Abstract
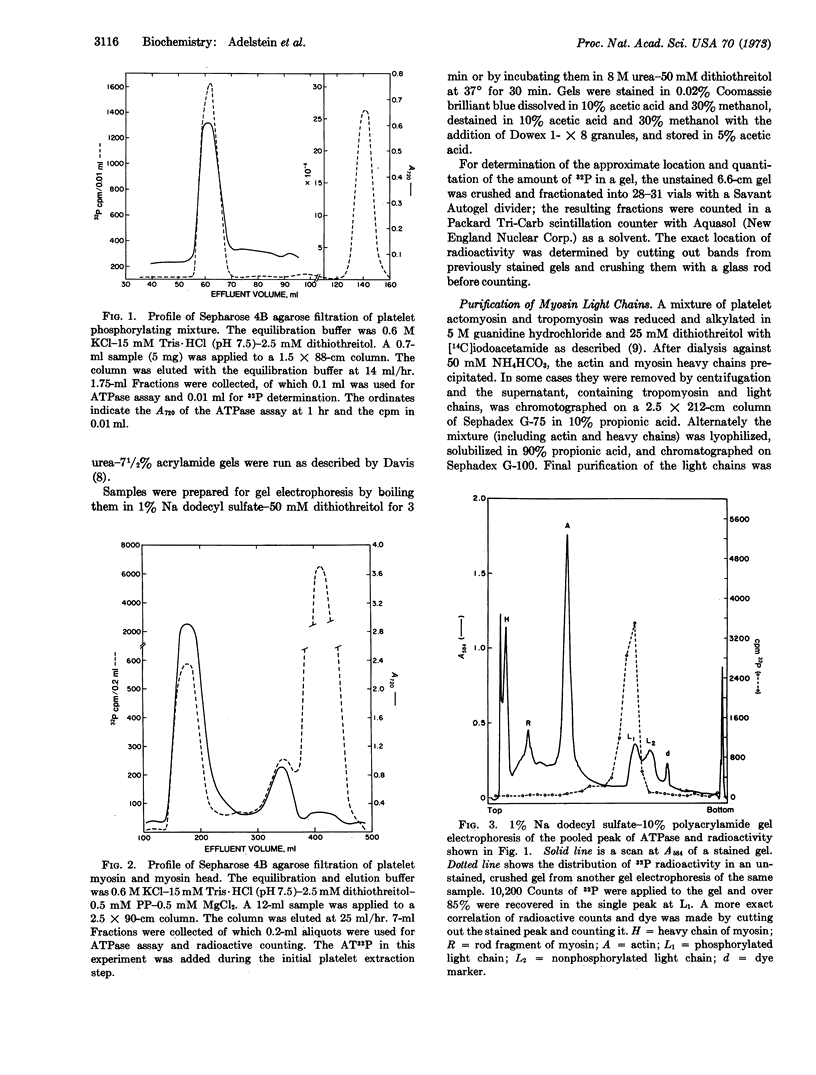
A preparation extracted from human blood platelets, which incorporates 32P from γ-labeled AT32P into one of the two light chains of platelet myosin and platelet myosin head is described. This phosphorylation, which appears to be due to an endogenous kinase, is specific for the myosin light chain in that no other protein extracted in 0.6 M KCl-15 mM Tris·HCl (pH 7.5) is phosphorylated. The phosphorylated light chain, which has been purified by gel filtration, releases the covalently bound phosphate after incubation in alkali and not after incubation in acid.
Keywords: myosin light chain, [γ-32P]ATP, contractile proteins, endogenous kinase
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adelstein R. S., Conti M. A., Johnson G. S., Pastan I., Pollard T. D. Isolation and characterization of myosin from cloned mouse fibroblasts. Proc Natl Acad Sci U S A. 1972 Dec;69(12):3693–3697. doi: 10.1073/pnas.69.12.3693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adelstein R. S., Kuehl W. M. Structural studies on rabbit skeletal actin. I. Isolation and characterization of the peptides produced by cyanogen bromide cleavage. Biochemistry. 1970 Mar 17;9(6):1355–1364. doi: 10.1021/bi00808a009. [DOI] [PubMed] [Google Scholar]
- Adelstein R. S., Pollard T. D., Kuehl W. M. Isolation and characterization of myosin and two myosin fragments from human blood platelets. Proc Natl Acad Sci U S A. 1971 Nov;68(11):2703–2707. doi: 10.1073/pnas.68.11.2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berl S., Puszkin S., Nicklas W. J. Actomyosin-like protein in brain. Science. 1973 Feb 2;179(4072):441–446. doi: 10.1126/science.179.4072.441. [DOI] [PubMed] [Google Scholar]
- Booyse F. M., Hoveke T. P., Rafelson M. E., Jr Human platelet actin. Isolation and properties. J Biol Chem. 1973 Jun 10;248(11):4083–4091. [PubMed] [Google Scholar]
- Cohen I., Cohen C. A tropomyosin-like protein from human platelets. J Mol Biol. 1972 Jul 21;68(2):383–387. doi: 10.1016/0022-2836(72)90220-3. [DOI] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- England P. J., Stull J. T., Krebs E. G. Dephosphorylation of the inhibitor component of troponin by phosphorylase phosphatase. J Biol Chem. 1972 Aug 25;247(16):5275–5277. [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Perrie W. T., Smillie L. B., Perry S. B. A phosphorylated light-chain component of myosin from skeletal muscle. Biochem J. 1973 Sep;135(1):151–164. doi: 10.1042/bj1350151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry S. V., Cole H. A. Phosphorylation of the "37000 component" of the troponin complex (troponin-t). Biochem J. 1973 Feb;131(2):425–428. doi: 10.1042/bj1310425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratje E., Heilmeyer L. M.G. Phosphorylation of rabbit muscle troponin and actin by a 3', 5'-c-AMP-dependent protein kinase. FEBS Lett. 1972 Oct 15;27(1):89–93. doi: 10.1016/0014-5793(72)80416-2. [DOI] [PubMed] [Google Scholar]
- Salzman E. W. Cyclic AMP and platelet function. N Engl J Med. 1972 Feb 17;286(7):358–363. doi: 10.1056/NEJM197202172860708. [DOI] [PubMed] [Google Scholar]
- Stull J. T., Brostrom C. O., Krebs E. G. Phosphorylation of the inhibitor component of troponin by phosphorylase kinase. J Biol Chem. 1972 Aug 25;247(16):5272–5274. [PubMed] [Google Scholar]
- Wolfe S. M., Shulman N. R. Adenyl cyclase activity in human platelets. Biochem Biophys Res Commun. 1969 Apr 29;35(2):265–272. doi: 10.1016/0006-291x(69)90277-0. [DOI] [PubMed] [Google Scholar]
- Yang Y. Z., Perdue J. F. Contractile proteins of cultured cells. I. The isolation and characterization of an actin-like protein from cultured chick embryo fibroblasts. J Biol Chem. 1972 Jul 25;247(14):4503–4509. [PubMed] [Google Scholar]