Abstract
Objectives
We aimed to evaluate the anti-inflammatory and inhibitory effects of Lithospermum erythrorhizon (LE) on cerulein-induced acute pancreatitis (AP) in a mouse model.
Methods
Acute pancreatitis was induced via intraperitoneal injection of cerulein (50 μg/kg) every hour for 6 times. In the LE, water extract (100, 250, or 500 mg/kg) was administered intraperitoneally 1 hour before the first injection of cerulein. Six hours after AP, blood, the pancreas, and the lung were harvested for further examination. In addition, pancreatic acinar cells were isolated using a collagenase method, and then, we investigated the acinar cell viability and cytokine productions.
Results
Treatment with LE reduced pancreatic damage and AP-associated lung injury and attenuated the severity of AP, as evidenced by the reduction in neutrophil infiltration, serum amylase and lipase levels, trypsin activity, and proinflammatory cytokine expression. In addition, treatment with LE inhibited high mobility group box 1 expression in the pancreas during AP. In accordance with in vivo data, LE inhibited the cerulein-induced acinar cell death, cytokine productions, and high-mobility group box 1 expression. Furthermore, LE also inhibited the activation of p38 mitogen-activated protein kinases.
Conclusions
These results suggest that LE plays a protective role during the development of AP by inhibiting the activation of p38.
Key Words: acute pancreatitis, cytokines, mitogen-activated protein kinases (MAPKs), Lithospermum erythrorhizon, high-mobility group box protein 1 (HMGB-1)
The incidence of acute pancreatitis (AP), a sudden inflammatory condition of the pancreas, has been increasing in recent years.1 In most patients with AP, the disease is mild and self-limited; however, approximately 25% of the patients with AP have a severe course, and approximately 30% of those with acute necrotizing pancreatitis die.2,3 In general, acinar cell response to the activation of trypsinogen in the pancreas is believed to play a role in initiating AP. Acinar cell responses leads to the recruitment of inflammatory cells and generation of inflammatory mediators.4–6 Despite the clinical importance of AP, specific and effective therapies are lacking because the pathogenesis of the disease is not yet fully understood. To verify the pathophysiology of AP, many cellular and animal models of AP have been used.7 Among them, cerulein-induced pancreatitis is one of the best characterized and widely used experimental models.8 The administration of cerulein, a cholecystokinin analog, takes advantage of a supramaximal induction of digestive enzyme secretion, which leads to premature enzyme activation, pancreatic cell injury, interstitial edema, and infiltration of inflammatory cells into the pancreas.8,9
Lithospermum erythrorhizon (LE) has long been used as a traditional Asian medicine for the treatment of rash, eczema, ulcers, and constipation.10 Recent in vitro and in vivo studies have shown beneficial pharmacological effects of LE root extract, such as anti-inflammatory and anticancer activity.11,12 However, the protective activities of LE on cerulein-induced AP have not yet been reported.
Therefore, our aim was to investigate the effect of LE on cerulein-induced AP and the underlying transduction mechanisms involved. To gain a better insight of the effects of LE, we investigated its activities of in vivo experimental pancreatitis as well as in vitro in isolated pancreatic acinar cells.
MATERIALS AND METHODS
Plant Materials
Lithospermum erythrorhizon was purchased from Omni Herb (Dae-Gu, South Korea). The identity of the herb was confirmed at Wonkwang University. Lithospermum erythrorhizon extract was prepared by decocting dried LE (100 g) with boiling distilled water (1 L). The decoction time was approximately 2 hours 30 minutes. The water extract was frozen at −80°C and then freeze dried to produce a powder (15.3 g). The extraction yield was 15.3%. The powder was extracted with distilled water and filtered. The final concentration of LE was 100 mg/mL. Filtrates were stored at 4°C until use.
Reagents
Avidin-peroxidase, cerulein, Tris–HCl, NaCl, hexadecyltrimethylammoniumbromide, and tetramethylbenzidine (TMB) were purchased from Sigma-Aldrich (St. Louis, Mo). TRIzol reagent and polymerase chain reaction (PCR) kits were purchased from Invitrogen Corporation (Carlsbad, Calif). IκBα, and β-actin antibody were purchased from Santa Cruz Biotechnology (Santa Cruz, Calif). Anti–high-mobility group box protein 1 (HMGB-1) and phospho-specific mitogen-activated protein kinase (MAPK) antibodies were purchased from Cell Signaling Technology (Beverly, Mass).
Animal Model
All experiments were performed according to the protocols approved by the Animal Care Committee of Wonkwang University. Female C57BL/6 mice (6-8 weeks old, weighing 15-20 g) were purchased from Orient Bio (Sungnam, KyungKiDo, South Korea). All animals were bred and housed in standard shoebox cages in a climate-controlled room with an ambient temperature of 23°C ± 2°C and a 12-hour light-dark cycle for 7 days. Animals were fed standard laboratory chow, allowed free access to water, and randomly assigned to control or experimental groups. The mice were fasted for 18 hours before the induction of AP.
Experimental Design
Acute pancreatitis was induced using intraperitoneal injections of supramaximal concentrations of the stable cholecystokinin analog cerulein (50 μg/kg); injections were performed hourly for 6 hours. In the pretreatment group, LE extract (100, 250, or 500 mg/kg; n = 6 per 1 experiment) or saline was administered intraperitoneally 1 hour before the first cerulein injection. Mice were killed 6 hours after the final cerulein injection. Blood samples were obtained to determine serum amylase and lipase levels. The pancreas was rapidly removed from each mouse for morphological examination. A portion of each pancreas was stored at −70°C and prepared for measurement of tissue myeloperoxidase (MPO) activity, an indicator of neutrophil sequestration, and for reverse transcription PCR (RT-PCR) and real-time RT-PCR analyses.
Histological Analysis
The entire pancreas was fixed in 4% neutral formaldehyde solution for 12 hours, embedded in paraffin, and cut into 4-mm thick sections, which were stained with hematoxylin and eosin (H&E) to observe the morphological changes under a light microscope. The assessment of edema and inflammation was previously described.13 One hundred microscopic fields were randomly chosen to be observed in each mouse. Histological scoring of pancreatic tissue was performed to grade the extent of acinar edema (0, no edema; 1, interlobular edema; 2, intralobular edema; 3, interacinar edema) and inflammation (0, no inflammation; 1, inflammatory cells present at intralobular; 2, inflammatory cells present at intralobular; 3, inflammatory cells present at interacini).
MPO Estimation
Neutrophil sequestration in the pancreas was quantified by measuring tissue MPO activity. Myeloperoxidase assay was modified according to a previous method.14 Tissue samples were thawed, homogenized in 20-mmol/L phosphate buffer (pH 7.4), and then centrifuged (10,000g, 10 minutes, 4°C). The resulting pellet was resuspended in 50-mmol/L phosphate buffer (pH 6.0) containing 0.5% hexadecyltrimethylammonium bromide and centrifuged (10,000g, 5 minutes, 4°C) to produce the supernatant used for the MPO assay. The reaction mixture consisted of the supernatant, 1.6-mmol/L TMB, 80-mmol/L sodium phosphate buffer (pH 5.4), and 0.3-mmol/L hydrogen peroxide. This mixture was incubated at 37°C for 110 seconds, the reaction was terminated with 2-mol/L H2SO4, and the absorbance was measured at 450 nm. Myeloperoxidase activity was expressed as unit per milligram of protein.
Measurement of Serum Amylase, Lipase, and Trypsin Levels
Blood samples for the determination of serum amylase, lipase, and trypsin levels were obtained 6 hours after the induction of pancreatitis. Mice were anesthetized with an intraperitoneal injection of ketamine (80 mg/kg) and xylazine (4 mg/kg). After anesthetization, blood was harvested using a syringe from the heart of each mouse. Amylase and lipase activities were measured using an assay kit from BioAssay Systems (Hayward, Calif). Trypsin activity was measured fluorometrically using Boc-Glu(OBzl)-Ala-Arg-AMC-HCl (Bachem, Heidelberg, Germany) as substrate and calculated from the slope determined using a standard curve generated with purified trypsin. Trypsin activity was expressed as a percentage of the activity in the control.
Messenger RNA Expression
Messenger RNA (mRNA) transcripts in mouse pancreatic tissues were analyzed by RT-PCR. Total RNA was isolated from the mouse pancreas using an RNeasy kit (Qiagen, Valencia, Calif), then the purity was confirmed by RNA calculator (Gene Quent Pro, biochrom) and subjected to reverse transcription using SuperScript II RT (Invitrogen). Taqman quantitative RT-PCR with a 7700 Sequence Detection System was performed according to the instructions of the manufacturer (Applied Biosystems, Foster City, Calif). Triplicate test reactions and a control reaction lacking reverse transcriptase were analyzed for the expression of the gene of interest for each sample. Results were normalized to those of the “housekeeping” hypoxanthine-guanine phosphoribosyltransferase mRNA. Arbitrary expression units were calculated by dividing the expression of the gene of interest by ribosomal protein hypoxanthine-guanine phosphoribosyltransferase mRNA expression. The forward, reverse, and probe oligonucleotide primers for multiplex real-time TaqMan PCR were as follows: mouse tumor necrosis factor α (TNF-α) (forward, 5′-TCT CTT CAA GGG ACA AGG CTG-3′; reverse, 5′-ATA GCA AAT CGG CTG ACG GT-3′; probe, 5′-CCC GAC TAC GTG CTC CTC ACC CA-3′), mouse interleukin 1β (IL-1β) (forward, 5′-TTG ACG GAC CCC AAA AGA T-3′; reverse, 5′-GAA GCT GGA TGC TCT CAT CTG-3′; universal probe, M15131.1-Roche Applied Science), and mouse IL-6 (forward, 5′-TTC ATT CTC TTT GCT CTT GAA TTA GA-3′; reverse, 5′- GTC TGA CCT TTA GCT TCA AAT CCT-3′; universal probe, M20572.1-Roche Applied Science).
Enzyme-Linked Immunosorbent Assay
Enzyme-linked immunosorbent assay (ELISA) for TNF-α, IL-6, and IL-1β (R&D Systems; Minneapolis, Minn) was performed in duplicate in 96-well plates (Nunc, Roskilde, Denmark) coated with 100-μL aliquots of anti-mouse TNF-α, IL-6, or IL-1β monoclonal antibodies (1.0 μg/mL) in phosphate-buffered saline (PBS) at pH 7.4 after an overnight incubation at 4°C. The plates were washed in PBS containing 0.05% Tween-20 and blocked with PBS containing 10% fetal bovine serum for 2 hours. After additional washes, the standards and the serum and acinar cell supernatants were added to the plates and incubated at room temperature for 3 hours. After incubation, the wells were washed and 0.2-μg/mL biotinylated anti-mouse TNF-α, IL-6, or IL-1β was added to each well. Incubation continued at room temperature for 1 hour. After the wells were washed, avidin-peroxidase was added, and the plates were incubated for 30 minutes at room temperature. The wells were washed again, and the TMB substrate was added. Color development was measured at 450 nm with an automated microplate ELISA reader. Standard curves were obtained for each sample through serial dilutions of recombinant TNF-α, IL-6, and IL-1β.
Histological and Immunohistochemical Analysis
Fixed pancreatic tissues were embedded in paraffin, cut into 4-mm sections, and stained with H&E for standard histological examination. Immunohistochemical (IHC) staining for HMGB-1 was performed using a DAB IHC kit (DAKO, Cytomation, Denmark).15 Relative intensity was measured using Leica microscope software (Wetzlar, Germany). The IHC results from all pancreatic tissues were analyzed semiquantitatively by 2 independent observers, followed by resolution of any differences by joint review and consultation with a third observer. A single intensity score was obtained because the intensity of staining within each core was mostly homogeneous. Intensity was scored on a scale of 0 to 10 (0 for absence of staining and 10 for strong staining). Scores were recorded for the different cell types as well as their nuclear and cytoplasmic compartments.
Acinar Cell Isolation
Pancreatic acini were isolated from the dissected pancreases of the C57BL/6 mice by using collagenase digestion. Pancreatic tissue was chopped with scissors and digested for 15 minutes in solution Q (120-mmol/L NaCl, 20-mmol/L HEPES, 5-mmol/L KCl, 1-mmol/L MgCl2, 1-mmol/L CaCl2, 10-mmol/L sodium pyruvate, 10-mmol/L ascorbate, 10-mmol/L glucose, 0.1% BSA, 0.01% soybean trypsinogen inhibitor, and 150-U collagenase per milliliter). The cells were continuously shaken and gassed with 100% O2 in a 37°C water bath and subsequently washed in fresh isolation medium. After collagenase digestion, the tissue was gently pipetted. Dispersed acini were filtered through a 150-μm nylon mesh, centrifuged 3 times each for 90 seconds at 720 rpm, resuspended with Waymouth medium (Invitrogen), and incubated in 95% O2 and 5% CO2 for 4 hours.
Cell Culture and Treatment
Acinar cells were cultured at 37°C in a 5% CO2 incubator. Acinar cells (2 × 105) were added to each well of a 24-well plate for 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. Acinar cells (1 × 106) were added to each well of a 6-well plate for ELISA and real-time RT-PCR. Acinar cells (5 × 106) were added to a 60-mm dish for Western blotting. After cell attachment (4 hours), LE or inhibitor was added at the indicated doses for 1 hour, followed by the addition of cerulein.
MTT Assay
Cell viability was assayed using a modified colorimetric technique on the basis of the ability of live cells to convert the tetrazolium compound MTT into purple formazan crystals. The MTT (5 mg/mL) was dissolved in Krebs-Henseleit buffer (115-mmol/L NaCl, 3.6-mmol/L KCl, 1.3-mmol/L KH2PO4, 25-mmol/L NaHCO3, 1-mmol/L CaCl2, and 1-mmol/L MgCl2), and 50 μL was added to each well. After incubation for 30 minutes at 37°C, the suspension was removed, and the purple formazan crystals were dissolved in 200 μL DMSO. Aliquots from each well were seeded in the wells of a 96-well plate in duplicate and assayed at 540 nm with the use of a microplate ELISA reader. The number of viable cells was expressed as a percentage of the control.
Western Blot Analysis
Mice were pretreated with LE (500 mg/kg) for 1 hour and then stimulated with cerulein (50 μg/kg) for the indicated times. Pancreatic tissues were homogenized, and the lysates were boiled in a sample buffer composed of 62.5-mmol/L Tris–HCl at pH 6.8, 2% sodium dodecyl sulfate (SDS), 20% glycerol, and 10% 2-mercaptoethanol. Proteins in the cell lysates were then separated by 10% SDS–polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to a nitrocellulose membrane. After transfer of proteins, the membrane was blocked with 5% skim milk in PBS–Tween-20 for 2 hours at room temperature, and then, we incubated anti–phospho–extracellular signal-regulated kinase 1/2 (ERK1/2), anti–phospho–c-Jun N-terminal kinase (JNK), anti–phospho-p38, anti-IκBα, and anti–HMGB-1 antibodies. After washing 3 times, each blot was incubated with peroxidase-conjugated secondary antibody for 1 hour, and the antibody-specific proteins were visualized using an enhanced chemiluminesence detection system (Amersham, Piscataway, NJ) according to the protocol recommended by the manufacturer.
High-Performance Liquid Chromatography Sample Preparation and Conditions
Aliquots of 0.1-mg shikonin, reported to be a major component of LE, and 2.5-mg LE water extract powder were separately dissolved with 1 mL of methanol and filtered through a 0.45-μm filter membrane before use. A volume of 20 μL was injected into the high-performance liquid chromatography (HPLC) sample injector system. The chromatographic experiments were performed on a YL9100 series HPLC instrument equipped with a sample injector and UV/Vis detector. For all the experiments, a YMC-Triart C18 analytical column (4.6 × 150 mm, 5 μm) was used as the stationary phase, and the injection volume was set at 20 μL. The mobile phase was composed of water (A) and MeOH (B), and a gradient program (0–50 minutes, 20%–100% B; 50–60 minutes, 100% B) was applied for 60 minutes. The column was cleaned with 20% B for 20 minutes, and then, the system was equilibrated for 20 minutes with the starting conditions. The flow rate was set at 0.7 mL/min, and the detection wavelength was adjusted to 254 nm.
Statistical Analysis
Results were normally distributed and expressed as mean ± SE. The significance of changes was evaluated using 1-way analysis of variance. Differences between the experimental groups were evaluated using analysis of variance. P < 0.05 was accepted as statistically significant. We used SPSS version 12.0 (IBM, Lancaster, CA).
RESULTS
Effect of LE on Pancreatic Histology During Cerulein-Induced AP
We investigated the histological architecture of the pancreas after administration of cerulein. In the control mice, the histological features of the pancreas were typical of normal pancreatic architecture. Histological examination of the pancreas of cerulein-treated mice conducted 6 hours after cerulein administration revealed tissue damage characterized by inflammatory cell infiltrate and interstitial edema. Interstitial edema, inflammation, and total histological scores were significantly reduced in the LE (100, 250, or 500 mg/kg) pretreatment group compared with the saline treated group (Fig. 1A, C).
FIGURE 1.
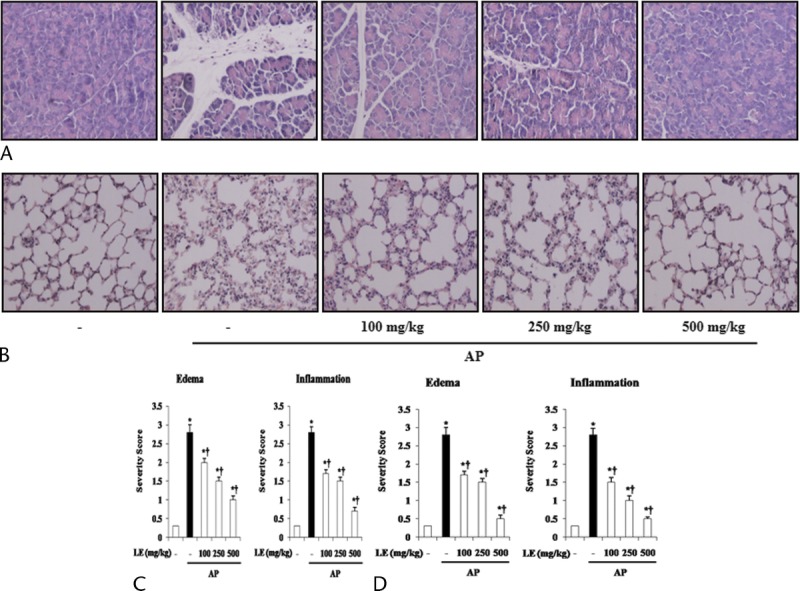
Effects of LE on pancreatic damage and AP-associated lung injury during cerulein-induced AP. A and B, Representative H&E-stained pancreas and lung sections of control mice and mice pretreated with LE (100, 250, or 500 mg/kg) 1 hour before the cerulein (50 μg/kg)–mediated induction of AP. C and D, Histological sections of the pancreas and lung were scored from 0 (normal) to 3 (severe) for edema and inflammation. Data are represented as mean ± SE for 6 mice for each group. The results were similar in 3 additional experiments. y axis represents the means, and the error bars represent the SE. *P < 0.05 versus control group, †P < 0.05 versus cerulein treatment alone. Original magnification ×400.
Effect of LE on Lung Histology During Cerulein-Induced AP
We assessed the histology of the lungs as well as the pancreas after cerulein administration. Lung injury during AP was evidenced by alveolar thickening and abundance of inflammatory cell infiltrates. Histological scores for lung edema and inflammation were significantly reduced in the LE (100, 250, or 500 mg/kg) pretreatment group compared with the saline treated group (Fig. 1B, D).
Effect of LE on MPO Activity in Cerulein-Induced AP
To examine the neutrophil infiltration into the damaged tissue, MPO activity in the pancreas and lung were examined. As shown in Figure 2, mice with AP showed increased MPO activity in both organs. However, mice pretreated with LE had less MPO activity in the pancreas and lungs (Fig. 2).
FIGURE 2.
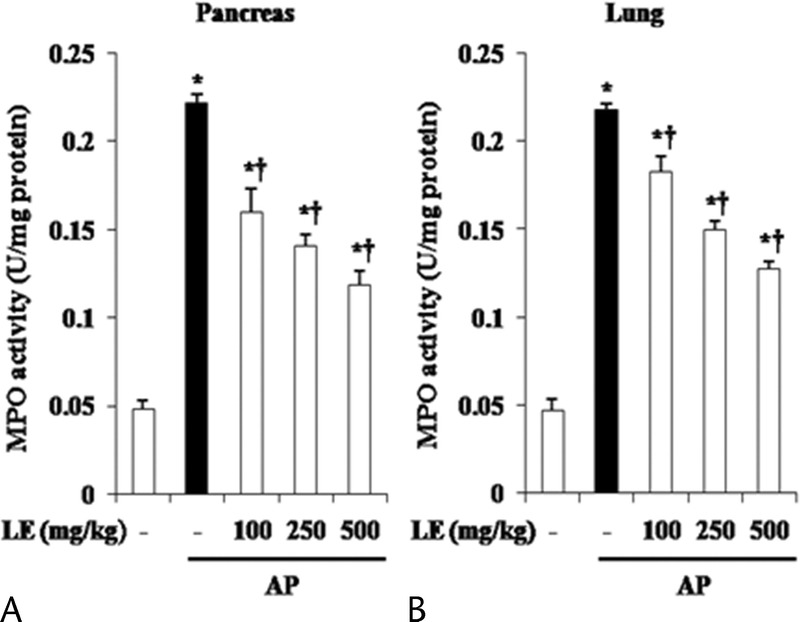
Effect of LE on MPO activity during cerulein-induced AP. Mice pretreated with LE (100, 250, or 500 mg/kg) were challenged with intraperitoneal injections of cerulein (50 μg/kg). Myeloperoxidase activity was measured in the pancreas (A) and lung (B) 6 hours after completion of cerulein injections. Details of methods are described in the experimental protocol. Data are expressed as unit per milligram of protein. Data are represented as mean ± SE for 6 mice for each group. The results were similar in 3 additional experiments. y axis represents the means, and the error bars represent the SE. *P < 0.05 versus control group, †P < 0.05 versus cerulein treatment alone.
Effect of LE on Pancreas Weight/Body Weight and Levels of Digestive Enzymes in Cerulein-Induced AP
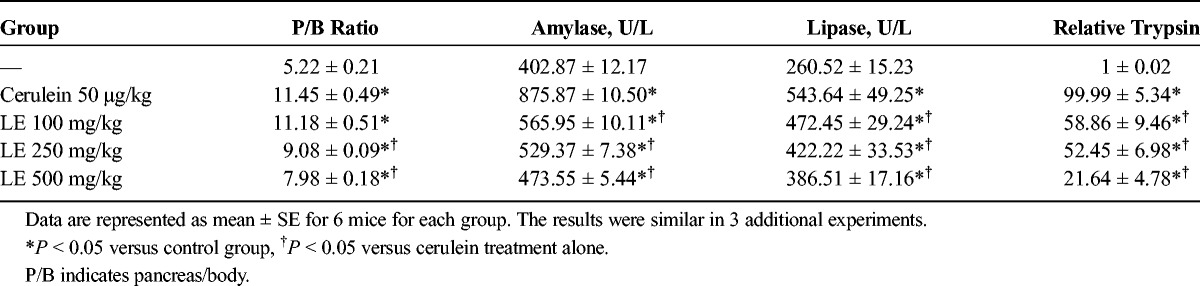
To assess the effect of LE on pancreatic edema, pancreas weight/body weight (PW/BW) was measured. As shown in Table 1, PW/BW was lower in the LE-treated mice compared with the saline-treated mice. Elevated serum levels of amylase and lipase are considered the most sensitive and specific markers of AP.16 Elevated trypsin is also an important marker of pancreatic acinar cell injury.17 Therefore, we examined serum amylase, lipase, and trypsin activity during cerulein-induced AP. As shown in Table 1, administration of LE significantly reduced the serum amylase and lipase levels as well as trypsin activity compared with the saline-treated mice.
TABLE 1.
Effects of LE on PW/BW and Production of Digestive Enzymes Such as Serum Amylase, Serum Lipase, and Trypsin During Cerulein-Induced AP
Effect of LE on Production of TNF-α, IL-6, and IL-1β in Cerulein-Induced AP
Cytokines, which are major inflammatory mediators, play a critical role in the pathogenesis of pancreatitis.2 To examine the effect of LE on the production of cytokines during AP, we measured the mRNA and protein levels of TNF-α, IL-6, and IL-1β. As shown in Figure 3, mRNA and protein levels of proinflammatory cytokines were increased during AP. However, this increase of inflammatory cytokines was significantly inhibited by the administration of LE (Fig. 3).
FIGURE 3.
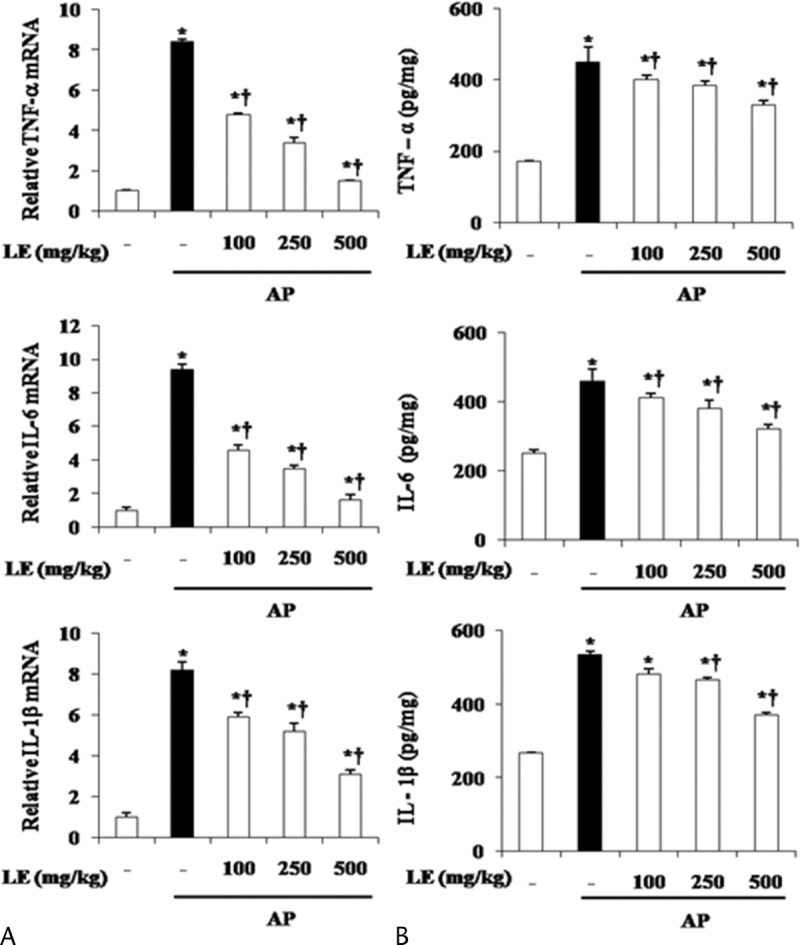
Effect of LE on TNF-α, IL-6, and IL-1β during cerulein-induced AP. Mice pretreated with LE (100, 250, or 500 mg/kg) were challenged with intraperitoneal injections of cerulein at supramaximal dose (50 μg/kg). Mice were killed 6 hours after the last cerulein injection. Levels of pancreatic TNF-α, IL-6, and IL-1β mRNA were measured by real-time RT-PCR (A), and the corresponding protein levels were measured in the pancreatic tissue by ELISA (B). Data are represented as mean ± SE for 6 mice for each group. The results were similar in 3 additional experiments. y axis represents the means, and the error bars represent the SE. *P < 0.05 versus control group, †P < 0.05 versus cerulein treatment alone.
Effect of LE on Pancreatic Expression of HMGB-1 in Cerulein-Induced AP
Recently, it has been reported that HMGB-1 plays a key role as a late-phase inflammatory mediator in the pathogenesis of AP.18 Therefore, we examined HMGB-1 expression in the pancreas using IHC method. As shown in Figure 4, IHC analysis revealed that HMGB-1, which is identified by the presence of a brown color, was rarely expressed in the pancreas of the normal mice but strongly expressed in the cerulein-treated mice. Pretreatment with LE (500 mg/kg) resulted in a significant decrease of HMGB-1 expression in the pancreatic tissue of cerulein-injected mice (Fig. 4A, B). Western blot analysis confirmed that the expression of HMGB-1 in the pancreas of cerulein-treated mice was markedly increased. However, the increase of HMGB-1 expression was reduced by LE treatment (Fig. 4C).
FIGURE 4.
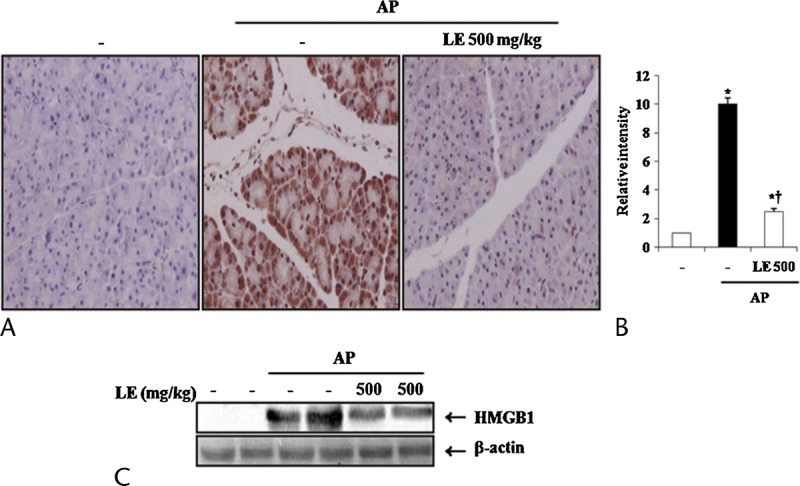
Effects of LE on pancreatic HMGB-1 expression during cerulein-induced AP. A, 400× magnification of representative IHC data. B, Relative intensity of HMGB-1 staining (DAB) was scored as described in the Materials and Methods section. C, Western blot data detecting HMGB-1 expression in pancreas. Data are represented as mean ± SE for 6 mice for each group. The results were similar in 3 additional experiments. y axis represents the means, and the error bars represent the SE. *P < 0.05 versus saline treatment, †P < 0.05 versus cerulein treatment alone.
Effect of LE on the Pancreatic Acinar Cell Responses Against Cerulein
Local inflammation caused by cerulein in pancreatic acinar cells results in acinar cell death and organ destruction.19 To assess whether LE could inhibit acinar cell death, we evaluated cell viability by using the MTT assay. The cells were pretreated with LE for 1 hour and then stimulated with cerulein for 6 hours. As shown in Figure 5A, LE significantly reduced cerulein-induced death in a dose-dependent manner (Fig. 5A). We also investigated whether LE could affect the production of inflammatory cytokines and found that LE inhibited cerulein-induced production of inflammatory cytokines such as TNF-α, IL-6, and IL-1β (Fig. 5B, C).
FIGURE 5.
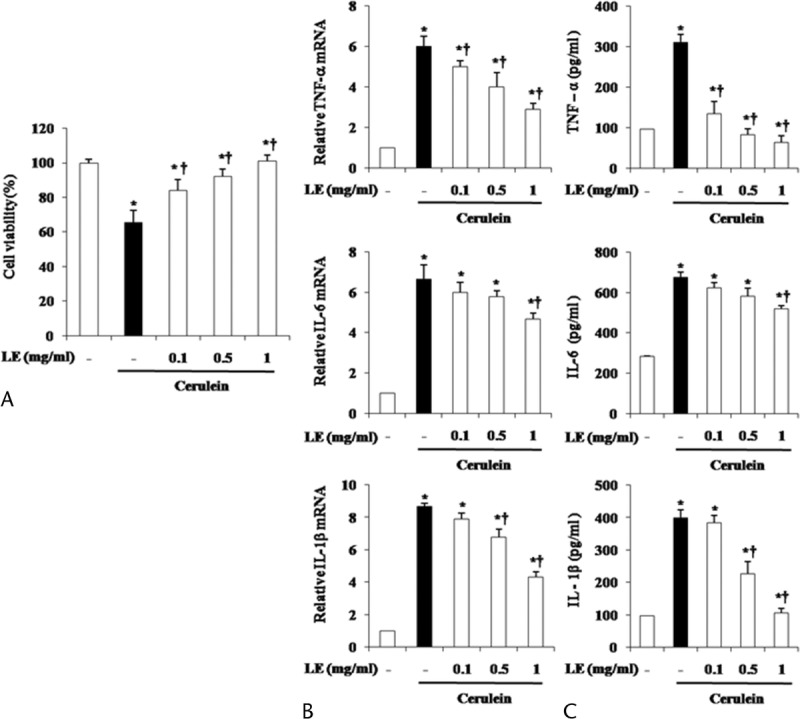
Effect of LE on cerulein-induced acinar cell death and the production of inflammatory mediators. The acinar cells were pretreated with LE for 1 hour at indicated doses. Six hours after cerulein stimulation, (A) the cell viability was measured, as described in the experimental protocol. After 24 hours of cerulein stimulation, cytokine levels in the isolated pancreatic acinar cells were examined using real-time RT-PCR (B) and ELISA (C). Data are represented as mean ± SE for 6 mice for each group. The results were similar in 3 additional experiments. y axis represents the means, and the error bars represent the SE. *P < 0.05 versus control group, †P < 0.05 versus cerulein treatment alone.
Effect of LE on MAPKs and Nuclear Factor κB Pathways in Cerulein-Induced AP
To evaluate the mechanisms by which LE regulates cytokine expression, we assessed the effect of LE on the activation of MAPKs and nuclear factor κB (NF-κB). In mice pretreated with LE, activation of p38 MAPK was significantly inhibited but ERK1/2 and JNK were not affected (Fig. 6). We also found that LE pretreatment did not inhibit the degradation of IκBα. To clarify whether down-regulation of the molecules in the p38 pathway by LE was responsible for the reduced inflammatory response, we used a p38 inhibitor (SB203580) and then measured pancreatic acinar cell death and inflammatory cytokine production. As shown in Figure 7, treatment with SB203580 inhibited cerulein-induced cell death and production of inflammatory cytokines (Fig. 7A–C). Moreover, p38 inhibition resulted in a decreased expression of HMGB-1 (Fig. 7D).
FIGURE 6.
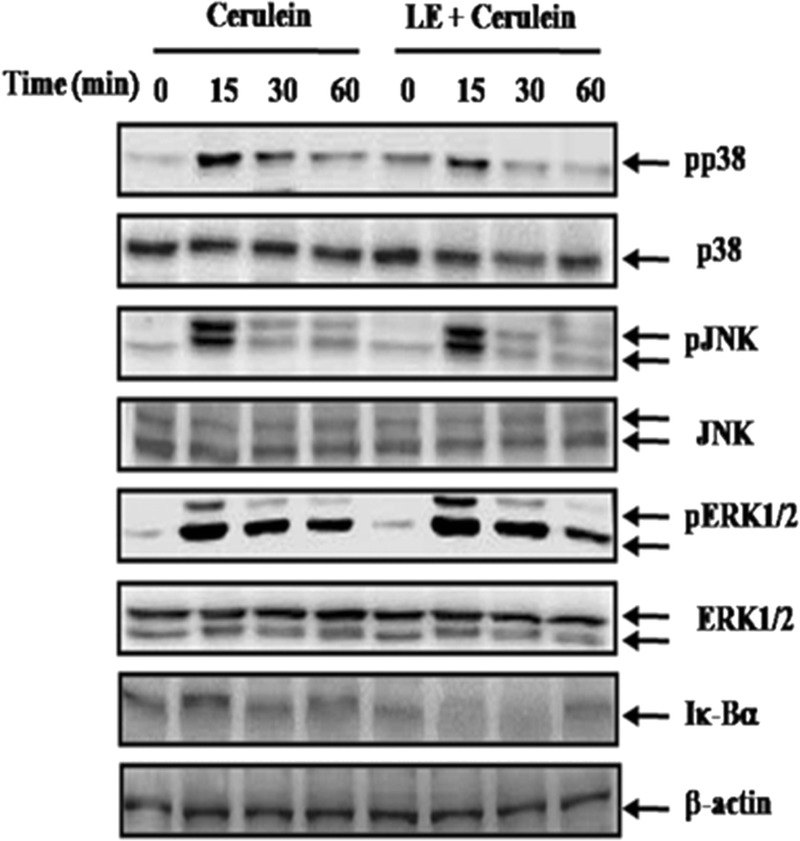
Effect of LE on activation of MAPKs and NF-κB. Mice pretreated with LE (500 mg/kg) were challenged with intraperitoneal injections of cerulein at supramaximal doses (50 μg/kg). Mice were killed at 0, 15, 30, and 60 minutes after the cerulein injection. Phosphorylation of MAPKs and degradation of IκBα were measured by Western blot. β-Actin and ERK1/2, JNK, and p38 were used as loading controls. The results were similar in the 3 additional experiments.
FIGURE 7.
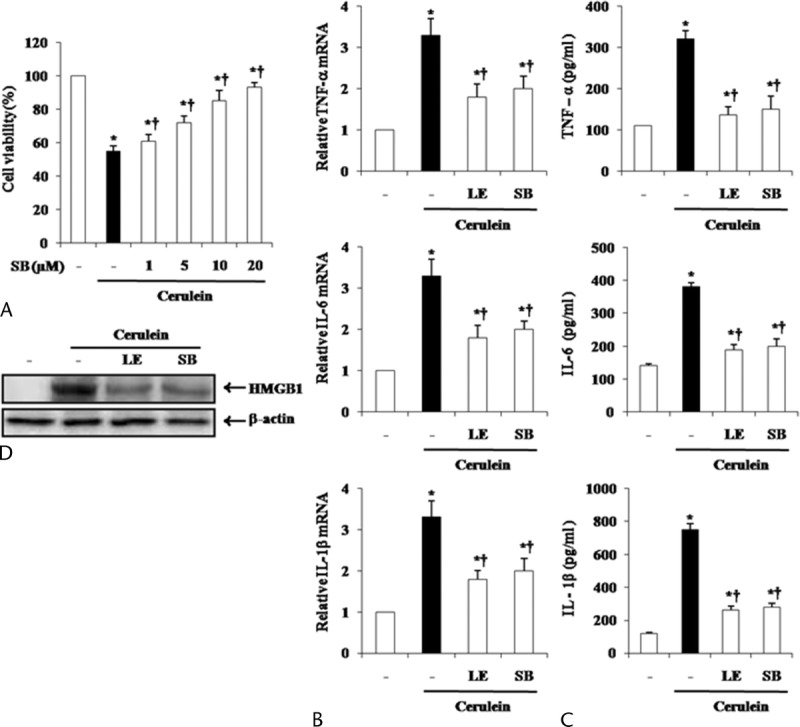
Effect of p38 inhibition by SB203580 on cerulein-induced acinar cell death and the production of inflammatory mediators. The acinar cells were pretreated with SB203580 for 1 hour at indicated doses. Six hours after cerulein stimulation, (A) the cell viability was measured, as described in the experimental protocol. SB203580 20 μmol/L or LE (1 mg/mL) were pretreated for 1 hour, and then, cerulein was added into isolated pancreatic acinar cells. After 24 hours of cerulein stimulation, cytokine levels in isolated pancreatic acinar cells were examined using real-time RT-PCR (B) and ELISA (C). D, HMGB-1 levels were measured by Western blot. Data are represented as mean ± SE for 6 mice for each group. The results were similar in 3 additional experiments. y axis represents the means, and the error bars represent the SE. *P < 0.05 versus control group, †P < 0.05 versus cerulein treatment alone.
Characterization of the Principal Component of LE
We analyzed LE by HPLC to determine its principal components. The peaks of the principal components of LE have not yet been identified. Further studies to evaluate the principal components of LE will be needed. A previous study reported that LE is characterized by high concentrations of shikonin.20 Therefore, we compared the peaks in the LE water extract chromatogram with the peaks in the shikonin chromatogram. As shown in Figure 8, the peaks for LE water extract did not match the peaks for shikonin, indicating that the LE water extract did not contain shikonin.
FIGURE 8.
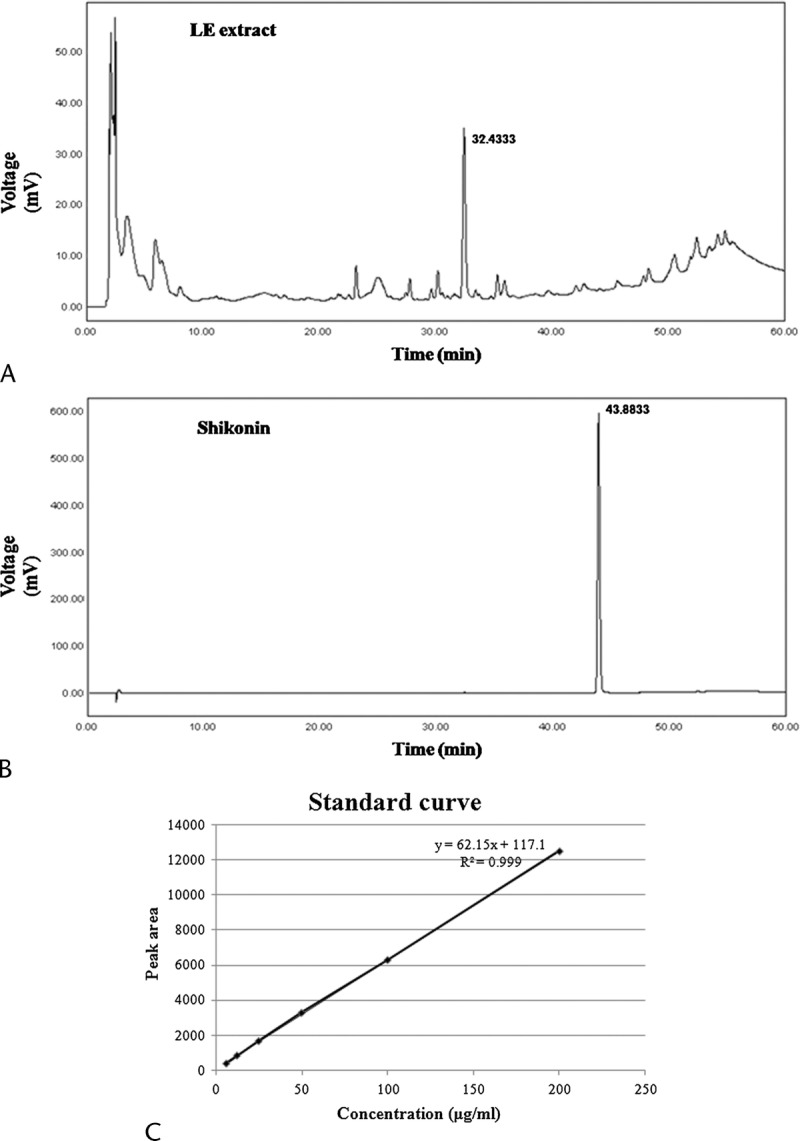
HPLC chromatogram of LE (A) and shikonin (B) at the length of 254 nm. C, Typical standard curve of shikonin.
DISCUSSION
This study investigated the effect of LE on cerulein-induced AP by using both in vivo and in vitro experiments. In this study, LE protects against AP and reduces pancreatic damages and associated lung injury. Administration of LE water extract inhibited MPO activity and led to decreased production of serum amylase, lipase, trypsin, and proinflammatory cytokines. In addition, we showed that pretreatment with LE inhibited HMGB-1 expression in the pancreas. In concordance with the results of in vivo experiments, LE also inhibited acinar cell death, cytokine production, and HMGB-1 expression in vitro. Furthermore, administration of LE inhibited the activation of p38 MAPKs against cerulein challenges.
Intracellular conversion of zymogens to their activated forms and release of cytokine are critical steps in the initiation of AP.21–25 Generally, pancreatic digestive enzymes including amylase and lipase contribute at an early stage to the damage of acinar cells and, consequently, to inflammatory processes and cytokine production into the pancreas and whole body.26 In our study, pretreatment with LE prevented the increased levels of digestive enzymes in serum (Table 1) and cytokines such as TNF-α, IL-6, and IL-1β in the pancreas and isolated pancreatic acinar cells (Figs. 3 and 5), suggesting that LE may be effective in inhibiting excessive exocrine enzyme secretion and inflammatory mediators during AP.
The signaling pathway responsible for regulating cytokine production during the course of AP has been of interest. One important signaling molecule, MAPK, has been identified as an important regulator of various inflammatory mediators in the pancreas.27 Accordingly, inhibition of MAPKs could possibly hinder cytokine production during AP.28,29 It was reported that activation of the p38 MAPK pathway was closely linked with AP.30,31 In accordance with these reports, our results showed that p38 inhibition protected pancreatic damages against cerulein-induced acinar cell death, cytokine release, and HMGB-1 production and suggest that p38 activation may play a crucial role during AP.
Shikonin is currently believed to be a major constituent of LE. Recently, Xiong et al20 reported the protective effects of shikonin in pancreatitis and showed that shikonin, similar to LE extract, ameliorates serum amylase and lipase activities, MPO activity, pancreatic damage, and cytokine levels. However, there is a difference between the regulating mechanisms of shikonin and LE water extract. Shikonin inhibits NF-κB activation. However, LE inhibited p38 activation but not NF-κB (Fig. 6). Because of this discrepancy, we investigated whether shikonin is actually a component of LE water extract. As shown in Figure 8, when we compared the HPLC peaks generated by LE water extract and shikonin, they did not coincide, suggesting that LE water extract does not contain shikonin. It may be conjectured that LE water extract contains large amounts of components other than shikonin. Further studies to evaluate the principal components of LE will be needed.
In conclusion, this study has shown that LE ameliorates the severity of cerulein-induced AP and pancreatitis-associated lung injury by inhibiting tissue injury, proinflammatory cytokine production, and HMGB-1 expression in mice by inhibiting the activation of the p38 MAPK pathway. These results suggest that LE may be an effective and beneficial candidate to ameliorate AP.
Footnotes
This research was supported by a National Research Foundation of Korea (NRF) grant funded by the Korean government (MEST) (NRF-2012M2B2B1055246, NRF-2012R1A1A2040906, and NRF-2012R1A1A2040916).
The authors declare no conflicts of interest.
REFERENCES
- 1.Talukdar R, Vege SS. Recent developments in acute pancreatitis. Clin Gastroenterol Hepatol. 2009; 7: S3– S9. [DOI] [PubMed] [Google Scholar]
- 2.Pandol SJ, Saluja AK, Imrie CW, et al. Acute pancreatitis: bench to the bedside. Gastroenterology. 2007; 132: 1127– 1151. [DOI] [PubMed] [Google Scholar]
- 3.Hu G, Shen J, Cheng L, et al. Reg4 protects against acinar cell necrosis in experimental pancreatitis. Gut. 2011; 60: 820– 828. [DOI] [PubMed] [Google Scholar]
- 4.Yu X, Li YG, He XW, et al. Hyperbaric oxygen reduces inflammatory response in acute pancreatitis by inhibiting NF-kappaB activation. Eur Surg Res. 2009; 42: 130– 135. [DOI] [PubMed] [Google Scholar]
- 5.Bhatia M, Wong FL, Cao Y, et al. Pathophysiology of acute pancreatitis. Pancreatology. 2005; 5: 132– 144. [DOI] [PubMed] [Google Scholar]
- 6.Vonlaufen A, Wilson JS, Apte MV. Molecular mechanisms of pancreatitis: current opinion. J Gastroenterol Hepatol. 2008; 23: 1339– 1348. [DOI] [PubMed] [Google Scholar]
- 7.Thrower EC, Osgood S, Shugrue CA, et al. The novel protein kinase C isoforms -delta and -epsilon modulate caerulein-induced zymogen activation in pancreatic acinar cells. Am J Physiol Gastrointest Liver Physiol. 2008; 294: G1344– G1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gorelick FS, Adler G, Kerin HF. Cerulein-induced pancreatitis. In: Go VW, DiMagno EP, Gardner JD, Lebenthal E, Reber HA, Scheele GA, eds. The Pancreas: Biology, Pathobiology, and Disease. 2nd ed New York, NY: Raven Press; 1993: 501– 526. [Google Scholar]
- 9.Willemer S, Elsasser HP, Adler G. Hormone-induced pancreatitis. Eur Surg Res. 1992; 24: S29– S49. [DOI] [PubMed] [Google Scholar]
- 10.Jin R, Wan LL, Mitsuishi T, et al. Immunomodulative effects of Chinese herbs in mice treated with anti-tumor agent cyclophosphamide [in Japanese]. Yakugaku Zasshi. 1994; 114: 533– 538. [DOI] [PubMed] [Google Scholar]
- 11.Han KY, Kwon TH, Lee TH, et al. Suppressive effects of Lithospermum erythrorhizon extracts on lipopolysaccharide-induced activation of AP-1 and NF-kappaB via mitogen-activated protein kinase pathways in mouse macrophage cells. BMB Rep. 2008; 41: 328– 333. [DOI] [PubMed] [Google Scholar]
- 12.Rajasekar S, Park da J, Park C, et al. In vitro and in vivo anticancer effects of Lithospermum erythrorhizon extract on B16F10 murine melanoma. J Ethnopharmacol. 2012; 144: 335– 345. [DOI] [PubMed] [Google Scholar]
- 13.Dembinski A, Warzecha Z, Ceranowicz P, et al. Dual, time-dependent deleterious and protective effect of anandamide on the course of cerulein-induced acute pancreatitis. Role of sensory nerves. Eur J Pharmacol. 2008; 591: 284– 292. [DOI] [PubMed] [Google Scholar]
- 14.Dawra R, Sharif R, Phillips P, et al. Development of a new mouse model of acute pancreatitis induced by administration of l-arginine. Am J Physiol Gastrointest Liver Physiol. 2007; 292 (4): G1009– G1018. [DOI] [PubMed] [Google Scholar]
- 15.Ulrika H, Heidi W, Nora B, et al. The novel inflammatory cytokine high mobility group box protein 1 (HMGB1) is expressed by human term placenta. Immunology. 2007; 122 (3): 430– 437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beger HG, Rau B, Mayer J, et al. Natural course of acute pancreatitis. World J Surg. 1997; 21: 130– 135. [DOI] [PubMed] [Google Scholar]
- 17.Kaiser AM, Saluja AK, Steer ML. Repetitive short-term obstructions of the common bile-pancreatic duct induce severe acute pancreatitis in the opossum. Dig Dis Sci. 1999; 44: 1653– 1661. [DOI] [PubMed] [Google Scholar]
- 18.Wang H, Bloom O, Zhang M, et al. HMG-1 as a late mediator of endotoxin lethality in mice. Science. 1999; 285: 248– 251. [DOI] [PubMed] [Google Scholar]
- 19.Gross V, Leser HG, Heinisch A, et al. Inflammatory mediators and cytokines—new aspects of the pathophysiology and assessment of severity of acute pancreatitis? Hepatogastroenterology. 1993; 40: 522– 530. [PubMed] [Google Scholar]
- 20.Xiong J, Ni J, Hu G, et al. Shikonin ameliorates cerulein-induced acute pancreatitis in mice. J Ethnopharmacol. 2013; 145: 573– 580. [DOI] [PubMed] [Google Scholar]
- 21.Gravante G, Garcea G, Ong SL, et al. Prediction of mortality in acute pancreatitis: a systematic review of the published evidence. Pancreatology. 2009; 9: 601– 614. [DOI] [PubMed] [Google Scholar]
- 22.Hofbauer B, Saluja AK, Lerch MM, et al. Intra-acinar cell activation of trypsinogen during caerulein-induced pancreatitis in rats. Am J Physiol. 1998; 275: G352– G362. [DOI] [PubMed] [Google Scholar]
- 23.Grewal HP, Mohey el Din A, Gaber L, et al. Amelioration of the physiologic and biochemical changes of acute pancreatitis using an anti-TNF-alpha polyclonal antibody. Am J Surg. 1994; 167: 214– 219. [DOI] [PubMed] [Google Scholar]
- 24.Norman JG, Fink GW, Franz MG. Acute pancreatitis induces intrapancreatic tumor necrosis factor gene expression. Arch Surg. 1995; 130: 966– 970. [DOI] [PubMed] [Google Scholar]
- 25.Mayer J, Rau B, Gansauge F, et al. Inflammatory mediators in human acute pancreatitis: clinical and pathophysiological implications. Gut. 2000; 47: 546– 552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Javier P, Luis S, Luis A, et al. Interaction between cytokines and oxidative stress in acute pancreatitis. Curr Med Chem. 2006; 13: 2775– 2787. [DOI] [PubMed] [Google Scholar]
- 27.Clemons AP, Holstein DM, Galli A, et al. Cerulein-induced acute pancreatitis in the rat is significantly ameliorated by treatment with MEK1/2 inhibitors U0126 and PD98059. Pancreas. 2002; 25: 251– 259. [DOI] [PubMed] [Google Scholar]
- 28.Jo IJ, Bae GS, Park KC, et al. Scolopendra subspinipes mutilans protected the cerulein-induced acute pancreatitis by inhibiting high-mobility group box protein-1. World J Gastroenterol. 2013; 19: 1551– 1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bae GS, Kim MS, Jeong J, et al. Piperine ameliorates the severity of cerulein-induced acute pancreatitis by inhibiting the activation of mitogen activated protein kinases. Biochem Biophys Res Commun. 2011; 410: 382– 388. [DOI] [PubMed] [Google Scholar]
- 30.Ren HB, Li ZS, Xu GM, et al. Dynamic changes of mitogen-activated protein kinase signal transduction in rats with severe acute pancreatitis. Chin J Dig Dis. 2004; 5: 123– 125. [DOI] [PubMed] [Google Scholar]
- 31.Samuel I, Zaheer S, Zaheer A. Bile-pancreatic juice exclusion increases p38MAPK activation and TNF-alpha production in ligation-induced acute pancreatitis in rats. Pancreatology. 2005; 5: 20– 26. [DOI] [PubMed] [Google Scholar]


