Abstract
This manuscript describes the early history of NK cell discovery, with emphasis on the events in the first decade of NK cell studies, 1972–1982. The authors highlight some of the earliest and most important observations that would later prove to be milestones in the study of NK cells and their activity.
Keywords: NK cells, discovery, characterization
I. INTRODUCTION
What were the earliest observations of natural killer (NK) activity or associated NK cells? Most important findings have their origins in the work of many people, and the description of NK activity and the associated cells was no different.
The earliest studies on NK function can be linked to several papers that described cellular assays performed in an attempt to elucidate immune reactivity to viral pathogens or tumor cells. In this review, we identify some of the seminal early reports that contributed significantly to the identification of a new immunological process that has become known as NK activity. Although this is not a complete review of all early NK cell studies, we highlight some of the earliest and most important observations that later proved to be milestones in the study of NK cells. We focus on the events in the first decade of NK studies, 1972–1982.
II. EARLY REPORTS
During the study of T-cell immune reactivity to tumor antigens in patients with cancer, several reports emerged of “non-specific” reactivity observed in vitro. One early example was a family study by E.B. Rosenberg, R.B. Herberman, P.H. Levine, R. Halterman, J. McCoy, and J.R. Wunderlich,1 which provided one of the first reports of unexplained “natural” cytotoxic reactivity. Even more importantly, this report was one of the first to suggest that this cytotoxicity was a real phenomenon, not just an in vitro artifact. Although the biological importance of the cytotoxicity was not clear at this point, the authors did state in their conclusions: “Positive cellular cytotoxicity reactions to leukemia-associated antigens by lymphocytes of family members and normal unrelated individuals are of great interest. Tumor surveillance mechanisms have been postulated which could explain why lymphocytes from normal individuals would destroy cells bearing tumor antigens.”
By early 1972, a number of research efforts were focused on attempting to define immune reactivity to cancer cells and determining the best methodology with which to evaluate this reactivity. In June of that year, the US National Cancer Institute held a “Conference & Workshop on Cellular Immune Reactions to Human Tumor-Associated Antigens” in Bethesda, Maryland.2 The program committee was composed of many noted researchers: Conference Chairman: Ronald B. Herberman; Program Committee: Ronald B. Herberman, Paul H. Levine, Clarice E. Gaylord, and Cathleen L. Baughman; with the monograph of the meeting being edited by Drs. Herberman and Gaylord. This conference focused on (1) cytotoxicity assays, (2) migration inhibition assays, (3) lymphocyte stimulation, and (4) skin tests. In this conference, the recognition of spontaneous cellular activity to tumors of various origins became evident. The participants included a variety of scientists from noted research institutions, including the Karolinska Institute (G. and E. Klein); the US National Institutes of Health (R. Herberman and R. Oldham); UCLA (M. Takasugi and S. Golub); and the MD Anderson Institute (J.G. Sinkovics). A brief summary of the presentations, assays, and key findings is provided in Table 1.
Table 1.
Summary of select reports from 1973 NCI Monograph
| Author | Test employed | Target(s) | Method of analysis | Major conclusion |
|---|---|---|---|---|
| J.S. Sinkovics, K. Tebbi, and J.R. Cabiness MD Anderson |
Microcytotoxicity | Rhabdomyosarcoma, Renal carcinoma, Breast carcinoma | Visual analysis | Established cultures of lymphocytes were nonspecifically cytotoxic to a battery of target tumor cells. |
| Charles F. McKhann, MD. Patrick H. Cleveland, Ph.D. and Martyn W. Burk, M.D Univ. Minnesota |
Cytotoxicity | Sarcoma | 51-Chromium 3H-Thymidinerelease |
Nonspecific lysis of target cells by normal lymphocytes is variable and should be considered when using this combination as a control for target cell killing by immune lymphoid cells. |
| Robert K. Oldham, David Siwarski, James L McCoy, Ernest J. Plata, and Ronald B. Herberman NCI & Litton Bionetics Research |
Cytotoxicity | Panel of breast carcinomas, melanomas, colon carcinomas, melanomas sarcomas | 125-IUDr release | Wide variation in the ability of lymphocytes from normal individuals to lyse these tissue-culture lines has been evident. Some, but not all, of this variability may be related to the method of lymphocyte purification. |
| James L. McCoy, Ronald B. Herberman, E.B. Rosenberg, F.C. Donnelly, Paul H. Levine, and C. Alford Litton Bionetics & NCI |
Cytotoxicity | Human lymphoid lines: NC-37 and F265 | 51-Chromium release | The results suggest that true cell-mediated immunologic reactions cause the lysis of human tissue-culture lymphoid cells by normal donor lymphocytes. The relevant antigen(s) appears neither to be “blast” associated nor to be related to antigens of fetal bovine serum. |
| M. Takasugi, M.R. Mickey, and P.I. Terasaki Univ. California |
Micro-cytotoxicity | Cultured human primary tumor cells | Electronic Image Analysis | The choice of a correct control in the test also became a problem when some normal individuals were found to react strongly and feeder effects were observed. A score was developed based on percent reduction from either medium controls or from negative lymphocyte tests with the maximum number of target cells. |
| Henryk Skurzak, Ladislaus Steiner, Eva Klein, and Ed Lamon Karolinska Institute |
Micro-cytotoxicity | Glioma lines; 118 & 158 MG; Glia lines: 587 & 622 CG, Osteosarcoma 2T | Visual Analysis | The pattern of cytotoxicity appeared to reflect target cell sensitivity rather than tumor specificity. Lymphocytes from the nontumor patients often produced cytotoxic effect against the target cells that was most pronounced. |
Selected summary from NCI Conference & Workshop on Cellular Immune Reactions to Human Tumor-Associated Antigens.2
Many key questions were proposed to be addressed at the conference: (1) What types of antigens are being detected in the various assays? (2) What are the phases of the immune response that these assays measure? (3) What is the nature of the reactive lymphocytes—are they T-lymphocytes or B-lymphocytes? (4) What role do lymphocyte-dependent antibodies play in the observed responses? (5) What are the relationships of these various assays to each other? (6) How reliable and reproducible are the results of these various assays? (7) Importantly, can the assays be used to differentiate patients with neoplastic diseases from those with benign disease or from normal individuals?
A large portion of the conference examined different in vitro assay systems, including; cytotoxicity, migration inhibition, and lymphocyte stimulation. Cytotoxicity assays discussed included microcytoxicity, 3H-proline release, 51-chromium release, and 125-iodine release assays. Using these assay systems many participants at this conference reported in vitro reactivity of “control” or “normal” lymphocytes in their assays. This activity was not well understood at the time but was consistently observed by many participants. Sinkovics et al. concluded, “Cultured lymphocytes were nonspecifically cytotoxic to a battery of target tumor cells. Purified lymphocytes were less cytotoxic.” Oldham et al. concluded, “Wide variations in the ability of lymphocytes from normal individual to lyse tissue-culture lines has been evident.” McCoy et al. from Litton Bionetics Research Laboratories concluded, “Normal human lymphocytes … directly lysed human tumor cell lines.”
Two general conclusions were drawn at the conclusion of this conference: (1) It is certainly possible that some or all normal individuals have immune reactivity against tumor cells or cell lines derived from tumors. (2) This could be activity against some cross-reactive antigens, e.g., bacterial or histocompatibility antigens.
Additional questions were raised in the summary of the meeting: (1) Does the activity seen with leukocytes from normal individuals represent real immunologic activity against tumor-associated antigens? (2) Is this activity just noise or problems with setting the baseline in the assays?
This important conference led to a critical increase in awareness regarding the “spontaneous” in vitro antitumor activity of normal leukocytes and the recognition that further studies were necessary to characterize this activity associated with unstimulated leukocytes. Thus began the first major push into the study of the “natural” or “non-specific” reactivity associated with normal, i.e., unstimulated, leukocytes.
III. EARLY DISCOVERIES AND CONTRIBUTIONS
By 1975, a series of key papers has been published that set the stage for important discoveries and the characterization of tumor cell killing by normal leukocytes. While previous immunologists had limited this in vitro function solely to sensitized T lymphocytes, two papers by Herberman et al. provided insight into the phenotype(s) responsible for natural cytotoxic activity of leukocytes in the mouse.3,4 The first paper demonstrated that the antitumor effector cells from non–tumor-bearing mice was mediated by a unique subpopulation of non-adherent lymphoid cells with no known T- or B-lymphocyte cell-surface markers. These cells were termed N cells.3 The second paper described the broad specificity associated with this lytic activity from normal mice, possibly associated with murine endogenous type-C viruses. This activity was not observed against normal cells or some tumor cell lines.4
At about the same time, Karolinska et al. reported similar results in the mouse.5 They concluded that the spontaneous cytotoxic activity of normal mouse spleen cells against Moloney leukemia cells was exerted by small “undefined” lymphocytes; they termed them natural killer (NK) cells. This name defined the cells based on their function, and the name has been preserved to this day.
In addition, Karolinska et al. also observed strong spontaneous lymphocyte-mediated cytotoxicity against a mouse lymphoma cell line (Yac-1), thus defining the prototype mouse NK target.6 Sendo et al. reported similar natural cytotoxicity using a Balb/c target, RLmale 1.7 Soon thereafter, Pross et al. reported the use of K562 to evaluate human spontaneous lymphocyte-mediated cytotoxicity, thus defining the prototype human NK target.8
All of these reports from the earliest studies of NK cells defined critical aspects of NK activity and set the stage for a later series of seminal discoveries and conferences (Fig. 1) regarding the nature and characteristics of these cells. The first NK Workshop was held in 1982 in North Carolina and was organized by Hillel Koren. The format of this meeting was very informal due the small attendance (~50 people). As can be seen in Fig. 1, many of the discussions occurred in small discussion groups. Formal presentations were few with many presenters merely stating a scientific observation followed by informal discussions. This initial meeting has been followed by a series of NK workshops that are still held today under the oversight of the Society of Natural Immunity starting in 1992.
FIG. 1.
First NK Workshop Collage – chaired by Hillel Koren in NC, USA. As shown, the first NK workshop was small and informal. The photo collage does not represent all of the key attendees’ but provides a flavor of these early meetings. Panel A – Herberman (left), Targan (center) Gorelik (right); Panel B – Bonivida (left), Goldfarb (center), Pollack (right); Panel C – Ortaldo (left), Bloom (right), Roder (right most); Panel D – Keissling (left), Pollack (center), Santoni (right); Panel E – Bennett (left), C. Lopez (2nd Left), J. Linna (right), Welsh (right most); Panel F – Keissling (left), Brunda (2nd Left), Wigzell (right).
IV. NATURE OF NK EFFECTOR CELLS
In spite of the relatively crude research tools available at the time, a number of early studies were able to begin to define the phenotype, specificity, and regulation of NK cells in both mouse and human. During the mid to late 1970s, NK cells were mostly defined by their lack of markers.9–16 NK cells lacked T- and B-lymphocyte cell-surface markers; most were Fc receptor (FcR) positive. But unlike monocytes they were non-adherent to plastic. Bolhuis et al. observed that human NK cells, which possessed a FcR for IgG, did not utilize this receptor in its NK killing function and concluded that NK cells must utilize an (as yet) undefined receptor(s) to mediate their function.16
However, the lack of unique markers on NK cells made their acceptance by the larger immunologic community difficult, and many investigators simply considered the spontaneous in vitro cytotoxicity seen with normal leukocytes to bean artifact.
This all changed in 1979 when the uniqueness of NK cells first became evident with the observations that human NK cell activity was highly associated with a relatively minor population of unique leukocytes called large granular lymphocytes (LGLs).17,18 This observation led to the identification of unique human and rodent markers on these cells,19–28 which allowed the enumeration, isolation, and functional analysis of purified NK cells. This discovery was a major milestone in the understanding of NK cells because most previous studies have not been able to not identify a unique cell mediating this in vitro function.
The presence of unique markers on NK cells also allowed the evaluation of NK cells in vivo during animal studies and in man during clinical trials. In the mouse, the ability to use antibodies against these unique markers for in vivo depletion of NK cells29–31 helped to identify new functions of murine NK cells.
Related to the finding that NK cells had a LGL morphology was the observation of LGL leukemias in both humans and rats.32–34 These leukemic cells provided a useful source of cellular materials for studies on the characteristics of these cells, including the use of perforin in NK-mediated killing.33,34
V. SPECIFICITY OF NK CELLS
Because of the lack of unique markers and the lack of appropriately definitive research tools, early experiments to study the specificity of NK cells were difficult to interpret. This is clearly demonstrated in early studies where some, but not all, lymphoma cells were highly susceptible to NK-mediated lysis.5,35,36 In addition, analysis of multiple inbred strains of mice added to the complexity of these observations, since different strains were classified as either “high” or “low” for the ability of their leukocytes to kill the prototype murine target Yac-1.5,6,8,35,36
In the mouse, one assay that was used to elucidate the specificity of NK-mediated lysis was the competitive cold-target inhibition assay. This assay allowed a panel of target cells to be used with specific indicator targets to identify common patterns of inhibition and perhaps common antigens.5,35 In humans, similar studies were done using competition assays which indicated a complex pattern of NK target-cell recognition.37,38
Another early technique used to study NK specificity was the effector cell adsorption assay, based on the early observations38,39 that NK cells form strong conjugates with tumor targets.17 This adsorption assay also demonstrated unique patterns of target cell recognition and suggested multiple “receptor-ligands” that might be involved in NK lysis.39,40 The conclusions of these studies were that “human NK cells may be heterogeneous, with each subpopulation recognizing different antigenic specificities on target cells.”
Additional early studies based on multiple-target monolayer adsorption analysis suggested that NK cells may utilize a minimum of seven antigenic specificities/receptors.39
The demonstration that NK cells can react selectively with some, but not all target cells, ruled out the frequent contention that in vitro NK activity simply represented a nonspecific binding or nonspecific membrane interaction. However, major issues remained: (1) Is the specificity of natural reactivity directed toward antigens that are common to a wide variety of cultured cell lines and tumor cells? (2) Does natural cell-mediated cytotoxicity represent a basic immune surveillance mechanism against tumors directed against a series of broadly distributed antigens on tumor cells? (3) How is this activity regulated? Finding the answers to these and other questions regarding NK cells will be the basis of studies for years to come.
VI. NK CELL REGULATION
Like other leukocytes, early studies on the regulation of NK activity indicated that NK cells were highly regulated. Their functional activity could be rapidly increased by a variety of natural agents and pathogens.41 Several laboratories made the observation that the cytotoxic activities of NK cells were rapidly activated early during virus infection.42–44 In addition, infection of target cells by a number of viruses rendered these cells susceptible to lysis by NK cells. These studies concluded that NK cells may be important factors in immune surveillance against both virus-induced tumors and virus infections. However, these observations also led to the important observation that virally-induced interferon was a major positive regulator of NK activity.45–51
The rapid and potent regulation of NK cells by interferons in the mouse led to early studies that evaluated the potential for interferon therapy in cancer using recombinant interferons.49 In these studies, “Patients received large doses of interferon to determine (1) whether interferon could induce NK lymphocytes in the peripheral blood of man, and (2) whether there are characteristic kinetics for the appearance, disappearance and reactivation of NK lymphocytes following interferon therapy.” These studies demonstrated that the “activation of human NK cells was observed by the systemic inoculation of human subjects with interferon.” This observation was followed by numerous additional clinical trials that attempted to manipulate in vivo human NK activity with a variety of recombinant proteins.
Another agent that emerged during this period of time, which was both a potent regulator of NK cell function but also a growth factor for NK cells, was interleukin-2 (IL-2). Early studies with T-cell growth factor (later named IL-2) demonstrated its potent effect on NK cells. These studies demonstrated that IL-2 could potently activate NK cells and broaden their range of target cell lysis.52–54 Where previous studies had focused on the lysis of leukemia and lymphoma targets by NK cells, IL-2-activated NK cells could lyse solid tumors,52–54 and IL-2-activated NK cells had potent in vivo activity.53
In addition to positive regulation, it became evident that NK activity could also be rapidly inhibited under certain stress conditions and after certain pathogen insults.55,56
A. Functions of NK cells
1. Anti-tumor Activity
The earliest reports of natural killer activity were in murine leukemia and lymphoma models using virus-induced targets. However, initial studies evaluating NK activity in man1–7 indicated that there was little evidence for direct killing of autologous tumors. This discrepancy led to a large number of studies, in both the human and a variety of animal models, to explain this difference. The total number of citations for studies that evaluated “natural killer” (NK) or “natural cytotoxic” function after 1975 rose rapidly after 1975 (Fig. 2a). During the decade from 1980 to 1990, there was an explosion of studies that evaluated NK cells in the tumor setting. Considering the total number of NK references by decade, a vast increase in the number of NK reports can be seen during the 1980s (Fig. 2b). Most of these citations were associated with the keyword “tumor”, with the keyword “virus” being a distant second.
FIG. 2.
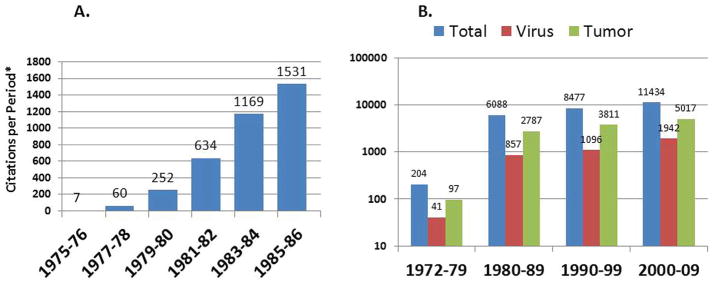
Graphic represention of PubMed articles published that contained the phrase “natural killer” or “natural cytotoxic”. Panel A depicts the period from 1975 thru 1986. Panel B depicts four decades of citations that are further subdivided into those also containing the word “tumor” or “virus”.
Early animal studies employed tumor cell lines to evaluate NK activity in vitro. However, evidence for the antitumor immune surveillance role of NK cells in vivo was only circumstantial, based primarily on strain variations for tumor incidence and in vitro tumor cell killing.41 Perhaps one of the most important factors leading to the dramatic increase in the number of references regarding NK antitumor function was the discovery that NK cells bear asialo-GM157 and express NK1.126 antigens on their surface. Ultimately, the use of antibodies to these antigens allowed for the direct evaluation of the role of NK cells in tumors.42–44,58 These reagents allowed for a large number of studies to be performed studying the in vivo role of NK cells in tumor surveillance, viral and parasitic infections, and non-pathogen systems as described in the following reviews.41,59–62
2. Anti-viral Activities
As noted above, the in vitro ability of murine NK cells to lyse tumor cells that were of viral origin led to the early hypothesis that non-tumor virus-infected cells may also be primary targets for NK cells. Anderson et al.42 noted, “… The presence of virus infection may be of prime importance in determining the susceptibility of cells to lysis by unsensitized NK lymphocytes. Indeed, preliminary results have been obtained indicating that infection of L cells with the Kunz strain of influenza A renders these cells similarly susceptible to innate cytotoxicity. The importance of NK cells in immune surveillance against both virus-induced tumors and virus infections generally is likely, therefore, to be considerable and worthy of further study.” These results were also observed with human NK cells where NK-resistant cells became susceptible NK targets after viral infection.58,63
In addition to the in vitro lysis of virus-infected targets, an in vivo role of NK cells in viral infection was suggested by Welsh et al. 43 in studies which concluded, “The advent of NK cell activity correlated with the synthesis of interferon in LCMV-infected mice. … These experiments suggest that LCMV induced NK cells via an interferon-dependent mechanism.” Further evidence for the role of NK cells in virus infection was provided by the studies of Biron et al. in a patient lacking NK cells that had recurring severe herpes virus infections.64
3. Response to Bacteria and Parasites
Another area of research, which was only partially appreciated from early NK studies, was the potential for NK cells to play a role in a variety of bacterial and parasitic diseases. Early studies with bacterial agents suggested a role for NK cells in the response to these agents.50 While there was little early evidence regarding the direct role of NK cells in controlling parasitic infections, there were reports of correlations of parasitic infection and NK cell activation. Eugui et al. demonstrated that in response to malaria, “…NK cells were recruited and activated by T lymphocyte-mediated immune responses to parasite antigens.”65,66 In addition, in hemoprotozoan infections, a possible correlation with NK activity was shown, in that “marked activation of NK cells occur, in resistant strains but not in susceptible ones.”65 In summary, these early studies examining the potential for NK cells to play a role in parasitic diseases concluded that “Of the nonspecific factors, macrophage activation, natural killer cells, and serum factors other than antibodies are critical in the battle against parasites.”66
4. Hybrid Resistance
Early studies on the phenomenon known as hybrid resistance (i.e., F1 anti-parent transplantation resistance) suggested that the effector cells, which mediate this resistance, share characteristics with NK cells.62 The in vivo regulation of F1 bone-marrow transplantation was later confirmed to be mediated by NK cells.67 As we know today, the receptors responsible for these reactions are the class I recognizing receptors (i.e., Ly49s in mouse and KIR in man). Evidence now suggests that these receptors play an important role in the innate resistance observed in human bone-marrow transplantation.67
5. Production of Cytokines
Another function that was associated early on with NK cells was the production of cytokines.68 Djeu et al. first reported that “The IFN produced by both LGL and monocytes were predominantly IFN-α, as assessed by neutralization assays with antisera …” These studies demonstrated that IFN-γ was also produced by NK cells, presumably for NK cell recognition of the virus used for stimulation. These data suggested an efficient positive self-regulatory mechanism in NK cells that may be readily switched on by viruses. NK cells secrete a high level of cytokines that regulate other leukocytes and NK function. Some important cytokines include but are not limited to IL-1-β, IL-8, TNF-α, IL-10, IL-13, GM-CSF, IFN-α, IFN-γ, and TGF-β.
VII. EARLY SEMINAL MILESTONES
Early studies of NK cells provided a number of key observations regarding the immunological community, which suggested a variety of functions for these cells. Summarized here are important milestone observations from early NK cell studies that have contributed to a better understanding, not only of NK cells, but of immune responses in general.
Resistance to tumors. Early reports of recognition of tumor cell lines by NK cells, which were later translated into the demonstration of the important role of NK cells in regulating the metastatic spread of tumors, placed NK cells as a key player of innate immunity in cancer.69–71
Control of virus infection. Early reports that NK cells were activated by virus infection, and could selectively recognize and kill virus-infected targets, placed NK cells as a critical component of the innate immune response to viral infections.72–74
Cytokine production. Early reports that activation of NK cells by tumors and viruses led to cytokine production placed NK cells as more than just a cytotoxic effector cell but as a major player in many aspects of the immune network.41,72,74 Today, we know that production of cytokines is an important immune-regulatory loop in the in vivo function of NK cells.
Unique patterns of recognition. Early reports regarding the broad specificity of NK cells led to the identification of a number of receptors used by NK cells.19–24 Unique among lymphocytes, the NK cells can recognize both pattern recognition domains as well as levels of MHC on target cells.
Identification of LGL morphology and LGL leukemias. The discovery of the LGL morphology associated with NK activity guided the initial isolation of purified NK cells and identification of unique markers on these cells that could be used to distinguish them from other lymphocytes.17,32 In addition, LGL leukemia cell lines provided a critical source of cells that resulted in the definition of lymphocyte perforin-mediated killing.33,34
Expression of IL2Rβ and response to IL2. The discovery that NK cells could rapidly respond to IL-2 to both proliferate and become activated spurred the discovery of the IL2Rβ chain.75
VIII. SUMMARY
In the early 1970s, the spontaneous in vitro antitumor activity of normal or unstimulated leukocytes was described as being “non-specific”, and possibly just an in vitro artifact. Since that time, a large number of studies have resulted in thousands of reports that have defined this activity (NK activity) and the cells associated with the activity (NK cells).
From these seminal early studies between 1970 and1980, it became clear that NK cells were a unique population of large granular lymphocytes (LGLs). We now know that they constitute a unique third major lymphocyte cell type and are a key member of the innate immune system. Today, it is also clear from these early studies that NK cells contribute to a number of immunological responses, including important and rapid responses to viral infection and significant antitumor protection, especially against the development of peripheral metastases. We have now identified many of the positive and negative factors that regulate these cells and their activity. These early studies helped us to understand the specificity of these cells and the basis for this specificity, including the identification of a number of different receptor families on NK cells that recognize MHC and unique pattern recognition domains.
These early studies provided many of the most important and critical observations about NK cells, which later proved to be milestones for understanding the biology of NK cells and their associated activities. While many laboratories contributed to these early studies, Ron Herberman and his laboratory must be considered one of the major contributors and visionary drivers of the NK field during this early period of discovery.
Acknowledgments
The authors recognize Ron Herberman for his vision and perseverance during the early days when NK activity was detected and the cells defined. His tenacity, often in the face of skepticism from the more traditional immunology community, helped to open a new and important aspect of science. The authors are also grateful to Ron for his leadership, mentoring, and staunch support for our own career development and advancement as well as that of many others.
ABBREVIATIONS
- FcR
Fc-receptor
- IFN
interferon
- Ig
Immunoglobulin
- IL-2
interleukin-2
- LGL
large granular lymphocytes
- NK
natural killer
References
- 1.Rosenberg EB, Herberman RB, Levine PH, Halterman RH, McCoy JL, Wunderlich JR. Lymphocyte cytotoxicity reactions to leukemia-associated antigens in identical twins. Int J Cancer. 1972 May 15;9(3):648–58. doi: 10.1002/ijc.2910090323. [DOI] [PubMed] [Google Scholar]
- 2.Herberman RB, Gaylord CE, editors. Nat Cancer Inst. Jun, 1973. Conference and Workshop on Cellular Immune Reactions to Human Tumor-Associated Antigens. Monograph 37. [PubMed] [Google Scholar]
- 3.Herberman RB, Nunn ME, Holden HT, Lavrin DH. Natural cytotoxic reactivity of mouse lymphoid cells against syngeneic and allogeneic tumors. II. Characterization of effector cells. Int J Cancer. 1975 Aug 15;16(2):230–9. doi: 10.1002/ijc.2910160205. [DOI] [PubMed] [Google Scholar]
- 4.Herberman RB, Nunn ME, Lavrin DH. Natural cytotoxic reactivity of mouse lymphoid cells against syngeneic acid allogeneic tumors. I. Distribution of reactivity and specificity. Int J Cancer. 1975 Aug 15;16(2):216–29. doi: 10.1002/ijc.2910160204. [DOI] [PubMed] [Google Scholar]
- 5.Kiessling R, Klein E, Pross H, Wigzell H. “Natural” killer cells in the mouse. II. Cytotoxic cells with specificity for mouse Moloney leukemia cells. Characteristics of the killer cell. Eur J Immunol. 1975 Feb;5(2):117–21. doi: 10.1002/eji.1830050209. [DOI] [PubMed] [Google Scholar]
- 6.Kiessling R, Petranyi G, Klein G, Wigzel H. Genetic variation of in vitro cytolytic activity and in vivo rejection potential of non-immunized semi-syngeneic mice against a mouse lymphoma line. Int J Cancer. 1975 Jun 15;15(6):933–40. doi: 10.1002/ijc.2910150608. [DOI] [PubMed] [Google Scholar]
- 7.Sendo F, Aoki T, Boyse EA, Buafo CK. Natural occurrence of lymphocytes showing cytotoxic activity to BALB/c radiation-induced leukemia RL male 1 cells. J Natl Cancer Inst. 1975 Sep;55(3):603–9. doi: 10.1093/jnci/55.3.603. [DOI] [PubMed] [Google Scholar]
- 8.Pross HF, Baines MG. Spontaneous human lymphocyte-mediated cytotoxicity againts tumour target cells. I. The effect of malignant disease. Int J Cancer. 1976 Nov 15;18(5):593–604. doi: 10.1002/ijc.2910180508. [DOI] [PubMed] [Google Scholar]
- 9.Habu S, Hayakawa K, Okumura K, Tada T. Surface markers on natural killer cells of the mouse. Eur J Immunol. 1979 Dec;9(12):938–42. doi: 10.1002/eji.1830091206. [DOI] [PubMed] [Google Scholar]
- 10.Haller O, Gidlund M, Hellström U, Hammarström S, Wigzell H. A new surface marker on mouse natural killer cells: receptors for Helix pomatia A hemagglutinin. Eur J Immunol. 1978 Nov;8(11):765–71. doi: 10.1002/eji.1830081103. [DOI] [PubMed] [Google Scholar]
- 11.Ojo E, Haller O, Wigzell H. Corynebacterium parvum-induced peritoneal exudate cells with rapid cytolytic activity against tumour cells are non-phagocytic cells with characteristics of natural killer cells. Scand J Immunol. 1978;8(3):215–22. doi: 10.1111/j.1365-3083.1978.tb00513.x. [DOI] [PubMed] [Google Scholar]
- 12.Shellam GR. Gross-virus-induced lymphoma in the rat. V. Natural cytotoxic cells are non-T cells. Int J Cancer. 1977 Feb 15;19(2):225–35. doi: 10.1002/ijc.2910190212. [DOI] [PubMed] [Google Scholar]
- 13.Glimcher L, Shen FW, Cantor H. Identification of a cell-surface antigen selectively expressed on the natural killer cell. J Exp Med. 1977 Jan 1;145(1):1–9. doi: 10.1084/jem.145.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Herberman RB, Holden HT, Ting CC, Lavrin DL, Kirchner H. Cell-mediated immunity to leukemia virus- and tumor-associated antigens in mice. Cancer Res. 1976 Feb;36(2 pt 2):615–21. [PubMed] [Google Scholar]
- 15.Herberman RB, Bartram S, Haskill JS, Nunn M, Holden HT, West WH. Fc receptors on mouse effector cells mediating natural cytotoxicity against tumor cells. J Immunol. 1977 Jul;119(1):322–6. [PubMed] [Google Scholar]
- 16.Bolhuis RL, Schuit HR, Nooyen AM, Ronteltap CP. Characterization of natural killer (NK) cells and killer (K) cells in human blood: discrimination between NK and K cell activities. Eur J Immunol. 1978 Oct;8(10):731–40. doi: 10.1002/eji.1830081012. [DOI] [PubMed] [Google Scholar]
- 17.Timonen T, Saksela E, Ranki A, Häyry P. Fractionation, morphological and functional characterization of effector cells responsible for human natural killer activity against cell-line targets. Cell Immunol. 1979 Nov;48(1):133–48. doi: 10.1016/0008-8749(79)90106-0. [DOI] [PubMed] [Google Scholar]
- 18.Timonen T, Ranki A, Saksela E, Häyry P. Human natural cell-mediated cytotoxicity against fetal fibroblasts. III. Morphological and functional characterization of the effector cells. Cell Immunol. 1979 Nov;48(1):121–32. doi: 10.1016/0008-8749(79)90105-9. [DOI] [PubMed] [Google Scholar]
- 19.Ortaldo JR, Sharrow SO, Timonen T, Herberman RB. Determination of surface antigens on highly purified human NK cells by flow cytometry with monoclonal antibodies. J Immunol. 1981 Dec;127(6):2401–9. [PubMed] [Google Scholar]
- 20.Reynolds CW, Sharrow SO, Ortaldo JR, Herberman RB. Natural killer activity in the rat. II. Analysis of surface antigens on LGL by flow cytometry. J Immunol. 1981 Dec;127(6):2204–8. [PubMed] [Google Scholar]
- 21.Timonen T, Ortaldo JR, Stadler BM, Bonnard GD, Sharrow SO, Herberman RB. Cultures of purified human natural killer cells: growth in the presence of interleukin 2. Cell Immunol. 1982 Sep 1;72(1):178–85. doi: 10.1016/0008-8749(82)90295-7. No abstract available. [DOI] [PubMed] [Google Scholar]
- 22.Phillips JH, Warner NL, Lanier LL. Correlation of biophysical properties and cell surface antigenic profile of Percoll gradient-separated human natural killer cells. Nat Immun Cell Growth Regul. 1983–1984;3(2):73–86. [PubMed] [Google Scholar]
- 23.Lanier LL, Le AM, Phillips JH, Warner NL, Babcock GF. Subpopulations of human natural killer cells defined by expression of the Leu-7 (HNK-1) and Leu-11 (NK-15) antigens. J Immunol. 1983 Oct;131(4):1789–96. [PubMed] [Google Scholar]
- 24.Lanier LL, Loken MR. Human lymphocyte subpopulations identified by using three-color immunofluorescence and flow cytometry analysis: correlation of Leu-2, Leu-3, Leu-7, Leu-8, and Leu-11 cell surface antigen expression. J Immunol. 1984 Jan;132(1):151–6. [PubMed] [Google Scholar]
- 25.Lanier LL, Le AM, Civin CI, Loken MR, Phillips JH. The relationship of CD16 (Leu-11) and Leu-19 (NKH-1) antigen expression on human peripheral blood NK cells and cytotoxic T lymphocytes. J Immunol. 1986 Jun 15;136(12):4480–6. [PubMed] [Google Scholar]
- 26.Jacobsen JB, Hammerling GJ, Hammerling U, Koo GC. Antigenic profile of murine natural killer cells. J Immunol. 1980;125:1003. [PubMed] [Google Scholar]
- 27.Stutman O, Cuttito MJ. Normal levels of natural cytotoxic cells against solid tumors in NK-deficient beige mice. Nature (Lond) 1981;290:254. doi: 10.1038/290254a0. [DOI] [PubMed] [Google Scholar]
- 28.Pollack SB, Hallenberg LA. In vivo reduction of NK activity with anti-NKl serum: direct evaluation of NK cells in tumor clearance. Int J Cancer. 1982;29:203. doi: 10.1002/ijc.2910290215. [DOI] [PubMed] [Google Scholar]
- 29.Young WW, Jr, Hakomori SI, Durdik JM, Henney CS. Identification of ganglio-N-tetraosylceramide as a new cell surface marker for murine natural killer (NK) cells. J Immunol. 1980 Jan;124(1):199–201. [PubMed] [Google Scholar]
- 30.Kasai M, Iwamori M, Nagai Y, Okumura K, Tada T. A glycolipid on the surface of mouse natural killer cells. Eur J Immunol. 1980 Mar;10(3):175–80. doi: 10.1002/eji.1830100304. [DOI] [PubMed] [Google Scholar]
- 31.Jacobsen JB, Hammerling GJ, Hammerling U, Koo GC. Antigenic profile of murine natural killer cells. J Immunol. 1980;125:1003. [PubMed] [Google Scholar]
- 32.Ward JM, Reynolds CW. Large granular lymphocyte leukemia. A heterogeneous lymphocytic leukemia in F344 rats. Am J Pathol. 1983 Apr;111(1):1–10. [PMC free article] [PubMed] [Google Scholar]
- 33.Henkart PA, Yue CC, Yang J, Rosenberg SA. Cytolytic and biochemical properties of cytoplasmic granules of murine lymphokine-activated killer cells. J Immunol. 1986 Oct 15;137(8):2611–7. [PubMed] [Google Scholar]
- 34.Podack ER, Konigsberg PJ. Cytolytic T cell granules. Isolation, structural, biochemical, and functional characterization. J Exp Med. 1984 Sep 1;160(3):695–710. doi: 10.1084/jem.160.3.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Herberman RB, Holden HT, Ting CC, Lavrin DL, Kirchner H. Cell-mediated immunity to leukemia virus- and tumor-associated antigens in mice. Cancer Res. 1976 Feb;36(2 pt 2):615–21. [PubMed] [Google Scholar]
- 36.Kiessling R, Klein E, Wigzell H. “Natural” killer cells in the mouse. I. Cytotoxic cells with specificity for mouse Moloney leukemia cells. Specificity and distribution according to genotype. Eur J Immunol. 1975 Feb;5(2):112–7. doi: 10.1002/eji.1830050208. [DOI] [PubMed] [Google Scholar]
- 37.Nunn ME, Herberman RB. Natural cytotoxicity of mouse, rat, and human lymphocytes against heterologous target cells. J Natl Cancer Inst. 1979 Apr;62(4):765–71. [PubMed] [Google Scholar]
- 38.Ortaldo JR, Oldham RK, Cannon GC, Herberman RB. Specificity of natural cytotoxic reactivity of normal human lymphocytes against a myeloid leukemia cell line. J Natl Cancer Inst. 1977 Jul;59(1):77–82. doi: 10.1093/jnci/59.1.77. [DOI] [PubMed] [Google Scholar]
- 39.Phillips WH, Ortaldo JR, Herberman RB. Selective depletion of human natural killer cells on monolayers of target cells. J Immunol. 1980 Nov;125(5):2322–7. [PubMed] [Google Scholar]
- 40.Jensen PJ, Koren HS. Depletion of NK by cellular immunoadsorption. J Immunol. 1979 Sep;123(3):1127–32. [PubMed] [Google Scholar]
- 41.Herberman RB. Immunoregulation and natural killer cells. Mol Immunol. 1982 Oct;19(10):1313–21. doi: 10.1016/0161-5890(82)90299-1. [DOI] [PubMed] [Google Scholar]
- 42.Anderson MJ. Innate cytotoxicity of CBA mouse spleen cells to Sendai virus-infected L cells. Infect Immun. 1978 Jun;20(3):608–12. doi: 10.1128/iai.20.3.608-612.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Welsh RM., Jr Cytotoxic cells induced during lymphocytic choriomeningitis virus infection of mice. I. Characterization of natural killer cell induction. J Exp Med. 1978 Jul 1;148(1):163–81. doi: 10.1084/jem.148.1.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Santoli D, Trinchieri G, Koprowski H. Cell-mediated cytotoxicity against virus-infected target cells in humans. II. Interferon induction and activation of natural killer cells. J Immunol. 1978 Aug;121(2):532–8. [PubMed] [Google Scholar]
- 45.Minato N, Reid L, Cantor H, Lengyel P, Bloom BR. Mode of regulation of natural killer cell activity by interferon. J Exp Med. 1980 Jul 1;152(1):124–37. doi: 10.1084/jem.152.1.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kiessling RW, Welsh RM., Jr Killing of normal cells by activated mouse natural killer cells: evidence for two patterns of genetic regulation of lysis. Int J Cancer. 1980 May 15;25(5):611–5. doi: 10.1002/ijc.2910250510. [DOI] [PubMed] [Google Scholar]
- 47.Senik A, Stefanos S, Kolb JP, Lucero M, Falcoff E. Enhancement of mouse natural killer cell activity by type II interferon. Ann Immunol (Paris) 1980 May-Jun;131C(3):349–61. [PubMed] [Google Scholar]
- 48.Herberman RB, Ortaldo JR, Djeu JY, Holden HT, Jett J, Lang NP, Rubinstein M, Pestka S. Role of interferon in regulation of cytotoxicity by natural killer cells and macrophages. Ann N Y Acad Sci. 1980;350:63–71. doi: 10.1111/j.1749-6632.1980.tb20608.x. [DOI] [PubMed] [Google Scholar]
- 49.Huddlestone JR, Merigan TC, Jr, Oldstone MB. Induction and kinetics of natural killer cells in humans following interferon therapy. Nature. 1979 Nov 22;282(5737):417–9. doi: 10.1038/282417a0. [DOI] [PubMed] [Google Scholar]
- 50.Herberman RB, Djeu J, Kay HD, Ortaldo JR, Riccardi C, Bonnard GD, Holden HT, Fagnani R, Santoni A, Puccetti P. Natural killer cells: characteristics and regulation of activity. Immunol Rev. 1979;44:43–70. doi: 10.1111/j.1600-065x.1979.tb00267.x. [DOI] [PubMed] [Google Scholar]
- 51.Djeu JY, Heinbaugh JA, Holden HT, Herberman RB. Augmentation of mouse natural killer cell activity by interferon and interferon inducers. J Immunol. 1979 Jan;122(1):175–81. [PubMed] [Google Scholar]
- 52.Grimm EA, Mazumder A, Zhang HZ, Rosenberg SA. Lymphokine-activated killer cell phenomenon. Lysis of natural killer-resistant fresh solid tumor cells by interleukin 2-activated autologous human peripheral blood lymphocytes. J Exp Med. 1982 Jun 1;155(6):1823–41. doi: 10.1084/jem.155.6.1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kedar E, Ikejiri BL, Gorelik E, Herbermann RB. Natural cell-mediated cytotoxicity in vitro and inhibition of tumor growth in vivo by murine lymphoid cells cultured with T cell growth factor (TCGF) Cancer Immunol Immunother. 1982;13(1):14–23. doi: 10.1007/BF00200195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hersey P, Bindon C, Edwards A, Murray E, Phillips G, McCarthy WH. Induction of cytotoxic activity in human lymphocytes against autologous and allogeneic melanoma cells in vitro by culture with interleukin 2. Int J Cancer. 1981 Dec;28(6):695–703. doi: 10.1002/ijc.2910280607. [DOI] [PubMed] [Google Scholar]
- 55.Saxena RK, Saxena QB, Adler WH. Regulation of natural killer activity in vivo: Part I—loss of natural killer activity during starvation. Indian J Exp Biol. 1980 Dec;18(12):1383–6. [PubMed] [Google Scholar]
- 56.Saxena QB, Mezey E, Adler WH. Regulation of natural killer activity in vivo. II. The effect of alcohol consumption on human peripheral blood natural killer activity. Int J Cancer. 1980 Oct 15;26(4):413–7. doi: 10.1002/ijc.2910260405. [DOI] [PubMed] [Google Scholar]
- 57.Kasai M, Iwamori M, Nagai Y, Okumura K, Tada T. A glycolipid on the surface of mouse natural killer cells. Eur J Immunol. 1980 Mar;10(3):175–80. doi: 10.1002/eji.1830100304. [DOI] [PubMed] [Google Scholar]
- 58.Trinchieri G, Granato D, Perussia B. Interferon-induced resistance of fibroblasts to cytolysis mediated by natural killer cells: specificity and mechanism. J Immunol. 1981 Jan;126(1):335–40. [PubMed] [Google Scholar]
- 59.Introna M, Mantovani A. Natural killer cells in human solid tumors. Cancer Metastasis Rev. 1983;2(4):337–50. doi: 10.1007/BF00048566. [DOI] [PubMed] [Google Scholar]
- 60.Herberman RB, Ortaldo JR. Natural killer cells: their roles in defenses against disease. Science. 1981 Oct 2;214(4516):24–30. doi: 10.1126/science.7025208. [DOI] [PubMed] [Google Scholar]
- 61.Kiessling R, Wigzell H. Surveillance of primitive cells by natural killer cells. Curr Top Microbiol Immunol. 1981;92:107–23. doi: 10.1007/978-3-642-68069-4_7. [DOI] [PubMed] [Google Scholar]
- 62.Cudkowicz G, Hochman PS. Do natural killer cells engage in regulated reactions against self to ensure homeostasis? Immunol Rev. 1979;44:13–41. doi: 10.1111/j.1600-065x.1979.tb00266.x. [DOI] [PubMed] [Google Scholar]
- 63.Weston PA, Jensen PJ, Levy NL, Koren HS. Spontaneous cytotoxicity against virus-infected cells: relationship to NK against uninfected cell lines and to ADCC. J Immunol. 1981 Mar;126(3):1220–4. [PubMed] [Google Scholar]
- 64.Biron CA, Byron KS, Sullivan JL. Severe herpesvirus infections in an adolescent without natural killer cells. N Engl J Med. 1989;320:1731–1735. doi: 10.1056/NEJM198906293202605. [DOI] [PubMed] [Google Scholar]
- 65.Eugui EM, Allison AC. Malaria infections in different strains of mice and their correlation with natural killer activity. Bull World Health Organ. 1979;57 (Suppl 1):231–8. [PMC free article] [PubMed] [Google Scholar]
- 66.Eugui EM, Allison AC. Differences in susceptibility of various mouse strains to haemoprotozoan infections: possible correlation with natural killer activity. Parasite Immunol. 1980 Winter;2(4):277–92. doi: 10.1111/j.1365-3024.1980.tb00059.x. [DOI] [PubMed] [Google Scholar]
- 67.Sentman CL, Hackett J, Jr, Kumar V, Bennett M. Identification of a subset of murine natural killer cells that mediates rejection of Hh-1d but not Hh-1b bone marrow grafts. J Exp Med. 1989 Jul 1;170(1):191–202. doi: 10.1084/jem.170.1.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Djeu JY, Stocks N, Zoon K, Stanton GJ, Timonen T, Herberman RB. Positive self-regulation of cytotoxicity in human natural killer cells by production of interferon upon exposure to influenza and herpes viruses. J Exp Med. 1982 Oct 1;156(4):1222–34. doi: 10.1084/jem.156.4.1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Herberman RB, Holden HT. Natural killer cells as antitumor effector cells. J Natl Cancer Inst. 1979 Mar;62(3):441–5. doi: 10.1093/jnci/62.3.441. [DOI] [PubMed] [Google Scholar]
- 70.Introna M, Mantovani A. Natural killer cells in human solid tumors. Cancer Metastasis Rev. 1983;2(4):337–50. doi: 10.1007/BF00048566. [DOI] [PubMed] [Google Scholar]
- 71.Herberman RB. Natural killer (NK) cells and their possible roles in resistance against disease. Clin Immunol Rev. 1981;1(1):1–65. [PubMed] [Google Scholar]
- 72.Welsh RM. Natural killer cells and interferon. Crit Rev Immunol. 1984;5(1):55–93. [PubMed] [Google Scholar]
- 73.Rager-Zisman B, Bloom BR. Natural killer cells in resistance to virus-infected cells. Springer Semin Immunopathol. 1982;4(4):397–414. doi: 10.1007/BF02053741. [DOI] [PubMed] [Google Scholar]
- 74.Welsh RM., Jr Mouse natural killer cells: induction specificity, and function. J Immunol. 1978 Nov;121(5):1631–5. [PubMed] [Google Scholar]
- 75.Ortaldo JR, Mason AT, Gerard JP, Henderson LE, Farrar W, Hopkins RF, 3rd, Herberman RB, Rabin H. Effects of natural and recombinant IL 2 on regulation of IFN gamma production and natural killer activity: lack of involvement of the Tac antigen for these immunoregulatory effects. J Immunol. 1984 Aug;133(2):779–83. [PubMed] [Google Scholar]



