Abstract
Purpose
To present pilot toxicity and survival outcomes for a prospective trial investigating adaptive radiotherapy (ART) for oropharyngeal squamous cell carcinoma.
Methods
Twenty-four patients enrolled onto an IRB-approved clinical trial. Twenty-two patients were analyzed. Daily CT-guided setup and deformable image registration permitted serial mapping of CTVs and avoidance structures for ART planning. Primary site was base of tongue in 15 patients, tonsil in 6, and glossopharyngeal sulcus in 1. Twenty (91%) patients had AJCC stage IV disease. T stage distribution was 2 T1, 12 T2, 3 T3, 5 T4 and N stage distribution was 1 N0, 2 N1, 5 N2a, 12 N2b, and 2 N2c. Twenty-one (95%) patients received systemic therapy.
Results
With 31 month median follow up (range: 13-45), there has been no primary site failure and 1 nodal relapse, yielding 100% local and 95% regional disease control at 2 years. Baseline tumor size correlated with absolute volumetric treatment response (p = 0.018). Parotid volumetric change correlated with duration of feeding tube placement (p = 0.025). Acute toxicity was comparable to conventional IMRT results. Chronic toxicity and functional outcomes beyond 1 year were tabulated.
Discussion
This is the first prospective evaluation of morbidity and survival outcomes in patients with locally advanced head and neck cancer treated with automated adaptive replanning. ART can provide dosimetric benefit with only 1 or 2 mid-treatment replanning events. Our preliminary clinical outcomes document functional recovery and preservation of disease control at one-year follow-up and beyond.
Keywords: Adaptive Radiotherapy, Head and Neck Cancer, Image-Guided, IMRT, Clinical Trial
INTRODUCTION
Emerging institutional data suggests promising locoregional tumor control (1, 2), as well as potential preservation of salivary function, swallowing, and overall quality-of-life (3-5) with intensity modulated radiotherapy (IMRT). Recent randomized data directly suggest that IMRT improves acute toxicity relative to conventional techniques (6). Nonetheless, IMRT continues to cause severe acute oral and pharyngeal side effects, as well non-traditional toxicities (7). IMRT depends upon imaging acquired several days prior to start of treatment; however, the location, geometry, and size of tumor and normal organs change continuously during a several week course of therapy (8-10).
Adaptive radiotherapy (ART) is an approach to correct for morphological changes in patient’s anatomy, such as tumor and normal tissue variations as a result of treatment. ART takes advantage of computed tomographic imaging, such as megavoltage CT (11), CT-on-rails (8, 12), or cone-beam CTs (13) performed in the treatment room to serially reassess current location and shape of target volumes and normal anatomy (14, 15). The technical and time resource requirements necessary for ART have hampered development. No routine use of a clinical ART procedure for treatment of H&N cancer has been described, and no ideal method to conduct ART has yet been validated.
This report presents initial toxicity and disease control outcomes from a prospective clinical trial investigating an automated ART approach for locally advanced oropharyngeal cancer designed to keep physician, physics, and dosimetry staff time requirements to a minimum.
METHODS
Patient cases
Twenty-four patients with histologically proven squamous cell carcinoma of the oropharynx were enrolled between 8/2007 and 4/2010 onto a prospective, IRB-approved trial. Inclusion criteria included: 1) patients older than 18 years of age with histologically proven squamous cell carcinoma of the oropharynx, 2) stage III, IVa, or IVb disease as defined by American Joint Committee on Cancer (AJCC) cancer staging criteria, and 3) ECOG performance status 0-2. Patients were excluded for any of the following: 1) resection of primary tumor or delivery of induction chemotherapy prior to radiation treatment, 2) prior cancer diagnosis, except appropriately treated localized epithelial skin cancer or cervical cancer, or 3) prior radiation therapy to the head and neck region. All potentially eligible patients were screened for enrollment; subjects who did not enter the trial typically did so at their own discretion or due to management decisions favoring induction chemotherapy. Two enrolled patients removed themselves from protocol; the remaining twenty-two patients were analyzed with at least 12 months follow-up.
Treatment
Baseline H&N IMRT planning was performed on an ADAC Pinnacle3 (Philips Medical Systems, Andover, MA). Patients were immobilized with a custom-fabricated thermoplastic mask. Plans incorporated a clinical target volume 1 (CTV1, gross disease and high risk regions) target treated to 66-70 Gy in 30-33 daily fractions, CTV2 (immediately adjacent lymph node levels and soft tissues) target treated to 60-63 Gy in 30-33 daily fractions, and a CTV3 (prophylactic cervical nodal coverage) target treated to 54-57 Gy in 30-33 daily fractions, as per our standard practice. Accelerated fractionation was used for bulky, high-risk primary disease: 3 patients received 70-72 Gy in 30 treatment days per published experience from DAHANCA (16), while 1 patient received concomitant boost to 71.8 Gy in 40 total fractions via separate primary and boost IMRT plans which were both adapted to anatomic changes. In all cases except one, which was treated with a whole-field IMRT technique, IMRT was matched at the superior aspect of the arytenoid cartilages to a conventional AP bilateral low neck field with a 3 × 3 cm larynx block, treated to 50 Gy in 25 daily fractions. Coned-down midneck AP photon and/or en face electron boost fields were used to boost low cervical neck nodal stations adjacent to or directly involved with nodal disease to 60-66 Gy in 2 Gy daily fractions. A 26 Gy mean parotid dose constraint was used for contralateral and ipsilateral parotid glands separated from target volumes. No constraints were employed for submandibular or sublingual gland sparing. Standard planning margins were used for baseline IMRT plans, with volumetric CTV-to-PTV expansions of 3-4 mm.
Twenty-one (95%) patients received concurrent systemic therapy with their radiation treatment. Sixteen patients received with single agent cisplatin either weekly or every three weeks × 2-3 cycles; one of these patients was switched to combination paclitaxel/carboplatin mid-treatment because of unfavorable early disease response. Four patients received weekly single agent cetuximab, while one patient received combination cisplatin and cetuximab.
Our ART planning process started with a baseline IMRT plan. Baseline simulation CT was used as a reference for quantifying subsequent corrections. Our procedure was a combined IGRT-ART approach with two levels of adaptation. First, daily on-line IGRT correction was performed for each treatment via simple couch shifts. This corrected first order systematic and random translational setup errors. In-room CT guidance was employed for each daily treatment session. The C2 vertebra was prioritized as an alignment target since it lies in geographic proximity to the oropharynx and the anatomical pivot point for axial and sagittal head rotation (9, 10). For the second level of adaptation, the original IMRT plan was re-calculated on a weekly basis or as needed when significant discrepancies were found between the contour overlay and the anatomy within the CT images.
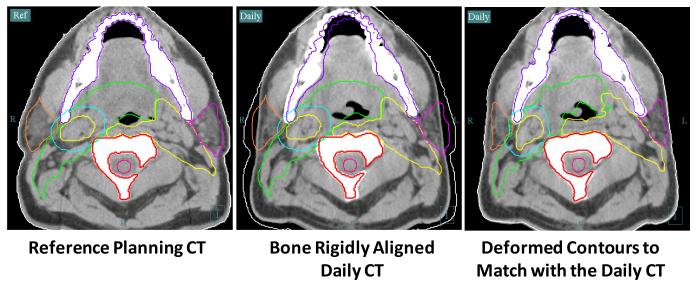
A validated in-house version of Thirion’s Demons algorithm (14, 15) deformably mapped baseline anatomic contours to the in-room CT image sets for dosimetric evaluation and ART replanning. An example of using deformable image registration for auto-segmentation is shown in Figure 1. The process starts with a rigid alignment of bony structure (C2 vertebra) between the reference planning CT (left) and the daily in-room CT (middle and right). The necessary planning contours are overlaid onto the daily CT to verify setup accuracy and to ascertain whether significant anatomic changes have occurred at interval. If changes are significant (for example, the original clinical target volumes no longer adequately cover gross disease visualized on daily CT images), deformable image registration can be performed to propagate the planning contours to the daily anatomy. The resultant contours are shown to the right. The entire transformation takes seconds, making it relevant to either online or offline IMRT replanning. The treating physician formally reviews all deformably mapped contours. Significant anatomic changes resulting in geographical miss of gross tumor or inadequate sparing of normal tissues (particularly parotid glands or larynx) prompts formal dosimetric evaluations and ART replanning. Zero-mm PTV margins are used for all ART planning. This is based on our experience from previous planning studies confirming that 3-4 mm PTV expansion margins become too generous with daily image guidance (12). Once the ART plan is approved, QA and data transfer remain identical to our conventional procedures.
Figure 1. Deformable Registration for ART.
Our ART process starts with rigid alignment between the reference planning CT and the daily in-room CT (left and middle). The planning contours are overlaid to the daily CT to verify setup accuracy and to evaluate changes in anatomy relative to baseline. If changes are significant, as illustrated in the middle picture, a deformable image registration can be performed to propagate original planning contours onto current anatomy. Final deformed contours are shown on the right.
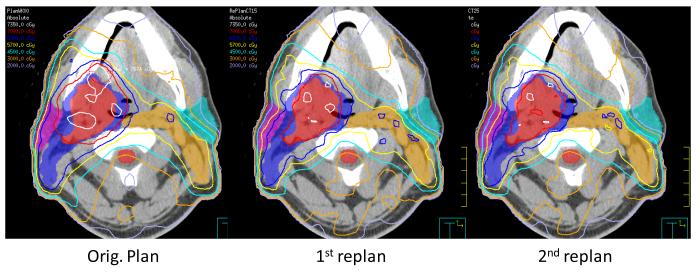
An example of ART dose recalculation and replanning is shown in Figure 2. On the left, the original plan is calculated onto current anatomy. Due to loss of weight and tissue separation, there is less attenuation of each IMRT beam. As a result, the original plan provides inappropriately large treatment margins and considerable dose heterogeneity within the high dose CTV. In the middle figure, a previous ART replan (ART1, designed at the 15th treatment fraction) is calculated onto current anatomy. The ART1 replan significantly improves dose conformality because PTV expansions are not used. On the right, a 2nd ART replan (ART2) is designed and calculated for the current daily image set. The ART2 plan provides further improvement of contralateral parotid sparing and a lower scattered body dose relative to the ART1 plan.
Figure 2. ART Replanning.
An example of serial ART dose recalculation using a daily CT image acquired at the 25th treatment fraction. On the left, the original plan is calculated on current anatomy. The original plan provides inappropriate treatment margins and dose heterogeneity within the high dose CTV. In the middle figure, an earlier ART replan (ART1, designed at the 15th treatment fraction) is calculated onto current anatomy. On the right, a 2nd ART replan (ART2) is designed and calculated for the current daily image set. The ART2 plan provides improved contralateral parotid sparing and a lower total body dose than the ART1 plan.
Toxicity
Acute treatment-associated toxicity was scored according to NCI Common Terminology Criteria for Adverse Events (CTCAE) v3.0. Toxicity was considered acute if it occurred within 90 days of treatment completion. For unstimulated resting sialometry, each patient expectorated accumulated saliva into a pre-weighed 100 mL vial after 60 seconds. The patient repeated this 4 more times for a total collection time of 5 minutes. Stimulated sialometry was then performed in the same fashion after 20 mL of citric acid solution was held in the mouth for 1 minute. The M.D. Anderson Dysphagia Inventory (MDADI) was self-administered at baseline, treatment completion, and at routine post-radiation surveillance appointments over the subsequent 24 months. At these time points, nutritional status was assessed by weight, Performance Status Scale for Head and Neck Cancer (PSS-HN), and percutaneous endoscopic gastrostomy (PEG) requirement. Patients underwent modified barium swallow (MBS) testing at baseline, 4-6, 12, and 24 months after the completion of ART. Studies were performed using standard radiographic systems. The order of bolus presentation included: two 5-ml Varibar thin liquid boluses, two 10-ml Varibar thin liquid boluses, two 20-ml Varibar thin liquid boluses, two cup sips of Varibar thin liquid, two pureed/Varibar pudding boluses, two solid boluses consisting of ¼ of a shortbread cookie or cracker coated with Varibar pudding, and 2 trials of the most difficult consistency in the A-P plane. Function was quantified by (1) Penetration-Aspiration Scale (PAS), a clinician rated 8-point, ordinal scale used to describe penetration and aspiration events with higher scores assumed to be a sign of worsening dysphagia; and (2) Oropharyngeal Swallow Efficiency (OPSE), a global measure of swallow function defined as the ratio of the percent swallowed into the esophagus divided by oropharyngeal transit time.
Volumetric treatment response
Median volumetric change for high-risk CTV and combined parotid glands relative to baseline were quantified at the time of the first ART replanning and at the end of treatment. Volumetric response of CTV was evaluated in lieu of GTV in order to avoid deformable registration errors in cases of complete GTV response. Also, CTV was felt to be more clinically relevant in light of our policy to not defer treatment to areas originally involved with disease via our ART process.
Statistics
Two tailed, non-parametric Spearman’s correlation was used to analyze any potential correlation among the percent of parotid volume shrinkage, the volume of the high risk CTV, age, weight loss, duration of feeding tube, acute and late treatment-associated toxicities etc. One-way ANOVA was used to determine the statistical significance between groups.
RESULTS
Study cohort and disease control outcomes
Study cohort characteristics are summarized in Table 1. The cohort was composed of 21 males and 1 female; median age was 55 (range: 42-75 years). Two patients had AJCC stage III disease and 20 patients had stage IVA disease, 19 of whom had N2 disease. Eight patients had T3-4 disease. Ten patients had a history of > 10 pack-year cigarette smoking (range: 10-72); 2 of these patients (with 20 and 38 pack-yr exposures) had HPV-positive disease by both p16 immunohistochemisty and high-risk HPV in situ hybridization. Eleven patients were lifetime non-smokers and one patient had a 1.5 pack-yr exposure; 3 of these patients had HPV-positive disease. Fifteen patients were not characterized for HPV infection status.
Table 1.
Study cohort and treatment characteristics.
| Case | Primary Site |
T Stage |
N Stage |
AJCC Stage |
Smoking (Pk-Yr) |
Drinks/ week |
RT Dose (Gy) |
RT Fractions |
Chemo | Post-RT Neck Dissection |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Base of tongue |
T1 | N2a | IVA | 0 | 2-5 | 66 | 30 | None | Y |
| 2 | Base of tongue |
T2 | N2b | IVA | 0 | >5 | 70 | 33 | Cetuximab | N |
| 3 | Tonsil | T2 | N2b | IVA | 72 | 0 | 70 | 33 | Cisplatin | N |
| 4 | Base of tongue |
T2 | N2a | IVA | 0 | 0 | 71.8 | 40 | Cetuximab | N |
| 5 | Base of tongue |
T4 | N2b | IVA | 50 | 2-5 | 70 | 33 | Cisplatin | N |
| 6 | Base of tongue |
T2 | N2b | IVA | 45 | 0 | 70 | 33 | Cisplatin | Y |
| 7 | Tonsil | T2 | N2b | IVA | 45 | >5 | 70 | 33 | Cisplatin | N |
| 8 | Base of tongue |
T3 | N2c | IVA | 0 | ≤1 | 70 | 33 | CDDP, Tax/Carbo |
N |
| 9 | Base of tongue |
T3 | N2b | IVA | 0 | 0 | 70 | 33 | Cisplatin | Y |
| 10 | Base of tongue |
T4 | N2b | IVA | 12 | ≤1 | 70 | Accelerated | Cisplatin | N |
| 11 | Base of tongue |
T4 | N2b | IVB | 0 | ≤1 | 70 | Accelerated | Cisplatin | N |
| 12 | Base of tongue |
T4 | N0 | IVA | 20 | 0 | 70 | 33 | Cisplatin | N |
| 13 | Base of tongue |
T4 | N2b | IVA | 0 | ≤1 | 70 | Accelerated | CDDP/Cet uximab |
Y |
| 14 | Tonsil | T2 | N2c | IVA | 38 | ≤1 | 70 | 33 | Cetuximab | Y |
| 15 | Tonsil | T2 | N2b | IVA | 0 | ≤1 | 70 | 33 | Cisplatin | N |
| 16 | Base of Tongue |
T2 | N1 | III | 10 | ≤1 | 70 | 33 | Cisplatin | N |
| 17 | GP Sulcus | T2 | N2a | IVA | 20 | >5 | 70 | 33 | Cisplatin | Y |
| 18 | Base of Tongue |
T2 | N2b | IVA | 0 | >5 | 70 | 33 | Cetuximab | Y |
| 19 | Base of Tongue |
T1 | N2a | IVA | 0 | ≤1 | 70 | 33 | Cisplatin | N |
| 20 | Tonsil | T2 | N2b | IVA | 55 | ≤1 | 70 | 33 | Cisplatin | N |
| 21 | Base of Tongue |
T2 | N2a | IVA | 0 | ≤1 | 70 | 33 | Cisplatin | Y |
| 22 | Tonsil | T3 | N1 | III | 1.5 | ≤1 | 70 | 33 | Cisplatin | Y |
With a median follow up of 31 months (range: 13-45), there has been 1 nodal relapse, yielding an estimated 100% local and 95% regional disease control rate at 2 years. The single case of neck failure was surgically salvaged and remains free of disease. Eight additional patients underwent consolidative neck dissection surgery for persistent adenopathy; none of these patients had residual viable disease in their surgical specimens. No distant disease relapses or deaths have occurred.
ART replanning
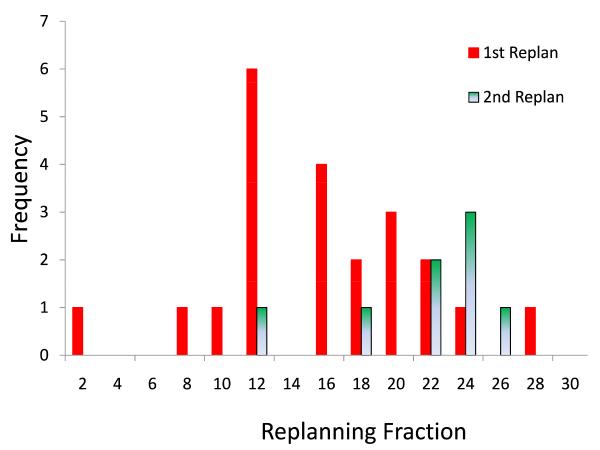
All patients required at least one replan (ART1) as specified by protocol due to CTV and normal tissue changes; 8 patients (36%) required a second replan (ART2). The median trigger point for first adaptive plan was the 16th treatment fraction (range: 2 to 28), at which point the median bilateral parotid volumes had shrunk by an average of 16% and the combined CTVs had shrunk by 5%. For ART2 patients, median trigger point for the first replan was the 11th fraction (range: 2 to 15) and for 2nd replan the 22th fraction (range: 11 to 25), at which point bilateral parotid volumes and CTVs had shrunk by 24% and 14%, respectively. The elapsed time interval from triggering in-room CT imaging to subsequent delivery of the prompted ART plan was 1.7 days (median: 2 days; range: 1-4 days), excluding weekends. Most ART replans were completed within one day. The timing distribution of the first replan and second replan is plotted in Figure 3.
Figure 3. Timing of ART Replanning.
Distribution of the triggering fraction for replanning is plotted for both 1st and 2nd ART events.
ART dosimetric impact
Standard image-guided IMRT (IGRT) and ART dosimetric results were tabulated from all daily in-room CT image sets for the first 22 patients enrolled on trial. Underdosing (e.g. greater than 5% dose loss in high-risk CTV) did not occur with either IGRT or ART treatment, confirming the adequacy of our 0-mm PTV expansion margin for ART replanning. Mean parotid dose sparing was improved with a single ART replanning (ART1) by 0.6 Gy (2.8%, p = 0.003) in the contralateral parotid, and by 1.3 Gy (3.9%, p = 0.002) in the ipsilateral parotid relative to standard IGRT. In patients who had an early response to treatment, two ART replans (ART2) provided sparing of 0.8 Gy or 3.8% (p = 0.026) for the contralateral parotid and 4.1 Gy or 9% (p= 0.001) for the ipsilateral parotid. We calculated the volumes of body dose at 60Gy, 40Gy, and 20Gy delivered either IGRT or ART. Initial ART replanning (ART1) reduced body dose over IGRT plans by 31cc at 60Gy (p = 0.019), 36cc at 40Gy (p = 0.007), and 13cc at 20Gy (p = 0.163). ART2 replanning marginally improved body dose beyond what initial ART had achieved.
Acute toxicity
Acute toxicity manifested predominantly as radiation mucositis (grade 3 in all patients), dermatitis (10 patients grade 1; 12 patients grade 2), and xerostomia (9 patients grade 1; 12 patients grade 2; 1 patient grade 3). Median percent weight loss from baseline at the time of treatment completion was 8.4% (range: 1.3-17.8%). Eighteen (82%) required gastrostomy tube (PEG) placement for nutritional support; these tubes remained in place for a median of 4.5 months (range: 1-13 months) following treatment, with removal eventually taking place in all cases. Older age (> 65 years) correlated with longer duration of feeding tube use (p = 0.02). Duration of PEG use correlated with the percent of parotid volume shrinkage at the end of treatment (p = 0.025), but not at the time of first adaptive replanning (p = 0.421).
Chronic toxicity
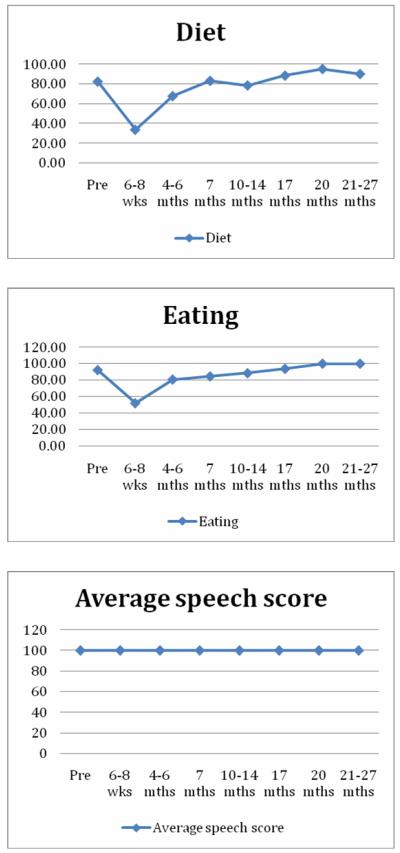
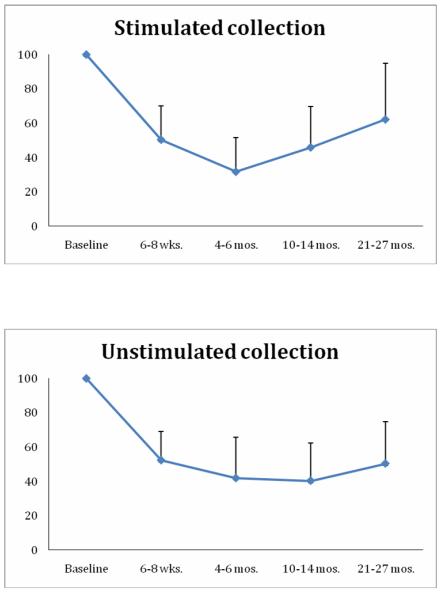
PSS-HN results (Figure 4) confirm full preservation or functional recovery of speech and eating function across tested domains by 20 months. Sialometry results (Figure 5) are notable for ongoing recovery of stimulated salivary production after 1 year follow-up despite persistent loss of unstimulated salivary production, consistent with institutional planning policy prioritizing parotid gland sparing at the cost of no formal sparing of submandibular glands. Additional swallowing outcomes are detailed in Table 1. No patient demonstrated aspiration after 12 months, although 2 patients did demonstrate a PAS score of 6.0-7.5 at 6 months or later. Modified barium swallow results were available for 17/19 patients at 12 months follow-up, with recovery of OPSE measures seen following a nadir at 6 month post-treatment. OPSE scores worsened at 24 months, but only 5 cases were available for this time point. Patient-defined MDADI measures of subjective swallowing function mirrored objective measures, with persistent recovery of function following a nadir at 6 months. As expected, standard deviations for mean MDADI scores for the cohort were relatively large.
Figure 4. PSS-HN Outcomes.
Serial mean scores plotted for diet, eating, and speech.
Figure 5. Sialometry Outcomes.
Serial mean stimulated and unstimulated measurements relative to the baseline.
Volumetric treatment response
Median volumetric change relative to the baseline at the time of first replan was −5.8% (range: −13.4% to 1.1%) for high risk CTV (combined primary and nodal disease) and −15.8% (range: −25.0% to −5.7%) for parotid glands. By the end of treatment, median volumetric change was −10.3% (range: −1.4% to −24.5%) and −24.3% (range: −15.7% to −47.7%) for high-risk CTV and parotid glands, respectively. Volumetric responses at either site did not correlate with smoking history, disease stage, or chemotherapy use.
Patient weight loss correlated with percent reduction in parotid gland volume at the time of first ART replan (p = 0.04), although no correlation was observed with percent parotid volume reduction measured at the end of treatment (p = 0.29). Patients who presented with large high-risk CTV at baseline demonstrated significantly greater response in CTV volume by the end of treatment (p < 0.0001). Baseline size of high-risk CTV at presentation did not correlate with any difference in acute or late toxicity.
DISCUSSION
Emerging institutional and cooperative group trial data suggest encouraging disease control outcomes with the use of IMRT for locoregional treatment of head and neck cancer (1, 2, 17, 18). These institutional series suggest improved preservation of salivary function with IMRT relative to conventional 3DRT, but also demonstrate continuing issues with toxicity. A recent randomized trial has confirmed significant improvement in salivary toxicity with IMRT relative to 3DRT, although this effort detected no additional benefits to IMRT with formal quality of life instruments (6).
Many of IMRT’s potential advantages remain unrealized when statically guided by pre-treatment imaging. Treatment failures may also result from unanticipated positional shifts of tumor across regions of sharp dose gradient. Adaptive radiotherapy (ART) refers to formal correction for daily tumor and normal tissue variations through online or offline modification of original IMRT target volumes and plans. Manual replanning can be pursued midway through treatment (19); however, direct staff input should be replaced by automated processes to make ART practical.
Our ART process starts with standard baseline IMRT planning, with volumetric CTV-to-PTV expansions of 3-4 mm. We use dedicated visualization techniques to monitor changes in patient’s anatomy through treatment, including skin contour, parotid volume, and non-rigid spine movement. Our deformable image registration process transfers baseline segmentation to daily CT image sets. If significant anatomic changes are noted on daily CT images, then formal dosimetry evaluations are instigated. Baseline treatment plans can be transferred directly to daily image sets for adaptive replanning based on current anatomy. IMRT planning constraints can be modified on an individualized basis.
Although our ART procedure begins initially with conventional CTV-to-PTV expansion margins, we must emphasize that we do not employ these PTV expansions for adaptive replans. Our experience has confirmed highly precise treatment set-up reproducibility with CT-guided IGRT once patients have acclimated to treatment. Additional experience has confirmed that standard 3-4 mm PTV expansion margins are too generous to maintain parotid dose sparing if daily image guidance is employed. Our ART planning does not differ from baseline planning in any other way. Therefore, the intent for ART is to recapitulate the treatment planning goals of the original IMRT plan as faithfully as possible.
Our initial dosimetric analysis confirmed that mean parotid dose sparing was improved with ART by 2.8% (p = 0.003) in the contralateral parotid, and by 3.9% (p = 0.002) in the ipsilateral parotid. This is consistent with Wu, et. al, who found a single mid-course ART replanning to provide parotid sparing of comparable magnitude (20). In patients undergoing two replans, sparing was 3.8% (p = 0.026) for the contralateral parotid and 9% (p = 0.001) for the ipsilateral parotid, which was also consistent with Wu’s finding of 5% combined parotid sparing when using two replans. Importantly, we were also able to spare dose to oral cavity, base of tongue, and glottic larynx over conventional IMRT or IGRT.
Do such improvements translate into clinically meaningful benefits that justify the added resource requirements of ART? Disease control outcomes from published head and neck IMRT series have been very promising. Therefore, the intent of this trial, beyond proof-of-principle demonstration of feasibility, was to detect whether ART could improve toxicity outcomes without sacrificing local disease control and overall survival. Our results suggest no significant change in acute toxicity relative to what we generally have observed for standard IMRT. Early chronic toxicity results suggest encouraging post-treatment functional recovery in our study cohort; however, these must be considered preliminary findings. Continued collection of toxicity data remains necessary, and a definitive head-to-head trial between IMRT and ART will eventually be required for conclusive validation. Although our ART platform is more automated than preceding systems, continued technical refinement of in-room imaging, deformable registration, and treatment planning processes will be necessary to further reduce equipment costs and staffing requirements to facilitate routine deployment into community-based centers with commercially available IGRT-capable systems.
Preservation of excellent survival outcomes in this series is notable on several levels. Our previously reported dosimetric analysis of this study cohort included composite dose calculations from in-room CT images obtained every day during treatment. This demonstrated universal development of unintended heterogeneous hotspots (>107% prescribed dose) within target volumes in the absence of adaptive correction. Preservation of disease control following removal of such incidental de facto dose escalation suggests feasibility to improve therapeutic ratio with oropharyngeal IMRT through rational dose de-intensification strategies, of which ART is an example. These findings are all the more striking given that 100% primary disease control was achieved in a study cohort with 9/24 (38%) cases of probable high-risk, HPV-unassociated disease by virtue of cigarette exposure history. This pilot trial started when universal HPV infection characterization of oropharyngeal cancer patients was not yet pursued by our center. Moving forward, we consider this information crucial to define the utility of ART for individual oropharyngeal cancer patients.
Important unresolved issues with ART include: 1) formulation of ideal dosimetric thresholds and timing for replanning, 2) definition of clinically relevant dose constraints to routine (e.g. parotid) and novel (e.g pharyngeal constrictor muscles) avoidance structures which could be potentially be better achieved through ART, 3) individualized prediction and localization of disease and normal tissue targets for mid-treatment dose adjustment through functional imaging, 4) feasibility and/or need for development of fully automated on-line dosimetric evaluation, replanning, and treatment modification, and 5) patient selection according to greatest need for ART to optimize cost effectiveness of deployment. Validation of predictive biomarkers for disease and normal tissue radiation responsiveness would promise to streamline patient selection for ART. In the absence of such predictive markers, younger patients with human papilloma virus-associated oropharyngeal disease with a high likelihood for radiation response may be the most logical population to attempt to reduce bystander dose with ART to minimize long-term functional deficits.
Adaptive radiotherapy (ART) is an approach to correct for changes in patient’s anatomy that occur during a course of treatment. The technical and staffing requirements necessary for ART, particularly for head and neck cancer, have hampered its development. In fact, no clinical outcomes resulting from standardized deployment of ART have yet been described. This report presents initial toxicity and disease control outcomes from the first prospective clinical trial investigating an automated ART approach for locally advanced oropharyngeal cancer using techniques specifically designed to minimize physician, physicist, and dosimetrist time demands.
Table 2.
Swallowing outcomes.
| Baseline | 6 mo.* | 12 mo.* | 24 mo.* | |
|---|---|---|---|---|
| n (MBS) | 17 | 17 | 17 | 5 |
| n (Questionnaire)* | 18 | 19 | 17 | 8 |
| OPSE, mean (SD) | ||||
| 10 mL liquid | 91.2 (14.0) | 88.0 (15.0) | 81.6 25.8) | 78.8 (20.7) |
| Pudding | 77.6 (29.0) | 59.9 (28.2) | 72.7 30.3) | 58.9 (20.4) |
| Cracker | 83.5 (36.4) | 45.3 (32.4) | 62.5 (34.5) | 49.6 (19.7) |
| All consistencies | 84.3 (21.3) | 65.2 (21.8) | 72.3 (22.5) | 63.3 (16.0) |
| Aspiration, No. (%) | ||||
| 10 mL liquid | -- | 1 (6%) | -- | -- |
| Pen-Asp Scale, median (Range) | ||||
| 10 mL liquid | 1 (1.0-1.5) | 1.5 (1.0-7.5) | 1.5 (1.0-6.0) | 1.5 (1.0-6.0) |
| MDADI, mean (SD) | ||||
| Global score | 89.4 (18.9) | 75.8 (22.7) | 80.0 (22.7) | 80.0 (26.2) |
| Composite score | 86.6 (12.9) | 67.0 (12.4) | 73.6 (19.2) | 75.9 (14.2) |
| Feeding Tube, No. (%) | ||||
| No | 18 (100%) | 12 (63%) | 16 (94%) | 8 (100%) |
| Yes | -- | 7 (37%) | 1 (6%) | -- |
| PSS-HN Normalcy of Diet | ||||
| Full diet (no restriction) | 10 | 2 | 4 | 3 |
| Full diet (liquid assist) | 5 | 7 | 9 | 3 |
| All meat | -- | -- | -- | -- |
| Raw carrots, celery | -- | 2 | -- | 1 |
| Dry bread and crackers | 1 | 3 | 1 | -- |
| Soft, chewable foods | 1 | 2 | 1 | 1 |
| Soft, non-chewable foods | -- | -- | 1 | -- |
| Pureed foods | 1 | 1 | -- | -- |
| Warm liquids | -- | -- | 1 | -- |
| Cold Liquids | -- | 1 | -- | -- |
| Non-oral feeding (NPO) | -- | 1 | -- | -- |
Questionnaires mailed to patients who missed MBS; OPSE = Oropharyngeal Swallow Efficiency; Pen-Asp Scale = Penetration-Aspiration Scale Score; MDADI = M.D. Anderson Dysphagia Inventory; PSS-HN = Performance Status Scale for H&N Cancer
Acknowledgements
Supported by CA139284 from the National Cancer Institute (to DLS) and by an unrestricted grant-in-aid from Varian Medical Systems (to LD). Presented in part at the Annual Meeting of the American Society for Radiation Oncology, Nov. 1-5, 2009, Chicago, IL.
Footnotes
The authors report no conflicts of interest with other involved parties in the process of conducting this study.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.de Arruda FF, Puri DR, Zhung J, Narayana A, Wolden S, Hunt M, et al. Intensity-modulated radiation therapy for the treatment of oropharyngeal carcinoma: the Memorial Sloan-Kettering Cancer Center experience. Int J Radiat Oncol Biol Phys. 2006 Feb 1;64(2):363–73. doi: 10.1016/j.ijrobp.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 2.Garden AS, Morrison WH, Wong PF, Tung SS, Rosenthal DI, Dong L, et al. Disease-control rates following intensity-modulated radiation therapy for small primary oropharyngeal carcinoma. Int J Radiat Oncol Biol Phys. 2007 Feb 1;67(2):438–44. doi: 10.1016/j.ijrobp.2006.08.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Feng FY, Kim HM, Lyden TH, Haxer MJ, Feng M, Worden FP, et al. Intensity-modulated radiotherapy of head and neck cancer aiming to reduce dysphagia: early dose-effect relationships for the swallowing structures. Int J Radiat Oncol Biol Phys. 2007 Aug 1;68(5):1289–98. doi: 10.1016/j.ijrobp.2007.02.049. [DOI] [PubMed] [Google Scholar]
- 4.Eisbruch A, Levendag PC, Feng FY, Teguh D, Lyden T, Schmitz PI, et al. Can IMRT or brachytherapy reduce dysphagia associated with chemoradiotherapy of head and neck cancer? The Michigan and Rotterdam experiences. Int J Radiat Oncol Biol Phys. 2007;69(2 Suppl):S40–2. doi: 10.1016/j.ijrobp.2007.04.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yao M, Karnell LH, Funk GF, Lu H, Dornfeld K, Buatti JM. Health-related quality-of-life outcomes following IMRT versus conventional radiotherapy for oropharyngeal squamous cell carcinoma. Int J Radiat Oncol Biol Phys. 2007 Dec 1;69(5):1354–60. doi: 10.1016/j.ijrobp.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 6.Clark CH, Miles EA, Urbano MT, Bhide SA, Bidmead AM, Harrington KJ, et al. Pre-trial quality assurance processes for an intensity-modulated radiation therapy (IMRT) trial: PARSPORT, a UK multicentre Phase III trial comparing conventional radiotherapy and parotid-sparing IMRT for locally advanced head and neck cancer. Br J Radiol. 2009 Jul;82(979):585–94. doi: 10.1259/bjr/31966505. [DOI] [PubMed] [Google Scholar]
- 7.Rosenthal DI, Chambers MS, Fuller CD, Rebueno NC, Garcia J, Kies MS, et al. Beam path toxicities to non-target structures during intensity-modulated radiation therapy for head and neck cancer. Int J Radiat Oncol Biol Phys. 2008 Nov 1;72(3):747–55. doi: 10.1016/j.ijrobp.2008.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barker JL, Jr., Garden AS, Ang KK, O’Daniel JC, Wang H, Court LE, et al. Quantification of volumetric and geometric changes occurring during fractionated radiotherapy for head-and-neck cancer using an integrated CT/linear accelerator system. International Journal of Radiation Oncology, Biology, Physics. 2004 July 15;59(4):960–70. doi: 10.1016/j.ijrobp.2003.12.024. [DOI] [PubMed] [Google Scholar]
- 9.Zhang L, Garden AS, Lo J, Ang KK, Ahamad A, Morrison WH, et al. Multiple regions-of-interest analysis of setup uncertainties for head-and-neck cancer radiotherapy. Int J Radiat Oncol Biol Phys. 2006;64(5):1559–69. doi: 10.1016/j.ijrobp.2005.12.023. [DOI] [PubMed] [Google Scholar]
- 10.van Kranen S, van Beek S, Rasch C, van Herk M, Sonke JJ. Setup Uncertainties of Anatomical Sub-Regions in Head-and-Neck Cancer Patients After Offline CBCT Guidance. Int J Radiat Oncol Biol Phys. 2009;73(5):1566–73. doi: 10.1016/j.ijrobp.2008.11.035. [DOI] [PubMed] [Google Scholar]
- 11.Schubert LK, Westerly DC, Tomé WA, Mehta MP, Soisson ET, Mackie TR, et al. A Comprehensive Assessment by Tumor Site of Patient Setup Using Daily MVCT Imaging From More Than 3,800 Helical Tomotherapy Treatments. Int J Radiat Oncol Biol Phys. 2009;73(4):1260–9. doi: 10.1016/j.ijrobp.2008.11.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.O’Daniel JC, Garden AS, Schwartz DL, Wang H, Ang KK, Ahamad A, et al. Parotid gland dose in intensity-modulated radiotherapy for head and neck cancer: is what you plan what you get? Int J Radiat Oncol Biol Phys. 2007 Nov 15;69(4):1290–6. doi: 10.1016/j.ijrobp.2007.07.2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jaffray DA, Siewerdsen JH, Wong JW, Martinez AA. Flat-panel cone-beam computed tomography for image-guided radiation therapy. International Journal of Radiation Oncology, Biology, Physics. 2002;53(5):1337–49. doi: 10.1016/s0360-3016(02)02884-5. [DOI] [PubMed] [Google Scholar]
- 14.Wang H, Dong L, O’Daniel J, Mohan R, Garden AS, Ang KK, et al. Validation of an accelerated ‘demons’ algorithm for deformable image registration in radiation therapy. Phys Med Biol. 2005 Jun 21;50(12):2887–905. doi: 10.1088/0031-9155/50/12/011. [DOI] [PubMed] [Google Scholar]
- 15.Wang H, Garden AS, Zhang L, Wei X, Ahamad A, Kuban DA, et al. Performance Evaluation of Automatic Anatomy Segmentation Algorithm on Repeat or Four-Dimensional Computed Tomography Images Using Deformable Image Registration Method. Int J Radiat Oncol Biol Phys. 2008;72(1):210–9. doi: 10.1016/j.ijrobp.2008.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Overgaard J, Hansen HS, Specht L, Overgaard M, Grau C, Andersen E, et al. Five compared with six fractions per week of conventional radiotherapy of squamous-cell carcinoma of head and neck: DAHANCA 6 and 7 randomised controlled trial. Lancet. 2003 Sep 20;362(9388):933–40. doi: 10.1016/s0140-6736(03)14361-9. [DOI] [PubMed] [Google Scholar]
- 17.Lee N, Harris J, Garden AS, Straube W, Glisson B, Xia P, et al. Intensity-modulated radiation therapy with or without chemotherapy for nasopharyngeal carcinoma: radiation therapy oncology group phase II trial 0225. J Clin Oncol. 2009 Aug 1;27(22):3684–90. doi: 10.1200/JCO.2008.19.9109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eisbruch A, Harris J, Garden AS, Chao CK, Straube W, Harari PM, et al. Multi-institutional trial of accelerated hypofractionated intensity-modulated radiation therapy for early-stage oropharyngeal cancer (RTOG 00-22) Int J Radiat Oncol Biol Phys. Apr;76(5):1333–8. doi: 10.1016/j.ijrobp.2009.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hansen EK, Bucci MK, Quivey JM, Weinberg V, Xia P. Repeat CT imaging and replanning during the course of IMRT for head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2006 Feb 1;64(2):355–62. doi: 10.1016/j.ijrobp.2005.07.957. [DOI] [PubMed] [Google Scholar]
- 20.Wu Q, Chi Y, Chen PY, Krauss DJ, Yan D, Martinez A. Adaptive replanning strategies accounting for shrinkage in head and neck IMRT. Int J Radiat Oncol Biol Phys. 2009 Nov 1;75(3):924–32. doi: 10.1016/j.ijrobp.2009.04.047. [DOI] [PubMed] [Google Scholar]