Abstract
Pathogenic fungi employ numerous mechanisms to flourish in the stressful environment encountered within their mammalian hosts. Central to this arsenal for filamentous fungi is invasive growth within the host microenvironment, mediated by establishment and maintenance of polarized hyphal morphogenesis. In Aspergillus fumigatus, the RasA signal transduction pathway has emerged as a significant regulator of hyphal morphogenesis and virulence, among other processes. The factors contributing to the regulation of RasA itself are not as thoroughly understood, although proper temporal activation of RasA and spatial localization of RasA to the plasma membrane are known to play major roles. Interference with RasA palmitoylation or prenylation results in mislocalization of RasA and is associated with severe growth deficits. In addition, dysregulation of RasA activation results in severe morphologic aberrancies and growth deficits. This review highlights the relationship between RasA signaling, hyphal morphogenesis, and virulence in Aspergillus fumigatus, and focuses on potential determinants of spatial and temporal RasA regulation.
Keywords: Aspergillus, Ras, signaling, hyphal morphogenesis, virulence, prenylation, palmitoylation
INTRODUCTION
Ras proteins are members of a family of small monomeric GTPases that is remarkably conserved in eukaryotes. Ras serves as a signal transducer between extracellular stimuli and intracellular signaling cascades. Control of Ras activity is mediated via guanosine nucleotide diphosphate (GDP) / guanosine nucleotide triphosphate (GTP) binding in a binary switch fashion [1]. Guanine nucleotide exchange factors (GEFs) exchange Ras-bound GDP for GTP, thereby activating Ras. GTPase activating proteins (GAPs) deactivate Ras by increasing the rate of its intrinsic GTPase activity, resulting in hydrolysis of Ras-bound GTP to GDP. A conformational difference in the switch regions of Ras toggles activity depending on whether GTP or GDP is bound [1]. Proper localization of Ras within the cell is essential for both Ras activation and Ras-mediated signal transduction [2]. Nascent Ras proteins are cytoplasmic before undergoing a series of post-translational modifications, including prenylation and palmitoylation. These modifications result in translocation of Ras to the appropriate membrane compartment, where signal transduction occurs..
Mammalian Ras proteins are heavily studied for their roles in tumor growth and development, and as targets for novel anti-cancer therapeutics [3]. Similarly, fugal Ras proteins have been implicated in such diverse cellular processes as polarized hyphal growth, cytoskeleton formation, and nitrogen metabolism. Although the human genome contains three functionally diverse forms of Ras (HRAS, KRAS, and NRAS), the budding yeast Saccharomyces cerevisiae has only two paralogs (Ras1p and Ras2p), likely having arisen from genome duplication [4]. The fission yeast Schizosaccharomyces pombe has a single Ras homolog (Ras1p), which is involved in regulation of cell morphology, mating, and nitrogen signaling [5,6]. Studies in these model yeasts have provided a framework for investigations into Ras-mediated processes regulating pathogenic growth of filamentous fungi such as Aspergillus fumigatus.
Two Ras homologs (RasA and RasB) have been identified in A. fumigatus, and each plays important roles in cellular processes including hyphal morphogenesis, polarized growth, and asexual development [7–9]. Whereas RasA homologs are present in both filamentous fungi and yeast, RasB homologs are unique to fungi that undergo filamentous growth during some phase of their lifecycle [7]. Expression of a dominant negative (DN) rasA (DNrasA) or deletion of rasA (ΔrasA) results in slowed germination rates [7], similar to an A. nidulans DNrasA strain [10]. In contrast, expression of DNrasB results in a delay in the initiation of conidial germination, but a germination rate equivalent to wild type [7]. Both rasA and rasB deletion mutants of A. fumigatus display irregularities in hyphal morphology and polarized growth, although the aberrancies in the ΔrasA mutant are more severe [8]. The nature of these aberrancies indicates that RasA and RasB play discrete albeit overlapping roles, and that RasA serves as the primary Ras homolog controlling hyphal growth and development in A. fumigatus [7–9].
SPATIAL REGULATION OF RAS: SUBCELLULAR LOCALIZATION OF RasA IN A. fumigatus
Spatial regulation of Ras signaling is a primary component in Ras protein functionality. Ras proteins are peripherally associated with membranes through a series of post-translational modifications [11]. RasA contains both the traditional “CAAX” (C = cysteine, A = any aliphatic amino acid, X = any amino acid) farnesylation motif and a dual cysteine palmitoylation motif at its C-terminus. A cytoplasmic farnesyltransferase enzyme is responsible for transferring a farnesyl group to the “CAAX” cysteine residue [12]. The “-AAX” residues are then cleaved and the C-terminus carboxymethylated [13,14]. The farnesylation step is sufficient for targeting Ras proteins to internal membranes, including the endoplasmic reticulum (ER) membrane, where it is then palmitoylated at the dual cysteine motif. It is this final palmitoylation modification that accomplishes plasma membrane-specific targeting [15]. In mammalian cells, palmitoylation of Ras is reversible, whereby a plasma membrane localized thioesterase cleaves the palmitoyl residues and Ras recycles to ER and Golgi membranes [16].
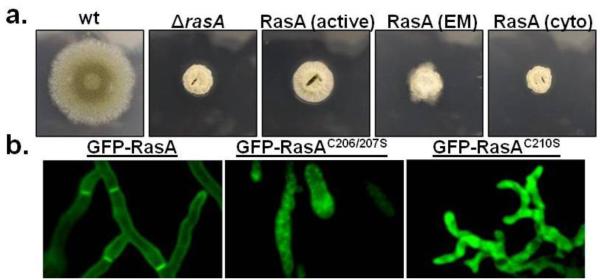
A GFP-RasA fusion protein was recently employed to show that RasA does indeed localize to the A. fumigatus plasma membrane when the above prenylation pathway is undisturbed [17]. However, ablation of the conserved dual cysteine palmitoylation motif mislocalizes RasA to internal membranes (Fig 1b). Furthermore, this RasA palmitoylation-deficient mutant displays defects in polarized hyphal growth, cell wall integrity, and virulence [17]. These phenotypes are similar to the ΔrasA mutant, indicating that RasA localization at the plasma membrane is essential for its full functionality in hyphal growth processes. In addition, recent results in our laboratory suggest that molecular blockade of RasA prenylation via mutation of the CAAX cysteine residue (C210) generates a completely non-functional Ras protein that is mislocalized to the cytoplasm (Fig 1b).
Fig 1. Spatiotemporal regulation of RasA activity is essential for growth and development.
a) Mutations affecting Ras activity or localization negatively affect hyphal development in A. fumigatus. wt = wild type (H237), ΔrasA = rasA deletion mutant [9], RasA (active) = constitutively active RasA [23], RasA (EM) = palmitoylation-deficient RasA mislocalized to endo-membranes [17], and RasA (cyto) = farnesylation-deficient RasA mislocalized to the cytoplasm. To measure radial growth of each strain, a 10 μL spot containing 1×104 conidia was placed in the center of glucose minimal media (GMM) agar plates and incubating at 37°C for 72 hr. b) Genetic abrogation of farnesylation or palmitoylation mislocalizes RasA to the cytoplasm or endomembranes, respectively. Left panel: GFP-RasA localizes primarily to the plasma membrane [17]; Middle panel: GFP-RasAC206/207S mislocalizes to endomembrane structures. Right panel: Expression of GFP-RasAC210S, mutated at a highly conserved cysteine residue required for farnesylation, mislocalizes RasA to the cytoplasm.
In light of this evidence, it is exciting to speculate that pharmacological interference with RasA palmitoylation and / or prenylation could make an effective treatment for fungal infections like invasive aspergillosis (IA). Inhibition of farnesyltransferase by the mammalian farnesyltransferase inhibitor (FTI) manumycin A has recently been shown to inhibit growth of A. fumigatus, albeit at a high minimum inhibitory concentration (MIC) [18]. Looking further down the Ras maturation pathway, inhibition of palmitoylation by 2-bromopalmitate causes RasA mislocalization and hyphal growth inhibition [17]. Therefore, continuing assessment of A. fumigatus susceptibility to prenyl- and palmitoyltransferase inhibitors is indicated, not only to bolster mechanistic knowledge of these processes but also to identify potential clinical advancements.
TEMPORAL REGULATION OF RAS: ACTIVATION OF RasA IN A. fumigatus
Spatial regulation is not the sole driver of RasA functionality; temporal regulation is no less critical. Control of RasA activation and deactivation in A. fumigatus is likely accomplished by interaction with GAPs and GEFs, in a similar fashion to mammalian processes. However, there is a paucity of knowledge specific to this regulation. In fact, one important difference between mammalian and fungal Ras activation lies in the regulation of the GEFs that activate Ras. In mammals, an activated receptor tyrosine kinase recruits the responsible GEFs to the plasma membrane, where they interact with and activate Ras [19]. This specific mechanism is almost certainly not the process in A. fumigatus, however; no identifiable receptor tyrosine kinase homologs have been identified in fungal genomes [20]. Although no Ras GEFs have been studied in Aspergillus, an Aspergillus nidulans Ras GAP, GapA, has been deleted and shown to play important roles in actin polarization and hyphal growth [21]. Interestingly, GapA localizes to the incipient germ tube site, implying a need for reduced Ras activity during polarity establishment [21].
The majority of knowledge regarding temporal regulation of Ras activity in filamentous fungi has been accomplished using mutational analyses in which Ras proteins have been either constitutively activated or inactivated and expressed in a wild type genetic background [10,22]. Because Ras proteins are known to toggle between active and inactive states, the presence of phenotypic abnormalities at progressive developmental stages in each of these mutants provides evidence that proper temporal regulation of Ras activity is crucial to normal hyphal growth and development. During initiation of germination, conidia of the dominant active (DA) rasA mutant exhibit a hyperswollen morphology, delayed germ tube formation, and hyperaccumulation of nuclei [23]. Mature hyphae of the DArasA mutant display swollen, extensively vacuolated subapical hyphal compartments. Taken together, these data indicate that loss of the ability to control RasA activity during development inhibits polarity establishment, inappropriately activates mitosis, and causes loss of polarity maintenance. Further, both constitutive activation and deletion of RasA lead to decreased radial growth [9,23]. The physical appearance of the two mutations differs, although both are grossly aberrant (Fig. 1a). These findings support a model in which regulation of RasA activity operates on a continuum during the developmental lifecycle, where either too little or too much activation of RasA at discrete times in the lifecycle negatively affects proper conidial germination and hyphal morphogenesis in A. fumigatus.
Although regulation of RasA localization and activation can be considered separately, these two levels of Ras protein regulation are likely to be intimately linked. For example, the palmitoylation-deficient RasA mutant described earlier exhibits more robust hyphal growth than the ΔrasA mutant [17], suggesting that plasma membrane localization of RasA is not an absolute requirement to retain some level of functionality. This finding supports two strong hypotheses: 1) RasA can be partially activated at the endo-membranes, albeit at a lower level than at the plasma membrane, due to limited interaction with GAPs and GEFs; or 2) RasA may have restricted access to its downstream effectors when mislocalized to endo-membranes. Currently, there are no data published establishing whether RasA activation and deactivation occur only at the plasma membrane. Accordingly, more comprehensive investigations into the mechanisms regulating RasA activity would be of great benefit in understanding how temporal and spatial requirements for RasA signaling intertwine.
RasA NETWORK SIGNALING IN A. fumigatus
The endpoint of proper spatiotemporal regulation of RasA activity is the appropriate transduction of developmental signals within the fungal cell. Although A. fumigatus has two Ras homologs (RasA and RasB), RasA is the major regulator of overall hyphal growth and development, likely due to orchestration of multiple cellular processes [7–9]. In filamentous fungi and yeast alike, many of these Ras-mediated processes are classically associated with aspects of fungal growth and stress response [24]. Thus, RasA signaling effectors are anticipated to be potentially important virulence determinants in the development of IA. In support of this hypothesis, the A. fumigatus rasA deletion mutant does exhibit decreased virulence compared with wild type when tested in a murine model of IA [9].
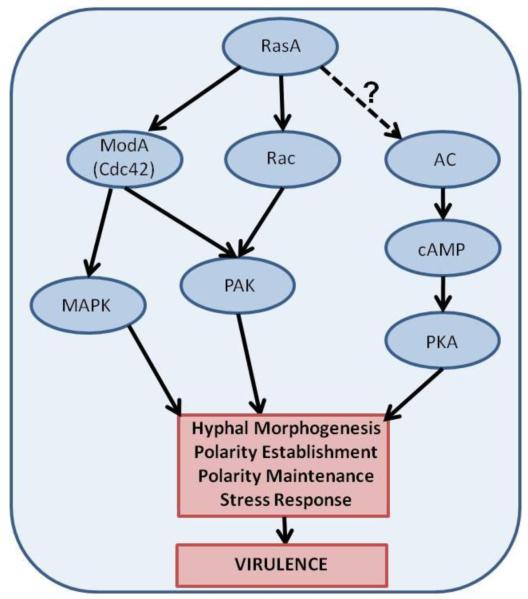
As has been demonstrated in other filamentous fungi and higher eukaryotes, Ras signaling in A. fumigatus is hypothesized to mainly proceed through the small GTPase Cdc42 and Rac networks [25] (Fig 2). Cdc42 and Rac activity, in turn, affect downstream mitogen-activated protein kinase (MAPK) and p21-activated kinase (PAK) activity. MAPK and PAK family proteins are serine/threonine protein kinases, and are well-conserved regulators of growth and differentiation in fungi [26][27]. The Aspergillus Cdc42 homolog, ModA, has not been studied in A. fumigatus. However, work in A. nidulans shows that, although the functions of Cdc42 and Rac partially overlap, Cdc42 plays a more crucial role than Rac in directing hyphal morphogenesis [28]. A racA deletion mutant (ΔracA) has been generated in A. fumigatus [29]. Studies with the ΔracA mutant reveal abnormalities in conidiophore development and hyphal morphology in the absence of RacA [29]. These phenotypes are potentially regulated via actin cytoskeleton modulation, as a ΔracA mutant in A. nidulans exhibits hyperpolarization of actin at hyphal tips and a reduction in actin concentration in subapical regions [30]. Indeed, the regulation of actin cytoskeletal rearrangement by Rac and Cdc42 signaling networks is likely a conserved theme throughout eukaryotic organisms. The extent to which A. fumigatus RasA signals to each of these essential morphogenetic networks is currently being elucidated in our lab.
Fig 2. Putative Ras signaling in Aspergillus fumigatus.
Downstream proteins controlled by RasA in A. fumigatus are hypothesized to include Cdc42 and Rac, which contribute to polarized hyphal morphogenesis via mitogen-activated protein kinase (MAPK) and p-21 activated kinase (PAK) pathways. At their endpoint, these pathways are predicted to activate nuclear transcription factors controlling gene expression related to hyphal growth and induce actin cytoskeletal rearrangements, respectively. Both of these activities are expected to be essential for the establishment and maintenance of normal cell polarity. Although a direct link between Ras and PKA activity is clear in budding yeast, this interaction has not been directly examined in A. fumigatus.
In contrast to Ras signaling in budding yeasts such as S. cerevisiae and Candida albicans, Ras signaling in filamentous fungi, including A. fumigatus, appears to be relatively independent of the cAMP/protein kinase A (PKA) pathway. Adenylate cyclase is bound and activated by Ras2p in S.cerevisiae, subsequently producing cAMP, which activates PKA [31,32]. The cAMP/PKA pathway is similarly activated in C. albicans, in which it has been shown to control growth and the yeast-to-hyphal morphological transition, both contributors to virulence [33,34]. Thus, PKA emerges as a major downstream effector of Ras in yeast. Although any direct connections between RasA and PKA in A.fumigatus have not been verified, exogenous addition of cAMP does not complement the growth deficit in the ΔrasA mutant [9]. Furthermore, Ras and PKA have been demonstrated to affect A. nidulans spore germination through separate pathways [35]. These results suggest that the most accurate model for Ras signaling in Aspergillus may be one in which Ras signaling and PKA signaling are largely independent of each other. However, direct examination of this relationship is needed.
CONCLUSION
Collectively, our work has shown that spatiotemporal regulation of Ras activity is essential for A. fumigatus growth and development (Fig 1a). The pathways through which RasA is hypothesized to act in A. fumigatus likely impact numerous potential virulence determinants. The current body of evidence suggesting that RasA is a critical link in the development of hyphal morphogenesis and virulence in A. fumigatus evokes many new and exciting questions about Ras regulation and signal transduction. Teasing out the mechanistic details of RasA interactions with the upstream effectors of its proper activation and localization is crucial to extending the current model of Ras-mediated growth and development. Of particular interest are the prenyltransferases and the GAP/GEF proteins. Identification of fungal-specific components of the Ras signaling and prenyl synthesis pathways will add an additional layer of translational applicability. Inhibition of fungal-specific participants in RasA localization, activation, and signaling is a promising focus for the future development of novel antifungal treatments.
ACKNOWLEDGEMENTS
Support for this work was provided by NIH K22 AI89786-01A1 and R01 AI106925-01A1 to JRF.
REFERENCES
- 1.Cox AD, Der CJ. Ras history. Small GTPases. 2010;1:2–27. doi: 10.4161/sgtp.1.1.12178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Prior IA, Hancock JF. Ras trafficking, localization and compartmentalized signalling. Semin. Cell Dev. Biol. 2012;23:145–53. doi: 10.1016/j.semcdb.2011.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gysin S, Salt M, Young A, McCormick F. Therapeutic Strategies for Targeting Ras Proteins. Genes Cancer. 2011;2:359–72. doi: 10.1177/1947601911412376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kellis M, Birren BW, Lander ES. Proof and evolutionary analysis of ancient genome duplication in the yeast Saccharomyces cerevisiae. Nature. 2004;428:617–24. doi: 10.1038/nature02424. [DOI] [PubMed] [Google Scholar]
- 5.Nielsen O, Davey J, Egel R. The ras1 function of Schizosaccharomyces pombe mediates pheromone-induced transcription. EMBO J. 1992;11:1391–5. doi: 10.1002/j.1460-2075.1992.tb05184.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Papadaki P, Pizon V, Onken B, Chang EC. Two ras pathways in fission yeast are differentially regulated by two ras guanine nucleotide exchange factors. Mol. Cell. Biol. 2002;22:4598–606. doi: 10.1128/MCB.22.13.4598-4606.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fortwendel JR, Panepinto JC, Seitz AE, Askew DS, Rhodes JC. Aspergillus fumigatus rasA and rasB regulate the timing and morphology of asexual development. Fungal Genet. Biol. 2004;41:129–39. doi: 10.1016/j.fgb.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 8.Fortwendel JR, Zhao W, Bhabhra R, Park S, Perlin DS, Askew DS, et al. A Fungus-Specific Ras Homolog Contributes to the Hyphal Growth and Virulence of Aspergillus fumigatus. Eukaryot. Cell. 2005;4:1982–9. doi: 10.1128/EC.4.12.1982-1989.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fortwendel JR, Fuller KK, Stephens TJ, Bacon WC, Askew DS, Rhodes JC. Aspergillus fumigatus RasA Regulates Asexual Development and Cell Wall Integrity. Eukaryot. Cell. 2008;7:1530–9. doi: 10.1128/EC.00080-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Som T, Kolaparthi VS. Developmental decisions in Aspergillus nidulans are modulated by Ras activity. Mol. Cell. Biol. 1994;14:5333–48. doi: 10.1128/mcb.14.8.5333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wright LP, Philips MR. Thematic review series: Lipid Posttranslational Modifications CAAX modification and membrane targeting of Ras. J. Lipid Res. 2006;47:883–91. doi: 10.1194/jlr.R600004-JLR200. [DOI] [PubMed] [Google Scholar]
- 12.Choy E, Chiu V, Silletti J, Feoktistov M, Morimoto T, Michaelson D, et al. Endomembrane Trafficking of Ras: The CAAX Motif Targets Proteins to the ER and Golgi. Cell. 1999;98:69–80. doi: 10.1016/S0092-8674(00)80607-8. [DOI] [PubMed] [Google Scholar]
- 13.Schmidt WK, Tam A, Fujimura-Kamada K, Michaelis S. Endoplasmic reticulum membrane localization of Rce1p and Ste24p, yeast proteases involved in carboxyl-terminal CAAX protein processing and amino-terminal a-factor cleavage. Proc. Natl. Acad. Sci. U. S. A. 1998;95:11175–80. doi: 10.1073/pnas.95.19.11175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Romano JD, Michaelis S. Topological and Mutational Analysis of Saccharomyces cerevisiae Ste14p, Founding Member of the Isoprenylcysteine Carboxyl Methyltransferase Family. Mol. Biol. Cell. 2001;12:1957–71. doi: 10.1091/mbc.12.7.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bartels DJ, Mitchell DA, Dong X, Deschenes RJ. Erf2, a Novel Gene Product That Affects the Localization and Palmitoylation of Ras2 in Saccharomyces cerevisiae. Mol. Cell. Biol. 1999;19:6775–87. doi: 10.1128/mcb.19.10.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dekker FJ, Rocks O, Vartak N, Menninger S, Hedberg C, Balamurugan R, et al. Small-molecule inhibition of APT1 affects Ras localization and signaling. Nat. Chem. Biol. 2010;6:449–56. doi: 10.1038/nchembio.362. [DOI] [PubMed] [Google Scholar]
- 17.Fortwendel JR, Juvvadi PR, Rogg LE, Asfaw YG, Burns KA, Randell SH, et al. Plasma Membrane Localization Is Required for RasA-Mediated Polarized Morphogenesis and Virulence of Aspergillus fumigatus. Eukaryot. Cell. 2012;11:966–77. doi: 10.1128/EC.00091-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Qiao J, Gao P, Jiang X, Fang H. In vitro antifungal activity of farnesyltransferase inhibitors against clinical isolates of Aspergillus and Candida. Ann. Clin. Microbiol. Antimicrob. 2013;12:37. doi: 10.1186/1476-0711-12-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Margolis B, Skolnik EY. Activation of Ras by receptor tyrosine kinases. J. Am. Soc. Nephrol. 1994;5:1288–99. doi: 10.1681/ASN.V561288. [DOI] [PubMed] [Google Scholar]
- 20.King N, Carroll SB. A receptor tyrosine kinase from choanoflagellates: Molecular insights into early animal evolution. Proc. Natl. Acad. Sci. U. S. A. 2001;98:15032–7. doi: 10.1073/pnas.261477698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harispe L, Portela C, Scazzocchio C, Penalva MA, Gorfinkiel L. Ras GTPase-Activating Protein Regulation of Actin Cytoskeleton and Hyphal Polarity in Aspergillus nidulans. Eukaryot. Cell. 2008;7:141–53. doi: 10.1128/EC.00346-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boyce KJ, Hynes MJ, Andrianopoulos A. The Ras and Rho GTPases genetically interact to co-ordinately regulate cell polarity during development in Penicillium marneffei. Mol. Microbiol. 2005;55:1487–501. doi: 10.1111/j.1365-2958.2005.04485.x. [DOI] [PubMed] [Google Scholar]
- 23.Fortwendel JR, Juvvadi PR, Rogg LE, Steinbach WJ. Regulatable Ras Activity Is Critical for Proper Establishment and Maintenance of Polarity in Aspergillus fumigatus. Eukaryot. Cell. 2011;10:611–5. doi: 10.1128/EC.00315-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weeks G, Spiegelman GB. Roles played by Ras subfamily proteins in the cell and developmental biology of microorganisms. Cell. Signal. 2003;15:901–9. doi: 10.1016/s0898-6568(03)00073-1. [DOI] [PubMed] [Google Scholar]
- 25.Harris SD. Cdc42/Rho GTPases in fungi: variations on a common theme. Mol. Microbiol. 2011;79:1123–7. doi: 10.1111/j.1365-2958.2010.07525.x. [DOI] [PubMed] [Google Scholar]
- 26.Xu J-R. MAP Kinases in Fungal Pathogens. Fungal Genet. Biol. 2000;31:137–52. doi: 10.1006/fgbi.2000.1237. [DOI] [PubMed] [Google Scholar]
- 27.Boyce KJ, Andrianopoulos A. Ste20-related kinases: effectors of signaling and morphogenesis in fungi. Trends Microbiol. 2011;19:400–10. doi: 10.1016/j.tim.2011.04.006. [DOI] [PubMed] [Google Scholar]
- 28.Virag A, Lee MP, Si H, Harris SD. Regulation of hyphal morphogenesis by cdc42 and rac1 homologues in Aspergillus nidulans. Mol. Microbiol. 2007;66:1579–96. doi: 10.1111/j.1365-2958.2007.06021.x. [DOI] [PubMed] [Google Scholar]
- 29.Li H, Barker BM, Grahl N, Puttikamonkul S, Bell JD, Craven KD, et al. The Small GTPase RacA Mediates Intracellular Reactive Oxygen Species Production, Polarized Growth, and Virulence in the Human Fungal Pathogen Aspergillus fumigatus. Eukaryot. Cell. 2011;10:174–86. doi: 10.1128/EC.00288-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kwon MJ, Arentshorst M, Roos ED, van den Hondel CAMJJ, Meyer V, Ram AFJ. Functional characterization of Rho GTPases in Aspergillus niger uncovers conserved and diverged roles of Rho proteins within filamentous fungi. Mol. Microbiol. 2011;79:1151–67. doi: 10.1111/j.1365-2958.2010.07524.x. [DOI] [PubMed] [Google Scholar]
- 31.Broek D, Samiy N, Fasano O, Fujiyama A, Tamanoi F, Northup J, et al. Differential activation of yeast adenylate cyclase by wild-type and mutant RAS proteins. Cell. 1985;41:763–9. doi: 10.1016/s0092-8674(85)80057-x. [DOI] [PubMed] [Google Scholar]
- 32.Toda T, Uno I, Ishikawa T, Powers S, Kataoka T, Broek D, et al. In yeast, RAS proteins are controlling elements of adenylate cyclase. Cell. 1985;40:27–36. doi: 10.1016/0092-8674(85)90305-8. [DOI] [PubMed] [Google Scholar]
- 33.Rocha CR, Schröppel K, Harcus D, Marcil A, Dignard D, Taylor BN, et al. Signaling through adenylyl cyclase is essential for hyphal growth and virulence in the pathogenic fungus Candida albicans. Mol. Biol. Cell. 2001;12:3631–43. doi: 10.1091/mbc.12.11.3631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hogan DA, Sundstrom P. The Ras/cAMP/PKA signaling pathway and virulence in Candida albicans. Future Microbiol. 2009;4:1263–70. doi: 10.2217/fmb.09.106. [DOI] [PubMed] [Google Scholar]
- 35.Fillinger S, Chaveroche M-K, Shimizu K, Keller N, d’ Enfert C. cAMP and ras signalling independently control spore germination in the filamentous fungus Aspergillus nidulans. Mol. Microbiol. 2002;44:1001–16. doi: 10.1046/j.1365-2958.2002.02933.x. [DOI] [PubMed] [Google Scholar]