Abstract
A low-molecular-weight protein synthesized in yeast mitochondria was purified. The protein was identified as a component of the rutamycin-sensitive ATPase. The amino-acid composition of the purified protein shows an extremely large preponderance of nonpolar residues, which may account for its solubility in chloroform-methanol. Indirect evidence suggests that this component is involved in the conferral of rutamycin sensitivity and may also have a function in the assembly of the ATPase complex.
Keywords: rutamycin, thin-layer chromatography
Full text
PDF
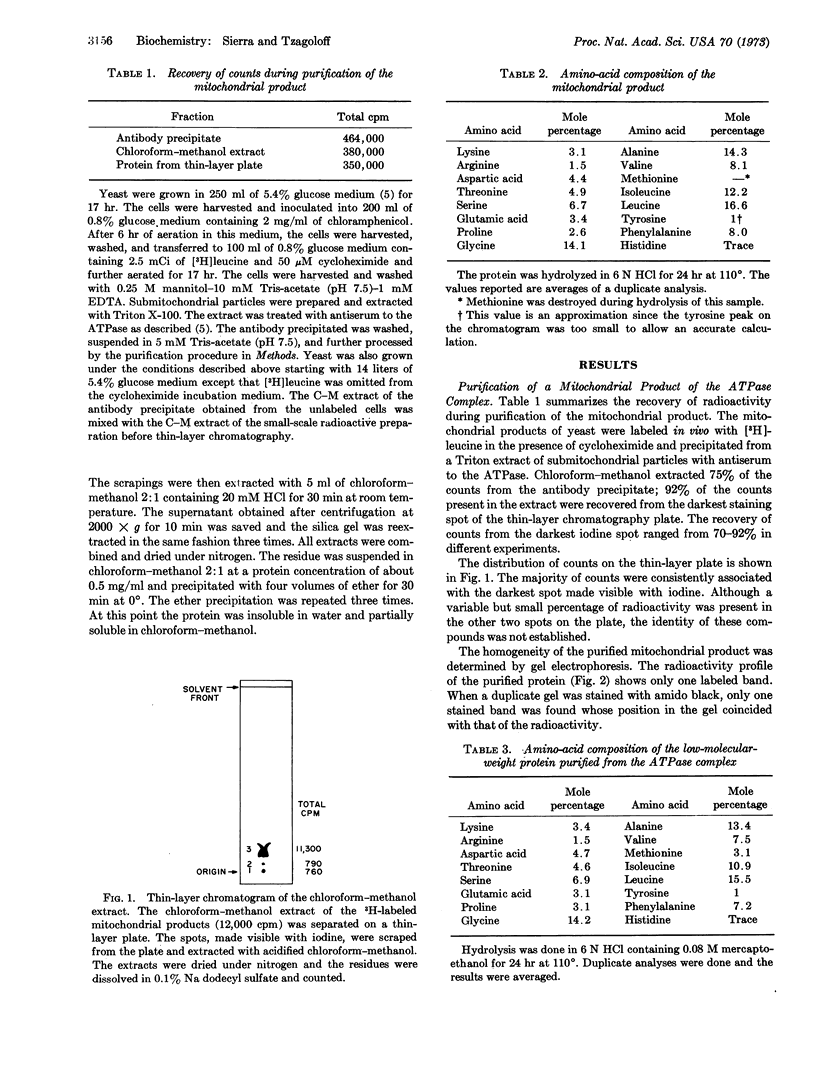
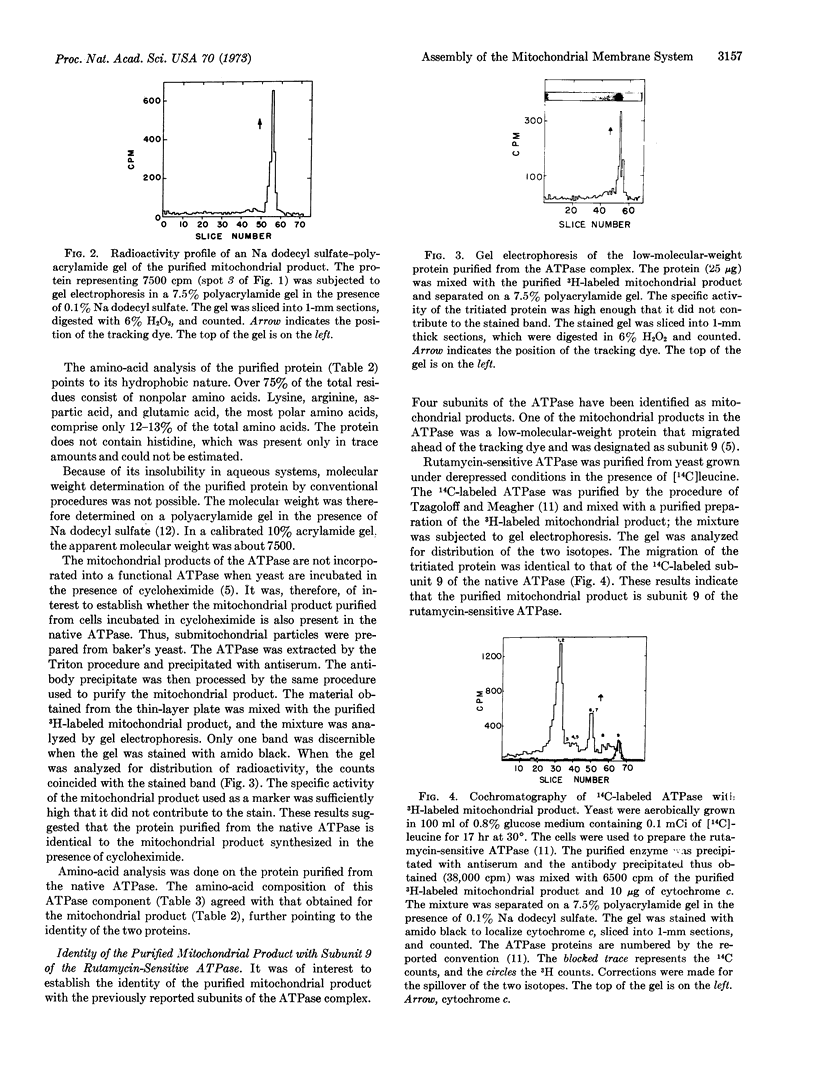


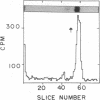
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burke J. P., Beattie D. S. The synthesis of proteolipid protein by isolated rat liver mitochondria. Biochem Biophys Res Commun. 1973 Mar 17;51(2):349–356. doi: 10.1016/0006-291x(73)91264-3. [DOI] [PubMed] [Google Scholar]
- Capaldi R. A., Vanderkooi G. The low polarity of many membrane proteins. Proc Natl Acad Sci U S A. 1972 Apr;69(4):930–932. doi: 10.1073/pnas.69.4.930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattell K. J., Lindop C. R., Knight I. G., Beechey R. B. The identification of the site of action of NN'-dicyclohexylcarbodi-imide as a proteolipid in mitochondrial membranes. Biochem J. 1971 Nov;125(1):169–177. doi: 10.1042/bj1250169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eng L. F., Chao F. C., Gerstl B., Pratt D., Tavaststjerna M. G. The maturation of human white matter myelin. Fractionation of the myelin membrane proteins. Biochemistry. 1968 Dec;7(12):4455–4465. doi: 10.1021/bi00852a042. [DOI] [PubMed] [Google Scholar]
- Kadenbach B., Hadváry P. Demonstration of two types of proteins synthesized in isolated rat-liver mitochondria. Eur J Biochem. 1973 Jan 15;32(2):343–349. doi: 10.1111/j.1432-1033.1973.tb02615.x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lamb A. J., Clark-Walker G. D., Linnane A. W. The biogenesis of mitochondria. 4. The differentiation of mitochondrial and cytoplasmic protein synthesizing systems in vitro by antibiotics. Biochim Biophys Acta. 1968 Jul 23;161(2):415–427. [PubMed] [Google Scholar]
- MacLennan D. H., Asai J. Studies on the mitochondrial adenosine triphosphatase system. V. Localization of the oligomycin-sensitivity conferring protein. Biochem Biophys Res Commun. 1968 Nov 8;33(3):441–447. doi: 10.1016/0006-291x(68)90592-5. [DOI] [PubMed] [Google Scholar]
- Mason T. L., Schatz G. Cytochrome c oxidase from bakers' yeast. II. Site of translation of the protein components. J Biol Chem. 1973 Feb 25;248(4):1355–1360. [PubMed] [Google Scholar]
- Murray D. R., Linnane A. W. Synthesis of proteolipid protein by yeast mitochondria. Biochem Biophys Res Commun. 1972 Nov 1;49(3):855–862. doi: 10.1016/0006-291x(72)90489-5. [DOI] [PubMed] [Google Scholar]
- PULLMAN M. E., PENEFSKY H. S., DATTA A., RACKER E. Partial resolution of the enzymes catalyzing oxidative phosphorylation. I. Purification and properties of soluble dinitrophenol-stimulated adenosine triphosphatase. J Biol Chem. 1960 Nov;235:3322–3329. [PubMed] [Google Scholar]
- Reynolds J. A., Tanford C. The gross conformation of protein-sodium dodecyl sulfate complexes. J Biol Chem. 1970 Oct 10;245(19):5161–5165. [PubMed] [Google Scholar]
- Rubin M. S., Tzagoloff A. Assembly of the mitochondrial membrane system. X. Mitochondrial synthesis of three of the subunit proteins of yeast cytochrome oxidase. J Biol Chem. 1973 Jun 25;248(12):4275–4279. [PubMed] [Google Scholar]
- Stekhovan F. S., Waitkus R. F., Van Moerkerk H. T. Identification of the dicyclohexylcarbodiimide-binding protein in the oligomycin-sensitive adenosine triphosphatase from bovine heart mitochondria. Biochemistry. 1972 Mar 28;11(7):1144–1150. doi: 10.1021/bi00757a005. [DOI] [PubMed] [Google Scholar]
- Tzagoloff A., Akai A., Sierra M. F. Assembly of the mitochondrial membrane system. VII. Synthesis and integration of F 1 subunits into the rutamycin-sensitive adenosine triphosphatase. J Biol Chem. 1972 Oct 25;247(20):6511–6516. [PubMed] [Google Scholar]
- Tzagoloff A. Assembly of the mitochondrial membrane system. 3. Function and synthesis of the oligomycin sensitivity-conferring protein of yeast mitochondria. J Biol Chem. 1970 Apr 10;245(7):1545–1551. [PubMed] [Google Scholar]
- Tzagoloff A. Assembly of the mitochondrial membrane system. IV. Role of mitochondrial and cytoplasmic protein synthesis in the biosynthesis of the rutamycin-sensitive adenosine triphosphatase. J Biol Chem. 1971 May 10;246(9):3050–3056. [PubMed] [Google Scholar]
- Tzagoloff A., Meagher P. Assembly of the mitochondrial membrane system. V. Properties of a dispersed preparation of the rutamycin-sensitive adenosine triphosphatase of yeast mitochondria. J Biol Chem. 1971 Dec 10;246(23):7328–7336. [PubMed] [Google Scholar]
- Tzagoloff A., Meagher P. Assesmbly of the mitochondrial membrane system. VI. Mitochondrial synthesis of subunit proteins of the rutamycin-sensitive adenosine triphosphatase. J Biol Chem. 1972 Jan 25;247(2):594–603. [PubMed] [Google Scholar]
- Tzagoloff A., Rubin M. S., Sierra M. F. Biosynthesis of mitochondrial enzymes. Biochim Biophys Acta. 1973 Feb 12;301(1):71–104. doi: 10.1016/0304-4173(73)90013-x. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]