Abstract
Increased levels of the matrix metalloproteinases-2 and -9 (also referred to gelatinase-A and -B, respectively) can be detected in intestinal inflammation. We have recently shown that selective gelatinase blockage by the synthetic compound RO28-2653 ameliorates acute murine ileitis and colitis. We here investigated whether RO28-2653 exerts anti-inflammatory effects in acute Campylobacter jejuni-induced enterocolitis of gnotobiotic IL-10−/− mice generated following antibiotic treatment. Mice were perorally infected with C. jejuni (day 0) and either treated with RO28-2653 (75 mg/kg body weight/day) or placebo from day 1 until day 6 post infection (p.i.) by gavage. Irrespective of the treatment, infected mice displayed comparable pathogen loads within the gastrointestinal tract. Following RO28-2653 administration, however, infected mice exhibited less severe symptoms such as bloody diarrhea as compared to placebo controls. Furthermore, less distinct apoptosis but higher numbers of proliferating cells could be detected in the colon of RO28-2653-treated as compared to placebo-treated mice at day 7 p.i. Remarkably, gelatinase blockage resulted in lower numbers of T- and B-lymphocytes as well as macrophages and monocytes in the colonic mucosa of C. jejuni-infected gnotobiotic IL-10−/− mice. Taken together, synthetic gelatinase inhibition exerts anti-inflammatory effects in experimental campylobacteriosis.
Keywords: acute ulcerative enterocolitis, apoptosis, Campylobacter jejuni, gelatinases, gnotobiotic IL-10 deficient mice, matrix metalloproteinases, pro-inflammatory immune cell responses, proliferating cells, RO28-2653, synthetic gelatinase blockage
Introduction
Campylobacter (C.) jejuni infections comprise a significant health and socioeconomic burden in humans with rising prevalences worldwide especially in industrialized countries [1, 2]. The highly motile Gram-negative bacteria are part of the commensal gut microbiota in a multitude of wild and domestic animals. Zoonotic transmission from livestock animals takes place via consumption of contaminated meat products or water [3, 4]. Infected humans present a broad range of clinical manifestations. Symptoms vary from mild malaise to severe ulcerative enterocolitis requiring hospitalization especially in severely immune-compromized patients [5]. In most cases, however, human campylobacteriosis is self-limiting [6]. In the acute stage of C. jejuni-induced enterocolitis, patients suffer from abdominal cramps, fever, watery or bloody diarrhea [5, 7]. Histological examination of inflamed intestinal tissues reveals apoptosis, crypt abscesses, ulcerations, and infiltration of the intestinal mucosa and lamina propria with pro-inflammatory immune cell populations such as lymphocytes, macrophages, and neutrophils [8, 9]. We have recently shown that gnotobiotic IL-10−/− mice generated by broad-spectrum antibiotic treatment are excellently suited as C. jejuni infection model to study host–pathogen interactions in vivo [10–12]. It is well known that rodents are 1000 times more resistant to Toll-like receptor-4 agonists such as lipopolysaccharide (LPS) and lipooligosacharide (LOS) than humans [13]. In addition, IL-10−/− mice are much more sensitive to LPS and LOS as compared to wildtype mice [10]. Given the key role of LOS in mediating C. jejuni-induced disease, gnotobiotic IL-10−/− mice develop acute ulcerative enterocolitis within 1 week following C. jejuni infection mimicking key features of severe human campylobacteriosis [10, 14]. Matrix metalloproteinases (MMPs) comprise a tightly controlled heterogenous family of zinc- and calcium-dependent matrix-degrading endopeptidases [15, 16]. With respect to their substrate specificity, MMPs are categorized into collagenases (MMP-1, -8, -13, and -18), gelatinases (MMP-2 and -9), stromelysins (MMP-3, -7, -10, and -11), elastase (MMP-12), and membrane-type matrix metalloproteinases (MT-MMP-1 to -5) [17]. MMPs are physiologically involved in embryonic development and differentiation, tissue proliferation, and regeneration [16, 18]. A dysbalance between activators and inhibitors of MMP expression, however, results in diseases such as arthritis, atherosclerosis, or cancer [19, 20]. In experimental models of intestinal inflammation [21–23] and in patients suffering from human inflammatory bowel diseases such as Crohn’s disease or ulcerative colitis [24–26], expression levels of the gelatinases A and B (MMP-2 and MMP-9, respectively) were shown to be upregulated. We have recently demonstrated that selective gelatinase blockage by the synthetic compound RO28-2653 ameliorated acute small intestinal inflammation [22] and acute colitis in mice [23]. The synthetic compound exerts its antigelatinase effects via direct binding to MMP-2 and MMP-9 in a manner that saturates all possible interactions with the pyrimidine core moiety to the protein [27]. Since RO28-2653 lacks anti-MMP-1 and -MMP-7 properties [27] – the major reasons for serious side effects exerted by nonselective MMP-blocking agent in clinical studies – the likelihood of unwanted adverse effects following gelatinase inhibition can be considered as rather low [28]. We were, therefore, interested in potential beneficial effects of the gelatinase-blocking compound RO28-2653 in acute C. jejuni-induced ulcerative enterocolitis. To address this, we here investigated 1) the gastrointestinal colonization properties of C. jejuni, 2) the clinical course of infection, and 3) the abundances of apoptotic, regenerating, and pro-inflammatory immune cell populations in the colonic mucosa and lamina propria of C. jejuni-infected gnotobiotic IL-10−/− mice following synthetic selective gelatinase blockage.
Materials and methods
Ethics statement
All animal experiments were conducted according to the European Guidelines for animal welfare (2010/63/EU) with approval of the commission for animal experiments headed by the “Landesamt für Gesundheit und Soziales” (LaGeSo, Berlin, Germany). Animal welfare was monitored twice daily by assessment of clinical conditions.
Mice
IL10−/− mice (in C57BL/10 background) were bred and maintained under specific pathogen-free (SPF) conditions in the Forschungseinrichtung für Experimentelle Medizin (FEM, Charité, Berlin, Germany). Gnotobiotic IL‑10−/− mice were generated by broad-spectrum antibiotic treatment as described previously [10, 29]. Briefly, to eradicate the commensal gut microbiota, mice were transferred to sterile cages and treated by adding a mix of ampicillin (1 g/l; Ratiopharm), vancomycin (500 mg/l; Cell Pharm), ciprofloxacin (200 mg/l; Bayer Vital), imipenem (250 mg/l; MSD), and metronidazole (1 g/l; Fresenius) to the drinking water ad libitum starting at 3 weeks of age immediately after weaning. Age-matched female mice were subjected to the quintuple antibiotic treatment for approximately 4 months before the infection experiments.
C. jejuni infection
Three days prior to infection, the antibiotic cocktail was withdrawn and replaced by sterile tap water. Immediately before infection, sterility of mice was confirmed by transferring individual fecal samples to thioglycollate enrichment broths (Oxoid, Wesel, Germany) and applying subsequent cultural analyses as described earlier [29]. Mice were then perorally infected with 109 colony forming units (CFU) of viable C. jejuni strain 81-176 in a volume of 0.3 ml phosphate buffered saline (PBS) by gavage on day 0.
Treatment with RO28-2653
Gnotobiotic IL-10−/− mice were treated perorally with RO28-2653 (75 mg/kg body weight/day; kindly provided by Dr. H.-W. Krell, Roche, Penzberg, Germany) in 0.3 ml PBS once daily by gavage starting at day 1 post infection (p.i.) following C. jejuni infection for 6 days until necropsy (day 7 p.i.). PBS-treated animals (0.3 ml perorally once daily) served as negative controls. A potential antibacterial effect of the compound was excluded as described previously [30].
Clinical scoring
To assess clinical signs of C. jejuni-induced infection on a daily basis, a standardized cumulative clinical score (maximum 12 points, addressing the occurrence of blood in feces [0 points: no blood; 2 points: microscopic detection of blood using Haemoccult, Beckman Coulter/PCD, Krefeld, Germany; 4 points: overt blood visible], diarrhea [0: formed feces, 2: pasty feces, 4: liquid feces], and the clinical aspect [0: normal; 2: ruffled fur, less locomotion; 4: isolation, severely compromized locomotion, prefinal aspect]) was used [10, 31].
Sampling procedures and histologic scoring
Mice were sacrificed by isofluran treatment (Abbott, Germany) 7 days following C. jejuni infection. Colon samples from each mouse were removed under sterile conditions and collected in parallel for histopathological, immunohistochemical, and microbiological analyses. For immunohistochemical stainings, colon samples were immediately fixed in 5% formalin and embedded in paraffin, and sections (5 μm) were stained with the respective antibodies as described below. Histopathology was investigated in paraffin-embedded hematoxylin and eosin (H&E) stained tissue sections. A published standardized histologic score ranging from 0 to 6 was used for blinded evaluation of the inflammatory processes in the colon [12].
Immunohistochemistry
In situ immunohistochemical analysis of 5 μm thin colonic paraffin sections was performed as described previously [10, 31–34]. Primary antibodies against cleaved caspase-3 (Asp175, Cell Signaling, USA, 1:200), Ki67 (TEC3, Dako, Denmark, 1:100), F4/80 (# 14-4801, clone BM8, eBioscience, 1:50), myeloperoxidase-7 (MPO-7, # A0398, Dako, 1:500), CD3 (M-20, Santa Cruz, dilution 1:1000), FOXP3 (FJK-16s, eBioscience, 1:100), and B220 (eBioscience, San Diego, CA, USA, 1:200) were used. For each animal, the average number of positively stained cells within at least six high power fields (HPF, 0.287 mm2; 400× magnification) was determined microscopically by two independent double-blinded investigators and subjected to statistical analysis as indicated below.
Statistical analysis
Mean values, medians, and levels of significance were determined using Mann–Whitney U test. Two-sided probability (P) values ≤ 0.05 were considered significant. All experiments were performed twice.
Results
Uncompromized C. jejuni infection of gnotobiotic IL-10−/− mice following selective gelatinase blockage
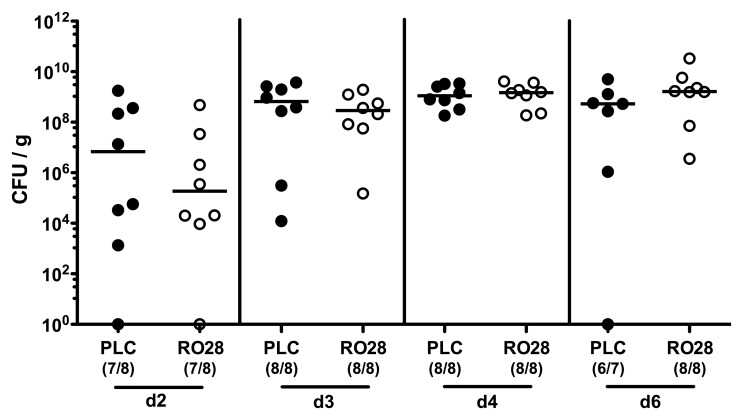
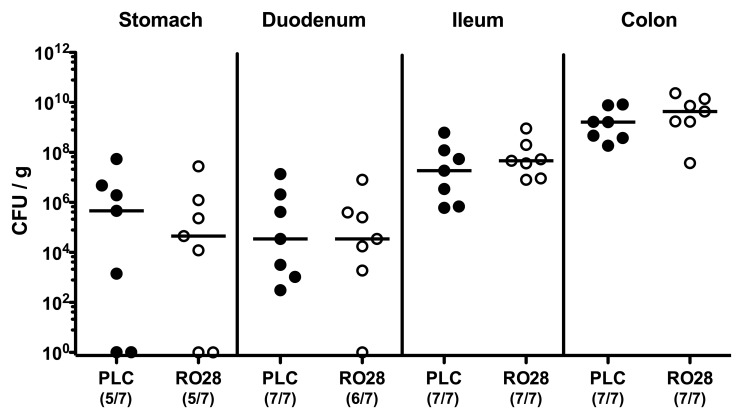
Given that selective gelatinase blockage by the synthetic compound RO28-2653 was shown effective in preventing and treating acute small intestinal as well as colonic inflammation [22, 23], we were interested whether RO28-2653 could affect murine acute ulcerative enterocolitis following C. jejuni infection. To address this, we applied our gnotobiotic IL-10−/− mouse infection model [10]. In order to eradicate the chronic colitogenic stimuli derived from the commensal intestinal microbiota, IL-10−/− mice were subjected to a quintuple antibiotic regimen for 4 months starting immediately after weaning [10]. The resulting gnotobiotic mice were then perorally infected with 109 CFU C. jejuni strain 81-176 on day 0. Starting at day 1, infected IL-10−/− mice were then perorally treated with RO28-2653 or placebo for 6 days on a daily basis. Notably, neither the synthetic compound nor placebo exerted any antimicrobial effects in respective disk diffusion assays (not shown) that might interfere with establishment of C. jejuni in the murine gastrointestinal tract. This was further confirmed by daily assessment of the C. jejuni loads in fecal samples demonstrating comparable pathogen burdens in the intestines of RO28-2653- and placebo-treated mice over time (Fig. 1). On day 7 post infection (p.i.), when gnotobiotic IL-10−/− mice suffered from severe C. jejuni-induced ulcerative enterocolitis, all animals displayed comparable pathogen loads throughout the entire gastrointestinal tract irrespective whether treated with RO28-2653 or placebo (Fig. 2). Hence, synthetic gelatinase blockage did not interfere with C. jejuni infection of gnotobiotic IL-10−/− mice.
Fig. 1.
Kinetic analysis of fecal C. jejuni loads following selective gelatinase blockage. Gnotobiotic IL-10−/− mice were generated by antibiotic treatment and orally infected with C. jejuni strain 81-176 on day 0. Starting at day 1 post infection, infected mice were either treated with RO28-2653 (RO28, open circles) or placebo (PLC, solid circles) once daily. C. jejuni loads were determined in fecal samples from day (d) 2 until d6 post infection by culture (CFU, colony forming units). Numbers of mice harboring the pathogen out of the total number of analyzed animals are given in parentheses. Medians (black bars) and significance levels (p values) determined by Mann–Whitney U test are indicated. Data are representative for two independent experiments
Fig. 2.
C. jejuni colonization of the gastrointestinal tract following selective gelatinase blockage. Gnotobiotic IL-10−/− mice were generated by antibiotic treatment and orally infected with C. jejuni strain 81-176 on day 0. Starting at day 1 post infection, infected mice were either treated with RO28-2653 (RO28, open circles) or placebo (PLC, solid circles) once daily. C. jejuni loads were determined in luminal samples of the gastrointestinal tract at day 7 post infection by culture (CFU, colony forming units). Numbers of mice harboring the pathogen out of the total number of analyzed animals are given in parentheses. Medians (black bars) and significance levels (p values) determined by Mann–Whitney U test are indicated. Data shown are representative for two independent experiments
Better clinical outcome of C. jejuni-infected mice following selective gelatinase blockage
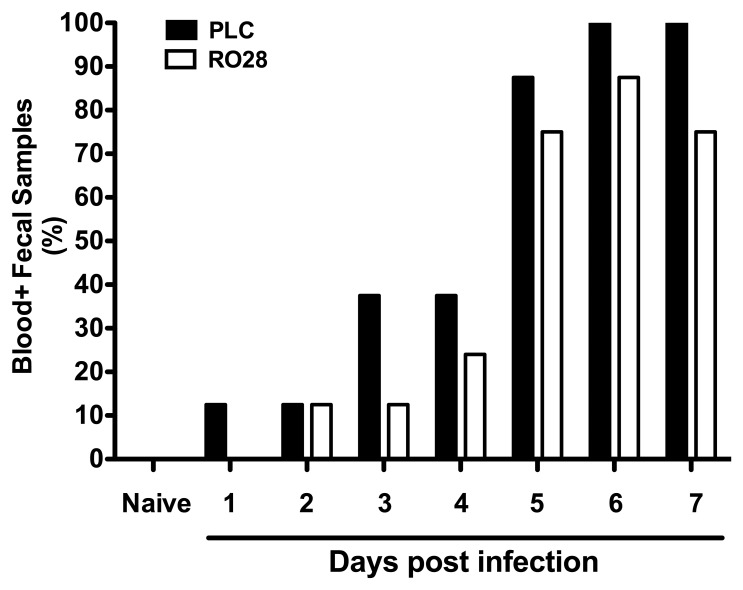
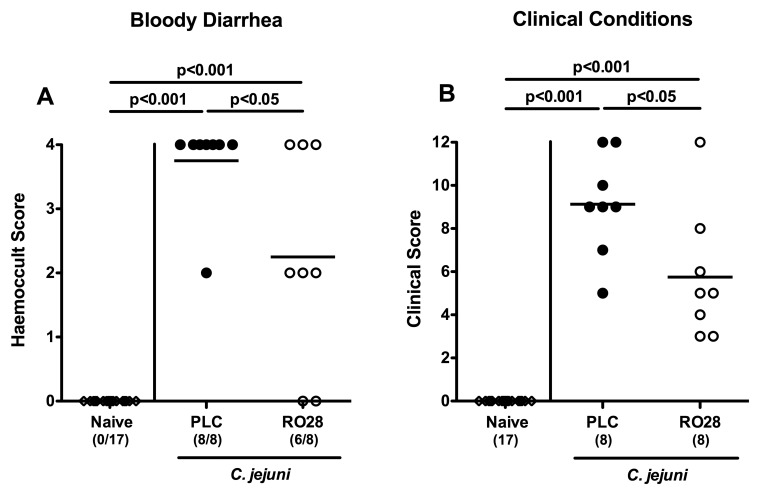
We next assessed clinical symptoms such as bloody diarrhea, a hallmark of severe campylobacteriosis [5, 7], in C. jejuni-infected mice upon selective gelatinase blockage over time. Remarkably, kinetic analyses of the occurrence of blood in fecal samples following C. jejuni infection revealed that RO28-2653-treated mice exhibited less frequently bloody diarrhea from day 3 until day 7 p.i. as compared to placebo control animals (Fig. 3 and Fig. 4A, p < 0.05). The beneficial effect of selective gelatinase blockage was further substantiated by assessing a more differential cumulative clinical score. At day 7 p.i., placebo control mice were severely compromised, whereas RO28-2653-treated mice exhibited approximately 50% lower clinical scores as compared to placebo animals (p < 0.05; Fig. 4B). Thus, selective gelatinase blockage resulted in better clinical outcomes of C. jejuni-infected gnotobiotic IL-10−/− mice.
Fig. 3.
Bloody diarrhea. Kinetic analysis of blood-positive fecal samples in C. jejuni-infected mice following selective gelatinase blockage. Gnotobiotic IL-10−/− mice were generated by antibiotic treatment and orally infected with C. jejuni strain 81-176 on day 0. Starting at day 1 post infection, infected mice were either treated with RO28-2653 (RO28, white bars) or placebo (PLC, black bars) once daily. Indicated relative rates of blood-positive fecal samples of uninfected (naive) and C. jejuni-infected IL-10−/− mice were determined by haemoccult test on a daily basis. Data shown are representative for two independent experiments
Fig. 4.
Clinical symptoms of C. jejuni-infected mice following selective gelatinase blockage. Gnotobiotic IL-10−/− mice were generated by antibiotic treatment and orally infected with C. jejuni strain 81-176 at day 0. Naive uninfected mice served as negative controls (open diamonds). Starting on day 1 post infection, infected mice were either treated with RO28-2653 (RO28, open circles) or placebo (PLC, solid circles) once daily. (A) The occurrence of blood in fecal samples and (B) clinical symptoms were assessed at day 7 post infection applying respective standardized scores as described in methods. Numbers of haemoccult-positive animals (A) out of the total numbers of analyzed mice (A, B) are given in parentheses. Means (black bars) and significance levels (p values) determined by Mann–Whitney U test are indicated. Data shown are representative for two independent experiments
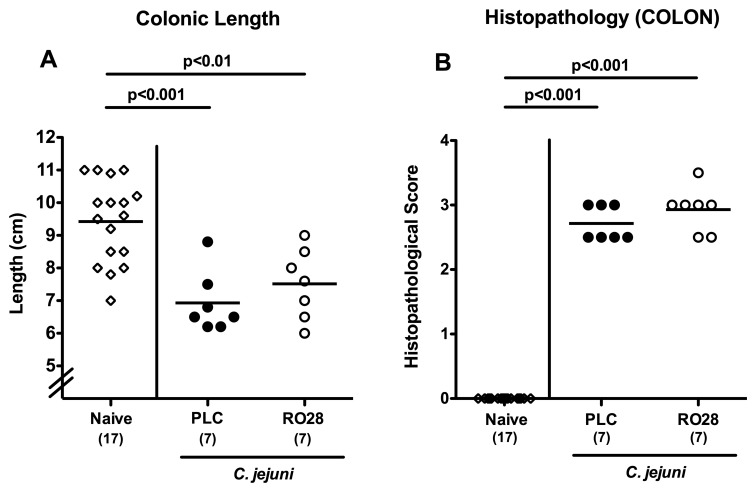
Macroscopic and histopathological colonic changes in C. jejuni-infected mice following selective gelatinase blockage
Given that intestinal inflammation results in a significant shortening of the intestines [10, 29, 33], we next determined the absolute colonic lengths in C. jejuni-infected mice following the respective treatment regimen. At day 7 p.i., placebo- and RO28-2653-treated animals exhibited significantly shorter large intestines as compared to naive controls (p < 0.001 and p < 0.01, respectively; Fig. 5A). Although not significantly different, infected mice subjected to selective gelatinase blockage displayed a trend towards higher large intestinal lengths as compared to the placebo group (Fig. 5A). We further determined the C. jejuni-induced colonic histopathological sequelae following gelatinase blockage. Irrespective of the treatment regimen, C. jejuni-infected gnotobiotic IL-10−/− mice displayed comparable histopathological scores determined in colonic H&E stained paraffin sections at day 7 p.i. (Fig. 5B).
Fig. 5.
Macroscopic and histopathological colonic changes in C. jejuni-infected mice following selective gelatinase blockage. Gnotobiotic IL-10−/− mice were generated by antibiotic treatment and orally infected with C. jejuni strain 81-176 on day 0. Naive uninfected mice served as negative controls (open diamonds). Starting at day 1 post infection, infected mice were either treated with RO28-2653 (RO28, open circles) or placebo (PLC, solid circles) once daily. (A) Absolute colonic lengths and (B) histopathological changes applying a standardized histopathological score in H&E-stained colonic paraffin sections were determined at day 7 post infection. Numbers of analyzed mice are given in parentheses. Means (black bars) and significance levels (p values) determined by Mann–Whitney U test are indicated. Data shown are representative for two independent experiments
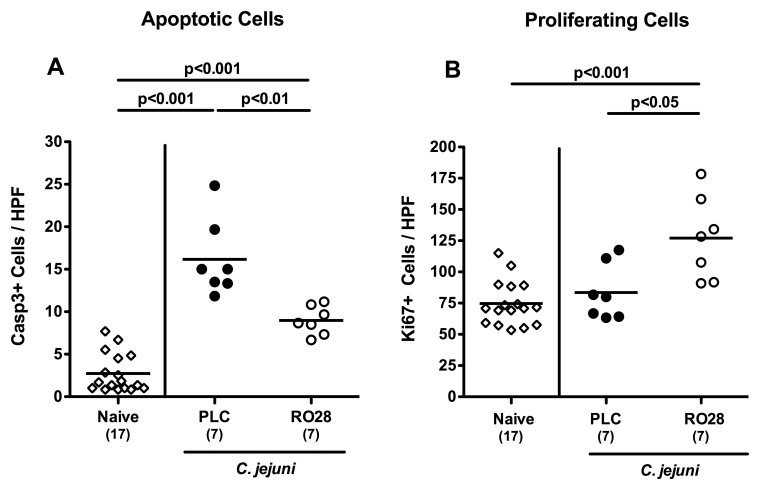
Colonic apoptotic and proliferating cells in C. jejuni-infected mice following selective gelatinase blockage
Given that apoptosis is a commonly used diagnostic marker in the histopathological evaluation and grading of intestinal diseases [33] and furthermore a key feature of C. jejuni-induced ulcerative enterocolitis in gnotobiotic IL-10−/− mice and infected humans [10], we next quantitatively assessed caspase-3+ cells within the colonic mucosa in C. jejuni-infected mice following selectice gelatinase inhibition. Seven days following C. jejuni infection numbers of apoptotic cells increased multifold (p < 0.001; Fig. 6A), but to a significantly lesser extent in RO28-2653-treated as compared to placebo-treated mice (p < 0.01; Fig. 6A). Conversely, numbers of colonic Ki67+ proliferating cells increased in C. jejuni-infected mice following selective gelatinase inhibition only as compared to naive or placebo treated animals at day 7 p.i. (p < 0.001 and p < 0.05, respectively; Fig. 6B). Hence, selective gelatinase blockage resulted in less apoptosis and more cell proliferation thereby counteracting mucosal inflammation in C. jejuni-infected gnotobiotic IL-10−/− mice.
Fig. 6.
Colonic apoptotic and proliferating cells in C. jejuni-infected mice following selective gelatinase blockage. Gnotobiotic IL-10−/− mice were generated by antibiotic treatment and orally infected with C. jejuni strain 81-176 on day 0. Naive, uninfected mice served as negative controls (open diamonds). Starting at day 1 post infection, infected mice were either treated with RO28-2653 (RO28, open circles) or placebo (PLC, solid circles) once daily. The average number of (A) apoptotic (positive for caspase-3, Casp3) and (B) proliferating cells (positive for Ki67) from at least six high power fields (HPF, 400× magnification) per animal was determined microscopically in immunohistochemically stained colonic paraffin sections at day 7 post infection. Means (black bars) and significance levels (p values) determined by Mann–Whitney U test are indicated. Data shown are representative for two independent experiments
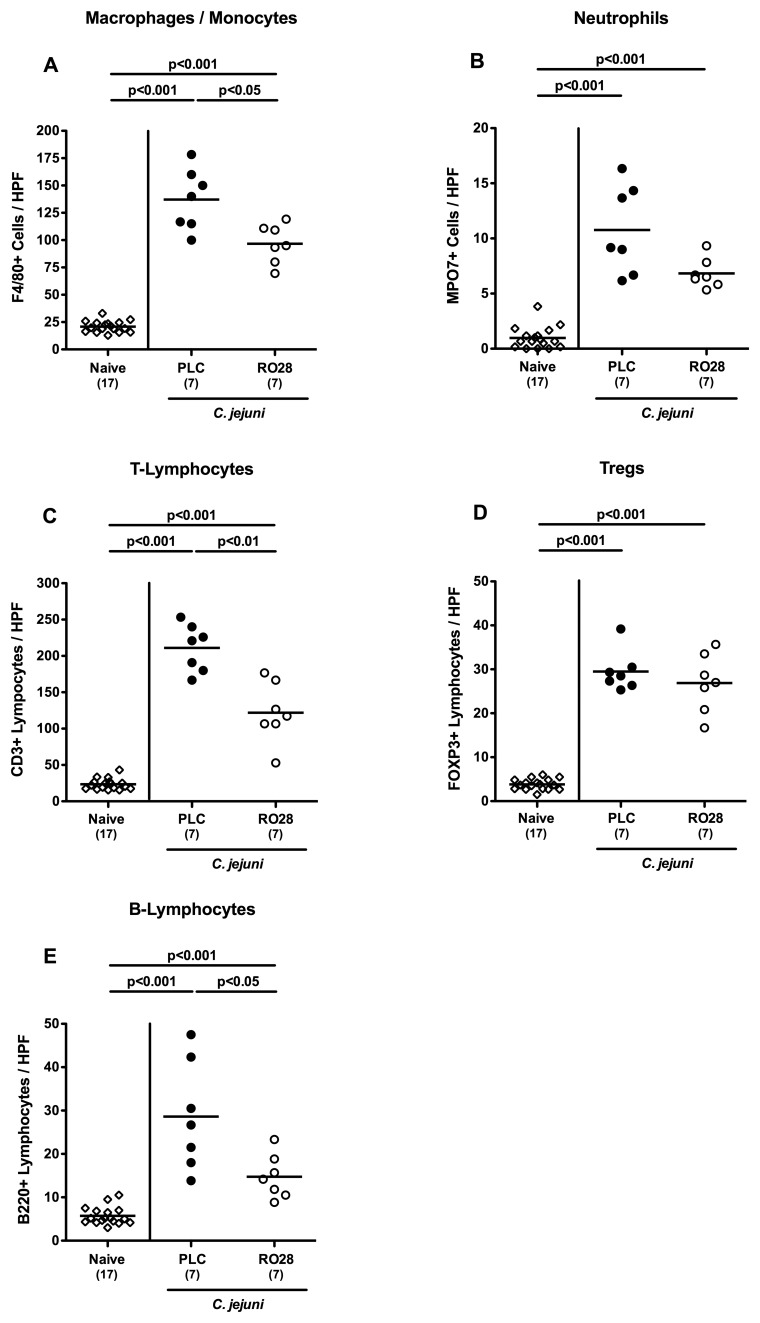
Less pronounced immune cell responses in the colonic mucosa of C. jejuni-infected mice following selective gelatinase blockage
Given that human colitis is accompanied by the recruitment of pro-inflammatory immune cell populations to sites of inflammation in the large intestine [23], we next quantitated inflammatory immune cells in situ by immunohistochemical staining of colonic paraffin sections of mice with antibodies against F4/80 (macrophages and monocytes), MPO7 (neutrophils), CD3 (T-lymphocytes), FOXP3 (regulatory T-cells, Treg), and B220 (B-lymphocytes). At day 7, following C. jejuni infection, placebo control mice displayed multifold increases in colonic macrophage, monocyte, and neutrophil numbers exerting oxidative stress to the epithelium (p < 0.001; Fig. 7A and B). The increase of F4/80+ macrophages and monocytes, however, was less pronounced in RO28-2653 treated mice (p < 0.05; Fig. 7A). Even though not statistically significant due to a relatively high standard deviation in the placebo group, a trend towards a less pronounced influx of MPO7+ neutrophils into the colonic lamina propria could be observed following RO28-2653 as compared to placebo treatment (Fig. 7B). We have recently shown that mucosal infiltration with CD3+ T-cells is a major characteristic feature of acute and chronic C. jejuni infection of mice [10, 31, 33–35]. Accordingly, a multifold increase of T-lymphoctes into the colonic mucosa and lamina propria could be observed 7 days following C. jejuni infection of gnotobiotic IL-10−/− mice (p < 0.001; Fig. 7C). This increase, however, was significantly less distinct following selective gelatinase blockage as compared to placebo treatment (p < 0.01; Fig. 7C). Interestingly, the C. jejuni-induced increase of FOXP3+ Tregs was similar in RO28-2653- and placebo-treated mice at day 7 p.i. (p < 0.001; Fig. 7D). Furthermore, numbers of colonic B220+ B-lymphocytes were lower in RO28-2653-treated as compared to placebo-treated mice seven day following C. jejuni infection (p < 0.05; Fig. 7E). Hence, selective gelatinase blockage resulted in reduced immune cell numbers within the colonic mucosa and lamina propria of C. jejuni-infected gnotobiotic IL-10−/− mice.
Fig. 7.
Colonic immune cell responses in C. jejuni-infected mice following selective gelatinase blockage. Gnotobiotic IL-10−/− mice were generated by antibiotic treatment and orally infected with C. jejuni strain 81-176 on day 0. Naive uninfected mice served as negative controls (open diamonds). Starting at day 1 post infection, infected mice were either treated with RO28-2653 (RO28, open circles) or placebo (PLC, solid circles) once daily. The average number of (A) macrophages and monocytes (positive for F4/80), (B) neutrophils (positive for MPO7), (C) T-lymphocytes (positive for CD3), (D) regulatory T-cells (Tregs, positive for FOXP3), and (E) B-lymphocytes (positive for B220) from at least six high power fields (HPF, 400× magnification) per animal was determined microscopically in immunohistochemically stained colonic paraffin sections at day 7 post infection. Means (black bars) and significance levels (p values) determined by Mann–Whitney U test are indicated. Data shown are representative for two independent experiments
Discussion
The gelatinases A and B play pivotal roles in intestinal inflammation in mice and men [21–26]. Given that MMPs are also essentially involved in physiological homeostasis of tissues including the cartilage and the gut [36, 37], compounds for nonselectively blocking MMPs (including the collagenases) can exert serious adverse effects such as muscle pain, arthralgia, and compromised wound healing limiting clinical application. Hence, selective and effective MMP blocking agents with limited side effects would be utmost desirable. Furthermore, MMPs are known to shed biologically active pro-inflammatory molecules such as TNF and IL-6 from surfaces of macrophages subsequently inducing MMP expression in epithelial, parenchymal, as well as immune cells [36, 38]. Therefore, from the therapeutic perspective, an effective MMP-blocking agent should cut this vicious pro-inflammatory cycle due to this positive MMP-feedback loop by downregulating expression and function of MMPs, immune cells such as macrophages, and pro-inflammatory cytokines all at once without compromising health-promoting MMP properties such as cell regeneration [23].
To date, no data exist regarding the roles of MMPs and particularly the gelatinases in C. jejuni infection. To address this, we applied the gnotobiotic IL-10−/− mouse infection model. Within 1 week, following C. jejuni infection, mice suffer from non-self-limiting acute ulcerative enterocolitis [10]. In the present study, selective gelatinase blockage by peroral treatment with the synthetic gelatinase blocking compound RO28-2653 effectively ameliorated C. jejuni-induced enterocolitis. We could show here that RO28-2653 treatment resulted in 1) better clinical conditions of infected mice, 2) less frequent bloody diarrhea (a clinical hallmark of human campylobacteriosis), 3) less colonic apoptosis, but 4) more cell proliferation counteracting the inflammatory process, and finally, 5) less distinct influx of pro-inflammatory immune cell populations such as T- and B-cells as well as macrophages and monocytes in C. jejuni-infected gnotobiotic IL-10−/− mice. Notably, RO28-2653 did not affect C. jejuni replication either in vitro or in vivo as indicated by comparable pathogen loads in the gastrointestinal tract of RO28-2653 as compared to placebo treated mice. These results are well in line with our two previous murine studies of acute intestinal inflammation. We could recently show that the selective gelatinase blocking compound RO28-2653 was effective in preventing and treating murine acute ileitis following oral Toxoplasma gondii infection [22]. In this hyper-acute inflammatory scenario, RO28-2653 could dampen the Th1-type immune responses and ameliorate small intestinal disease. In addition, RO28-2653 treatment could also ameliorate acute DSS-induced colitis [23]. Like in our C. jejuni infection study, influx of immune cells such as T- and B-lymphocytes, macrophages, and monocytes as well as neutrophils into the colonic mucosa and lamina propria (perpetuating the inflammatory process) was diminished upon selective gelatinase blockage. Notably, RO28-2653 did neither show significant adverse effects in rat and monkey toxological studies [27] nor any antimicrobial effects that might affect the commensal microbiota compositon upon treatment [23]. Notably, regenerative properties of the colonic epithelium were not compromised by gelatinase inhibition in C. jejuni-mediated enterocolitis. Instead, numbers of Ki67+ proliferating cells in the colonic mucosa were even higher in RO28-2653-treated and C. jejuni-infected mice thereby counteracting the inflammatory process.
Conclusion
In conclusion, we propose the selective gelatinase blocker RO28-2653 as a promising future option for prophylaxis and treatment of intestinal inflammation in humans including campylobacteriosis.
Outlook
We have recently shown that MMP-2, but not MMP-9, is essentially involved in mediating acute T. gondii-induced ileitis [22] and acute DSS-induced colitis in mice [23]. In an ongoing study, we currently unravel whether MMP-2, MMP-9, or both are essentially involved in C. jejuni-induced inflammation.
Acknowledgements
This work was supported by grants from the German Research Foundation (DFG) to S.B. and A.F. (SFB633, TP A7), A.K. (SFB633, TP Z1), M.M.H. (SFB633, TP B6), M.E.A., and U.G. (SFB633, Immuco Graduate School) and from the German Federal Ministry of Education and Research (BMBF) to S.B. (TP1.1). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the article.
We thank Michaela Wattrodt, Ulrike Hagen, Ursula Rüschendorf, Alexandra Bittroff-Leben, Ines Puschendorf, Silvia Schulze, Gernot Reifenberger, Uwe Lohmann, and the staff of the animal research facility for excellent technical assistance, animal breeding, and genotyping of mice. We are grateful to Simone Spieckermann for immunohistochemistry staining of colon sections.
Contributor Information
M. E. Alutis, 1Department of Microbiology and Hygiene, Charité – University Medicine Berlin, Berlin, Germany.
U. Grundmann, 1Department of Microbiology and Hygiene, Charité – University Medicine Berlin, Berlin, Germany.
A. Fischer, 1Department of Microbiology and Hygiene, Charité – University Medicine Berlin, Berlin, Germany.
A. A. Kühl, 2Department of Internal Medicine, Rheumatology and Clinical Immunology/Research Center ImmunoSciences (RCIS), Charité – University Medicine Berlin, Berlin, Germany.
S. Bereswill, 1Department of Microbiology and Hygiene, Charité – University Medicine Berlin, Berlin, Germany.
M. M. Heimesaat, 1Department of Microbiology and Hygiene, Charité – University Medicine Berlin, Berlin, Germany.
References
- 1.Hermans D, Pasmans F, Messens W, Martel A, Van Immerseel F, Rasschaert G, Heyndrickx M, Van Deun K, Haesebrouck F. Poultry as a host for the zoonotic pathogen Campylobacter jejuni. Vector Borne Zoonotic Dis. 2012 Feb;12(2):89–98. doi: 10.1089/vbz.2011.0676. [DOI] [PubMed] [Google Scholar]
- 2.Man SM. The clinical importance of emerging Campylobacter species. Nat Rev Gastroenterol Hepatol. 2011 Oct 25;8(12):669–685. doi: 10.1038/nrgastro.2011.191. [DOI] [PubMed] [Google Scholar]
- 3.Lane JA, Mehra RK, Carrington SD, Hickey RM. The food glycome: a source of protection against pathogen colonization in the gastrointestinal tract. Int J Food Microbiol. 2010 Aug 15;142(1-2):1–13. doi: 10.1016/j.ijfoodmicro.2010.05.027. [DOI] [PubMed] [Google Scholar]
- 4.Guerry P, Szymanski CM. Campylobacter sugars sticking out. Trends Microbiol. 2008 Sep;16(9):428–435. doi: 10.1016/j.tim.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 5.Kist M, Bereswill S. Campylobacter jejuni. Contrib Microbiol. 2001;8:150–165. doi: 10.1159/000060405. [DOI] [PubMed] [Google Scholar]
- 6.Havelaar AH, van Pelt W, Ang CW, Wagenaar JA, van Putten JP, Gross U, Newell DG. Immunity to Campylobacter: its role in risk assessment and epidemiology. Crit Rev Microbiol. 2009;35(1):1–22. doi: 10.1080/10408410802636017. [DOI] [PubMed] [Google Scholar]
- 7.Janssen R, Krogfelt KA, Cawthraw SA, van Pelt W, Wagenaar JA, Owen RJ. Host-pathogen interactions in Campylobacter infections: the host perspective. Clin Microbiol Rev. 2008 Jul;21(3):505–518. doi: 10.1128/CMR.00055-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van Spreeuwel JP, Duursma GC, Meijer CJ, Bax R, Rosekrans PC, Lindeman J. Campylobacter colitis: histological immunohistochemical and ultrastructural findings. Gut. 1985 Sep;26(9):945–951. doi: 10.1136/gut.26.9.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Walker RI, Caldwell MB, Lee EC, Guerry P, Trust TJ, Ruiz-Palacios GM. Pathophysiology of Campylobacter enteritis. Microbiol Rev. 1986 Mar;50(1):81–94. doi: 10.1128/mr.50.1.81-94.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haag LM, Fischer A, Otto B, Plickert R, Kühl AA, Göbel UB, Bereswill S, Heimesaat MM. Campylobacter jejuni induces acute enterocolitis in gnotobiotic IL-10-/- mice via Toll-like-receptor-2 and -4 signaling. PLoS One. 2012;7(7):e40761. doi: 10.1371/journal.pone.0040761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heimesaat MM, Lugert R, Fischer A, Alutis M, Kühl AA, Zautner AE, Tareen AM, Göbel UB, Bereswill S. Impact of Campylobacter jejuni cj0268c knockout mutation on intestinal colonization, translocation, and induction of immunopathology in gnotobiotic IL-10 deficient mice. PLoS One. 2014;9(2):e90148. doi: 10.1371/journal.pone.0090148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heimesaat MM, Alutis M, Grundmann U, Fischer A, Tegtmeyer N, Böhm M, Kühl AA, Göbel UB, Backert S, Bereswill S. The role of serine protease HtrA in acute ulcerative enterocolitis and extra-intestinal immune responses during Campylobacter jejuni infection of gnotobiotic IL-10 deficient mice. Front Cell Infect Microbiol. 2014;4:77. doi: 10.3389/fcimb.2014.00077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Warren HS, Fitting C, Hoff E, Adib-Conquy M, Beasley-Topliffe L, Tesini B, Liang X, Valentine C, Hellman J, Hayden D, Cavaillon JM. Resilience to bacterial infection: difference between species could be due to proteins in serum. J Infect Dis. 2010 Jan 15;201(2):223–232. doi: 10.1086/649557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Masanta WO, Heimesaat MM, Bereswill S, Tareen AM, Lugert R, Groß U, Zautner AE. Modification of intestinal microbiota and its consequences for innate immune response in the pathogenesis of campylobacteriosis. Clin Dev Immunol. 2013;2013:526860. doi: 10.1155/2013/526860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Birkedal-Hansen H, Moore WG, Bodden MK, Windsor LJ, Birkedal-Hansen B, DeCarlo A, Engler JA. Matrix metalloproteinases: a review. Crit Rev Oral Biol Med. 1993;4(2):197–250. doi: 10.1177/10454411930040020401. [DOI] [PubMed] [Google Scholar]
- 16.Brinckerhoff CE, Matrisian LM. Matrix metalloproteinases: a tail of a frog that became a prince. Nat Rev Mol Cell Biol. 2002 Mar;3(3):207–214. doi: 10.1038/nrm763. [DOI] [PubMed] [Google Scholar]
- 17.Nelson AR, Fingleton B, Rothenberg ML, Matrisian LM. Matrix metalloproteinases: biologic activity and clinical implications. J Clin Oncol. 2000 Mar;18(5):1135–1149. doi: 10.1200/JCO.2000.18.5.1135. [DOI] [PubMed] [Google Scholar]
- 18.Goetzl EJ, Banda MJ, Leppert D. Matrix metalloproteinases in immunity. J Immunol. 1996 Jan 1;156(1):1–4. [PubMed] [Google Scholar]
- 19.Crawford HC, Matrisian LM. Mechanisms controlling the transcription of matrix metalloproteinase genes in normal and neoplastic cells. Enzyme Protein. 1996;49(1-3):20–37. doi: 10.1159/000468614. [DOI] [PubMed] [Google Scholar]
- 20.Sarén P, Welgus HG, Kovanen PT. TNF-alpha and IL-1beta selectively induce expression of 92-kDa gelatinase by human macrophages. J Immunol. 1996 Nov 1;157(9):4159–4165. [PubMed] [Google Scholar]
- 21.Salmela MT, MacDonald TT, Black D, Irvine B, Zhuma T, Saarialho-Kere U, Pender SL. Upregulation of matrix metalloproteinases in a model of T cell mediated tissue injury in the gut: analysis by gene array and in situ hybridisation. Gut. 2002 Oct;51(4):540–547. doi: 10.1136/gut.51.4.540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Muñoz M, Heimesaat MM, Danker K, Struck D, Lohmann U, Plickert R, Bereswill S, Fischer A, Dunay IR, Wolk K, Loddenkemper C, Krell HW, Libert C, Lund LR, Frey O, Hölscher C, Iwakura Y, Ghilardi N, Ouyang W, Kamradt T, Sabat R, Liesenfeld O. Interleukin (IL)-23 mediates Toxoplasma gondii-induced immunopathology in the gut via matrixmetalloproteinase-2 and IL-22 but independent of IL-17. J Exp Med. 2009 Dec 21;206(13):3047–3059. doi: 10.1084/jem.20090900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Heimesaat MM, Dunay IR, Fuchs D, Trautmann D, Fischer A, Kühl AA, Loddenkemper C, Batra A, Siegmund B, Krell HW, Bereswill S, Liesenfeld O. Selective gelatinase blockage ameliorates acute DSS colitis. Eur J Microbiol Immunol (Bp) 2011 Sep;1(3):228–236. doi: 10.1556/EuJMI.1.2011.3.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.von Lampe B, Barthel B, Coupland SE, Riecken EO, Rosewicz S. Differential expression of matrix metalloproteinases and their tissue inhibitors in colon mucosa of patients with inflammatory bowel disease. Gut. 2000 Jul;47(1):63–73. doi: 10.1136/gut.47.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bailey CJ, Hembry RM, Alexander A, Irving MH, Grant ME, Shuttleworth CA. Distribution of the matrix metalloproteinases stromelysin, gelatinases A and B, and collagenase in Crohn's disease and normal intestine. J Clin Pathol. 1994 Feb;47(2):113–116. doi: 10.1136/jcp.47.2.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baugh MD, Perry MJ, Hollander AP, Davies DR, Cross SS, Lobo AJ, Taylor CJ, Evans GS. Matrix metalloproteinase levels are elevated in inflammatory bowel disease. Gastroenterology. 1999 Oct;117(4):814–822. doi: 10.1016/s0016-5085(99)70339-2. [DOI] [PubMed] [Google Scholar]
- 27.Lein M, Jung K, Ortel B, Stephan C, Rothaug W, Juchem R, Johannsen M, Deger S, Schnorr D, Loening S, Krell HW. The new synthetic matrix metalloproteinase inhibitor (Roche 28-2653) reduces tumor growth and prolongs survival in a prostate cancer standard rat model. Oncogene. 2002 Mar 27;21(13):2089–2096. doi: 10.1038/sj.onc.1205267. [DOI] [PubMed] [Google Scholar]
- 28.Bernardo MM, Brown S, Li ZH, Fridman R, Mobashery S. Design, synthesis, and characterization of potent, slow-binding inhibitors that are selective for gelatinases. J Biol Chem. 2002 Mar 29;277(13):11201–11207. doi: 10.1074/jbc.M111021200. [DOI] [PubMed] [Google Scholar]
- 29.Heimesaat MM, Bereswill S, Fischer A, Fuchs D, Struck D, Niebergall J, Jahn HK, Dunay IR, Moter A, Gescher DM, Schumann RR, Göbel UB, Liesenfeld O. Gram-negative bacteria aggravate murine small intestinal Th1-type immunopathology following oral infection with Toxoplasma gondii. J Immunol. 2006 Dec 15;177(12):8785–8795. doi: 10.4049/jimmunol.177.12.8785. [DOI] [PubMed] [Google Scholar]
- 30.Bereswill S, Muñoz M, Fischer A, Plickert R, Haag LM, Otto B, Kühl AA, Loddenkemper C, Göbel UB, Heimesaat MM. Anti-inflammatory effects of resveratrol, curcumin and simvastatin in acute small intestinal inflammation. PLoS One. 2010 Dec 3;5(12):e15099. doi: 10.1371/journal.pone.0015099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Haag LM, Fischer A, Otto B, Plickert R, Kühl AA, Göbel UB, Bereswill S, Heimesaat MM. Intestinal microbiota shifts towards elevated commensal Escherichia coli loads abrogate colonization resistance against Campylobacter jejuni in mice. PLoS One. 2012;7(5):e35988. doi: 10.1371/journal.pone.0035988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Heimesaat MM, Nogai A, Bereswill S, Plickert R, Fischer A, Loddenkemper C, Steinhoff U, Tchaptchet S, Thiel E, Freudenberg MA, Göbel UB, Uharek L. MyD88/TLR9 mediated immunopathology and gut microbiota dynamics in a novel murine model of intestinal graft-versus-host disease. Gut. 2010 Aug;59(8):1079–1087. doi: 10.1136/gut.2009.197434. [DOI] [PubMed] [Google Scholar]
- 33.Bereswill S, Fischer A, Plickert R, Haag LM, Otto B, Kühl AA, Dasti JI, Zautner AE, Muñoz M, Loddenkemper C, Gross U, Göbel UB, Heimesaat MM. Novel murine infection models provide deep insights into the "ménage à trois" of Campylobacter jejuni, microbiota and host innate immunity. PLoS One. 2011;6(6):e20953. doi: 10.1371/journal.pone.0020953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Heimesaat MM, Haag LM, Fischer A, Otto B, Kühl AA, Göbel UB, Bereswill S. Survey of extra-intestinal immune responses in asymptomatic long-term Campylobacter jejuni-infected mice. Eur J Microbiol Immunol (Bp) 2013 Sep;3(3):174–182. doi: 10.1556/EuJMI.3.2013.3.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Haag LM, Fischer A, Otto B, Grundmann U, Kühl AA, Göbel UB, Bereswill S, Heimesaat MM. Campylobacter jejuni infection of infant mice: acute enterocolitis is followed by asymptomatic intestinal and extra-intestinal immune responses. Eur J Microbiol Immunol (Bp) 2012 Mar;2(1):2–11. doi: 10.1556/EuJMI.2.2012.1.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Naito Y, Takagi T, Kuroda M, Katada K, Ichikawa H, Kokura S, Yoshida N, Okanoue T, Yoshikawa T. An orally active matrix metalloproteinase inhibitor, ONO-4817, reduces dextran sulfate sodium-induced colitis in mice. Inflamm Res. 2004 Sep;53(9):462–468. doi: 10.1007/s00011-004-1281-1. [DOI] [PubMed] [Google Scholar]
- 37.Parks WC, López-Boado YS, Wilson CL. Matrilysin in epithelial repair and defense. Chest. 2001 Jul;120(1 Suppl):36S–41S. doi: 10.1378/chest.120.1_suppl.s36. [DOI] [PubMed] [Google Scholar]
- 38.Wang M, Qin X, Mudgett JS, Ferguson TA, Senior RM, Welgus HG. Matrix metalloproteinase deficiencies affect contact hypersensitivity: stromelysin-1 deficiency prevents the response and gelatinase B deficiency prolongs the response. Proc Natl Acad Sci U S A. 1999 Jun 8;96(12):6885–6889. doi: 10.1073/pnas.96.12.6885. [DOI] [PMC free article] [PubMed] [Google Scholar]