Quality assurance with cervicography and specialist review significantly improved the sensitivity of visual inspection with acetic acid to detect cervical intraepithelial neoplasia grade 2 or worse.
Key Words: quality assurance, visual inspection of the cervix, digital cervicography
Abstract
Objective
To determine whether a quality assurance (QA) program using digital cervicography improved the performance of a visual inspection with acetic acid (VIA) to detect cervical intraepithelial neoplasia grade 2 or worse (CIN 2+) in HIV-infected women in Johannesburg, South Africa.
Materials and Methods
Visual inspection with acetic acid was performed among HIV-infected women, aged 18 to 65 years, in Johannesburg, South Africa. Nurses received 2 weeks of training on the VIA procedure. The VIA interpretation was performed in real time. The VIA results were then photographed using a retail available digital camera. A gynecologist and medical officer reviewed the VIA digital images within 2 weeks of the procedure. Colposcopic biopsy was performed on all women with positive VIA and 25% negative VIA results. Sensitivity and specificity of VIA for the detection of CIN 2+ were compared between the nurses and physicians at the beginning and at the end of the study.
Results
Positive VIA results were found in 541 (45%) of the 1,202 participating women. The sensitivity of VIA to predict CIN 2+ was improved from 65% to 75% (p = .001) with the addition of digital cervicography and specialist review. There was no statistical difference in the sensitivity of the VIA readings when comparing the first 600 participants to the final 593 participants between the nurses (p = .613) and physicians (p = .624).
Conclusions
Quality assurance performed by specialists using digital cervicography improved the sensitivity of VIA. There was no difference in sensitivity in interpreting VIA between the beginning and the end of the study. Quality assurance should form a cornerstone of any VIA program to improve sensitivity in detecting CIN 2+ lesions.
Cervical cancer is a leading cause of cancer death among women in sub-Saharan Africa [1]. Access to cytology-based screening programs is limited in many African countries owing to the lack of skills, capacity, financial resources, and the inability of women to return for results because of several reasons such as limited transport, work, and childcare issues. Visual inspection with acetic acid (VIA) has been suggested as an alternative method of screening for cervical disease and has similar sensitivity to Pap smears for the detection of high-grade cervical lesions in HIV-infected women [2–4]. Several resource-limited countries are expanding cervical cancer screening using the VIA screening method [5]. A quality assurance (QA) program is crucial to the successful implementation of a cervical cancer screening program. However, data evaluating the effect of QA programs in improving the sensitivity of VIA for the diagnosis of cervical intraepithelial neoplasia 2 or worse (CIN 2+), in HIV-infected women, is limited. We present the results of an evaluation of a QA program for VIA, which was nested within a large screening study comparing human papillomavirus (HPV), cytology, and VIA performed in 1,202 HIV-infected women in South Africa [2]. In addition, we evaluated the level of improved skill among nurses and specialists in correctly interpreting VIA as an indicator for CIN 2+ during the course of the study.
METHODS
Study Procedures
In the parent screening study, a total of 1,202 women were recruited and enrolled between November 2009 and August 2011 in an HIV clinic located in a tertiary hospital in Johannesburg, South Africa [2]. Women were screened with Digene Hybrid Capture 2 High-Risk HPV DNA Test (Qiagen GmbH, Hilden, Germany), a conventional Pap smear, and VIA. Experienced South African primary health care nurses were trained to perform VIA for 2 weeks at the Cervical Cancer Prevention Program in Zambia—a large PEPFAR-funded VIA + television (TV) monitor-linked digital cervicography screening program, in operation since 2006, that is linked to HIV care and treatment with both theoretical and practical hands-on skills training developed and supervised by professor Groesbeck Parham [6]. Training and study implementation were conducted in accordance with the standard operating procedures of the Cervical Cancer Prevention Program in Zambia. Visual inspection with acetic acid was conducted by applying 5% acetic acid to the cervix and waiting for 3 minutes [7, 8]. Visual inspection with acetic acid was then interpreted by the study nurse in real time without the aid of digital cervicography, and results were recorded. A digital photograph was then taken using a PowerShot A590 IS 8-megapixel (3264 × 2448) camera (Canon, Chiba, Japan) with LA-DC52G lens adaptor (Canon) and 250D close-up lens digital camera (Canon) available at retail level in South Africa. Digital images were stored electronically on a laptop computer under the confidential patient identifier assigned by the study, backed-up on an external hard drive, and stored on an institutional server. In addition, all VIA results were also recorded manually as a picture drawn in the patient’s paper file.
All photographs were reviewed within 2 weeks of the VIA procedure at a weekly QA meeting attended by the study team. The digital photographs were placed on a computer and projected onto a screen for visual interpretation by the study physicians. Two physicians reviewed the images; 1 physician per protocol was required to be a registered gynecologist registered by the Health Professions Council of South Africa. The other physician was a medical officer trained and active in colposcopic interpretation and biopsy. The VIA real time (nurse) and digital review (physician) interpretations of VIA were then compared. All medical staff were blinded to Pap smear and HPV results at the time of interpreting the digital image.
To evaluate whether VIA interpretation improved with experience of reading VIA, the first 300 VIA study readings were compared with the last 300 VIA results and, in addition, a comparison of the first 600 VIA readings versus the last 593 VIA readings for CIN 2+ was conducted for approximately 18 months. Similar analyses were also undertaken for the digital images, and interpretation was performed by the study physicians.
All women with a positive VIA or abnormal Pap smear result were referred for a confirmatory colposcopy and biopsy. Approximately 25% of women with negative VIA results and negative Pap smear also received a colposcopic biopsy at the 6- and 12-o’clock positions of the cervix to adjust for verification bias [9]. The HPV results in the parent study were for research purposes only and were not used for clinical decisions for treatment. The protocol was approved by the Human Research Ethics Committee (Medical) of the University of Witwatersrand and, for secondary data analyses, by the Institutional Review Board of the University of North Carolina.
Statistical Analyses
Sensitivity and specificity estimates for VIA interpretation for the detection of CIN 2+ were estimated using maximum likelihood estimators [9]. Sensitivity and specificity for VIA interpretation for physicians and nurses were then compared using a κ statistic. Positive predictive values and negative predictive values were calculated; 95% confidence intervals (CIs) were derived from the asymptotic normalities of maximum likelihood estimators and their asymptotic variances.
RESULTS
The parent screening study recruited 1,202 women, of whom 1,193 were evaluable. Nine women were excluded from the analysis because they did not have a complete set of results (6 had inadequate or no cytology and 3 had invalid HPV or VIA results). The median age and CD4 count were 38 years (interquartile range [IQR] = 32–43 y) and 394 cells/mm3 (IQR = 252.5–572 cells/mm3), respectively. Overall, 528 (45%) of the women had positive VIA results on final review. There was a higher number (n = 872, 73%) of abnormal cytology results. Almost half of the abnormal Pap smear results (n = 399) were high-grade lesions. Confirmative colposcopic biopsy was obtained from 94.4% (878/930) of the women requiring biopsy per the study protocol. There were 2 histologically diagnosed cases of invasive cervical carcinoma. A discrepancy was found between cytology and histology in 14% of cases. Only 3% (2/63) of the verification biopsies showed histologic results of CIN 2 cases. Histologic review of the follow-up loop electrical excision procedure results for these 2 CIN 2 cases revealed no high-grade cervical precancer and only minimal changes consistent with the presence of HPV infection [2].
There was substantial agreement between the VIA real-time readings of the nurse and that of the physician with digital cervicography (κ statistic = 0.69, 95% CI = 0.64–0.73). Nurses achieved a sensitivity of 65.4% (95% CI = 59.7%–71.1%) with real-time VIA interpretation; however, the sensitivity achieved through the physician’s/specialist physician’s review using digital cervicography secondary review was notably higher (75.5%, p = .001). The specificity for CIN 2+ between the nurses and the physicians was similar at 68% (see Table 1). By comparing the VIA interpretations to detect CIN 2+ at the beginning of the study to the end of the study, it was observed that there was no statistical difference between the ability of nurses or physicians to detect CIN 2+ at the beginning and at the end of the study (see Figure 1).
Table 1.
Sensitivity and Specificity for VIA to Determine CIN 2+
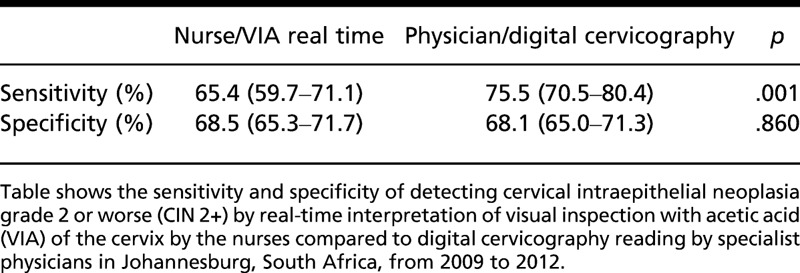
Figure 1.
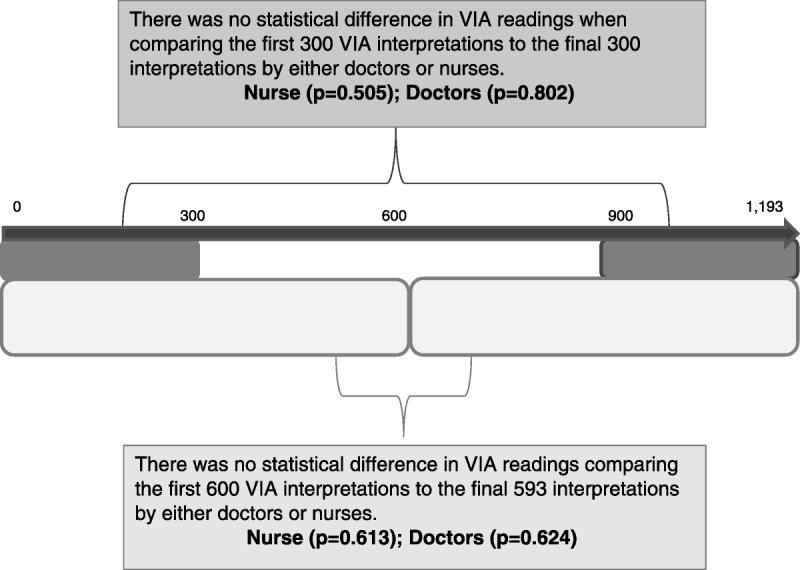
Comparison of visual inspection with acetic acid (VIA) of the cervix for both nurses and physicians in Johannesburg, South Africa, during the study period from 2009 to 2012.
DISCUSSION
At present, there are few published studies on the requirements and/or benefits of QA within a VIA screening program in HIV-infected women in resource-limited countries where this type of program is most likely to be implemented. Digital cervicography has been proposed as a valuable adjunctive procedure to VIA in improving diagnostic capability, and it can also be used in QA and teaching within a VIA screening program [6–8]. Our QA program with initial VIA readings followed by expert review using digital cervicography showed a higher sensitivity in detecting CIN 2+ similar to results from a study conducted among the general population of women in Northeast Brazil (mean age = 27.6 y) [10].
Although digital cervicography may seem incongruous for resource-limited countries because of additional technical equipment, costs, and increased personnel requirements, QA programs have been successfully implemented in Zambia [6, 7] and in rural South Africa (Dr Bridgette Goeieman, personal communication). These 2 programs involve a review of digital photographs that are brought to the specialist physicians trained in colposcopy. Both programs are successfully based on commercially available digital cameras and images that are stored on laptop computers and transferred to experts for review through a multitude of reasonably affordable technology such as e-mail zip files or mobile phone technology. If the Internet is not available, we have downloaded the images to USB/memory sticks, and these have been couriered to the specialists for their review. In addition, if projectors and/or monitors are not available, the review can also occur by viewing the image available on the digital camera itself. The Zambian program has termed this electronic cervical cancer control or “eC3” [11]. While there are always problems and hurdles such as equipment costs, technical failure, additional personal time, and training of staff, this system has been able to successfully screen more than 145,000 women in Zambia using VIA with digital cervicography using a digital camera linked to a TV monitor (professor Groesbeck Parham, personal communication).
Human papillomavirus testing–, histology-, and cytology-based screening programs throughout the world are required to have both internal and external QA to ensure the best screening outcomes for women. For example, the cytology-based program in Johannesburg has both internal and external QA modalities. Internal QA involves the examination of positive results (atypical squamous cells of undetermined significance and worse) by 2 cytotechnologists (one of whom is a senior cytotechnologist or a physician), 100% rapid review of all reportedly negative Pap smears, retrospective review in the case of previous and current Pap smears that have different diagnoses, and cytologic-histologic correlation. External QA includes proficiency testing using the Royal College of Pathologists of Australasia’s QA program for cytology. A QA program should be required for VIA-based screening programs.
Another interesting finding of this substudy was that there seemed to be no difference in the ability to determine CIN 2+ at the beginning and at the end of the study for both nurses and physicians. These results suggest that VIA may be learned by qualified nursing staff within a 2-week rigorous training course, with consistent readings over time, but that a QA program does not improve the interpretative skill of clinicians over time. A 2-week training course allows for a reasonable training implementation time within a country’s cervical cancer screening program where staffing and funds for training are often limited. A review of the Alliance for Cervical Cancer Prevention training program showed that trainees looking at digital cervical images agreed with an expert assessment greater than 60% of the time after approximately 10 days of training [12]. This review also showed that the level of agreement between different experts looking at a set of static photographs of cervices had a κ value of 0.57 or overall agreement of 67% [13]. Our study team, working together, had a higher κ score.
This is the first study to report data indicating that a QA system seems to notably improve the sensitivity of a VIA screening program in HIV-infected women in sub-Saharan Africa. In this substudy, it is difficult to determine whether the improved sensitivity was due to (1) the digital cervicography, (2) the specialist’s review, (3) the second review, or (4) the combination of all these specific interventions. Reviewing the digital photographs is a quick process that can easily be incorporated into a routine staff meeting.
The cost of implementing a QA program using a digital camera (US$ 800), a TV monitor (US$ 300), and a laptop computer (US$ 1,000) pales in comparison to the cost of treatment of 1 cervical cancer case, which involves highly trained physicists, radiation oncologists, gynecologist surgeons, oncologists, cisplatin, and purchase and continual maintenance and calibration of expensive radiation machines. Access to this necessary equipment and the expertise to treat invasive cervical cancer are extremely limited in sub-Saharan Africa. The process of a second review using digital cervicography showed an additional 10 women with CIN 2+ (of every 100 women screened) whose disease could be treated early and cervical cancer could be prevented. The cost and time to implement a QA program for VIA screening are well worth the resources required to prevent cervical cancer.
Implementation of a wide-scale cervical cancer screening program in sub-Saharan Africa using HPV testing, VIA with digital cervicography, or cytology will not occur overnight and will take significant engagement and political will of the government, health care providers, and funders to improve access.
The most important take-home lesson is that QA programs are needed for any screening modality. This study shows that a QA program involving a second review with digital photographs improves the sensitivity of the VIA procedure. With the VIA method now being implemented in many African countries, a well-developed QA program is imperative to ensure the most successful screening outcome for the individual woman and the program as a whole.
Acknowledgments
The study team would like to thank Prof Groesbeck Parham for his advice, training, and mentorship within this study and for the implementation of the cervical cancer VIA screening program. The study team to would also like to thank Andrea Teagle for help in preparing the tables for this article.
Footnotes
Funding was provided by US Agency for International Development (USAID) under the terms of agreement USAID-674-A-12-00029, 674-A-00-08-00007-00, PHE ZA-09.0265 with Right to Care.
The contents are the responsibility of the authors and do not necessarily reflect the views of USAID, National Institutes of Health, or the US government.
The authors have declared they have no conflicts of interest.
REFERENCES
- 1.Available at: http://globocan.iarc.fr/Pages/fact_sheets_cancer.aspx. Accessed December 19, 2013.
- 2. Firnhaber C, Mayisela N, Mao L, Williams S, Swarts A, Faesen M, et al. Validation of cervical cancer screening methods in HIV positive women from Johannesburg, South Africa. PLoS One 2013; 8: e53494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Akinwuntan AL, Adesina OA, Okolo CA, Oluwasola OA, Oladokun A, Ifemeje AA, et al. Correlation of cervical cytology and visual inspection with acetic acid in HIV-positive women. J Obstet Gynaecol 2008; 28: 638– 41. [DOI] [PubMed] [Google Scholar]
- 4. Mabeya H, Khozaim K, Liu T, Orango O, Chumba D, Pisharodi L, et al. Comparison of conventional cervical cytology versus visual inspection with acetic acid (VIA) among HIV-infected women in western Kenya. J Low Genit Tract Dis 2012; 16: 92– 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pink Ribbon Red Ribbon. 2012 Annual Report: Partnering for Progress and Purpose. Pink Ribbon Red Ribbon; 2012: 6– 8. [Google Scholar]
- 6.Cervical Cancer Prevention Program in Zambia. Available at: http://www.acewcc.org/. Accessed September 30, 2013.
- 7. Mwanahamuntu M, Sahasrabuddhe V, Kapambwe S, Pfaendler KS, Chibwesha C, Mkumba G, et al. Advancing cervical cancer prevention initiatives in resource-constrained settings: insights from the Cervical Cancer Prevention Program in Zambia. PLoS Med 2011; 8: e1001032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mwanahamuntu M, Sahasrabuddhe V, Pfaendler KS, Mudenda V, Hicks ML, Vermund SH, et al. Implementation of ‘see-and-treat’ cervical cancer prevention services linked to HIV care in Zambia. AIDS 2009; 23: N1– 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhou XH, Higgs RE. Assessing the relative accuracies of two screening tests in the presence of verification bias. Stat Med 2000; 19: 1697– 705. [DOI] [PubMed] [Google Scholar]
- 10. Bomfim-Hyppólito S, Franco ES, Franco RG, de Albuquerque CM, Nunes GC. Cervicography as an adjunctive test to visual inspection with acetic acid in cervical cancer detection screening. Int J Gynaecol Obstet 2006; 92: 58– 63. [DOI] [PubMed] [Google Scholar]
- 11. Parham GP, Mwanahamuntu MH, Pfaendler KS, Sahasrabuddhe VV, Myung D, Mkumba G, et al. eC3—a modern telecommunications matrix for cervical cancer prevention in Zambia. J Low Genit Tract Dis 2010; 14: 167– 73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Blumenthal PD, Lauterbach M, Sellors JW, Sankaranarayanan R. Training for cervical cancer prevention programs in low-resource settings: focus on visual inspection with acetic acid and cryotherapy. Int J Gynaeco Obstet 2005; 89 (suppl 2): S30– 7. [DOI] [PubMed] [Google Scholar]
- 13. Sellors JW, Jeronimo J, Sankaranarayanan R, Wright TC, Howard M, Blumenthal PD. Assessment of the cervix after acetic acid wash: inter-rater agreement using photographs. Obstet Gynecol 2002; 99: 635– 40. [DOI] [PubMed] [Google Scholar]


