Abstract
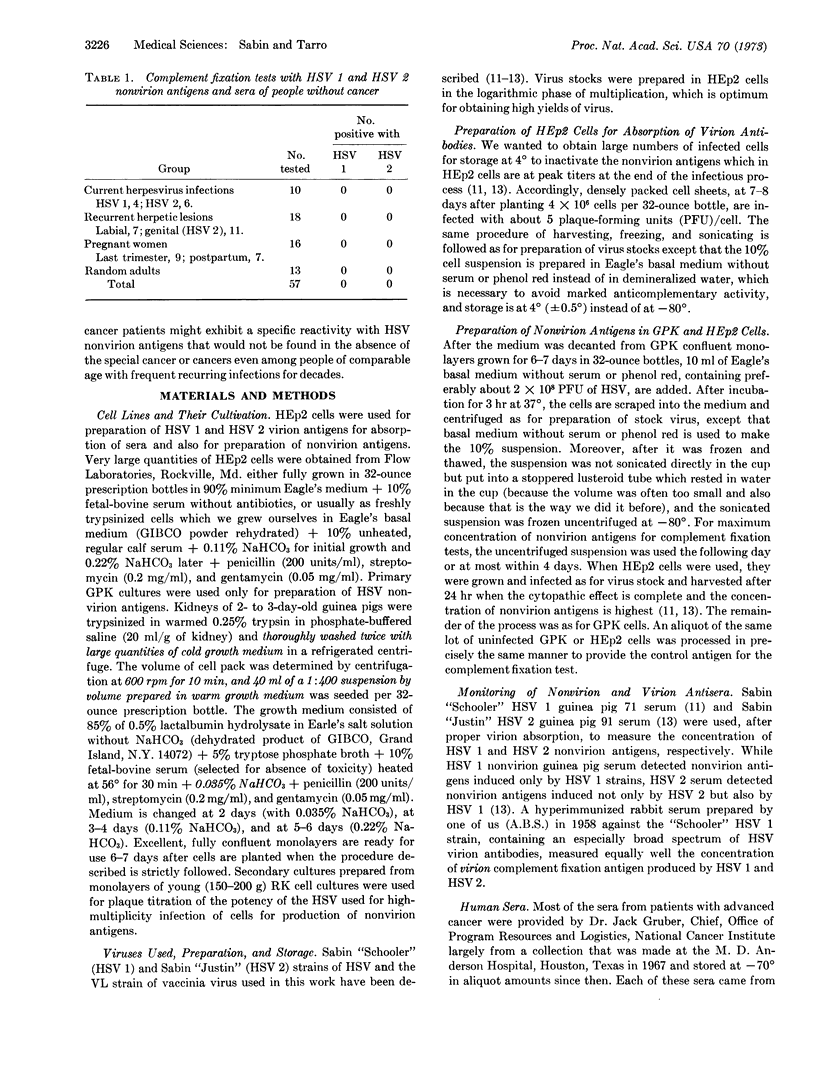
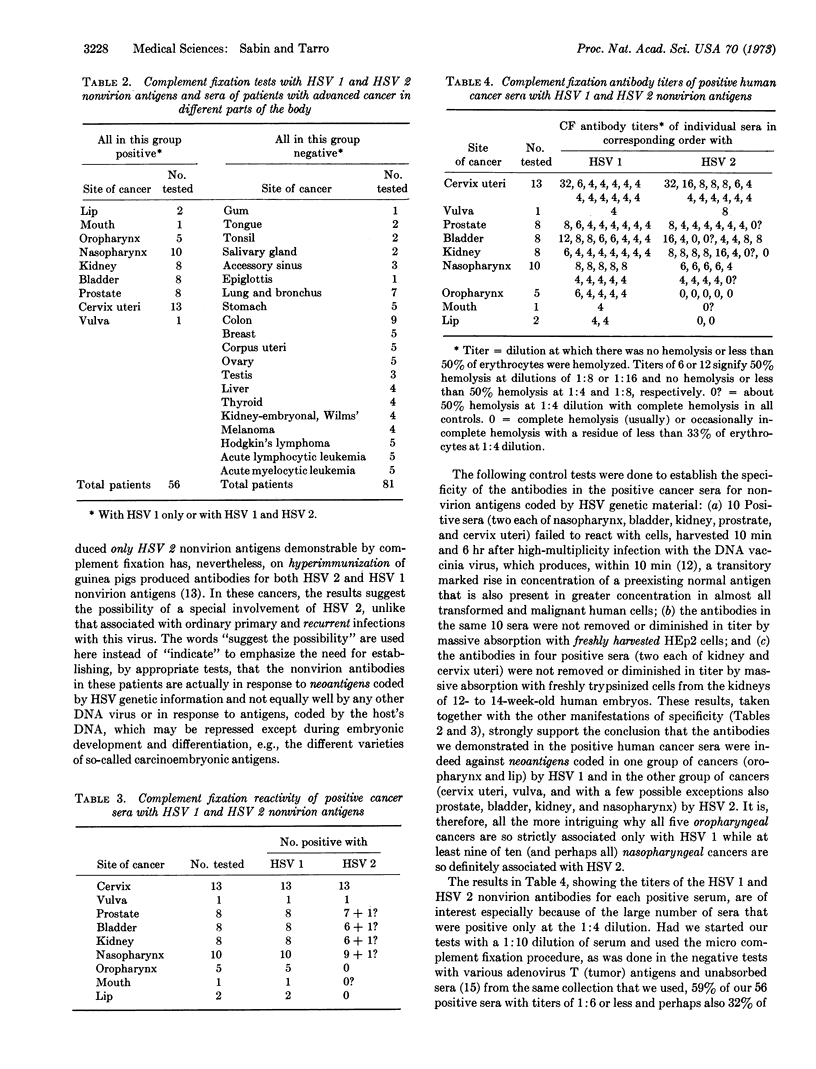
The results of complement fixation tests on 202 sera from people without cancer and from patients with cancer in 29 different areas of the body indicated that only those with nine varieties of advanced cancer (lip, mouth, oropharynx, nasopharynx, kidney, urinary bladder, prostate, cervix uteri, and vulva-all of 56 tested) gave positive specific reactions with nonvirion antigens induced by the DNA herpes simplex (HSV 1) and herpes genitalis (HSV 2) viruses. None of 57 people without cancer (including 10 with current and 18 with recurrent HSV 1 or HSV 2 infections), none of 81 patients with 20 other varieties of advanced cancer (gum, tongue, tonsil, salivary gland, accessory sinus, epiglottis, lung-bronchus, stomach, colon, breast, corpus uteri, ovary, testis, liver, thyroid, Wilms' embryonal kidney, melanoma, Hodgkin's disease, acute lymphocytic leukemia, and acute myelocytic leukemia), and none of four women with early malignant changes in the cervix uteri gave positive results. The seven patients with advanced cancer of the lip or oropharynx gave positive reactions with HSV 1 but not with HSV 2 nonvirion antigens (compatible with involvement of only HSV 1), all of the 13 women with advanced cancer of the cervix uteri and the one woman with advanced cancer of the vulva gave positive reactions with both HSV 1 and HSV 2 nonvirion antigens (compatible with involvement of only HSV 2), while among the 35 other positive patients only two (one with cancer of the kidney and one with cancer of the bladder) reacted with HSV 1 and not at all with HSV 2 nonvirion antigens. Positive sera failed to react with cells harvested at different times after high-multiplicity infection with the DNA vaccinia virus. Massive absorption of positive sera with trypsinized, uninfected human embryonic kidney cells failed to remove, or lower the titer of, the HSV 1 and HSV 2 nonvirion antibodies.
All of these data taken together are interpreted as indicating that HSV 1 and HSV 2 play an etiologic role in certain human cancers, because they provide the kind of evidence by which virus-free experimental cancers can be proved to have been originally induced by such DNA viruses as polyoma, Simian Virus 40, or certain types of adenovirus.
Keywords: DNA tumor viruses, nonvirion antigens, complement fixation, HeLa cells, HEp2 cells
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Frenkel N., Roizman B., Cassai E., Nahmias A. A DNA fragment of Herpes simplex 2 and its transcription in human cervical cancer tissue. Proc Natl Acad Sci U S A. 1972 Dec;69(12):3784–3789. doi: 10.1073/pnas.69.12.3784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilden R. V., Kern J., Lee Y. K., Rapp F., Melnick J. L., Riggs J. L., Lennette E. H., Zbar B., Rapp H. J., Turner H. C. Serologic surveys of human cancer patients for antibody to adenovirus T antigens. Am J Epidemiol. 1970 May;91(5):500–509. doi: 10.1093/oxfordjournals.aje.a121160. [DOI] [PubMed] [Google Scholar]
- HOGGAN M. D., ROWE W. P., BLACK P. H., HUEBNER R. J. PRODUCTION OF "TUMOR-SPECIFIC" ANTIGENS BY ONCOGENIC VIRUSES DURING ACUTE CYTOLYTIC INFECTIONS. Proc Natl Acad Sci U S A. 1965 Jan;53:12–19. doi: 10.1073/pnas.53.1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUEBNER R. J., ROWE W. P., TURNER H. C., LANE W. T. SPECIFIC ADENOVIRUS COMPLEMENT-FIXING ANTIGENS IN VIRUS-FREE HAMSTER AND RAT TUMORS. Proc Natl Acad Sci U S A. 1963 Aug;50:379–389. doi: 10.1073/pnas.50.2.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollinshead A. C., Tarro G. Soluble membrane antigens of lip and cervical carcinomas: reactivity with antibody for herpesvirus nonvirion antigens. Science. 1973 Feb 16;179(4074):698–700. doi: 10.1126/science.179.4074.698. [DOI] [PubMed] [Google Scholar]
- POPE J. H., ROWE W. P. IMMUNOFLUORESCENT STUDIES OF ADENOVIRUS 12 TUMORS AND OF CELLS TRANSFORMED OR INFECTED BY ADENOVIRUSES. J Exp Med. 1964 Oct 1;120:577–588. doi: 10.1084/jem.120.4.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAPP F., BUTEL J. S., FELDMAN L. A., KITAHARA T., MELNICK J. L. DIFFERENTIAL EFFECTS OF INHIBITORS ON THE STEPS LEADING TO THE FORMATION OF SV40 TUMOR AND VIRUS ANTIGENS. J Exp Med. 1965 Jun 1;121:935–944. doi: 10.1084/jem.121.6.935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAPP F., KITAHARA T., BUTEL J. S., MELNICK J. L. SYNTHESIS OF SV40 TUMOR ANTIGEN DURING REPLICATION OF SIMIAN PAPOVAVIRUS (SV40). Proc Natl Acad Sci U S A. 1964 Nov;52:1138–1142. doi: 10.1073/pnas.52.5.1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SABIN A. B., KOCH M. A. BEHAVIOR OF NONINFECTIOUS SV 40 VIRAL GENOME IN HAMSTER TUMOR CELLS: INDUCTION OF SYNTHESIS OF INFECTIOUS VIRUS. Proc Natl Acad Sci U S A. 1963 Sep;50:407–417. doi: 10.1073/pnas.50.3.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SABIN A. B., KOCH M. A. SOURCE OF GENETIC INFORMATION FOR SPECIFIC COMPLEMENT-FIXING ANTIGENS IN SV40 VIRUS-INDUCED TUMORS. Proc Natl Acad Sci U S A. 1964 Nov;52:1131–1138. doi: 10.1073/pnas.52.5.1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarro G., Sabin A. B. Increase in preexisting cellular antigen-combining groups at different times after infection with different viruses. Proc Natl Acad Sci U S A. 1970 Oct;67(2):731–737. doi: 10.1073/pnas.67.2.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarro G., Sabin A. B. Nonvirion antigens produced by herpes simplex viruses 1 and 2. Proc Natl Acad Sci U S A. 1973 Apr;70(4):1032–1036. doi: 10.1073/pnas.70.4.1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarro G., Sabin A. B. Virus-specific, labile, nonvirion antigen in herpesvirus-infected cells. Proc Natl Acad Sci U S A. 1970 Mar;65(3):753–760. doi: 10.1073/pnas.65.3.753. [DOI] [PMC free article] [PubMed] [Google Scholar]



