Abstract
Background
Patients diagnosed with fulminant hepatic failure face high mortality rates. A potential therapeutic approach for these patients is the use of extracorporeal porcine liver perfusion, to serve as a form of “liver dialysis”. Previously, our laboratory has shown that, during a 72 hour extracorporeal perfusion with human blood, porcine Kupffer cells bind to and phagocytose human erythrocytes causing the hematocrit to fall to 2.5% of the original value. Subsequently, erythrocyte binding has been shown to involve N-acetylneuraminic acid (Neu5Ac) on the surface of human erythrocytes and sialoadhesin on the surface of the porcine Kupffer cells.
Methods
Given that no primate other than the human is known to express the majority of its sialic acid as Neu5Ac, we evaluated whether nonhuman primates would provide adequate evaluation of the loss of erythrocytes that might be expected in a clinical trial of extracorporeal porcine liver perfusion.
Results
We found that while porcine macrophages readily bound human erythrocytes, binding of nonhuman primate erythrocytes was significantly reduced (p < 0.001).
Conclusions
This study suggests that nonhuman primates may fail to serve as an adequate model for studying extracorporeal porcine liver perfusion, due to the fact that porcine macrophages do not bind nonhuman primate erythrocytes.
Keywords: xenograft rejection, macrophages, sialic acid and nonhuman primates
Introduction
Xenotransplantation offers the possibility of supplying sufficient organs to meet current transplantation demands. Given the scarce organ supply and the rapid progression of fulminant hepatic failure, one approach considered as an early clinical application of xenotransplantation is extracorporeal porcine liver xenoperfusion (1). Extracorporeal porcine liver perfusion could serve as “bridge therapy” for patients in liver failure awaiting liver transplantation. To date, several porcine anti-human graft-vs.-host responses have been identified involving the destruction of human platelets and erythrocytes, which limit the use of this therapy (2, 3).
Experiments investigating extracorporeal liver xenoperfusion have spanned nearly 50 years, and almost without exception, investigators have noted significant thrombocytopenia occurring very shortly after the initiation of liver xenoperfusion (4–6). Platelet counts rapidly fall following reperfusion of dog-to-pig liver xenografts, and in pig-to-nonhuman primate liver transplantation, nearly all platelets are removed from the circulation within 1 hour (4, 6). Extracorporeal perfusion of whole human blood through porcine livers leads to near complete loss of platelets within minutes (5). Burlak et al. have recently shown that the mechanism of human platelet destruction by porcine livers involves direct phagocytosis by both Kupffer cells and liver sinusoidal endothelial cells (3). Several investigators have now observed disseminated intravascular coagulation with thrombocytopenia following xenotransplantation (7–10). As clinical support of patients in hepatic failure using extracorporeal porcine livers has been limited to durations of 6–8 hours, and the most significant coagulopathies have been observed only after the xenograft has been engrafted for 48 hours, this problem has not yet been fully evaluated in the pig-to-human combination.
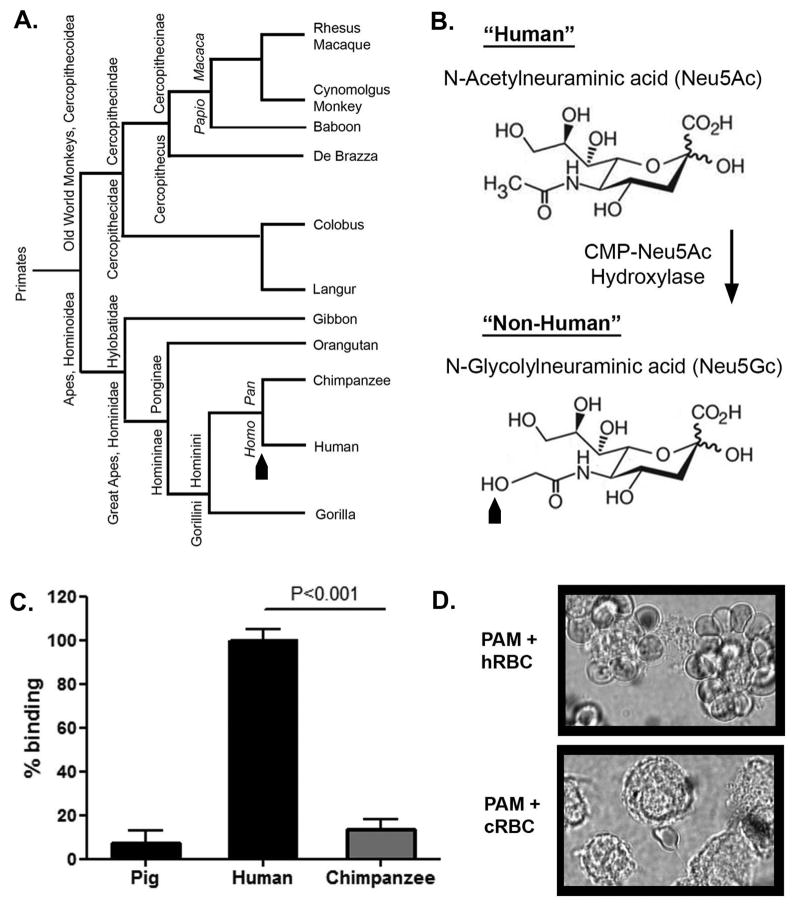
During a 72-hour ex vivo perfusion with isolated human blood, porcine livers consume the equivalent of three units of erythrocytes (5). This anti-human erythrocyte reaction is mediated by porcine Kupffer cells (resident liver macrophages) utilizing the lectin sialoadhesin to bind the carbohydrate N-acetylneuraminic acid (Neu5Ac) on the surface of human erythrocytes (11–16). Although more than 32 natural glycoforms of sialic acid are known (17), two main forms are found in mammals, Neu5Ac and N-glycolylneuraminic acid (Neu5Gc) (18). These two glycoforms differ from one another by a single hydroxyl group, a difference produced by the activity of cytidine monophosphate-N-acetylneuraminic acid hydroxylase (CMAH, Fig. 1B) (19–22). This subtle distinction between Neu5Ac and Neu5Gc brings to light a rare genotypic difference between humans and all other nonhuman primates. Due to an Alu-mediated deletion in the CMAH gene, which took place sometime after the divergence of the Homo Pan genera (Fig. 1A), humans do not express CMAH and thus express the Neu5Ac form of sialic acid to the exclusion of Neu5Gc (19–22). On the contrary, great apes, as well as all other primates excluding humans, have retained the ability to hydroxylate the Neu5Ac precursor. As a result, the majority of the sialic acid expressed on nonhuman primate cell surfaces is expressed as the Neu5Gc form (23).
Figure 1.
Effect of CMP-Neu5Ac hydroxylase (CMAH) on macrophage recognition of xenogeneic erythrocytes susceptibility to porcine macrophages. (A) Phylogenetic tree showing evolutionary differences of species within the Ape and Old World monkey families. Black arrow indicates time of an Alu-mediated disruption of the CMAH gene. (B) Structure of the “human form” of sialic acid (Top), Neu5Ac; and the hydroxylated “nonhuman primate form” arising from CMP-Neu5Ac hydroxylase activity. The black arrow represents the addition of the hydroxyl group and the formulation of Neu5Gc resulting from the activity of CMP-Neu5Ac hydroxylase. (C) Quantification of porcine macrophage binding to human, chimpanzee, and autologous porcine erythrocytes was determined using a colorimetric rosetting assay. Samples were prepared in triplicate. Data are representative of three independent experiments performed using human positive control erythrocytes from three different individuals. The means and standard errors are shown. Percentage of binding is presented relative to human erythrocyte binding, which was set at 100%. Significantly more human erythrocytes were bound as compared to chimpanzee erythrocytes (P<0.001). (D) Representative microscopy images of porcine alveolar macrophages (PAM) illustrate human erythrocyte (top, hRBC) and chimpanzee erythrocyte (bottom, cRBC) binding patterns.
Lutz et al. recently produced double knockout pigs deficient in both Neu5Gc and galactose α-1,3-galactose (Gal α-1,3-Gal), and demonstrated a significant reduction in the human anti-pig immune response to cells from these animals as compared with cells derived from Gal α-1,3-Gal single knockouts (24). Given that humans cannot synthesize Neu5Gc and are known to have a significant natural antibody response directed against Neu5Gc, it is not surprising that removing Neu5Gc from the surface of porcine cells would reduce the human immune response directed against Neu5Gc-deficient porcine cells. But this research has a further implication. If one wished to use an animal model to examine the benefit of removing Neu5Gc that might be expected in the pig-to-human model, one would require an animal model in which the recipient animal lacked Neu5Gc-expression and had an inherent humoral response directed against Neu5Gc. Such a model does not exist. As humans are the only mammal deficient in CMAH, thus lacking Neu5Gc expression, no large animal model would be expected to recapitulate the human anti-Neu5Gc response. Thus, it is unlikely that any nonhuman primate model will demonstrate the benefit that might be expected from a CMAH-deficient porcine organ when transplanted into a human recipient.
As a means of testing this inability to evaluate the benefit of CMAH-deficient pigs in any model other than pig-to-human, we have exploited the fact that porcine macrophages bind to and destroy human erythrocytes through a mechanism dependent on Neu5Ac expression (12). We hypothesized that porcine macrophages would fail to recognize nonhuman primate erythrocytes because, like pigs, nonhuman primates express the majority of their sialic acid in the Neu5Gc form. To test this hypothesis, we performed a series of in vitro assays assessing the ability of porcine macrophages to bind erythrocytes from various nonhuman primates. The data suggests that the difference in sialic acid expression present when the species combination is pig and human, and not present when the combination is pig and nonhuman primate, limits the ability of porcine macrophages to bind nonhuman primate erythrocytes. We propose that this limitation of the nonhuman primate erythrocytes to provide useful information about what porcine Kupffer cells will do to human erythrocytes is harbinger of the lack of efficacy of nonhuman primates to serve as an appropriate pre-clinical model for either extracorporeal porcine liver xenoperfusion or the evaluation of CMAH/Gal α-1,3-Gal double knockout porcine xenografts.
Results
Porcine macrophages bind human erythrocytes but not chimpanzee erythrocytes
First, given their genetic proximity to humans (Fig. 1A), we reasoned that erythrocytes from a chimpanzee would serve as the best model for determining whether the retained CMAH function seen in nonhuman primates decreased their susceptibility to porcine macrophages. To test porcine macrophage recognition of chimpanzee erythrocytes, primary porcine macrophages previously shown to have high sialoadhesin expression (data not shown), were co-incubated with chimpanzee erythrocytes; binding was quantified using a colorimetric rosetting assay. Human and autologous porcine erythrocytes were used as controls. Previous data collected in our laboratory demonstrate that approximately 70–80 percent of cultured porcine macrophages bind human erythrocytes (15). Compared to porcine macrophage recognition of human erythrocytes (100%), the percent binding of chimpanzee erythrocytes was significantly reduced (13.4%, p<0.001) (Fig. 1C). Of note, porcine macrophages bound autologous erythrocytes (7.1%) to a similar extent as xenogeneic chimpanzee erythrocytes (13.4%). Light microscopy of co-cultured cells confirmed this observation (Fig. 1D).
Porcine macrophage binding of erythrocytes from commonly used primate pre-clinical models is reduced as compared to porcine macrophage binding of human erythrocytes
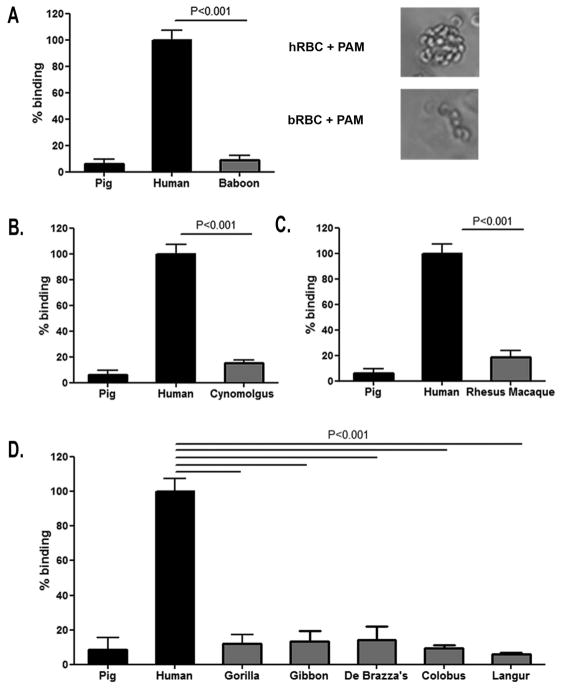
Next, we evaluated if erythrocytes from other nonhuman primates likely to be used in pre-clinical trials of extracorporeal porcine liver perfusion showed a lack of binding by porcine macrophages as compared to human erythrocytes. Baboon erythrocyte binding (9.1%), Cynomolgus monkey erythrocyte binding (15.2%), and Rhesus macaque erythrocyte binding (18.5%) was significantly less than the binding of human erythrocytes (100%, p<0.001) (Fig. 2A,B,C).
Figure 2.
Porcine macrophage binding to xenogeneic erythrocytes obtained from nonhuman primates most commonly used in xenotransplantation pre-clinical studies. (A) The ability of porcine macrophages to recognize erythrocytes from baboons was determined using a quantitative colorimetric erythrocyte rosetting assay. Baboon erythrocytes binding was significantly less than human erythrocyte binding (P<0.001). Phase contrast microscopy results further supported the findings obtained with the quantitative rosetting assay. Binding of Cynomolgus monkey (B) and Rhesus macaque erythrocytes (C) to porcine macrophages was quantified relative to human erythrocyte binding. Binding of erythrocytes from Cynomolgus monkeys and Rhesus macaques was significantly less than human erythrocyte binding (P<0.001). Data are representative of three independent experiments performed using human positive control erythrocytes from three different individuals. (D) The ability of porcine macrophages to recognize erythrocytes from various nonhuman primates was quantified. Binding of erythrocytes from all five nonhuman primate species tested was significantly less than that of human erythrocyte binding (P<0.001). Three independent experiments were done using erythrocytes from three individuals for the human positive control and nonhuman primate erythrocytes from single animals.
Porcine macrophages fail to recognize nonhuman primate erythrocytes
Given that neither erythrocytes from the chimpanzee, nor erythrocytes from nonhuman primate species commonly used in pre-clinical research were bound by porcine macrophages, we determined if this phenomenon was consistent across a wider array of primate species. We tested porcine macrophage binding of erythrocytes derived from five additional nonhuman primate species representing two different super families, Hominoidea and Cercopithecoidea. In no case did porcine macrophages bind nonhuman primate erythrocytes to the same degree to which they recognize human erythrocytes, p<0.001 (Fig. 2D).
Discussion
Extracorporeal porcine liver xenoperfusion as a treatment for fulminant hepatic failure has been suggested as a reasonable first step in the clinical evaluation of xenotransplantation. This approach has been advocated for multiple reasons: 1) the shortage of human livers available for transplantation; 2) the high mortality rate associated with fulminant hepatic failure; 3) the lack of a means of temporary support for hepatic failure; 4) that exposure to the porcine organ will be limited to a few days rather than permanent implantation; and 5) long-term immunosuppression may be avoided if the patient is “bridged” to recovery. Although pre-clinical animal studies sometimes fail to accurately predict human responses (25), current FDA requirements for xenotransplantation to advance to clinical trial include evidence for efficacy of the therapy using nonhuman primates in a pre-clinical setting (26). The data in this study provide evidence from nine different nonhuman primate species, including the three most commonly used in pre-clinical studies, suggesting that nonhuman primates will not recapitulate the graft-vs.-host response seen when human blood is perfused through a porcine liver. In addition, Lutz et al. recently produced double knockout pigs deficient in both Neu5Gc and galactose α-1,3-galactose (Gal α-1,3-Gal), and demonstrated a significant reduction in the human anti-pig immune response to cells from these animals as compared with cells derived from Gal α-1,3-Gal single knockouts (24). Data in this study, along with data published by Lutz et al., suggest that, as a result of retained CMAH activity and Neu5Gc expression, nonhuman primates will fail as a model to evaluate the graft-vs.-host response seen when human blood is perfused through a porcine liver as in extracorporeal porcine liver perfusion; and will fail to illustrate the benefit of eliminating Neu5Gc in the host-vs.-graft response seen in pig-to-human whole organ xenotransplantation.
This would not be the first instance where nonhuman primates failed to predict clinical outcomes in transplantation medicine. For example, sirolimus administered at a clinically relevant dose, was found to cause profound gastrointestinal toxicity in rhesus macaques, that viewed in isolation, would have suggested sirolimus would not be tolerated in humans (27). Furthermore, the primate model was unsuccessful at predicting efficacy of anti-CD40 ligand therapy in early pre-clinical studies given the subsequent platelet effects observed in humans (28, 29). While most new therapies in transplantation are studied for safety and efficacy in at least one large animal model prior to phase one studies in humans, studies in large animal models should be designed with the limitations of these models in mind (30).
This study offers insight into a potential pitfall of the use of nonhuman primates in preclinical studies of extracorporeal porcine liver perfusion. The data also allude to the inadequacy of nonhuman primates to demonstrate the benefit that might be expected from a CMAH-deficient porcine organ when transplanted into a human recipient. Given the data presented in the present study and by Lutz et al. (24), as a precondition for clinical trials, we propose that the efficacy of extracorporeal porcine liver perfusion and transplantation of porcine organs from Neu5Gc/Gal α-1,3-Gal double knockouts be evaluated in brain-dead patients determined not to be suitable candidates for organ donation, rather than in nonhuman primates. The concept of using brain dead individuals as research subjects has been called a variety of terms in the literature, but consensus appears to be evolving that such research be referred to as “research on the recently dead” (31). Not surprisingly, research of this nature has been controversial (32–34). However, consensus panel-derived guidelines for the use of brain-dead subjects in medical research have been established, and one institution developed a separate and specialized institutional review committee for this purpose (31, 35). To our knowledge, there are only two reported cases in which a patient has been determined to be brain-dead and subsequently studied as a research subject prior to the removal of life support (36, 37). The present study suggests that the use of brain-dead human subjects in preclinical xenotransplantation studies may be more useful than the current standard of primate experimentation.
Materials and Methods
Cells
All animal experiments were approved by the University of Toledo Institutional Animal Care and Use Committee and completed in the Department of Laboratory Animal Research at the University of Toledo Medical Center, Toledo, OH. Porcine macrophages were obtained via bronchoalveolar lavage (BAL) according to Wensvoort et al. and Brock et al. (38). In short, porcine lungs were carefully excised, and lavaged with approximately 1500 mL (2 × 750 mL flushes) of cold 0.9% saline (Baxter Healthcare, Deerfield, IL). Lungs were massaged in order to facilitate the release of resident alveolar macrophages. All animals were treated in accordance with the Institute for Laboratory Animal Research (ILAR) publication, The Guide for the Care and Use of Laboratory Animals (National Research Council, National Academy Press, Washington, D.C., 1996) and the Animal Welfare Act. Under sterile conditions, the procedure was done quickly in order to preserve cell viability. The BAL fluid was then aliquoted into 50 mL conical tubes and centrifuged at 200 x g for 5 min. The supernatants were decanted and cells were suspended in Roswell Park Memorial Institute Medium (RPMI-1640, Mediatech, Manasses, VA). Cells were then layered onto lymphocyte separation media (Mediatech,) and spun at 500 x g for 40 min, at which time mononuclear cells were extracted from the mononuclear cell monolayer. Cells were then washed in Hanks Balanced Salt Solution (HBSS, Life Technologies, Carlsbad, CA) and re-suspended at a concentration of 10×106 in chilled RPMI 1640 and supplemented with 10% fetal bovine serum (FBS, Life Technologies), 1% penicillin/streptomycin (100 U/mL, 100 mg/mL, Life Technologies), and 5% dimethyl sulfoxide (DMSO, Sigma, St. Louis, MO). Samples remained frozen in liquid nitrogen until used. They were removed and placed into culture for 4 days before being utilized in experiments.
Nonhuman primate whole blood samples were obtained from the Toledo Zoo (Toledo, OH), the Southwest National Primate Research Center (San Antonio, TX), or the Yerkes National Primate Research Center (Atlanta, GA) through a protocol approved by the University of Toledo Institutional Animal Care and Use Committee. Type O blood was used for whole human blood used in experiments. Written informed consent was obtained for all human volunteers under a University of Toledo Institutional Review Board approved protocol. Human and nonhuman primate whole blood was collected into a lithium-heparin tube and mixed (Thermo Fisher Scientific, Waltham, MA). The whole blood was washed twice with HBSS. A 0.1% red blood cell solution to be applied to cultured macrophages was prepared with RPMI.
Rosetting Assay
Binding
Determination of porcine macrophage recognition of xenogeneic erythrocytes was performed using an erythrocyte rosetting assay as previously described (15). Data represent three independent experiments performed using human and nonhuman primate erythrocytes from three separate individuals per species, except where noted in figure legend. In brief, after 4 days in culture, macrophages were removed from culture flasks and seeded into a 96-well plate at 3×104 cells per well in RPMI culturing medium. After 2 h of incubation, RPMI was replaced and macrophages were incubated with a 0.1% erythrocyte solution. After one hour of erythrocyte/macrophage co-incubation, wells were rinsed with RPMI in order to remove unbound erythrocytes. Wells were then allowed to dry and cells were subsequently fixed with cold methanol.
Quantification
After xenogeneic erythrocyte binding, rosettes were quantified using a tetramethylbenzidine (TMB) reaction in which erythrocyte binding is measured based on the presence of oxygen via oxidation and subsequent color change (39). For the TMB reaction, 100 μl of solution A and B (R & D systems, Minneapolis, MN) was added to each well and allowed to incubate in the dark for 20 minutes. Following incubation, the reaction was stopped with 2M sulfuric acid and the plate was quantified with a spectrophotometer at a wave length of 450 nm. Data were calculated as percent human erythrocyte binding.
Microscopy
In order to verify quantitative data, rosettes were visualized using bright field microscopy. Porcine macrophages were seeded into a 24-well plate and cultured as previously discussed. After co-incubation, unbound erythrocytes were removed and cells were fixed with 4% paraformaldehyde and then visualized by bright field microscopy using an Olympus FSX100 microscope (Olympus, Tokyo, Japan).
Statistical Analysis
Significant differences between groups were determined using a student’s t-test. A P-value of <0.05 was considered to be significant. Data are representative of three individual experiments.
Acknowledgments
Support:
This work was supported by a grant from the National Institutes of Health (R01-DK066160), monies provided by Life Connection of Ohio, and start-up funds from the University of Toledo. LG Brock was supported, in part, by a Matthews Pre-doctoral Research Fellowship Grant (N-101162-01) from the Kidney Foundation of Northwest Ohio.
The authors wish to thank the following individuals at the University of Toledo for their contributions to this study: Karen Domenico, for flow cytometry guidance; Rebecca Wynn and Lisa Twining, for technical assistance in the procurement of primary macrophages; and Katherine Goans and Richard Rulman, for animal surgical assistance.
Abbreviation
- CMAH
cytidine monophosphate-N-acetylneuraminic acid hydroxylase
- ECLP
Extracorporeal liver perfusion
- Gal α-1
3-Gal, galactose α-1,3-galactose
- hRBC
human red blood cells
- KC
Kupffer cells
- Neu5Ac
N-acetylneuraminic acid
- Neu5Gc
N-glycolylneuraminic acid
- NHP
nonhuman primate
- pSn
porcine sialoadhesin
Footnotes
Conflicts of Interest:
The authors have no conflicts of interest to disclose
Author contributions:
1Participated in research design, data collection and analysis, and writing of the paper
2Participated in research design, data collection and analysis, and editing of the paper
3Gained funding for the project, participated in research design, and editing of the paper
References
- 1.Pascher A, Sauer IM, Hammer C, Gerlach JC, Neuhaus P. Extracorporeal liver perfusion as hepatic assist in acute liver failure: a review of world experience. Xenotransplantation. 2002 Sep;9(5):309–24. doi: 10.1034/j.1399-3089.2002.01076.x. [DOI] [PubMed] [Google Scholar]
- 2.Rees MA, Butler AJ, Davies HF, Bolton E, Wight DG, Skepper J, et al. Porcine livers perfused with human blood mount a graft-versus-“host” reaction. Transplantation. 2002 May 15;73(9):1460–7. doi: 10.1097/00007890-200205150-00016. [DOI] [PubMed] [Google Scholar]
- 3.Burlak C, Paris LL, Chihara RK, Sidner RA, Reyes LM, Downey SM, et al. The fate of human platelets perfused through the pig liver: implications for xenotransplantation. Xenotransplantation. 2010 Sep-Oct;17(5):350–61. doi: 10.1111/j.1399-3089.2010.00605.x. [DOI] [PubMed] [Google Scholar]
- 4.Ekser B, Long C, Echeverri GJ, Hara H, Ezzelarab M, Lin CC, et al. Impact of thrombocytopenia on survival of baboons with genetically modified pig liver transplants: clinical relevance. Am J Transplant. 2010 Feb;10(2):273–85. doi: 10.1111/j.1600-6143.2009.02945.x. [DOI] [PubMed] [Google Scholar]
- 5.Rees MA, Butler AJ, Chavez-Cartaya G, Wight DG, Casey ND, Alexander G, Khuder SA, White DG, Friend PJ. Prolonged function of extracorporeal hDAF transgenic pig livers perfused with human blood. Transplantation. 2002;73:1194–202. doi: 10.1097/00007890-200204270-00003. [DOI] [PubMed] [Google Scholar]
- 6.Tector AJ, Fridell JA, Elias N, Watanabe T, Salazar J, Greinke D, et al. Aberrations in hemostasis and coagulation in untreated discordant hepatic xenotransplantation: studies in the dog-to-pig model. Liver Transpl. 2002 Feb;8(2):153–9. doi: 10.1053/jlts.2002.30881. [DOI] [PubMed] [Google Scholar]
- 7.Kozlowski T, Shimizu A, Lambrigts D, Yamada K, Fuchimoto Y, Glaser R, et al. Porcine kidney and heart transplantation in baboons undergoing a tolerance induction regimen and antibody adsorption. Transplantation. 1999 Jan 15;67(1):18–30. doi: 10.1097/00007890-199901150-00004. [DOI] [PubMed] [Google Scholar]
- 8.Cowan PJ, Aminian A, Barlow H, Brown AA, Chen CG, Fisicaro N, et al. Renal xenografts from triple-transgenic pigs are not hyperacutely rejected but cause coagulopathy in non-immunosuppressed baboons. Transplantation. 2000 Jun 27;69(12):2504–15. doi: 10.1097/00007890-200006270-00008. [DOI] [PubMed] [Google Scholar]
- 9.Buhler L, Basker M, Alwayn IP, Goepfert C, Kitamura H, Kawai T, et al. Coagulation and thrombotic disorders associated with pig organ and hematopoietic cell transplantation in nonhuman primates. Transplantation. 2000 Nov 15;70(9):1323–31. doi: 10.1097/00007890-200011150-00010. [DOI] [PubMed] [Google Scholar]
- 10.Ierino FL, Kozlowski T, Siegel JB, Shimizu A, Colvin RB, Banerjee PT, et al. Disseminated intravascular coagulation in association with delayed rejection of pig-to-baboon renal xenografts. Transplantation. 1998;66(11):1436–50. doi: 10.1097/00007890-199812150-00006. [DOI] [PubMed] [Google Scholar]
- 11.Burlak C, Twining L, Rees M. Carbohydrates borne on human glycophorin A are recognized by porcine kupffer cells. Transplantation. 2005a;80(1):66–74. doi: 10.1097/01.tp.0000162975.88938.d2. [DOI] [PubMed] [Google Scholar]
- 12.Burlak C, Twining LM, Rees MA. Terminal sialic acid residues on glycophorin A are recognized by porcine kupffer cells. Transplantation. 2005b;80(3):344–52. doi: 10.1097/01.tp.0000162974.94890.9f. [DOI] [PubMed] [Google Scholar]
- 13.Brock L, Delputte P, Waldman J, Nauwynck H, Rees M. Porcine Sialoadhesin: A Newly Identified Xenogeneic Innate Immune Receptor. American Journal of Transplantation. 2012;12(12):3272–82. doi: 10.1111/j.1600-6143.2012.04247.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rees M, Butler A, Negus M, Davies H, Friend P. Classical pathway complement destruction is not responsible for the loss of human erythrocytes during porcine liver perfusion. Transplantation. 2004;77:1416–23. doi: 10.1097/01.tp.0000121135.24688.a3. [DOI] [PubMed] [Google Scholar]
- 15.Rees M, Butler A, Brons I, Negus M, Skepper J, Friend P. Evidence of macrophage receptors capable of direct recognition of xenogeneic epitopes without opsonization. Xenotransplantation. 2005;12(1):13–9. doi: 10.1111/j.1399-3089.2004.00195.x. [DOI] [PubMed] [Google Scholar]
- 16.Waldman JP, Vogel T, Bulak C, Coussios C, Dominguez J, Friend P, et al. Blocking porcine sialoadhesin improves extracorporeal porcine liver perfusion with human blood. Xenotransplantation. 2013;20(4):239–51. doi: 10.1111/xen.12043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schauer R. Analysis of Sialic Acids. Methods in Enzymology: ScienceDirect. 1987:132–61. doi: 10.1016/0076-6879(87)38012-7. [DOI] [PubMed] [Google Scholar]
- 18.Brinkman-Van der Linden EC, Sjoberg ER, Juneja LR, Crocker PR, Varki N, Varki A. Loss of N-glycolylneuraminic acid in human evolution. Implications for sialic acid recognition by siglecs. J Biol Chem. 2000 Mar 24;275(12):8633–40. doi: 10.1074/jbc.275.12.8633. [DOI] [PubMed] [Google Scholar]
- 19.Irie A, Suzuki A. CMP-N-Acetylneuraminic acid hydroxylase is exclusively inactivated in humans. Biochemical and Biophysical Research Communications. 1998;248(2):330–3. doi: 10.1006/bbrc.1998.8946. [DOI] [PubMed] [Google Scholar]
- 20.Irie A, Koyama S, Kozutsumi Y, Kawasaki T, Suzuki A. The molecular basis for the absence of N-glycolylneuraminic acid in humans. Journal of Biological Chemistry. 1998;273(25):15866–71. doi: 10.1074/jbc.273.25.15866. [DOI] [PubMed] [Google Scholar]
- 21.Chou HH, Takematsu H, Diaz S, Iber J, Nickerson E, Wright KL, Muchmore EA, Nelson DL, Warren ST, Varki A. A mutation in human CMP-sialic acid hydroxylase occured after the Homo-Pan divergence. Proceedings of the National Academy of Science USA. 1998;95(20):11751–6. doi: 10.1073/pnas.95.20.11751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hayakawa T, Satta Y, Gagneux P, Varki A, Takahata N. Alu-mediated inactivation of human CMP-N-acetylneuraminic acid hydroxylase gene. Proceedings of the National Academy of Science USA. 2001;98(20):11399–404. doi: 10.1073/pnas.191268198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Muchmore EA, Diaz S, Varki A. A Structural Differance Between the Cell Surfaces of Human and the Great Apes. American Journal of Physical Anthropology. 1998;107(2):187–98. doi: 10.1002/(SICI)1096-8644(199810)107:2<187::AID-AJPA5>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 24.Lutz AJ, Li P, Estrada JL, Sidner RA, Chihara RK, Downey SM, et al. Double knockout pigs deficient in N-glycolylneuraminic acid and Galactose alpha-1,3-Galactose reduce the humoral barrier to xenotransplantation. Xenotransplantation. 2013 Jan;20(1):27–35. doi: 10.1111/xen.12019. [DOI] [PubMed] [Google Scholar]
- 25.Seok J, Warren HS, Cuenca AG, Mindrinos MN, Baker HV, Xu W, et al. Genomic responses in mouse models poorly mimic human inflammatory diseases. Proc Natl Acad Sci U S A. 2013 Feb 26;110(9):3507–12. doi: 10.1073/pnas.1222878110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.FDA US. Guidance for Industry: Source Animal, Product, Preclinical, and Clinical Issues Concerning the Use of Xenotransplantation Products in Humans. 2012. updated 4/19/2012; cited 2013 March 7. [Google Scholar]
- 27.Montgomery SP, Mog SR, Xu H, Tadaki DK, Hirshberg B, Berning JD, et al. Efficacy and toxicity of a protocol using sirolimus, tacrolimus and daclizumab in a nonhuman primate renal allotransplant model. Am J Transplant. 2002 Apr;2(4):381–5. doi: 10.1034/j.1600-6143.2002.20415.x. [DOI] [PubMed] [Google Scholar]
- 28.Kirk AD, Harlan DM, Armstrong NN, Davis TA, Dong Y, Gray GS, et al. CTLA4-Ig and anti-CD40 ligand prevent renal allograft rejection in primates. Proc Natl Acad Sci U S A. 1997 Aug 5;94(16):8789–94. doi: 10.1073/pnas.94.16.8789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kirk AD, Knechtle SJ, Sollinger H, Vincenti FG, Stecher S, KN Preliminary results of the use of humanized anti-CD154 in human renal allotransplantation. Am J Transplant. 2001;1(S191) [Google Scholar]
- 30.Kirk AD. Crossing the bridge: large animal models in translational transplantation research. Immunol Rev. 2003 Dec;196:176–96. doi: 10.1046/j.1600-065x.2003.00081.x. [DOI] [PubMed] [Google Scholar]
- 31.Pentz RD, Cohen CB, Wicclair M, DeVita MA, Flamm AL, Youngner SJ, et al. Ethics guidelines for research with the recently dead. Nature Medicine. 2005 Nov;11(11):1145–9. doi: 10.1038/nm1105-1145. [DOI] [PubMed] [Google Scholar]
- 32.Tomasini F. Research on the recently dead: an historical and ethical examination. British Medical Bulletin. 2008;85(1):7–16. doi: 10.1093/bmb/ldn006. [DOI] [PubMed] [Google Scholar]
- 33.Wicclair MR. Informed consent and research involving the newly dead. Kennedy Inst Ethics J. 2002 Dec;12(4):351–72. doi: 10.1353/ken.2002.0028. [DOI] [PubMed] [Google Scholar]
- 34.Wicclair MR. Ethics and research with deceased patients. Camb Q Healthc Ethics. 2008 Winter;17(1):87–97. doi: 10.1017/S0963180108080092. [DOI] [PubMed] [Google Scholar]
- 35.DeVita MA, Wicclair M, Swanson D, Valenta C, Schold C. Research involving the newly dead: an institutional response. Critical Care Medicine. 2003 May;31(5 Suppl):S385–90. doi: 10.1097/01.CCM.0000065142.41379.5C. [DOI] [PubMed] [Google Scholar]
- 36.Arap W, Kolonin MG, Trepel M, Lahdenranta J, Cardo-Vila M, Giordano RJ, et al. Steps toward mapping the human vasculature by phage display. Nature Medicine. 2002 Feb;8(2):121–7. doi: 10.1038/nm0202-121. [DOI] [PubMed] [Google Scholar]
- 37.Coller BS, Scudder LE, Berger HJ, Iuliucci JD. Inhibition of human platelet function in vivo with a monoclonal antibody. With observations on the newly dead as experimental subjects. Ann Intern Med. 1988 Oct 15;109(8):635–8. doi: 10.7326/0003-4819-109-8-635. [DOI] [PubMed] [Google Scholar]
- 38.Wensvoort G, Terpstra C, Pol J, ter Laak E, Bloemraad M, EPdK, et al. Mystery swine disease in The Netherlands: the isolation of Lelystad virus. The Veterinary Quarterly. 1991;13(3):121–30. doi: 10.1080/01652176.1991.9694296. [DOI] [PubMed] [Google Scholar]
- 39.Martin TL, Mufson EJ, Mesulam MM. The light side of horseradish peroxidase histochemistry. J Histochem Cytochem. 1984 Jul;32(7):793. doi: 10.1177/32.7.6736628. [DOI] [PubMed] [Google Scholar]