Abstract
Background
Noninvasive diagnosis of giant cell arteritis (GCA) remains challenging, particularly with regard to evaluation of extracranial arterial disease.
Objectives
The objective of the study was to retrospectively review extracranial involvement in patients with GCA and/or polymyalgia rheumatica (PMR), evaluated with magnetic resonance imaging (MRI), especially 3-dimensional contrast-enhanced magnetic resonance angiography images of the aortic arch and its branches.
Methods
Clinical information, biopsy status, and MRI examinations of 28 patients with GCA/PMR were reviewed. Patient images were mixed randomly with 20 normal control images and were independently reviewed by 2 radiologists. Interobserver agreement for detection of arterial stenosis was determined by the k coefficient.
Results
Both readers described vascular alterations in keeping with extracranial GCA in 19 of 28 patients (67%) with good interobserver agreement (k = 0.73) and with even higher agreement on diagnosing nonocclusive versus occlusive disease (k = 1.00). The most common lesions were bilateral axillary stenosis or obstructions, observed by both readers in 8 patients (28%). Among the 19 patients with magnetic resonance angiography lesions in the subclavian/axillary arteries, 12 (75%) had biopsy-proven GCA, but only 5 (41%) of these patients had clinical features of large artery disease.
Conclusions
In our series review, MRI could provide accurate information on involvement of the aortic arch and its branches in extracranial GCA, depicting different degrees of stenosis. Our analysis also illustrates that occult large artery vasculitis should be considered in patients without biopsy-proven GCA, patients with classic GCA but without clinical signs of large artery disease, and in patients initially diagnosed as having PMR.
Keywords: large artery vasculitis, giant cell arteritis, MRI, MRA
Temporal arteritis, or giant cell arteritis (GCA), is a granulomatous vasculitis that manifests preferentially in medium and large arteries and involves characteristically the superficial cranial arteries.1 The pathogenesis of GCA is only partially understood, but important risk factors include advanced age, Northern European ancestry, and genetic risk determinants.1 Incidence rates reach 15 to 25 cases per 100,000 persons older than 50 years, and two thirds of the affected are women. Polymyalgia rheumatica (PMR) is a disorder characterized by signs of systemic inflammation with severe myalgia and stiffness of the muscles of the neck, shoulder, girdle, and pelvic girdle. Giant cell arteritis and PMR are related conditions that seem to represent different clinical expressions of the same underlying vascular disorder.2
Giant cell arteritis typically involves the extracranial branches of the aorta and spares intracranial vessels. Branches of the external and internal carotid arteries are particularly susceptible, and their involvement leads to the classic manifestations of blindness, headache, scalp tenderness, and jaw claudication. Involvement of the subclavian, axillary, and proximal brachial arteries leads to the aortic arch syndrome, characterized by arm claudication, stenotic bruits on auscultation, and absent or asymmetric pulses. Involvement of the vertebral arteries can cause stroke in the posterior cerebral circulation, vertigo, and dizziness. Aortitis is less frequent, but associated with serious potential consequences, including aortic dissection, aneurysmal dilatation, and rupture. Coronary, mesenteric, and lower-leg arteries are rarely affected.3,4
Noninvasive diagnosis of GCA remains challenging, particularly with regard to evaluation of extracranial arterial disease. The American College of Rheumatology presented a GCA classification system in 1990, which included temporal artery biopsy showing necrotizing arteritis.5 Recent studies investigated the role of magnetic resonance imaging (MRI) as a non-invasive tool for diagnosing GCA. High-resolution 3-T MRI may show signs of mural inflammation in the small temporal and occipital arteries and may be useful as a substitute for biopsy. However, not all patients will have temporal artery involvement, and patients with temporal arteritis and prior biopsies are no longer amenable to imaging analysis of the biopsied artery. There is also evidence to suggest that MRI may be useful for detecting GCA involvement in extracranial arteries.6–9
This study objective was to review clinical data, biopsy status, and MRI examinations in a series of patients with GCA or PMR, describing arterial lesions in keeping with extracranial involvement of GCA, mainly analyzing the 3-dimensional contrast-enhanced magnetic resonance angiography (3DCE-MRA) images of the thoracic aorta and major branches.
PATIENTS AND METHODS
Patients
This study was Health Insurance Plan Portability and Accountability compliant and approved by our institutional review board. Patients had been referred for an MRI examination from the rheumatology clinic as part of a clinical routine imaging protocol at our center. The study design was retrospective and based on approval for chart and imaging review. Retrospective search identified a total of 28 rheumatology patients who had an MRI. These patients had a diagnosis of either GCA or PMR, and all were referred for the following indications: the presence of signs or symptoms suggestive of aortic arch syndrome; to investigate the possibility of subclinical large artery involvement as the cause of unexplained persistence of elevated inflammatory markers in treated patients; and in PMR patients who failed to respond to habitual doses of corticosteroids. Clinical and laboratory information and biopsy results were obtained from review of the electronic medical records. The diagnosis of GCA was based on the 1990 American College of Rheumatology criteria,5 whereas PMR was mostly diagnosed based on the criteria proposed by Healey.10 Magnetic resonance imaging examinations of 20 normal subjects were also reviewed to serve as a control group. These were subjects included into a concurrent study for cardiac disease and who were well characterized as having no risk factors for atherosclerosis, cardiac disease, or any form of vasculitis. This control group was composed of individuals older than 50 years, and no sex preference.
Magnetic Resonance Angiography
All MRI studies were performed on a 1.5-T system equipped with cardiac performance gradients (Avanto [Siemens Medical Solutions, Erlangen, Germany] or Intera [Philips Medical Systems, Eindhoven, the Netherlands]) and using torso phased array surface coils. Gadolinium chelate (gadobenate dimeglumine [Gd-BOPTA], Multi-Hance; Bracco Imaging SpA, Milan, Italy) was administered intravenously at a dose of 0.1 mmol/kg, using a power injector at a flow rate of 2 mL/s, followed by a 15 mL saline flush at the same rate. Three-dimensional contrast-enhanced magnetic resonance angiography was performed in the coronal plane by using a fast spoiled 3D gradient echo sequence. Typical sequence parameters were as follows: repetition time/echo time/flip = 3.3 milliseconds/1.1 milliseconds/30 degrees and a 320 × 290 matrix. All studies were performed with breath-hold, centric-reordered K space acquisition, no interpolation, partial Fourier acquisition, and parallel acceleration, with an acquisition time of 18 seconds or less. A 350-mm field of view comprising the aortic arch, its proximal branches, and the axillary vessels was used.
Image Analysis
Images of the 28 patients and 20 controls were independently reviewed on a computer workstation by 2 radiologists who were blinded to all clinical information. The images were arranged by study accession numbers to achieve random ordering of presentation to the reviewers. Revision results were charted independently for later statistical evaluation. The image review focused mainly on the source 3DCE-MRA images, the background-subtracted source images and the maximum intensity projection (MIP) images. For qualitative analysis, the arterial tree was divided into the following 11 segments: aortic arch, brachiocephalic trunk, left prevertebral subclavian, right subclavian, left subclavian (postvertebral), right common carotid, left common carotid, right vertebral, left vertebral, right axillary, and left axillary. Degree of stenosis in each vascular segment was characterized by using a 5-point grading scale: grade 0 indicated a normal segment (no stenosis); grade 1, mild arterial disease (wall irregularity or G50% luminal narrowing); grade 2, moderate arterial stenosis (50%–69% luminal stenosis); grade 3, severe arterial stenosis (70%–99% luminal stenosis); and grade 4, complete arterial occlusion. When 2 or more areas of narrowing were present in the same segment, the most severe lesion was used for grading. Significant luminal alteration was considered when stenosis grade higher than 2 was identified.
Statistical Analysis
The degree of interobserver agreement for detection of arterial stenosis was determined by calculating the k coefficient (poor agreement, k = 0; slight agreement, k = 0.01–0.20; fair agreement, k = 0.21–0.40; moderate agreement, k = 0.41–0.60; good agreement, k = 0.61–0.80; and excellent agreement, k = 0.81–1.00).11
RESULTS
Clinical Data
Among the 28 patients included in the study (22 women, 6 men; age range, 36–84 years; average age, 69 years), 16 (57%) had biopsy-proven GCA (15 with temporal artery biopsy and 1 with aortic biopsy). Between the 12 patients without biopsy evidence of GCA, 4 (14% of all patients) were initially diagnosed as having only isolated PMR. The remaining 8 patients, without biopsy, initially presented with classic symptoms of GCA (blindness, headache, scalp tenderness, and jaw claudication) at the time of presumptive diagnosis and had a prompt response to corticosteroid therapy, with normalization of the acute inflammatory markers. Among the 16 patients with biopsy-proven GCA, 6 (37%) had clinical features suggestive of large artery disease, including arm claudication, stenotic bruits on auscultation, and absent or asymmetric forearm pulses.
Magnetic Resonance Angiography
Both readers found vascular lesions in 19 (67%) of 28 patients, with significant luminal alteration (stenosis grade 92) observed mainly in the subclavian and/or axillary arteries. Besides the characteristic distribution, these lesions were also described in the absence of diffuse arterial irregularity more characteristic of atherosclerotic disease; therefore, they were considered suspect for inflammatory involvement, in keeping with extracranial GCA. The overall interobserver agreement for detection of arterial disease was good (k = 0.73; 95% confidence interval, 0.67–0.79). The agreement between the 2 readers for determination of nonocclusive versus occlusive disease was excellent (k = 1.00).
Reader 1 identified alterations in 77 of 528 arterial segments observed in all patients and control subjects, which included 45 segments with wall irregularity or less than 50% luminal narrowing, 11 segments with 50% to 69% luminal stenosis, 16 segments with 70% to 99% luminal stenosis, and 5 segmental occlusions. Reader 2 identified alterations in 73 arterial segments, which included 34 segments with wall irregularity or less than 50% luminal narrowing, 23 segments with 50% to 69% luminal stenosis, 11 segments with 70% to 99% luminal stenosis, and 5 segmental occlusions (Table 1).
TABLE 1.
Distribution of Arterial Stenosis Detected Independently by 2 Readers With 3DCE-MRA of the Aortic Arch and Its Branches, in 48 Subjects (528 Segments), Including 28 Patients With GCA or PMR and 20 Control Subjects
| Reader 1
|
Reader 2
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Grade 0 | Grade 1 | Grade 2 | Grade 3 | Grade 4 | Grade 0 | Grade 1 | Grade 2 | Grade 3 | Grade 4 | |
| Aortic arch | 38 | 10 | 0 | 0 | 0 | 42 | 6 | 0 | 0 | 0 |
| Brachiocephalic trunk | 44 | 3 | 1 | 0 | 0 | 44 | 3 | 1 | 0 | 0 |
| Left prevertebral subclavian | 41 | 5 | 1 | 1 | 0 | 41 | 4 | 2 | 1 | 0 |
| Right subclavian | 37 | 7 | 2 | 2 | 0 | 38 | 5 | 3 | 2 | 0 |
| Left postvertebral subclavian | 36 | 8 | 2 | 2 | 0 | 37 | 4 | 5 | 2 | 0 |
| Right common carotid | 46 | 2 | 0 | 0 | 0 | 48 | 0 | 0 | 0 | 0 |
| Left common carotid | 46 | 2 | 0 | 0 | 0 | 48 | 0 | 0 | 0 | 0 |
| Right vertebral | 45 | 1 | 0 | 1 | 1 | 43 | 3 | 1 | 0 | 1 |
| Left vertebral | 45 | 1 | 1 | 1 | 0 | 44 | 1 | 2 | 1 | 0 |
| Right axillary | 37 | 4 | 3 | 3 | 1 | 33 | 6 | 6 | 2 | 1 |
| Left axillary | 36 | 2 | 1 | 6 | 3 | 37 | 2 | 3 | 3 | 3 |
| Total | 451 | 45 | 11 | 16 | 5 | 455 | 34 | 23 | 11 | 5 |
Degree of stenosis was characterized by using a 5-point grading scale: grade 0 = normal segment, grade 1 = mild arterial disease, grade 2 = moderate arterial stenosis (50%–69% luminal stenosis), grade 3 = severe arterial stenosis or subocclusion (70%–99% luminal stenosis), and a grade 4 = occlusion.
Data are number of segments. Analysis with the k coefficient revealed good interobserver agreement (k = 0.73; 95% confidence interval, 0.67–0.79).
The most common abnormality was bilateral axillary stenosis or obstruction (grades 2–4), observed by both readers in 8 patients (28%) (Fig. 1). The subclavian arteries were the second most involved vessels, with 9 lesions observed by reader 1 and 11 lesions identified by reader 2 (Fig. 2). Both readers described mild disease of the aorta in a few patients, some also having lesions in subclavian or axillary arteries. Carotidal stenosis or occlusion was not observed. Vertebral stenosis was identified in 5 patients by reader 2 and in 4 patients by reader 1, with a right vertebral occlusion described by both readers in the same patient.
FIGURE 1.
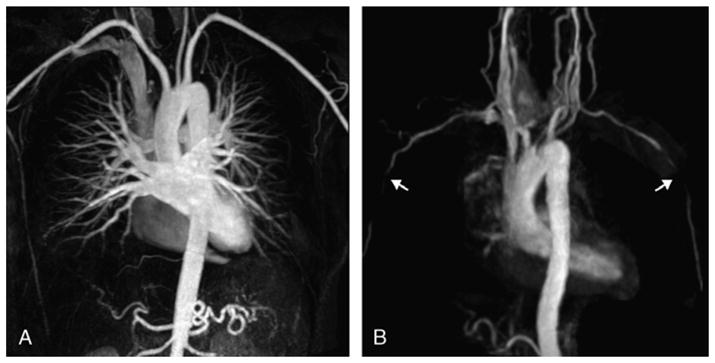
Maximum intensity projection reformations of the aortic arch and supra-aortic branches of a control subject (A) and a patient (B) with biopsy-proven GCA, obtained from contrast-enhanced MRA (fast spoiled 3D gradient echo sequence, breath held, coronal field of view, ECG gated, with 1.0 mmol/kg of gadolinium at a flow rate of 2 mL/s). This patient presented the most found lesions, bilateral stenosis in the axillary arteries (arrows in B). The control subject has normal vessels.
FIGURE 2.
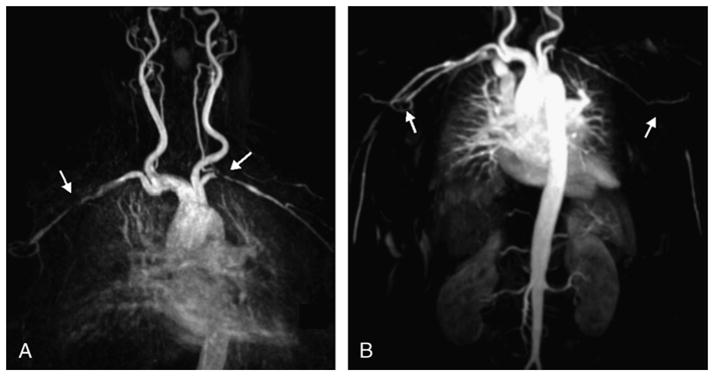
Image (A) in a 70-year-old woman with negative temporal biopsy and symptoms of PMR with aortic arch syndrome and (B) in a 79-year-old woman with biopsy-proven GCA. In both cases, there are lesions in the subclavian and axillary arteries, with bilateral subclavian stenosis in the first patient (arrows in A) and axillary bilateral obstruction in the second patient (arrows in B). Both images are MIP reconstructions from contrast-enhanced MRA (fast spoiled 3D gradient echo sequence, breath held, coronal field of view, ECG gated, with 1.0 mmol/kg of gadolinium at a flow rate of 2 mL/s).
Clinical Data and MRI Correlation
Among the 19 patients with MR images showing significant lesions in subclavian or axillary arteries, 12 had biopsy-proven GCA (75% of this group), and within these 12 cases, only 5 (41%) had clinical features of thoracic/upper limb large artery disease including bruits and or symptoms. Seven patients with MRI lesions in keeping with GCA were in the group of 12 patients without biopsy-proven disease or with negative/inconclusive biopsy (58% of this group), and within these 7 cases, 6 (85%) did not have clinical features of thoracic/upper limb large artery disease including no evidence of bruits.
Patients described with vertebral arteries stenosis also had biopsy-proven GCA, including 2 patients with no lesions in the subclavian or axillary arteries. Aortic wall irregularity was described in patients with and without positive biopsy and in no control cases. No control study was scored to have any vascular abnormality greater than grade 1 (wall irregularity or G50% luminal narrowing). In addition, no cases, either in the patient or control group, were found to have any features of atherosclerotic wall thickening or luminal narrowing involving the aortic arch vessels.
DISCUSSION
In this study, we reviewed MRI examinations of patients with GCA or PMR, especially the 3DCE-MRA used to outline the entire supra-aortic circulation and the axillary arteries, looking for arterial lesions in keeping with extracranial involvement of GCA. Magnetic resonance imaging showed the typical distribution of arterial stenosis and obstructions in patients with GCA in a higher incidence than previously expected. Lesions were described in patients with temporal biopsy-proven GCA without aortic arch syndrome, patients with negative or inconclusive biopsy, and patients with previous diagnosis of PMR only. Two readers retrospectively reviewed, independently and blinded to the diagnosis, images from 28 patients mixed with images from 20 control subjects, and both readers depicted arterial lesions with a good interobserver agreement.
Whereas the indication and limitations of MRI have been increasingly investigated in Takayasu arteritis, more limited data are available regarding the usefulness of vascular MRI studies in GCA. As in GCA, few reports have been devoted to exploration of the role of vascular MRI studies in PMR.6 It is well established that, like digital subtraction angiography, 3DCE-MRA provides detailed lumen information with high sensitivity and specificity for detection of stenosis and occlusions. Images are obtained noninvasively in a multitude of planes, avoiding the risks of arterial puncture, iodinated contrast load, and radiation exposure.12
Initial clinical studies showed signs of stenotic involvement of the upper-extremity arteries in 4% to 15% of patients with GCA.13,14 Angiographic studies described the typical pattern of bilateral stenosis or occlusions with a smooth, tapered appearance, in the subclavian, axillary, and proximal brachial arteries, with good correlation with the clinical symptoms. Because of the characteristic findings, the authors suggested that GCA should be considered in elderly women with occlusive disease of the upper extremities, even if typical features of GCA are lacking, and especially if elevated erythrocyte sedimentation rate is present.15–18
Extracranial involvement in GCA has been increasingly recognized, especially because of newer diagnostic procedures and higher attention to involvement of other vessels besides the temporal arteries, in search of alternative diagnostic tools to substitute the invasive biopsy.19 Temporal artery ultrasound seems to have a very high specificity, with the characteristic halo sign, but also have a very low sensitivity, so that it cannot be used as an effective substitute for biopsy nor a reliable screening test.20 Color Doppler sonography has also been used to show peripheral artery involvement (upper and lower limb) in GCA, and few studies likewise showed a higher-than-expected incidence of extracranial involvement of the disease.21,22 New imaging modalities, such as positron emission tomography and positron emission tomography/computed tomography, have also been used to identify vascular inflammatory diseases such as GCA, allowing the evaluation of disease activity and vessel morphology, as well as the localization of the inflammatory process, in a single examination. In GCA, it has also shown extracranial involvement in patients who had predominantly systemic symptoms.23,24
We found vascular lesions in keeping with extracranial GCA in 19 (68%) of 28 patients. No significant arterial lesion was described in the control group, used for the blinded imaging review. We found positive MRI findings in less expected patients, so that characteristic subclavian and axillary lesions were demonstrated in patients without positive biopsy, those without upper-extremity clinical manifestations, and patients with initial diagnosis of isolated PMR.
In our study, we mostly reviewed the imaging data for the presence of lumen alteration, especially evaluating MIP reconstructions obtained from the 3DCE-MRA. Recent advances in imaging technique, especially in 3-T systems, seem to make it possible to evaluate inflammatory activity of the vascular lesions and provide a useful guide for treatment of GCA and Takayasu arteritis, especially in the aorta and its proximal branches.25 Vessel wall thickness and edema, as well as mural contrast enhancement, are features of vasculitis that can be present before lumen changes are apparent angiographically, and MRI can also provide this complementary information.7,26 Imaging with 3-T MRI with high submillimeter spatial resolution can reveal the aortic arch, supra-aortic vessels, neck, and the entire head vascular involvement pattern within a single comprehensive MRI examination. These new techniques may be more sensitive and show an even higher incidence of extracranial GCA. Magnetic resonance imaging may also be used to monitor corticosteroid treatment effects and to indicate relapse of disease, but these observations need to be evaluated in larger patient studies.7–9,26,27 In our study, we did not intend to describe possible vessel wall disease characteristics; our MRA technique emphasized the evaluation only of the luminal morphology and not the changes within the wall of the vessels.
Another limitation of our study is that we could not compare MRI findings with other imaging modalities, such as digital subtraction angiography. However, as previously mentioned, previous angiographic studies in GCA showed the typical multiple stenotic areas and occlusions seen most frequently in the subclavian, axillary, and brachial arteries, very similar to the pattern of findings in our case series. Moreover, previous authors described that such arteriographic abnormalities are considered suggestive of GCA in a female patient older than 50 years.15,16 It is very uncharacteristic for atherosclerosis to affect the distal subclavian and axillary arteries, especially in the absence of aortic and proximal disease; none of our patients or control cases showed these changes of atherosclerotic disease.
It is estimated that about 10% of patients initially presenting with isolated PMR have vasculitis on histologic examination, requiring a change in diagnosis to GCA.1 Moreover, some investigators have suggested that GCA may take on a large-vessel form associated with PMR, with a low frequency of temporal artery involvement.4 In our case series, we found that MRI could demonstrate the typical GCA arterial lesions in patients originally diagnosed with isolated PMR and patients considered as having GCA but with negative temporal artery biopsy.
Giant cell arteritis is a heterogeneous disease with more than a single clinical presentation, including a variant in which vascular occlusive disease of the large upper-extremity arteries dominates the clinical presentation. This disease variant, characterized by symptoms referable to obstruction of large arteries (aortic arch syndrome), can be distinguished from classic temporal arteritis and can occur in the absence of positive findings in temporal artery biopsy. Large artery GCA shall not be representative of more aggressive disease and is not a late complication of cranial GCA.2 Therefore, the diagnosis of GCA has to be considered and pursued in patients lacking evidence of temporal artery involvement, and MRI, with the advantages of a noninvasive examination without iodinated contrast agents, can show the characteristic vascular occlusive involvement of the subclavian and axillary arteries, so that it might help in the diagnosis of GCA in a timely fashion. Our retrospective analysis illustrated that occult large artery vasculitis should be considered in patients without biopsy-proven GCA, patients with classic GCA in apparent clinical remission and unexplained persistence of raised acute-phase reactants, and in patients presenting only a syndrome of systemic inflammation, initially diagnosed as having PMR.
Footnotes
The authors declare no conflict of interest.
References
- 1.Weyand CM, Goronzy JJ. Medium- and large-vessel vasculitis. N Engl J Med. 2003;349:160–169. doi: 10.1056/NEJMra022694. [DOI] [PubMed] [Google Scholar]
- 2.Weyand CM, Goronzy JJ. Giant-cell arteritis and polymyalgia rheumatica. Ann Intern Med. 2003;139:505–515. doi: 10.7326/0003-4819-139-6-200309160-00015. [DOI] [PubMed] [Google Scholar]
- 3.Evans JM, O’Fallon WM, Hunder GG. Increased incidence of aortic aneurysm and dissection in giant cell (temporal) arteritis. A population-based study. Ann Intern Med. 1995;122:502–507. doi: 10.7326/0003-4819-122-7-199504010-00004. [DOI] [PubMed] [Google Scholar]
- 4.Brack A, Martinez-Taboada V, Stanson A, et al. Disease pattern in cranial and large-vessel giant cell arteritis. Arthritis Rheum. 1999;42:311–317. doi: 10.1002/1529-0131(199902)42:2<311::AID-ANR14>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 5.Hunder GG, Bloch DA, Michel BA, et al. The American College of Rheumatology 1990 criteria for the classification of giant cell arteritis. Arthritis Rheum. 1990;33:1122–1128. doi: 10.1002/art.1780330810. [DOI] [PubMed] [Google Scholar]
- 6.Narvaez J, Narvaez JA, Nolla JM, et al. Giant cell arteritis and polymyalgia rheumatica: usefulness of vascular magnetic resonance imaging studies in the diagnosis of aortitis. Rheumatology (Oxford) 2005;44:479–483. doi: 10.1093/rheumatology/keh513. [DOI] [PubMed] [Google Scholar]
- 7.Markl M, Uhl M, Wieben O, et al. High resolution 3T MRI for the assessment of cervical and superficial cranial arteries in giant cell arteritis. J Magn Reson Imaging. 2006;24:423–427. doi: 10.1002/jmri.20639. [DOI] [PubMed] [Google Scholar]
- 8.Bley TA, Uhl M, Markl M, et al. MRI in giant cell (temporal) arteritis. Rofo. 2007;179:703–711. doi: 10.1055/s-2007-963123. [DOI] [PubMed] [Google Scholar]
- 9.Bley TA, Uhl M, Venhoff N, et al. 3-T MRI reveals cranial and thoracic inflammatory changes in giant cell arteritis. Clin Rheumatol. 2007;26:448–450. doi: 10.1007/s10067-005-0160-7. [DOI] [PubMed] [Google Scholar]
- 10.Healey LA. Long-term follow-up of polymyalgia rheumatica: evidence for synovitis. Semin Arthritis Rheum. 1984;13:322–328. doi: 10.1016/0049-0172(84)90012-x. [DOI] [PubMed] [Google Scholar]
- 11.Landis JR, Koch GG. An application of hierarchical kappa-type statistics in the assessment of majority agreement among multiple observers. Biometrics. 1977;33:363–374. [PubMed] [Google Scholar]
- 12.Meaney JFM. MR angiography of peripheral arteries: lower extremities. In: Schneider G, Prince MR, Meaney JFM, et al., editors. Magnetic Resonance Angiography Techniques, Indications and Practical Applications. 1. Milan, Italy: Springer-Verlag; 2005. pp. 269–284. [Google Scholar]
- 13.Klein RG, Hunder GG, Stanson AW, et al. Large artery involvement in giant cell (temporal) arteritis. Ann Intern Med. 1975;83:806–812. doi: 10.7326/0003-4819-83-6-806. [DOI] [PubMed] [Google Scholar]
- 14.Leu HJ, Garzoli G. Extracranial giant cell arteritis. Pathol Microbiol (Basel) 1975;43:187–191. doi: 10.1159/000162818. [DOI] [PubMed] [Google Scholar]
- 15.Stanson AW, Klein RG, Hunder GG. Extracranial angiographic findings in giant cell (temporal) arteritis. AJR Am J Roentgenol. 1976;127:957–963. doi: 10.2214/ajr.127.6.957. [DOI] [PubMed] [Google Scholar]
- 16.Stanson AW. Imaging findings in extracranial (giant cell) temporal arteritis. Clin Exp Rheumatol. 2000;18:S43–S48. [PubMed] [Google Scholar]
- 17.Ninet JP, Bachet P, Dumontet CM, et al. Subclavian and axillary involvement in temporal arteritis and polymyalgia rheumatica. Am J Med. 1990;88:13–20. doi: 10.1016/0002-9343(90)90121-s. [DOI] [PubMed] [Google Scholar]
- 18.Lambert M, Weber A, Boland B, et al. Large vessel vasculitis without temporal artery involvement: isolated form of giant cell arteritis? Clin Rheumatol. 1996;15:174–180. doi: 10.1007/BF02230336. [DOI] [PubMed] [Google Scholar]
- 19.Both M, Aries PM, Muller-Hulsbeck S, et al. Balloon angioplasty of arteries of the upper extremities in patients with extracranial giant-cell arteritis. Ann Rheum Dis. 2006;65:1124–1130. doi: 10.1136/ard.2005.048470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maldini C, Dépinay-Dhellemmes C, Tra TT, et al. Limited value of temporal artery ultrasonography examinations for diagnosis of giant cell arteritis: analysis of 77 subjects. J Rheumatol. 2010;37:2326–2330. doi: 10.3899/jrheum.100353. [DOI] [PubMed] [Google Scholar]
- 21.Schmidt WA, Natusch A, Moller DE, et al. Involvement of peripheral arteries in giant cell arteritis: a color Doppler sonography study. Clin Exp Rheumatol. 2002;20:309–318. [PubMed] [Google Scholar]
- 22.Aschwanden M, Kesten F, Stern M, et al. Vascular involvement in patients with giant cell arteritis determined by duplex sonography of 2 × 11 arterial regions. Ann Rheum Dis. 2010;69:1356–1359. doi: 10.1136/ard.2009.122135. [DOI] [PubMed] [Google Scholar]
- 23.Rehak Z, Fojtik Z, Stanicek J, et al. 18F-FDG PET in the diagnosis of large vessel vasculitis. Vnitr Lek. 2006;52:1037–1044. [PubMed] [Google Scholar]
- 24.Henes JC, Müller M, Krieger J, et al. [18F] FDG-PET/CT as a new and sensitive imaging method for the diagnosis of large vessel vasculitis. Clin Exp Rheumatol. 2008;26(3 suppl 49):S47–S52. [PubMed] [Google Scholar]
- 25.Seko Y. Giant cell and Takayasu arteritis. Curr Opin Rheumatol. 2007;19:39–43. doi: 10.1097/BOR.0b013e3280119866. [DOI] [PubMed] [Google Scholar]
- 26.Geiger J, Bley T, Uhl M, et al. Diagnostic value of T2-weighted imaging for the detection of superficial cranial artery inflammation in giant cell arteritis. J Magn Reson Imaging. 2010;31:470–474. doi: 10.1002/jmri.22047. [DOI] [PubMed] [Google Scholar]
- 27.Takekawa H, Daimon Y, Takashima R, et al. Giant cell arteritis associated with lesion of the internal carotid artery: assessment of response to steroid therapy by magnetic resonance angiography. Intern Med. 2008;47:1285–1286. doi: 10.2169/internalmedicine.47.1137. [DOI] [PubMed] [Google Scholar]


