Abstract
The production of hydrogen (H2) is an inherent component of biological dinitrogen (N2) fixation, and there have been several studies quantifying H2 production relative to N2 fixation in cultures of diazotrophs. However, conducting the relevant measurements for a field population is more complex as shown by this study of N2 fixation, H2 consumption and dissolved H2 concentrations in the oligotrophic North Pacific Ocean. Measurements of H2 oxidation revealed microbial consumption of H2 was equivalent to 1–7% of ethylene produced during the acetylene reduction assay and 11–63% of 15N2 assimilation on a molar scale. Varying abundances of Crocosphaera and Trichodesmium as revealed by nifH gene abundances broadly corresponded with diel changes observed in both N2 fixation and H2 oxidation. However, no corresponding changes were observed in the dissolved H2 concentrations which remained consistently supersaturated (147–560%) relative to atmospheric equilibrium. The results from this field study allow the efficiency of H2 cycling by natural populations of diazotrophs to be compared to cultured representatives. The findings indicate that dissolved H2 concentrations may depend not only on the community composition of diazotrophs but also upon relevant environmental parameters such as light intensity or the presence of other H2-metabolizing microorganisms.
Introduction
In the surface waters of the tropical and subtropical open ocean, dissolved H2 concentrations typically range from 1–3 nmol l−1, equivalent to 300–900% supersaturation relative to atmospheric equilibrium (Herr et al., 1984; Conrad and Seiler, 1988; Moore et al., 2009). The magnitude of the dissolved H2 pool is determined by the ‘oceanic H2 cycle’ which reflects the balance between production and loss processes. As such, the main source of H2 is considered to be biological dinitrogen (N2) fixation (Herr et al., 1984; Scranton et al., 1987; Moore et al., 2009), whereby N2 is reduced to ammonia (NH3), as shown in Eq. 1:
| 1 |
where ADP and ATP are adenosine-5′-diphosphate and adenosine-5′-triphosphate respectively, H+ is hydrogen ion, e- is electron and Pi is inorganic phosphorus (Simpson and Burris, 1984). While N2 fixation is more commonly measured than H2 production, it is unwise to use the theoretical stoichiometry predicted in Eq. 1 to provide an estimate of H2 production associated with nitrogenase activity. This is due to several inherent issues associated with H2 cycling linked to N2 fixation, as listed below:
-
(i)
Measurements of H2 production alongside measurements of N2 fixation are always less than the equimolar stoichiometry predicted in Eq. 1 (Schubert and Evans, 1987; Wilson et al., 2010). This is because all diazotrophs contain uptake hydrogenases that re-assimilate a variable portion of H2 released during N2 fixation to conserve energy (Burns and Hardy, 1975, Tamagnini et al., 2007).
-
(ii)
Rates of net H2 production by diazotrophs appear to be highly species-specific. Laboratory-maintained cultures of two diazotrophs, Crocosphaera and Trichodesmium produce H2 at approximately 1% and 25% of their respective rates of N2 fixation, as measured by the acetylene reduction (AR) assay (Wilson et al., 2010). The comparatively high rates of net H2 production by Trichodesmium are a consequence of the cells fixing N2 during the daytime as the supply of photosynthetically derived energy and reductant decreases the need to re-assimilate the H2 as an energy source, resulting in an increase of net H2 production (Wilson et al., 2012b). By comparison, Crocosphaera fixes N2 during the dark period restricting the supply of cellular energy to nitrogenase from the respiration of photosynthetically fixed carbon (Waterbury et al., 1988; Berman-Frank et al., 2007). This causes a greater demand for the energy and reductant obtained from oxidizing H2 and therefore decreases the net H2 production (Wilson et al., 2010).
-
(iii)
Field measurements of N2 fixation can be conducted using the 15N2 assimilation technique or the AR assay. The 15N2 tracer technique is considered to be a measure of net N2 fixation as it does not account for dissolved organic and inorganic material released from cells (Montoya et al., 1996; Mulholland et al., 2004). The AR assay measures total nitrogenase activity by quantifying the reduction of acetylene (C2H2) to ethylene (C2H4) and therefore represents an indirect assay of N2 fixation (Burris, 1975). Because H2 production is equimolar with N2 fixation (Eq. 1), the AR assay should represent a better measurement when estimating the total amount of H2 produced by nitrogenase.
Due to the issues listed above, to define the role of N2 fixation in the global H2 cycle (e.g. Price et al., 2007), it is imperative to conduct field measurements of both N2 fixation and H2 production. In this study, simultaneous measurements of N2 fixation, biological H2 consumption and dissolved H2 concentrations were conducted in the surface waters of the open ocean where diazotrophs are present. Results are presented showing the diazotrophic community composition (as measured by nifH gene abundance and diversity), rates of net and gross N2 fixation (as measured by 15N2 tracer assimilation and AR assay respectively), H2 concentrations and H2 oxidation rates (using 3H2 as a tracer). Quantitative interpretation of the field data is aided by the recent measurement of net H2 production and N2 fixation in laboratory cultures of diazotrophs to infer the relative contribution of the representative marine N2-fixing microorganisms to the oceanic H2 cycle.
Results and discussion
Sampling overview
The oceanographic cruise was located approximately 250 km north of Oahu, Hawaii in the North Pacific Subtropical Gyre (NPSG) and occurred between 6 and 21 September 2011. The sampling stations were occupied along the north-western edge of an anti-cyclonic eddy spanning a total distance of 90 km and the subsequent westward section of the cruise track which spanned 80 km. Vertical profiles of dissolved H2 were conducted daily alongside biogeochemical and hydrographic measurements. Biological rate measurements of N2 fixation and H2 consumption were conducted at three sampling stations: Station (Stn) 3, 7 and 13 which were sampled on 7, 9 and 18 September 2011 respectively. Descriptions of the hydrographic conditions and biogeochemical properties of the water column are available in the accompanying Supporting Information and also online at http://hahana.soest.hawaii.edu/cmorebiolincs/biolincs.html.
Dissolved H2 concentrations
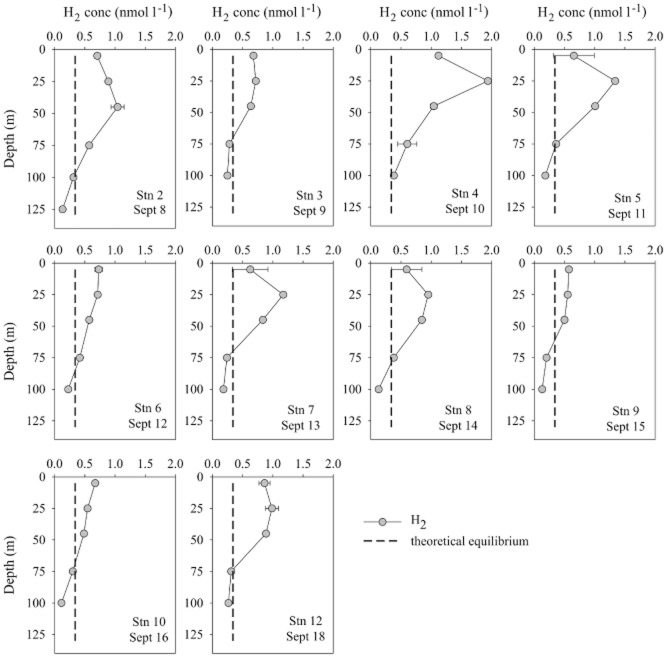
Dissolved H2 concentrations were supersaturated with respect to atmospheric equilibrium in the upper 75 m of the water column (Fig. 1). Overall, dissolved H2 concentrations in the surface mixed layer (0–45 m) ranged from 0.5–1.9 nmol l−1, with an average concentration of 0.83 nmol l−1, equivalent to 250% supersaturation. Dissolved H2 concentrations in seawater were calculated using the Bunsen solubility coefficients provided by Wiesenburg and Guinasso (1979). On four separate occasions, the concentrations of dissolved H2 in the mixed layer exceeded 1 nmol l−1 (Fig. 1). The concentrations of H2 measured in surface seawater during this cruise are consistent with measurements in other marine environments (e.g. the Mediterranean Sea, Atlantic and Pacific Ocean) revealing a persistent supersaturation of dissolved H2 in the near-surface seawater (Scranton et al., 1982; Herr et al., 1984; Conrad and Seiler, 1988; Moore et al., 2009). At depths exceeding 75 m, a progressive depletion in H2 concentrations was observed with values approaching undersaturation with respect to atmospheric equilibrium by a depth of 100 m. Vertical profiles of N2 fixation in the NPSG measured on previous occasions (Grabowski et al., 2008; Church et al., 2009) similarly show a decrease at 75 m, consistent with the hypothesis that the dissolved H2 is derived from nitrogenase activity.
Fig 1.
Dissolved H2 concentrations (nmol l−1) between depths of 5–125 m in the North Pacific Ocean. For each sampling occasion, seawater samples were collected at 1300 h. The theoretical value of dissolved H2 concentrations in seawater at atmospheric equilibrium (with an atmospheric concentration of 0.5 ppmv) is represented by the dashed line. Error bars where shown represent standard deviation (n = 3).
N2 fixation
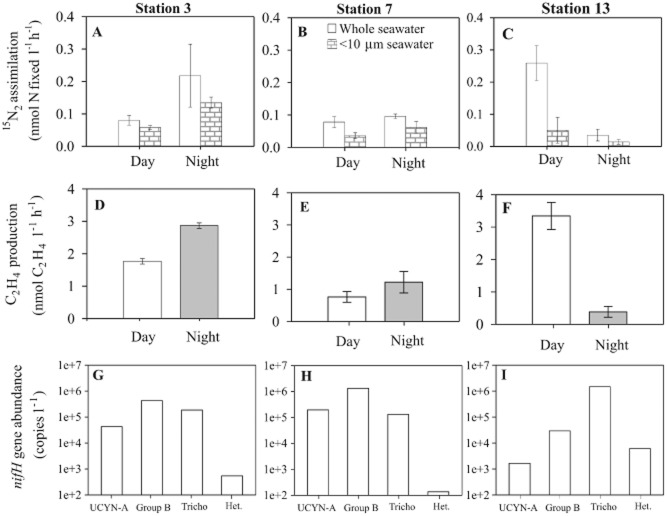
N2 fixation rate measurements, determined by both the 15N2 tracer assimilation and the AR assay, were conducted at Stn 3, 7 and 13. The overall temporal pattern of N2 fixation changed between the stations from an initial prevalence during the night-time, to a subsequent dominance during the day-time. Specifically, rates of 15N2 assimilation during the night-time (0.22 nmol l−1 h−1) exceeded the day-time (0.08 nmol l−1 h−1) at Stn 3 (Fig. 2A). In contrast, at Stn 13, rates of 15N2 assimilation in whole seawater were highest (0.26 nmol l−1 h−1) during the day-time, compared to the rates during the night-time (0.04 nmol l−1 h−1) (Fig. 2C). No significant difference was observed between the daytime and night-time measurements of N2 fixation at Stn 7. At all sampling stations, the rate of 15N2 assimilation in whole seawater samples exceeded the comparative rates in the accompanying < 10 μm size-fractionated seawater samples. Comparison of the < 10 μm size-fraction across the three stations reveals low variability in the rate of 15N2 assimilation (0.04–0.06 nmol l−1 h−1) during the daytime. In contrast, night-time rates of 15N2 assimilation for the < 10 μm size fraction varied by an order of magnitude, decreasing from 0.14 nmol l−1 h−1 at Stn 3, to 0.01 nmol l−1 h−1 at Stn 13 (Fig. 2A–C).
Fig 2.
N2 fixation rates as measured by (A–C) 15N2 tracer assimilation and (D–F) the AR assay for seawater samples collected at 25 m and incubated onboard the ship during either the day or night period. Post-incubation size fractionation was conducted for replicate 15N2 tracer additions and not for the AR assay. The error bars in A–F represent standard error (n = 3). The nifH gene abundances collected from the same depth on the same date are shown for UCYN-A, Group B (Crocosphaera spp.), (Tricho) Trichodesmium and (Het) heterocystous cyanobacteria (G–I).
AR was measured on whole seawater samples, and a significant increase in C2H4 concentrations was always detected during the 3–4 h incubations (Fig. 2D–F). The rates of C2H4 production support the 15N2 assimilation measurements with higher rates during the night-time (2.9 nmol l−1 h−1) compared to the daytime (1.8 nmol l−1 h−1) at Stn 3. Furthermore, at Stn 13, the diel pattern of C2H4 production changed with daytime (3.3 nmol l−1 h−1) exceeding night-time (0.4 nmol l−1 h−1) (Fig. 2F). Overall, the ratio of C2H4 to 15N2 assimilation varied from 9–22 which exceeds the theoretical ratio of 3:1 (Capone, 1993) by 3- to 7-fold. It should be noted that the theoretical ratio of 3:1 is based on the difference between two electrons required to reduce C2H2 to C2H4, and six electrons needed to reduce N2 to 2NH3. The reasons for the discrepancies between the theoretical and observed ratios have previously been discussed (e.g. Graham et al., 1980) and focus mainly on the excretion of N from the cell and the role of H2. There is insufficient data in this study to contribute to this discussion; however we do note from our work and the relevant literature that there is a greater difference in the AR:15N2 assimilation ratio in field measurements compared to culture-based analyses. Furthermore, there is a lack of experimental testing on the effect of key environmental parameters on the AR:15N2 assimilation ratio, e.g. light intensity or nutrient concentrations (Mague et al., 1977).
Diazotroph community structure
Representative N2 fixing microorganisms in the open ocean include: (i) the filamentous, non-heterocystous cyanobacterium Trichodesmium, (ii) the heterocystous cyanobacteria (e.g. Richelia and Calothrix) that form symbioses with eukaryotic algae, and (iii) unicellular cyanobacteria including Group A (termed UCYN-A) and Group B (e.g. Crocosphaera) (Mague et al., 1977; Carpenter and Romans, 1991; Zehr et al., 2001). The analysis of nifH gene abundances revealed Group B was the most abundant diazotroph for the first two sampling occasions (Stns 3 and 7), with 4.3 × 105 and 1.3 × 106 gene copies l−1. At the third sampling site (Stn 13), nifH gene copies of Group B decreased to 2.9 × 104 gene copies l−1, in contrast to Trichodesmium nifH gene copies which increased to a maximum of 1.6 × 106 gene copies l−1 (Fig. 2). The shift from a Group B-dominated to a Trichodesmium-dominated diazotroph community between Stn 3 and Stn 13 respectively, could account for the change in the diel pattern of N2 fixation. The unicellular Crocosphaera fixes N2 during the night, and rates of N2 fixation were highest during the night-time in waters where Crocosphaera gene copies were most abundant. Two other groups of diazotrophs were present at lower abundances throughout the cruise; UCYN-A nifH abundance ranged from 1.6 × 103 to 1.9 × 105 gene copies l−1, and the total heterocystous cyanobacterial gene copies were the lowest of all nifH gene groups measured with a maximum abundance of 6.2 × 103 gene copies l−1 at Stn 13.
Microbial consumption of H2
Biological 3H2 oxidation was measured during the day-time and night-time, alongside N2 fixation rate measurements at Stns 3 and 13. Overall, the rates of biological 3H2 oxidation ranged from 15 to 42 pmol H2 l−1 h−1 (Table 1). At Stn 3, night-time rates of biological 3H2 oxidation (25 pmol H2 l−1 h−1) exceeded daytime rates (15 pmol H2 l−1 h−1) by 66%. In contrast, at Stn 13 the daytime rates of biological 3H2 oxidation (42 pmol H2 l−1 h−1) were 68% higher than night-time (25 pmol H2 l−1 h−1) (Table 1). In this respect, the diel variability in biological 3H2 oxidation rates reflect the diel patterns observed in the rate of 15N2 assimilation and the AR assay. The measured rates of 3H2 oxidation were equivalent to 11–63% of 15N2 assimilation and 1–7% of C2H4 production as measured by the AR assay.
Table 1.
Rates of biological 3H2 oxidation conducted on whole seawater samples collected at 25 m (the error bars represent standard deviation of replicate samples, n = 3). The rate measurements are compared with the 15N2 assimilation and C2H4 production values in whole seawater (Fig. 1) to calculate the percentage of N2 fixation accounted for by biological oxidation
| Station sampled | Water-column 3H2 oxidation (pmol H2 L−1 h−1) | % of AR assay accounted for by 3H2 oxidation | % of 15N2 assimilation accounted for by 3H2 oxidation | Turnover time of dissolved H2 pool (h) |
|---|---|---|---|---|
| Stn 3 (Day) | 15 ± 1 | 0.8 | 18.8 | 40 |
| Stn 3 (Night) | 25 ± 4 | 0.9 | 11.4 | 23 |
| Stn 13 (Day) | 42 ± 6 | 1.3 | 16.2 | 22 |
| Stn 13 (Night) | 25 ± 2 | 6.6 | 62.5 | 36 |
Previous measurements of biological H2 consumption have been reported from other aquatic habitats including coastal seawater (Punshon et al., 2007), shallow lakes (Conrad et al., 1983) and river systems (Paerl, 1982). These previous studies have revealed H2 turnover times ranging from < 1 h in a eutrophic shallow lake (Conrad et al., 1983) to 2–3 days in high-latitude coastal seawater (Punshon et al., 2007). In comparison, the H2 turnover times measured in this study at two sampling stations ranged from 22–40 h (Table 1).
Estimation of the production and consumption of H2 associated with N2 fixation
The measured rates of N2 fixation using the AR assay at Stns 3 and 13 were used to estimate the production of H2 derived from nitrogenase (Table 2). We use laboratory-derived measurements of net H2 production by Trichodesmium and Crocosphaera cultures described in the Introduction to provide upper and lower boundaries for net H2 production. Therefore in contrast to Price and colleagues (2007) who estimated net H2 production at 55% of N2 fixation in the marine environment, we set maximum and minimum net H2 production rates at 25% and 1% of C2H4 production respectively. The resulting estimates of net H2 production range from 0.004 to 0.84 nmol H2 l−1 h−1 in the upper water column. Furthermore, the calculations indicate that N2 fixation can replenish the dissolved H2 pool in as little as 1 h and extending up to 34 h, with the exception of 19 September during the night-time which has an excessively long upper estimate of 245 h (Table 2).
Table 2.
Estimation of H2 production in the open ocean water column at a depth of 25 m. The minimum and maximum values are based on 1% and 25% of C2H4 production
| Date sampled | Water-column H2 concentration (nmol H2 L−1) | AR assay (nmol C2H4 L−1 h−1) | Estimated H2 prod. (nmol H2 L−1 h−1) | Estimated time to replenish H2 stock (h) | |
|---|---|---|---|---|---|
| Min. | Max. | ||||
| Stn 3 (day) | 0.6 | 1.77 | 0.018 | 0.44 | 1–34 |
| Stn 3 (night) | 0.6 | 2.87 | 0.029 | 0.72 | 1–21 |
| Stn 13 (day) | 0.93 | 3.34 | 0.033 | 0.84 | 1–28 |
| Stn 13 (night) | 0.93 | 0.38 | 0.004 | 0.10 | 10–245 |
The estimates of net H2 production in surface seawater as listed in Table 2 can be compared with the biological 3H2 oxidation measurements which were conducted on the same seawater samples (Table 1). The rates of 3H2 oxidation were equivalent to 0.8–6.6% of the AR assay (Table 1) indicating biological consumption was equivalent to the lower end of estimated rates of net H2 production (i.e. comparable to rates of net H2 production by Crocosphaera). This suggests that concentrations of dissolved H2 may increase in the presence of Trichodesmium and stimulate the diel cycle of H2 in surface seawater as observed by Herr and colleagues (1984) in the South Atlantic. However in this study, the increase in Trichodesmium abundance was not matched by an increase in net H2 concentrations (Fig. 1) suggesting that field populations of Trichodesmium may re-assimilate more of the H2 produced via nitrogenase compared to their cultured counterparts and are therefore more energetically efficient. Alternatively, other sinks of H2 in the upper ocean may contribute to the loss of dissolved H2, and these are considered in the next section.
H2 cycling in the open ocean
The oceanic H2 cycle depends not only on biological production and consumption as discussed with reference to diazotrophs, but also physical forcing mechanisms. The physical processes can be considered with respect to the sink terms for H2, comparing estimates of air–sea gas exchange and downwards diffusion with biological oxidation. The downward diffusion of H2 can be estimated from the concentration gradient between depths of 45 m and 75 m, using the vertical eddy diffusion coefficient reported by Ledwell et al. (1993) (Table 3). The flux of H2 to the atmosphere can be estimated according to Eq. 2, where S is the Bunsen solubility coefficient (Wiesenburg and Guinasso, 1979), Δp is the difference in partial pressure (p) between the atmosphere and ocean, and k is the transfer velocity. An atmospheric H2 concentration of 0.53 parts per million by volume (ppmv) was used in the flux calculations (Novelli et al., 1999). The transfer velocity (k) was calculated according to Wanninkhof (1992) (Eq. 3) where U is the wind speed (m sec−1) normalized to 10 m above the sea surface and Sc represents the Schmidt number for H2 at in situ seawater temperature and salinity (Jähne et al., 1987).
| 2 |
| 3 |
Table 3.
Depth integrated (0–45 m) inventories of dissolved H2 concentrations in comparison with sea-air gas flux, downward diffusion, and estimated biological consumption.
| Date | Depth-integrated (0–45 m) H2 inventories (μmol m−2) | Water column sea–air H2 flux (μmol H2 m−2 h−1) | Downward diffusion (μmol H2 m−2 h−1) | Biological consumption (μmol H2 m−2 h−1) |
|---|---|---|---|---|
| Stn 3 (day) | 30.6 | 0.03–0.06 | 0.42 | 0.03–5.17 |
| Stn 3 (night) | 30.6 | 0.04–0.08 | 0.42 | 0.31–3.87 |
| Stn 13 (day) | 41.0 | 0.11–0.37 | 0.68 | 0.47–5.80 |
| Stn 13 (night) | 41.0 | 0.08–0.33 | 0.68 | 1.69–16.05 |
To obtain depth-integrated estimates of H2 consumption, we used recent measurements of N2 fixation profiles at Stn ALOHA (HOT cruises #202–213, corresponding to June 2008–July 2009) to calculate the relationship between N2 fixation measurements at 25 m and 0–45 m depth integrated values (y = 46.12x + 23.8, r2 = 0.82). The conversion factor was applied to the rates of N2 fixation (Fig. 1) using the percentage of AR assay and 15N2 assimilation (Table 1) to provide a lower and upper estimate of biological H2 consumption respectively, integrated across the 0–45 m depth horizon. While there is approximately an order of magnitude difference between the upper and lower estimates of biological consumption (Table 3), the median values for turnover times compare favourably with the rates of H2 consumption calculated from the 3H2 oxidation measurements for discrete seawater samples collected from 25 m (Table 1). It is evident that for this time period, biological consumption and downward diffusion represented the main loss pathways for dissolved H2 in the upper ocean. The estimated flux of H2 to the atmosphere ranged from 0.03–0.33 μmol m−2 h−1 (Table 3) and should be considered a low estimate of H2 loss to the overlying atmosphere due to the predominantly low wind speeds (< 5 m sec−1) during the cruise.
Conclusion
During a 10-day sampling period in the NPSG, dissolved H2 concentrations were 147–560% supersaturated with respect to atmospheric equilibrium. Measured rates of 15N2 assimilation and AR revealed a change in the prevalence of N2 fixation from night-time to day-time, which was accompanied by a decrease in the abundance in Group B nifH gene copies, and an increase in the abundance of Trichodesmium nifH gene copies. Prior to this study, it was hypothesized that varying abundance of larger, daytime N2 fixing microorganisms (e.g. Trichodesmium) might influence the dissolved pool of H2 in surface seawater due to their relatively high rates of net H2 production (Wilson et al., 2010). However, the absence of varying dissolved H2 concentrations indicate that field populations of Trichodesmium may be more efficient at recycling H2 compared to laboratory cultures. Biological H2 oxidation measurements in seawater sampled from 25 m depth indicate that H2 production needed to exceed 1–6% of C2H4 production to cause an increase in the ambient pool of dissolved H2 (Table 1). This is considerably lower than in laboratory-maintained Trichodesmium cultures where the rate of net H2 production was equivalent to 25% of C2H4 production (Wilson et al., 2012b). Using either the AR assay or the 15N2 assimilation technique caused approximately one order of magnitude variability when calculating the efficiency of H2 cycling. We consider the AR assay to be more representative of nitrogenase activity but recognize that it is an indirect measurement and not widely used in oceanographic studies on non-concentrated seawater samples. Comparison of the loss mechanisms for dissolved H2 in the upper ocean indicated that biological oxidation represented the most prevalent sink compared to downward diffusion and flux to the atmosphere (Table 3).
It should be noted that oceanic H2 cycling is not limited to diazotrophs, and opportunistic H2-oxidizing microorganisms (e.g. aerobic anoxygenic photosynthetic bacteria and heterotrophic bacteria) will also metabolize H2. Furthermore, other sources of H2 such as photochemical degradation of dissolved organic matter (Punshon and Moore, 2008) and fermentation (Schropp et al., 1987) should be considered when studying H2 cycling in the upper water column. Nonetheless, this study provides an important contribution to our understanding on the role of diazotrophs in dissolved H2 cycling and reveals it to be more restrained than measurements conducted using laboratory cultures of diazotrophs.
Experimental procedures
Dissolved H2 concentrations were measured with a reduced gas analyzer (Peak Laboratories, Mountain View) adapting the method of Moore and colleagues (2009). The rate of H2 consumption was quantified by measuring the production of 3H2O from tracer additions of 3H2 as previously used in laboratory cultures of diazotrophs (Chan et al., 1980) and environmental microbial assemblages (Paerl, 1983). To determine the rate of N2 fixation, measurements of 15N2 assimilation and AR were carried out as described in Wilson and colleagues (2012a). The nifH gene abundance was quantified using the methodological protocols previously published by Moisander and colleagues (2010). Full descriptions of all the analytical methods for measuring H2 and N2 fixation and also the accompanying hydrographic datasets are in the Supporting Information (see Appendix S1).
Acknowledgments
We are grateful to the numerous scientists who contributed to the success of the C-MORE BioLINCS cruise, and in particular to Blake Watkins, Tara Clemente, Ben Rubin, Ariel Rabines, Daniela Böttjer and Susan Curless who assisted with sample collection and analysis. We also thank the R/V Kilo Moana captain and crew for their support. This research was supported by the National Science Foundation supported Center for Microbial Oceanography: Research and Education (C-MORE) (EF0424599 to D.M.K., P.I.), NSF Grant OCE-1153656 (D.M.K., P.I.) and the Gordon and Betty Moore Foundation Marine Microbiology Investigator awards to J.P.Z and D.M.K., including the MEGAMER facility grant by the Gordon and Betty Moore Foundation.
Supporting Information
Additional Supporting Information may be found in the online version of this article at the publisher's website
Appendix S1. The relevant hydrographic and biogeochemical datasets together with full descriptions of the analytical methods for measuring dissolved H2 and N2 fixation are in the Supporting Information.
Figure S1. 14-day composite of satellite derived SSHA 100 km north of the Hawaiian Islands in the Pacific Ocean between 7 and 21 September 2011 (data from Moderate Resolution Imaging Spectroradiometer). A summary of the cruise transect is indicated by the solid black line and the labeled white circles represent the sampling stations discussed in the text. Station ALOHA, the long-term sampling station for the Hawaii Ocean Time-series (HOT) programme, located at 22°45′N, 158°W is also highlighted.
Figure S2. Representative water column profiles for the two sections of the cruise track, (A-B) Stn 3 and (C-D) Stn 13.
References
- Berman-Frank I, Quigg A, Finkel ZV, Irwin AJ. Haramaty L. Nitrogen-fixation strategies and Fe requirements in cyanobacteria. Limnol Oceanogr. 2007;52:2260–2269. [Google Scholar]
- Burns RC. Hardy RWF. Nitrogen Fixation in Bacteria and Higher Plants. Molecular Biology, Biochemistry, and Biophysics. Vol. 21. Heidelberg, Berlin, Germany: Springer Verlag; 1975. pp. 1–189. [DOI] [PubMed] [Google Scholar]
- Burris RH. The acetylene-reduction technique. In: Stewart WDP, editor. Nitrogen Fixation by Free-Living Microorganisms. New York, USA: Cambridge University Press; 1975. pp. 249–257. [Google Scholar]
- Capone DG. Determination of nitrogenase activity in aquatic samples using the acetylene reduction procedure. In: Cole JJ, editor; Kemp PF, Sherr BF, Sherr EB, editors. Handbook of Microbial Methods in Aquatic Microbial Ecology. Boca Raton, FL, USA: Lewis Publishers; 1993. pp. 621–631. [Google Scholar]
- Carpenter EJ. Romans K. Major role of the cyanobacterium Trichodesmium in nutrient cycling in the North Atlantic Ocean. Science. 1991;254:1356–1358. doi: 10.1126/science.254.5036.1356. [DOI] [PubMed] [Google Scholar]
- Chan YK, Nelson LM. Knowles R. Hydrogen metabolism of Axospirillum brasilense in nitrogen-free medium. Can J Microbiol. 1980;26:1126–1131. doi: 10.1139/m80-186. [DOI] [PubMed] [Google Scholar]
- Church MJ, Mahaffey C, Letelier RM, Lukas R, Zehr JP. Karl DM. Physical forcing of nitrogen fixation and diazotroph community structure in the North Pacific subtropical gyre. Global Biogeochem Cycles. 2009;23:GB2020. doi: 10.1029/2008GB003418. [Google Scholar]
- Conrad R. Seiler W. Methane and hydrogen in seawater (Atlantic Ocean) Deep Sea Res. 1988;35:1903–1917. [Google Scholar]
- Conrad R, Aragno M. Seiler W. Production and consumption of hydrogen in a eutrophic lake. Appl Environ Microbiol. 1983;45:502–510. doi: 10.1128/aem.45.2.502-510.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabowski MNW, Church MJ. Karl DM. Nitrogen fixation rates and controls at Stn ALOHA. Aquat Microb Ecol. 2008;52:175–183. [Google Scholar]
- Graham BM, Hamilton RD. Campbell NER. Comparison of the nitrogen-15 uptake and acetylene reduction methods for estimating the rates of nitrogen fixation by freshwater blue-green algae. Can J Microbiol. 1980;37:488–493. [Google Scholar]
- Herr FL, Frank EC, Leones GM. Kennicutt MC. Diurnal variability of dissolved molecular hydrogen in the tropical South Atlantic Ocean. Deep Sea Res. 1984;31:13–20. [Google Scholar]
- Jähne B, Heinz G. Dietrich W. Measurement of the diffusion coefficients of sparingly soluble gases in water. J Geophys Res. 1987;92:10767–10776. [Google Scholar]
- Ledwell JR, Watson AJ. Law CS. Evidence for slow mixing across the pycnocline from an open-ocean tracer-release experiment. Nature. 1993;364:701–703. [Google Scholar]
- Mague TH, Mague FC. Holm-Hansen O. Physiology and chemical composition of nitrogen-fixing phytoplankton in the central North Pacific Ocean. Mar Biol. 1977;41:213–227. [Google Scholar]
- Moisander PH, Beinart RA, Hewson I, White AE, Johnson KS, Carlson CA, et al. Unicellular cyanobacterial distributions broaden the oceanic N2 fixation domain. Science. 2010;327:1512–1514. doi: 10.1126/science.1185468. [DOI] [PubMed] [Google Scholar]
- Montoya JP, Voss M, Kähler P. Capone DG. A simple, high-precision, high-sensitivity tracer assay for N2 fixation. Appl Environ Microbiol. 1996;62:986–993. doi: 10.1128/aem.62.3.986-993.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore RM, Punshon S, Mahaffey C. Karl DM. The relationship between dissolved hydrogen and nitrogen fixation in ocean waters. Deep Sea Res. 2009;56:1449–1458. [Google Scholar]
- Mulholland MR, Bronk DA. Capone DG. Dinitrogen fixation and release of ammonium and dissolved organic nitrogen by Trichodesmium IMS101. Aquat Microb Ecol. 2004;37:85–94. [Google Scholar]
- Novelli PC, Lang PM, Masarie KA, Hurst DF, Myers R. Elkins JW. Molecular hydrogen in the troposphere: Global distribution and budget. J Geophys Res. 1999;104:427–430. [Google Scholar]
- Paerl HW. In situ H2 production and utilization by natural populations of N2-fixing blue-green algae. Can J Bot. 1982;60:2542–2546. [Google Scholar]
- Paerl HW. Environmental regulation of H2 utilization (3H2 exchange) among natural and laboratory populations of N2 and non-N2 fixing phytoplankton. Microb Ecol. 1983;9:79–97. doi: 10.1007/BF02015124. [DOI] [PubMed] [Google Scholar]
- Price H, Jaeglé L, Rice A, Quay P, Novelli PC. Gammon R. Global budget of molecular hydrogen and its deuterium content: Constraints from ground station, cruise, and aircraft observations. J Geophys Res. 2007;112:D22108. doi: 10.1029/2006JD008152. [Google Scholar]
- Punshon S. Moore RM. Photochemical production of molecular hydrogen in lake water and coastal seawater. Mar Chem. 2008;108:215–220. [Google Scholar]
- Punshon S, Moore RM. Xie H. Net loss rates and distribution of molecular hydrogen (H2) in mid-latitude coastal waters. Mar Chem. 2007;105:129–139. [Google Scholar]
- Schropp SJ, Scranton MI. Schwarz JR. Dissolved hydrogen, facultatively anaerobic, hydrogen-producing bacteria, and potential hydrogen production rates in the western North Atlantic Ocean and Gulf of Mexico. Limnol Oceanogr. 1987;32:396–402. [Google Scholar]
- Schubert KR. Evans HJ. Hydrogen evolution: A major factor affecting the efficiency of nitrogen fixation in nodulated symbionts. Proc Natl Acad Sci U S A. 1976;73:1207–1211. doi: 10.1073/pnas.73.4.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scranton MI, Jones MM. Herr FL. Distribution and variability of dissolved hydrogen in the Mediterranean Sea. J Mar Res. 1982;40:873–891. [Google Scholar]
- Scranton MI, Novelli PC, Michaels A, Horrrigan SG. Carpenter EJ. Hydrogen production and nitrogen fixation by Oscillatoria thiebautii during in situ incubations. Limnol Oceanogr. 1987;32:998–1006. [Google Scholar]
- Simpson FB. Burris RH. A nitrogen pressure of 50 atmospheres does not prevent evolution of hydrogen by nitrogenase. Science. 1984;224:1095–1097. doi: 10.1126/science.6585956. [DOI] [PubMed] [Google Scholar]
- Tamagnini P, Leitão E, Oliveira P, Ferreira D, Pinto F, Harris DJ, et al. Cyanobacterial hydrogenases: Diversity, regulation and applications. FEMS Microbiol Rev. 2007;31:692–720. doi: 10.1111/j.1574-6976.2007.00085.x. [DOI] [PubMed] [Google Scholar]
- Wanninkhof R. Relationship between gas exchange and wind speed over the ocean. J Geophys Res. 1992;97:7373–7381. [Google Scholar]
- Waterbury JB, Watson SW. Valois FW. Temporal separation of photosynthesis and dinitrogen fixation in the marine unicellular cyanobacterium: Erythrosphaera marina. EOS Trans Am Geophys Union. 1988;69:1089. [Google Scholar]
- Wiesenburg DA. Guinasso NL. Equilibrium solubilities of methane, carbon monoxide and hydrogen in water and seawater. J Chem Eng Data. 1979;24:356–360. [Google Scholar]
- Wilson ST, Foster RA, Zehr JP. Karl DM. Hydrogen production by Trichodesmium erythraeum, Cyanothece sp. and Crocosphaera watsonii. Aquat Microb Ecol. 2010;59:197–206. [Google Scholar]
- Wilson ST, Böttjer D, Church MJ. Karl DM. Comparative assessment of nitrogen fixation methodologies conducted in the oligotrophic North Pacific Ocean. Appl Environ Microbiol. 2012a;78:6516–6523. doi: 10.1128/AEM.01146-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson ST, Kolber ZS, Tozzi S, Zehr JP. Karl DM. Nitrogen fixation, hydrogen production and electron transport kinetics in Trichodesmium erythraeum strain IMS101. J Phycol. 2012b;48:595–506. doi: 10.1111/j.1529-8817.2012.01166.x. [DOI] [PubMed] [Google Scholar]
- Zehr JP, Waterbury JB, Turner PJ, Montoya JP, Omoregie E, Steward GF, et al. Unicellular cyanobacteria fix N2 in the subtropical North Pacific Ocean. Nature. 2001;412:635–638. doi: 10.1038/35088063. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. The relevant hydrographic and biogeochemical datasets together with full descriptions of the analytical methods for measuring dissolved H2 and N2 fixation are in the Supporting Information.
Figure S1. 14-day composite of satellite derived SSHA 100 km north of the Hawaiian Islands in the Pacific Ocean between 7 and 21 September 2011 (data from Moderate Resolution Imaging Spectroradiometer). A summary of the cruise transect is indicated by the solid black line and the labeled white circles represent the sampling stations discussed in the text. Station ALOHA, the long-term sampling station for the Hawaii Ocean Time-series (HOT) programme, located at 22°45′N, 158°W is also highlighted.
Figure S2. Representative water column profiles for the two sections of the cruise track, (A-B) Stn 3 and (C-D) Stn 13.