Abstract
Tau, a microtubule-associated protein with multiple phosphorylation sites, forms aggregates that correlate with neurodegeneration in Alzheimer Disease and several other neurodegenerative diseases, termed tauopathies. Hsc70 is a highly-expressed constitutive chaperone that can drive conformational change in proteins, prevent the aggregation of its substrates, recognize misfolded substrates, and facilitate their degradation. Here, we show that hsc70 binds to the microtubule-binding domain of tau in vitro and in vivo, without an absolute requirement for tau phosphorylation. Binding requires a carboxy-terminal region of hsc70 comprising its peptide-binding and variable domains. We have identified two hsc70-binding sites on tau and hydrophobic amino acids crucial for hsc70-binding. Interestingly, these hsc70-binding sites correspond to the β-structure elements that have been previously reported to facilitate tau aggregation. Thus, it is possible that hsc70 binding might directly inhibit tau-tau interactions that precede tau oligomerization and aggregation. Our results provide an important stimulus for research into how the hsc70-tau interaction might affect tau fate in normal cells and in disease.
Keywords: tau, chaperone, heat shock, PHF6, MTBR, SPR
Introduction
Tau aggregation into neurofibrillary tangles is a common denominator in neurodegenerative tauopathies [reviewed in (Goedert and Jakes 2005)]. In the human central nervous system, alternative splicing gives rise to six major tau isoforms, all of which are found in tangles. These isoforms can have zero, one or two N-terminal inserts (0N, 1N, 2N), and either three or four C-terminal “repeat” sequences (3R or 4R). These 30–32 amino acid (aa) C-terminal sequences, viz. R1, R2, R3 and R4 together constitute the tau microtubule-binding repeat region (MTBR). While this region has long been implicated in tau aggregation, recent advances have identified specific areas in R2–R4 that tend to adopt β-structure and might facilitate tau aggregation (Giannetti et al. 2000; Li and Lee 2006; Mukrasch et al. 2005; Santa-Maria et al. 2006; von Bergen et al. 2001; von Bergen et al. 2000).
In vivo, tau aggregation involves a complex interplay of phosphorylation, conformational change and proteolytic cleavage, though the exact mechanisms are unclear. Hyperphosphorylation (i.e., excessive phosphorylation at physiological sites and at additional sites) is thought to dissociate tau from microtubules, causing tau accumulation in the somatodendritic compartment and an increased risk of aggregation. It was previously reported that tau hyperphosphorylation in cell cultures was mitigated by heat shock protein (HSP) chaperones (Kirby et al. 1994; Wallace et al. 1993). Subsequently, associations between tau and HSP chaperones from several classes, including hsp90 (Dickey et al. 2006; Dou et al. 2003), hsp70 (Dou et al. 2003; Sahara et al. 2007), hsp27 (Shimura et al. 2004b), and alphaB-crystallin (Bauer and Richter-Landsberg 2006; Richter-Landsberg and Bauer 2004) have been reported. Moreover, elevated HSP levels have been proposed to attenuate tau aggregation [reviewed in (Smith et al. 2005)].
Heat Shock Protein 70 (HSP70) chaperones are an important part of the cellular protein quality control and degradation systems [reviewed in (Brodsky and Chiosis 2006; Mayer and Bukau 2005)]. The HSP70 family includes the heat shock cognate 70 protein hsc70 (also known as hsp73) and the heat shock (inducible) protein hsp70 (also known as hsp72). Hsc70 and hsp70 share many similarities: both bind magnesium and ATP through their highly conserved N-terminal ATPase domains and bind polypeptides through a substrate-binding domain located just upstream of a variable/regulatory domain [reviewed in (Mayer and Bukau 2005)]. Hsp70 has low expression in unstressed mammalian cell lines while hsc70 is constitutively expressed in all mammalian cells. Mild stress elicits hsc70 upregulation in cells whereas relatively more severe stress induces hsp70. Hsc70 is enriched in the nervous system early in postnatal development (D'Souza and Brown 1998; Morrison et al. 2000) and has been calculated to comprise as much as 1% of soluble brain protein (Schlossman et al. 1984). A cytoprotective role for hsc70 may be particularly relevant in neurons where an attenuated hsp70 response has been correlated with high hsc70 expression (Manzerra et al. 1997). Also, there is evidence that hsc70 may be upstream of hsp70 activation (Ahn et al. 2005).
In cells, hsc70 facilitates protein degradation through the proteasomal pathway (Bercovich et al. 1997) and the lysosomal pathway (Chiang et al. 1989). Hsc70 has been implicated in the ubiquitin-dependent degradation of tau (Shimura et al. 2004b), as well as the ubiquitin-independent degradation of tau (Elliott et al. 2007). However, a direct association between tau and hsc70 (or any HSP70 protein) has not been demonstrated. Here we describe our identification of a tau-hsc70 interaction from an unbiased screen and demonstrate the direct binding between tau and hsc70. Our work reveals that hsc70 binds tau at two sites which are involved in tau aggregation and one of which is regulated by alternative splicing.
Materials and Methods
Plasmids and constructs
The 0N3R and 0N4R human tau isoforms (thereafter 3R tau and 4R tau, respectively) were cloned into pRc/CMV vector for eukaryotic expression (Invitrogen, Inc.) as previously described (Hall et al. 1997). All tau deletion and substitution mutants were generated by site-directed mutagenesis (Stratagene QuikChange kit). All mutations were verified by DNA sequencing.
For expressing glutathione sepharose transferase (GST) fusion proteins in E. coli, a pET-GST vector was constructed by inserting the GST sequence from pGEX-4T-1 (Pharmacia Biotech, Inc.) into pET-17b (Novagen, Inc.). Wild-type bovine hsc70 cDNA (DeLuca-Flaherty and McKay 1990; Newmyer and Schmid 2001) was inserted into the pET-GST vector, resulting in full length hsc70 tagged at its amino terminus with GST. Truncations in the GST-bovine hsc70 construct were made by inserting stop codons through site-directed mutagenesis. Bovine hsc70 is identical to the human hsc70 aa sequence with the exception of an extra GGMP repeat downstream of aa 614 in the variable domain, i.e., bovine hsc70 is 650 aa while human hsc70 is 646 aa.
His-tagged human 2N4R wt tau and its mutants #2–6 (Fig. 5) in pT7c vector constructs have been previously described (Carmel et al. 1996). Bacterial expression pET constructs encoding untagged 3R, 4R human wild-type tau, and truncation mutants #11 and #14 (Fig. 5) have been described previously (Bhaskar et al. 2005; Brandt and Lee 1993). The truncation mutants #12 and #13 (Fig. 5) were constructed by replacing aa 274 and 336, respectively, with stop codons using site directed mutagenesis. Mutants #8 and #9 (Fig. 5) were synthesized according to Makarova et al. (Makarova et al. 2000), making internal deletions of 93 bp and 96 bp, respectively, in 0N4R tau.
Fig. 5. The microtubule-binding domain of tau contains its hsc70-binding sites.
A panel of recombinant tau proteins containing internal deletions and/or truncations was tested for binding to GST-Hsc70. Bolded numbers at the extreme left are for ease of reference in Results. Tau isoforms and deletions are listed under “Tau constructs”. Amino acid numbering is per the 441 aa isoform (2N4R). Only constructs derived from 2N4R tau had His tags. The + and − under “Binding” summarize the results of at least 3 binding reactions. The deletions revealed two hsc70 binding sites, shown by two pairs of dashed vertical lines. A binding site is located between amino acids 274–282, within R2, encoded by exon 10. A second binding site lies between amino acids 306–335, within R3, encoded by exon 11. Binding requires the presence of at least one site.
Yeast two-hybrid assay
A Hela cell cDNA library was screened by the yeast two-hybrid system as described by Gyuris et al. (Gyuris et al. 1993), using 3R tau as “bait”. cDNA libraries were constructed in the pJG vector and tau was expressed by the pEG202 vector. Positive interactors were identified by growing transformants on leucine-deficient medium. Confirmed positives were sequenced, then subcloned into pRc/CMV with an HA tag.
Cell culture and transfections
Simian COS7 cells were grown in DMEM with 10% bovine serum (Hyclone or Benchmark). COS7 cells were transfected using Lipofectamine Plus (Invitrogen) according to the manufacturer’s protocol and harvested 48 hours after transfection. SH-SY5Y human neuroblastoma cells were grown in RPMI as previously described (Lee et al. 1998). SH-SY5Y cells were transfected with 3R wild-type or mutant human tau in pRcCMV vector, using Lipofectamine 2000 (Invitrogen). Stably-transfected SH-SY5Y lines were selected by 200 µg/ml G418. Immunofluorescence and Western blots identified clonal SH-SY5Y lines with robust tau expression. Cell lines with roughly equivalent expression of tau were used for immunoprecipitation.
Co-immunoprecipitation
Cells were harvested in lysis buffer containing 50mM Tris-HCl pH 7.5, 150 mM NaCl, 1% Triton-X 100, 0.25% sodium deoxycholate, 1 mM sodium vanadate, 10 mM sodium fluoride, 1 mM AEBSF, 2.16 mM leupeptin, 1.46 mM pepstatin, and 0.15 mM aprotinin. Following centrifugation to remove insoluble debris, cell lysates were pre-cleared by 1 hr incubation with non-specific IgG (Jackson ImmunoResearch). Tau was immunoprecipitated using rabbit polyclonal anti-tau antibody (Lee et al. 2004) or tau13 monoclonal antibody (gift from Dr. Lester Binder). Immunoprecipitations used either Protein A Sepharose (GE Healthcare) or Protein G Plus-Agarose (Calbiochem) and were resolved by SDS-PAGE.
To increase cellular levels of the inducible hsp70 prior to immunoprecipitation, SH-SY5Y cells were subjected to thermal conditioning, such that cells received 2–3 “heat shock” treatments, interspersed with 24 h recovery periods, over consecutive days. In each treatment, cells were incubated in a 43°C water bath (“heat shocked”) for 40–60 min. On the final day, cells were heat shocked for 60 min, allowed to recover 5 h, and harvested for immunoprecipitation as above.
SDS-PAGE and Western Blotting
Proteins in denaturing SDS loading buffer were resolved on Tris-Glycine SDS-PAGE gels containing 8, 9 or 10% acrylamide (National Diagnostics) and transferred to Immobilon-P PVDF membranes (Millipore). PVDF blots were blocked using 2% gelatin (Bio-Rad) in Tris buffered saline containing 0.05% (v/v) Tween-20 detergent (TBS-T). Following incubation with primary antibodies, blots were incubated with HRP-conjugated secondary antibodies (Jackson Immuno Research, eBioscience). Blots were developed with either Western Lightning ECL reagent (Perkin Elmer) or Amersham ECL Plus reagent (GE Healthcare). The following primary antibodies were used: tau5, tau1 (gifts of Dr. Lester Binder), 5A6 (Developmental Studies Hybridoma Bank, University of Iowa), anti-hsc70 SPA 815 at 1:10,000 (Assay Designs), anti-hsc70 sc-7298 at 1:4000 (Santa Cruz Biotechnology), anti-hsc70 sc-1059 at 1:1000 (Santa Cruz Biotechnology), anti-hemagglutinin 3F10 at 1:1000 (Roche) and anti-GST at 1:1000 (GE Healthcare). For re-probing, if necessary, antibodies were stripped from membranes by washing in TBS containing 100 mM β-mercaptoethanol, 0.01% SDS and 0.1% Tween-20 for 40 min at 50 °C.
Recombinant protein preparation
All recombinant proteins were prepared by expression in E.coli BL21(DE3)LysS cells (Studier et al. 1990). Bacterial cultures were grown in M9ZB broth with selection antibiotics and induced by 0.4 mM IPTG at OD600 ~ 0.8. After 30 minutes of induction, 200 µg/ml of rifampicin was added and the culture incubated for another 2.5 hours at 37 °C before harvesting. For GST-Hsc70 expression, IPTG-induction was performed at 30 °C.
Purification of untagged tau protein from E. coli has been described previously (Brandt and Lee 1993). Briefly, bacteria were lysed in 30 mM Tris pH 8.0, 0.1% Triton X-100, 4 mM β-mercaptoethanol with protease inhibitors. After centrifugation, the bacterial lysate was subjected to DE52 (Whatman) and phosphocellulose (Whatman) chromatography. Tau was eluted from the phosphocellulose by 0.3 and 0.4 M KCl steps. His-tagged tau proteins were purified from bacteria by a Nickel-NTA Spin Kit (Qiagen) according to the manufacturer’s protocol. Prior to surface plasmon resonance or GST-Hsc70 binding assays, tau was desalted and buffer-exchanged using Micro Bio-Spin chromatography columns (Bio-Rad).
GST-Hsc70 protein was affinity-purified from bacterial lysates using Glutathione Sepharose 4B (GE Healthcare). GST-Hsc70 complexed beads were washed at least 3 times, including a 0.5 mM ATP wash, and stored at 4 °C until further use. Protein concentrations were determined by the Bradford assay (Bradford 1976) using bovine serum albumin as a standard.
Surface Plasmon Resonance
Surface plasmon resonance (SPR) experiments were performed with a BIAcore 3000 instrument (GE Healthcare). A carboxymethylated dextran-coated Pioneer B1 chip was activated according to the manufacturer’s protocol. To the activated dextran matrix, we coupled either purified nucleotide-free recombinant wild-type bovine hsc70 (Assay Designs) (hsc70 flow cell) or no protein (control flow cell). Hsc70 immobilization was evident from a 1300 Response Units (R.U.) increase in SPR signal. Excess uncoupled carboxymethylated dextran sites were blocked by subsequent ethanolamine injection. 3R and 4R tau analytes were dissolved in 10 mM HEPES, pH 7.4, 150 mM sodium chloride, 3 mM EDTA, 0.005% (v/v) Tween-20 and 1mM DTT. Tau interaction with immobilized hsc70 was measured at 25°C by injecting 60 µl of different concentrations of tau (25nM, 250 nM, 500 nM, 750 nM, 1000 nM and 1250 nM) at a flow-rate of 40 µl/min. SPR data was obtained throughout the association phase (90 sec) and the dissociation phase (at least 180 sec). Between analyte injections, the flow cell surfaces were regenerated by two consecutive injections of 10mM Glycine pH 2.2. The responses in the control flow cell were subtracted from those of the hsc70 flow cell. Using BIAevaluation 3.0 software (GE Healthcare), sensorgrams were globally fitted to a 1:1 Langmuir binding model. Quality of fit was assessed from residuals, which show the difference between the calculated and the experimental curve at each time point, and the chi square value, χ2, which measures the closeness of fit. The kon (association) and koff (dissociation) rate constants were calculated using the 1:1 Langmuir binding model. The KD (equilibrium dissociation constant) was calculated as koff / kon.
In vitro binding assays
For GST-mediated sedimentation assays, GST-Hsc70 was pre-complexed to glutathione sepharose. In addition, prior to incubation with tau, the GST-Hsc70 was “primed” by brief incubation with 1 mM ATP in binding buffer (50 mM Tris pH 7.5, 150 mM sodium chloride, 2 mM magnesium chloride, 2 mM β-mercaptoethanol and 0.1–0.2% Triton X-100), followed by a wash in binding buffer without ATP. Priming was employed since ATP binding is thought to switch the hsc70 substrate-binding pocket to an open conformation, allowing it to be more receptive to substrates [reviewed in (Mayer and Bukau 2005)]. Also, priming would dislodge any bacterial proteins or degradation products that might have occupied the hsc70 substrate binding pocket during preparation or storage. Binding reactions proceeded 60 min at 4 °C, using purified recombinant human tau (150–200 ng) and a molar excess of GST-Hsc70 (8–12 ug) in binding buffer. GST-Hsc70 binding experiments were repeated at least thrice with different preps of each protein.
Results
Tau-hsc70 binding is direct and requires the hsc70 C-terminus
The yeast two-hybrid system was used to identify proteins that interact with tau. 5×105 members of a HeLa cell cDNA library were screened using 3R tau and 30 clones were identified. Restriction analysis of the 30 clones indicated that there were 9 unique cDNAs. Following sequence analysis of the 9 clones, one clone, which accounted for 2 of the original 30 isolates, was determined to be the heat shock cognate protein (hsc70). Sequencing determined that the clone encoded a carboxy-terminal fragment of human hsc70 comprising aa 360–646, which contained the hsc70 substrate/peptide-binding domain (aa 385–544) and a variable domain extending to the end of the molecule (Chappell et al. 1987; Hu and Wang 1996; Wang et al. 1993). Thus, the tau-binding hsc70 fragment excluded most of the nucleotide-binding domain (aa 1–384) of hsc70.
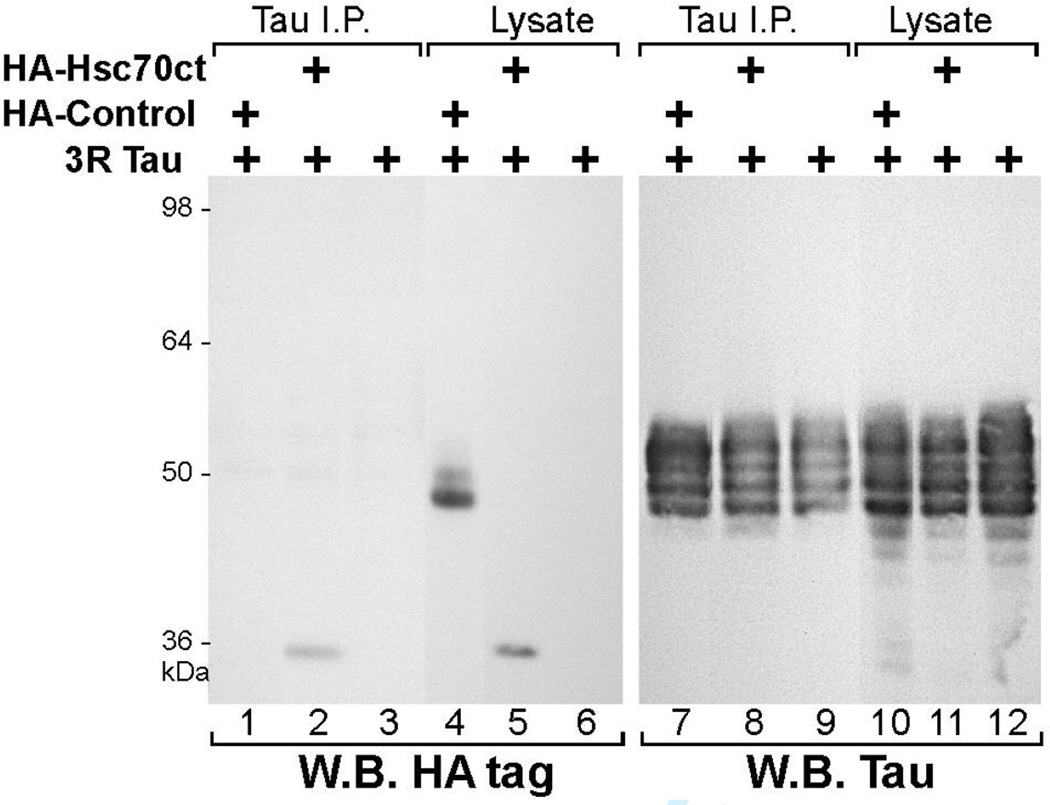
To determine if tau and hsc70 interacted in mammalian cells, we expressed the hsc70 fragment (aa 360–646) and 3R tau in COS7 cells (Fig. 1). When we immunoprecipitated (IPed) tau from the co-transfected COS7 cell lysates, the HA-tagged Hsc70 carboxy-terminal fragment (HA-Hsc70ct) co-immunoprecipitated (co-IPed) with tau (Fig. 1, lane 2). The HA tag did not bind tau or the anti-tau antibody, since a control HA-tagged 46 kDa protein failed to co-IP with tau (Fig. 1, lane 1). The multiple band pattern of tau, ranging ~45–55 kDa is due to differential phosphorylation of tau (Medina et al. 1995). The HA-Hsc70ct consistently co-IPed with tau under a variety of detergent and salt conditions (data not shown).
Fig. 1. A carboxy-terminal fragment of hsc70 associates with tau.
COS7 cells were transiently transfected with plasmid encoding human 3R tau, with or without plasmids encoding either HA-tagged hsc70 carboxy-terminal fragment spanning aa 360–646 of human hsc70 (HA-Hsc70ct), or a control HA-tagged protein (HA-Control). Tau was immunoprecipitated from cell lysates and the immunoprecipitates were analyzed by Western blotting. Immunoblotting with anti-HA antibody showed HA-Hsc70ct (35 kDa) co-immunoprecipitated with tau (lane 2). HA-Control (46 kDa, lane 4) was not immunoprecipitated by the anti-tau antibody (lane 1). Lanes 7–12 show the same blot re-probed for tau.
As hsc70 protein is abundant in cells and its sequence is very well conserved, we asked if endogenous simian hsc70 in COS7 would bind transfected human tau. Endogenous hsc70 co-IPed with 3R tau (Fig. 2A, lane 1). Hsc70 was not detected if tau was absent or if tau was not IPed (Fig. 2A, lanes 2, 3). Similarly, we confirmed the binding of endogenous hsc70 to 4R tau (Fig. 2B, lane 1). We have noted that although tau can be expressed at high levels, the associating hsc70 was a very small fraction of the large pool of cytosolic hsc70 (typically less than 0.5%).
Fig. 2. Wild type tau isoforms associate with endogenous hsc70 in vivo.
A. Human 3R tau was expressed in COS7 cells by transient transfection and immunoprecipitated from cell lysates. Co-immunoprecipitating endogenous hsc70 was detected by immunoblotting (lane 1, arrowhead). Hsc70 was not detected if tau was absent (lane 2) or if non-specific IgG (lane 3) was used in immunoprecipitation. The ~98 kDa species (marked by *) in the lysate lane is a background band.
B. Human 4R tau was expressed in COS7 cells by transient transfection. As above, immunoprecipitation showed that endogenous hsc70 co-immunoprecipitated with the 4R tau. Tau signal in the lysate (lane 6), upon longer exposure, displayed a multi-band tau pattern similar to the tau immunoprecipitate (lane 4).
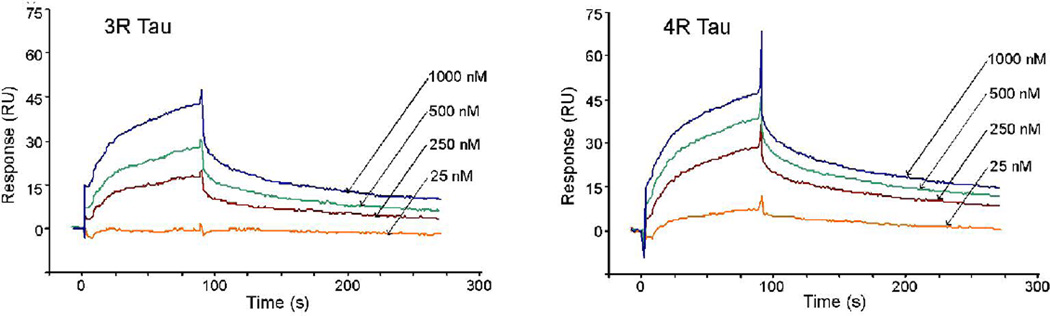
To test for binding between purified preparations of tau and hsc70 and to assess real-time binding kinetics we utilized surface plasmon resonance (SPR). When different concentrations of recombinant 3R or 4R tau (analyte) were injected over immobilized recombinant bovine hsc70 (ligand), the BIAcore sensorgrams provided evidence of specific binding between tau and hsc70 (Fig. 3). The sensorgrams showed a good fit to a 1:1 Langmuir interaction model, as judged by the residuals being within 2 R.U. and the chi square values being under 10 (Table I). Kinetic analysis suggested that the 4R tau-hsc70 association proceeded faster than the 3R tau-hsc70 association and that the 4R tau-hsc70 complexes dissociated more slowly. SPR data suggested that hsc70 affinity for 4R tau was two-fold higher than affinity for 3R tau, with equilibrium dissociation constants of 0.31 and 0.16 µM, respectively. These data confirmed the yeast two-hybrid results in vitro and indicated that phosphorylation of tau was not required for the interaction.
Fig. 3. Tau and hsc70 show specific, high-affinity binding measured by surface plasmon resonance (SPR).
A range of 3R or 4R tau analyte concentrations was injected over a test surface containing immobilized bovine hsc70. The relative changes in SPR signal, as obtained by subtracting the signal of a control dextran surface from that of the hsc70 test surface, are shown. Analyte injection lasted 90 sec (association phase) and the dissociation phase, starting at time = 90 sec, was monitored for at least 180 sec. Sensorgrams shown are from a single representative experiment; however, multiple injections with a wider range of tau concentrations were used calculate the values shown in Table I
Table I.
Surface plasmon resonance analysis of tau-hsc70 binding
| Tau | kon ± S.D. (M−1s−1) | koff ± S.D. (s−1) | KD ± S.D. (M) | n | Residuals (R.U.) | Chi-square |
|---|---|---|---|---|---|---|
| 3R | 15.13 ± 1.36 × 103 | 4.66 ± 0.71 × 10−3 | 0.31 ± 0.05 × 10−6 | 3 | −2 to +2 | 0.21; 0.71; 0.78 |
| 4R | 21.60 ± 3.54 × 103 | 3.44 ± 1.40 × 10−3 | 0.16 ± 0.04 × 10−6 | 2 | −2 to +2 | 0.22; 0.27 |
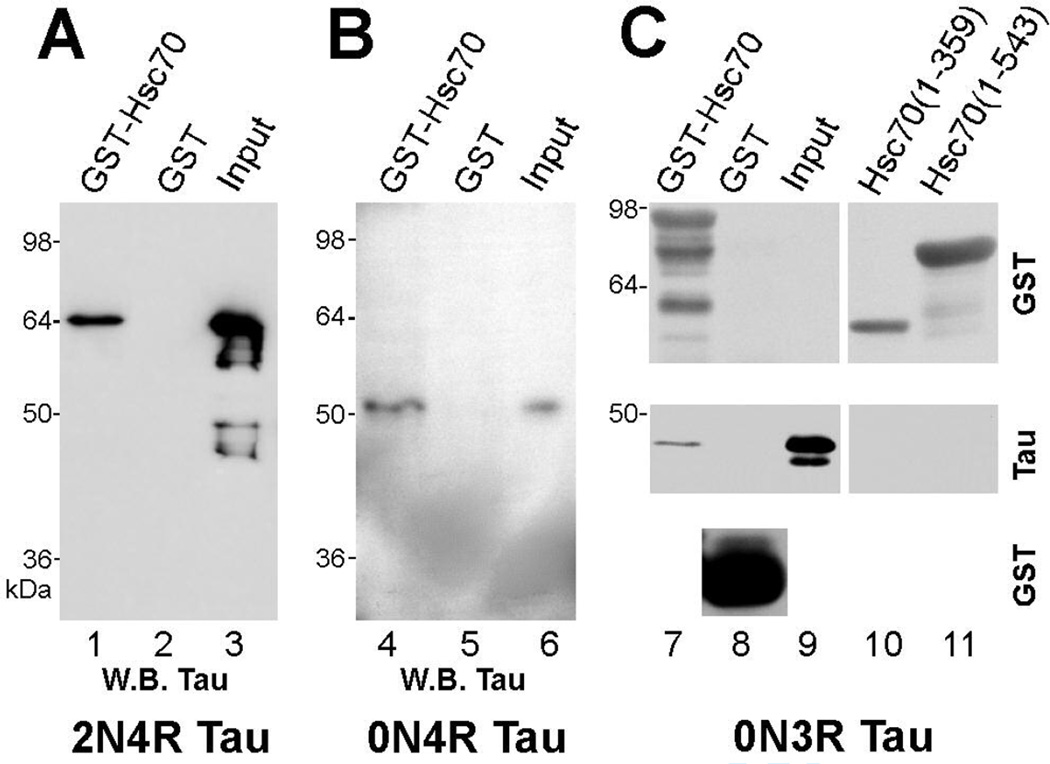
To further investigate the hsc70 interaction with tau, we conducted in vitro sedimentation assays using GST-Hsc70 and recombinant human tau. Hsc70 bound each of the human wild type tau isoforms tested: 2N4R, 4R and 3R (Fig. 4, lanes 1, 4, 7). In addition, we tested the smallest binding isoform (3R) for interaction with two fragments of hsc70. Consistent with our previous results, GST-Hsc70(1–359), which lacked the hsc70 sequence identified in the yeast two-hybrid screen, did not bind tau (Fig. 4, lane 10). GST-Hsc70(1–543), which contained the ATPase and peptide-binding domains of hsc70, did not bind tau either, suggesting that the variable domain, downstream of the hsc70 peptide-binding domain, was required for tau binding (Fig. 4, lane 11). This finding is consistent with previous reports implicating the variable domain in the retention of bound substrate (see Discussion).
Fig. 4. Tau binding requires the carboxy-terminal variable domain of hsc70.
Recombinant human tau isoforms 2N4R, 0N4R (4R) and 0N3R (3R) were tested for binding to GST-Hsc70 using GST protein as a negative control (lanes 1–9). Protein complexes were resolved by SDS-PAGE and tau binding was confirmed by immunoblotting. As controls, top and bottom panels in lanes 7 and 8 show the GST proteins as detected by anti-GST. Lanes 10 and 11 test the association between 3R tau and two deletion mutants of GST-Hsc70 that lack hsc70 carboxy-terminal sequence; the two truncated GST-Hsc70 proteins were detected by anti-GST (lanes 10 and 11, top panel). Full length hsc70 bound all wild-type tau isoforms tested (lanes 1, 4, 7) while two hsc70 deletion mutants lacking carboxy-terminal sequence did not bind tau (lanes 10 and 11, middle panel). Some recombinant protein preps showed some degradation, as in lanes 3 and 7. Input lanes depict the tau input: lane 3 = 10% (50 ng); lane 6 = 0.5% (1 ng); lane 9 = 1% (2 ng).
Short hydrophobic amino acid sequences in the tau MTBR bind to hsc70
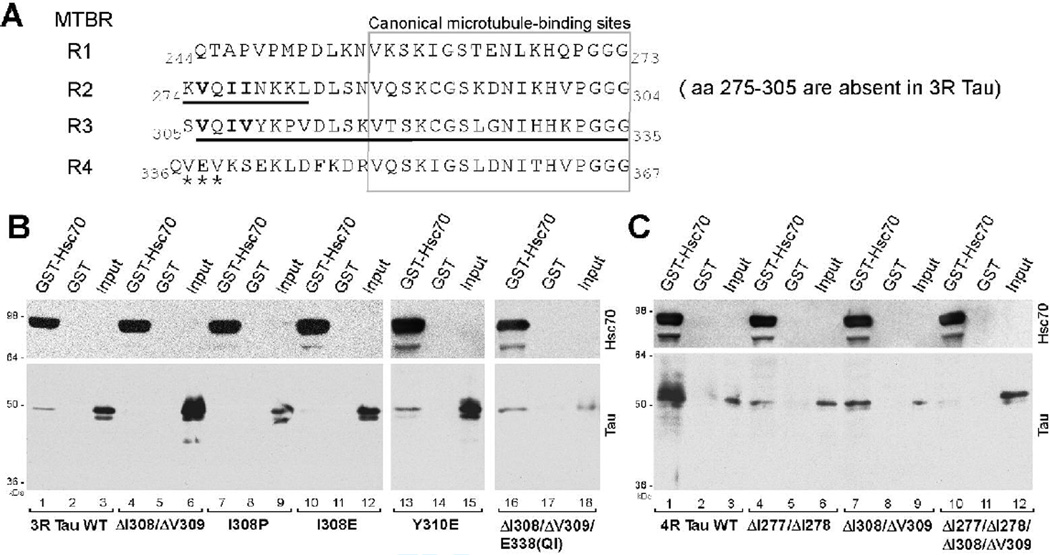
To map the binding site on tau, we tested a panel of tau mutants for binding to GST-Hsc70 (Fig. 5). For ease of reference, tau constructs analyzed for GST-Hsc70 binding are numbered in Fig. 5 (extreme left). As indicated, the tau MTBR comprises sequences R1, R2, R3 and R4; alternative splicing removes aa 275–305 (R2) to generate 3R tau isoforms. The wild-type 2N4R, 4R and 3R tau proteins have been included for reference, as #1, #7, and #10, respectively. Hsc70 binding of 3R tau (#10) and 4R tau (#7) had already shown that the tau sequence encoded by exons 2 and 3 was not needed for binding. In fact, a large part of tau, immediately upstream of the microtubule binding region (aa 50–244), was not needed for hsc70 binding, as shown by #2. Further, the inability of #3 and #4 to bind hsc70 also excluded the amino terminus of tau as a binding site and, by comparison with #2, suggested that the microtubule binding repeats might be involved. The binding of #5 and #6, in light of the results from #3 and #4, excluded the extreme carboxy terminus of tau from our analyses and focused our attention on the tau repeats R1, R2 and R3. Additionally, the binding of #8 and #9 to hsc70 suggested that repeats R1 and R2 were sufficient for binding. The inability of #11 to bind hsc70 was not surprising, in the light of previous results from #2. Importantly, the inability of #12 to bind suggested that the binding site lay downstream of R1, while a comparison of #12 and #5 allowed us to narrow down the binding site to aa 274–282, i.e. the beginning of tau R2. However, this finding that aa 274–282 within R2 could endow tau with hsc70-binding ability was complicated by the fact that #10 (3R isoform), which lacked R2 (aa 275–305), still bound hsc70. This suggested that there could be more than one hsc70 binding site in the tau microtubule binding domain. Comparing #12 and #13 indicated that another hsc70 binding site was located within aa 306–335 of R3 (aa 305–335). This would also explain why #14, lacking R2 but containing R3, bound hsc70. Though #3 and #4 had indicated that the carboxy-terminal half of R4 lacked hsc70 binding activity, we could not entirely exclude the possibility of R4 having additional hsc70 binding activity within 336–355. Similarly, we had not excluded the possibility of hsc70 binding activity within 283–304. Subsequently, our analysis showed that sites at the beginning of R2 and R3 account entirely for tau’s binding to hsc70 (see below).
As indicated in Fig. 6A, each of R1, R2, R3 and R4 contains a canonical microtubule-binding site (Butner and Kirschner 1991; Ennulat et al. 1989) located downstream of a 12–14 mer sequence. The 12–14 mer sequences at the beginning of R2, R3 and R4 were initially referred to as “inter-repeat” sequences, and those in R2 and R3 have been shown to influence microtubule binding (Goode et al. 2000; Goode and Feinstein 1994; Mukrasch et al.). Our deletion analysis had resolved a hsc70 binding site to one such region at the beginning of R2, aa 274–282 (Fig. 6A, underlined). At the same time, we had evidence of another hsc70 binding site within R3, aa 306–335 (Fig. 6A, underlined). We noted that aa 306–311 displayed considerable homology to aa 275–280. Specifically, 275VQII278 constituted a cluster of strongly hydrophobic residues (with the exception of Q) at the beginning of R2, and 306VQIV309 had a similar characteristic and location in R3. It has been shown that heat shock 70 proteins, including hsc70, can bind 5–7-mer sequences rich in hydrophobic amino acids (Fourie et al. 1994; Rudiger et al. 1997; Takenaka et al. 1995). We therefore hypothesized that a ΦQΦΦ site, where Φ represented a strongly hydrophobic amino acid, might mediate tau-hsc70 binding, as in the case of 275VQII278 and 306VQIV309. Noticeably, no part of R1, which was incapable of hsc70 binding (Fig. 5, #12), was homologous to 275VQII278 or 306VQIV309, while the beginning of R4 had only limited homology (337VEV339, marked by asterisks in Fig. 6A).
Fig. 6. VQI(I/V) motifs in tau repeats R2 and R3 mediate hsc70 binding.
A. Primary structure of the tau microtubule binding repeat domain, showing R1, R2, R3 and R4 (boundaries do not necessarily correspond to the exon boundaries). Amino acids 275–305, which are encoded by exon 10, are absent in three-repeat tau (3R tau). Therefore, in 3R tau, K274 is followed by V306, and R2 is omitted. Bold underlining marks the two hsc70-binding areas identified by previous deletion analysis (Fig. 5). The hydrophobic amino acids clustered in VQI(I/V) are represented by bold font in R2 and R3. A less hydrophobic cluster containing VEV in R4 is labeled with asterisks.
B. Disruption of hydrophobic residues within 306VQIV309 impairs 3R tau-hsc70 binding. Tau mutants were tested for binding to GST-Hsc70 or GST control. Upper panels show GST-Hsc70 detected by a hsc70 antibody. Lower panels show sedimented tau and input tau. Input lanes represent about 1% (~ 2 ng) of the total tau input, except lane 18 which shows 0.5% (~ 0.9 ng). Note that the 3R Y310E mutation, positioned immediately downstream of 306VQIV309, did not impair mutant tau-hsc70 binding (lane 13). GST-Hsc70 also bound 3R ΔI308/ΔV309/E338(QI), a tau mutant in which the loss of I308/V309 in R3 was compensated by the creation of a VQIV cluster in R4 (lane 16).
C. Coincident disruption of hydrophobic residues within both hsc70 binding sites impairs 4R tau-hsc70 binding. GST-Hsc70 bound 4R tau (lane 1) and also bound the 4R ΔI277/ΔI278 (lane 4) and ΔI308/ΔV309 (lane 7) tau mutants, in which only one of the two hsc70 binding sites had been disrupted. Hsc70 binding was severely impaired in the 4R ΔI277/ΔI278/ΔI308/ΔV309 mutant, which contained deletions in both hsc70 binding sites (lane 10). Input lanes show ~ 0.4% (0.8 ng) of the total tau input, except lane 9, which shows ~0.2% (0.4 ng).
To test our hypothesis, we disrupted the hydrophobic clusters in 275VQII278 and 306VQIV309 by site directed mutagenesis and tested the behavior of the tau mutants in GST-Hsc70 binding assays. Per our hypothesis, since 3R tau lacks 275VQII278, disruption of the 306VQIV309 site should be sufficient to affect hsc70 binding. We found that deletion of 308IV309 (ΔI308/ΔV309, Fig. 6B, lane 4) or deletion of 306VQI308 (data not shown) reduced hsc70 binding to 3R tau. We found that I308P and I308E point mutations also reduced hsc70 binding to 3R tau (Fig. 6B, lanes 7, 10). We did not test any amino acids downstream of Y310, since the Y310E 3R mutant retained its binding to hsc70 (Fig. 6B lane 13). Further, the inability of 3R tau to bind hsc70 following disruption of the 306VQIV309 site in R3 indicated that 337VEV339 in R4 could not serve as an alternative binding site. We next asked whether the disruption of the ΦQΦΦ in R3 could be compensated by insertion of a ΦQΦΦ motif into a different location. To test this possibility, we chose R4, since it already had two valine residues in close proximity (337VEV339, Fig. 6A). We modified our 3R tau ΔI308/ΔV309 mutant to create a VQIV site in R4, by replacing the E338 with (QI). In contrast to the ΔI308/ΔV309 mutant, this ΔI308/ΔV309 /E338(QI) mutant bound hsc70 (Fig. 6B lane 16), suggesting that the VQIV was sufficient for hsc70 binding. Turning to 4R tau, since 4R tau contains two binding sites for hsc70, disruption of any one site alone should not abolish hsc70 binding. Consistent with our hypothesis, hsc70 bound both the 4R ΔI277/ΔI278 and the 4R ΔI308/ΔV309 mutants (Fig. 6C, lanes 4,7). However, when both ΦQΦΦ sites were mutated (4R ΔI277/ΔI278/ΔI308/ΔV309), binding to hsc70 was clearly affected (Fig. 6C, lane 10).
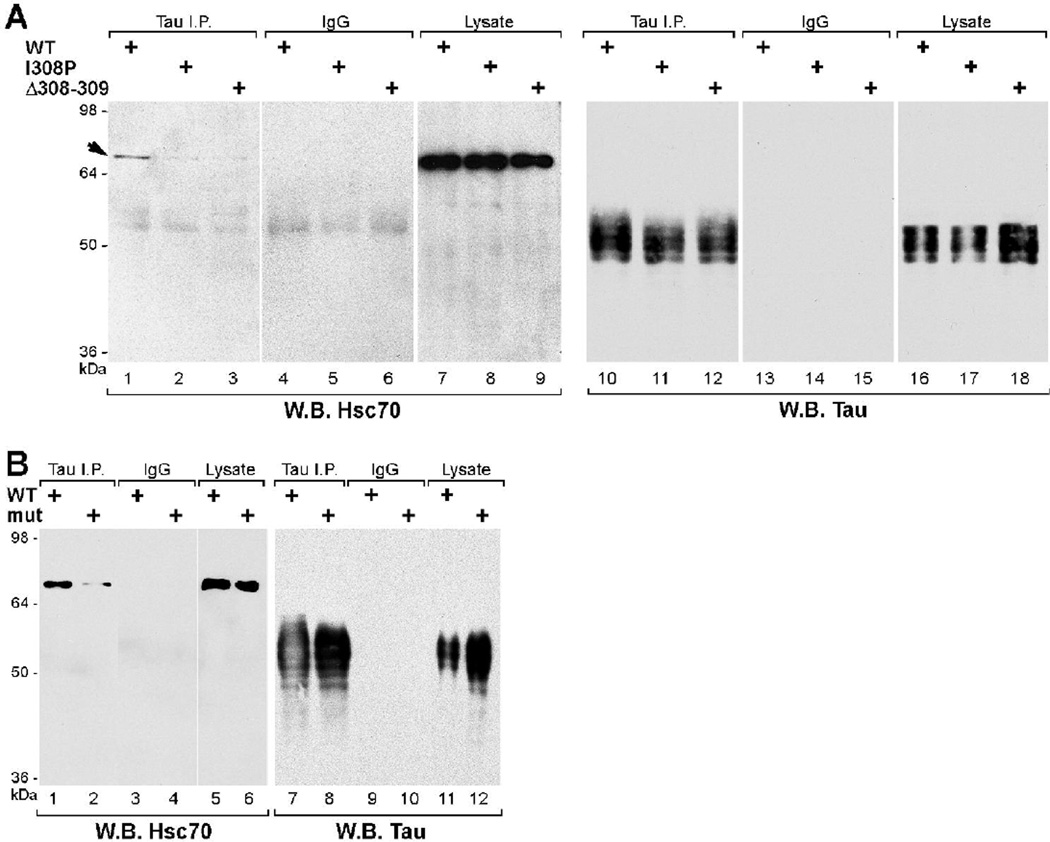
We investigated the effects of tau mutations, which decreased tau-hsc70 association in vitro, on the properties of tau in mammalian cells. Using COS7 cells, we expressed human 3R and 4R tau proteins with mutations in the hsc70-binding sites and tested mutant tau immunoprecipitates for the presence of hsc70. Endogenous hsc70 did not co-IP with 3R I308P or ΔI308/ΔV309 (Fig. 7A, lanes 2, 3). Also, there was a clear reduction in the amount of hsc70 associating with 4R ΔI277/ΔI278/ΔI308/ΔV309 mutant tau, as compared to 4R wild-type tau (Fig. 7B, lane 2 versus lane 1); mutant tau proteins were expressed and IPed similarly to wild-type (Fig.7A and 7B, panels on the right). As the mutations were located close to the canonical microtubule-binding sites in the tau MTBR, we also determined the localization pattern of mutant tau in transiently transfected mouse NIH/3T3 fibroblasts using indirect immunofluorescence: both 3R and 4R tau mutants showed co-localization with microtubules, similar to wild-type tau (data not shown).
Fig. 7. VQI(I/V) motifs mediate tau association with hsc70 in COS cells.
Human wild-type tau or tau with mutations that decreased tau-hsc70 binding in vitro were transiently expressed in COS7 cells. Tau was immunoprecipitated and associating endogenous hsc70 was analyzed by Western blotting.
A. Hsc70 co-immunoprecipitated with 3R wt (lane 1), but not with 3R mutant I308P (lane 2) or ΔI308/ΔV309 (lane 3). Lanes 10–12 show that wild type and mutant tau immunoprecipitations were equivalent. Lanes 16–18 show comparable expression levels of wild type and mutant tau proteins.
B. Hsc70 co-immunoprecipitated with 4R wt (lane 1) and, by comparison, weakly immunoprecipitated with the 4R tau ΔI277/ΔI278/ΔI308/ΔV309 mutant (lane 2). In this particular experiment, the difference in tau levels (lane 7–8 and 11–12) showed that despite higher levels of protein present the 4R mutant tau was less efficient in associating with hsc70.
Hsp70 and hsc70 binding is mediated by the same site in tau
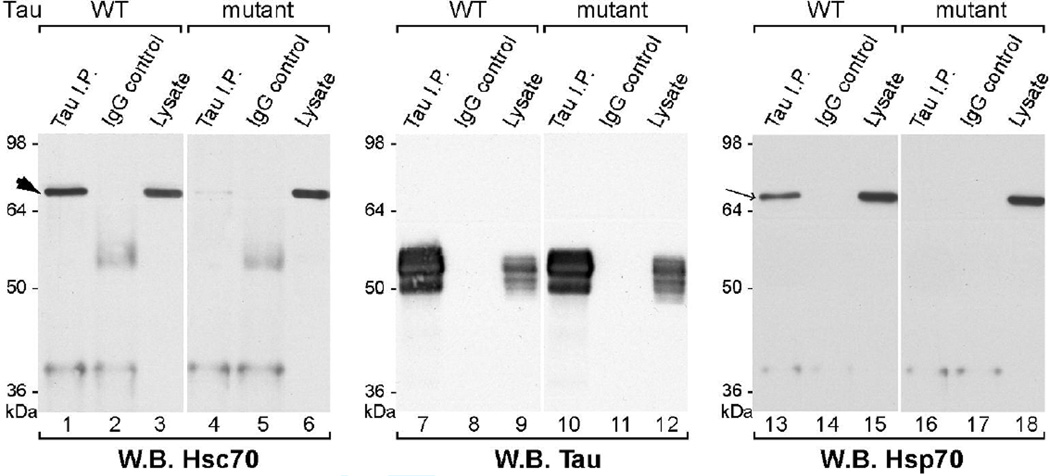
We also analyzed tau mutations affecting tau-hsc70 binding in neuronal cell lines. We established human neuroblastoma SH-SY5Y cells lines with stable expression of 3R wild-type or 3R ΔI308/ΔV309 tau. Tau immunoprecipitation confirmed the association of wild-type tau with endogenous hsc70 and the lack of association between the tau mutant and endogenous hsc70 (Fig. 8, lanes 1, 4). Lastly, as hsc70 and the inducible hsp70 show similarities in their substrate-binding properties (Mayer and Bukau 2005), we investigated the association between tau and hsp70. To facilitate detection of hsp70 in the SH-SY5Y cells, thermal conditioning was used to elevate hsp70 levels, as described in Materials and Methods. While hsp70 associated with wild-type tau in these neuronal cells, the association was noticeably reduced by the ΔI308/ΔV309 mutation (Fig. 8, lanes 13, 16). Similar results were obtained using other pairs of clonal cell lines (data not shown). These results strongly suggest that hsp70 and hsc70 utilize either common or overlapping sites for binding to tau.
Fig. 8. VQI(I/V) motif mediates tau association with hsc70 and hsp70 in neuronal cells.
Transfected SH-SY5Y human neuroblastoma cell lines, stably expressing 3R wild-type or ΔI308/ΔV309 (mutant) tau, were subjected to thermal conditioning to increase hsp70 protein levels. Tau immunoprecipitates were analyzed by probing Western blots for hsc70 (lanes 1–6), hsp70 (lanes 13–18) or tau (lanes 7–12). Hsp70 (arrow) migrated slightly faster than hsc70 (arrowhead). Similar to hsc70, little or no hsp70 co-immunoprecipitated with the mutant tau.
Discussion
An association between tau and hsp70/hsc70 was first reported in PC12 cells (Wallace et al. 1993). Later, hsc70 was identified in a group of brain lysate proteins that associated preferentially with phosphorylated tau (Shimura et al. 2004b). In the same study, in vitro phosphorylation of tau by glycogen synthase kinase (GSK)-3β enhanced the subsequent ubiquitination of tau, in the presence of hsc70, by the E3 ligase CHIP (carboxyl terminus of Hsc70-interacting protein). Thus, the authors proposed that tau phosphorylation increased its association with a hsc70-CHIP complex, which facilitated tau polyubiquitination and degradation (Shimura et al. 2004b). Recently, an association between tau and hsc70 has also been inferred from an hsc70-dependent interaction between BAG-1 and tau (Elliott et al. 2007). Our findings reveal a direct association between hsc70 and tau that does not require phosphorylation and, as discussed below, suggest that hsc70/hsp70 interaction with tau may impact on tau aggregation.
Our first indication that hsc70 could bind to tau stemmed from an unbiased screen for tau interactors using the yeast two-hybrid assay. Upon verifying that endogenous hsc70 and tau complexes existed in mammalian cells, we proceeded to characterize the interaction further. Surface plasmon resonance data suggested a high-affinity direct interaction that did not require an ATP-induced conformational change in hsc70. Though ATP-induced conformational changes are generally considered an integral part of the hsc70-substrate cycle in vivo, substrate binding can occur without ATP, as shown for hsc70-clathrin binding (Prasad et al. 1994). SPR analysis yielded submicromolar KD values for tau-hsc70 binding, which compare favorably with other reported KD values for HSP70-substrate binding (de Crouy-Chanel et al. 1999; Rudiger et al. 1997). The SPR data demonstrated that compared to 3R tau, 4R tau showed a two-fold higher affinity for hsc70. The difference in affinity is consistent with our subsequent identification of two hsc70-binding sites in 4R and one in 3R.
In mapping the hsc70 binding site on tau, we employed sedimentation assays using GST-Hsc70 proteins (Ungewickell et al. 1997; Wang et al. 1993). However, the degree of tau binding observed in the sedimentation assays did not correlate well with the submicromolar KD yielded by SPR analysis. The lower binding efficiency of the sedimentation assay might be due to the higher detergent and lower temperature conditions employed relative to the SPR conditions. Also, the hsc70 in the sedimentation assays carried an N-terminal GST tag and N-terminal tagging has been shown to affect the substrate binding affinities of hsc70 (Boice and Hightower 1997). Nevertheless, the presence of the GST tag did not interfere with our ability to map hsc70 interaction sites on tau.
The VQII and VQIV hsc70-binding motifs identified here agree with previous reports of peptide recognition by hsc70. Hsc70 has been shown to preferentially bind short peptides enriched in hydrophobic/basic or hydrophobic/aromatic amino acids (Fourie et al. 1994; Takenaka et al. 1995), which are characteristics of the KVQIINKK and VQIVYK sequences in the tau R2 and R3, respectively (Fig. 6A). While VQIVYK is present in all tau isoforms, KVQIINKK is present only in 4R isoforms due to alternative splicing. The importance of the isoleucine and valine in the interaction was proven by mutating or deleting the I308/V309, and by conversion of aa 337–339 (VEV) to VQIV. Previously, Rudiger et al. had proposed that 2–4 consecutive hydrophobic amino acids might constitute DnaK binding sites (Rudiger et al. 1997). Our data indicate that 1 or 2 consecutive hydrophobic amino acids can critically affect hsc70 (or hsp70) binding. Presumably, the flanking residues may control specificity. Our data also showed that formation of stable tau-hsc70 complexes requires both the peptide-binding domain and the variable domain of hsc70. This is in agreement with the general view that although the substrate-binding domain of hsc70 can independently interact with peptides, part of the variable domain forms a cap or lid which is required to retain larger polypeptides in the peptide-binding domain (Boice and Hightower 1997; Hu and Wang 1996).
The sequences 275VQIINK280 (also known as PHF6*) and 306VQIVYK311 (also known as PHF6) have previously been identified as polypeptide regions involved in tau aggregation (Giannetti et al. 2000; Li and Lee 2006; Santa-Maria et al. 2006; von Bergen et al. 2001; von Bergen et al. 2000). Our data suggest that both sequences contain hsc70-binding sites as well. Interestingly, the KD values for tau-hsc70 binding are about two orders of magnitude lower than the recently reported SPR-derived KD for tau-tau binding (46 µM) (Guo et al. 2006). Thus, if the hsc70-tau interaction is stronger than the tau-tau interaction, hsc70 might competitively inhibit tau-tau binding. We hypothesize that hsc70 or hsp70 binding can interfere with tau aggregation. Consistent with this hypothesis, Sahara and co-workers have recently demonstrated that hsp70 decreases the heparin-induced in vitro aggregation of recombinant human tau (Sahara et al. 2007). The loss of tau aggregation activity, though, may not necessarily lead to a loss of hsc70 binding. For example, the Y310E mutation, which suppressed tau fibrillization in previous reports (Scaramozzino et al. 2006; von Bergen et al. 2000), did not decrease hsc70 binding with 3R tau.
The location of the hsc70-binding site in the tau MTBR suggests that hsc70 cannot bind microtubule-associated tau. This is supported by the finding that hsc70 does not fractionate with tau-microtubule complexes (Elliott et al. 2007). The location of the hsc70-binding site also suggests that hsc70 might compete with tubulin or microtubules for binding to tau. The cellular level of hsc70 is reported to be 1% of total protein (Schlossman et al. 1984) while tubulin is estimated to be 2–5% of total protein in cell cultures and even higher in neural tissues (Hiller and Weber 1978). Therefore, both are present in excess over tau, which has been estimated to be a modest 0.25% of cellular protein (Drubin et al. 1985). The equilibrium KD for tau-hsc70 binding was between 0.16 – 0.31 µM in our SPR experiments whereas the reported KD for tau-microtubule binding has ranged from 0.15 – 1.10 µM (Goode et al. 2000; Goode and Feinstein 1994; Gustke et al. 1994). Thus, a close competition between hsc70 and microtubules for tau may be possible, with tau phosphorylation adding yet another layer of complexity.
Our data implicating I308/V309 in tau’s binding to hsp70 similarly suggest that tau cannot bind simultaneously to hsp70 and microtubules. However, unlike hsc70, which does not associate directly with tubulin or microtubules (Gache et al. 2005), hsp70 binds microtubules, presumably through its N-terminus (Sanchez et al. 1994). This would allow hsp70 to simultaneously bind to tau through its C-terminal substrate-binding domain and to microtubules through its N-terminus. Consequently, hsp70 induction might enhance the partitioning of tau to microtubules, as previously observed (Dou et al. 2003).
Hsc70 and hsp70, in the role of molecular chaperones, bind numerous newly-synthesized proteins (Beckmann et al. 1990; Frydman et al. 1994; Qian et al. 2006; Thulasiraman et al. 1999). Therefore, hsc70 may bind nascent or newly-synthesized tau, as previously hypothesized (Kirby et al. 1994). Newly-synthesized tau would comprise a small fraction of total cellular tau, which would be consistent with the very small fraction of cellular hsc70 that co-immunoprecipitated with tau from cultured cells.
Our findings imply that hsc70 and/or hsp70 can bind all tau isoforms in the nervous system. Since hsc70 is ubiquitous and highly expressed in the cytosol, hsc70 would be in a position to complement, though not necessarily imitate, other chaperones acting on tau (Dickey et al. 2006; Dou et al. 2003; Sahara et al. 2007; Shimura et al. 2004a). In summary, we have provided evidence for a direct interaction between tau and hsc70. We have also shown that tau binding by hsc70/hsp70 chaperones requires hydrophobic motifs implicated in tau aggregation. This finding advances our understanding of the interaction between tau and molecular chaperones and may contribute towards therapeutic strategies based on leveraging well-developed intracellular chaperone systems to delay, prevent or even reverse tau accumulation and aggregation in disease.
Acknowledgment
We are grateful to Dr. Henry Paulson for his gifts of hsc70 cDNA and antibodies, to Dr. Kiran Bhaskar for valuable guidance with BIAcore and protein purification, to Aaron Hobbs for the tau Δ305–335 and Δ336–367 plasmids, to Drs. Lester Binder and Dawn Quelle for their gifts of tau and GST antibodies, and to Dr. Jason Schuman (BIAcore) for useful comments. We thank Dr. Roger Brent and Stephanie Kwei for aid in yeast two-hybrid library screening. This study was performed in partial fulfillment of the requirements for a Ph.D. in The University of Iowa Neuroscience Graduate Program (M.S.).
Authors acknowledge the support of NIH grants NS32100 (G.L.) and AG14452 (J.K.).
References
- Ahn SG, Kim SA, Yoon JH, Vacratsis P. Heat-shock cognate 70 is required for the activation of heat-shock factor 1 in mammalian cells. Biochem J. 2005;392(Pt 1):145–152. doi: 10.1042/BJ20050412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer NG, Richter-Landsberg C. The dynamic instability of microtubules is required for aggresome formation in oligodendroglial cells after proteolytic stress. J Mol Neurosci. 2006;29(2):153–168. doi: 10.1385/JMN:29:2:153. [DOI] [PubMed] [Google Scholar]
- Beckmann RP, Mizzen LE, Welch WJ. Interaction of Hsp 70 with newly synthesized proteins: implications for protein folding and assembly. Science. 1990;248(4957):850–854. doi: 10.1126/science.2188360. [DOI] [PubMed] [Google Scholar]
- Bercovich B, Stancovski I, Mayer A, Blumenfeld N, Laszlo A, Schwartz AL, Ciechanover A. Ubiquitin-dependent degradation of certain protein substrates in vitro requires the molecular chaperone Hsc70. J Biol Chem. 1997;272(14):9002–9010. doi: 10.1074/jbc.272.14.9002. [DOI] [PubMed] [Google Scholar]
- Bhaskar K, Yen SH, Lee G. Disease-related modifications in tau affect the interaction between Fyn and Tau. J Biol Chem. 2005;280(42):35119–35125. doi: 10.1074/jbc.M505895200. [DOI] [PubMed] [Google Scholar]
- Boice JA, Hightower LE. A mutational study of the peptide-binding domain of Hsc70 guided by secondary structure prediction. J Biol Chem. 1997;272(40):24825–24831. doi: 10.1074/jbc.272.40.24825. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brandt R, Lee G. Functional organization of microtubule-associated protein tau. Identification of regions which affect microtubule growth, nucleation, and bundle formation in vitro. J Biol Chem. 1993;268(5):3414–3419. [PubMed] [Google Scholar]
- Brodsky JL, Chiosis G. Hsp70 molecular chaperones: emerging roles in human disease and identification of small molecule modulators. Curr Top Med Chem. 2006;6(11):1215–1225. doi: 10.2174/156802606777811997. [DOI] [PubMed] [Google Scholar]
- Butner KA, Kirschner MW. Tau protein binds to microtubules through a flexible array of distributed weak sites. J Cell Biol. 1991;115(3):717–730. doi: 10.1083/jcb.115.3.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmel G, Mager EM, Binder LI, Kuret J. The structural basis of monoclonal antibody Alz50's selectivity for Alzheimer's disease pathology. J Biol Chem. 1996;271(51):32789–32795. doi: 10.1074/jbc.271.51.32789. [DOI] [PubMed] [Google Scholar]
- Chappell TG, Konforti BB, Schmid SL, Rothman JE. The ATPase core of a clathrin uncoating protein. J Biol Chem. 1987;262(2):746–751. [PubMed] [Google Scholar]
- Chiang HL, Terlecky SR, Plant CP, Dice JF. A role for a 70-kilodalton heat shock protein in lysosomal degradation of intracellular proteins. Science. 1989;246(4928):382–385. doi: 10.1126/science.2799391. [DOI] [PubMed] [Google Scholar]
- de Crouy-Chanel A, Kohiyama M, Richarme G. Interaction of DnaK with native proteins and membrane proteins correlates with their accessible hydrophobicity. Gene. 1999;230(2):163–170. doi: 10.1016/s0378-1119(99)00083-9. [DOI] [PubMed] [Google Scholar]
- DeLuca-Flaherty C, McKay DB. Nucleotide sequence of the cDNA of a bovine 70 kilodalton heat shock cognate protein. Nucleic Acids Res. 1990;18(18):5569. doi: 10.1093/nar/18.18.5569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickey CA, Dunmore J, Lu B, Wang JW, Lee WC, Kamal A, Burrows F, Eckman C, Hutton M, Petrucelli L. HSP induction mediates selective clearance of tau phosphorylated at proline-directed Ser/Thr sites but not KXGS (MARK) sites. Faseb J. 2006;20(6):753–755. doi: 10.1096/fj.05-5343fje. [DOI] [PubMed] [Google Scholar]
- Dou F, Netzer WJ, Tanemura K, Li F, Hartl FU, Takashima A, Gouras GK, Greengard P, Xu H. Chaperones increase association of tau protein with microtubules. Proc Natl Acad Sci U S A. 2003;100(2):721–726. doi: 10.1073/pnas.242720499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drubin DG, Feinstein SC, Shooter EM, Kirschner MW. Nerve growth factor-induced neurite outgrowth in PC12 cells involves the coordinate induction of microtubule assembly and assembly-promoting factors. J Cell Biol. 1985;101(5 Pt 1):1799–1807. doi: 10.1083/jcb.101.5.1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Souza SM, Brown IR. Constitutive expression of heat shock proteins Hsp90, Hsc70, Hsp70 and Hsp60 in neural and non-neural tissues of the rat during postnatal development. Cell Stress Chaperones. 1998;3(3):188–199. doi: 10.1379/1466-1268(1998)003<0188:ceohsp>2.3.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott E, Tsvetkov P, Ginzburg I. BAG-1 associates with Hsc70/tau complex and regulates the proteasomal degradation of tau protein. J Biol Chem. 2007 doi: 10.1074/jbc.M706379200. [DOI] [PubMed] [Google Scholar]
- Ennulat DJ, Liem RK, Hashim GA, Shelanski ML. Two separate 18-amino acid domains of tau promote the polymerization of tubulin. J Biol Chem. 1989;264(10):5327–5330. [PubMed] [Google Scholar]
- Fourie AM, Sambrook JF, Gething MJ. Common and divergent peptide binding specificities of hsp70 molecular chaperones. J Biol Chem. 1994;269(48):30470–30478. [PubMed] [Google Scholar]
- Frydman J, Nimmesgern E, Ohtsuka K, Hartl FU. Folding of nascent polypeptide chains in a high molecular mass assembly with molecular chaperones. Nature. 1994;370(6485):111–117. doi: 10.1038/370111a0. [DOI] [PubMed] [Google Scholar]
- Gache V, Louwagie M, Garin J, Caudron N, Lafanechere L, Valiron O. Identification of proteins binding the native tubulin dimer. Biochem Biophys Res Commun. 2005;327(1):35–42. doi: 10.1016/j.bbrc.2004.11.138. [DOI] [PubMed] [Google Scholar]
- Giannetti AM, Lindwall G, Chau MF, Radeke MJ, Feinstein SC, Kohlstaedt LA. Fibers of tau fragments, but not full length tau, exhibit a cross beta-structure: implications for the formation of paired helical filaments. Protein Sci. 2000;9(12):2427–2435. doi: 10.1110/ps.9.12.2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goedert M, Jakes R. Mutations causing neurodegenerative tauopathies. Biochim Biophys Acta. 2005;1739(2–3):240–250. doi: 10.1016/j.bbadis.2004.08.007. [DOI] [PubMed] [Google Scholar]
- Goode BL, Chau M, Denis PE, Feinstein SC. Structural and functional differences between 3-repeat and 4-repeat tau isoforms. Implications for normal tau function and the onset of neurodegenetative disease. J Biol Chem. 2000;275(49):38182–38189. doi: 10.1074/jbc.M007489200. [DOI] [PubMed] [Google Scholar]
- Goode BL, Feinstein SC. Identification of a novel microtubule binding and assembly domain in the developmentally regulated inter-repeat region of tau. J Cell Biol. 1994;124(5):769–782. doi: 10.1083/jcb.124.5.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo JP, Arai T, Miklossy J, McGeer PL. Abeta and tau form soluble complexes that may promote self aggregation of both into the insoluble forms observed in Alzheimer's disease. Proc Natl Acad Sci U S A. 2006;103(6):1953–1958. doi: 10.1073/pnas.0509386103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustke N, Trinczek B, Biernat J, Mandelkow EM, Mandelkow E. Domains of tau protein and interactions with microtubules. Biochemistry. 1994;33(32):9511–9522. doi: 10.1021/bi00198a017. [DOI] [PubMed] [Google Scholar]
- Gyuris J, Golemis E, Chertkov H, Brent R. Cdi1, a human G1 and S phase protein phosphatase that associates with Cdk2. Cell. 1993;75(4):791–803. doi: 10.1016/0092-8674(93)90498-f. [DOI] [PubMed] [Google Scholar]
- Hall GF, Yao J, Lee G. Human tau becomes phosphorylated and forms filamentous deposits when overexpressed in lamprey central neurons in situ. Proc Natl Acad Sci U S A. 1997;94(9):4733–4738. doi: 10.1073/pnas.94.9.4733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiller G, Weber K. Radioimmunoassay for tubulin: a quantitative comparison of the tubulin content of different established tissue culture cells and tissues. Cell. 1978;14(4):795–804. doi: 10.1016/0092-8674(78)90335-5. [DOI] [PubMed] [Google Scholar]
- Hu SM, Wang C. Involvement of the 10-kDa C-terminal fragment of hsc70 in complexing with unfolded protein. Arch Biochem Biophys. 1996;332(1):163–169. doi: 10.1006/abbi.1996.0328. [DOI] [PubMed] [Google Scholar]
- Kirby BA, Merril CR, Ghanbari H, Wallace WC. Heat shock proteins protect against stress-related phosphorylation of tau in neuronal PC12 cells that have acquired thermotolerance. J Neurosci. 1994;14(9):5687–5693. doi: 10.1523/JNEUROSCI.14-09-05687.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee G, Newman ST, Gard DL, Band H, Panchamoorthy G. Tau interacts with src-family non-receptor tyrosine kinases. J Cell Sci. 1998;111(Pt 21):3167–3177. doi: 10.1242/jcs.111.21.3167. [DOI] [PubMed] [Google Scholar]
- Lee G, Thangavel R, Sharma VM, Litersky JM, Bhaskar K, Fang SM, Do LH, Andreadis A, Van Hoesen G, Ksiezak-Reding H. Phosphorylation of tau by fyn: implications for Alzheimer's disease. J Neurosci. 2004;24(9):2304–2312. doi: 10.1523/JNEUROSCI.4162-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Lee VM. Characterization of Two VQIXXK Motifs for Tau Fibrillization in Vitro. Biochemistry. 2006;45(51):15692–15701. doi: 10.1021/bi061422+. [DOI] [PubMed] [Google Scholar]
- Makarova O, Kamberov E, Margolis B. Generation of deletion and point mutations with one primer in a single cloning step. Biotechniques. 2000;29(5):970–972. doi: 10.2144/00295bm08. [DOI] [PubMed] [Google Scholar]
- Manzerra P, Rush SJ, Brown IR. Tissue-specific differences in heat shock protein hsc70 and hsp70 in the control and hyperthermic rabbit. J Cell Physiol. 1997;170(2):130–137. doi: 10.1002/(SICI)1097-4652(199702)170:2<130::AID-JCP4>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Mayer MP, Bukau B. Hsp70 chaperones: cellular functions and molecular mechanism. Cell Mol Life Sci. 2005;62(6):670–684. doi: 10.1007/s00018-004-4464-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina M, Montejo de Garcini E, Avila J. The role of tau phosphorylation in transfected COS-1 cells. Mol Cell Biochem. 1995;148(1):79–88. doi: 10.1007/BF00929506. [DOI] [PubMed] [Google Scholar]
- Morrison AJ, Rush SJ, Brown IR. Heat shock transcription factors and the hsp70 induction response in brain and kidney of the hyperthermic rat during postnatal development. J Neurochem. 2000;75(1):363–372. doi: 10.1046/j.1471-4159.2000.0750363.x. [DOI] [PubMed] [Google Scholar]
- Mukrasch MD, Biernat J, von Bergen M, Griesinger C, Mandelkow E, Zweckstetter M. Sites of tau important for aggregation populate {beta}-structure and bind to microtubules and polyanions. J Biol Chem. 2005;280(26):24978–24986. doi: 10.1074/jbc.M501565200. [DOI] [PubMed] [Google Scholar]
- Newmyer SL, Schmid SL. Dominant-interfering Hsc70 mutants disrupt multiple stages of the clathrin-coated vesicle cycle in vivo. J Cell Biol. 2001;152(3):607–620. doi: 10.1083/jcb.152.3.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad K, Heuser J, Eisenberg E, Greene L. Complex formation between clathrin and uncoating ATPase. J Biol Chem. 1994;269(9):6931–6939. [PubMed] [Google Scholar]
- Qian SB, Princiotta MF, Bennink JR, Yewdell JW. Characterization of rapidly degraded polypeptides in mammalian cells reveals a novel layer of nascent protein quality control. J Biol Chem. 2006;281(1):392–400. doi: 10.1074/jbc.M509126200. [DOI] [PubMed] [Google Scholar]
- Richter-Landsberg C, Bauer NG. Tau-inclusion body formation in oligodendroglia: the role of stress proteins and proteasome inhibition. Int J Dev Neurosci. 2004;22(7):443–451. doi: 10.1016/j.ijdevneu.2004.07.003. [DOI] [PubMed] [Google Scholar]
- Rudiger S, Germeroth L, Schneider-Mergener J, Bukau B. Substrate specificity of the DnaK chaperone determined by screening cellulose-bound peptide libraries. Embo J. 1997;16(7):1501–1507. doi: 10.1093/emboj/16.7.1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahara N, Maeda S, Yoshiike Y, Mizoroki T, Yamashita S, Murayama M, Park JM, Saito Y, Murayama S, Takashima A. Molecular chaperone-mediated tau protein metabolism counteracts the formation of granular tau oligomers in human brain. J Neurosci Res. 2007;85(14):3098–3108. doi: 10.1002/jnr.21417. [DOI] [PubMed] [Google Scholar]
- Sanchez C, Padilla R, Paciucci R, Zabala JC, Avila J. Binding of heat-shock protein 70 (hsp70) to tubulin. Arch Biochem Biophys. 1994;310(2):428–432. doi: 10.1006/abbi.1994.1188. [DOI] [PubMed] [Google Scholar]
- Santa-Maria I, Perez M, Hernandez F, Munoz V, Moreno FJ, Avila J. In vitro tau fibrillization: mapping protein regions. Biochim Biophys Acta. 2006;1762(7):683–692. doi: 10.1016/j.bbadis.2006.06.003. [DOI] [PubMed] [Google Scholar]
- Scaramozzino F, Peterson DW, Farmer P, Gerig JT, Graves DJ, Lew J. TMAO promotes fibrillization and microtubule assembly activity in the C-terminal repeat region of tau. Biochemistry. 2006;45(11):3684–3691. doi: 10.1021/bi052167g. [DOI] [PubMed] [Google Scholar]
- Schlossman DM, Schmid SL, Braell WA, Rothman JE. An enzyme that removes clathrin coats: purification of an uncoating ATPase. J Cell Biol. 1984;99(2):723–733. doi: 10.1083/jcb.99.2.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimura H, Miura-Shimura Y, Kosik KS. Binding of tau to heat shock protein 27 leads to decreased concentration of hyperphosphorylated tau and enhanced cell survival. J Biol Chem. 2004a;279(17):17957–17962. doi: 10.1074/jbc.M400351200. [DOI] [PubMed] [Google Scholar]
- Shimura H, Schwartz D, Gygi SP, Kosik KS. CHIP-Hsc70 complex ubiquitinates phosphorylated tau and enhances cell survival. J Biol Chem. 2004b;279(6):4869–4876. doi: 10.1074/jbc.M305838200. [DOI] [PubMed] [Google Scholar]
- Smith RC, Rosen KM, Pola R, Magrane J. Stress proteins in Alzheimer's disease. Int J Hyperthermia. 2005;21(5):421–431. doi: 10.1080/02656730500133165. [DOI] [PubMed] [Google Scholar]
- Studier FW, Rosenberg AH, Dunn JJ, Dubendorff JW. Use of T7 RNA polymerase to direct expression of cloned genes. Methods Enzymol. 1990;185:60–89. doi: 10.1016/0076-6879(90)85008-c. [DOI] [PubMed] [Google Scholar]
- Takenaka IM, Leung SM, McAndrew SJ, Brown JP, Hightower LE. Hsc70-binding peptides selected from a phage display peptide library that resemble organellar targeting sequences. J Biol Chem. 1995;270(34):19839–19844. doi: 10.1074/jbc.270.34.19839. [DOI] [PubMed] [Google Scholar]
- Thulasiraman V, Yang CF, Frydman J. In vivo newly translated polypeptides are sequestered in a protected folding environment. Embo J. 1999;18(1):85–95. doi: 10.1093/emboj/18.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungewickell E, Ungewickell H, Holstein SE. Functional interaction of the auxilin J domain with the nucleotide- and substrate-binding modules of Hsc70. J Biol Chem. 1997;272(31):19594–19600. doi: 10.1074/jbc.272.31.19594. [DOI] [PubMed] [Google Scholar]
- von Bergen M, Barghorn S, Li L, Marx A, Biernat J, Mandelkow EM, Mandelkow E. Mutations of tau protein in frontotemporal dementia promote aggregation of paired helical filaments by enhancing local beta-structure. J Biol Chem. 2001;276(51):48165–48174. doi: 10.1074/jbc.M105196200. [DOI] [PubMed] [Google Scholar]
- von Bergen M, Friedhoff P, Biernat J, Heberle J, Mandelkow EM, Mandelkow E. Assembly of tau protein into Alzheimer paired helical filaments depends on a local sequence motif ((306)VQIVYK(311)) forming beta structure. Proc Natl Acad Sci U S A. 2000;97(10):5129–5134. doi: 10.1073/pnas.97.10.5129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace W, Johnson G, Sugar J, Merril CR, Refolo LM. Reversible phosphorylation of tau to form A68 in heat-shocked neuronal PC12 cells. Brain Res Mol Brain Res. 1993;19(1–2):149–155. doi: 10.1016/0169-328x(93)90160-q. [DOI] [PubMed] [Google Scholar]
- Wang TF, Chang JH, Wang C. Identification of the peptide binding domain of hsc70. 18-Kilodalton fragment located immediately after ATPase domain is sufficient for high affinity binding. J Biol Chem. 1993;268(35):26049–26051. [PubMed] [Google Scholar]