Abstract
Introduction
In patients with refractory ACTH-dependent Cushing’s syndrome (CS), we evaluated steroidogenesis inhibition (SI) and bilateral adrenalectomy (BA) to predict which patients might benefit most from each treatment modality.
Methods
Clinical data from patients treated 1970-2012 were retrospectively reviewed by treatment group (SI or SI+BA). Validated severity scales were used to calculate metabolic (M) score (hypokalemia, hyperglycemia, hypertension, proximal muscle weakness) and adverse events (AE) score (thrombosis, fracture, infection).
Results
65 patients (16 pituitary, 49 ectopic) were treated with SI+BA (n=21,32%) or SI alone (n=44,68%). Presenting M scores and source of ACTH excess (ectopic vs. pituitary) were similar. Both groups improved metabolically after treatment. 39% of AEs in the SI+BA group occurred within 12 months of presentation. 24(55%) SI patients died (median survival 24.0 months); steroid excess contributed to 71%. Six SI+BA patients died (29%), including all 3 patients with recurrent CS after BA. Minor perioperative complications occurred in 7 patients (33%).
Conclusions
Post-treatment M and AE scores improved for all patients and 70% of AEs occurred in SI+BA patients within 12 months of presentation, emphasizing the importance of early surgical intervention. These data argue for the safety and efficacy of early BA in selected patients with uncontrollable CS.
Introduction
Cushing’s syndrome can be caused by an ACTH producing pituitary or ectopic tumor (ACTH-dependent) or an adrenal adenoma/carcinoma (ACTH-independent). Classic metabolic disturbances include hypertension, diabetes mellitus, hypokalemia, alkaosis, bone loss, fractures, and psychiatric problems. Morbidity and mortality most commonly result from infection, myocardial infarction, and venous thromboembolism. (1)
First-line treatment should address the primary source of ACTH secretion whenever possible. However, in ACTH-dependent Cushing’s syndrome, the source of ACTH overproduction may not be controllable in cases of occult, unresectable, or metastatic tumors, or persistent/recurrent pituitary Cushing’s syndrome despite multiple targeted interventions. Medical steroidogenesis inhibition (SI) is generally adjunctive and can cause significant side effects including nausea, vomiting, elevated liver enzymes, dizziness, and hirsutism. SI normalizes cortisol levels in only half of patients, and relieves symptoms of cortisol excess in just one-third.(2)
Bilateral adrenalectomy (BA) can eliminate the end-organ effects of ACTH hypersecretion, but requires lifetime, daily hormone replacement and careful dose monitoring to avoid life-threatening adrenal insufficiency. BA can be used in addition to SI therapy (SI+BA) to treat ACTH-dependent Cushing's syndrome, though specific criteria do not exist to guide use of this modality. (3-6)
This observational study reviewed the treatment of patients with uncontrollable ACTH-dependent Cushing’s syndrome from an ectopic or pituitary source to characterize the changes in metabolic profiles and occurrence of adverse events after SI and SI+BA. We aimed to evaluate the use of each modality in our patient population to identify predictors of which patients might benefit from each intervention.
Methods
We conducted an institutional review board-approved retrospective review of patients with refractory ACTH-dependent Cushing’s syndrome from an ectopic or pituitary source who had primary medical and surgical treatment at MD Anderson Cancer Center from 9/1970-9/2012. Many of these patients were included in a previous report from our institution.(7) Patients with an occult primary were analyzed with the ectopic group.
The Common Terminology Criteria for Adverse Events (CTCAE) Version 4 (Table 1) was used to calculate a metabolic score (hypokalemia, hyperglycemia, hypertension and proximal muscle weakness) and an adverse events score (thrombosis, fracture and infection).(8) A normalized score was derived from adding the grades of event a patient experienced in each category (0-3 or 0-4), divided by the total possible points (based on available data), multiplied by 100. For example, a patient with potassium 2.7 requiring hospitalization (grade 3), glucose 170 mg/dL (grade 1), blood pressure 110/70 (grade 0), and no proximal muscle weakness (grade 0) would have a normalized metabolic score of 4/15 x 100 = 26.7. Grade 5 was excluded from the scoring as this category represents death and would have biased the results in the SI group.
Table 1.
Common Terminology Criteria for Adverse Events (CTCAE) Version 4, categories used for metabolic and adverse events scores.
| 1 | 2 | 3 | 4 | |
|---|---|---|---|---|
| METABOLIC SCORE | ||||
| Hypokalemia | <3-3.5 mEq/L | <3-3.5 mEq/L; symptomatic; intervention indicated |
<3.0-2.5 mEq/L; hospitalization indicated |
<2.5 mEq/L; life- threatening consequences |
| Hyperglycemia | 150-200 mg/dL | >200-250 mg/dL | >250-500 mg/dL; hospitalization indicated |
Glucose >500 mg/dL; life-threatening consequences |
| Hypertension | SBP 120-139 mmHg or DBP 80- 89 mmHg |
SBP 140-159 or DBP 90-99; medical intervention indicated |
SBP ≥ 160 or DBP ≥ 100 |
Life threatening consequences |
| Generalized muscle weakness |
Symptomatic; weakness perceived by patient but not evident on physical exam |
Symptomatic; weakness on physical exam limiting instrumental ADL |
Disabling weakness limiting self-care |
Not applicable |
| ADVERSE EVENTS SCORE | ||||
| Fracture | Asymptomatic; clinical or diagnostic observations only |
Symptomatic but non-displaced; immobilization indicated |
Severe symptoms; displaced or open wound with bone exposure; operative intervention indicated |
Life-threatening consequences; urgent intervention indicated |
| Thromboembolic event |
Superficial venous thrombosis |
Uncomplicated DVT, medical intervention indicated |
Thrombosis (e.g., uncomplicated PE, non-embolic cardiac mural thrombus), medical intervention indicated |
Life-threatening (e.g., PE, stroke, arterial insufficiency); hemodynamic or neurologic instability; urgent intervention indicated |
| Infection | Asymptomatic or mild symptoms; clinical or diagnostic observations only |
Local or non- invasive intervention indicated (e.g., oral antibiotics) |
Medically significant requiring hospitalization, radiologic, or operative intervention |
Life-threatening consequences; urgent intervention indicated |
Abbreviations: DVT = deep vein thrombosis, PE = pulmonary embolus, ADL = activities of daily living, SBP = systolic blood pressure, DBP = diastolic blood pressure
We evaluated data from 2 time points in SI patients (at initial presentation to MD Anderson Cancer Center/start of SI therapy and after SI therapy), and 3 time points in SI+BA patients (at initial presentation to MD Anderson Cancer Center/start of SI therapy, after SI therapy, and after BA).
Fracture and thrombosis were diagnosed via imaging studies. Bloodstream or urinary tract infection was defined by positive cultures. Respiratory infection was defined by positive cultures or radiographic evidence. A score for proximal muscle weakness was assigned retrospectively based on clinical documentation. Resolution of Cushing’s syndrome was defined as undetectable cortisol with concomitant clinical improvement in Cushing’s-related metabolic manifestations.
Cause of death was ascertained by the death note or autopsy report. Deaths classified as a direct consequence of Cushing’s syndrome occurred in patients who had persistent hypercortisolism or non-resolution of clinical sequelae of Cushing’s syndrome and included sepsis, electrolyte derangements, or pulmonary embolus (PE). Patients with an overwhelming tumor burden whose malignancy may have contributed to infection (e.g., post-obstructive pneumonia in small cell lung cancer) or PE/thrombosis were classified as a mixed cause of death (Cushing’s syndrome and cancer). Patients who died at home where no autopsy was performed were classified as unknown.
We included any patient with this diagnosis for whom the majority of analyzed data were available. We excluded any patient with this diagnosis who had primary medical treatment, BA, or long-term follow up outside our institution.
Descriptive statistics were used for demographic, clinical characteristics, and outcomes. Categorical measures were summarized using frequencies and percentages and assessed using Fisher’s exact tests. Continuous measures were summarized using means, medians, and ranges and were assessed using Wilcoxon signed rank tests (within group) and Wilcoxon rank sum tests (between groups). Overall survival was calculated from the date of presentation. Patients alive at the last follow-up date were censored. For patients receiving SI+BA, time to specific adverse events was computed from date of presentation to date of adverse event. The Kaplan-Meier method was used to estimate overall survival and time to specific adverse events. A two-sided significance level of 0.05 was used for all statistical tests. All statistical analyses were performed using SAS 9.3 for Windows (Copyright © 2011 by SAS Institute Inc., Cary, NC).
Results
Sixty-five patients were included; 49 patients (75%) had Cushing’s syndrome from an ectopic ACTH source (ectopic Cushing’s syndrome) and 16 (25%) from a pituitary source (pituitary Cushing’s syndrome). All patients were prescribed steroidogenesis inhibitors (SI) – ketoconazole and/or metyrapone. Forty-four patients (68%) were treated with SI alone while 21 patients (32%) were also treated with bilateral adrenalectomy (SI+BA). Median follow up time from initiation of SI therapy was 7.6 years.
Patients presented to MD Anderson a median of 2 (mean 19) months after diagnosis. However, patients with pituitary Cushing’s syndrome presented at a median of 30 (mean 49) months after diagnosis while patients with ectopic Cushing’s syndrome presented at a median of 2 (mean 10) months after diagnosis.
Compared to patients with ectopic Cushing’s syndrome, those with pituitary Cushing’s syndrome were younger (median 38.2 vs. 50.1 years old, p=0.007) and did not often die (overall survival 93.8% for pituitary Cushing’s syndrome and 40.8% for ectopic Cushing’s syndrome, p<0.001) (Table 2). While there was not an association between ectopic and pituitary Cushing’s syndrome and type of treatment received (p=0.357), there was a significant difference in tumor type among ectopic Cushing’s syndrome patients treated with SI only versus SI+BA (p=0.003, Table 3). None of the patients with small cell lung cancer (n=11) or medullary thyroid cancer (n=5) whereas all of the patients with thymic neuroendocrine tumors (n=3) underwent BA.
Table 2.
Patient characteristics and outcomes by diagnosis (pituitary versus ectopic Cushing’s syndrome (CS)).
| Characteristics | Diagnosis | p-value | |
|---|---|---|---|
| Ectopic CS (n=49) |
Pituitary CS (n=16) |
||
| Gender, n (%) | |||
| Male | 18 (36.7) | 4 (25.0) | 0.546 |
| Female | 31 (63.3) | 12 (75.0) | |
| Age at MD Anderson Cancer Center Presentation (years), median (range) |
50.1 (11.7 – 77.3) | 38.2 (8.9 – 53.3) | 0.007 |
| Treatment, n (%) | |||
| SI+BA | 14 (28.6) | 7 (43.8) | 0.357 |
| SI only | 35 (71.4) | 9 (56.3) | |
| Overall Survival, n (%) | |||
| Dead | 29 (59.2) | 1 (6.3) | <0.001 |
| Alive | 20 (40.8) | 15 (93.8) | |
| Time on SI Therapy before BA (months), median (range) – (SI+BA patients only) |
7.1 (0.8 – 26.4) | 13.5 (1.6 – 46.4) | 0.410 |
Abbreviations: SI = steroidogenesis inhibition, BA = bilateral adrenalectomy
Table 3.
Demographic and primary disease information for 65 patients with uncontrolled Cushing’s syndrome treated 1970-2012 with steroidogenesis inhibitors (SI) or bilateral adrenalectomy (BA).
| Characteristics | All patients (n=65) | SI+BA (n=21) |
SI only (n=44) |
p-value |
|---|---|---|---|---|
| Gender, n (%) | ||||
| Male | 22 (33.8) | 4 (19.0) | 18 (40.9) | 0.099 |
| Female | 43 (66.2) | 17 (81.0) | 26 (59.1) | |
| Age at presentation (years), median (range) |
43.6 (8.9-77.3) | 39.8 (11.7-1.3) | 49.8 (8.9-77.3) | 0.082 |
| Cushing’s syndrome source, n (%) | ||||
| Ectopic | 49 (75.4) | 14 (66.7) | 35 (79.5) | 0.357 |
| Pituitary | 16 (24.6) | 7 (33.3) | 9 (20.5) | |
| Source of ACTH, n (%) | ||||
| Unresectable/locally invasive |
31 (47.7) | 9 (42.9) | 22 (50.0) | 0.24 |
| Metastatic disease | 23 (35.4) | 6 (28.6) | 17 (38.6) | |
| Unknown source | 11 (16.9) | 6 (28.6) | 5 (11.4) | |
| Post-SI mean metabolic score | -- | 18.6 | 19.3 | 0.665 |
| Post-SI mean adverse event score | -- | 13.3 | 11.9 | 0.605 |
| ECOG at presentation, n (%) 0 1 2 3 4 |
24 (38.1) 18 (28.6) 12 (19.0) 7 (11.1) 2 (3.2) |
10 (47.6) 8 (38.1) 3 (14.3) 0 (0.0) 0 (0.0) |
14 (33.3) 10 (23.8) 9 (21.4) 7 (16.7) 2 (4.8) |
0.025 |
| Tumor Type, n (%) | ||||
| Pituitary | 16 (24.6) | 7 (33.3) | 9 (20.5) | 0.003 |
| Lung NET | 6 (9.2) | 2 (9.5) | 4 (9.1) | |
| Small cell lung cancer | 11 (16.9) | 0 (0.0) | 11 (25.0) | |
| Pancreatic NET | 6 (9.2) | 2 (9.5) | 4 (9.1) | |
| Thymic NET | 3 (4.6) | 3 (14.3) | 0 (0.0) | |
| Medullary thyroid cancer | 5 (7.7) | 0 (0.0) | 5 (11.4) | |
| Metastatic NET of unknown Primary |
1 (1.5) | 1 (4.8) | 0 (0.0) | |
| Other NET* | 8 (12.3) | 1 (4.8) | 7 (15.9) | |
| Occult | 9 (13.8) | 5 (23.8) | 4 (9.1) |
Abbreviations: ECOG = Eastern Cooperative Oncology Group, NET = neuroendocrine tumor
Other neuroendocrine tumor included bladder, nasopharyngeal, ovarian, and prostate.
Metabolic and adverse event scores
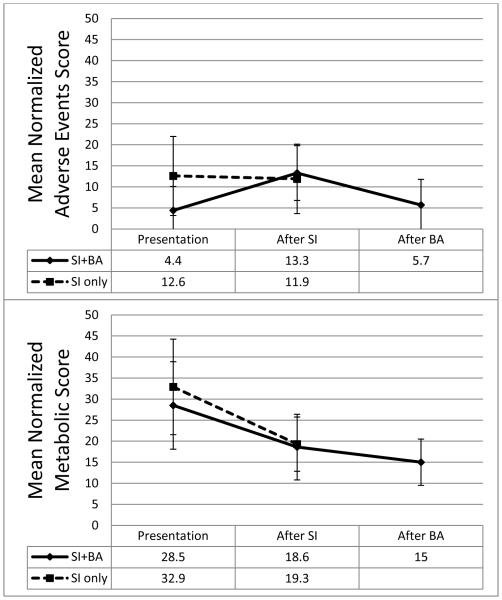
At presentation, both continuous variables including glucose, potassium, bicarbonate, and diastolic blood pressure, and metabolic scores (components discussed in methods) were similar between treatment groups. Average metabolic scores improved after SI treatment (median 4.1 months between measurements) in both groups (Figure 1). Only SI patients experienced a significant decrease in mean metabolic score between presentation and SI therapy (mean change −13.2, p<0.001). SI+BA patients experienced a more modest decline in metabolic scores between presentation and SI therapy (mean change −9.3, p=0.114), and a significant decline in metabolic scores between SI therapy and BA (mean change −13.9, p=0.032).
Figure 1.
Normalized metabolic and adverse events scores at presentation and after treatment for patients undergoing steroidogenesis inhibition (SI) treatment only versus SI+bilateral adrenalectomy (BA) (with sustained resolution of Cushing’s syndrome).
The adverse event score was significantly higher at presentation in the SI compared to the SI+BA group (12.6 versus 4.4, p=0.038, Figure 1), likely due to higher grade infections in the SI group (p=0.03, Figure 2). 36% of SI patients (16/44) compared to 9.5% of SI+BA patients (2/21) had an infection at presentation to MD Anderson Cancer Center.
Figure 2.

Summary of adverse events by Common Terminology Criteria for Adverse Events (CTCAE) grade at presentation for steroidogenesis inhibition (SI) only and SI+ bilateral adrenalectomy (BA) treatment groups. There was a significant difference in the grade and occurrence of infection in the SI only versus the SI+BA group (p=0.034).
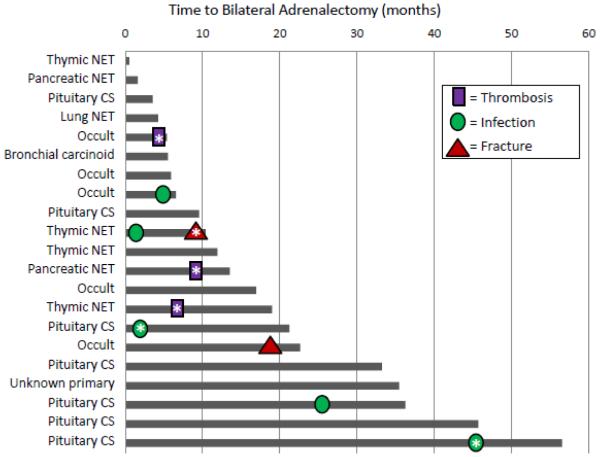
In SI patients, the average adverse event score remained stable through SI therapy. However, in SI+BA patients, the average adverse event score increased between presentation and SI treatment, then declined towards baseline after BA (Figure 1). 48% (10/21) of patients who underwent BA experienced adverse events requiring treatment between presentation and BA (Figure 3). None of the BA patients experienced more than 1 adverse event preoperatively.
Figure 3.
Time to adverse events for individual patients with uncontrolled Cushing’s syndrome patients undergoing bilateral adrenalectomy (BA). Each line represents a single patient by diagnosis and the months from presentation to the date of BA. Adverse events are represented symbolically for each patient. AEs marked with an asterisk (*) are Common Terminology Criteria for Adverse Events (CTCAE) grade 3 (requires invasive treatment). All other adverse events are CTCAE grade 2 (requires medical treatment).
Eastern Cooperative Oncology Group Performance Status
86% of patients undergoing BA had performance status of 0 or 1 at presentation. In comparison, 18/42 patients (43%) in the SI only group had performance status of 2-4 (p=0.025, Table 3). Twenty-four patients with performance status 0 or 1 were treated with SI only. Reasons for not operating on these patients included disease-specific limited life expectancy (n=6), patient refusal (n=4), good response to SI therapy (n=5), and pursuit of additional treatment for primary disease (n=9).
Timing of BA
43% of patients were evaluated by a surgeon for BA; most often, the decision to operate was made at the first visit (mean 1.9 months, range 0-18.8 months). The median time between the surgeon’s decision for BA and the operative date was 0.59 months (range 0.1 – 14.4 months). Ultimately, 21 of 28 patients initially evaluated by a surgeon underwent BA. Reasons why 7 patients referred for BA did not undergo the procedure included patient refusal (2), primary tumor treatment (3), and limited life expectancy (2).
We performed a time-to-event analysis for adverse events that occurred between presentation and BA. For patients who would undergo BA, 11% had experienced an adverse event by 2.5 months after presentation and 39% had experienced an adverse event by 12 months after presentation (Figure 3).
Operative Approach and Outcomes
Operative approach to BA was open (n=8, transabdominal (n=5) or posterior approach (n=3)) or laparoscopic [anterior transperitoneal laparoscopic (n=1) or posterior retroperitoneoscopic adrenalectomy (PRA, n=12)]. The lap/PRA approach was associated with less blood loss compared to the open approach (median 10 ml versus 300 ml), but equivalent dissection time. Lap/PRA patients stayed in the hospital a median of 3 days postoperatively, compared to 7 days in the open group.
All 3 patients who developed recurrent Cushing’s syndrome after BA underwent BA via the PRA approach. Recurrences included a single left adrenal bed nodule in 2 patients and bilateral adrenal bed nodules in 1 patient. Of the 12 patients who underwent PRA, the 3 patients with recurrent Cushing’s syndrome underwent operation during the first 5 procedures performed in patients with ACTH-dependent Cushing’s syndrome requiring BA using this technique. All 3 of these patients died at a median of 46.5 months follow up.
There was no operative mortality in this series. Eight perioperative events occurred in 7 patients: arrhythmia (2), blood transfusion (2), C. difficile colitis (1), subcostal nerve injury (1), urinary retention (1), pneumothorax (1). No patient presented with adrenal crisis during follow up. One patient developed Nelson’s syndrome.
The use of BA increased over time. Evaluating our series by decade, the percent of patients who underwent BA out of all the patients diagnosed during the decade increased from 16.7% in the 1980s to 27.3% in the 1990s to 36.1% in the 2000s. Furthermore, the number of BA performed for unmanageable ACTH-dependent Cushing’s syndrome was steady at 0.1-0.3 procedures per year during the first 3 decades of the study, then increased to 1.3 per year during the 2000s when the transition to a laparoscopic approach occurred. After the first PRA was performed in 2005, 7 additional PRAs were performed within the next 5 years.
Overall Outcomes
Of the 65 patients, 30 died. Nine deaths were a direct consequence of Cushing’s syndrome and an additional 10 deaths were related to both malignancy and Cushing’s syndrome. Five patients died as a consequence of their malignancy (Table 4). Over half (24/44) of the SI patients died; median survival was 24.0 months. Steroid excess contributed to 71% of deaths in the SI only group (41% directly related to Cushing’s syndrome). Six deaths (29%) occurred in the SI+BA group: in addition to the 3 patients with recurrent Cushing’s syndrome who died, 3 of the 18 patients (17%) with sustained resolution of Cushing’s syndrome died. These latter 3 patients all died of cancer-related causes at a median of 7.1 months after BA. The others were alive at median follow up of 49.2 months.
Table 4.
Median overall survival by disease type for 65 patients with uncontrolled Cushing’s syndrome (CS) with an overall median follow up of 7.6 years.
| Tumor type | No. died n/N (%) |
Overall Survival (mo) | Cause of Death | ||||
|---|---|---|---|---|---|---|---|
| Median (95% CI) |
Min, Max |
CS | CS+ Cancer |
Cancer | Unknown/O ther |
||
| Small cell lung | 10/11 (91) | 1.4 (0.3, 5.9) |
0.3, 11.2 | 4 | 3 | 2 | 1 |
| Medullary thyroid | 5/5 (100) | 14.7 (3.0, 32.2) |
3.0, 32.2 | 1 | 3 | 1 | |
| Thymic NET | 1/3 (33) |
NA (23.4, NA) |
19.7, 69.6 |
1 | |||
| Lung NET | 4/6 (67) |
23.6 (9.2, 30.6) |
9.2, 30.6 | 1 | 1 | 1 | 1 |
| Other NET | 5/8 (63) |
24.2 (2.1, 64.5) |
2.1, 64.5 | 2 | 2 | 1 | |
| Pancreatic NET | 4/6 (67) |
40.7 (6.7, NA) |
6.7, 49.8 | 1 | 2 | 1 | |
| Unknown primary | 0/1 (0) |
NA | 121.7, 121.7 |
||||
| Occult | 0/9 (0) |
NA | 3.1, 133.6 |
||||
| Pituitary | 1/16 (6) |
NA (65.6, NA) |
15.8, 227.5 |
1 | |||
| TOTAL | 30/65 (46) | 64.5 (23.4, NA) |
0.3, 227.5 |
10 | 10 | 5 | 5 |
Abbreviations: NET = neuroendocrine tumor, CI: = confidence interval, NA = not attained, CS = Cushing’s syndrome.
Outcomes by Primary Disease
Patients with pituitary Cushing’s syndrome who were treated with BA (7/16; 44%) underwent a median of 3 failed therapies of their primary tumor prior to BA. Pituitary resection was the initial treatment in all patients and 9/16 (56%) underwent at least 1 reoperative pituitary resection. 12/16 (75%) patients were also treated with radiation. 50% (8/16) of pituitary Cushing’s syndrome patients experienced complications associated with treatment of their disease or with SI while on SI therapy, including 2 patients with grade 5 CTCAE adverse events (radiation-induced brain necrosis and optic chiasm neuritis and blindness). Table 5 describes reasons why pituitary Cushing’s syndrome patients did not undergo BA.
Table 5.
Reasons why 44 patients with Cushing’s syndrome (CS) in this series were not treated with bilateral adrenalectomy (BA) (steroidogenesis inhibition only).
| Diagnosis | |||||
|---|---|---|---|---|---|
| Reasons for no BA | Ectopic CS (n=35) | Pituitary CS (n=9) | Total | ||
| Occult (n=4) |
MTC or SCLC (n=16) |
Other NET (n=15) |
|||
| Prohibitive operative risk |
2 | 1 | 1 | 4 | |
| Patient refusal | 1 | 1 | 2 | 4 | |
| Primary tumor treatment |
6 | 4 | 10 | ||
| Well controlled symptoms |
1 | 2 | 2 | 5 | |
| Limited life expectancy | 16 | 5 | 21 | ||
Abbreviations: MTC = medullary thyroid cancer, SCLC = small cell lung cancer, NET = neuroendocrine tumor
Nine of the 49 patients with ectopic Cushing’s syndrome had an occult source of ACTH production (no imagable tumor); 5 underwent BA. Of those, two patients’ tumors were subsequently localized (thymic and well-differentiated lung neuroendocrine tumors) and both underwent successful resection 19 and 23 months, respectively, after BA. The other three patients are in good health with no evident source of ACTH production. The four patients who did not undergo BA were medically managed with persistent Cushing’s syndrome (Table 5).
Fourteen patients with a metastatic or unresectable source of ACTH excess died within the first 6 months of presentation to MD Anderson, including 9 of the 11 patients with small cell lung cancer, 2 of the 5 patients with medullary thyroid cancer, and 3 patients with other neuroendocrine tumors. Table 4 demonstrates median overall survival by disease type for all patients.
Discussion
This retrospective study describes the medical and surgical treatment of patients with ACTH-dependent Cushing’s syndrome from an unmanageable primary source who were treated within a single institution over the last four decades. From this relatively large cohort with an informative follow-up period, we are able to comment upon the key issues of: 1) whether medical and surgical treatment of Cushing’s syndromeprovides meaningful benefit for these patients, 2) which patients benefit from surgical intervention, 3) the importance of surgical timing, and 4) the selection of operative approach.
1) Benefit of treating Cushing’s syndrome
Two-thirds of patient deaths in this study were related to Cushing’s syndrome, suggesting that controlling cortisol excess is as meaningful as addressing the challenging primary disease in this population. We were able to quantify a beneficial metabolic response and a decrease in adverse events in both treatment groups. However, patients treated only medically (SI) were more likely to die from Cushing’s-related sequelae while those who underwent adrenalectomy (SI+BA) were not. While patients in the surgical group had a better performance status and increased predicted longevity compared to the medical group, BA may have helped decrease morbidity and mortality in this highly selected population.
These complex patients require a coordinated, multidisciplinary team-based approach to their disease. Initially, they may be seen by an endocrinologist, medical oncologist, radiation oncologist, and neurosurgeon. We recommend early referral to a surgical endocrinologist to allow inclusion of potential BA in the overall treatment algorithm. Only ¼ of patients in this study were ever referred for consideration of BA. Noting this selection bias serves not only as a reminder of an inherent weakness of this retrospective study but also how BA is often not considered in standard care in this disease.
2) Selecting patients for BA
When selecting therapy, one must consider the source of the ACTH excess. Evaluating the initial response to SI therapy is also critical. Additionally, attention to the biology of the underlying tumor process in patients with advanced cancer, and an understanding of the cancer-directed therapies available, is essential in developing the most effective integrated treatment strategy.
In agreement with others’ work, patients in this study with pituitary Cushing’s syndrome were young and experienced very little mortality. However, half of these patients experienced complications as a result of the treatment for their recalcitrant primary disease or SI therapy, including radiation-induced brain necrosis and optic chiasm neuritis and blindness. Severe treatment-related complications in this group can dramatically impact long-term quality of life. (9, 10) One quality of life analysis in a similar group of pituitary Cushing’s syndrome patients who underwent BA revealed that postoperatively 86% of patients felt that their health status was good to excellent compared with 1 year before BA.(9) In our retrospective study, BA was not proposed in pituitary Cushing’s syndrome patients mainly due to pursuit of further treatment of primary disease or because the patient was felt to be well controlled on medical management. This reasoning may not be appropriate in all patients; early surgical consultation for BA as part of a team-based approach in patients with persistent pituitary Cushing’s syndrome may be warranted.
While patients with ectopic sources of ACTH excess are older and have a greater potential to die from their primary disease than their pituitary counterparts, over a fourth of these patients underwent SI+BA with excellent response. Type of ectopic tumor and malignancy-related outcome is important in considering whether BA is appropriate. The reluctance to operate on patients with small cell lung cancer or medullary thyroid cancer due to abbreviated survival has been validated by others.(11, 12) Of the 10 patients with ACTH-secreting medullary thyroid cancer from the French Endocrine Tumor Registry, 8 died within 2 to 30 months after diagnosis; 4 died of Cushing’s-related complications and 4 from progressive disease.(11)
In contrast, patients with ACTH-secreting thymic neuroendocrine tumors can often be treated with resection of their primary tumor and many have prolonged life expectancy (>5 years).(13) Two of our patients with thymic neuroendocrine tumors who were not initially candidates for sternotomy due to uncontrolled Cushing’s syndrome (inappropriately high surgical risk for median sternotomy) underwent BA followed by curative resection of their primary tumor.
Based on our clinical experience, in patients with a reasonable life expectancy from their primary disease, BA may enable those with initially unresectable primary disease to become candidates for surgical resection or other treatments or, at least aid in preventing the development of adverse events. Early BA may be warranted in patients with malignant and metastatic ACTH-producing tumors after careful consideration of the patient’s clinical condition, comorbidies, and plans for systemic chemotherapy.
Patients with an occult source of ACTH production represent a challenging group. In our series, 6 of 9 patients with an occult source have never had any radiological localization of an ACTH source. Those who underwent BA have excellent quality of life and are followed with annual imaging studies. The 17 patients in the NIH series with ACTH-dependent Cushing’s syndrome and occult primary tumors had prolonged survival compared to those with identifiable tumors.(12) We emphasize that appropriate patients with an occult source of ACTH excess be referred for surgical consult after an initial thorough search for a source is performed.
Response to initial SI therapy is critical to selecting future therapies. Use of both the graded CTCAE system and a composite score allowed us to assess accurately the severity of the patient’s comorbidities and overall metabolic status. Patients treated with SI alone had a significant improvement in metabolic score after SI treatment. Those treated with SI+BA had only a modest improvement in metabolic score after SI therapy and developed adverse events. Suboptimal improvements in metabolic parameters or worsening adverse events after SI alone are indications to strongly consider BA. Performance status should also be used as an objective assessment of these patients. All surgical patients in this study had a performance status < 2; the lack of perioperative morbidity and mortality likely reflect this fact.
3) Timing of BA
Between presentation and BA, adverse events occurred at a steady rate and 70% occurred within the first 10 months. Thus, delaying BA to ascertain a response to SI therapy must be balanced with the risks of developing adverse events.
Delay in surgical referral or decision-making can result in the development of adverse events that prohibit BA. Two patients who initially refused BA experienced a series of adverse events that led to multiple emergency room visits, repeated hospitalizations, and severely diminished quality of life. Unfortunately, they never again became candidates for BA due to declining performance status. One patient whose operative date for BA was delayed due to administrative issues experienced a similar outcome. Loss of treatment opportunity in patients with ectopic tumors is another reason to consider immediate surgical consultation while initiating SI therapy as part of the team-based decision-making and treatment approach.
4) Safety and Efficacy of Operative Techniques
Several groups have shown that BA for uncontrollable ACTH-dependent Cushing’s syndrome can routinely be accomplished safely in a minimally invasive fashion with acceptably low morbidity.[8,9] Our operative approach varied by surgeon and era. The PRA approach has allowed more direct access to the adrenal glands in Cushing’s syndrome patients with truncal obesity and hepatomegaly.[10,11] However, all 3 patients with recurrent Cushing’s syndrome after BA underwent PRA during the first five BA for ACTH-dependent Cushing’s syndrome using this approach. Although we use the PRA approach routinely to resect benign adrenal tumors, these data suggest a learning curve for this technique in these challenging patients with morbid obesity and adrenal hyperplasia. Patients with recurrent Cushing’s syndrome had significantly worse postoperative metabolic scores and all 3 died during follow up. These results stress the importance of complete surgical resection of all adrenal tissue in this population by whichever approach allows the greatest chance of attaining that goal.
This study has several limitations. Retrospectively evaluating performance status, proximal muscle weakness, and cause of death is inexact. In addition, due to the rarity of ACTH-dependent Cushing’s syndrome, we included patients treated over a >40 year period. Survival is likely underestimated in the group treated several decades ago due to modern antineoplastic therapies.
In conclusion, selected patients with ACTH-dependent Cushing’s syndrome from unmanageable primary sources with a reasonable life expectancy from their primary disease may benefit from SI plus BA more than from SI alone. The surgical endocrinologist should be involved at an early stage in the treatment course. Source of ACTH production as well as response to initial medical therapy are important factors in determination of surgery. BA can be performed safely and effectively by those with expertise in the operative techniques and complete resection of all adrenal tissue is imperative to long-term success.
Footnotes
Disclosures: We have no financial or commercial interests to disclose.
References
- 1.Dekkers OM, Horvath-Puho E, Jorgensen JO, Cannegieter SC, Ehrenstein V, Vandenbroucke JP, et al. Multisystem Morbidity and Mortality in Cushing's Syndrome: a Cohort Study. The Journal of clinical endocrinology and metabolism. 2013 doi: 10.1210/jc.2012-3582. Epub 2013/03/28. [DOI] [PubMed] [Google Scholar]
- 2.Valassi E, Crespo I, Gich I, Rodriguez J, Webb SM. A reappraisal of the medical therapy with steroidogenesis inhibitors in Cushing's syndrome. Clinical endocrinology. 2012;77(5):735–42. doi: 10.1111/j.1365-2265.2012.04424.x. Epub 2012/04/27. [DOI] [PubMed] [Google Scholar]
- 3.Alberda WJ, van Eijck CH, Feelders RA, Kazemier G, de Herder WW, Burger JW. Endoscopic bilateral adrenalectomy in patients with ectopic Cushing's syndrome. Surgical endoscopy. 2012;26(4):1140–5. doi: 10.1007/s00464-011-2020-7. Epub 2011/11/03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chow JT, Thompson GB, Grant CS, Farley DR, Richards ML, Young WF., Jr. Bilateral laparoscopic adrenalectomy for corticotrophin-dependent Cushing's syndrome: a review of the Mayo Clinic experience. Clinical endocrinology. 2008;68(4):513–9. doi: 10.1111/j.1365-2265.2007.03082.x. Epub 2007/11/01. [DOI] [PubMed] [Google Scholar]
- 5.Porterfield JR, Thompson GB, Young WF, Jr., Chow JT, Fryrear RS, van Heerden JA, et al. Surgery for Cushing's syndrome: an historical review and recent ten-year experience. World journal of surgery. 2008;32(5):659–77. doi: 10.1007/s00268-007-9387-6. Epub 2008/01/16. [DOI] [PubMed] [Google Scholar]
- 6.Alesina PF, Hommeltenberg S, Meier B, Petersenn S, Lahner H, Schmid KW, et al. Posterior retroperitoneoscopic adrenalectomy for clinical and subclinical Cushing's syndrome. World journal of surgery. 2010;34(6):1391–7. doi: 10.1007/s00268-010-0453-0. Epub 2010/02/10. [DOI] [PubMed] [Google Scholar]
- 7.Ejaz S, Vassilopoulou-Sellin R, Busaidy NL, Hu MI, Waguespack SG, Jimenez C, et al. Cushing syndrome secondary to ectopic adrenocorticotropic hormone secretion: the University of Texas MD Anderson Cancer Center Experience. Cancer. 2011;117(19):4381–9. doi: 10.1002/cncr.26029. Epub 2011/03/18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.CTCAE Cancer Therapy Evaluation Program, Common Terminology Criteria for Adverse Events, Version 4.0. 2010 DCTD, NCI, NIH, DHHS. [Google Scholar]
- 9.Smith PW, Turza KC, Carter CO, Vance ML, Laws ER, Hanks JB. Bilateral adrenalectomy for refractory Cushing disease: a safe and definitive therapy. Journal of the American College of Surgeons. 2009;208(6):1059–64. doi: 10.1016/j.jamcollsurg.2009.02.054. Epub 2009/05/30. [DOI] [PubMed] [Google Scholar]
- 10.Biller BM, Grossman AB, Stewart PM, Melmed S, Bertagna X, Bertherat J, et al. Treatment of adrenocorticotropin-dependent Cushing's syndrome: a consensus statement. The Journal of clinical endocrinology and metabolism. 2008;93(7):2454–62. doi: 10.1210/jc.2007-2734. Epub 2008/04/17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barbosa SL, Rodien P, Leboulleux S, Niccoli-Sire P, Kraimps JL, Caron P, et al. Ectopic adrenocorticotropic hormone-syndrome in medullary carcinoma of the thyroid: a retrospective analysis and review of the literature. Thyroid : official journal of the American Thyroid Association. 2005;15(6):618–23. doi: 10.1089/thy.2005.15.618. Epub 2005/07/21. [DOI] [PubMed] [Google Scholar]
- 12.Ilias I, Torpy DJ, Pacak K, Mullen N, Wesley RA, Nieman LK. Cushing's syndrome due to ectopic corticotropin secretion: twenty years' experience at the National Institutes of Health. The Journal of clinical endocrinology and metabolism. 2005;90(8):4955–62. doi: 10.1210/jc.2004-2527. Epub 2005/05/26. [DOI] [PubMed] [Google Scholar]
- 13.Neary NM, Lopez-Chavez A, Abel BS, Boyce AM, Schaub N, Kwong K, et al. Neuroendocrine ACTH-producing tumor of the thymus--experience with 12 patients over 25 years. The Journal of clinical endocrinology and metabolism. 2012;97(7):2223–30. doi: 10.1210/jc.2011-3355. Epub 2012/04/18. [DOI] [PMC free article] [PubMed] [Google Scholar]