Abstract
Lung cancer screening with computed tomography has demonstrated a significant reduction in mortality. While these findings are important for the lung cancer research field, the most important risk factor for lung cancer, i.e. smoking, should not be ignored. We performed a pilot study to examine the feasibility of delivering a program that included both tobacco dependence treatment and lung cancer screening. The objectives of this study were to: (1) estimate the proportion of smokers who complied with a 12-week treatment protocol that included both tobacco dependence treatment and lung cancer screening, (2) obtain preliminary estimates of abstinence and quit attempts at 4 and 6 months, and (3) obtain preliminary estimates of the cognitive social health information processing (C-SHIP) constructs and how they change following the intervention. In this randomized pilot study, 18 volunteers completed a 12-week protocol: half received the tobacco dependence treatment program before a CT scan (BCT) and the other received the CT scan first, followed by the treatment program (ACT). The treatment protocol included both nurse-delivered telephone counseling and either nicotine replacement therapy or varenicline. Only one person did not complete all follow-up evaluations. At 4 months post enrollment, the carbon monoxide confirmed quit rates were 33.3% in the BCT arm and 22.2% in the ACT arm (27.8% overall), and all but one had made a 24-h attempt to quit. At 6 months the confirmed abstinence decreased to 22.1% in the BCT arm and 11.1% in the ACT arm (16.7% overall), and 72.2% of participants had made a 24-h quit attempt. These preliminary results suggest that it might be better to deliver treatment before the screening test. Future randomized trials with a larger sample size are needed to confirm these findings.
Keywords: Lung cancer screening, Smoking cessation, Nicotine replacement therapy, Varenicline, Epidemiology, Public health
1. Introduction
Until very recently, there was no clinical trial evidence that a lung cancer screening test would result in lower cancer-related mortality. Findings from the National Lung Screening Trial (NLST) showed that individuals who were randomized to the low-dose helical computed tomography (CT) scan arm of the trial experienced a 20% reduction in lung cancer mortality and 7% reduction in all cause mortality compared to those randomized to the X-ray arm [1]. These individuals were at high risk for lung cancer and therefore age and smoking history criteria were used in the selection of participants. Specifically, participants were age 55 or older, a current smoker or former smoker who quit within the past 15 years, and had at least a 30 pack-year smoking history. The NLST finally demonstrated that a lung cancer screening test could result in decreased mortality, which has a potential to change clinical practice and the way in which high risk individuals are diagnosed with lung cancer. However, the authors caution that the NLST data alone should not be used to establish recommendations for screening given the uncertainty of the cost-effectiveness of screening [1]. The cost-effectiveness of screening with CT is currently being estimated by the NLST group.
The lung cancer screening setting appears to be a good environment in which to introduce tobacco dependence treatment. First, several studies indicate that the majority of smokers are interested having a lung cancer screening test [2,3]. Second, in a study using data from two lung cancer screening programs, the majority of smokers stated they were interested in receiving smoking cessation counseling or pharmacotherapy [4]. Third, 74% of smokers in the Early Lung Cancer Action Program (ELCAP) reported that the screening trial had made them think about abstinence [5]. Fourth, results from some screening trials indicate that smokers who enroll in a screening trial may be more motivated to quit than the general population of smokers [4,6]. Fifth, several studies suggest that smokers who have an abnormal CT result are more likely to quit smoking [7–9] or to be ready to quit smoking [4] compared to smokers with a normal scan. However, there are some reports of no relation between an abnormal CT result and abstinence [5,6,10]. Overall, the evidence suggests that smokers who undergo lung cancer screening are interested in quitting and are perhaps more likely to succeed.
What is lacking in the literature is a description of how effective a formal tobacco dependence program can be in this setting. Only a few studies have reported the results from minimal interventions delivered at the time of lung cancer screening [11,12]. Additional studies are needed to test a combination of sustained counseling and pharmacotherapy, which is the recommendation provided in the United States Public Health Service (USPHS) Clinical Practice Guideline [13]. The optimal timing of the delivery of the intervention is another question that has not been examined. It is not known whether delivering the intervention before the spiral CT or after leads to a greater rate of abstinence. Further, it is not entirely clear how the results of the test will affect abstinence following a tobacco dependence treatment program.
The cognitive-social health information processing (C-SHIP) model is an information processing model that has been developed to guide cancer prevention research [14,15]. Constructs of the C-SHIP are the following: (1) health relevant cognitive strategies for selecting and processing health threats (perception of lung cancer risk), (2) health beliefs and expectancies (self-efficacy), (3) affects (worry, depressive symptoms), and (4) health goals and values (decisional balance). This model served as the theoretical framework for the intervention and guided the decision to examine the two programs that differed only in their timing of the CT scan.
The objectives of this study were to: (1) estimate the proportion of smokers who comply with a 12-week treatment protocol that included both tobacco dependence treatment and lung cancer screening, (2) obtain preliminary estimates of abstinence and quit attempts at 4 and 6 months, and (3) obtain preliminary estimates of the C-SHIP constructs and how they change following the intervention.
2. Methods
The protocol was approved by the Institutional Review Board at The Ohio State University. Participants for this pilot study were recruited from volunteers who responded to advertisements placed on our institution’s listserves and in medical clinics. Recruitment occurred during a one-month period. Eligible participants were the following: (1) age 50 years or older, (2) self-reported current smokers with a 20 pack-year history, (3) not currently enrolled in tobacco dependence treatment, (4) no personal history of cancer other than skin cancer, and (5) no symptoms suggestive of lung cancer. The smoking history and age criteria were identical to the criteria used by Swensen and colleagues in their 5-year prospective study of CT screening [16]. Individuals who provided consent were then randomized to one of two study arms: a tobacco dependence treatment intervention before CT (BCT) or an intervention after CT (ACT) (Fig. 1).
Fig. 1.
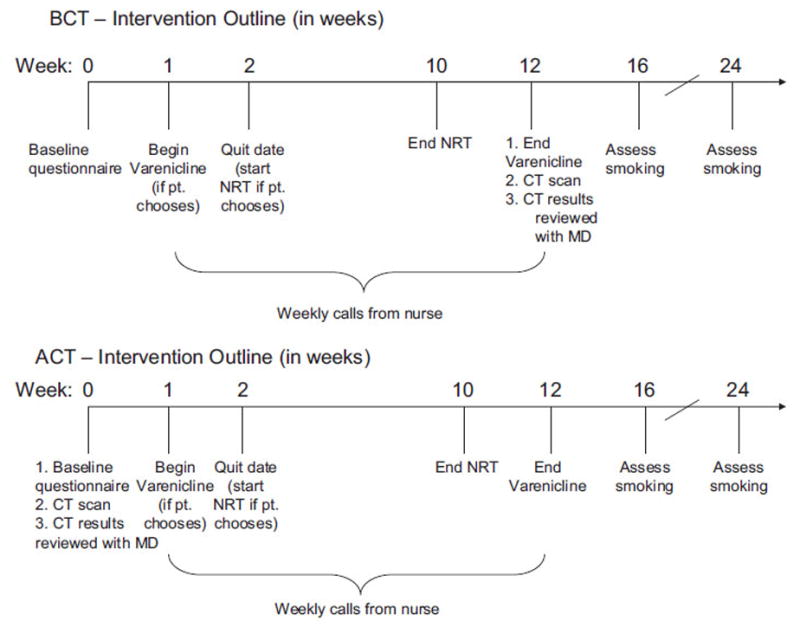
Outline of the tobacco dependence treatment protocol for each intervention arm.
2.1. Assessments
All participants completed the baseline assessment that included an expired carbon monoxide (CO) reading and a questionnaire that contained tobacco-related items and scales, including the Fagerström Test of Nicotine Dependence [17]. Standardized scales were administered in order to measure constructs in the C-SHIP model. Perception of lung cancer risk as it relates to smoking was assessed using two items that addressed absolute and comparative risk [18]. The absolute risk question asked respondents to rate how likely they are to get cancer if they continue to smoke and the comparative risk question asked respondents to rate their likeliness of getting lung cancer compared to others with the same age, sex, and who smoke as much. Response options ranged on a five-point scale from “very likely” to “very unlikely”. High perceived risk was defined as a “very likely” response. Self-efficacy was measured with the Smoking Situations Confidence scale [19]. This nine-item scale assessed how tempted smokers may be to smoke in different situations. Each item was rated on a five-point scale from “not at all tempted” to “extremely tempted.” Three subscales were created with the nine items: positive affect situations, negative affect situations, and habitual situations. The average scores to individual items on each subscale were calculated as the subscale scores. The published alpha coefficients for these scales range from 0.80 (habitual situations) to 0.95 (negative affect situations) [19]. Worry about lung cancer was assessed using an item that addressed concern about getting cancer in one’s lifetime, rated on a four-point scale from “not at all” to “very much” [18]. Those who responded “very much” were considered to have a high level of worry. Depressive symptoms were measured with the Center for Epidemiologic Studies Depression (CES-D) scale [20]. This scale has been studied in a variety of samples, and all coefficient alphas were 0.80 or higher [20]. Decisional balance was measured and defined as the pros of smoking scale score minus the cons of smoking scale score [21]. Thus, a positive score implies the pros of smoking outweigh the cons of smoking. Coefficient alphas for the pros and cons scales have been reported to be 0.87 and 0.90, respectively [21].
Study outcomes included point prevalence abstinence and number of quit attempts. First, self-report of 7-day point prevalence abstinence with carbon monoxide (CO) confirmation was assessed. Those reporting no smoking in the past 7 days were asked to provide a breath sample for CO analysis. Participants with ≤8 ppm were categorized as abstinent [22]. The number of serious quit attempts, defined as no smoking for a period of 24 h in an attempt to quit, was also included as a primary endpoint. These study outcomes were assessed for 4 months and 6 months after the start of the tobacco dependence treatment protocol.
Lung cancer screening was performed with a Siemens Biograph 16 PET/CT scanner. All lung scans were read by a board certified radiologist. A positive CT result was defined as containing at least one non-calcified nodule. Participants with non-calcified nodules were followed-up with a diagnostic CT scan of the chest with high-resolution imaging. Any abnormal finding was communicated directly to the patient, and with their consent, to their primary care physician.
2.2. Treatment conditions
ACT arm
Following the baseline assessment, participants in the ACT arm completed a CT scan and then had a clinic appointment with a medical oncologist specializing in lung cancer where they received advice to quit smoking, the scan results, and a prescription for cessation pharmacotherapy. Following this visit, the 12-week tobacco dependence treatment protocol was started, which included varenicline or nicotine replacement therapy (NRT patch + gum) and weekly telephone counseling delivered by a nurse.
BCT arm
Following the baseline assessment, participants in the BCT arm visited the same medical oncologist where they received advice to quit smoking and a prescription for cessation pharmacotherapy. Following this visit, the same 12-week tobacco dependence treatment protocol was started. At the end of the 12-week period, participants in this arm completed the CT scan and then received the scan results during a clinic visit with the medical oncologist.
Pharmacotherapy and weekly counseling
The cessation pharmacotherapy included 12 weeks of varenicline or 8 weeks of NRT. Participants on varenicline were instructed to take 0.5 mg/day for days 1–3, 0.5 mg twice per day for days 4–7, and 1 mg twice per day starting on day 8 and continuing until the end of week 12. Participants were instructed not to use tobacco or other NRT products after their quit date (week 2). Participants on NRT were instructed to apply a 21 mg NRT patch on their skin, starting on quit day and continuing for 8 weeks. Participants were instructed not to use any tobacco products while on the patch. However, they were told that they could chew up to 20 pieces of 2 mg NRT gum to reduce nicotine cravings.
One trained nurse delivered counseling weekly throughout the protocol; the first visit was in-person and the remaining sessions were on the phone. A standard script was used to guide the discussion, and the topics included: self-monitoring of tobacco consumption behaviors; ability to integrate tobacco abstinence into daily life; ability to cope with triggers and withdrawal; and mood management. The behavioral therapy techniques were based on the recommendations in the USPHS Clinical Practice Guideline [13]. The nurse also asked questions about use of pharmacotherapy and side effects associated with pharmacotherapy.
2.3. Data analysis
The analysis involved calculating baseline descriptive statistics by treatment arm. Quit attempts and abstinence estimates at 4 and 6 months by treatment arm and scan result were also estimated. Due to the small sample size for each treatment arm, statistical tests were not performed. The baseline and 4-month (i.e. end of treatment) C-SHIP construct values were compared, both groups combined, to determine if there was a change over time using either a paired t-test or McNemar’s test for paired frequency data. Finally, abstainers at 6 months were compared to continued smokers with respect to baseline C-SHIP construct values. No statistical tests were performed due to the small sample size among the abstainers.
3. Results
Of the 64 calls received in response to the advertisements, 25 individuals were eligible to participate and 18 enrolled in the study. Table 1 contains the baseline characteristics of the participants; over two-thirds were female and white. The current cigarette consumption was 15 cigarettes per day and the average Fagerström Test of Nicotine Dependence score indicated moderate dependence. Half of the participants chose varenicline while the other half chose NRT (38.9%) or no drug (11.1%). Adherence to the protocol was high: 76% of participants on pharmacotherapy took 80% or more of the scheduled doses and 83% of all participants completed 80% or more of the scheduled calls. Only one person did not complete all study visits (94% compliance).
Table 1.
Baseline characteristics of study participants by group.
| Before CT (n = 9) | After CT (n = 9) | Total (n = 18) | |
|---|---|---|---|
| Sociodemographic characteristics | |||
| Age | 58.1 ± 6.5 | 54.7 ± 3.8 | 56.4 ± 5.5 |
| Female | 66.7% | 77.8% | 72.2% |
| White race | 66.7% | 77.8% | 72.2% |
| Married/living together | 55.5% | 55.5% | 55.5% |
| Household Income = $50,000 | 55.6% | 66.7% | 61.1% |
| Greater than HS education | 66.7% | 100% | 83.3% |
| Tobacco related characteristics | |||
| Years smoked | 38.9 ± 6.0 | 32.7 ± 4.4 | 35.6 ± 6.0 |
| Age at initiation | 18.3 ± 3.2 | 18.7 ± 4.2 | 18.5 ± 3.7 |
| Cigarettes/day | 16.5 ± 5.4 | 14.6 ± 6.0 | 15.5 ± 5.6 |
| Fagerström score | 4.3 ± 1.7 | 5.0 ± 1.5 | 4.6 ± 1.6 |
| At least one 24-h quit attempt in past year | 62.5% | 22.2% | 41.2% |
| Selected chantix | 55.6% | 44.5% | 50.0% |
Table 2 contains the outcome data. At 4 months post enrollment, 33.3% of participants in the BCT arm and 22.2% in the ACT arm had quit (27.8% overall), and all but one had made a 24-h attempt to quit. At 6 months, abstinence decreased to 22.1% in the BCT arm and 11.1% in the ACT arm (16.7% overall), and 72.2% of participants had made at least one 24-h quit attempt.
Table 2.
Outcome data by intervention group.
| Before CT (n = 9) | After CT (n = 9) | Total (n = 18) | |
|---|---|---|---|
| 4-month abstinence | 33.3% | 22.2% | 27.8% |
| 4-month quit attempts | 88.9% | 100% | 94.4% |
| 6-month abstinence | 22.2% | 11.1% | 16.7% |
| 6-month quit attempts | 66.7% | 77.8% | 72.2% |
Seven participants had a positive CT result defined by the presence of a nodule or the need to follow up a suspicious finding; however, none had cancer. At 4 and 6 months abstinence estimates were similar for participants who had a positive scan (28.6% and 14.3%, respectively) compared to those with a negative scan (27.3% and 18.2%, respectively) (data not shown).
Table 3 contains the baseline and 4-month values of the C-SHIP construct scales. Except for comparative risk and CES-D score, the baseline and 4-month values were similar between the two groups. Therefore, data from the two groups were combined and the values at the two time periods were compared. Perceived absolute risk significantly decreased following the intervention (p = 0.046), whereas perceived comparative risk did not change (combined, or among each arm separately). Self-efficacy improved following the intervention (positive affect situations p = 0.0015, negative affect situations p = 0.0016, and habitual situations p = 0.004). The decrease in the scores indicates that participants were less tempted to smoke in these various situations. The mean CES-D score did not change following the intervention (combined, or among each arm separately). Worry about lung cancer did not significantly change following the intervention. Decisional balance significantly decreased over time (p = 0.0017), indicating that the cons of smoking outweighed the pros of smoking following the intervention.
Table 3.
Pre- and post-intervention C-SHIP construct values by group.
| Before CT
|
After CT
|
|||
|---|---|---|---|---|
| Baseline | 4-months | Baseline | 4-months | |
| Health relevant cognitive strategies | ||||
| High perceived absolute risk | 66.7% | 44.4% | 55.6% | 33.3% |
| High perceived comparative risk | 22.2% | 55.6% | 66.7% | 33.3% |
| Health beliefs and expectancies | ||||
| Self-efficacy in positive affect situations | 3.96 ± 0.75 | 3.37 ± 1.17 | 3.50 ± 1.11 | 2.81 ± 1.23 |
| Self-efficacy in negative affect situations | 4.26 ± 0.62 | 3.63 ± 0.84 | 4.29 ± 0.65 | 3.70 ± 0.72 |
| Self-efficacy in habitual situations | 3.56 ± 1.07 | 2.96 ± 0.96 | 3.42 ± 0.79 | 2.52 ± 0.65 |
| Affect – worry | ||||
| High level of worry about getting lung CA | 66.7% | 66.7% | 77.8% | 66.7% |
| Affect – depressive symptoms | ||||
| CES-D score | 13.1 ± 11.3 | 20.6 ± 13.1 | 13.0 ± 8.0 | 13.9 ± 11.2 |
| Health goals and values | ||||
| Decisional balance (Pros–Cons of smoking) | 0.89 ± 2.26 | −1.67 ± 4.79 | 0 ± 3.38 | −3.0 ± 2.45 |
While no statistical tests were performed due to the small sample size, some findings appear to suggest possible relations between abstinence at 6 months and the C-SHIP constructs (see Table 4). Specifically, absolute and comparative risk estimates, as well as worry, were greater in participants who were abstinent at 6 months. Additionally, smokers who quit had lower scores on the subscales that addressed being tempted in positive and habitual situations, but they had higher scores on the negative situations subscale. Finally, smokers who quit had lower CES-D scores and a lower decisional balance score, indicating that pros of smoking outweighed the cons of smoking to a lesser extent.
Table 4.
Pre-intervention C-SHIP construct values by quit status at 6 months.
| Abstinent at 6 months (n = 3) | Continued smoking at 6 months (n = 15) | |
|---|---|---|
| Health relevant cognitive strategies | ||
| High perceived absolute risk (%) | 100% | 53.3% |
| High perceived comparative risk (%) | 100% | 33.3% |
| Health beliefs and expectancies | ||
| Temptation in positive affect situations | 2.3 ± 1.2 | 4.0 ± 0.6 |
| Temptation in negative affect situations | 4.4 ± 0.7 | 4.3 ± 0.6 |
| Temptation in habitual situations | 3.2 ± 1.0 | 3.5 ± 0.6 |
| Affect – worry | ||
| High level of worry about getting lung CA | 100% | 67.7% |
| Affect – depressive symptoms | ||
| CES-D Score | 9.3 ± 1.2 | 13.8 ± 10.3 |
| Health goals and values | ||
| Decisional balance (Pros–Cons of smoking) | 0.3 ± 5.5 | 0.5 ± 2.2 |
4. Discussion
This is one of the first studies to examine the feasibility of delivering a combined lung cancer screening and tobacco dependence treatment intervention to smokers. The protocol included both counseling and pharmacotherapy, which are recommended by the USPHS Clinical Practice Guideline Treating Tobacco Dependence [13]. The results suggest that smokers are interested in participating in a program that includes lung cancer screening combined with tobacco dependence treatment. The compliance rate for this 6-month study was high (94%) and at 6 months close to 17% of smokers was abstinent, which on a population level would translate to a large number of high risk smokers. The purpose of spiral CT screening is to detect lung cancer tumors when they are still in an early stage in order to optimize survival. Because patients with early stage lung cancer have a greater 5-year survival compared to patients with late stage cancer (53% versus 4%; [23]), treating tobacco dependence at the time of screening has the potential to further impact survival, as the literature suggests that continued smoking after diagnosis of early stage cancer is associated with an increased risk of mortality and recurrence [24].
While we did not have enough power to test for differences between the two arms, the preliminary data suggest that receiving tobacco dependence treatment prior to the CT scan, compared to after the scan, could result in a higher abstinence rate (22.2% versus 11.1% abstinence at 6 months).
Some additional interesting findings were noted in this study. First, abstinence rates did not appear to vary with type of CT scan result which is contrary to previous findings [8,9]. Second, abstinence rates did appear to vary with some of the C-SHIP constructs, such as perceived risk, worry, self efficacy, depressive symptoms, and decisional balance. A high perceived risk for an abnormal CT result among smokers undergoing screening has been positively associated with being more ready to quit smoking [4]. Perceived risk for complications associated with continued smoking has been positively associated with abstinence in cancer patients [25]. Worry about the health effects of smoking has been related to abstinence [26] and motivation to quit smoking [27]. However, at least one study reported that increased worry about lung cancer decreased readiness to quit smoking [4]. Self-efficacy is a good predictor of abstinence in healthy [28,29] and diseased populations [25]. Depressive symptoms have been negatively associated with abstinence among participants in a tobacco dependence treatment program [30]. Finally, decisional balance (operationalized as the pros and cons of smoking) has predicted abstinence among patients with cancer [25] and cardiovascular disease [31].
The limitations of this study include a small sample size, a volunteer sample, and the overrepresentation of insured, working, and highly educated smokers. The goals of the project were to examine feasibility issues and obtain preliminary estimates of abstinence rates for this type of program. As a result we chose to only enroll smokers who were somehow affiliated with our institution and hence all of them were working and insured. It is not clear how successful such a program would be in a different setting, such as with low-income minority or rural smokers.
In conclusion, our results suggest that delivering a tobacco dependence treatment program that includes recommendations provided by the USPHS Clinical Practice Guideline [13] is feasible at the time of lung cancer screening. There appears to be interest among smokers, based on the call rate during the recruitment period, and the compliance with the protocol was high. We believe this study provides support for a larger more definitive study testing these two strategies of tobacco dependence treatment in the setting of a lung cancer screening program.
Acknowledgments
Funding source
This study was funded by the Ohio Department of Development (grant ODOD AGMT TECH 04-049).
Footnotes
Conflict of interest
The authors have no conflicts of interest to report.
References
- 1.The NLST Research Team. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365(5):395–409. doi: 10.1056/NEJMoa1102873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hahn EJ, Rayens MK, Hopenhayn C, Christian WJ. Perceived risk and interest in screening for lung cancer among current and former smokers. Res Nurs Health. 2006;29:359–70. doi: 10.1002/nur.20132. [DOI] [PubMed] [Google Scholar]
- 3.Schnoll RA, Bradley P, Miller SM, Unger M, Babb J, Cornfeld M. Psychological issues related to the use of spiral CT for lung cancer early detection. Lung Cancer. 2003;39:315–25. doi: 10.1016/s0169-5002(02)00501-9. [DOI] [PubMed] [Google Scholar]
- 4.Taylor KL, Sanderson L, Zincke N, Mehta L, McGuire C, Gelmann E. Lung cancer screening as a teachable moment for smoking cessation. Lung Cancer. 2007;56:125–34. doi: 10.1016/j.lungcan.2006.11.015. [DOI] [PubMed] [Google Scholar]
- 5.Ostroff JS, Buckshee N, Mancuso CA, Yankelevitz DF, Henschke CI. Smoking cessation following CT screening for early detection of lung cancer. Prev Med. 2001;33:613–21. doi: 10.1006/pmed.2001.0935. [DOI] [PubMed] [Google Scholar]
- 6.Van der Aalst CM, van Klaveren RJ, van den Bergh KAM, Willemsen MC, de Koning HJ. The impact of a lung cancer CT screening result on smoking abstinence. Eur Respir J Express. 2010 doi: 10.1183/09031936.00035410. [DOI] [PubMed] [Google Scholar]
- 7.Ashraf H, Tonnesen P, Pedersen JH, Dirksen A, Thorsen H, Dossing M. Effect of CT screening on smoking habits at 1-year follow-up in the Danish Lung Cancer Screening Trial (DLCST) Thorax. 2009 doi: 10.1136/thx.2008.102475. [DOI] [PubMed] [Google Scholar]
- 8.Styn MA, Land SR, Perkins KA, Wilson DO, Romkes M, Weissfeld JL. Smoking behavior 1 year after computed tomography screening for lung cancer: effect of physician referral for abnormal CT findings. Cancer Epidemiol Biomarkers. 2009;18(12):3484–9. doi: 10.1158/1055-9965.EPI-09-0895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Townsend CO, Clark MM, Jett JR, Patten CA, Schroeder DR, Nirelli LM, et al. Relation between smoking cessation and receiving results from three annual spiral chest computed tomography scans for lung carcinoma screening. Cancer. 2005;103:2154–62. doi: 10.1002/cncr.21045. [DOI] [PubMed] [Google Scholar]
- 10.Cox LS, Clark MM, Jett JR, Patten CA, Schroeder DR, Nirelli LM, et al. Change in smoking status after spiral chest computed tomography scan screening. Cancer. 2003;98(11):2495–501. doi: 10.1002/cncr.11813. [DOI] [PubMed] [Google Scholar]
- 11.Clark MM, Cox LS, Jett JR, Patten CA, Schroeder DR, Nirelli LM, et al. Effectiveness of smoking cessation self-help materials in a lung cancer screening population. Lung Cancer. 2004;44:13–21. doi: 10.1016/j.lungcan.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 12.Schnoll RA, Miller SM, Unger M, McAleer C, Halbherr T, Bradley P. Characteristics of female smokers attending a lung cancer screening program: a pilot study with implications for program development. Lung Cancer. 2002;37:257–65. doi: 10.1016/s0169-5002(02)00106-x. [DOI] [PubMed] [Google Scholar]
- 13.Fiore MC, Jaén CR, Baker TB, Bailey WC, Benowitz NL, Curry SJ, et al. Treating tobacco use and dependence: 2008 update clinical practice guideline. Rockville, MD: U.S. Department of Health and Human Services, Public Health Service; 2008. [Google Scholar]
- 14.Miller SM, Diefenbach MA. C-SHIP: a cognitive-social health information processing approach to cancer. In: Krantz D, editor. Perspectives in behavioral medicine. Manwah, NJ: Lawrence Erlbaum Associates, Inc; 1998. pp. 219–44. [Google Scholar]
- 15.Miller SM, Hurley K, Shoda Y. Applying cognitive-social theory to health-protective behavior: breast self-examination in cancer screening. Psychol Bull. 1996;119:70–94. doi: 10.1037/0033-2909.119.1.70. [DOI] [PubMed] [Google Scholar]
- 16.Swensen SJ, Jett JR, Hartman TE, Midthun DE, Mandrekar SJ, Hillman SL, et al. CT screening for lung cancer: five-year prospective experience. Radiology. 2005;235:259–65. doi: 10.1148/radiol.2351041662. [DOI] [PubMed] [Google Scholar]
- 17.Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The Fagerström test for nicotine dependence: a revision of the Fagerström tolerance questionnaire. Br J Addict. 1991;86:1119–27. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- 18.Lyna P, McBride C, Samsa G, Pollak KI. Exploring the association between perceived risks of smoking and benefits to quitting: who does not see the link? Addict Behav. 2002;27:293–307. doi: 10.1016/s0306-4603(01)00175-7. [DOI] [PubMed] [Google Scholar]
- 19.Velicer WF, DiClemente CC, Rossi JS, Prochaska JO. Relapse situations and self-efficacy: an integrative model. Addict Behav. 1990;15:271–83. doi: 10.1016/0306-4603(90)90070-e. [DOI] [PubMed] [Google Scholar]
- 20.Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1:385–401. [Google Scholar]
- 21.Velicer WF, DiClemente CC, Prochaska JO, Brandenberg N. A decisional balance measure for assessing and predicting smoking status. J Personal Soc Psychol. 1985;48:1279–89. doi: 10.1037//0022-3514.48.5.1279. [DOI] [PubMed] [Google Scholar]
- 22.SRNT Subcommittee on Biochemical Verification. Biochemical verification of tobacco use and cessation. Nicotine Tob Res. 2002;4:149–59. doi: 10.1080/14622200210123581. [DOI] [PubMed] [Google Scholar]
- 23.American Cancer Society. Cancer facts & figures 2010. Atlanta: American Cancer Society; 2010. [Google Scholar]
- 24.Parsons A, Daley A, Begh R, Aveyard P. Influence of smoking cessation after diagnosis of early stage lung cancer on prognosis: systematic review of observational studies with meta-analysis. Br Med J. 2010;340:b5569. doi: 10.1136/bmj.b5569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schnoll RA, James C, Malstrom M, Rothman RL, Wang H, Babb J, et al. Longitudinal predictors of continued tobacco use among patients diagnosed with cancer. Ann Behav Med. 2003;25:214–21. doi: 10.1207/S15324796ABM2503_07. [DOI] [PubMed] [Google Scholar]
- 26.Dijkstra A, Brosschot J. Worry about health in smoking behaviour change. Behav Res Ther. 2003;41:1081–92. doi: 10.1016/s0005-7967(02)00244-9. [DOI] [PubMed] [Google Scholar]
- 27.McCaul KD, Hockemeyer JR, Johnson RJ, Zetocha K, Quinlan K, Glasgow RE. Motivation to quit using cigarettes: a review. Addict Behav. 2006;31:42–56. doi: 10.1016/j.addbeh.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 28.Blissmer B, Prochaska JO, Velicer WF, Redding CA, Rossi JS, Greene GW, et al. Common factors predicting long-term changes in multiple health behaviors. J Health Psychol. 2010;15(2):205–14. doi: 10.1177/1359105309345555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Haug S, Meyer C, Ulbrichg S, Schorr G, Ruge J, Rumpf HJ, et al. Predictors and moderators of outcome in different brief interventions for smoking cessation in general medical practice. Patient Educ Couns. 2010;78:57–64. doi: 10.1016/j.pec.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 30.Wewers ME, Ferketich AK, Harness J, Paskett ED. Effectiveness of a nurse-managed, lay-led tobacco cessation intervention among Ohio Appalachian women. Cancer Epidemiol Biomarkers. 2009;18(12):3451–8. doi: 10.1158/1055-9965.EPI-09-0952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chouinard MD, Robichaud-Ekstrand S. Predictive value of the transtheoretical model to smoking cessation in hospitalized patients with cardiovascular disease. Eur J Cardiovasc Prev Rehabil. 2007;14:51–8. doi: 10.1097/HJR.0b013e328014027b. [DOI] [PubMed] [Google Scholar]


