Abstract
When females mate with multiple males both pre- and post-copulatory sexual selections occur. It has been suggested that females benefit from polyandry when better-quality males are successful in sperm competition and sire high-quality offspring. Indeed, studies of experimental evolution have confirmed that sperm competition selects for both increased ejaculate quality and elevated offspring viability. Fewer investigations have explored whether these fitness benefits are evident beyond early life-history stages. Here, I used house mice (Mus domesticus) from selection lines that had been evolving for 25 generations under either polygamy or monogamy to test whether females preferred males from lines that had evolved with sperm competition. Males from the polygamous lines had previously been shown to achieve a fitness advantage under semi-natural conditions, deeming them to be of high genetic quality and leading to the a priori expectation that females would prefer males that had evolved with sperm competition compared with males that had not. Contrary to expectation, the data showed that sexually receptive females spent more time associating with males from the monogamous lines. This unexpected but interesting result is discussed in relation to sperm competition theory that predicts a trade-off between male investment in pre- and post-copulatory sexually selected traits.
Keywords: polyandry, experimental evolution, sperm competition, female choice, house mice
1. Introduction
A male's fitness is almost exclusively dependent upon his genetic quality. Males of the highest quality are expected to be successful in acquiring both mates and resources [1] and are predicted to be superior competitors in the post-copulatory arena [2]. Females have been shown to prefer to mate with males that exhibit high-quality secondary sexual traits, which leads to the production of offspring with good genes [1]. Elaborate traits may allow females to use cues in mate assessment, and usually reliably reflect condition-dependent male quality [3]. The females of many species rely on olfactory cues in mate quality assessment [4]. For example, in male house mice the frequency of urine scent-marking is associated with access to resources and territory defence [5].
The urine of male house mice contains major urinary proteins (MUPs), which function to mediate the release of pheromones [6]. Despite being costly to synthesize, MUPs have been detected at high concentrations within scent marks [6]. This inherent cost ensures that scent-marking is a reliable indicator of male quality, and male house mice are known to modulate their scent-marking display depending on their health condition and perceived mating opportunities [7]. Consequently, female house mice may use information in scent marks to gauge the quality of potential mates [8]. High-frequency scent markers experience greater reproductive success compared with low-frequency scent markers when females are given free choice [9], and odour preference tests have shown that females are more attracted to the scent of males that exclusively defend territories, and are more likely to interact sexually with these males, compared with males that are unable to exclude intruders from territories [10].
Females may also exploit post-copulatory mechanisms to acquire ‘good’ and/or compatible genes for her offspring. For example, when a female mates with multiple males, her reproductive tract functions as a selective arena whereby specific sperm haplotypes will be favoured via mechanisms of sperm competition and/or cryptic choice [11]. The good sperm hypothesis predicts that females mate with multiple males to incite sperm competition, allowing males of high genetic quality with increased competitive fertilization success to sire the offspring [2]. Recent support for this hypothesis has come from studies of experimentally evolving populations of house mice; males that had evolved with sperm competition were shown to have increased competitive fertilization success and sire embryos with increased viability over males that had evolved under monogamy [12,13]. Here, I assessed whether the increased fitness among males that had evolved with sperm competition was evident in their ability to attract mates. Thus, I compared males from selection lines that had evolved with and without sperm competition for biases in female preferences, and the rate of scent-marking.
2. Material and methods
(a). Selection lines
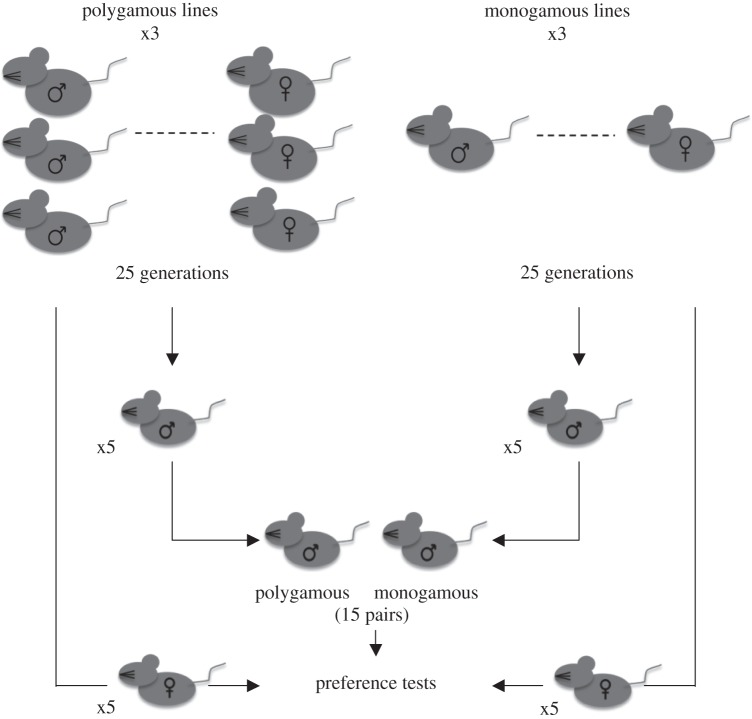
A detailed description of the experimental design and mating regimes of the selection lines is provided elsewhere [14]. Briefly, four monogamous and four polygamous lines were established and maintained for 25 generations. In the monogamous lines, males (18) and females (18) mated with a single partner so that 36 individuals contributed to each generation. In the polygamous lines, the same three females mated with the same three males, such that 18 females but potentially fewer than 18 males contributed to each generation. Animals were randomly selected from three monogamous and three polygamous lines and used in this experiment (figure 1).
Figure 1.
A schematic representation of the experimental design.
(b). Female preference test
To assess whether females show a preference for males with either a monogamous or polygamous selection history, a series of tests were performed using a preference apparatus ([15]; described in the electronic supplementary material). Females were monitored for oestrus condition via inspection of the vaginal tissue; a method proved to be reliable for predicting female receptiveness [16]. Only females deemed to be in oestrus were used in preference tests. Five females from three monogamous and three polygamous replicate lines were used. Males in each monogamous–polygamous testing pair (15) were matched for body weight. Males and females from the same replicate line were not used in the same test.
Preference tests were conducted under a red light during the hours of the dark phase when mating typically occurs [14]. The males were placed in the apparatus for a 30-min acclimation period. Following this, a female was placed in the third chamber for a testing period of 60 min. The animals were continually observed during the testing period, and female behaviour was scored as either (i) a male association or (ii) neutral.
(c). Male scent-marking rate
Urine marking frequency of males from the monogamous (30) and polygamous (30) selection lines was measured as detailed in the electronic supplementary material [17].
3. Results
(a). Female preference test
I used a GLM with a logit link function and a binomial error distribution to analyse variation in female preferences based on female selection history. Time spent with the ‘monogamous' male was included as the response variable, and time spent with both males was included as the binomial denominator in the GLM. As the data were overdispersed, the dispersion parameter was estimated from the scaled deviance. Thus, I present F-tests rather than χ2. The analysis revealed that there was no effect of female selection history on the proportion of time spent with the monogamous males (F1,28 = 0.750, p = 0.394).
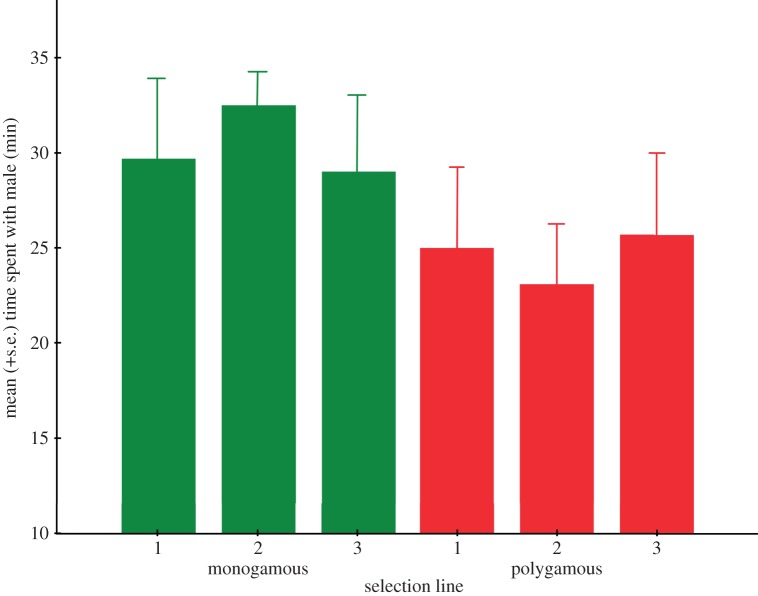
I performed a nested ANOVA on the time spent with each male to assess whether females showed a preference towards males with different selection histories. The analysis revealed that females spent more time associating with males from the monogamous lines (30.4 ± 2.0 min) compared with males from the polygamous lines (24.6 ± 2.0 min) (F1,4 = 10.969, p = 0.029; figure 2; full model: electronic supplementary material, table S1).
Figure 2.
The amount of time (mean + s.e.) females spent associating with males from the monogamous and polygamous selection lines. (Online version in colour.)
(b). Male scent-marking rate
Although there was a significant effect of body weight on male marking frequency, there was no difference between males from the monogamous (54.6 ± 4.2 cm2) and polygamous (50.9 ± 4.7 cm2) selection lines (electronic supplementary material, table S2 and figure S3).
4. Discussion
The reproductive success of males largely depends on the outcome of female mate selection [1] and sperm competition when females choose to mate with multiple partners [11]. Experimental populations evolving under a polygamous regime have provided evidence that sperm competition is a persuasive force influencing both the evolution of the sperm traits and the genetic quality of offspring [13,18]. Few studies have explored whether fitness benefits associated with polyandry are evident beyond an early life-history stage.
I assessed differences in pre-copulatory processes of house mice from selection lines that had evolved with and without sperm competition. Previously, it had been shown that males from the polygamous lines produced embryos of increased viability, leading to increased litter sizes [13,14]. It was concluded that the enhanced male fitness was likely attributable to sperm competition and the removal of deleterious mutation loads, and/or selection for ‘good genes' [14]. These results lead to a priori expectation that females would prefer males that had evolved with sperm competition versus males that had evolved under a monogamous regime. Indeed, when released into free-ranging enclosures and forced to compete for mates, males from the polygamous lines experienced high reproductive success at the expense of ‘monogamous' males (sired 80% of the offspring [19]). In this study, I was unable to directly examine mating preferences and behaviour. However, I did find that sexually receptive females spent more time associating with males that had evolved under a monogamous regime, and it is possible that this social preference, which contradicted expectation, forms the basis of a mating preference [10]. If this is the case, and if female house mice experience greater fitness returns when they reproduce with a preferred versus a non-preferred mating partner [20], why would females exhibit a preference for males who are ostensibly of inferior quality?
Sperm competition theory predicts that there will be a trade-off between male investment in traits associated with mate attraction and those that influence the competitiveness of the ejaculate [21]. Negative relationships between secondary sexual traits and ejaculate quality have been reported among different taxa [22,23]. It is known that males from the polygamous lines had evolved to produce competitively superior ejaculates characterized by high sperm numbers and greater sperm motility [12,14]. Thus, an elevated investment in traits that ensure success in sperm competition could have traded-off against investment in pre-copulatory sexual traits. It has been shown that high-status male mice invest more in scent marking [17]. Here, while body size accounted for variation in the frequency of male scent-marking, there was no difference among individuals from the different selection lines. However, there is the potential that scent quality differed among males that had evolved under different selection regimes. The positioning of males side-by-side in the preference arena would have allowed for protracted interaction between males via chemical cues. If males from the monogamous lines were able to produce higher-quality scents, or scent mark more frequently in the ‘presence’ of a rival male, then they would be preferred over males that had evolved with sperm competition. Alternatively, or additionally, variation in male ultrasonic vocalization could have influenced female social interest and inspection behaviour [24,25]. The ability of ‘monogamous' males to better attract females could explain why they gained paternity representation in ca 50% of litters that were produced in free-ranging enclosures [19]. However, the ultimate success of males that had evolved with sperm competition suggests that high-quality ejaculates provide a greater fitness return than investments in pre-copulatory traits [19]. Future research will directly explore trade-offs between pre-copulatory traits (e.g. scent qualities and ultrasonic vocalizations) and ejaculate qualities in house mice.
Supplementary Material
Acknowledgement
I thank Shresta Lobind for technical assistance.
Ethics statement
This work was approved by the UWA Animal Ethics Committee (approval no. 07/100/607).
Data accessibility
Data is available through Dryad (doi:10.5061/dryad.2v4h6).
Funding statement
This research was funded by the Australian Research Council.
References
- 1.Andersson M, Simmons LW. 2006. Sexual selection and mate choice. Trends Ecol. Evol. 21, 296–302. ( 10.1016/j.tree.2006.03.015) [DOI] [PubMed] [Google Scholar]
- 2.Yasui Y. 1997. A ‘good sperm’ model can explain the evolution of costly multiple mating by females. Am. Nat. 149, 573–584. ( 10.1086/286006) [DOI] [Google Scholar]
- 3.Møller AP. 1992. Female swallow preference for symmetrical male sexual ornaments. Nature 357, 238–240. ( 10.1038/357238a0) [DOI] [PubMed] [Google Scholar]
- 4.Roberts SC. 2007. Scent marking. In Rodent societies. An ecological and evolutionary perspective (eds Wolff JO, Sherman PW.), pp. 255–266. Chicago, IL: The University of Chicago Press. [Google Scholar]
- 5.Hurst JL. 1990. Urine marking in populations of wild house mice Mus domesticus Rutty. I. Communication between males. Anim. Behav. 40, 209–222. ( 10.1016/S0003-3472(05)80916-9) [DOI] [Google Scholar]
- 6.Hurst JL, Robertson DHL, Tolladay U, Beynon RJ. 1998. Proteins in urine scent marks of male house mice extend the longevity of olfactory signals. Anim. Behav. 55, 1289–1297. ( 10.1006/anbe.1997.0650) [DOI] [PubMed] [Google Scholar]
- 7.Penn DJ, Potts WK. 1998. Chemical signals and parasite-mediated sexual selection. Trends Ecol. Evol. 13, 391–396. ( 10.1016/S0169-5347(98)01473-6) [DOI] [PubMed] [Google Scholar]
- 8.Wolff RJ. 1985. Mating behaviour and female choice: their relation to social status in wild caught house mice Mus musculus housed in a semi-natural environment. J. Zool. 207, 43–51. ( 10.1111/j.1469-7998.1985.tb04914.x) [DOI] [Google Scholar]
- 9.Thonhauser K, Raveh S, Hettyey A, Beismann H, Penn DJ. 2013. Scent marking increases male reproductive success in house mice. Anim. Behav. 86, 1013–1021. ( 10.1016/j.anbehav.2013.09.004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rich TJ, Hurst JL. 1998. Scent marks as reliable signals of the competitive ability of mates. Anim. Behav. 56, 727–735. ( 10.1006/anbe.1998.0803) [DOI] [PubMed] [Google Scholar]
- 11.Birkhead TR, Møller AP. 1998. Sperm competition and sexual selection. London, UK: Academic Press. [Google Scholar]
- 12.Firman RC, Simmons LW. 2011. Experimental evolution of sperm competitiveness in a mammal. BMC Evol. Biol. 11, 19 ( 10.1186/1471-2148-11-19) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Firman RC, Simmons LW. 2012. Male house mice evolving with post-copulatory sexual selection sire embryos with increased viability. Ecol. Lett. 15, 42–46. ( 10.1111/j.1461-0248.2011.01706.x) [DOI] [PubMed] [Google Scholar]
- 14.Firman RC, Simmons LW. 2010. Experimental evolution of sperm quality via postcopulatory sexual selection in house mice. Evolution 64, 1245–1256. ( 10.1111/j.1558-5646.2009.00894.x) [DOI] [PubMed] [Google Scholar]
- 15.Ryan KK, Altmann J. 2001. Selection for male choice based primarily on mate compatibility in the oldfield mouse, Peromyscus polionotus rhoadsi. Behav. Ecol. Sociobiol. 50, 436–440. ( 10.1007/s002650100385) [DOI] [Google Scholar]
- 16.Byers SL, Wiles MV, Dunn SL, Taft RA. 2012. Mouse estrous cycle identification tool and images. PLoS ONE 7, e35538 ( 10.1371/journal.pone.0035538) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Drickamer LC. 2001. Urine marking and social dominance in male house mice (Mus musculus domesticus). Behav. Proc. 53, 113–120. ( 10.1016/S0376-6357(00)00152-2) [DOI] [PubMed] [Google Scholar]
- 18.Hosken DJ, Garner TWJ, Tregenza T, Wedell N, Ward PI. 2003. Superior sperm competitors sire higher-quality young. Proc. R. Soc. Lond. B 270, 1933–1938. ( 10.1098/rspb.2003.2443) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Firman RC. 2011. Polyandrous females benefit by producing sons that achieve high reproductive success in a competitive environment. Proc. R. Soc. B 278, 2823–2831. ( 10.1098/rspb.2010.2791) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Raveh S, Sutalo S, Thonhauser KE, Thoß M, Hettyey A, Winkelser F, Penn DJ. 2014. Female partner preferences enhance offspring ability to survive an infection. BMC Evol. Biol. 14, 14 ( 10.1186/1471-2148-14-14) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Parker GA. 1998. Sperm competition and the evolution of ejaculates: towards a theory base. In Sperm competition and sexual selection (eds Birkhead TR, Møller AP.), pp. 3–49. San Diego, CA: Academic Press. [Google Scholar]
- 22.Thomas ML, Simmons LW. 2007. Male dominance influences pheromone expression, ejaculate quality, and fertilization success in the Australian field cricket, Teleogryllus oceanicus. Behav. Ecol. 20, 1118–1124. ( 10.1093/beheco/arp105) [DOI] [Google Scholar]
- 23.Rowe M, Swaddle JP, Pruett-Jones S, Webster MS. 2010. Plumage coloration, ejaculate quality and reproductive phenotype in the red-backed fairy-wren. Anim. Behav. 79, 1239–1246. ( 10.1016/j.anbehav.2010.02.020) [DOI] [Google Scholar]
- 24.Pomerantz SM, Nunez AA, Bean NJ. 1983. Female behavior is affected by male ultrasonic vocalizations in house mice. Physiol. Behav. 31, 91–96. ( 10.1016/0031-9384(83)90101-4) [DOI] [PubMed] [Google Scholar]
- 25.Musolf K, Hoffman F, Penn DJ. 2010. Ultrasonic courtship vocalizations in wild house mice, Mus musculus musculus. Anim. Behav. 79, 757–764. ( 10.1016/j.anbehav.2009.12.034) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data is available through Dryad (doi:10.5061/dryad.2v4h6).