Summary
Owing to the need of lifelong immunosuppression, solid-organ transplant recipients are known to have an increased risk of posttransplant malignancies including lung cancer. Posttransplant neoplastic transformation of donor-derived cells giving rise to hematopoietic malignancies, Kaposi sarcoma, and basal cell carcinoma in nongraft tissues has been reported. The goal of this study was to assess the cell origin (donor versus recipient derived) of posttransplant non–small cell lung carcinomas (NSCLCs) in kidney and heart transplant recipients. An institutional database search identified 2557 kidney and heart transplant recipients in 8 consecutive years. Among this cohort, 20 (0.8%) renal and 18 (0.7%) heart transplant recipients developed NSCLC. The study cohort comprised 6 of 38 NSCLCs arising in donor-recipient sex-mismatched transplant patients. The tumor cell origin was evaluated by chromogenic in situ hybridization with Y-chromosome probe on formalin-fixed, paraffin-embedded tissues. Y-chromosome was identified in 97% ± 1% (range from 92% to 99%) of all types of nucleated cells in male control tissues. In all 5 NSCLCs from male recipients of female donor organ, Y-chromosome was identified in 97% ± 2% (range from 92% to 100%) of tumor cells, statistically equivalent to normal control (P < .001). No Y-chromosome was identified in NSCLC cells from a female recipient of male kidney. These findings suggest a recipient derivation of NSCLC arising in kidney and heart transplant recipients. A combination of histologic evaluation and chromogenic in situ hybridization with Y-chromosome analysis allows reliable determination of tissue origin in sex-mismatched solid-organ transplant recipients and may aid in management of posttransplant malignancy in such cases.
Keywords: Post–solid-organ transplantation lung cancer, Chromogenic in situ hybridization for Y-chromosome
1. Introduction
Posttransplant malignancy (PTM) is one of the common causes of death among long-term survivors of kidney and heart transplantation [1,2]. The frequency of de novo malignant tumors in transplant recipients ranges from 4% to 18%, and is up to several hundred-fold greater than in the general population [3-5]. The cause of PTM is believed to be multifactorial, and chronic immunosuppression is thought to play an important role [4,6]. Long-term immunosuppression impairs the transplant recipient’s immunosurveillance for neoplastic cells and depresses host antiviral immune activity, leading to increased risk for viral-related malignancies [7]. In addition, with improvement of transplant recipient survival, the risk for development of PTM increases in aging patients. Moreover, expanded donor criteria to include older donors due to donor shortage also increase the risk of donor-derived malignancies [8].
The most frequent malignancies after transplantation are mucocutaneous carcinomas, lymphoproliferative disorders, and Kaposi sarcoma [4,6]. The study by de Perrot et al [9] has estimated that there is a 0.3% risk of developing lung cancer after solid-organ transplant. In terms of PTM location, the tumor could be present either in the graft itself, or in other organs outside the graft. The recognized pathways of PTM include (1) acquisition of an occult donor malignancy, (2) development of de novo malignancy, (3) recurrence of recipient’s previous malignancy, and (4) propagation of oncogenic viral infection leading to de novo malignancy [8,10]. Recent publications have suggested another (5) intriguing pathway where de novo malignancy is thought to develop from circulating donor cells in nongraft organs [11-14].
Determination of a tumor origin is important for clinical management. If a tumor is of donor origin, the recipient’s immune system sees it as “foreign” and may provide more robust immune response once the immunosuppression is reduced or stopped [8,15,16].
Because blood from the entire body is being filtered through the pulmonary capillary bed, we hypothesized that lung may serve as ideal organ for circulating donor cells engraftment and development of PTM. To test this hypothesis, we evaluated the tumor cell origin of non–small cell lung carcinomas (NSCLCs) in sex-mismatched heart and kidney transplant recipients using chromogenic in situ hybridization with Y-chromosome (Y-CISH) probe.
2. Materials and methods
2.1. Material selection
An institutional database search from January 2002 to September 2010 identified 2557 kidney or heart transplant cases. Among these, 38 patients presented with PTM. Six of 38 patients who underwent sex-mismatched solid-organ transplantation comprised the study cohort. The histologic slides including special stains were rereviewed to assure that the tumor classification meets the current criteria [17]. The tumor tissue, normal male lung, and kidney tissue (positive control) and normal female lung and kidney tissue (negative control) were evaluated for the presence of Y chromosome by Y-CISH.
2.2. Chromogenic in situ hybridization for Y-chromosome
Y-CISH was performed on formalin-fixed, paraffin-embedded tissue using 10-μm sections placed on positively charged slides. Slides with specimens were baked in a 6°C oven for 1 hour, cooled, and deparaffinized/rehydrated through xylenes and graded ethanol solutions to deionized (DI) water. Pretreatment was performed by heat-induced epitope retrieval, in which the slides were placed in Heat Pretreatment Solution EDTA (ZytoVision, Bremerhaven, Germany) for 15 minutes at greater than 95°C using a vegetable steamer and cooled for 20 minutes in solution. Slides were then washed in DI water 3 times, for 2 minutes each wash. Proteolysis was performed by applying 1 to 2 drops of the Pepsin Solution (ZytoVision CISH Kit) on each tissue section and incubating slides at 37°C, for 10 minutes, in a humidity chamber. After proteolysis, the slides were washed 3 times, for 2 minutes each wash, in DI water. Tissue sections were then dehydrated through graded alcohols and air-dried.
Once the slides were thoroughly dry, 10 to 20 μL of the ZytoDot CEN Y probe (ZytoVision) was applied to the tissue on each slide. The slides were subsequently coverslipped and sealed with rubber cement, to keep the tissue from drying out during the denaturing and hybridization process. Slides were then denatured at 95°C for 5 minutes and hybridized overnight at 37°C. After overnight hybridization, rubber cement was gently removed from each slide. Slides were washed at room temperature in Wash Buffer SSC (RTU; ZytoVision CISH Kit) for 5 minutes, to remove coverslips. After the short room temperature incubation, slides were then transferred to Wash Buffer SSC (ZytoVision CISH Kit) that had been preheated to 75°C to 80°C and washed for an additional 5 minutes. Slides were removed from the heated SSC solution and rinsed for 2 minutes, 3 times each, in DI water. Tissue sections were then incubated for 10 minutes in 3% H2O2 solution and washed for 2 minutes, 3 times each in phosphate-buffered saline/Tween (ZytoVision CISH Kit). Blocking solution (ZytoVision CISH Kit) was applied (1-2 drops/slide) and incubated for 10 minutes at room temperature. Blocking solution was then blotted off, but slides were not rinsed. A labeled polymer (ZytoVision CISH Kit) was used as detection system.
Counterstain was performed using Mayer hematoxylin solution (ZytoVision CISH Kit) for approximately 5 to 10 seconds, to avoid dark counterstaining that may obscure positive staining signals. The slides were then transferred to staining jar and washed for 2 minutes in tap water. Slides were then dehydrated through graded ethanol solutions and then incubated in 2 changes of xylene, 2 minutes each. Sections were then air-dried for approximately 15 minutes and coverslipped using alcoholic Mounting Solution (Zyto-Vision CISH Kit). Stained and coverslipped tissue sections were air-dried overnight and sent for evaluation by light microscopy.
At least 10 high-power fields within tumors and nonneoplastic lung and kidney tissues were evaluated for the presence of hybridization signals at ×500 magnification. The average percentage of positive cells between groups was assessed using equivalence test at an α a level of .05 (version 9.2; SAS, Cary, NC).
3. Results
3.1. Clinicopathological findings
Thirty-eight of 2557 (1.5%) kidney or heart transplant recipients developed NSCLC, with 20 (0.8%) cases arising in kidney and 18 (0.7%) cases arising in heart transplant recipients. The kidney transplant cohort included 16 men and 4 women (mean age, 63 years; range, 51-72 years); the heart transplant cohort included 17 men and 1 woman (mean age, 64 years; range, 55-73 years). Twenty-two (58%) of 38 cases were squamous cell carcinomas, 10 (26%) were poorly differentiated NSCLC without light microscopic or immunohistochemical features of glandular or squamous differentiation, and 6 (16%) were adenocarcinomas. Mean time from transplant to diagnosis of carcinoma was 93 months (range from 20 to 228 months). Six of the 38 cases were donor-recipient sex mismatched, including 5 kidney transplant cases and 1 heart transplant case (Table). These included 5 squamous cell carcinomas and 1 adenocarcinoma. The mean age of recipients at the time of NSCLC diagnosis was 66 years (range from 57 to 74 years); the mean time from transplant to NSCLC diagnosis was 127 months (range from 115 to 150 months). The tumor stage varied at presentation; however, eventually all patients died of lung cancer within 1 to 94 months (mean survival, 25.8 months).
Table.
Clinicopathological findings
| Age (y) | Recipient/Donor | Transplant | Time a (mo) | Diagnosis | Stage | DOD (mo) | Y-CISH |
|---|---|---|---|---|---|---|---|
| 57 | Male/Female | Kidney | 125 | SQC | IV | 5 | + |
| 71 | Female/Male | Kidney | 126 | SQC | IV | 40 | − |
| 76 | Male/Female | Kidney | 115 | SQC | Ia | 2 | + |
| 61 | Male/Female | Kidney | 117 | ADC | IIIa | 13 | + |
| 68 | Male/Female | Kidney | 131 | SQC | Ib | 94 | + |
| 74 | Male/Female | Heart | 150 | SQC | IIIa | 1 | + |
Abbreviations: DOD, dead of disease; SQC, squamous cell carcinoma; ADC, adenocarcinoma.
Time from transplantation to diagnosis of carcinoma.
3.2. Y-CISH in control tissues
All types of normal male kidney nucleated cells including tubular and glomerular epithelial cells, endothelial cells, stromal, and inflammatory cells (Fig. 1A) showed nuclear dot-like signals (96% ± 1%, range from 92% to 99%). All types of normal male lung nucleated cells (Fig. 1B) including pneumocytes, alveolar macrophages, endothelial, stromal, smooth muscle, and inflammatory cells showed nuclear dot-like signals (97% ± 1%, range from 94% to 99%). Noticeably, the hybridization signals differed from anthracotic dust commonly present in lung parenchyma and appeared as distinct similar in size and brown nuclear dots (Fig. 1A and B, arrows), whereas the anthracotic dust comprised variable in shape and size gray-black intracytoplasmic and interstitial particles (Fig. 1B and D, arrowheads). No hybridization signals were observed in normal female kidney (Fig. 1C) and lung (Fig. 1D).
Fig. 1.
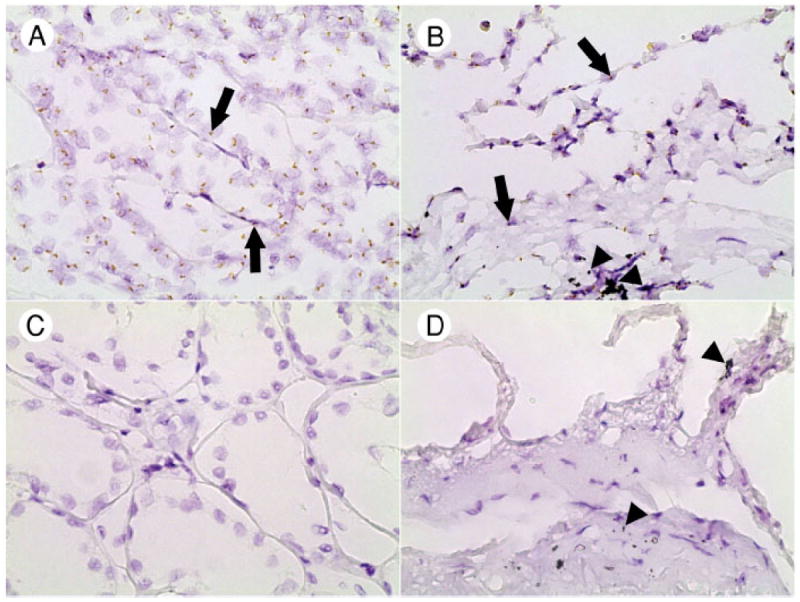
Sex-specific hybridization with Y-chromosome probe in normal kidney and lung tissues. Distinct nuclear hybridization signals are seen in kidney (A) and lung (B) parenchyma from a man; the hybridization signals are lacking in kidney (C) and lung (D) parenchyma from a woman. Note the difference in color between hybridization signals (brown, arrows) and anthracotic dust (black, arrowheads). Original magnification ×600.
3.3. Y-CISH in lung carcinomas
Six NSCLCs were evaluated for the presence of Y-chromosome (Table). Five NSCLCs from male recipient/female donor cases showed high levels of hybridization in both the tumor and adjacent nonneoplastic lung (Fig. 2), with 97% ± 2% (range from 92% to 100%) NSCLC cells showing the presence of Y-chromosome and 97% ± 1% (range from 94% to 99%) pulmonary parenchyma cells showing the presence of Y-chromosome (P < .001, equivalence test). One NSCLC from a female recipient/male donor case showed no Y-chromosome in either the tumor cells or the adjacent nonneoplastic lung parenchyma.
Fig. 2.
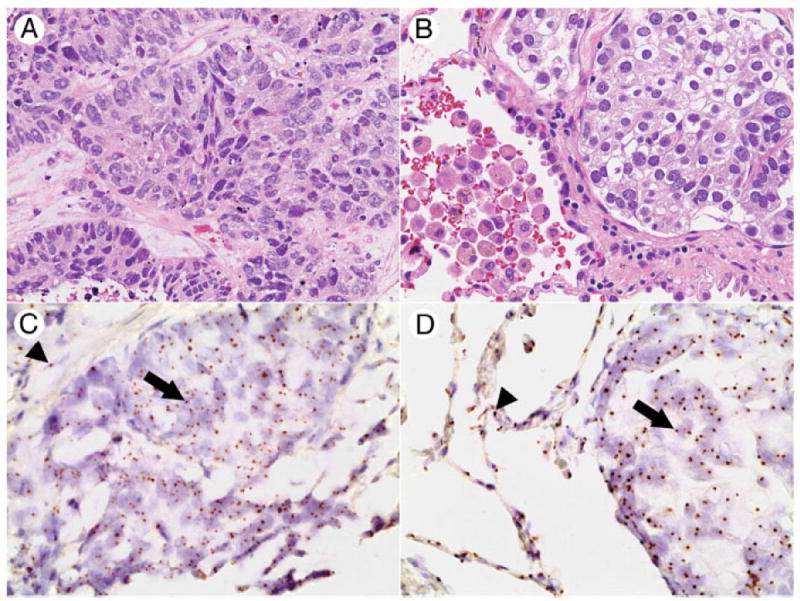
Y-chromosome status of NSCLCs and normal lung parenchyma in patients with sex-mismatched kidney transplant. Squamous cell carcinoma from case 3: hematoxylin and eosin (A) and corresponding section subjected to chromogenic in situ hybridization for Y chromosome (C). Adenocarcinoma from case 4: hematoxylin and eosin (B) and corresponding section subjected to chromogenic in situ hybridization for Y chromosome (D). Distinct nuclear hybridization signals indicating male sex are seen in neoplastic (arrows) and nonneoplastic (arrowheads) components. A and C, ×400 and ×600; B and D, ×400 and ×600.
4. Discussion
Previous studies have demonstrated that donor cells could relocate to nongraft tissues and give rise to PTM outside the graft. Aractingi et al [12] suggested that stem cells originating from a grafted kidney, in rare occasions, may give rise to skin carcinoma. Donor-derived bone marrow and blood stem cells were found to contribute to a recipient’s solid-organ cancers [13,14]. The concept of foreign donor cells coexisting with “self” recipient cells, known as mixed allogenic chimerism, may play a role in these scenarios. Mixed allogenic chimerism has been observed in pregnancy. The presence of gestation-derived male fetal cells in maternal organs is seen long after gestation [18-20]. In lung allograft recipients surviving more than 1 month after transplantation, donor cells have been identified in multiple nongraft organs, including recipient’s native lung, heart, lymph node, skin, liver, spleen, and kidney [11].
In this study, to establish the origin of PTM, we identified 6 cases of NSCLC in patients with sex-mismatched heart or kidney transplants and tested their tissues for the presence of Y-chromosome. We showed that 6 of 6 sex-mismatched posttransplant NSCLC cases had the concordant Y-chromosome status between the tumor and nonneoplastic lung, suggesting a recipient origin of their tumors.
The results of our assessment of NSCLC are different from what was previously reported in nonmelanoma skin cancer, where 48 cutaneous lesions developed in 14 women grafted with a male kidney were analyzed for the tumor cell origin [12]. Using quantitative polymerase chain reaction (PCR) for Y-chromosome, the authors showed that a significant proportion of cutaneous lesions contained male cells, whereas 1 basal cell carcinoma had male cells at high levels. Based on the results of immunohistochemical and fluorescent in situ hybridization analysis in selected cases, they proposed that stem cells originating from a grafted kidney may migrate to the skin, differentiate, or fuse as keratinocytes that could, rarely undergo cancer transformation. As in our set of NSCLC cases, we found no confirmation of that hypothesis; our findings suggest that in contrast to nonmelanoma skin cancer, where immunosuppression is a recognized risk factor for malignant transformation, it may present a lesser risk in NSCLC. Nevertheless, it is possible that NSCLCs do show a low level of mixed allogenic chimerisms beyond sensitivity of CISH or at a low frequency that would only be detected in a setting of much larger series.
One of 6 cases in the study cohort was a female recipient of male kidney who developed squamous cell carcinoma. Y-CISH testing showed complete lack of Y-chromosome signals, and thus, the tumor was interpreted as of a recipient origin. Because Y-chromosome loss is not uncommon in NSCLC [21,22], it lays ground for false-positive Y-CISH results where lack of Y-chromosome signals is a sign of cytogenetic alterations and not a female sex. Complete absence of Y-chromosome would be more in keeping with a female sex, as was seen in our case; however, additional studies may be needed to confirm the Y-CISH assessment in male to female transplants.
In light of the assay methodology, Y-CISH could only be used for assessment of sex-mismatched cases. Microsatellite molecular analysis with use of capillary electrophoresis and PCR-based DNA analysis may be used to study tumor cell origin in sex-matched transplant cases [23,24]. Other molecular techniques to establish donor versus recipient origin include quantitative real-time PCR for Y-chromosome [12].
Y-CISH testing to assess the tumor origin in sex-mismatched cases is a novel approach offering certain advantages over other methods. It permits use of formalin-fixed, paraffin-embedded archival tissue, shows good preservation of cell morphology for subsequent histologic evaluation, and generates an end-product that can be permanently archived [25,26]. In contrast, more commonly used fluorescent in situ hybridization is more difficult to correlate with cell morphology and has limited working time window due to photobleaching [27,28]. In addition, CISH is a relatively inexpensive technique that could be performed in most routine histopathology laboratories, and the slides can be read with regular light microscopes.
In the present study, a small percentage (~3%) of the normal male tissues lacked Y-chromosome signals in the planes of examination, probably due to nuclear truncation. Furthermore, because the procedure requires 10-μm-thick tissue sections, it is likely that the chromosome loci targeted by the hybridization probe are left out of the plane of sectioning, representing a technical artifact.
Among particles that may mimic CISH hybridization signals and potentially lead to false-positive results are anthracotic dust, ferruginous bodies, hemosiderin granules, and Hamazaki-Wesenberg bodies [29,30]. The main feature that helps to separate Y-CISH signals from the mimickers is their intranuclear location. Furthermore, the Y-CISH hybridization signals are small uniformed and brown as opposed to anthracotic dust, which is represented by black particulate matter. Hemosiderin granules show greater size and shape variability, whereas ferruginous bodies have a complex composition associated with particular or fibrous core. Hamazaki-Wesenberg bodies are larger and are commonly found in mediastinal lymph nodes, not lung parenchyma.
In conclusion, Y-CISH analysis suggests a recipient derivation of posttransplant NSCLC in our cohort of heart and kidney transplant patients. A combination of histologic evaluation and high levels of probe hybridization by Y-CISH allows reliable determination of tissue origin in sex-mismatched solid-organ transplant recipients and may aid in management of PTM in such cases.
Acknowledgments
The authors are thankful to Jennifer Lundy for assistance with database searches, Kristin Schwartz for help with in situ hybridization testing, and Shawn Scully for help with illustrative materials.
Footnotes
Competing interests: No conflict of interest to disclose.
The research was approved by Ohio State University Institutional Review Board.
References
- 1.Briggs JD. Causes of death after renal transplantation. Nephrol Dial Transplant. 2001;16:1545–9. doi: 10.1093/ndt/16.8.1545. [DOI] [PubMed] [Google Scholar]
- 2.Kirklin JK, Naftel DC, Bourge RC, et al. Evolving trends in risk profiles and causes of death after heart transplantation: a ten-year multi-institutional study. J Thorac Cardiovasc Surg. 2003;125:881–90. doi: 10.1067/mtc.2003.168. [DOI] [PubMed] [Google Scholar]
- 3.Penn I. Neoplastic complications of transplantation. Semin Respir Infect. 1993;8:233–9. [PubMed] [Google Scholar]
- 4.Penn I. Post-transplant malignancy: the role of immunosuppression. Drug Saf. 2000;23:101–13. doi: 10.2165/00002018-200023020-00002. [DOI] [PubMed] [Google Scholar]
- 5.Bellil Y, Edelman MJ. Bronchogenic carcinoma in solid organ transplant recipients. Curr Treat Options Oncol. 2006;7:77–81. doi: 10.1007/s11864-006-0034-5. [DOI] [PubMed] [Google Scholar]
- 6.Genebes C, Brouchet L, Kamar N, et al. Characteristics of thoracic malignancies that occur after solid-organ transplantation. J Thorac Oncol. 2010;5:1789–95. doi: 10.1097/JTO.0b013e3181f19226. [DOI] [PubMed] [Google Scholar]
- 7.Buell JF, Gross TG, Woodle ES. Malignancy after transplantation. Transplantation. 2005;80:S254–64. doi: 10.1097/01.tp.0000186382.81130.ba. [DOI] [PubMed] [Google Scholar]
- 8.Gandhi MJ, Strong DM. Donor derived malignancy following transplantation: a review. Cell Tissue Bank. 2007;8:267–86. doi: 10.1007/s10561-007-9036-1. [DOI] [PubMed] [Google Scholar]
- 9.de Perrot M, Wigle DA, Pierre AF, et al. Bronchogenic carcinoma after solid organ transplantation. Ann Thorac Surg. 2003;75:367–71. doi: 10.1016/s0003-4975(02)04379-5. [DOI] [PubMed] [Google Scholar]
- 10.Mathew J, Kratzke RA. Lung cancer and lung transplantation: a review. J Thorac Oncol. 2009;4:753–60. doi: 10.1097/JTO.0b013e31819afdd9. [DOI] [PubMed] [Google Scholar]
- 11.Kubit V, Sonmez-Alpan E, Zeevi A, et al. Mixed allogeneic chimerism in lung allograft recipients. Hum Pathol. 1994;25:408–12. doi: 10.1016/0046-8177(94)90151-1. [DOI] [PubMed] [Google Scholar]
- 12.Aractingi S, Kanitakis J, Euvrard S, et al. Skin carcinoma arising from donor cells in a kidney transplant recipient. Cancer Res. 2005;65:1755–60. doi: 10.1158/0008-5472.CAN-04-2783. [DOI] [PubMed] [Google Scholar]
- 13.Avital I, Moreira AL, Klimstra DS, et al. Donor-derived human bone marrow cells contribute to solid organ cancers developing after bone marrow transplantation. Stem Cells. 2007;25:2903–9. doi: 10.1634/stemcells.2007-0409. [DOI] [PubMed] [Google Scholar]
- 14.Munakata W, Nomoto J, Takahashi N, et al. Carcinoma of donor origin after allogeneic peripheral blood stem cell transplantation. Am J Surg Pathol. 2012;36:1376–84. doi: 10.1097/PAS.0b013e318261089c. [DOI] [PubMed] [Google Scholar]
- 15.Wilson RE, Hager EB, Hampers CL, Corson JM, Merrill JP, Murray JE. Immunologic rejection of human cancer transplanted with a renal allograft. N Engl J Med. 1968;278:479–83. doi: 10.1056/NEJM196802292780904. [DOI] [PubMed] [Google Scholar]
- 16.Desai R, Collett D, Watson CJ, Johnson P, Evans T, Neuberger J. Cancer transmission from organ donors—unavoidable but low risk. Transplantation. 2012;94:1200–7. doi: 10.1097/TP.0b013e318272df41. [DOI] [PubMed] [Google Scholar]
- 17.Travis WD, Brambilla E, Noguchi M, et al. International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society International Multidisciplinary Classification of Lung Adenocarcinoma. J Thorac Oncol. 2011;6:244–85. doi: 10.1097/JTO.0b013e318206a221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johnson KL, Samura O, Nelson JL, McDonnell MWM, Bianchi DW. Significant fetal cell microchimerism in a nontransfused woman with hepatitis C: evidence of long-term survival and expansion. Hepatology. 2002;36:1295–7. doi: 10.1053/jhep.2002.35622. [DOI] [PubMed] [Google Scholar]
- 19.Khosrotehrani K, Johnson KL, Cha DH, Salomon RN, Bianchi DW. Transfer of fetal cells with multilineage potential to maternal tissue. JAMA. 2004;292:75–80. doi: 10.1001/jama.292.1.75. [DOI] [PubMed] [Google Scholar]
- 20.O’Donoghue K, Chan J, de la Fuente J, et al. Microchimerism in female bone marrow and bone decades after fetal mesenchymal stem-cell trafficking in pregnancy. Lancet. 2004;364:179–82. doi: 10.1016/S0140-6736(04)16631-2. [DOI] [PubMed] [Google Scholar]
- 21.Berrieman HK, Ashman JN, Cowen ME, Greenman J, Lind MJ, Cawkwell L. Chromosomal analysis of non–small-cell lung cancer by multicolour fluorescent in situ hybridisation. Br J Cancer. 2004;90:900–5. doi: 10.1038/sj.bjc.6601569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Center R, Lukeis R, Vrazas V, Garson OM. Y chromosome loss and rearrangement in non–small-cell lung cancer. Int J Cancer. 1993;55:390–3. doi: 10.1002/ijc.2910550309. [DOI] [PubMed] [Google Scholar]
- 23.Bilal M, Eason JD, Das K, Sylvestre PB, Dean AG, Vanatta JM. Donor-derived metastatic melanoma in a liver transplant recipient established by DNA fingerprinting. Exp Clin Transplant. 2013;11:458–63. doi: 10.6002/ect.2012.0243. [DOI] [PubMed] [Google Scholar]
- 24.Gomez-Roman JJ, Del Valle CE, Zarrabeitia MT, et al. Recurrence of bronchioloalveolar carcinoma in donor lung after lung transplantation: microsatellite analysis demonstrates a recipient origin. Pathol Int. 2005;55:580–4. doi: 10.1111/j.1440-1827.2005.01872.x. [DOI] [PubMed] [Google Scholar]
- 25.Mayr D, Heim S, Weyrauch K, et al. Chromogenic in situ hybridization for Her-2/neu-oncogene in breast cancer: comparison of a new dual-colour chromogenic in situ hybridization with immunohistochemistry and fluorescence in situ hybridization. Histopathology. 2009;55:716–23. doi: 10.1111/j.1365-2559.2009.03427.x. [DOI] [PubMed] [Google Scholar]
- 26.Gupta D, Middleton LP, Whitaker MJ, Abrams J. Comparison of fluorescence and chromogenic in situ hybridization for detection of HER-2/neu oncogene in breast cancer. Am J Clin Pathol. 2003;119:381–7. doi: 10.1309/p40p2ead42pukdmg. [DOI] [PubMed] [Google Scholar]
- 27.Hruban RH, Long PP, Perlman EJ, et al. Fluorescence in situ hybridization for the Y-chromosome can be used to detect cells of recipient origin in allografted hearts following cardiac transplantation. Am J Pathol. 1993;142:975–80. [PMC free article] [PubMed] [Google Scholar]
- 28.Wollensak G, Green WR. Analysis of sex-mismatched human corneal transplants by fluorescence in situ hybridization of the sex-chromosomes. Exp Eye Res. 1999;68:341–6. doi: 10.1006/exer.1998.0611. [DOI] [PubMed] [Google Scholar]
- 29.Churg AM, Warnock ML. Asbestos and other ferruginous bodies: their formation and clinical significance. Am J Pathol. 1981;102:447–56. [PMC free article] [PubMed] [Google Scholar]
- 30.Tudway AJ. Yellow bodies in superficial and deep lymph nodes. J Clin Pathol. 1979;32:52–5. doi: 10.1136/jcp.32.1.52. [DOI] [PMC free article] [PubMed] [Google Scholar]


