Abstract
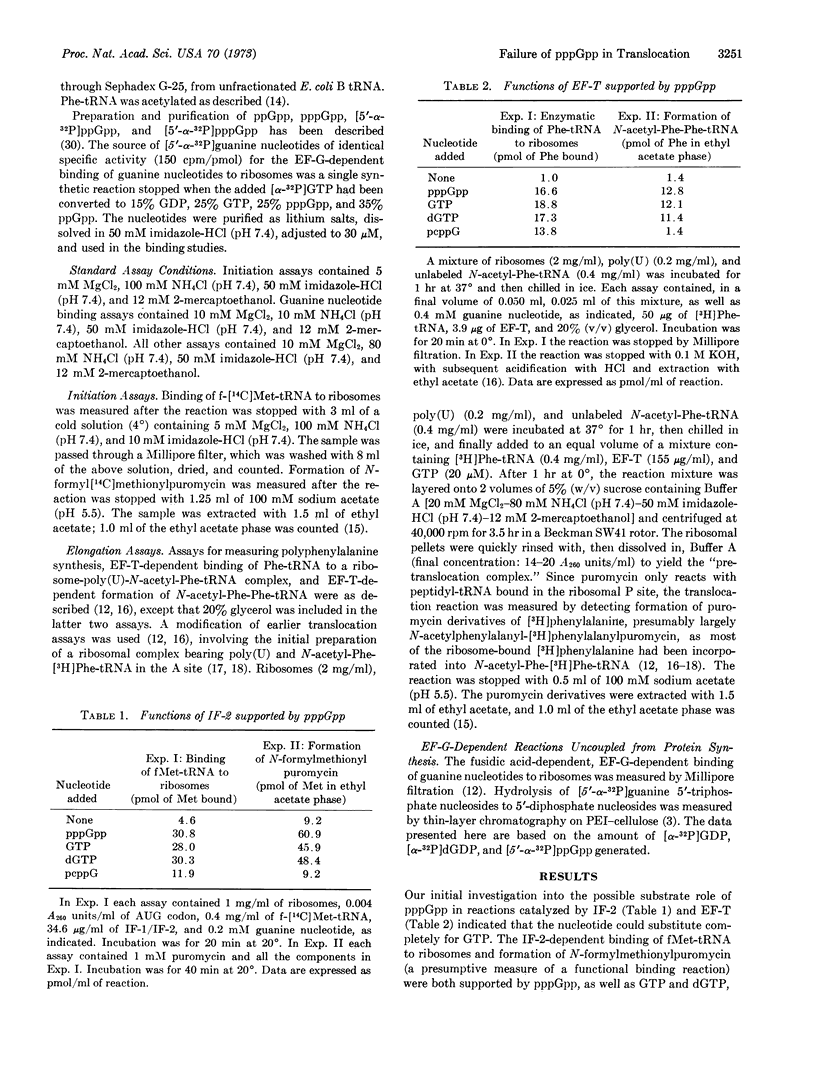
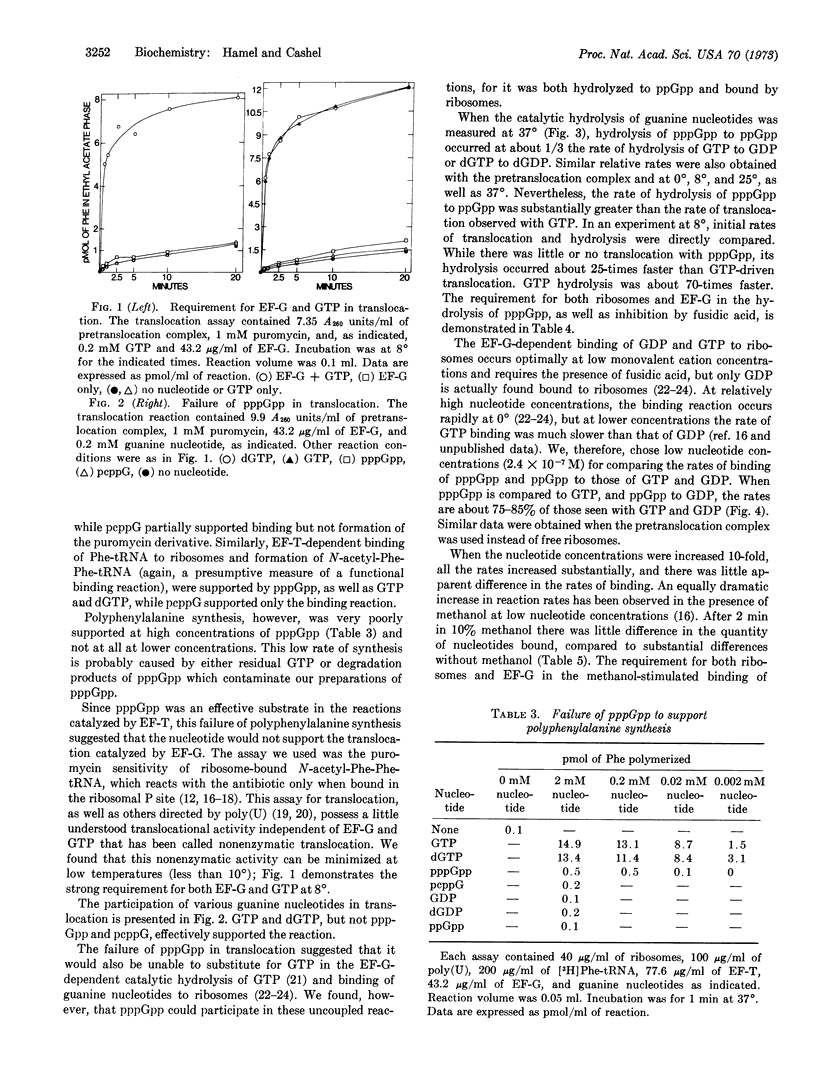
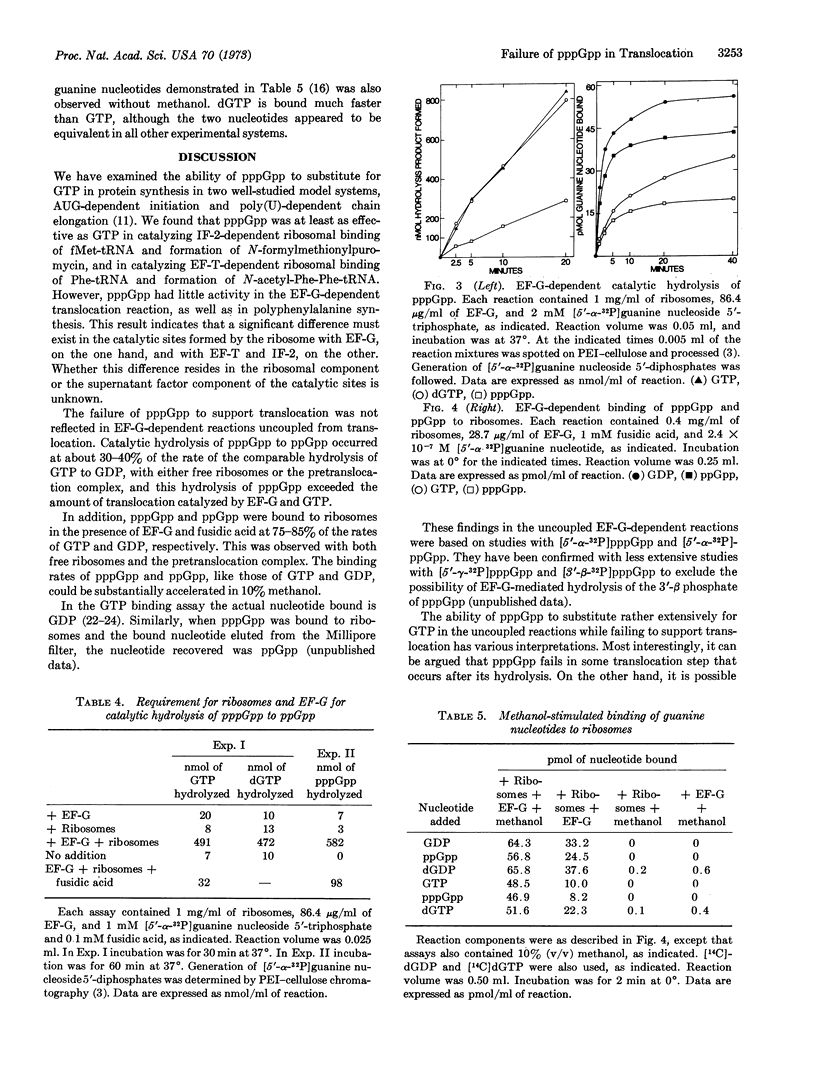
The possible role of guanosine 5′-triphosphate,3′-diphosphate (pppGpp) in protein synthesis by Escherichia coli ribosomes and protein factors was examined. Although pppGpp could effectively substitute for GTP in reactions catalyzed by initiation factor 2 (ribosomal binding of fMet-tRNA and formation of N-formylmethionylpuromycin) and elongation factor T (ribosomal binding of Phe-tRNA and formation of dipeptidyl-tRNA), pppGpp poorly supported polyphenylalanine synthesis. The interaction of elongation factor G with pppGpp was, therefore, examined in detail. The nucleotide was found to be almost without activity in the translocation reaction, as measured by formation of N-acetylphenylalanyl-phenylalanylpuromycin. Nevertheless, the rate of the catalytic hydrolysis of pppGpp to guanosine 5′-diphosphate,3′-diphosphate by elongation factor G and ribosomes was about 30% of the rate of hydrolysis of GTP, a rate of hydrolysis that significantly exceeded the rate of translocation with GTP. Moreover, the rates of the fusidic acid-dependent, elongation factor G-dependent binding of pppGpp and ppGpp to ribosomes were about 75 to 85% the rates of GTP and GDP binding, respectively. We also found that dGTP could substitute for GTP in all reactions examined.
Keywords: E. coli, pppGpp, translocation
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arai K., Arai N., Kawakita M., Kaziro Y. Interaction of guanosine 5'-diphosphate, 2'-(or 3'-) diphosphate(ppGpp) with elongation factors from E. coli. Biochem Biophys Res Commun. 1972 Jul 11;48(1):191–196. doi: 10.1016/0006-291x(72)90361-0. [DOI] [PubMed] [Google Scholar]
- Baliga B. S., Munro H. N. Evidence of formation of a complex between GTP and elongation factor two and the binding of the complex to a specific site on mammalian ribosomes. Biochim Biophys Acta. 1972 Aug 25;277(2):368–383. doi: 10.1016/0005-2787(72)90418-2. [DOI] [PubMed] [Google Scholar]
- Blumenthal T., Landers T. A., Weber K. Bacteriophage Q replicase contains the protein biosynthesis elongation factors EF Tu and EF Ts. Proc Natl Acad Sci U S A. 1972 May;69(5):1313–1317. doi: 10.1073/pnas.69.5.1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodley J. W., Zieve F. J., Lin L., Zieve S. T. Studies on translocation. 3. Conditions necessary for the formation and detection of a stable ribosome-G factor-guanosine diphosphate complex in the presence of fusidic acid. J Biol Chem. 1970 Nov 10;245(21):5656–5661. [PubMed] [Google Scholar]
- Brot N., Spears C., Weissbach H. The interaction of transfer factor G, ribosomes, and guanosine nucleotides in the presence of fusidic acid. Arch Biochem Biophys. 1971 Mar;143(1):286–296. doi: 10.1016/0003-9861(71)90211-6. [DOI] [PubMed] [Google Scholar]
- CONWAY T. W., LIPMANN F. CHARACTERIZATION OF A RIBOSOME-LINKED GUANOSINE TRIPHOSPHATASE IN ESCHERICHIA COLI EXTRACTS. Proc Natl Acad Sci U S A. 1964 Dec;52:1462–1469. doi: 10.1073/pnas.52.6.1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cashel M., Gallant J. Two compounds implicated in the function of the RC gene of Escherichia coli. Nature. 1969 Mar 1;221(5183):838–841. doi: 10.1038/221838a0. [DOI] [PubMed] [Google Scholar]
- Cashel M., Kalbacher B. The control of ribonucleic acid synthesis in Escherichia coli. V. Characterization of a nucleotide associated with the stringent response. J Biol Chem. 1970 May 10;245(9):2309–2318. [PubMed] [Google Scholar]
- Cashel M. The control of ribonucleic acid synthesis in Escherichia coli. IV. Relevance of unusual phosphorylated compounds from amino acid-starved stringent strains. J Biol Chem. 1969 Jun 25;244(12):3133–3141. [PubMed] [Google Scholar]
- Edlin G., Broda P. Physiology and genetics of the "ribonucleic acid control" locus in escherichia coli. Bacteriol Rev. 1968 Sep;32(3):206–226. doi: 10.1128/br.32.3.206-226.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavrilova L. P., Spirin A. S. Stimulation of "non-enzymic" translocation in ribosomes by p-chloromercuribenzoate. FEBS Lett. 1971 Oct 1;17(2):324–326. doi: 10.1016/0014-5793(71)80177-1. [DOI] [PubMed] [Google Scholar]
- Haenni A. L., Chapeville F. The behaviour of acetylphenylalanyl soluble ribonucleic acid in polyphenylalanine synthesis. Biochim Biophys Acta. 1966 Jan 18;114(1):135–148. doi: 10.1016/0005-2787(66)90261-9. [DOI] [PubMed] [Google Scholar]
- Haenni A. L., Lucas-Lenard J. Stepwise synthesis of a tripeptide. Proc Natl Acad Sci U S A. 1968 Dec;61(4):1363–1369. doi: 10.1073/pnas.61.4.1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamel E., Koka M., Nakamoto T. Requirement of an Escherichia coli 50 S ribosomal protein component for effective interaction of the ribosome with T and G factors and with guanosine triphosphate. J Biol Chem. 1972 Feb 10;247(3):805–814. [PubMed] [Google Scholar]
- Hamel E., Nakamoto T. Effect of methanol on the partial reactions of polypeptide chain elongation. Biochemistry. 1972 Oct 10;11(21):3933–3938. doi: 10.1021/bi00771a016. [DOI] [PubMed] [Google Scholar]
- Haseltine W. A., Block R., Gilbert W., Weber K. MSI and MSII made on ribosome in idling step of protein synthesis. Nature. 1972 Aug 18;238(5364):381–384. doi: 10.1038/238381a0. [DOI] [PubMed] [Google Scholar]
- Kaziro Y., Inoue N., Kuriki Y., Mizumoto K., Tanaka M., Kawakita M. Purification and properties of factor G. Cold Spring Harb Symp Quant Biol. 1969;34:385–393. doi: 10.1101/sqb.1969.034.01.045. [DOI] [PubMed] [Google Scholar]
- Lazzarini R. A., Cashel M., Gallant J. On the regulation of guanosine tetraphosphate levels in stringent and relaxed strains of Escherichia coli. J Biol Chem. 1971 Jul 25;246(14):4381–4385. [PubMed] [Google Scholar]
- Leder P., Bursztyn H. Initiation of protein synthesis II. A convenient assay for the ribosome-dependent synthesis of N-formyl-C14-methionylpuromycin. Biochem Biophys Res Commun. 1966 Oct 20;25(2):233–238. doi: 10.1016/0006-291x(66)90586-9. [DOI] [PubMed] [Google Scholar]
- Lucas-Lenard J. Protein biosynthesis. Annu Rev Biochem. 1971;40:409–448. doi: 10.1146/annurev.bi.40.070171.002205. [DOI] [PubMed] [Google Scholar]
- Miller D. L., Cashel M., Weissbach H. The interaction of guanosine 5'-diphosphate, 2' (3')-diphosphate with the bacterial elongation factor Tu. Arch Biochem Biophys. 1973 Feb;154(2):675–682. doi: 10.1016/0003-9861(73)90022-2. [DOI] [PubMed] [Google Scholar]
- Modolell J., Vazquez D. Inhibition by aminoacyl transfer ribonucleic acid of elongation factor G-dependent binding of guanosine nucleotide to ribosomes. J Biol Chem. 1973 Jan 25;248(2):488–493. [PubMed] [Google Scholar]
- Ono Y., Skoultchi A., Waterson J., Lengyel P. Stoichiometry of aminoacyl-transfer RNA binding and GTP cleavage during chain elongation and translocation. Nature. 1969 Aug 16;223(5207):697–701. doi: 10.1038/223697a0. [DOI] [PubMed] [Google Scholar]
- Pestka S. Studies on the formation of transfer ribonucleic acid-ribosome complexes. VI. Oligopeptide synthesis and translocation on ribosomes in the presence and absence of soluble transfer factors. J Biol Chem. 1969 Mar 25;244(6):1533–1539. [PubMed] [Google Scholar]
- Que L., Jr, Willie G. R., Cashel M., Bodley J. W., Gray G. R. Guanosine 5'-diphosphate, 3'-diphosphate: assignment of structure by 13C nuclear magnetic resonance spectroscopy. Proc Natl Acad Sci U S A. 1973 Sep;70(9):2563–2566. doi: 10.1073/pnas.70.9.2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter D. Competition between the elongation factors 1 and 2, and phenylalanyl transfer ribonucleic acid for the ribosomal binding sites in a polypeptide-synthesizing system from brain. J Biol Chem. 1973 Apr 25;248(8):2853–2857. [PubMed] [Google Scholar]
- Sy J., Lipmann F. Identification of the synthesis of guanosine tetraphosphate (MS I) as insertion of a pyrophosphoryl group into the 3'-position in guanosine 5'-diphosphate. Proc Natl Acad Sci U S A. 1973 Feb;70(2):306–309. doi: 10.1073/pnas.70.2.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida M., Travers A., Clark B. F. Inhibition of translation initiation complex formation by MS1. FEBS Lett. 1972 Jun 15;23(2):163–166. doi: 10.1016/0014-5793(72)80331-4. [DOI] [PubMed] [Google Scholar]



