Abstract
Geckos employ their adhesive system when moving up an incline, but the directionality of the system may limit function on downhill surfaces. Here, we use a generalist gecko to test whether limb modulation occurs on downhill slopes to allow geckos to take advantage of their adhesive system. We examined three-dimensional limb kinematics for geckos moving up and down a 45° slope. Remarkably, the hind limbs were rotated posteriorly on declines, resulting in digit III of the pes facing a more posterior direction (opposite to the direction of travel). No significant changes in limb orientation were found in any other condition. This pes rotation leads to a dramatic shift in foot function that facilitates the use of the adhesive system as a brake/stabilizer during downhill locomotion and, although this rotation is not unique to geckos, it is significant for the deployment of adhesion. Adhesion is not just advantageous for uphill locomotion but can be employed to help deal with the effects of gravity during downhill locomotion, highlighting the incredible multi-functionality of this key innovation.
Keywords: adhesive system, gecko, inclines, declines
1. Introduction
Geckos are incredibly diverse; both in terms of morphology [1] and habitat [2]. They thrive in habitats that require climbing, such as trees and rocks, utilizing three-dimensional components of their habitat. The gecko adhesive system is a key innovation that facilitates climbing vertically, and even in inverted positions, on smooth surfaces. This has resulted in increased rates of diversification [1] and allows them to occupy areas of the habitat that are unsuitable for other animals.
Gecko adhesion occurs via a combination of van der Waals and frictional forces [3–5]. Setae, on the ventral side of their toes, provide the increased surface area and close contact between the foot and the substrate that permits successful attachment [6]. Digital hyperextension provides the attachment/release mechanism for the adhesive system, which is only employed on inclines of 10° or greater [7]. Gecko adhesion is directional [3], acting primarily along the long axis of the digit. This constrains how the foot must be orientated during locomotion. Although the adhesive system facilitates inclined locomotion, its function may be limited to uphill surfaces given that the manus or pes would have to be rotated posteriorly for adhesion to be effective on declines. This leads to a fundamental question about gecko adhesion: can geckos employ their adhesive system when moving downhill?
We use Chondrodactylus bibronii (Smith 1846) to investigate the modulation of fore- and hindlimb kinematics during downhill locomotion. We chose C. bibronii due to its varied habitat preference [8]; they also lack functional claws, so any advantage on declines would be from the adhesive system [9]. Although we cannot discount unique specializations for a non-terrestrial habitat, which could lead to unique kinematics, using an animal that is a generalist provides us with initial insight into how animals move downhill.
2. Material and methods
Six adult C. bibronii (body mass = 13.4 ± 6.9 g [average ± s.d.]; snout-vent length = 72.8 ± 8.0 mm) were used. Prior to running trials, each animal had white markers placed on joint locations using nail polish (figure 1). Animals were run on a flat runway, covered in 60-grit sandpaper (Ra-value: 254 µm) that was inclined at 0°, +45° and −45°. Three synchronized high-speed video cameras (2 Phantom Miro M150, Vision Research Inc., NJ, USA; 1 Photron APX-RS, Photron, San Diego, CA, USA) were located to one side of the runway; one recording a direct lateral view, while the other two were set obliquely to the runway. Three good steady strides per limb, condition and individual were collected.
Figure 1.
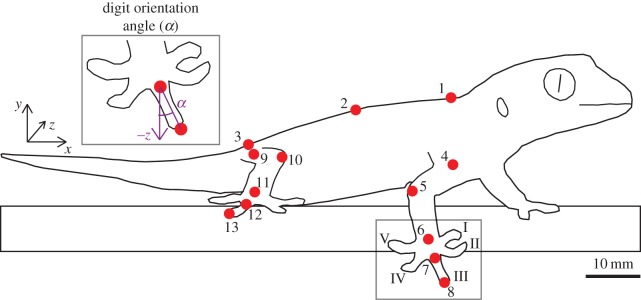
Schematic for marker locations, data coordinate system and calculation of digit orientation. 1–3: body markers; 4: shoulder; 5: elbow; 6: wrist; 7: metacarpalphalangeal joint (MCP); 8: digit III tip; 9: hip; 10: knee; 11: ankle; 12: metatarsalphalangeal joint (MTP); 13: digit III tip; letters I–V represent the digits.
Markers were digitized using DLT_dv5 [10] and the data were processed using custom-written code in Matlab (R2013b, The MathWorks, Natick, MA, USA). Joint angles were calculated following methods previously published [11–13]. Statistics and graphs were also produced using custom codes in Matlab. ANOVAs were run using condition as a fixed factor and individual as a random factor, with the F-values subsequently adjusted using the interaction term between individual and condition [14].
3. Results
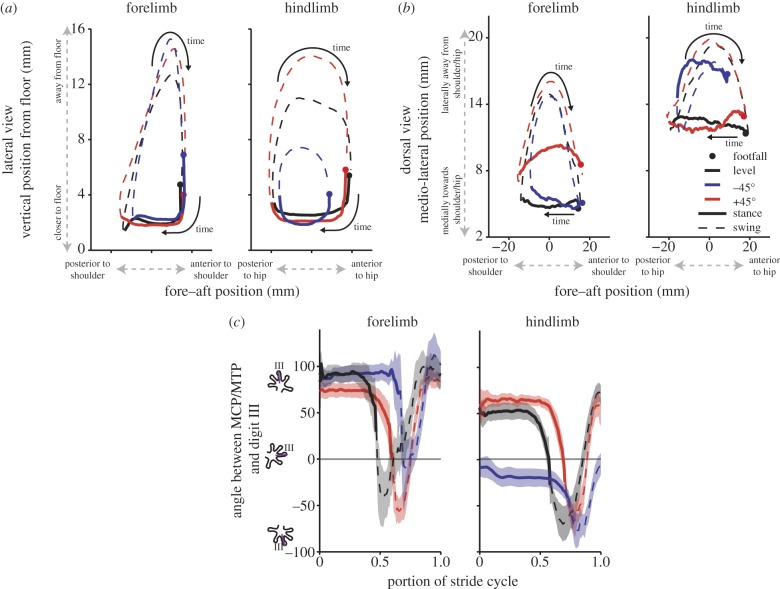
The forelimbs were more sprawled on inclines than during level locomotion (figure 2b; electronic supplementary material, table S1). Forelimb motion was only slightly adjusted during downhill locomotion; the digits did not move as far posteriorly nor as close to the floor compared with the other conditions (figure 2a,b; electronic supplementary material, table S1). The hindlimb digit trajectory was similar for the level and uphill conditions (figure 2a,b). However, digit III on the pes had significant changes in the fore–aft and vertical planes when moving downhill (electronic supplementary material, table S1). The total excursion in both the fore–aft and vertical direction around the hip was greatly reduced (figure 2a). Overall, the cyclical motion of the hindlimb exhibited a reduced circular arc on the decline.
Figure 2.
Digit III position and angle relative to MCP/MTP joint. (a) Average digit III position in vertical (relative to the floor) and in the fore–aft plane (relative to shoulder/hip marker). (b) Average digit III position in the medio-lateral and fore–aft planes relative to the shoulder/hip marker. (c) Average angle between MCP/MTP and digit III (figure 1 for calculation). Shaded regions represent the 95% confidence intervals.
Despite changes in incline, the orientation of the digits on the manus were unaffected during stance; with the tendency for digit III to be pointed nearly in line with the fore–aft plane—therefore, pointing anteriorly (figure 2c). On the other hand, the pes on the level and uphill conditions faced slightly laterally away from the body midline (figure 2c). The pes was rotated posteriorly (70° compared to level) and away from the direction of travel on declines (figure 2c; ANOVA at footfall angle between metatarsalphalangeal joint (MTP) and digit: F2,10 = 27.74; p < 0.005; post hoc Dunn–Sidak both level and uphill versus downhill p < 0.005). Proximal femur rotation accounted for 14° (table 1) indicating that the remaining rotation occurred at distal joints.
Table 1.
Average femur rotation for level condition, and average difference from level (negative values indicate increased clockwise rotation) on incline and decline conditions.
| condition | level (degrees ± s.e.m.) | difference from level (degrees ± s.e.m.) |
d.f. numerator | d.f. denominator | |
|---|---|---|---|---|---|
| +45° | −45° | ||||
| footfall | −28.94 ± 3.56 | −12.12 ± 3.38a | −13.58 ± 3.03a | 2 | 10 |
| mid-stance | −38.16 ± 2.88 | −10.87 ± 2.92a | −10.74 ± 3.05a | 2 | 10 |
| end stance | −67.29 ± 1.68 | 8.49 ± 7.14 | 2.53 ± 2.35 | 2 | 10 |
aSignificant difference from the level condition. d.f.: degrees of freedom.
4. Discussion
Geckos are extremely capable of climbing up steep, smooth surfaces. Here, we demonstrate that geckos have a remarkable ability to reverse the position of their hind feet in order to use adhesion as a brake and/or stabilizer when moving on steep downhill surfaces. Specifically, on the 45° downhill slope, geckos rotated their hindlimb up to 70° posteriorly (relative to level conditions—figure 2c). Use of the adhesive system on declines has previously been suggested [15], but not tested experimentally. We provide evidence for the multi-functionality of the gecko adhesive system, permitting effective locomotion on both uphill and downhill slopes. Without this ability, geckos would be effective at going up, but would be constrained by not being able to descend as easily. This critical component of locomotion (i.e. what goes up, must come down) has been achieved effectively using extreme degrees of joint rotation.
Animals often exhibit differential limb function on level and sloped terrain [16–20], and the amount of bodyweight support per limb often shifts between the fore- and hindlimbs depending on the type of terrain. Here, the significant rotation of the pes likely results in the forelimbs accounting for a much greater component of bodyweight support on the downhill condition than in other conditions. The forelimbs probably adopt a more significant role in braking [16,19], whereas the hindlimbs likely act as stabilizers (see the electronic supplementary video), analogous to stabilizer wheels on a child's bike to prevent them from toppling medio-laterally. It is probable that, although the hindlimbs may be providing medio-lateral stability, the adhesive system is also engaged to supply some braking, via increased friction, and fore–aft stability. Our data highlight the significant and context-dependent decoupling between limbs, as well as the large shifts in limb modulation. This shift has probably led to significant changes in the neural control of locomotion, but this requires further research. In addition, future studies could confirm our interpretations by quantifying the patterns of force generation by the fore- and hindlimbs simultaneously.
The posterior rotation of the pes during downhill locomotion is not unique to C. bibronii. Several studies have noted this capability in mammals (e.g. [21–24]), but this potential functional change in the hindlimb has not been previously quantified during locomotion. For mammals that reverse their foot orientation, femoral rotation accounts for a very small portion of the total rotation. The majority of rotation stems from the crurotalar and subtalar joints [22–24]. This is similar to C. bibronii, where femoral rotation accounts for only 20% of the full foot reversal (table 1). As in mammals, we expect that the astragalocalcaneum (fused ankle bones) of the mesotarsal joint are providing the majority of the posterior rotation in geckos. Lizards, however, exhibit differences in morphology of their astragalocalcaneum, which, in some cases, may preclude separate rotations between distal limb elements and the foot [25]. However, climbing geckos appear to possess an astragalocalcaneum that may allow greater rotation at the articulation with the tibia and fibula [26], particularly as there has been no mention of significant hindlimb reversal in lizards in the literature, although some rotation at the mesotarsal joint appears common among lizards [27]. Future work detailing ankle–foot morphology in Gekkota and other lizards will determine the extent of foot reversal capabilities and the link with an adhesive system.
In conclusion, we highlight the ability of climbing geckos to rotate their adhesive system to deal with declines. The directionality of the system, in that adhesion only works when loaded in shear opposite to the direction of travel, has the potential to constrain locomotion in geckos, but the extreme rotation discovered in our study ameliorates this constraint and avoids a trade-off.
Data accessibility
Kinematic data are accessible on Dryad at http://dx.doi.org/10.5061/dryad.mb180.
Supplementary Material
Acknowledgements
We thank Amy Cheu and Shayan Amiri for help with digitising videos.
Funding statement
This work is supported by an NSF grant (NSF IOS-1147043) to T.E.H.
References
- 1.Gamble T, Greenbaum E, Jackman TR, Russell AP, Bauer AM. 2012. Repeated origin and loss of adhesive toepads in geckos. PLoS ONE 7, e39429 ( 10.1371/journal.pone.0039429) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Johnson MK, Russell AP, Bauer AM. 2005. Locomotor morphometry of the Pachydactylus radiation of lizards (Gekkota: Gekkonidae): a phylogenetically and ecologically informed analysis. Can. J. Zool. 83, 1511–1524. ( 10.1139/z05-112) [DOI] [Google Scholar]
- 3.Autumn K, Liang YA, Hsieh ST, Zesch W, Chan WP, Kenny TW, Fearing R, Full RJ. 2000. Adhesive force of a single gecko foot-hair. Nature 405, 681–685. ( 10.1038/35015073) [DOI] [PubMed] [Google Scholar]
- 4.Autumn K, et al. 2002. Evidence for van der Waals adhesion in gecko setae. Proc. Natl Acad. Sci. USA 99, 12 252–12 256. ( 10.1073/pnas.192252799) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Autumn K, Dittmore A, Santos D, Spenko M, Cutkosky M. 2006. Frictional adhesion: a new angle on gecko attachment. J. Exp. Biol. 209, 3569–3579. ( 10.1242/jeb.02486) [DOI] [PubMed] [Google Scholar]
- 6.Ruibal R, Ernst V. 1965. The structure of the digital setae of lizards. J. Morphol. 117, 271–293. ( 10.1002/jmor.1051170302) [DOI] [PubMed] [Google Scholar]
- 7.Russell AP, Higham TE. 2009. A new angle on clinging in geckos: incline, not substrate, triggers the deployment of the adhesive system. Proc. R. Soc. B 276, 3705–3709. ( 10.1098/rspb.2009.0946) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Branch WR. 1998. Field guide to snakes and other reptiles of southern Africa. Cape Town, South Africa: Struik. [Google Scholar]
- 9.Hora SL. 1923. The adhesive apparatus on the toes of certain geckos and tree frogs. J. Proc. Asiatic Soc. 9, 137–145. [Google Scholar]
- 10.Hedrick TL. 2008. Software techniques for two- and three-dimensional kinematic measurements of biological and biomimetic systems. Bioinspir. Biomim. 3, 034001 ( 10.1088/1748-3182/3/3/034001) [DOI] [PubMed] [Google Scholar]
- 11.Foster KL, Higham TE. 2012. How forelimb and hindlimb function changes with incline and perch diameter in the green anole, Anolis carolinensis. J. Exp. Biol. 215, 2288–2300. ( 10.1242/jeb.069856) [DOI] [PubMed] [Google Scholar]
- 12.Jayne BC, Irschick DJ. 1999. Effects of incline and speed on the three-dimensional hindlimb kinematics of a generalized iguanian lizard (Dipsosaurus dorsalis). J. Exp. Biol. 202, 143–159. [DOI] [PubMed] [Google Scholar]
- 13.Spezzano LC, Jayne BC. 2004. The effects of surface diameter and incline on the hindlimb kinematics of an arboreal lizard (Anolis sagrei). J. Exp. Biol. 207, 2115–2131. ( 10.1242/jeb.00995) [DOI] [PubMed] [Google Scholar]
- 14.Zar JH. 1996. Biostatistical analysis. Upper Saddle River, NJ: Prentice Hall. [Google Scholar]
- 15.Russell AP. 1975. A contribution to the functional analysis of the foot of the Tokay, Gekko-Gecko (Reptilia: Gekkonidae). J. Zool. 176, 437–476. ( 10.1111/j.1469-7998.1975.tb03215.x) [DOI] [Google Scholar]
- 16.Lee DV. 2011. Effects of grade and mass distribution on the mechanics of trotting in dogs. J. Exp. Biol. 214, 402–411. ( 10.1242/jeb.044487) [DOI] [PubMed] [Google Scholar]
- 17.Chen JJ, Peattie AM, Autumn K, Full RJ. 2006. Differential leg function in a sprawled-posture quadrupedal trotter. J. Exp. Biol. 209, 249–259. ( 10.1242/jeb.01979) [DOI] [PubMed] [Google Scholar]
- 18.Autumn K, Hsieh ST, Dudek DM, Chen J, Chitaphan C, Full RJ. 2006. Dynamics of geckos running vertically. J. Exp. Biol. 209, 260–272. ( 10.1242/jeb.01980) [DOI] [PubMed] [Google Scholar]
- 19.Lammers AR. 2007. Locomotor kinetics on sloped arboreal and terrestrial substrates in a small quadrupedal mammal. Zoology 110, 93–103. ( 10.1016/j.zool.2006.12.002) [DOI] [PubMed] [Google Scholar]
- 20.Lammers AR, Earls KD, Biknevicius AR. 2006. Locomotor kinetics and kinematics on inclines and declines in the gray short-tailed opossum Monodelphis domestica. J. Exp. Biol. 209, 4154–4166. ( 10.1242/jeb.02493) [DOI] [PubMed] [Google Scholar]
- 21.Cartmill M. 1974. Pads and claws in Arboreal locomotion. In Primate locomotion (ed. Jenkins FAJ.), pp. 45–84. New York, NY: Academic Press. [Google Scholar]
- 22.Jenkins FA, McClearn D. 1984. Mechanisms of hind foot reversal in climbing mammals. J. Morphol. 182, 197–219. ( 10.1002/jmor.1051820207) [DOI] [PubMed] [Google Scholar]
- 23.Trapp GR. 1972. Some anatomical and behavioral adaptations of ringtails, Bassariscus astutus. J. Mammal. 53, 549–557. ( 10.2307/1379044) [DOI] [Google Scholar]
- 24.Meldrum DJ, Dagosto M, White J. 1997. Hindlimb suspension and hind foot reversal in Varecia variegata and other arboreal mammals. Am. J. Phys. Anthropol. 103, 85–102. () [DOI] [PubMed] [Google Scholar]
- 25.Landsmeer JMF. 1990. Functional-morphology of the hindlimb in some Lacertilia. Eur. J Morphol. 28, 3–34. [PubMed] [Google Scholar]
- 26.Zaaf A, Herrel A, Aerts P, De Vree F. 1999. Morphology and morphometrics of the appendicular musculature in geckoes with different locomotor habits (Lepidosauria). Zoomorphology 119, 9–22. ( 10.1007/s004350050077) [DOI] [Google Scholar]
- 27.Rewcastle SC. 1980. Form and function in lacertilian knee and mesotarsal joints; a contribution to the analysis of sprawling locomotion. J. Zool. 191, 147–170. ( 10.1111/j.1469-7998.1980.tb01454.x) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Kinematic data are accessible on Dryad at http://dx.doi.org/10.5061/dryad.mb180.