trimethylases or by Pol2, but rather maintained in bivalent state (Fig 3). However, Gli3, a key component of the Shh signaling that results in formation of additional digits during limb bud development and acts as a gatekeeper for the exit to chondrogenic differentiation,27 was marked for H3K4me3 and Pol2 (Fig 3). FGF10 exhibited H3K4me3 marks, since its expression remains in the expanding mesenchyme at this stage (Fig 3). BMP4 is expressed in limb mesoderm and regulates digit number and identity 28, and thus was marked for both for H3K4me3 and Pol2 (Fig 3). Grem1 is a BMP antagonist and contributes to maintain FGF expression in AER29 was poised with low marks for H3K4me3 and H3K27me3 (data not shown).
Figure 3. Chromatin State of Signaling Molecules.
Cartoon illustrates the expression profile of key regulators of limb patterning and cell specification at E12.5, including signaling molecules Fgf8, Shh and BMP4 and SSTFs Hoxb10, Hoxa10 and Pax3. Black bars above peaks indicate the “called peaks” as determined with MACS 2.0. Thick bar segments are subpeaks, with adjoining lines indicating a merge of subpeaks that makes up a marked region. Shh and Fgf8 are bivalently marked, while Fgf10 was marked for H3K4me3. Gli3 and BMP4 were actively transcribed.
SSTFs in Mouse Embryonic Forelimb
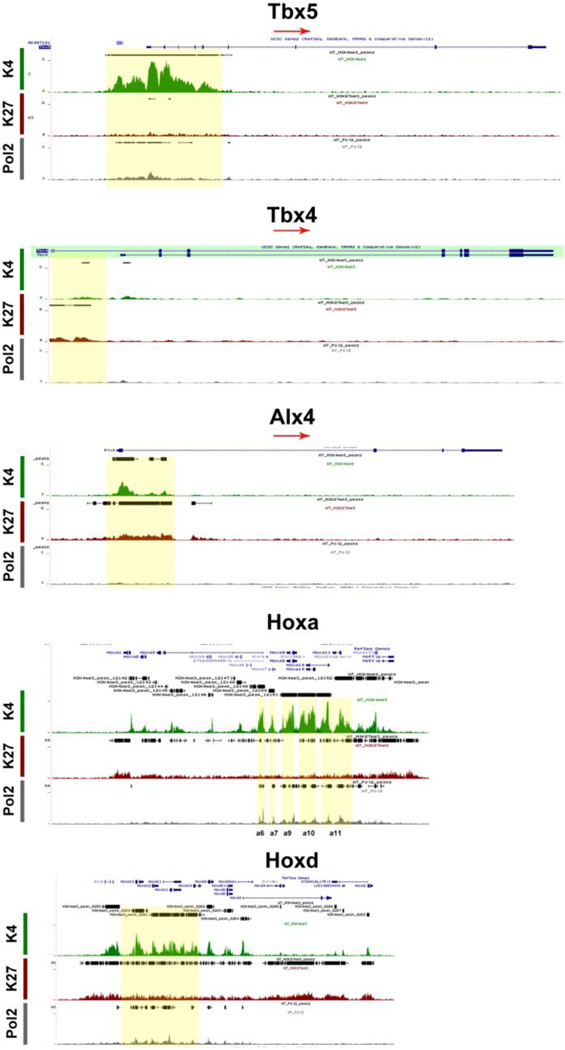
The limb bud emerges from the mesenchymal cells covered with a layer of ectodermal cells. Within days these cell populations give rise to skeletal elements, muscles and connective tissues. The T-box transcription factors Tbx5 and Tbx4 are expressed and regulate the formation in fore-and hindlimbs respectively. 30 Our Chip-seq data have shown that Tbx5 exhibited H3K4me3 with small traces of Pol2 while Tbx4 exhibited HeK27me3 (Fig 3) further supporting the limb specification properties of these markers. The aristaless-like homeobox 4, Alx4, is involved in pattering the structures of the forelimb at earlier stages, exhibited marks for both H3K4me3 and H3K27me3 (Fig 4). Two families of Hox genes, Hoxa and Hoxd, are involved in forelimb development and their mutations display a range of phenotypes including prenatal and postnatal lethality and developmental defects in skeletal, muscular and other systems. Our ChIP-seq data indicated that members of the Hoxa cluster, a9, a10, a11, 13, and the Hoxd cluster, d3, d8, d9, d10, d11, d12, d13, exhibited both H3K4me3 and Pol2 (Fig 4) suggesting that at E12.5 the initial patterning of forelimb is still an ongoing process to further define the anterior-posterior and dorsal-ventral borders during cell growth and specification.
Figure 4. Chromatin State of Forelimb specific SSTFs.
Tbx5 that marks and regulates forelimb development was marked for HeK4me3 and Pol2, but Tbx4 that regulates hindlimb development was repressed. Alx4, a forelimb patterning factors was bivalent. Hoxa and Hoxd family members were marked for He3K4me3 and Pol2.
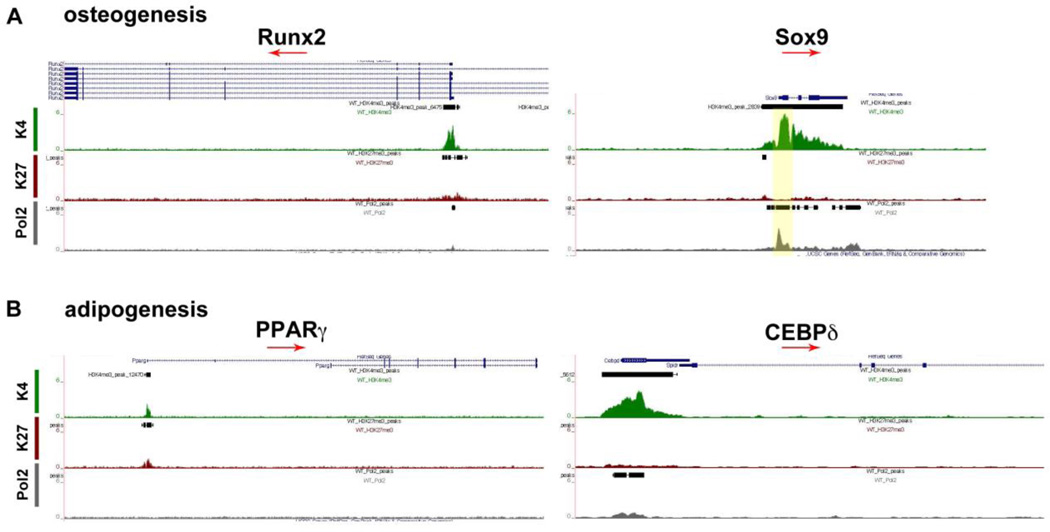
Osteogenesis is a highly regulated developmental process with Runx2 as a master regulator of the transcriptional program essential for bone formation.31 Runx2 was marked for H3K4me3 but not Pol2 (Fig 5A) suggesting that the osteogenic program has been initiated before E12.5. The Sry-related transcription factor Sox9 is essential for chondrogenesis of the mesenchymal stem cells in the limb.32 Sox9 was marked with both H3K4me3 and Pol2 (Fig 5A) suggesting that chondrogenic program has been activated. Formation of adipocytes will follow and gene regulators including peroxisome proliferator activated receptor gamma (PPARγ) and the CCAAT/enhancer binding protein (CEBPδ) only H3K4me3 marks (Fig 5B). Both genes were poised for activation in later developmental stages.
Figure 5. Chromatin State of SSTFs involved in Osteogenesis and Adipogenesis.
Osteogenic marker Runx2 was marked for H3K4me3 and chondrogenic marker Sox9 was marked with H3K4me3 and Pol2. Red arrows indicate the direction of the gene, and yellow highlighted areas are regions of interest with marks for H3K4me3 and Pol2. Adipogenesis is initiated at this stage with PPARγ in a bivalent state and CEBPδ in active state.
Muscle SSTFs in Mouse Embryonic Forelimb
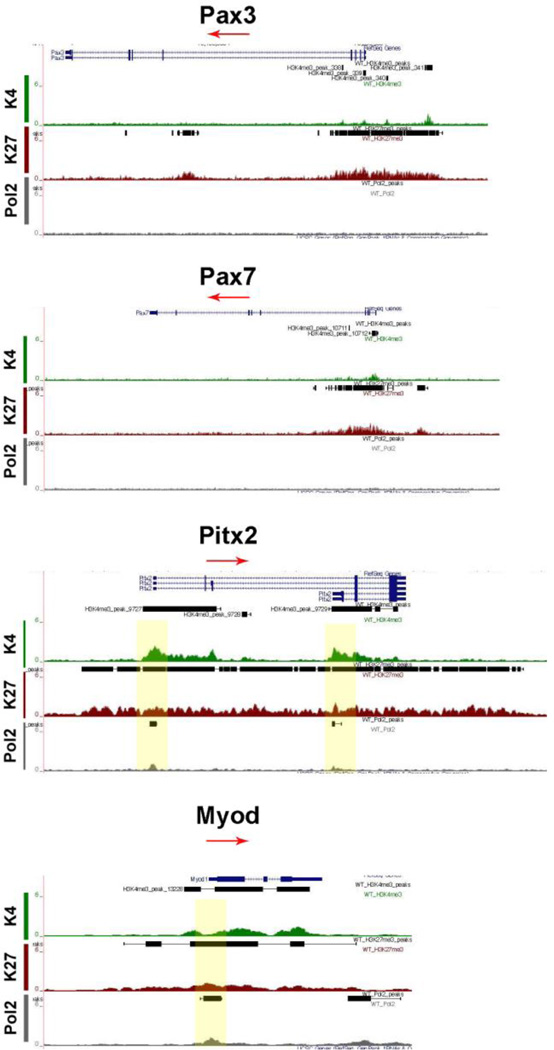
Specific transcription factors are required for molecular specification, movement and myogenic progression during muscle development. The expression of the homeo-paired domain Pax3 transcription factor in the dermomyotome precedes the expression of the Muscle Regulatory Factors (MRFs) a family of four transcription factors, including Myod, Myf5, Myog, and Mrf4. Myog expression is required for classic muscle differentiation and precedes the expression of proteins of the contractile apparatus. Pax3 and Pax733 or Myod and Myf534 can contribute to different muscle lineages. The homeodomain transcription factor Pitx2 marks all muscles35, while Pitx1 is expressed only in the hindlimb muscles36 and Pitx3 marks muscle cells at later developmental stages37. Cells expressing Pax3 and Pax7 mark the muscle progenitor pool 38,39 and were bivalently marked (Fig 6). Pax3 is required for the delamination of muscle precursors from the dermomyotome, and at this stage of development, it is likely that Pax3 is no longer actively transcribed, but rather repressed for the next stage of myogenesis to proceed. Similarly, Pax7 begins to be expressed at E12.5 and is essential for postnatal myogenesis.40 The bivalent marks indicate that Pax7 was poised for repression during embryonic myogenesis (E9-E12) and activation during fetal myogenesis (E13-E16). The bivalently marked regions upstream of Pax3 and Pax7 likely lie on enhancer or regulatory regions. The marked region 7 kb upstream of Pax3 is a known enhancer/regulator region involved in Pax3 expression in the ventral-lateral lip of interlimb somites.41 The H3K27me3 mark in the 4th intron of Pax3 also lies on a known regulatory region.42
Figure 6. Chromatin State of SSTFs involved in Myogenesis.
Pax3 and Pax7 were bivalently marked, but not marked by Pol2, indicating they are likely regulated nodes. Pax3 was poised for repression and Pax7 for activation. The bivalently marked regions upstream of Pax3 and Pax7 likely lie on regulatory regions. Pitx2 is bivalently marked and bound by Pol2. Myod was regulated and likely actively transcribed. Myod is the primary and initial MRF to be expressed in hypaxial musculature.
Mapped reads on the UCSC genome browser indicated that all four isoforms of Pitx2 were bivalently marked and occupied by Pol2 (Fig 6). Pitx1 was highly marked with H3K27me3, concurrent with its specific expression in the hindlimb and, thus, would not be available in the forelimbs (data not shown). Pitx3 was bivalently marked for H3K4me3 and H3K27me3 but not Pol2 (data not shown).37 Of the four MRFs, only Myod was bivalently marked with Pol2 mark indicating the cells have entered and being specified to the myogenic program (Fig 6). The other three factors were marked with H3K4me3, indicating the genes could be available for transcription at later developmental stages, which is in accord with their expression profiles.43
Discussion
Chromatin profiling is a powerful indicator for detection of the genome’s regulatory activity. Genome-wide analysis of all cell types of an organ, the forelimb in our studies, at different developmental stages will provide a detailed map of transcriptional activity and generate predictions of the cell fate. Developmental regulatory genes have both activating (H3K4me3) and repressive (HeK27me3) histone modifications in embryonic forelimb. This bivalent configuration can maintain cell state commitment in a poised state. We have applied ChIP-seq approach to investigate the chromatin in mouse forelimbs at E12.5, a critical stage for growth and cell lineage specification. We report that almost one third (31%) of the mapped genes were transcriptionally active by exhibiting H3K4me3 and Pol2 marks, 4% were repressed by exhibiting H3K27me3 marks, 9% were poised by exhibiting H3K4me3 marks, 7% were in a bivalent state exhibiting HeK4me3 and H3K27me3 marks, while only 2% were trivalent state by exhibiting all three marks HeK4me3, H3K27me3 and Pol2. The active presence (50%) of H3K4me3 in the promoters can be explained by the active remodeling of the limb at this stage. The repressed genes are not expressed in the forelimb. The patterning genes have already established all axes, anterior-posterior, dorsal-ventral, distal-proximal, and signaling molecules and SSTFS involved in lineage determination including osteogenesis/chondrogenesis, myogenesis and adipogenesis have been activated or remained poised for the next regulatory kernel to be established. The small percentage of bivalent and the even smaller trivalent, domains suggests that the cells have lost their plasticity and pluripotency that characterizes the ES cells. Limb cells at E12.5 are prepared for a fine-tune gene expression to produce more specific lineages with unique characteristics and functions rather that a stand-by bivalent state that allows them to be self-renewed and differentiated. Our observations suggest that mesoderm-, mesenchyme- and ectoderm- derived cells could be altered though modulation of distinct chromatin states.
Acknowledgements
We thank Mark Dasenko for CGRB Core Lab support, Roy Brown for computational support and Denny Weber for help with editing the manuscript. This research was supported by the College of Pharmacy at Oregon State University, the NIH-NIAMS AR054406 and MRF grants to CK.
Footnotes
Author Contributions
DE performed experiments and data analysis and contributed to drafting the article; WKV contributed to data acquisition; NSF contributed to data interpretation; MKG contributed to analysis and data interpretation, CK contributed to the conception, design, analysis and interpretation of data and drafting the article.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Davidson EH, Rast JP, Oliveri P, et al. A genomic regulatory network for development. Science. 2002 Mar 1;295(5560):1669–1678. doi: 10.1126/science.1069883. [DOI] [PubMed] [Google Scholar]
- 2.Longabaugh WJ, Davidson EH, Bolouri H. Computational representation of developmental genetic regulatory networks. Developmental biology. 2005 Jul 1;283(1):1–16. doi: 10.1016/j.ydbio.2005.04.023. [DOI] [PubMed] [Google Scholar]
- 3.Bolouri H, Davidson EH. The gene regulatory network basis of the "community effect," and analysis of a sea urchin embryo example. Developmental biology. 2010 Apr 15;340(2):170–178. doi: 10.1016/j.ydbio.2009.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Robertson G, Hirst M, Bainbridge M, et al. Genome-wide profiles of STAT1 DNA association using chromatin immunoprecipitation and massively parallel sequencing. Nat Methods. 2007 Aug;4(8):651–657. doi: 10.1038/nmeth1068. [DOI] [PubMed] [Google Scholar]
- 5.Johnson DS, Mortazavi A, Myers RM, Wold B. Genome-wide mapping of in vivo protein-DNA interactions. Science. 2007 Jun 8;316(5830):1497–1502. doi: 10.1126/science.1141319. [DOI] [PubMed] [Google Scholar]
- 6.Barski A, Cuddapah S, Cui K, et al. High-resolution profiling of histone methylations in the human genome. Cell. 2007 May 18;129(4):823–837. doi: 10.1016/j.cell.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 7.Mikkelsen TS, Ku M, Jaffe DB, et al. Genome-wide maps of chromatin state in pluripotent and lineage-committed cells. Nature. 2007 Aug 2;448(7153):553–560. doi: 10.1038/nature06008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Birney E, Stamatoyannopoulos JA, Dutta A, et al. Identification and analysis of functional elements in 1% of the human genome by the ENCODE pilot project. Nature. 2007 Jun 14;447(7146):799–816. doi: 10.1038/nature05874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Park PJ. ChIP-seq: advantages and challenges of a maturing technology. Nat Rev Genet. 2009 Oct;10(10):669–680. doi: 10.1038/nrg2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dunham I, Kundaje A, Aldred SF, et al. An integrated encyclopedia of DNA elements in the human genome. Nature. 2012 Sep 6;489(7414):57–74. doi: 10.1038/nature11247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heintzman ND, Stuart RK, Hon G, et al. Distinct and predictive chromatin signatures of transcriptional promoters and enhancers in the human genome. Nat Genet. 2007 Mar;39(3):311–318. doi: 10.1038/ng1966. [DOI] [PubMed] [Google Scholar]
- 12.Ringrose L, Paro R. Epigenetic regulation of cellular memory by the Polycomb and Trithorax group proteins. Annu Rev Genet. 2004;38:413–443. doi: 10.1146/annurev.genet.38.072902.091907. [DOI] [PubMed] [Google Scholar]
- 13.Boyer LA, Plath K, Zeitlinger J, et al. Polycomb complexes repress developmental regulators in murine embryonic stem cells. Nature. 2006 May 18;441(7091):349–353. doi: 10.1038/nature04733. [DOI] [PubMed] [Google Scholar]
- 14.Bernstein BE, Mikkelsen TS, Xie X, et al. A bivalent chromatin structure marks key developmental genes in embryonic stem cells. Cell. 2006 Apr 21;125(2):315–326. doi: 10.1016/j.cell.2006.02.041. [DOI] [PubMed] [Google Scholar]
- 15.Young MD, Willson TA, Wakefield MJ, et al. ChIP-seq analysis reveals distinct H3K27me3 profiles that correlate with transcriptional activity. Nucleic Acids Res. 2011 Sep 1;39(17):7415–7427. doi: 10.1093/nar/gkr416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang Y, Liu T, Meyer CA, et al. Model-based analysis of ChIP-Seq (MACS) Genome Biol. 2008;9(9):R137. doi: 10.1186/gb-2008-9-9-r137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heinz S, Benner C, Spann N, et al. Simple combinations of lineage-determining transcription factors prime cis-regulatory elements required for macrophage and B cell identities. Mol Cell. 2010 May 28;38(4):576–589. doi: 10.1016/j.molcel.2010.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang da W, Sherman BT, Lempicki RA. Bioinformatics enrichment tools: paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res. 2009 Jan;37(1):1–13. doi: 10.1093/nar/gkn923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang da W, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nature protocols. 2009;4(1):44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- 20.Li H, Durbin R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics. 2009 Jul 15;25(14):1754–1760. doi: 10.1093/bioinformatics/btp324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Voigt P, Tee WW, Reinberg D. A double take on bivalent promoters. Genes & development. 2013 Jun 15;27(12):1318–1338. doi: 10.1101/gad.219626.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xu X, Weinstein M, Li C, et al. Fibroblast growth factor receptor 2 (FGFR2)-mediated reciprocal regulation loop between FGF8 and FGF10 is essential for limb induction. Development. 1998 Feb;125(4):753–765. doi: 10.1242/dev.125.4.753. [DOI] [PubMed] [Google Scholar]
- 23.Sekine K, Ohuchi H, Fujiwara M, et al. Fgf10 is essential for limb and lung formation. Nat Genet. 1999 Jan;21(1):138–141. doi: 10.1038/5096. [DOI] [PubMed] [Google Scholar]
- 24.Maden M. Intercalary regeneration in the amphibian limb and the rule of distal transformation. Journal of embryology and experimental morphology. 1980 Apr;56:201–209. [PubMed] [Google Scholar]
- 25.Harfe BD, Scherz PJ, Nissim S, Tian H, McMahon AP, Tabin CJ. Evidence for an expansion-based temporal Shh gradient in specifying vertebrate digit identities. Cell. 2004 Aug 20;118(4):517–528. doi: 10.1016/j.cell.2004.07.024. [DOI] [PubMed] [Google Scholar]
- 26.Pizette S, Niswander L. BMPs negatively regulate structure and function of the limb apical ectodermal ridge. Development. 1999 Feb;126(5):883–894. doi: 10.1242/dev.126.5.883. [DOI] [PubMed] [Google Scholar]
- 27.Lopez-Rios J, Speziale D, Robay D, et al. GLI3 constrains digit number by controlling both progenitor proliferation and BMP-dependent exit to chondrogenesis. Dev Cell. 2012 Apr 17;22(4):837–848. doi: 10.1016/j.devcel.2012.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bandyopadhyay A, Tsuji K, Cox K, Harfe BD, Rosen V, Tabin CJ. Genetic analysis of the roles of BMP2, BMP4, and BMP7 in limb patterning and skeletogenesis. PLoS genetics. 2006 Dec;2(12):e216. doi: 10.1371/journal.pgen.0020216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zuniga A, Haramis AP, McMahon AP, Zeller R. Signal relay by BMP antagonism controls the SHH/FGF4 feedback loop in vertebrate limb buds. Nature. 1999 Oct 7;401(6753):598–602. doi: 10.1038/44157. [DOI] [PubMed] [Google Scholar]
- 30.Hasson P, DeLaurier A, Bennett M, et al. Tbx4 and tbx5 acting in connective tissue are required for limb muscle and tendon patterning. Developmental cell. 2010 Jan 19;18(1):148–156. doi: 10.1016/j.devcel.2009.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Long F. Building strong bones: molecular regulation of the osteoblast lineage. Nature reviews. Molecular cell biology. 2012 Jan;13(1):27–38. doi: 10.1038/nrm3254. [DOI] [PubMed] [Google Scholar]
- 32.Cheng A, Genever PG. SOX9 determines RUNX2 transactivity by directing intracellular degradation. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2010 Dec;25(12):2680–2689. doi: 10.1002/jbmr.174. [DOI] [PubMed] [Google Scholar]
- 33.Relaix F, Rocancourt D, Mansouri A, Buckingham M. Divergent functions of murine Pax3 and Pax7 in limb muscle development. Genes & development. 2004 May 1;18(9):1088–1105. doi: 10.1101/gad.301004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kablar B, Krastel K, Ying C, Asakura A, Tapscott SJ, Rudnicki MA. MyoD and Myf-5 differentially regulate the development of limb versus trunk skeletal muscle. Development. 1997 Dec;124(23):4729–4738. doi: 10.1242/dev.124.23.4729. [DOI] [PubMed] [Google Scholar]
- 35.Shih HP, Gross MK, Kioussi C. Expression pattern of the homeodomain transcription factor Pitx2 during muscle development. Gene expression patterns : GEP. 2007 Feb;7(4):441–451. doi: 10.1016/j.modgep.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 36.Szeto DP, Rodriguez-Esteban C, Ryan AK, et al. Role of the Bicoid-related homeodomain factor Pitx1 in specifying hindlimb morphogenesis and pituitary development. Genes & development. 1999 Feb 15;13(4):484–494. doi: 10.1101/gad.13.4.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.L'Honore A, Coulon V, Marcil A, et al. Sequential expression and redundancy of Pitx2 and Pitx3 genes during muscle development. Developmental biology. 2007 Jul 15;307(2):421–433. doi: 10.1016/j.ydbio.2007.04.034. [DOI] [PubMed] [Google Scholar]
- 38.Kassar-Duchossoy L, Giacone E, Gayraud-Morel B, Jory A, Gomes D, Tajbakhsh S. Pax3/Pax7 mark a novel population of primitive myogenic cells during development. Genes & development. 2005 Jun 15;19(12):1426–1431. doi: 10.1101/gad.345505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Relaix F, Rocancourt D, Mansouri A, Buckingham M. A Pax3/Pax7-dependent population of skeletal muscle progenitor cells. Nature. 2005 Jun 16;435(7044):948–953. doi: 10.1038/nature03594. [DOI] [PubMed] [Google Scholar]
- 40.Murphy M, Kardon G. Origin of vertebrate limb muscle: the role of progenitor and myoblast populations. Curr Top Dev Biol. 2011;96:1–32. doi: 10.1016/B978-0-12-385940-2.00001-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brown CB, Engleka KA, Wenning J, Min Lu M, Epstein JA. Identification of a hypaxial somite enhancer element regulating Pax3 expression in migrating myoblasts and characterization of hypaxial muscle Cre transgenic mice. Genesis. 2005 Apr;41(4):202–209. doi: 10.1002/gene.20116. [DOI] [PubMed] [Google Scholar]
- 42.Degenhardt KR, Milewski RC, Padmanabhan A, et al. Distinct enhancers at the Pax3 locus can function redundantly to regulate neural tube and neural crest expressions. Developmental biology. 2010 Mar 15;339(2):519–527. doi: 10.1016/j.ydbio.2009.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hasty P, Bradley A, Morris JH, et al. Muscle deficiency and neonatal death in mice with a targeted mutation in the myogenin gene. Nature. 1993 Aug 5;364(6437):501–506. doi: 10.1038/364501a0. [DOI] [PubMed] [Google Scholar]