Abstract
Pseudomonas aeruginosa (PA) is commonly isolated from the respiratory secretions of antibody deficiency patients, but the significance of this has not been well studied. We have reviewed our adult antibody deficiency cohort of 179 patients and assessed the prevalence and characteristics of PA infection and the effects of early antibiotic eradication treatments. Of the 34 patients with PA, 55.9% (19) underwent successful eradication and were infection-free, 38.2% (13) had intermittent infection, and 5.9% (2) had chronic PA. PA infection was significantly associated with bronchiectasis (p < 0.0001), with 36.1% (22 out of 61) of patients with bronchiectasis developing a PA infection. Infection status was also significantly associated with chronic sinusitis (p < 0.0001). Most treated PA exacerbations were symptomatic and with colony counts of ≥1000 cfu/ml. Current eradication protocols used at our center involve early treatment at first positive isolate with ciprofloxacin for 3 weeks and nebulized colomycin for 3 months, and if eradication fails, intravenous ceftazidime and gentamycin or colomycin is administered for 2 weeks. Continued sputum surveillance and early eradication treatments upon positive PA culture may help to limit chronic PA infection in antibody deficiency patients.
Keywords: bronchiectasis, immunodeficiency, infection, Pseudomonas
Introduction
Cystic fibrosis (CF) patients experience a high burden of infection with Pseudomonas aeruginosa (PA), with 56–75% becoming chronically infected by early adulthood with a further 13.8% intermittently infected [1, 2]. PA infection can become chronic which has been associated with an accelerated decline in lung function and a reduction in quality of life in CF patients as well as in patients with noncystic fibrosis bronchiectasis (NCFB) [3–5]. Antibody deficient patients are also at risk, with PA being one of the most frequently isolated organisms from the sputum of immunodeficient patients [6, 7]. For this patient population, a background of immune deficiency and the presence of bronchiectasis may promote PA infection which, if sub-optimally treated, can become chronic. Early aggressive antibiotic eradication treatments in CF patients delays PA chronic colonization and reduces the decline in lung function [8–10], but evidence for the utility of such approaches in antibody deficiency patients is lacking. Several criteria exist for the classification of chronic PA infection, some of which depend on the presence of PA-antibodies, which could not be used in this immunodeficient cohort [11, 12]; thus, we have used the Leeds criteria [13, 14]. Here, we have assessed the prevalence of PA infection in our adult antibody deficiency cohort and have identified the proportion of patients never infected, infection-free, and with intermittent or chronic PA infection. Factors associated with infection status, characteristics of treated episodes, and features of the eradication protocol used are described.
Methods
A retrospective longitudinal review of the 179 adult antibody deficient patients receiving immunoglobulin-replacement at Barts Health NHS Trust, London, UK as of October 2013 was carried out. Local practice is for microbiology culture of sputum or cough swab samples at every routine visit and additionally if symptomatic. Patients were categorised as: “never” (never having a PA-positive culture), “infection-free” (previously PA-positive, but no positive cultures in the past 12 months), “intermittent” (≤50% of monthly PA-positive cultures in the past 12 months) or “chronic” (>50% of monthly PA-positive cultures in the past 12 months) [13]. All previous sputum sample results and data on the presence of bronchiectasis (high-resolution computed tomography confirmed), chronic sinusitis, immunoglobulin levels, length of infection, and antibiotic treated PA episodes were collected. The retrospective period of sampling for all patients covered all available medical records, with a median follow-up of 4.1 years (range 1.2–10.4) since first positive culture for the PA-infected patients. Annual decline in lung function (before first PA-positive culture to current date) and the number of serious infections (requiring IV antibiotics or hospitalisation) and nonserious infections (all other infections) were used as outcome measures.
The eradication regimen used at our center is oral ciprofloxacin (750 mg BD) for 3 weeks with nebulized colomycin (1 MU BD) for 3 months. If PA is not eradicated (defined as three successive negative cultures over 6 months), then 2 weeks of IV ceftazidime (1 g thrice daily) and gentamycin 80 mg tds, adjusted according to serum level, is initiated. Chronically PA infected and some intermittently infected patients are on long-term nebulized colomycin or tobramycin, as recommended for CF patients [1]. Data was collected after obtaining written informed consent and in accordance with approval by the City and East London Research Ethics Committee. Analysis was carried out using GraphPad Prism 5.0 software; p values of <0.05 were considered significant.
Results
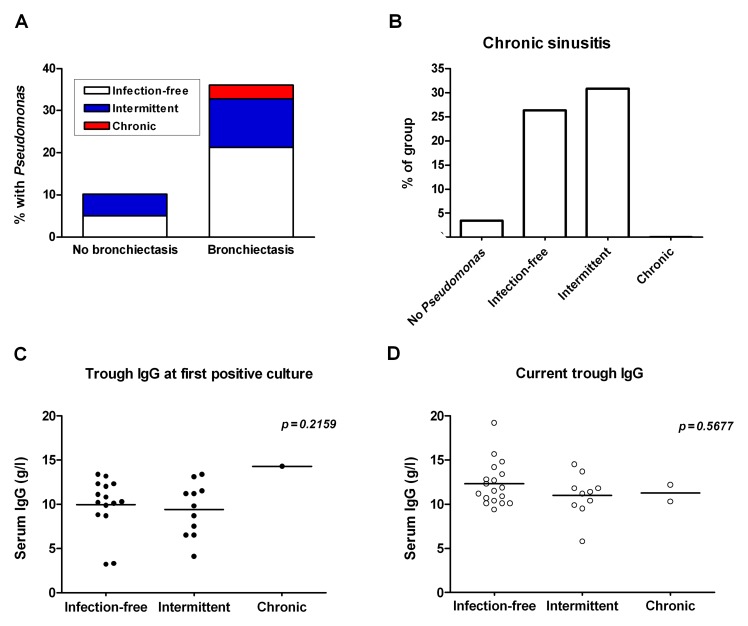
The majority of the antibody deficiency cohort had never had PA isolated (81.0%), with 10.6% of the cohort currently being infection-free (55.9% of PA-infected), 7.3% having intermittent PA (38.2% of PA-infected), and only 1.1% with chronic PA (5.9% of PA-infected) (Table 1). The type of immunodeficiency was not associated with the presence of PA infection, but bronchiectasis was significantly associated with PA infection (Table 1). Of all the patients with bronchiectasis, 36.1% (22/61) had PA compared to 10.2% (12/118) of patients without bronchiectasis (Fig. 1A). Chronic sinusitis was more prevalent in the intermittent group (4/13; 30.8%) and the infection-free (5/19; 26.3%) groups than in the never-infected group (5/145; 3.4%), with a significant difference between the infection-status groups (Chi-square value 22.53, p < 0.0001) (Fig. 1B). Serum IgG levels at the time of first PA-positive culture or the current trough IgG level did not differ significantly between the groups (Fig. 1C and D).
Table 1.
Pseudomonas aeruginosa infection in immunodeficiency cohort
| Number of patients | Mean age (range) | Male/female | Type of immunodeficiency |
Bronchiectasis | |||||
|---|---|---|---|---|---|---|---|---|---|
| Agamma-globulinemia | CVID | Specific/subclass deficiency | Other PID | Secondary antibody deficiency | |||||
| No Pseudomonas | 145 | 53 (17–91) |
62/83 | 11† | 82† | 7† | 16† | 29† | 39‡ |
| Pseudomonas | 34 | 56 (22–82) |
15/19 | 3† | 17† | 4† | 2† | 8† | 22‡ |
| Infection-free | 19 | 52 (22–82) |
8/11 | 2 | 11 | 1 | 2 | 3 | 13 |
| Intermittent | 13 | 59 (24–73) |
6/7 | 1 | 4 | 3 | 0 | 5 | 7 |
| Chronic | 2 | 63 (58–68) |
1/1 | 0 | 2 | 0 | 0 | 0 | 2 |
|
†Type of immunodeficiency: Chi-square test: 3.317,
n.s. p = 0.5062 ‡Presence of bronchiectasis: Chi-square test: 17.53, ***p < 0.0001 CVID, common variable immune deficiency; PID, primary immune deficiency | |||||||||
Fig. 1.
Bronchiectasis, sinusitis, and trough IgG levels in patients with Pseudomonas infection. The proportion of patients with or without bronchiectasis that had PA is shown in (A). The proportion of each infection-group with chronic sinusitis (B) was determined. Serum IgG levels at the time of first positive culture (C) and current trough IgG levels (D) are shown. Data in (C) and (D) were analyzed using the Kruskal–Wallis test; n.s., nonsignificant (p > 0.05)
Local policy is for eradication treatment upon first positive culture in these at-risk patients, although other centers may consider PA positive isolates as contamination and not always treat. To address whether the positive isolates were a result of contamination or genuine infection we looked at the characteristics of the treated episodes. All treated episodes were on the basis of a positive culture from sputum rather than cough swab samples, which have a lower positive predictive value. Although C-reactive protein (CRP) was only raised in 41.9% of episodes, the majority of episodes were symptomatic (85.5%), defined as change in sputum or cough, with a significant association with infection status. The majority (79.7%) of treated episodes were associated with colony counts of ≥1000 cfu/ml, making contamination less likely in this study (Table 2). The infection-free patients were most likely to have received treatment for a non-symptomatic episode associated with normal CRP levels and a low colony count PA isolate.
Table 2.
Characteristics of the treated Pseudomonas episodes
| Infection-free | Intermittent | Chronic | Total | |
|---|---|---|---|---|
| Number of episodes | 44 | 28 | 14 | 84 |
| Sputum samples | 100% | 100% | 100% | 100% |
| Symptomatic† | 75% (33/44) | 100% (27/27) | 91.7% (11/12) | 85.5% (71/83) |
| Raised CRP‡ | 38.5% (10/26) | 46.7% (7/15) | 50.0% (1/2) | 41.9% (18/43) |
| Number of episodes with ≥1000 cfu/ml§ | 75% (24/32) | 77.8% (14/18) | 100% (9/9) | 79.7% (47/59) |
|
†Chi-square test: 8.881,
*p = 0.0118 ‡Chi-square test: 0.3202, n.s. p = 0.8520 §Chi-square test: 2.766, n.s. p = 0.2508 | ||||
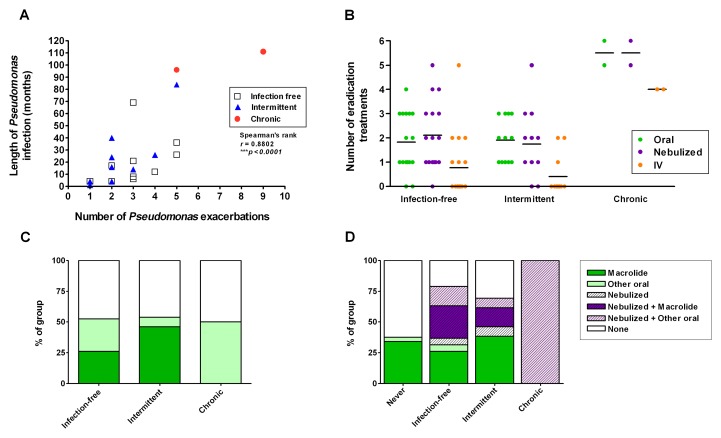
The number of PA exacerbations (positive culture and treated) was significantly correlated with the duration of PA infection (time between first positive culture and last positive culture or current date). The two chronically infected patients experienced the longest duration of infection, although two infection-free patients had as many exacerbations as one of the chronically infected patients (Fig. 2A). Local eradication regimens employ first-line treatment with oral ciprofloxacin for 3 weeks with nebulized colomycin for 3 months, and second-line treatment of IV ceftazidime and gentamycin for 2 weeks if failed to eradicate. The chronic group had the greatest number of eradication treatments, with the intermittent and infection-free groups having similar numbers of oral and nebulized eradication treatments (Fig. 2B). One infection-free patient underwent five IV antibiotic treatments before successful eradication. The median length of infection-free status in this group was 26 months (range 12–90 months), leading us to believe that, in most patients, this is genuine eradication, although a subset may become PA-positive again over time. Half of each of the PA-infected groups were on oral antibiotic prophylaxis at the time of first positive culture, with the majority on a macrolide (azithromycin 500 mg thrice weekly) (Fig. 2C). Currently, 47.3% of the infection-free group, 20.8% of the intermittent group, and 100% of the chronic group are on nebulized antibiotics (Fig. 2D).
Fig. 2.
Pseudomonas exacerbations and eradication treatments. The number of PA exacerbations (positive culture and treated) relative to the length of infection (months between first and last positive culture) is shown (A). The number of oral, nebulized, and IV antibiotic eradication treatments used in each patient is shown in (B). The proportion of each group treated with oral and/or nebulized prophylactic antibiotics at the time of first positive culture (C) and currently (D) is shown. Data in (A) were analyzed by Spearman’s rank correlation coefficient test
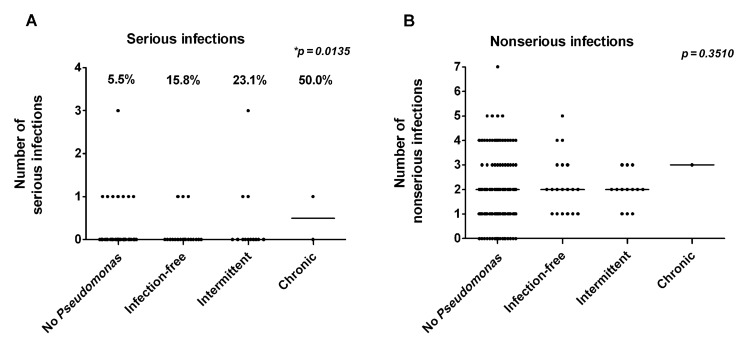
There was no significant difference in decline in lung function (% predicted FEV1 and FVC) from first patient-contact to most recent values between the PA-infected groups (data not shown). Respiratory infection frequency over the past one-year period was reviewed. There was no significant difference in nonserious infection frequency between the groups, but 50% of the chronic group, 23.1% of the intermittent group, and 15.8% of the infection-free group had a serious infection, compared to 5.5% of the never-infected group (Fig. 3A and B).
Fig. 3.
Number of serious and nonserious respiratory infections in a year. The number of serious respiratory infections (PA-negative culture, requiring hospitalization and/or IV antibiotics) (A) and nonserious respiratory infections (all other patient-reported infections) (B) in a year is shown for each group. The percentage of each group that had a serious infection is shown in (A). Data were analyzed using the Kruskal–Wallis test, *p < 0.05, n.s., nonsignificant
Discussion
The importance of PA infection in antibody deficiency patients may have been previously underestimated, despite evidence for serious consequences in CF and NCFB patients. In this antibody deficiency cohort, ~20% experienced PA infection, with more than half successfully eradicating PA with early treatment.
PA infection was associated with the presence of bronchiectasis, as described for patients with NCFB [4] and may be more prevalent in those with chronic sinusitis which could have a role in bacterial seeding of the lower respiratory tract from a sinus PA reservoir not reached by oral colomycin nebulizers. PA infection was not associated with IgG levels, which may be due to the low levels of circulating anti-PA antibodies in the donor pool used for immunoglobulin-replacement products. Anti-PA antibodies are traditionally associated with chronic infection and poorer outcomes in CF patients [12, 15]. As such, their protective effect and their effect if replaced in antibody deficient patients are unknown. Several definitions use anti-PA antibodies as part of the criteria of chronic infection [11] which would not be possible in these antibody deficient patients. We have used the Leeds CF criteria for infection status [13], which recommends a sampling frequency of at least 3 monthly. Due to less frequent follow-up compared to CF patients, sampling frequency was slightly lower in our cohort with a median of 3.5/year in PA-infected patients.
It is likely that patients would be reclassified with regards to infection status over time. As such, some of the patients in the intermittent group may become infection-free and some may become chronic. However, given the very low number of chronically infected patients in this study, we believe that with continued sputum surveillance and early eradication treatments, the majority will not become chronically infected. Treatment of intermittent infection with oral ciprofloxacin and nebulized colomycin prevented 78–80% of CF patients progressing to chronic infection [8, 9]. Once chronic colonisation becomes established, it is more difficult to eradicate, so preventing this step is essential in preventing PA-associated morbidity [16].
The proportion of antibody deficient patients that are chronically infected is much lower than that observed in adult CF patients, with studies (some using different criteria) reporting 56% [1] or 31% [14] of CF cohorts and 19.7% of NCFB cohorts [4] being chronically infected. However, even in CF patients, the proportion with chronic PA infection is decreasing with aggressive treatment and infection control practice, such that chronic PA has decreased from 23.8% to 4.3% between 1980 and 2000 in one regional paediatric center [10]. We did not observe any significant difference in lung function, as observed in some studies [4, 10] but not in others [14, 17], which may be due to shorter follow-up time, shorter duration of infection, and early treatment in this study.
There is currently no consensus on when treatment is appropriate and what the treatment regimen should be for PA infection in immunodeficient patients. The prevalence of bronchiectasis in immunodeficient patients is as high as 23% [18], suggesting that PA infection could become problematic in a proportion of these patients. Further studies and discussion are required to establish the extent of PA infection in this population and to determine a consensus on the best approach for management.
Acknowledgements
We are grateful to Drs. Lieske Kuitert and Siobhan Carr for assistance in patient management and advice on treatment protocols based on their extensive experience in cystic fibrosis.
Funding Statement
S.D.’s salary was funded by CSL Behring. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the article.
Footnotes
Author contributions: S.D. collected, analysed, and interpreted data, performed statistical analysis, and wrote the article; S.H. collected data; M.B. cared for the involved patients, and critically read the article; S.G. cared for the involved patients and provided clinical data, designed the study, and critically read the article; H.J.L. cared for the involved patients and provided clinical data, designed and organized the study, interpreted data, and wrote the article.
Conflicts of interest: Dr. Hilary Longhurst and members of her department have received education funding and/or taken part in advisory meetings for the following immunoglobulin manufacturers: CSL Behring and Baxter. S.G. has received support for meeting attendances from BPL and CSL Behring and has been the Chief Investigator/Lead Investigator/Co-investigator for several immunoglobulin clinical trials with the following companies: Baxter, CSL Behring, and Octapharma.
Contributor Information
Sai S. Duraisingham, 1Immunology Department, Barts Health NHS Trust, London, UK.
Steven Hanson, 2Department of Immunological Medicine, King’s College Hospital, London, UK.
Matthew Buckland, 1Immunology Department, Barts Health NHS Trust, London, UK.
Sofia Grigoriadou, 1Immunology Department, Barts Health NHS Trust, London, UK.
Hilary J. Longhurst, 1Immunology Department, Barts Health NHS Trust, London, UK.
References
- 1.Cysticfibrosis.org. UK Cystic Fibrosis registry 2012 Annual Data Report. Kent: Bromley; 2012. [Google Scholar]
- 2.Foundation CF. Cystic Fibrosis Foundation patient registry 2012 Annual Data Report. Bethesda, Maryland: 2012. [Google Scholar]
- 3.Li Z, Kosorok MR, Farrell PM, Laxova A, West SE, Green CG, Collins J, Rock MJ, Splaingard ML. Longitudinal development of mucoid Pseudomonas aeruginosa infection and lung disease progression in children with cystic fibrosis. JAMA. 2005 Feb 2;293(5):581–588. doi: 10.1001/jama.293.5.581. [DOI] [PubMed] [Google Scholar]
- 4.Martínez-García MA, Soler-Cataluña JJ, Perpiñá-Tordera M, Román-Sánchez P, Soriano J. Factors associated with lung function decline in adult patients with stable non-cystic fibrosis bronchiectasis. Chest. 2007 Nov;132(5):1565–1572. doi: 10.1378/chest.07-0490. [DOI] [PubMed] [Google Scholar]
- 5.Wilson CB, Jones PW, O'Leary CJ, Hansell DM, Cole PJ, Wilson R. Effect of sputum bacteriology on the quality of life of patients with bronchiectasis. Eur Respir J. 1997 Aug;10(8):1754–1760. doi: 10.1183/09031936.97.10081754. [DOI] [PubMed] [Google Scholar]
- 6.Pettit SJ, Bourne H, Spickett GP. Survey of infection in patients receiving antibody replacement treatment for immune deficiency. J Clin Pathol. 2002 Aug;55(8):577–580. doi: 10.1136/jcp.55.8.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Winkelstein JA, Marino MC, Lederman HM, Jones SM, Sullivan K, Burks AW, Conley ME, Cunningham-Rundles C, Ochs HD. X-linked agammaglobulinemia: report on a United States registry of 201 patients. Medicine (Baltimore) 2006 Jul;85(4):193–202. doi: 10.1097/01.md.0000229482.27398.ad. [DOI] [PubMed] [Google Scholar]
- 8.Frederiksen B, Koch C, Høiby N. Antibiotic treatment of initial colonization with Pseudomonas aeruginosa postpones chronic infection and prevents deterioration of pulmonary function in cystic fibrosis. Pediatr Pulmonol. 1997 May;23(5):330–335. doi: 10.1002/(sici)1099-0496(199705)23:5<330::aid-ppul4>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 9.Hansen CR, Pressler T, Høiby N. Early aggressive eradication therapy for intermittent Pseudomonas aeruginosa airway colonization in cystic fibrosis patients: 15 years experience. J Cyst Fibros. 2008 Nov;7(6):523–530. doi: 10.1016/j.jcf.2008.06.009. [DOI] [PubMed] [Google Scholar]
- 10.Lee TW, Brownlee KG, Denton M, Littlewood JM, Conway SP. Reduction in prevalence of chronic Pseudomonas aeruginosa infection at a regional pediatric cystic fibrosis center. Pediatr Pulmonol. 2004 Feb;37(2):104–110. doi: 10.1002/ppul.10401. [DOI] [PubMed] [Google Scholar]
- 11.Pressler T, Bohmova C, Conway S, Dumcius S, Hjelte L, Høiby N, Kollberg H, Tümmler B, Vavrova V. Chronic Pseudomonas aeruginosa infection definition: EuroCareCF Working Group report. J Cyst Fibros. 2011 Jun;10(Suppl 2):S75–S78. doi: 10.1016/S1569-1993(11)60011-8. [DOI] [PubMed] [Google Scholar]
- 12.Ratjen F, Walter H, Haug M, Meisner C, Grasemann H, Döring G. Diagnostic value of serum antibodies in early Pseudomonas aeruginosa infection in cystic fibrosis patients. Pediatr Pulmonol. 2007 Mar;42(3):249–255. doi: 10.1002/ppul.20562. [DOI] [PubMed] [Google Scholar]
- 13.Lee TW, Brownlee KG, Conway SP, Denton M, Littlewood JM. Evaluation of a new definition for chronic Pseudomonas aeruginosa infection in cystic fibrosis patients. J Cyst Fibros. 2003 Mar;2(1):29–34. doi: 10.1016/S1569-1993(02)00141-8. [DOI] [PubMed] [Google Scholar]
- 14.Proesmans M, Balinska-Miskiewicz W, Dupont L, Bossuyt X, Verhaegen J, Høiby N, de Boeck K. Evaluating the "Leeds criteria" for Pseudomonas aeruginosa infection in a cystic fibrosis centre. Eur Respir J. 2006 May;27(5):937–943. doi: 10.1183/09031936.06.00100805. [DOI] [PubMed] [Google Scholar]
- 15.Pressler T, Karpati F, Granström M, Knudsen PK, Lindblad A, Hjelte L, Olesen HV, Meyer P, Høiby N. Diagnostic significance of measurements of specific IgG antibodies to Pseudomonas aeruginosa by three different serological methods. J Cyst Fibros. 2009 Jan;8(1):37–42. doi: 10.1016/j.jcf.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 16.Ballmann M, Rabsch P, von der Hardt H. Long-term follow up of changes in FEV1 and treatment intensity during Pseudomonas aeruginosa colonisation in patients with cystic fibrosis. Thorax. 1998 Sep;53(9):732–737. doi: 10.1136/thx.53.9.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Döring G, Conway SP, Heijerman HG, Hodson ME, Høiby N, Smyth A, Touw DJ. Antibiotic therapy against Pseudomonas aeruginosa in cystic fibrosis: a European consensus. Eur Respir J. 2000 Oct;16(4):749–767. doi: 10.1034/j.1399-3003.2000.16d30.x. [DOI] [PubMed] [Google Scholar]
- 18.Gathmann B, Grimbacher B, Beauté J, Dudoit Y, Mahlaoui N, Fischer A, Knerr V, Kindle G. The European internet-based patient and research database for primary immunodeficiencies: results 2006-2008. Clin Exp Immunol. 2009 Sep;157(Suppl 1):3–11. doi: 10.1111/j.1365-2249.2009.03954.x. [DOI] [PMC free article] [PubMed] [Google Scholar]