Abstract
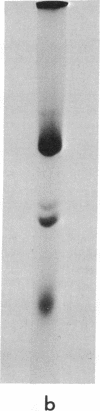
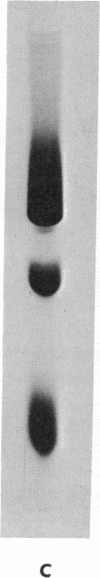
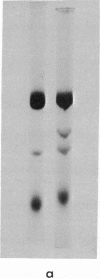
52 Spontaneous nonsense mutants in the lac i gene of Escherichia coli were isolated and characterized. All mutants located early in the gene show negative complementation in vivo with a wild-type i gene in a recA diploid strain. In vitro studies show that those mutants that display negative complementing activity in vivo also make lac repressor fragments retaining inducer binding and immunological crossreactivity with wild-type repressor. Amino-acid sequence analysis of these fragments shows that they arise by reinitiation at internal sities of the i message after chain termination at a prior amber or ochre codon.
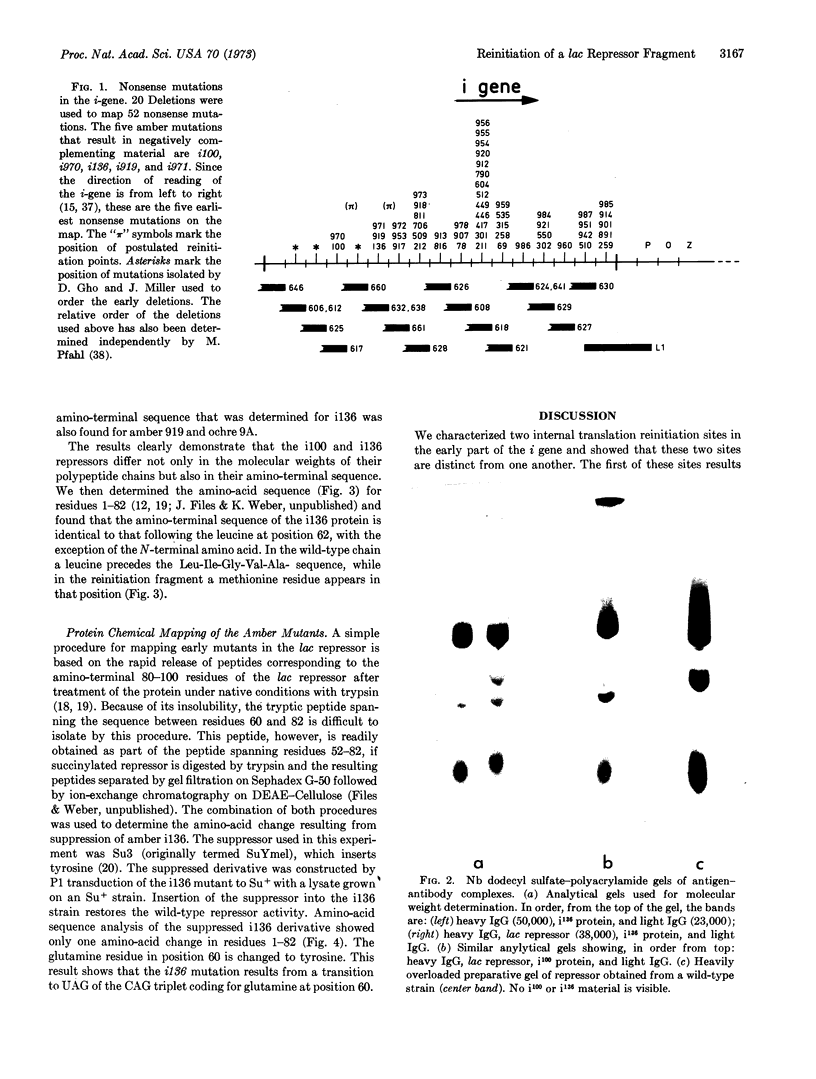
There are at least two different internal reinitiation sites in the first 200 nucleotides of the translated part of the i message. The first site corresponds to the first internal in phase AUG codon, which specifies the methionine residue at position 42 of the repressor protein. This site can be activated by an amber codon, 45 nucleotides before the AUG codon. The second site is only 60 nucleotides past the first site and can be activated by an amber mutation derived from residue 60 of the protein. The second initiation codon specifies the amino-acid leucine in the wild-type repressor, but the reinitiated fragment shows an amino-terminal methionine residue at this position. Therefore, the second initiation site seems to involve an in vivo ambiguity of the genetic code in that the same codon can be translated into two different amino acids depending on the recognition of this codon during initiation (when methionine is inserted) or elongation of protein synthesis (when leucine is inserted). The possibility that a codon other than AUG can act as an initiation codon in vivo is discussed.
Keywords: protein synthesis, antibody, gel electrophoresis, amber mutants, negative complementation, E. coli
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BECKWITH J. RESTORATION OF OPERON ACTIVITY BY SUPPRESSORS. Biochim Biophys Acta. 1963 Sep 17;76:162–164. [PubMed] [Google Scholar]
- Brown J. C., Smith A. E. Initiator codons in eukaryotes. Nature. 1970 May 16;226(5246):610–612. doi: 10.1038/226610a0. [DOI] [PubMed] [Google Scholar]
- Capecchi M. R. Initiation of E. coli proteins. Proc Natl Acad Sci U S A. 1966 Jun;55(6):1517–1524. doi: 10.1073/pnas.55.6.1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark B. F., Marcker K. A. The role of N-formyl-methionyl-sRNA in protein biosynthesis. J Mol Biol. 1966 Jun;17(2):394–406. doi: 10.1016/s0022-2836(66)80150-x. [DOI] [PubMed] [Google Scholar]
- Dennert G., Henning U. Tyrosine-incorporating amber suppressors in Escherichia coli K12. J Mol Biol. 1968 Apr 14;33(1):327–329. doi: 10.1016/0022-2836(68)90300-8. [DOI] [PubMed] [Google Scholar]
- Dube S. K., Rudland P. S., Clark B. F., Marcker K. A. A structural requirement for codon-anticodon interaction on the ribosome. Cold Spring Harb Symp Quant Biol. 1969;34:161–166. doi: 10.1101/sqb.1969.034.01.023. [DOI] [PubMed] [Google Scholar]
- Ghosh H. P., Söll D., Khorana H. G. Studies on polynucleotides. LXVII. Initiation of protein synthesis in vitro as studied by using ribopolynucleotides with repeating nucleotide sequences as messengers. J Mol Biol. 1967 Apr 28;25(2):275–298. doi: 10.1016/0022-2836(67)90142-8. [DOI] [PubMed] [Google Scholar]
- Gorini L. Informational suppression. Annu Rev Genet. 1970;4:107–134. doi: 10.1146/annurev.ge.04.120170.000543. [DOI] [PubMed] [Google Scholar]
- Grodzicker T., Zipser D. A mutation which creates a new site for the re-initiation of polypeptide synthesis in the z gene of the lac operon of Escherichia coli. J Mol Biol. 1968 Dec;38(3):305–314. doi: 10.1016/0022-2836(68)90388-4. [DOI] [PubMed] [Google Scholar]
- Gupta S. L., Chen J., Schaefer L., Lengyel P., Weissman S. M. Nucleotide sequence of a ribosome attachment site of bacteriophage f2 RNA. Biochem Biophys Res Commun. 1970 Jun 5;39(5):883–888. doi: 10.1016/0006-291x(70)90406-7. [DOI] [PubMed] [Google Scholar]
- Hindley J., Staples D. H. Sequence of a ribosome binding site in bacteriophage Q-beta-RNA. Nature. 1969 Dec 6;224(5223):964–967. doi: 10.1038/224964a0. [DOI] [PubMed] [Google Scholar]
- Kumar S., Szybalski W. Orientation of transcription of the lac operon and its repressor gene in Escherichia coli. J Mol Biol. 1969 Feb 28;40(1):145–151. doi: 10.1016/0022-2836(69)90303-9. [DOI] [PubMed] [Google Scholar]
- Lengyel P., Söll D. Mechanism of protein biosynthesis. Bacteriol Rev. 1969 Jun;33(2):264–301. doi: 10.1128/br.33.2.264-301.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michels C. A., Zipser D. Mapping of polypeptide reinitiation sites within the beta-galactosidase structural gene. J Mol Biol. 1969 May 14;41(3):341–347. doi: 10.1016/0022-2836(69)90280-0. [DOI] [PubMed] [Google Scholar]
- Miller J. H., Beckwith J., Muller-Hill B. Direction of transcription of a regulatory gene in E. coli. Nature. 1968 Dec 28;220(5174):1287–1290. doi: 10.1038/2201287a0. [DOI] [PubMed] [Google Scholar]
- Min Jou W., Haegeman G., Ysebaert M., Fiers W. Nucleotide sequence of the gene coding for the bacteriophage MS2 coat protein. Nature. 1972 May 12;237(5350):82–88. doi: 10.1038/237082a0. [DOI] [PubMed] [Google Scholar]
- Müller-Hill B., Crapo L., Gilbert W. Mutants that make more lac repressor. Proc Natl Acad Sci U S A. 1968 Apr;59(4):1259–1264. doi: 10.1073/pnas.59.4.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton A. Effect of nonsense mutations on translation of the lactose operon of Escherichia coli. Cold Spring Harb Symp Quant Biol. 1966;31:181–187. doi: 10.1101/sqb.1966.031.01.026. [DOI] [PubMed] [Google Scholar]
- Pfahl M. Genetic map of the lactose repressor gene (i) of Escherichia coli. Genetics. 1972 Nov;72(3):393–410. doi: 10.1093/genetics/72.3.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platt T., Files J. G., Weber K. Lac repressor. Specific proteolytic destruction of the NH 2 -terminal region and loss of the deoxyribonucleic acid-binding activity. J Biol Chem. 1973 Jan 10;248(1):110–121. [PubMed] [Google Scholar]
- Platt T., Weber K., Ganem D., Miller J. H. Translational restarts: AUG reinitiation of a lac repressor fragment. Proc Natl Acad Sci U S A. 1972 Apr;69(4):897–901. doi: 10.1073/pnas.69.4.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RajBhandary U. L., Kumar A. A formylatable methionine transfer ribonucleic acid from yeast: comparison of coding properties and sequences around the anticodon with Escherichia coli formylatable methionine transfer RNA. J Mol Biol. 1970 Jun 28;50(3):707–711. doi: 10.1016/0022-2836(70)90095-1. [DOI] [PubMed] [Google Scholar]
- Sarabhai A., Brenner S. A mutant which reinitiates the polypeptide chain after chain termination. J Mol Biol. 1967 Jul 14;27(1):145–162. doi: 10.1016/0022-2836(67)90357-9. [DOI] [PubMed] [Google Scholar]
- Scaife J., Beckwith J. R. Mutational alteration of the maximal level of Lac operon expression. Cold Spring Harb Symp Quant Biol. 1966;31:403–408. doi: 10.1101/sqb.1966.031.01.052. [DOI] [PubMed] [Google Scholar]
- Smith A. E., Marcker K. A. Cytoplasmic methionine transfer RNAs from eukaryotes. Nature. 1970 May 16;226(5246):607–610. doi: 10.1038/226607a0. [DOI] [PubMed] [Google Scholar]
- Steitz J. A. Polypeptide chain initiation: nucleotide sequences of the three ribosomal binding sites in bacteriophage R17 RNA. Nature. 1969 Dec 6;224(5223):957–964. doi: 10.1038/224957a0. [DOI] [PubMed] [Google Scholar]
- Stewart J. W., Sherman F., Shipman N. A., Jackson M. Identification and mutational relocation of the AUG codon initiating translation of iso-1-cytochrome c in yeast. J Biol Chem. 1971 Dec 25;246(24):7429–7445. [PubMed] [Google Scholar]
- Sundararajan T. A., Thach R. E. Role of the formylmethionine codon AUG in phasing translation of synthetic messenger RNA. J Mol Biol. 1966 Aug;19(1):74–90. doi: 10.1016/s0022-2836(66)80051-7. [DOI] [PubMed] [Google Scholar]
- Takeishi K., Ukita T., Nishimura S. Characterization of two species of methionine transfer ribonucleic acid from bakers' yeast. J Biol Chem. 1968 Nov 10;243(21):5761–5768. [PubMed] [Google Scholar]
- Uhlenbeck O. C., Baller J., Doty P. Complementary oligonucleotide binding to the anticodon loop of fMet-transfer RNA. Nature. 1970 Feb 7;225(5232):508–510. doi: 10.1038/225508a0. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Weber K., Platt T., Ganem D., Miller J. H. Altered sequences changing the operator-binding properties of the Lac repressor: colinearity of the repressor protein with the i-gene map. Proc Natl Acad Sci U S A. 1972 Dec;69(12):3624–3628. doi: 10.1073/pnas.69.12.3624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner A. M., Platt T., Weber K. Amino-terminal sequence analysis of proteins purified on a nanomole scale by gel electrophoresis. J Biol Chem. 1972 May 25;247(10):3242–3251. [PubMed] [Google Scholar]
- Zipser D., Zabell S., Rothman J., Grodzicker T., Wenk M. Fine structure of the gradient of polarity in the z gene of the lac operon of Escherichia coli. J Mol Biol. 1970 Apr 14;49(1):251–254. doi: 10.1016/0022-2836(70)90392-x. [DOI] [PubMed] [Google Scholar]