Abstract
Cyclooxygenase-2 (COX-2) overexpression is associated with a poor prognosis in non–small-cell lung cancer (NSCLC) and may promote resistance to epidermal growth factor receptor inhibitors. This randomized phase 2 trial evaluated apricoxib, a novel COX-2 inhibitor, in combination with erlotinib in biomarker-selected patients. Patients with stage IIIB/IV NSCLC previously treated with platinum-based chemotherapy were randomized (2:1) to 400 mg/day apricoxib plus 150 mg/day erlotinib (AP/E) or placebo plus erlotinib (P/E) in 21-day cycles until disease progression or unacceptable toxicity. The primary endpoint was time to progression (TTP). A decrease of 50% or more from baseline urinary prostaglandin E2 metabolite after a 5-day, open-label, run-in period was used to select eligible patients. One hundred twenty patients (median age 64 years) were randomized (78 to AP/E and 42 to P/E). Overall median TTP was 1.8 months in the AP/E group and 2.1 months in the P/E group, with a 12% objective response rate in both groups (intent-to-treat analysis). A subgroup analysis in patients aged 65 years or younger demonstrated a statistically significant TTP benefit for AP/E (hazard ratio 0.5 [95% confidence interval: not applicable–0.9]; p=0.018) and overall survival advantage at minimum 1-year follow-up (median 12.2 versus 4.0 months; hazard ratio=0.5; p=0.021). The most common adverse events were rash, diarrhea, fatigue, and nausea. Toxicity contributed to early discontinuations in patients aged more than 65 years treated with AP/E. This is the first randomized placebo-controlled study of a COX-2 inhibitor in NSCLC to use a prospective patient-selection strategy. Although AP/E seemed to improve TTP and overall survival in a subset of patients aged 65 years or younger, the primary endpoint of the trial was not met.
Keywords: Non–small-cell lung cancer, Apricoxib, Erlotinib, Cyclooxygenase-2 inhibitor, Prostaglandin E2 metabolite
Epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors such as erlotinib and gefitinib have demonstrated clinical activity in NSCLC patients with activating EGFR mutations,1 and after platinum-based chemotherapy in unselected patients.2
Preclinical and clinical evidence suggest that hyperactivity of cyclooxygenase-2 (COX-2) may confer resistance to EGFR inhibitors.3,4 COX-2 is overexpressed in 70% to 80% of patients with NSCLC and is associated with a poor prognosis.3 To date, however, phase 2 studies combining celecoxib with either erlotinib or gefitinib in unselected patients with previously treated NSCLC have not demonstrated improvements in efficacy over an EGFR inhibitor alone.5
Apricoxib is a novel, selective COX-2 inhibitor that has demonstrated potent antitumor effects in animal models. Only those tumors with elevated COX-2 activity, which produced high levels of prostaglandin E2 (PGE2), were responsive to the antitumor effects of apricoxib6,7; suggesting that a biomarker-driven patient-selection strategy might improve efficacy in the clinic. Intratumoral PGE2 levels have been shown to correlate with the stable urinary metabolite of PGE2 (PGE-M).8 Moreover, an association has been observed between a decrease from baseline in urinary PGE-M and response to celecoxib plus chemotherapy.8,9 A phase I trial demonstrated that apricoxib at daily doses up to 400 mg was well tolerated in combination with erlotinib (150 mg/day) in patients with advanced NSCLC.10
The current, prospective, randomized, double-blind, phase II study was designed to test whether the addition of apricoxib (400 mg/day) to erlotinib would improve time to disease progression (TTP) in biomarker-selected patients with recurrent stage IIIB/IV NSCLC. Selection of patients for this study was based on a 50% decrease from baseline urinary PGE-M in response to apricoxib.
PATIENTS AND METHODS
Patient Selection
Adult patients (≥18 years of age) with stage IIIB (pleural effusion; 6th edition of the American Joint Committee on Cancer) or IV NSCLC and measurable disease by Response Evaluation Criteria in Solid Tumors who had failed at least 1 prior platinum-based chemotherapy regimen were enrolled in this study. Eligible patients also had an Eastern Cooperative Oncology Group (ECOG) performance status (PS) of 0 to 2 and adequate renal, hepatic, and bone marrow function. By protocol amendment, patients with ECOG PS 2 were subsequently excluded after the Data Safety Monitoring Committee detected increased toxicity in these patients (n=6). Patients with central nervous system metastases were eligible if asymptomatic, and off steroids after radiotherapy for 2 weeks or more. Patients were ineligible if they had received prior treatment with an EGFR tyrosine kinase inhibitor or had a history of significant cardiovascular disease or upper gastrointestinal bleeding.
Study Design and Treatment
This was a multi-institutional phase II trial. Patients entered an open-label run-in period where they received single-agent apricoxib (400 mg/day) for 5 consecutive days. Urinary PGE-M was measured on the first and last day of the run-in period. Patients with at least a 50% decrease from baseline were randomized 2:1 to apricoxib (400 mg/day) plus erlotinib (150 mg/day) or placebo plus erlotinib on 21-day cycles. The primary efficacy endpoint was TTP. Secondary endpoints included overall response, progression-free survival (PFS), overall survival (OS), safety, and biomarker analysis (COX-2 expression and urinary PGE-M).
Patients were evaluated at baseline, on day 1 of every even-numbered cycle, for tumor response according to Response Evaluation Criteria in Solid Tumors version 1.0.11 Safety was assessed using National Cancer Institute Common Toxicity Criteria version 3.0. Urinary PGE-M was assessed at baseline and on day 1 of cycles 2 and 3.
The sample size was determined to achieve 80% power to detect a 40% improvement in TTP corresponding to a Cox proportional hazard ratio (HR) of 1.4 by one-sided log-rank test with an α error of 0.20. The original sample size was 115, and this was increased to 122 by amendment excluding enrollment of patients with an ECOG PS of 2.
RESULTS
Patients
A total of 176 patients were enrolled into the 5-day open-label run-in period (Fig. 1). Of these, 120 patients (68%) who exhibited a decrease of 50% or more from baseline urinary PGE-M on day 5 were randomized to treatment with apricoxib plus erlotinib (AP/E group; n = 78) or placebo plus erlotinib (P/E group; n = 42). Baseline patient and disease characteristics for all randomized patients are shown in Table 1. Median age was 64 years. The primary reason for study discontinuation was disease progression (59% of patients in the AP/E group and 74% of patients in the P/E group). During the double-blind period, 16 patients (20%) in the AP/E group discontinued because of adverse events compared with three (7%) in the P/E group. Overall, 41% of AP/E-treated patients and 33% of P/E-treated patients had a delay or dose modification.
FIGURE 1.

Consolidated Standards of Reporting Trials (CONSORT) diagram. P/E, placebo plus erlotinib; AP/E, apricoxib plus 150 mg/day erlotinib; ITT, intent-to-treat; PS, performance status.
TABLE 1.
Baseline Patient and Disease Characteristics
| P/E (n = 42) | AP/E (n = 78) | All (N = 120) | |
|---|---|---|---|
| Median age, years (range) | 65 (36–84) | 63 (35–81) | 64 (35–84) |
| Age category, n (%) | |||
| ≤ 65 years | 23 (55) | 46 (59) | 69 (58) |
| > 65 years | 19 (45) | 32 (41) | 51 (42) |
| Sex, n (%) | |||
| Male | 25 (60) | 42 (56) | 67 (56) |
| Female | 17 (40) | 36 (44) | 53 (44) |
| ECOG performance status, n (%) | |||
| 0 or 1 | 39 (93) | 75 (96) | 114 (95) |
| 2 | 3 (7) | 3 (4) | 6 (5) |
| Never smoker, n (%) | 6 (14) | 9 (12) | 15 (13) |
| Histology, n (%) | |||
| Adenocarcinoma | 24 (57) | 45 (58) | 69 (57) |
| Squamous cell carcinoma | 11 (26) | 21 (27) | 32 (27) |
| Bronchioalveolar carcinoma | 0 | 3 (4) | 3 (3) |
| Other or unknown | 7 (17) | 9 (11) | 16 (13) |
| Disease stage, n (%) | |||
| IIIB (pleural effusion) | 2 (5) | 13 (17) | 15 (13) |
| IV | 40 (95) | 65 (83) | 105 (88) |
| Baseline urinary | |||
| PGE-M, n (%) | |||
| Normal | 6 (14) | 12 (15) | 18 (15) |
| Elevated | 36 (86) | 66 (85) | 102 (85) |
| EGFR mutation, n (%) | |||
| Yes | 0 | 2 (3) | 2 (2) |
| No | 13 (31) | 20 (26) | 33 (27) |
| Unknown | 29 (69) | 56 (72) | 85 (71) |
| KRAS mutation, n (%) | |||
| Yes | 1 (2) | 8 (10) | 9 (7) |
| No | 14 (33) | 18 (23) | 32 (27) |
| Unknown | 27 (64) | 52 (67) | 79 (66) |
| COX-2 IHC index | |||
| N | 9 | 14 | 23 |
| Mean (SD) | 7.0 (3.1) | 5.8 (3.2) | 6.3 (3.2) |
ECOG, Eastern Cooperative Oncology Group; PGE-M, prostaglandin E2 metabolite; EGFR, epidermal growth factor receptor; KRAS, Kirsten rat sarcoma viral oncogene homolog; COX-2, cyclooxygenase-2; IHC, immunohistochemistry.
Efficacy Analysis
The intent-to-treat (ITT) analysis included 75 patients in the AP/E group and 39 patients in the P/E group with an ECOG PS of 0 or 1. Prespecified covariate analyses of TTP, PFS, OS, best overall response, and disease control rate (DCR) were conducted using factors such as sex, age, ECOG PS, smoking status, disease stage, and biomarkers. Among these covariates, younger age (≤60 versus >60 years; protocol specified) emerged as a significant factor (interaction p = 0.012) associated with longer TTP in the AP/E group, and this was also consistently observed for secondary endpoints. However, on extending the analysis it was observed that patients up to 65 years of age seemed to benefit from treatment with AP/E; therefore, efficacy analyses are also reported for the subgroups (age ≤65 years and >65 years; interaction p = 0.009). Baseline characteristics in these subgroups were balanced between treatment groups and similar to those of the ITT population.
At the time of the primary analysis of TTP, all randomized patients had been followed up for at least 5 months; median TTP was 1.8 months in the AP/E group and 2.1 months in the P/E group (Table 2 and Fig. 2). In contrast, among patients aged 65 years or younger, median TTP was 2.7 months in the AP/E group compared with 1.4 months in the P/E group (HR = 0.5 [95% confidence interval: not applicable (NA)–0.9]; p = 0.018). Results of the PFS analysis were nearly identical and are not reported. At the time of the OS analysis, all patients had at least 1 year of follow-up, and median OS in the subset of patients aged 65 years or younger was 12.2 months in the AP/E group compared with 4.0 months in the P/E group (HR = 0.5 [95% confidence interval: NA–0.9]; p = 0.021). In contrast, patients more than 65 years of age randomized to AP/E had worse outcomes compared with the P/E group for both TTP and OS (Table 2).
TABLE 2.
Time to Progression and Overall Survival for Intent-to-Treat Population and Prespecified Subgroups by Age
| Median
|
HR (95% CI) | Log-Rank p Value | ||
|---|---|---|---|---|
| P/E | AP/E | |||
| Intent-to-treat populationa | n = 39 | n = 75 | ||
| Median TTP, months | 2.1 | 1.8 | 1.0 (NA, 1.4) | 0.438 |
| Median OS,b months | 6.4 | 7.4 | 0.9 (NA, 1.4) | 0.390 |
| Patients ≤65 years of age | n = 20 | n = 45 | ||
| Median TTP, months | 1.4 | 2.7 | 0.5 (NA, 0.9) | 0.018 |
| Median OS,b months | 4.0 | 12.2 | 0.5 (NA, 0.9) | 0.021 |
| Patients >65 years of age | n = 19 | n = 30 | ||
| Median TTP, months | 4.7 | 1.4 | 2.0 (NA, 3.9) | 0.958 |
| Median OS,b months | 9.1 | 4.3 | 1.7 (NA, 3.0) | 0.949 |
Intent-to-treat population included only patients with Eastern Cooperative Oncology Group performance status of 0 or 1.
OS analysis conducted when all patients had at least 1 year of follow-up.
P/E, placebo plus erlotinib; AP/E, apricoxib plus 150 mg/day erlotinib; HR, hazard ratio; CI, confidence interval; TTP, time to progression; OS, overall survival; NA, not applicable.
FIGURE 2.
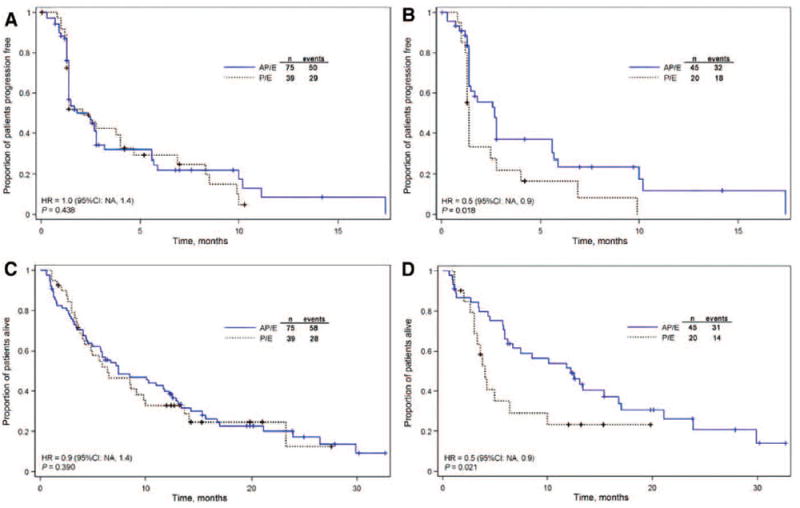
Kaplan–Meier estimate of time to progression by treatment group in the intent-to-treat population (A) and among patients ≤65 years of age (B). Kaplan–Meier estimate of overall survival by treatment group in the intent-to-treat population (C) and among patients ≤65 years of age (D). P/E, placebo plus erlotinib; AP/E, apricoxib plus 150 mg/day erlotinib; HR, hazard ratio; CI, confidence interval, NA, not applicable.
Best overall response and DCR is shown in Table 3. In the ITT population, nine patients (12%) in the AP/E group had a partial response with a median duration of 8.6 months, and five patients (13%) in the P/E group had either a complete or partial response with a median duration of 7.4 months. The DCR was also similar in both treatment groups. In contrast, among patients aged 65 years or younger, best overall response was significantly higher in the AP/E group compared with the P/E group (p = 0.036), and DCR was also significantly higher in the AP/E group (p = 0.018).
TABLE 3.
Best Overall Response and Tumor Control Rate
| Patients, n (%)
|
||
|---|---|---|
| P/E | AP/E | |
| Intent-to-Treat Populationa | n = 39 | n = 75 |
| CR | 1 (3) | 0 |
| PR | 4 (10) | 9 (12) |
| SD ≥6 weeks | 13 (33) | 29 (39) |
| Disease control (CR+PR+SD ≥6 weeks) | 18 (46) | 38 (51) |
| Evaluable patients ≤65 years of age | n = 20 | n = 45 |
| CR | 0 | 0 |
| PR | 1 (5) | 8 (18)b |
| SD | 6 (30) | 19 (42) |
| Disease control | 7 (35) | 27 (60)c |
Intent-to-treat population included only patients with Eastern Cooperative Oncology Group performance status of 0 or 1.
p = 0.036 based on a proportional odds model (adjusted for smoking status) comparing tumor responses between P/E and AP/E.
p = 0.018 based on a logistic regression model (adjusted for smoking status) comparing the disease control rate between P/E and AP/E.
P/E, placebo plus erlotinib; AP/E, apricoxib plus 150 mg/day erlotinib; CR, complete response; PR, partial response; SD, stable disease.
Safety
The incidence of adverse events (AEs) was similar in both treatment groups (Table 4), but those events of grade 3 or higher were more frequent in the AP/E arm. Patients in the AP/E group had a higher incidence of fatigue, cough, and increased serum creatinine (observed in the AP/E group only), whereas patients in the P/E group had a higher incidence of anorexia, acneiform rash, and pruritus.
TABLE 4.
Treatment Emergent Adverse Events Occurring in >10% of Patients During Double-Blind Period
| Preferred Term | Patients, n (%)
|
|||
|---|---|---|---|---|
| P/E (n = 42)
|
AP/E (n = 78)
|
|||
| All Grades | Grade ≥3 | All Grades | Grade ≥3 | |
| Rash | 23 (55) | 2 (5) | 42 (54) | 3 (4) |
| Diarrhea | 25 (60) | 1 (2) | 41 (53) | 5 (6) |
| Fatigue | 12 (29) | 1 (2) | 33 (42) | 3 (4) |
| Nausea | 12 (29) | 0 | 27 (35) | 0 |
| Dry skin | 11 (26) | 0 | 20 (26) | 0 |
| Cough | 5 (12) | 0 | 18 (23) | 0 |
| Anorexia | 15 (36) | 1 (2) | 17 (22) | 2 (3) |
| Dyspnea | 11 (26) | 3 (7) | 16 (21) | 4 (5) |
| Vomiting | 4 (10) | 0 | 16 (21) | 1 (1) |
| Mucositis | 4 (10) | 0 | 15 (19) | 2 (3) |
| Acneform rash | 11 (26) | 1 (2) | 13 (17) | 2 (3) |
| Constipation | 5 (12) | 0 | 12 (15) | 0 |
| Insomnia | 7 (17) | 0 | 11 (14) | 0 |
| Dizziness | 4 (10) | 0 | 10 (13) | 0 |
| Dyspepsia | 6 (14) | 0 | 10 (13) | 1 (1) |
| Pruritis | 9 (21) | 0 | 10 (13) | 2 (3) |
| Anemia | 2 (5) | 0 | 9 (12) | 4 (5) |
| Increased serum creatinine | 0 | 0 | 9 (12) | 1 (1) |
| Weight loss | 6 (14) | 0 | 9 (12) | 0 |
| Peripheral edema | 6 (14) | 0 | 8 (10) | 0 |
| Back pain | 5 (12) | 3 (7) | 7 (9) | 2 (3) |
| Abdominal pain | 5 (12) | 2 (5) | 5 (6) | 2 (3) |
P/E, placebo plus erlotinib; AP/E, apricoxib plus 150 mg/day erlotinib.
Seventeen patients (14%) had a treatment-related serious adverse event (SAE), including 13 patients (17%) in the AP/E group and four patients (10%) in the P/E group. Although there were more gastrointestinal SAEs in the AP/E group (6 versus 1), only one patient in each treatment group had gastrointestinal hemorrhage, and two patients in the AP/E group had a gastric or intestinal ulcer perforation. Seven patients in the AP/E group died while on study treatment compared with one death in the P/E group, and three of those deaths were attributed to SAEs of cerebral infarction, cerebrovascular accident, or pulmonary fibrosis.
Patients more than 65 years of age had more SAEs and more early discontinuations because of AEs than patients aged 65 years or younger. In the AP/E group, 62% of patients aged more than 65 years compared with 35% of patients aged 65 years or younger had an SAE during double-blind treatment. Similarly, in the P/E group 42% and 22% had an SAE, respectively. The majority of patients who discontinued study drug because of AEs were more than 65 years of age (13 in the AP/E group and 3 in the P/E group).
Biomarker Analysis
Among 35 patients tested for EGFR mutations, 33 were EGFR wild type, and two patients in the AP/E group had EGFR mutations. Among 41 patients successfully tested for K-ras mutations, 32 were wild type and nine had a mutation. K-ras mutations were present in eight patients in the AP/E group and one patient in the P/E group. Notably, among patients with K-ras mutations in the AP/E group, two had PR. The majority of tumors tested (n = 23) expressed high levels of COX-2 as indicated by an immunohistochemistry index 4 or more, and 85% of patients screened had elevated baseline urinary PGE-M.
DISCUSSION
Selecting patients for treatment with targeted agents based on tumor biology can enrich the population with patients who are most likely to benefit. This concept was recently validated in NSCLC patients with activating EGFR mutations, where treatment with erlotinib or gefitinib produced higher response rates and improved PFS than first-line chemotherapy.1,12 Selection of patients for the current study based on modulation of urinary PGE-M was implemented based on evidence that patients who exhibited a decrease from baseline PGE-M in response to treatment with celecoxib plus chemotherapy were more likely to receive clinical benefit.8,9,13 In addition, increased intratumoral COX-2 expression was significantly associated with improved PFS in NSCLC patients treated with celecoxib plus erlotinib.14 Taken together, these studies suggest that overexpression of COX-2 and/or a biochemical response to a COX-2 inhibitor may identify NSCLC patients who are more likely to benefit from addition of a COX-2 inhibitor to standard agents. COX-2 activity has also been linked to epithelial mesenchymal transition,12-14 which is associated with increased metastasis and resistance to EGFR TKIs.6,7 Strikingly, addition of apricoxib to an erlotinib regimen abolished all metastatic spread in two COX-2–overexpressing orthotopic pancreatic cancer models but did not improve outcomes in COX-2–independent models.14 Therefore, apricoxib may inhibit tumor progression and overcome resistance to erlotinib in tumors with hyperactive COX-2.
This is the first randomized placebo-controlled study of a COX-2 inhibitor in NSCLC to use a prospective patient-selection strategy. This is a unique patient population, able to modulate urinary PGE-M in response to a COX-2 inhibitor that has never before been studied. The primary endpoint of the study was not met, with no difference between treatment groups in the ITT analysis with respect to TTP or secondary endpoints. In a subset analysis of patients aged 65 years or younger, the combination of AP/E demonstrated statistically significant benefit based on TTP, OS, and DCR compared with P/E. In contrast, patients aged more than 65 years randomized to AP/E had worse toxicity, TTP, and OS compared with the P/E group.
In general, the combination of apricoxib plus erlotinib was tolerated in the majority of patients; however, there were more discontinuations because of AEs and three deaths because of SAEs in the AP/E group. The increase in serum creatinine observed in the AP/E group is consistent with the toxicology of COX-2 inhibitors. However, patients aged more than 65 years had a higher incidence of SAEs and accounted for 75% of early discontinuations because of AEs, which may have contributed to the lack of clinical benefit in that subset. Our trial suggests that apricoxib may add to the toxicity of erlotinib, particularly in older patients and in those who had poor PS. Similar findings were reported in the BR.21 study of erlotinib in advanced NSCLC. In that study, a retrospective analysis by age (≥70 years and <70 years) showed that older patients were more likely to discontinue because of treatment-related toxicity and received a lower dose intensity compared with younger patients.15
Even though this trial does not allow us to draw a definitive conclusion about the role of a particular clinical characteristic predictive of benefit, a phase 3 trial is being considered in a biomarker-selected population of NSCLC patients aged 65 years or younger and with a PS lower than 2.
Acknowledgments
The authors thank the patients and their families for participating in this study and the Apricoxib in combination Oncology Treatment - Lung (APRiCOT-L) investigators for their contributions. Editorial support was provided by SyNova Medical Communications, and funding for editorial support was provided by Tragara Pharmaceuticals, Inc.
Footnotes
Conception and design: B. Gitlitz and S. Zaknoen; administrative support: M. Syto and S. Zaknoen; provision of study materials or patients: B. Gitlitz, E. Bernstein, E. Santos, G.A. Otterson, G. Milne, M. Syto, and S. Zaknoen; collection and assembly of data: B. Gitlitz, G. Milne, M. Syto, and S. Zaknoen; data analysis and interpretation: B. Gitlitz, M. Syto, and S. Zaknoen; and article writing: B. Gitlitz, E. Santos, G.A. Otterson, M. Syto, F. Burrows, and S. Zaknoen.
Disclosure: MS and FB are employees of Tragara. SZ was employed at Tragara during this study and is currently an employee of Polynoma, LLC, San Diego, CA. The other authors declare no conflict of interest.
References
- 1.Zhou C, Wu YL, Chen G, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 2011;12:735–742. doi: 10.1016/S1470-2045(11)70184-X. [DOI] [PubMed] [Google Scholar]
- 2.Shepherd FA, Rodrigues Pereira J, Ciuleanu T, et al. National Cancer Institute of Canada Clinical Trials Group. Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med. 2005;353:123–132. doi: 10.1056/NEJMoa050753. [DOI] [PubMed] [Google Scholar]
- 3.Csiki I, Johnson DH. Did targeted therapy fail cyclooxygenase too? J Clin Oncol. 2006;24:4798–4800. doi: 10.1200/JCO.2006.08.0622. [DOI] [PubMed] [Google Scholar]
- 4.Lippman SM, Gibson N, Subbaramaiah K, Dannenberg AJ. Combined targeting of the epidermal growth factor receptor and cyclooxygenase-2 pathways. Clin Cancer Res. 2005;11:6097–6099. doi: 10.1158/1078-0432.CCR-05-1217. [DOI] [PubMed] [Google Scholar]
- 5.Gadgeel SM, Ali S, Philip PA, Ahmed F, Wozniak A, Sarkar FH. Response to dual blockade of epidermal growth factor receptor (EGFR) and cycloxygenase-2 in nonsmall cell lung cancer may be dependent on the EGFR mutational status of the tumor. Cancer. 2007;110:2775–2784. doi: 10.1002/cncr.23100. [DOI] [PubMed] [Google Scholar]
- 6.Kirane A, Toombs JE, Larsen JE, et al. Epithelial-mesenchymal transition increases tumor sensitivity to COX-2 inhibition by apricoxib. Carcinogenesis. 2012;33:1639–1646. doi: 10.1093/carcin/bgs195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kirane A, Toombs JE, Ostapoff K, et al. Apricoxib, a novel inhibitor of COX-2, markedly improves standard therapy response in molecularly defined models of pancreatic cancer. Clin Cancer Res. 2012;18:5031–5042. doi: 10.1158/1078-0432.CCR-12-0453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Csiki I, Morrow JD, Sandler A, et al. Targeting cyclooxygenase-2 in recurrent non-small cell lung cancer: a phase II trial of celecoxib and docetaxel. Clin Cancer Res. 2005;11:6634–6640. doi: 10.1158/1078-0432.CCR-05-0436. [DOI] [PubMed] [Google Scholar]
- 9.Mutter R, Lu B, Carbone DP, et al. A phase II study of celecoxib in combination with paclitaxel, carboplatin, and radiotherapy for patients with inoperable stage IIIA/B non-small cell lung cancer. Clin Cancer Res. 2009;15:2158–2165. doi: 10.1158/1078-0432.CCR-08-0629. [DOI] [PubMed] [Google Scholar]
- 10.Reckamp K, Gitlitz B, Chen LC, et al. Biomarker-based phase I dose-escalation, pharmacokinetic, and pharmacodynamic study of oral apricoxib in combination with erlotinib in advanced nonsmall cell lung cancer. Cancer. 2011;117:809–818. doi: 10.1002/cncr.25473. [DOI] [PubMed] [Google Scholar]
- 11.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 12.Rosell R, Carcereny E, Gervais R, et al. Spanish Lung Cancer Group in collaboration with Groupe Français de Pneumo-Cancérologie and Associazione Italiana Oncologia Toracica. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012;13:239–246. doi: 10.1016/S1470-2045(11)70393-X. [DOI] [PubMed] [Google Scholar]
- 13.Edelman MJ, Watson D, Wang X, et al. Eicosanoid modulation in advanced lung cancer: cyclooxygenase-2 expression is a positive predictive factor for celecoxib + chemotherapy–Cancer and Leukemia Group B Trial 30203. J Clin Oncol. 2008;26:848–855. doi: 10.1200/JCO.2007.13.8081. [DOI] [PubMed] [Google Scholar]
- 14.Csiki I, Johnson DH. Did targeted therapy fail cyclooxygenase too? J Clin Oncol. 2006;24:4798–4800. doi: 10.1200/JCO.2006.08.0622. [DOI] [PubMed] [Google Scholar]
- 15.Wheatley-Price P, Ding K, Seymour L, Clark GM, Shepherd FA. Erlotinib for advanced non-small-cell lung cancer in the elderly: an analysis of the National Cancer Institute of Canada Clinical Trials Group Study BR.21. J Clin Oncol. 2008;26:2350–2357. doi: 10.1200/JCO.2007.15.2280. [DOI] [PubMed] [Google Scholar]


