Abstract
A common feature of progeria syndromes is a premature aging phenotype and an enhanced accumulation of DNA damage arising from a compromised repair system. HGPS (Hutchinson–Gilford progeria syndrome) is a severe form of progeria in which patients accumulate progerin, a mutant lamin A protein derived from a splicing variant of the lamin A/C gene (LMNA). Progerin causes chromatin perturbations which result in the formation of DSBs (double-strand breaks) and abnormal DDR (DNA-damage response). In the present article, we review recent findings which resolve some mechanistic details of how progerin may disrupt DDR pathways in HGPS cells. We propose that progerin accumulation results in disruption of functions of some replication and repair factors, causing the mislocalization of XPA (xeroderma pigmentosum group A) protein to the replication forks, replication fork stalling and, subsequently, DNA DSBs. The binding of XPA to the stalled forks excludes normal binding by repair proteins, leading to DSB accumulation, which activates ATM (ataxia telangiectasia mutated) and ATR (ATM- and Rad3-related) checkpoints, and arresting cell-cycle progression.
Keywords: DNA repair, genome instability, Hutchinson–Gilford progeria syndrome (HGPS), laminA, premature aging, xeroderma pigmentosum group A (XPA)
Introduction
The aging process represents progressive cellular changes which culminate in death due to accumulated deficiencies in enzymes and proteins necessary for maintaining cell metabolism and replicative fidelity of the genome [1-4]. Mutations to genes directly involved in basic genome metabolism understandably would cause an accelerated aging phenotype and/or shortened lifespan (e.g. Werner’s, Bloom’s or Cockayne syndromes) [5,6].
Unlike many other progeria syndromes which are caused by mutations of genes involved in DNA metabolism or DNA repair, HGPS (Hutchinson–Gilford progeria syndrome) is a laminopathy-based disease that arises from a mutation causing altered processing/maturation of lamin A, an intermediate-filament protein component of the nuclear lamina [6-13]. Nevertheless, HGPS is one of the most severe forms of progeria; individuals have an average lifespan of 13.5 years [9,11,14,15]. Although lamin A is not involved directly in DNA metabolism, particularly DNA-repair and-damage responses, DSBs (double-strand breaks) accumulate in HGPS cells [16-18]. Similar progerin-induced DSB accumulation also occurs in older healthy aging individuals [19]. Thus an interesting question concerns how progerin disrupts normal genome organization to cause deficiencies in DNA-repair processes and cell-cycle regulation. In the present paper, the effects of lamin A abnormalities will be considered relative to the perturbation of DNA-damage recognition and its repair, leading to the loss of genome function in HGPS patients.
Laminopathy in HGPS
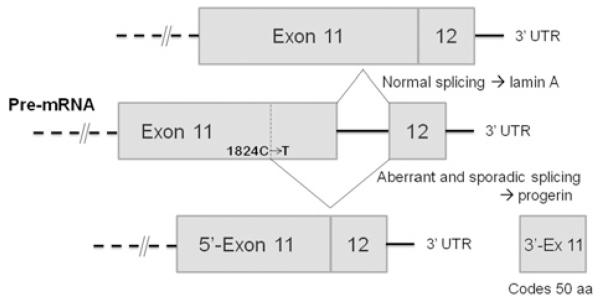
The lamins are structural filamentous proteins in the nuclear lamina and also form nucleoplasmic foci which perform dynamic organizational roles in the nucleus [20-23]. Lamin proteins also interact with histone H2A [24,25]. Prelamin A, the normal translation product of LMNA (lamin A/C) mRNA, is post-translationally processed into lamin A by two transfer reactions and two proteolytic cleavages [26]. Restrictive dermopathy arises from a deficiency of Zmpste24 (zinc metalloprotease Ste24 homologue) which performs the proteolytic cleavages, resulting in intact, but farnesylated, prelamin A [13,27,28]. The HGPS laminopathy arises from a deficiency in these post-translational modifications due to a heterozygous mutation within the LMNA gene. The dominant mutation is a base substitution (1824C>T) within exon 11, creating a cryptic splice donor site (Figure 1). Sporadic use of this cryptic site for splicing removes a 150-base sequence, leading to a 50-amino-acid deletion within prelamin A. The deletion disrupts normal prelamin A processing and produces progerin, a smaller farnesylated and carboxymethylated mutant protein. The hydrophobic farnesyl chain gives progerin a greater affinity for the inner nuclear membrane, deforming the membrane and causing dysmorphic interphase nuclei and a loss of heterochromatin and nucleoplasmic lamin A foci [29]. These foci normally contain the replicative proteins PCNA (proliferating-cell nuclear antigen) and DNA polymerase δ and appear to be critical for ordered initiation of S-phase replication [30,31]. Functionally, nucleocytoplasmic transport is disrupted [32], histone modification and gene expression patterns change [33-36], and DNA damage increases with a loss of repair efficiency [8,16,37]. Lamina dissolution at M-phase and reformation in G1-phase also are perturbed, delaying nuclear reformation and functionally disrupting G1 interphase chromatin [38,39]. These changes lead to increased genome instability and cytotoxicity as progerin accumulates in aging HGPS cells [7,13,15,20].
Figure 1. In HGPS, a C>T point mutation at position 1824 in exon 11 of the lamin A gene creates a new donor splice sequence.
Sporadic splicing can occur between the mutation site and the 5′-end of exon 12, producing a protein (progerin) which is 50 amino acids (aa) shorter than the wild-type lamin A protein. UTR, untranslated region.
DNA-damage accumulation and DDR (DNA-damage response) signalling in HGPS cells
HGPS cells accumulate endogenous DNA damage, in particular DSBs, with passage in culture [8,16,17]. The laminopathy-based progeroid cells are also sensitive to various DNA-damaging agents, including DSB inducers [ionizing radiation, CPT (camptothecin) and etoposide], mitomycin C, which induces interstrand cross-links, and the alkylating agent methyl methanesulfonate [8,37]. HGPS cells also exhibit a delayed cytotoxicity to UV radiation [40]. These cytotoxicity phenotypes reflect a deficiency in genome maintenance in progeroid cells, possibly involving components of homologous recombination, NHEJ (non-homologous end-joining) and NER (nucleotide excision repair).
HGPS cells in culture exhibit limited growth potential relative to BJ cells, normal human primary fibroblasts. Young HGPS cells grow quite well, but senesce quickly relative to BJ cells [16], with an increase in dysmorphic nuclei and the number of γ H2AX (phosphorylated histone H2AX) foci (a marker of DNA DSBs) [7,17,41,42]. H2AX, a minor histone H2A variant [43], is phosphorylated to γ H2AX in response to DSBs [44,45]. γ H2AX is used to cytologically mark nuclear sites of DSBs and biochemically to isolate chromatin containing DSBs [17,46].
Liu et al. [16] examined culture-aged HGPS and found higher levels of γ H2AX than in normal BJ cells, and increased phosphorylated Chk1 and Chk2 (checkpoint kinase 1 and 2) owing to ATM (ataxia telangiectasia mutated) and ATR (ATM- and Rad3-related) activation. Phosphorylated p53, a downstream product of Chk1 and Chk2 activation, was also increased [16], demonstrating that ATR and ATM checkpoints were persistently activated, as confirmed by others [47,48]. In addition, ATM and ATR were clustered into distinct nuclear foci in HGPS cells [16], identical with those observed in BJ cells treated with UV irradiation or CPT [8]. Caffeine inhibition or siRNA (small interfering RNA) knockdown of ATM and ATR confirmed biochemically that these checkpoint activities were responsible for the extended cell cycle and reduced replicative capacity of HGPS cells [16]. Thus DNA-damage-activated ATM and ATR checkpoint pathways mediated the decreased cell cycling in aged progeroid cells.
Is the activation and subnuclear clustering of ATM and ATR in progeroid cells directly related to progerin accumulation? Liu et al. [16] observed that HeLa cells transfected with a progerin-expressing plasmid exhibited ATR nuclear focus formation, demonstrating that foci formation is progerin-dependent. Inhibition of prenylation of the G608G mutant prelamin A with an FTI (farnesyltransferase inhibitor) restored normal nuclear shape, but the levels of γ H2AX and phosphorylated Chk1 and Chk2 in HGPS cells were not reduced. Disrupted or abnormal processing of prelamin A is a significant factor in the development of other progeroid symptoms, only some of which can be reversed by FTI treatment [27,29,35,37,49]. Thus reversal of dysmorphic nuclei formation may have limited effect on cell-cycle checkpoint activations from existing DNA DSBs. The more complete inhibition of lamin and progerin prenylation by statin and bisphosphonate drugs may be a more effective therapy [50].
Deficiencies in DNA-damage recognition and DDR in HGPS
Genome instability can arise from an increased sensitivity to DNA damage due to genetic or epigenetic deficiencies in DNA repair. The persistent activation of ATM/ATR in HGPS reflects a delay in DNA repair efficiency in these cells [16]. The DSB accumulation is particularly puzzling since HGPS cells are genetically defective in prelamin A and related processing pathways rather than in DNA-repair proteins.
Multiple proteins are normally recruited to DNA-damage sites for repair during the damage response. Surprisingly, such was not the case in HGPS cells. Zou’s group observed a significant parallel increase in nuclear γ H2AX foci and DSB frequency in HGPS cells relative to BJ fibroblasts [17]. Although elements of the damage-response system (i.e. ATR, ATM, Chk1, Chk2 and p53) were activated [16], immunofluorescence studies indicated that nuclear foci of Rad50 or Rad51 did not co-localize with the γ H2AX foci inHGPS cells [17]. This was unexpected, since Rad50 and Rad51 are early components of the damage response and are critical for repair of DNA DSBs [51-54] and for the restart of stalled replication forks [55]. In contrast, DSBs induced in normal BJ cells by CPT did show co-localization of γ H2AX with Rad50 or Rad51 foci, validating the immunofluorescence assay. The failed recruitment of repair factors to the laminopathy-induced DSBs made the DNA damage unrepairable in HGPS cells [17]. Impaired recruitment to DSB foci of Rad51 and 53BP1 (p53-binding protein 1) also was observed in bone marrow cells of Zmpste24−/−> mice and in HGPS cells treated with γ -irradiation [8]. A delay in the recruitment of repair factors phospho-NBS1 and MRE11 of the MRN (MRE11–Rad50–NBS1) complex to the sites of radiation-induced DNA DSBs also has been reported in HGPS cells [37].
The data cited above raise the question of why these repair proteins were not recruited to the DSB sites. XPA (xeroderma pigmentosum group A) protein is a specific and essential factor for NER, but not for repair of DNA DSBs [54]. In NER, XPA functions in DNA-damage recognition, nuclease recruitment and stabilization of intermediates [54,56-59]. NER does not process DSBs, neither does it introduce DSB intermediates during the repair process. Surprisingly, XPA co-localized with the γ H2AX sites of DNA DSBs in HGPS cells [17]. In HGPS cells treated with CPT, XPA did not colocalize to CPT-induced DSBs, although it still co-localized to the endogenous laminopathy-induced DSB foci. Also, the CPT-induced foci were repaired in HGPS cells, although at a lower rate than in the BJ cells, demonstrating that the DNA DSB repair system in HGPS cells is functional, and, also that XPA behaves normally in not binding to genotoxin-induced DNA DSBs.
How does the binding of XPA to laminopathy-generated DSBs relate to the lack of Rad50 and Rad51 binding? Is XPA binding sufficient to exclude these proteins? Zou’s group employed the ChIP (chromatin immunoprecipitation) assay and siRNA knockdown of XPA to resolve these questions. XPA was found in the γ H2AX-associated chromatin from HGPS cells, but not from normal BJ cells, even when DNA DSBs were induced in BJ cells by CPT [17]. Nuclease treatment of the chromatin before immunoprecipitation released the XPA from the γ H2AX chromatin complex. Thus DNA mediates the association of XPA and γ H2AX-marked chromatin containing DNA DSBs.
Liu et al. [17,60] observed that XPA depletion by siRNA knockdown partially restored the recruitment of Rad50, Rad51 and Ku70 to γ H2AX chromatin containing DNA DSBs and reduced the level of DSBs in HGPS cells. This confirms that the binding of XPA to laminopathy-induced DSBs in HGPS cells disrupted recruitment of repair factors. Thus XPA binding to DNA DSBs in progeroid cells may explain the absence of appropriate repair proteins and the observed genome instability due to faulty DNA repair.
Bomgarden at al. [61] found that XPA was needed for ATR signalling during S-phase and that XPA knockdown compromised the normal response to UV damage. The proportion of HGPS cells in S-phase increases with cell age as does the level of accumulated DNA DSBs. Thus it is not surprising that XPA localization to DSB sites [17] causes persistent activation of ATM, ATR, Chk1, Chk2 and p53, and cell growth becomes arrested in aged HGPS cells, although the major cause of checkpoint activation is triggered by DSB accumulation [16].
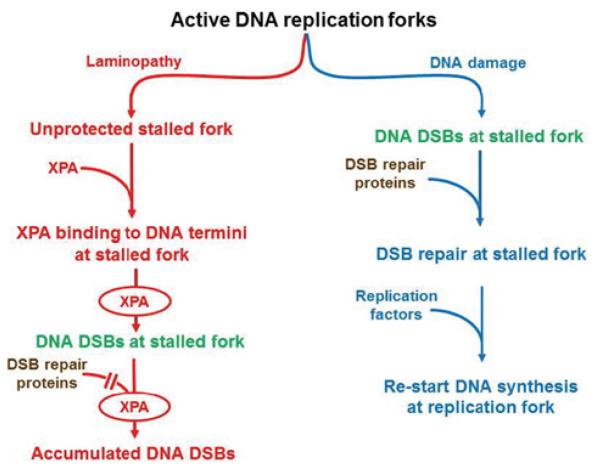
Lamin A and C proteins form nucleoplasmic foci containing proteins that initiate early S-phase replication, including the co-localization of PCNA and RFC (replication factor C) [31]. The chromatin lamin A/C granules normally contain PCNA and DNA polymerase δ [30]; these proteins are required for fork progression at replication centres [62]. Progerin interferes with the formation of these lamin A/C granules in HGPS cells, disrupting the normal distribution of PCNA, RFC and DNA polymerase δ. This redistribution of PCNA and/or RFC would also cause replication fork stalling and DNA DSBs. During this process, the replication fork and its damage intermediates, probably caused by PCNA and RFC deficiencies, may become accessible for XPA binding which blocks access to repair proteins such as Rad50, Rad51 and 53BP1 [8,17] (Figure 2, left-hand side). PCNA forms discrete nuclear foci in early-passage HGPS cells [63] when no XPA foci were seen, but were absent from late-passage cells (H. Tang, B. Hilton, P.R. Musich and Y. Zou, unpublished work) when XPA foci co-localize with γ H2AX and DNA DSBs [17].
Figure 2. A proposed model showing that DNA DSB repair activity is impaired in HGPS cells.
Unlike the replication fork collapse induced by genotoxins (right-hand side), laminopathy induced dysfunctional replication factors at replication forks characterized by the loss of PCNA, unprotected ds–ssDNA junctions, binding of XPA to the ‘naked’ replication forks, and collapse of replication forks (left-hand side). XPA binding denies the access of DSB repair proteins to the formed DSBs for repair.
Why does XPA co-localize with the laminopathy-induced DSBs in aging progeroid cells? XPA binds to ds (doublestranded)–ss (single stranded) DNA junctions with a higher affinity than it has for the DNA damage processed by NER [59]. In HGPS cells, progerin aggregation and PCNA sequestration at functioning replication forks may leave the ds–ssDNA junctions rich in Okazaki fragments unprotected, allowing access to XPA for binding (Figure 2). Thus progerin increases with age in progeroid cells, as do nuclear γ H2AX foci and measurable DSBs as well as XPA foci [17]. In addition, the translocation of XPA to the DSB sites in progeroid cells may sequester this NER protein, subsequently reducing NER activity for repair of bulky DNA adducts. This may explain the observed hypersensitivity of progeroid cells to UV damage in addition to DSB damage [8,40].
Therapeutic strategies for treatment of HPGS
FTIs were able to block the prenylation of prelamin A in progeroid cells [10,57] and reduce the farnesylated form of progerin and correct the nuclear dysmorphology [64,65]. However, FTI treatment did not reduce the frequency of DSBs nor the levels of γ H2AX protein and its nuclear foci [8,17,60]. Statins and aminobisphosphonates, common anti-hypercholesterolaemia drugs [66], appear more effective than FTIs in reducing phenotypic markers of laminopathy in model mice and HGPS cellular assays because alternative prenylation was found in mice treated with FTIs [50,66,67]. Future studies should benefit significantly from the availability of iPSCs (induced pluripotent stem cells) derived from HGPS fibroblasts [68-71]. For example, using iPSCs, it was shown recently that progerin levels were correlated with autophagic activity in HGPS cells [69], but inversely correlated with telomere length/telomerase activity [68,72]. It will be of interest to determine whether and how these factors moderate XPA distribution and integrity of DNA repair in HGPS cells.
HGPS and normal aging
Recent findings link normal aging to laminopathy diseases. Cells from healthy aged individuals also express low levels of progerin [19], resulting in similar phenotypes. For instance, the levels of γ H2AX, DNA DSBs and abnormal nuclei increase with an individual’s age. Also, as in HGPS, this DNA-damage accumulation is not caused by a genetic deficiency in DNA repair. Finally, like in HPGS, DSBs formed in normal human aging also are unrepairable, although genotoxin-induced DSBs in the same cells can be repaired efficiently [4]. Preliminary studies indicate that the level of chromatin-bound XPA is much higher in older HGPS cells. Interestingly, chromatin-bound XPA also was higher in the cells from normal older individuals than in cells from younger individuals (H. Tang, B. Hilton, P.R. Musich and Y. Zou, unpublished work). Thus HGPS or related laminopathies are an excellent model for the study of normal human aging.
Conclusions
Genome instability caused by cellular accumulation of DNA damage, particularly DNA DSBs, is a common cause of systemic aging and premature aging [3,4,73,74]. However, how and why DNA damage accumulates in healthy aging cells and laminopathy-based premature aging cells is far from clear. Recent studies have shed new light on the molecular basis of genome instability and the DDR in these cells. These findings indicate that DSBs accumulate in HGPS cells as well as normal aging cells which also express low levels of progerin. This DNA damage is unrepairable. As part of the DDR, ATM and ATR checkpoints are persistently activated in progeroid cells, leading to accelerated replicative arrest. Importantly, the inability to repair the DSBs is in part due to a ‘murder–suicide’ action mediated by the NER protein XPA which is unexpectedly trapped to DSB sites. This XPA sequestration blocks the access of DSBs to DSB-repair factors and also abolishes the NER activity of XPA. This mechanism also represents the first known case in which a protein from one DNA repair pathway disrupts another DNA repair pathway. Owing to the common involvement of progerin in both HGPS and normal aging, it will be of great interest to see whether the same mechanism is also true in normal aging. In addition, outstanding questions as to what is the cause for XPA mislocalization to the DSB sites and what is the epigenetic role of progerin in this process remain to be addressed in the future.
Acknowledgments
Funding
Our work is sponsored by National Cancer Institute (NCI) of the National Institutes of Health (NIH) [grant number CA86927 (to Y.Z.)], the National Institute on Aging (NIA) of the NIH [grant number: AG031503 (to Y.Z.)] and the Progeria Research Foundation
Abbreviations used
- 53BP1
p53-binding protein 1
- ATM
ataxia telangiectasia mutated
- ATR
ATM- and Rad3-related
- Chk
checkpoint kinase
- CPT
camptothecin
- DDR
DNA-damage response
- ds
double-stranded
- DSB
double-strand break
- FTI
farnesyltransferase inhibitor
- γ H2AX
phosphorylated histone H2AX
- HGPS
Hutchinson–Gilford progeria syndrome
- iPSC
induced pluripotent stem cell
- LMNA
lamin A/C
- NER
nucleotide excision repair
- PCNA
proliferating-cell nuclear antigen
- RFC
replication factor C
- siRNA
small interfering RNA
- ss
single-stranded
- XPA
xeroderma pigmentosum group A
- Zmpste24
zinc metalloprotease Ste24 homologue
References
- 1.Gorbunova V, Seluanov A, Mao Z, Hine C. Changes in DNA repair during aging. Nucleic Acids Res. 2007;35:7466–7474. doi: 10.1093/nar/gkm756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kirkwood TB. Understanding the odd science of aging. Cell. 2005;120:437–447. doi: 10.1016/j.cell.2005.01.027. [DOI] [PubMed] [Google Scholar]
- 3.Lombard DB, Chua KF, Mostoslavsky R, Franco S, Gostissa M, Alt FW. DNA repair, genome stability, and aging. Cell. 2005;120:497–512. doi: 10.1016/j.cell.2005.01.028. [DOI] [PubMed] [Google Scholar]
- 4.Sedelnikova OA, Horikawa I, Zimonjic DB, Popescu NC, Bonner WM, Barrett JC. Senescing human cells and ageing mice accumulate DNA lesions with unrepairable double-strand breaks. Nat. Cell Biol. 2004;6:168–170. doi: 10.1038/ncb1095. [DOI] [PubMed] [Google Scholar]
- 5.Ellis NA, German J. Molecular genetics of Bloom’s syndrome. Hum. Mol. Genet. 1996;5:1457–1463. doi: 10.1093/hmg/5.supplement_1.1457. [DOI] [PubMed] [Google Scholar]
- 6.Kudlow BA, Kennedy BK, Monnat RJ., Jr Werner and Hutchinson–Gilford progeria syndromes: mechanistic basis of human progeroid diseases. Nat. Rev. Mol. Cell Biol. 2007;8:394–404. doi: 10.1038/nrm2161. [DOI] [PubMed] [Google Scholar]
- 7.Goldman RD, Shumaker DK, Erdos MR, Eriksson M, Goldman AE, Gordon LB, Gruenbaum Y, Khuon S, Mendez M, Varga R, Collins FS. Accumulation of mutant lamin A causes progressive changes in nuclear architecture in Hutchinson–Gilford progeria syndrome. Proc. Natl. Acad. Sci. U.S.A. 2004;101:8963–8968. doi: 10.1073/pnas.0402943101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu B, Wang J, Chan KM, Tjia WM, Deng W, Guan X, Huang JD, Li KM, Chau PY, Chen DJ, et al. Genomic instability in laminopathy-based premature aging. Nat. Med. 2005;11:780–785. doi: 10.1038/nm1266. [DOI] [PubMed] [Google Scholar]
- 9.Misteli T, Scaffidi P. Genome instability in progeria: when repair gets old. Nat. Med. 2005;11:718–719. doi: 10.1038/nm0705-718. [DOI] [PubMed] [Google Scholar]
- 10.Pereira S, Bourgeois P, Navarro C, Esteves-Vieira V, Cau P, De Sandre-Giovannoli A, Lévy N. HGPS and related premature aging disorders: from genomic identification to the first therapeutic approaches. Mech. Ageing Dev. 2008;129:449–459. doi: 10.1016/j.mad.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 11.Smith ED, Kudlow BA, Frock RL, Kennedy BK. A-type nuclear lamins, progerias and other degenerative disorders. Mech. Ageing Dev. 2005;126:447–460. doi: 10.1016/j.mad.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 12.Wiesel N, Mattout A, Melcer S, Melamed-Book N, Herrmann H, Medalia O, Aebi U, Gruenbaum Y. Laminopathic mutations interfere with the assembly, localization, and dynamics of nuclear lamins. Proc. Natl. Acad. Sci. U.S.A. 2008;105:180–185. doi: 10.1073/pnas.0708974105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dechat T, Adam SA, Taimen P, Shimi T, Goldman RD. Nuclear Lamins. Cold Spring Harbor Perspect. Biol. 2010;2:1–22. doi: 10.1101/cshperspect.a000547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Scaffidi P, Gordon L, Misteli T. The cell nucleus and aging: tantalizing clues and hopeful promises. PLoS Biol. 2005;3:e395. doi: 10.1371/journal.pbio.0030395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gonzalez JM, Pla D, Perez-Sala D, Andres V. A-type lamins and Hutchinson–Gilford progeria syndrome: pathogenesis and therapy. Front. Biosci. (Schol. Ed.) 2011;3:1133–1146. doi: 10.2741/216. [DOI] [PubMed] [Google Scholar]
- 16.Liu Y, Rusinol A, Sinensky M, Wang Y, Zou Y. DNA damage responses in progeroid syndromes arise from defective maturation of prelamin A. J. Cell Sci. 2006;119:4644–4649. doi: 10.1242/jcs.03263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu Y, Wang Y, Rusinol AE, Sinensky MS, Liu J, Shell SM, Zou Y. Involvement of xeroderma pigmentosum group A (XPA) in progeria arising from defective maturation of prelamin A. FASEB J. 2008;22:603–611. doi: 10.1096/fj.07-8598com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Scaffidi P, Misteli T. Reversal of the cellular phenotype in the premature aging disease Hutchinson–Gilford progeria syndrome. Nat. Med. 2005;11:440–445. doi: 10.1038/nm1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Scaffidi P, Misteli T. Lamin A-dependent nuclear defects in human aging. Science. 2006;312:1059–1063. doi: 10.1126/science.1127168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dechat T, Pfleghaar K, Sengupta K, Shimi T, Shumaker DK, Solimando L, Goldman RD. Nuclear lamins: major factors in the structural organization and function of the nucleus and chromatin. Genes Dev. 2008;22:832–853. doi: 10.1101/gad.1652708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Houben F, Ramaekers FC, Snoeckx LH, Broers JL. Role of nuclear lamina–cytoskeleton interactions in the maintenance of cellular strength. Biochim. Biophys. Acta. 2007;1773:675–686. doi: 10.1016/j.bbamcr.2006.09.018. [DOI] [PubMed] [Google Scholar]
- 22.Hutchison CJ. Lamins: building blocks or regulators of gene expression? Nat. Rev. Mol. Cell Biol. 2002;3:848–858. doi: 10.1038/nrm950. [DOI] [PubMed] [Google Scholar]
- 23.Navarro CL, De Sandre-Giovannoli A, Bernard R, Boccaccio I, Boyer A, Genevieve D, Hadj-Rabia S, Gaudy-Marqueste C, Smitt HS, Vabres P, et al. Lamin A and ZMPSTE24 (FACE-1) defects cause nuclear disorganization and identify restrictive dermopathy as a lethal neonatal laminopathy. Hum. Mol. Genet. 2004;13:2493–2503. doi: 10.1093/hmg/ddh265. [DOI] [PubMed] [Google Scholar]
- 24.Goldberg M, Harel A, Brandeis M, Rechsteiner T, Richmond TJ, Weiss AM, Gruenbaum Y. The tail domain of lamin Dm0 binds histones H2A and H2B. Proc. Natl. Acad. Sci. U.S.A. 1999;96:2852–2857. doi: 10.1073/pnas.96.6.2852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schirmer EC. The epigenetics of nuclear envelope organization and disease. Mutat. Res. 2008;647:112–121. doi: 10.1016/j.mrfmmm.2008.07.012. [DOI] [PubMed] [Google Scholar]
- 26.Rusinol AE, Sinensky MS. Farnesylated lamins, progeroid syndromes and farnesyl transferase inhibitors. J. Cell Sci. 2006;119:3265–3272. doi: 10.1242/jcs.03156. [DOI] [PubMed] [Google Scholar]
- 27.Columbaro M, Mattioli E, Schena E, Capanni C, Cenni V, Lévy N, Navarro CL, Coco R, Squarzoni S, Camozzi D, et al. Prelamin A processing and functional effects in restrictive dermopathy. Cell Cycle. 2010;9:4766–4768. doi: 10.4161/cc.9.23.14210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Corrigan DP, Kuszczak D, Rusinol AE, Thewke DP, Hrycyna CA, Michaelis S, Sinensky MS. Prelamin A endoproteolytic processing in vitro by recombinant Zmpste24. Biochem. J. 2005;387:129–138. doi: 10.1042/BJ20041359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang SH, Chang SY, Ren S, Wang Y, Andres DA, Spielmann HP, Fong LG, Young SG. Absence of progeria-like disease phenotypes in knock-in mice expressing a non-farnesylated version of progerin. Hum. Mol. Genet. 2011;20:436–444. doi: 10.1093/hmg/ddq490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barbie DA, Kudlow BA, Frock R, Zhao J, Johnson BR, Dyson N, Harlow E, Kennedy BK. Nuclear reorganization of mammalian DNA synthesis prior to cell cycle exit. Mol. Cell. Biol. 2004;24:595–607. doi: 10.1128/MCB.24.2.595-607.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kennedy BK, Barbie DA, Classon M, Dyson N, Harlow E. Nuclear organization of DNA replication in primary mammalian cells. Genes Dev. 2000;14:2855–2868. doi: 10.1101/gad.842600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kelley JB, Datta S, Snow CJ, Chatterjee M, Ni L, Spencer A, Yang C-S, Cubenas-Potts C, Matunis MJ, Paschal BM. The defective nuclear lamina in Hutchinson–Gilford progeria syndrome disrupts the nucleocytoplasmic Ran gradient and inhibits nuclear localization of Ubc9. Mol. Cell. Biol. 2011;31:3378–3395. doi: 10.1128/MCB.05087-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kyng KJ, Bohr VA. Gene expression and DNA repair in progeroid syndromes and human aging. Ageing Res. Rev. 2005;4:579–602. doi: 10.1016/j.arr.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 34.Shumaker DK, Dechat T, Kohlmaier A, Adam SA, Bozovsky MR, Erdos MR, Eriksson M, Goldman AE, Khuon S, Collins FS, et al. Mutant nuclear lamin A leads to progressive alterations of epigenetic control in premature aging. Proc. Natl. Acad. Sci. U.S.A. 2006;103:8703–8708. doi: 10.1073/pnas.0602569103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Candelario J, Borrego S, Reddy S, Comai L. Accumulation of distinct prelamin A variants in human diploid fibroblasts differentially affects cell homeostasis. Exp. Cell Res. 2010;317:319–329. doi: 10.1016/j.yexcr.2010.10.014. [DOI] [PubMed] [Google Scholar]
- 36.O’Sullivan RJ, Kubicek S, Schreiber SL, Karlseder J. Reduced histone biosynthesis and chromatin changes arising from a damage signal at telomeres. Nat. Struct. Mol. Biol. 2010;17:1218–1225. doi: 10.1038/nsmb.1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Constantinescu D, Csoka AB, Navara CS, Schatten GP. Defective DSB repair correlates with abnormal nuclear morphology and is improved with FTI treatment in Hutchinson–Gilford progeria syndrome fibroblasts. Exp. Cell Res. 2010;316:2747–2759. doi: 10.1016/j.yexcr.2010.05.015. [DOI] [PubMed] [Google Scholar]
- 38.Cao K, Capell BC, Erdos MR, Djabali K, Collins FS. A lamin A protein isoform overexpressed in Hutchinson–Gilford progeria syndrome interferes with mitosis in progeria and normal cells. Proc. Natl. Acad. Sci. U.S.A. 2007;104:4949–4954. doi: 10.1073/pnas.0611640104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dechat T, Shimi T, Adam SA, Rusinol AE, Andres DA, Spielmann HP, Sinensky MS, Goldman, R Alterations in mitosis and cell cycle progression caused by a mutant lamin A known to accelerate human aging. Proc. Natl. Acad. Sci. U.S.A. 2007;104:4955–4960. doi: 10.1073/pnas.0700854104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Johnson MB. Honors Thesis in Biology. East Tennessee State University; Johnson City, TN, U.S.A.: 2011. The Effect of Ultraviolet Light on Cell Viability, DNA Damage and Repair in Hutchinson–Gilford Progeria Syndrome and BJ Fibroblasts. [Google Scholar]
- 41.Bonner WM, Redon CE, Dickey JS, Nakamura AJ, Sedelnikova OA, Solier S, Pommier Y. γ H2AX and cancer. Nat. Rev. Cancer. 2008;8:957–967. doi: 10.1038/nrc2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sedelnikova OA, Horikawa I, Redon C, Nakamura A, Zimonjic DB, Popescu NC, Bonner WM. Delayed kinetics of DNA double-strand break processing in normal and pathological aging. Aging Cell. 2008;7:89–100. doi: 10.1111/j.1474-9726.2007.00354.x. [DOI] [PubMed] [Google Scholar]
- 43.Redon C, Pilch D, Rogakou E, Sedelnikova O, Newrock K, Bonner W. Histone H2A variants H2AX and H2AZ. Curr. Opin. Genet. Dev. 2002;12:162–169. doi: 10.1016/s0959-437x(02)00282-4. [DOI] [PubMed] [Google Scholar]
- 44.Kinner A, Wu W, Staudt C, Iliakis G. γ -H2AX in recognition and signaling of DNA double-strand breaks in the context of chromatin. Nucleic Acids Res. 2008;36:5678–5694. doi: 10.1093/nar/gkn550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rogakou EP, Boon C, Redon C, Bonner WM. Megabase chromatin domains involved in DNA double-strand breaks in vivo. J. Cell Biol. 1999;146:905–916. doi: 10.1083/jcb.146.5.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lobrich M, Shibata A, Beucher A, Fisher A, Ensminger M, Goodarzi AA, Barton O, Jeggo PA. γ -H2AX foci analysis for monitoring DNA double-strand break repair: strengths, limitations and optimization. Cell Cycle. 2010;9:662–669. doi: 10.4161/cc.9.4.10764. [DOI] [PubMed] [Google Scholar]
- 47.Nam EA, Cortez D. ATR signalling: more than meeting at the fork. Biochem. J. 2011;436:527–536. doi: 10.1042/BJ20102162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ugalde AP, Ramsay AJ, de la Rosa J, Varela I, Marino G, Cadiñanos J, Lu J, Freije JM, López-Otín C. Aging and chronic DNA damage response activate a regulatory pathway involving miR-29 and p53. EMBO J. 2011;30:2219–2232. doi: 10.1038/emboj.2011.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ragnauth CD, Warren DT, Liu Y, McNair R, Tajsic T, Figg N, Shroff R, Skepper J, Shanahan CM. Prelamin A acts to accelerate smooth muscle cell senescence and is a novel biomarker of human vascular aging. Circulation. 2010;121:2200–2210. doi: 10.1161/CIRCULATIONAHA.109.902056. [DOI] [PubMed] [Google Scholar]
- 50.Varela I, Pereira S, Ugalde AP, Navarro CL, Suárez MF, Cau P, Cadiñanos J, Osorio FG, Foray N, Cobo J, et al. Combined treatment with statins and aminobisphosphonates extends longevity in a mouse model of human premature aging. Nat. Med. 2008;14:767–772. doi: 10.1038/nm1786. [DOI] [PubMed] [Google Scholar]
- 51.Lee JH, Paull TT. Direct activation of the ATM protein kinase by the Mre11/Rad50/Nbs1 complex. Science. 2004;304:93–96. doi: 10.1126/science.1091496. [DOI] [PubMed] [Google Scholar]
- 52.Lee JH, Paull TT. ATM activation by DNA double-strand breaks through the Mre11–Rad50–Nbs1 complex. Science. 2005;308:551–554. doi: 10.1126/science.1108297. [DOI] [PubMed] [Google Scholar]
- 53.Paull TT, Lee JH. The Mre11/Rad50/Nbs1 complex and its role as a DNA double-strand break sensor for ATM. Cell Cycle. 2005;4:737–740. doi: 10.4161/cc.4.6.1715. [DOI] [PubMed] [Google Scholar]
- 54.Sancar A, Lindsey-Boltz LA, Unsal-Kacmaz K, Linn S. Molecular mechanisms of mammalian DNA repair and the DNA damage checkpoints. Annu. Rev. Biochem. 2004;73:39–85. doi: 10.1146/annurev.biochem.73.011303.073723. [DOI] [PubMed] [Google Scholar]
- 55.Trenz K, Smith E, Smith S, Costanzo V. ATM and ATR promote Mre11 dependent restart of collapsed replication forks and prevent accumulation of DNA breaks. EMBO J. 2006;25:1764–1774. doi: 10.1038/sj.emboj.7601045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Guzder SN, Sommers CH, Prakash L, Prakash S. Complex formation with damage recognition protein Rad14 is essential for Saccharomyces cerevisiae Rad1–Rad10 nuclease to perform its function in nucleotide excision repair in vivo. Mol. Cell. Biol. 2006;26:1135–1141. doi: 10.1128/MCB.26.3.1135-1141.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liu Y, Liu Y, Yang Z, Utzat C, Wang G, Basu AK, Zou Y. Cooperative interaction of human XPA stabilizes and enhances specific binding of XPA to DNA damage. Biochemistry. 2005;44:7361–7368. doi: 10.1021/bi047598y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wu X, Shell SM, Yang Z, Zou Y. Phosphorylation of nucleotide excision repair factor xeroderma pigmentosum group A by ataxia telangiectasia mutated and Rad3-related-dependent checkpoint pathway promotes cell survival in response to UV irradiation. Cancer Res. 2006;66:2997–3005. doi: 10.1158/0008-5472.CAN-05-3403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yang Z, Roginskaya M, Colis LC, Basu AK, Shell SM, Liu Y, Musich PR, Harris CM, Harris TM, Zou Y. Specific and efficient binding of xeroderma pigmentosum complementation group A to double-strand/single-strand DNA junctions with 3′- and/or 5′-ssDNA branches. Biochemistry. 2006;45:15921–15930. doi: 10.1021/bi061626q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Liu Y. Ph.D. Thesis. East Tennessee State University; Johnson City, TN, U.S.A: 2006. 1, Structural and Functional Studies of Human Replication Protein A; 2, DNA Damage Responses and DNA Repair Defects in Laminopathy-Based Premature Aging. [Google Scholar]
- 61.Bomgarden RD, Lupardus PJ, Soni DV, Yee MC, Ford JM, Cimprich KA. Opposing effects of the UV lesion repair protein XPA and UV bypass polymerase η on ATR checkpoint signaling. EMBO J. 2006;25:2605–2614. doi: 10.1038/sj.emboj.7601123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mirkin EV, Mirkin SM. Replication fork stalling at natural impediments. Microbiol. Mol. Biol. Rev. 2007;71:13–35. doi: 10.1128/MMBR.00030-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Leonhardt H, Rahn HP, Weinzierl P, Sporbert A, Cremer T, Zink D, Cardoso MC. Dynamics of DNA replication factories in living cells. J. Cell Biol. 2000;149:271–280. doi: 10.1083/jcb.149.2.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Glynn MW, Glover TW. Incomplete processing of mutant lamin A in Hutchinson–Gilford progeria leads to nuclear abnormalities, which are reversed by farnesyltransferase inhibition. Hum. Mol. Genet. 2005;14:2959–2969. doi: 10.1093/hmg/ddi326. [DOI] [PubMed] [Google Scholar]
- 65.Young SG, Meta M, Yang SH, Fong LG. Prelamin A farnesylation and progeroid syndromes. J. Biol. Chem. 2006;281:39741–39745. doi: 10.1074/jbc.R600033200. [DOI] [PubMed] [Google Scholar]
- 66.Navarro CL, Cau P, Levy N. Molecular bases of progeroid syndromes. Hum. Mol. Genet. 2006;15:R151–R161. doi: 10.1093/hmg/ddl214. [DOI] [PubMed] [Google Scholar]
- 67.Meshorer E, Gruenbaum Y. Rejuvenating premature aging. Nat. Med. 2008;14:713–715. doi: 10.1038/nm0708-713. [DOI] [PubMed] [Google Scholar]
- 68.Cao K, Blair CD, Faddah DA, Kieckhaefer JE, Olive M, Erdos MR, Nabel EG, Collins FS. Progerin and telomere dysfunction collaborate to trigger cellular senescence in normal human fibroblasts. J. Clin. Invest. 2011;121:2833–2844. doi: 10.1172/JCI43578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cao K, Graziotto JJ, Blair CD, Mazzulli JR, Erdos MR, Krainc D, Collins FS. Rapamycin reverses cellular phenotypes and enhances mutant protein clearance in Hutchinson–Gilford progeria syndrome cells. Sci. Transl. Med. 2011;3:89ra58. doi: 10.1126/scitranslmed.3002346. [DOI] [PubMed] [Google Scholar]
- 70.Liu G-H, Barkho BZ, Ruiz S, Diep D, Qu J, Yang S-L, Panopoulos AD, Suzuki K, Kurian L, Walsh C, et al. Recapitulation of premature ageing with iPSCs from Hutchinson–Gilford progeria syndrome. Nature. 2011;472:221–225. doi: 10.1038/nature09879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhang J, Lian Q, Zhu G, Zhou F, Sui L, Tan C, Mutalif RA, Navasankari R, Zhang Y, Tse HF, et al. A human iPSC model of Hutchinson Gilford progeria reveals vascular smooth muscle and mesenchymal stem cell defects. Cell Stem Cell. 2011;8:31–45. doi: 10.1016/j.stem.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 72.Jaskelioff M, Muller FL, Paik JH, Thomas E, Jiang S, Adams AC, Sahin E, Kost-Alimova M, Protopopov A, Cadiñanos J, et al. Telomerase reactivation reverses tissue degeneration in aged telomerase-deficient mice. Nature. 2011;469:102–106. doi: 10.1038/nature09603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Musich PR, Zou Y. Genomic instability and DNA damage responses in progeria arising from defective maturation of prelamin A. Impact Aging. 2009;1:28–37. doi: 10.18632/aging.100012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gorbunova V, Seluanov A. Making ends meet in old age: DSB repair and aging. Mech. Ageing Dev. 2005;126:621–628. doi: 10.1016/j.mad.2005.02.008. [DOI] [PubMed] [Google Scholar]