Abstract
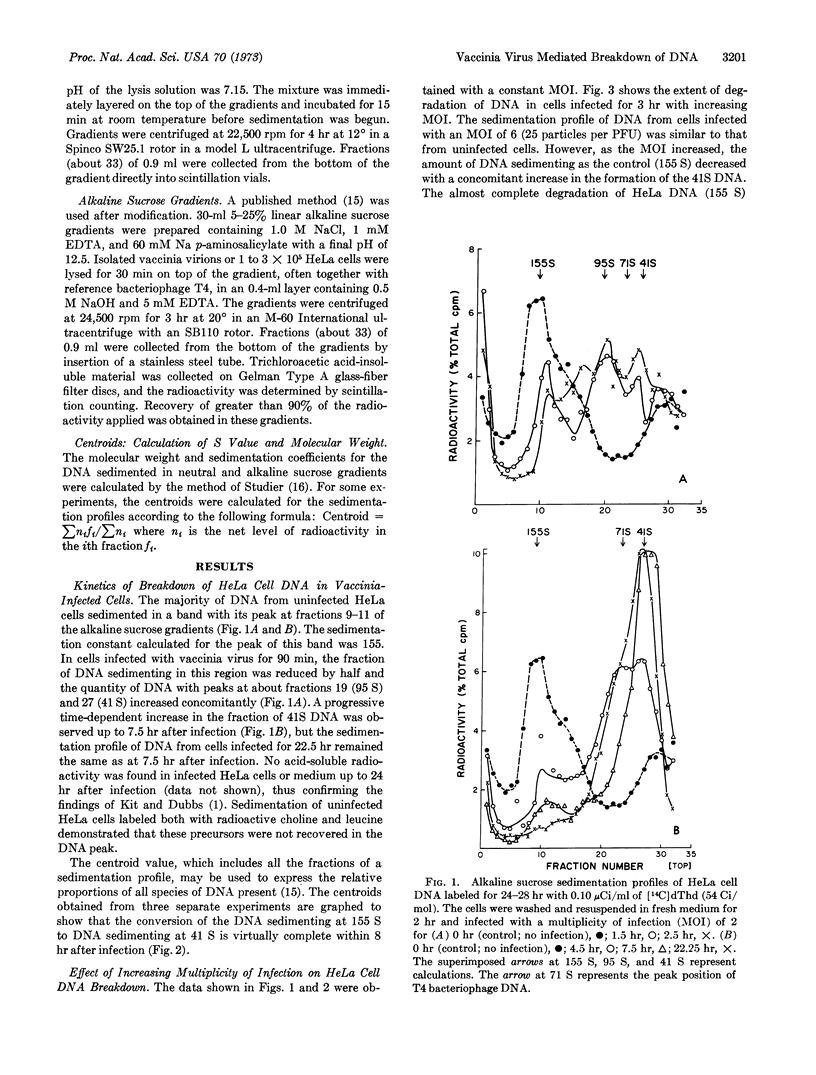
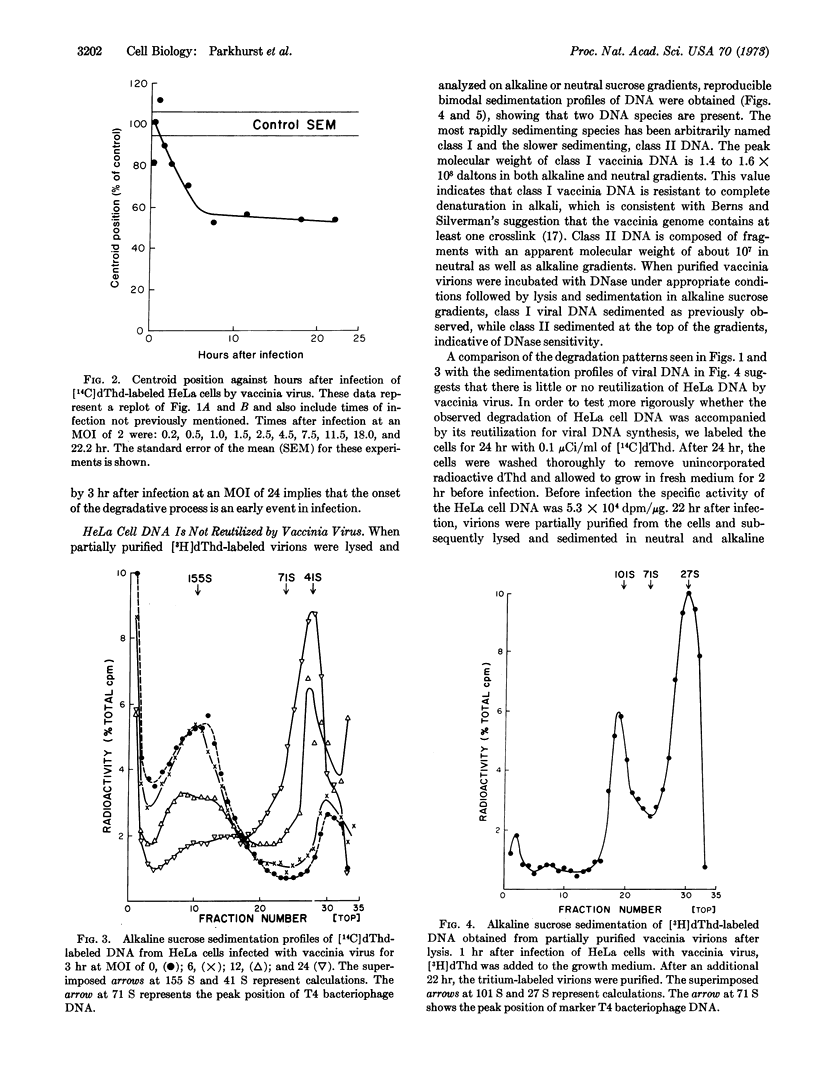
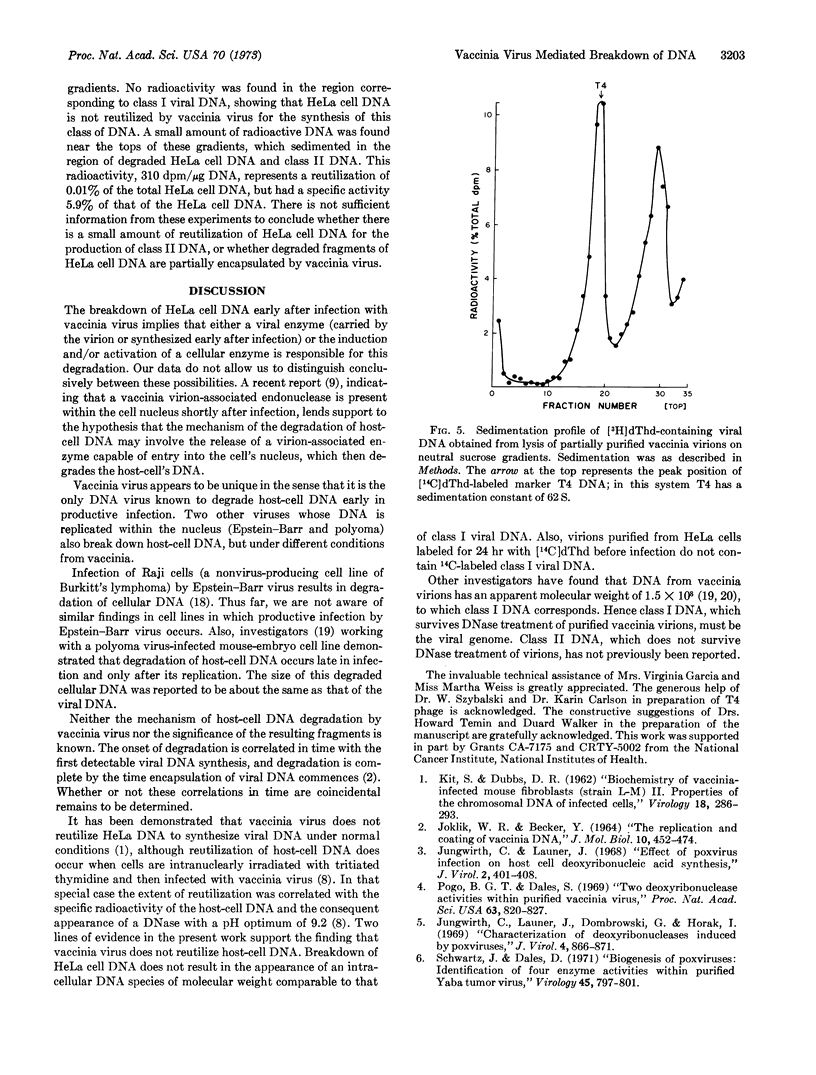
Breakdown of HeLa cell DNA begins within 90 min after infection with vaccinia virus at a multiplicity of infection of 2-plaque-forming units per cell, and ends about 7.5 hr after infection. HeLa cell DNA is degraded to a uniform size of 1 to 2 × 107 daltons, as judged by alkaline sucrose sedimentation analysis. The rate of host-cell DNA degradation by vaccinia virus increased directly with the multiplicity of infection. Sedimentation patterns in neutral and alkaline sucrose gradients of viral DNA from infected cells, as well as from partially purified virions, indicated that two size classes of DNA were present. Class 1 DNA sediments like T4 DNA in neutral gradients and has a molecular weight twice that of T4 DNA in alkaline gradients. Class II DNA sediments as a molecule of lower molecular weight than T4 DNA in both types of gradients. Infection of prelabeled HeLa cells with vaccinia virus did not result either in formation of trichloroacetic acid-soluble radioactivity or, upon purification of the virions, radioactivity associated with class I DNA, indicating that vaccinia virus does not reutilize HeLa cell DNA.
Keywords: viral DNA, alkaline sucrose gradients
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aubertin A. M., McAuslan B. R. Virus-associated nucleases: evidence for endonuclease and exonuclease activity in rabbitpox and vaccinia viruses. J Virol. 1972 Mar;9(3):554–556. doi: 10.1128/jvi.9.3.554-556.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Porat T., Kaplan A. S. Correlation between replication and degradation of cellular DNA in polyoma virus-infected cells. Virology. 1967 Jul;32(3):457–464. doi: 10.1016/0042-6822(67)90297-8. [DOI] [PubMed] [Google Scholar]
- Berns K. I., Silverman C. Natural occurrence of cross-linked vaccinia virus deoxyribonucleic acid. J Virol. 1970 Mar;5(3):299–304. doi: 10.1128/jvi.5.3.299-304.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahl R., Kates J. R. Intracellular structures containing vaccinia DNA: isolation and characterization. Virology. 1970 Oct;42(2):453–462. doi: 10.1016/0042-6822(70)90288-6. [DOI] [PubMed] [Google Scholar]
- Fujiwara Y., Heidelberger C. Fluorinated pyrimidines. 38. The incorporation of 5-trifluoromethyl-2'-deoxyuridine into the deoxyribonucleic acid of vaccinia virus. Mol Pharmacol. 1970 May;6(3):281–291. [PubMed] [Google Scholar]
- Hayflick L. Tissue cultures and mycoplasmas. Tex Rep Biol Med. 1965 Jun;23(Suppl):285+–285+. [PubMed] [Google Scholar]
- JOKLIK W. K., BECKER Y. THE REPLICATION AND COATING OF VACCINIA DNA. J Mol Biol. 1964 Dec;10:452–474. doi: 10.1016/s0022-2836(64)80066-8. [DOI] [PubMed] [Google Scholar]
- Jungwirth C., Launer J., Dombrowski G., Horák I. Characterization of deoxyribonucleases induced by poxviruses. J Virol. 1969 Dec;4(6):866–871. doi: 10.1128/jvi.4.6.866-871.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jungwirth C., Launer J. Effect of poxvirus infection on host cell deoxyribonucleic acid synthesis. J Virol. 1968 May;2(5):401–408. doi: 10.1128/jvi.2.5.401-408.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KIT S., DUBBS D. R. Biochemistry of vaccinia-infected mouse fibroblasts (strain L-M). II. Properties of the chromosomal DNA of infected cells. Virology. 1962 Oct;18:286–293. doi: 10.1016/0042-6822(62)90015-6. [DOI] [PubMed] [Google Scholar]
- KOZINSKI A. W. Fragmentary transfer of P32-labeled parental DNA to progeny phage. Virology. 1961 Jan;13:124–134. doi: 10.1016/0042-6822(61)90039-3. [DOI] [PubMed] [Google Scholar]
- Nonoyama M., Pagano J. S. Replication of viral deoxyribonucleic acid and breakdown of cellular deoxyribonucleic acid in Epstein-Barr virus infection. J Virol. 1972 Apr;9(4):714–716. doi: 10.1128/jvi.9.4.714-716.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oki T., Fujiwara Y., Heidelberger C. Utilization of host-cell DNA by vaccinia virus replicating in HeLa cells irradiated intranuclearly with tritium. J Gen Virol. 1971 Dec;13(3):401–413. doi: 10.1099/0022-1317-13-3-401. [DOI] [PubMed] [Google Scholar]
- Peterson A. R., Fox B. W., Fox M. Alkaline sucrose sedimentation studies of DNA from P388F lymphoma cells treated with disfunctional alkylating agents. Biochim Biophys Acta. 1973 Mar 28;299(3):385–396. doi: 10.1016/0005-2787(73)90263-3. [DOI] [PubMed] [Google Scholar]
- Pogo B. G., Dales S. Two deoxyribonuclease activities within purified vaccinia virus. Proc Natl Acad Sci U S A. 1969 Jul;63(3):820–827. doi: 10.1073/pnas.63.3.820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STUDIER F. W. SEDIMENTATION STUDIES OF THE SIZE AND SHAPE OF DNA. J Mol Biol. 1965 Feb;11:373–390. doi: 10.1016/s0022-2836(65)80064-x. [DOI] [PubMed] [Google Scholar]
- Sarov I., Becker Y. Studies on vaccinia virus DNA. Virology. 1967 Nov;33(3):369–375. doi: 10.1016/0042-6822(67)90112-2. [DOI] [PubMed] [Google Scholar]
- Schwartz J., Dales S. Biogenesis of poxviruses: identification of four enzyme activities within purified Yaba tumor virus. Virology. 1971 Sep;45(3):797–801. doi: 10.1016/0042-6822(71)90198-x. [DOI] [PubMed] [Google Scholar]
- Walen K. H. Nuclear involvement poin xvirus infection. Proc Natl Acad Sci U S A. 1971 Jan;68(1):165–168. doi: 10.1073/pnas.68.1.165. [DOI] [PMC free article] [PubMed] [Google Scholar]



