Abstract
Background
Recent studies describe a unique subset of obese individuals with normal metabolic profiles despite having excess weight called “metabolically healthy but obese (MHO)”.
Objective
Our aim was to determine the prevalence of individuals without diabetes and hypertension and risk factors associated with the MHO phenotype among bariatric surgery patients.
Methods
We conducted a retrospective study of 710 adults who underwent bariatric surgery at Johns Hopkins between 2008 and 2010. In the first analysis of 523 individuals, we identified 150 individuals without diabetes and hypertension; in the second analysis of 260 individuals, we identified 44 individuals without diabetes, hypertension and hypertriglyceridemia. We used multivariable logistic regression to examine the association between each group and potential risk factors including age, sex, race, BMI (body mass index) and presence of liver disease on liver biopsy.
Results
The prevalence of individuals without diabetes and hypertension was 28.7%; among these individuals 88.7% had liver steatosis, 7.3% nonalcoholic steatohepatitis (NASH), and 19.3% liver fibrosis. These individuals were significantly more likely to be White OR=1.9 (95% CI: 1.1–3.1), younger OR= 4.1 (95% CI=2.6–6.3), and female OR=2.1, (95% CI=1.2–3.6) and less likely to have liver steatosis OR=0.4 (95% CI=0.2–0.9) or NASH OR=0.3 (95% CI=0.2–0.6).
Conclusion
Among bariatric surgery patients, almost a third of patients do not have diabetes and hypertension and could be probably considered “MHO” and were more likely to be white, young, female and have less liver injury. The high prevalence of liver steatosis in MHO individuals among bariatric surgery patients challenges the notion of MHO as a truly metabolically healthy entity.
Keywords: obesity phenotypes, bariatric surgery, epidemiology, prevalence, metabolically healthy obese
Introduction
The presence of metabolic complications varies widely among obese individuals. Recently, a unique subset of obese individuals with normal metabolic profiles (normal insulin sensitivity, absent hypertension, and favorable lipid and inflammation profiles) despite obesity has been described as “metabolically healthy obese (MHO)” [1,2]. Additionally, MHO individuals have been characterized by less visceral and ectopic fat in the liver and lower liver enzymes compared to non-MHO individuals [2–4].
The prevalence of MHO individuals varies between 10 to 40% depending on its definition 5). The prevalence of MHO individuals among obese patients undergoing bariatric surgery is unknown. Similarly, there is little known about the characteristics behind the metabolically favorable profile of the MHO phenotype among morbidly obese or bariatric surgery patients. Preliminary data suggest that decrease in visceral fat distribution and increase in weight and adiponectin at birth may lead to MHO phenotype [2,6,7].
In this retrospective study, our objectives were to determine the prevalence of individuals without diabetes and hypertension and to identify potential risk factors associated with the MHO phenotype among bariatric surgery patients.
METHODS
Study Population
This is a single center, retrospective cross-sectional study. The study population is comprised of 710 consecutive patients above the age of 18, presenting for bariatric surgery at The Johns Hopkins Center for Bariatric Surgery between July 2008 and June 2010. The study was approved by the Johns Hopkins Institutional Review Boards.
We excluded persons with frequent alcohol use, positive Hepatitis B surface antigen, Hepatitis C antibody or detectable Hepatitis C viral RNA, hemochromatosis, autoimmune liver disease, Wilson’s disease, alpha 1 antitrypsin deficiency, primary biliary cirrhosis, primary sclerosing cholangitis, and toxic liver and pregnancy. Subjects with missing data on diabetes status, hypertension status, race, smoking status, liver enzymes, liver biopsy, or those undergoing revisional bariatric surgeries were also excluded from analysis. We performed two analyses: 1) we analyzed 523 individuals with complete data on diabetes and hypertension status as well as covariates mentioned above and 2) we analyzed 260 individuals from the first analysis who had additional data on fasting serum triglycerides.
Data collection and definitions
A single clinical investigator reviewed all patient charts and recorded patient data collected within 6 months prior to surgery. Data collected included age, gender, ethnicity/race, body mass index (BMI kg/m2), blood pressure, fasting glucose (mg/dl), lipid panel and hepatic aminotransferases (alanine aminotransferase (ALT) and aspartate aminotransferase (AST) U/L). Ethnicity and race were self-reported. One to three blood pressure measurements were recorded and we used the average value or one reading value if only one was available. All patients underwent a routine intraoperative wedge liver biopsy at the start of bariatric surgery. Biopsy specimens were fixed and stained with hematoxylin and eosin and Masson’s trichrome. Biopsy specimens were read by 1 expert hepatopathologist. For the current analyses the following liver biopsy features were considered: steatosis, nonalcoholic steatohepatitis (NASH), any inflammation, and any fibrosis.
Diabetes was identified by self-report of prior diagnosis, diagnosis in the medical record, use of diabetes medications, or fasting glucose greater than 126 mg/dl. Similarly, we identified hypertension from self-report, diagnosis in the medical record, use of antihypertensive medications or measured systolic blood pressure greater than 130 mmHg and diastolic blood pressure greater than 85 mmHg.
We defined MHO phenotype as obesity without diabetes, hypertension and hypertriglyceridemia (serum triglycerides > 150 mg/dl).
Statistical Analysis
The differences in characteristics between those with and without diabetes and hypertension in the first analysis and between MHO and non-MHO groups in the second analysis were evaluated with Chi-squared test or t-test. We used multivariable logistic regression model to evaluate the independent correlates of absence of diabetes and hypertension in the first analysis and MHO in the second analysis and included the following variables as covariates: age, race, sex, steatosis and NASH. The model’s fit was tested using Likelihood-ratio test. Stratified analyses were performed by gender and race. Data were analyzed using STATA version 12.1 (College Station, Texas).
RESULTS
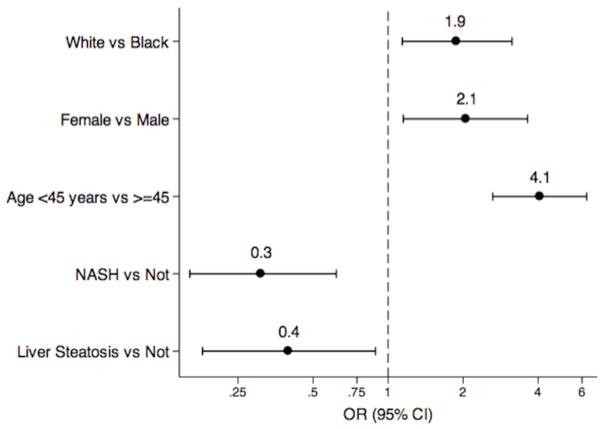
Among 523 subjects included in the first analysis, 78% were female, 77% were White and 48% were less than 45 years old. A total of 28.7% of the subjects did not have diabetes and hypertension and had a mean BMI of 49 kg/m2. The physical and metabolic characteristics of individuals with and without diabetes and hypertension are shown in Table 1. Gender, age, prevalence of NASH, liver fibrosis and steatosis were significantly different between the two groups (P< 0.05). No differences were noted between the two groups for race, BMI, smoking status or liver enzymes. The unadjusted results of the analyses examining the association between the potential correlates and absence of diabetes and hypertension are shown in Table 2. After multivariable adjustment, our results showed that White race, female gender and younger age were factors independently associated with absence of diabetes and hypertension (Figure 1 and Table 2): White race OR=1.9 (95% CI=1.1–3.1), female OR=2.1 (95% CI=1.2–3.6), and younger than 45 years old OR=4.1 (95% CI=2.6–6.3). In addition, patients with liver steatosis were 60% less likely (OR 0.4, 95% CI=0.2–0.9) and those with NASH 69% less likely (OR =0.3, CI=0.2–0.6) to be in this group of obese without diabetes and hypertension.
Table 1.
Physical and Metabolic Characteristics of 523 Consecutive Bariatric Surgery Patients according to Absence or Presence of Diabetes and Hypertension
| Absence of DM and HTN | Presence of DM and HTN | P value | |
|---|---|---|---|
| Variables | 150 (28.7%) | 373 (71.3%) | |
| Race | 0.494 | ||
| White | 118 (78.7%) | 283 (75.9%) | |
| Black | 32 (21.3%) | 90 (24.1%) | |
| Sex | <0.001 | ||
| Female | 132 (88.0%) | 276 (74.0%) | |
| Male | 18 (12.0%) | 97 (26.0%) | |
| Age (years) | <0.001 | ||
| <45 | 109 (72.7%) | 144 (38.6%) | |
| ≥45 | 41 (27.3%) | 229 (61.4%) | |
| BMI (kg/m2) | 0.301 | ||
| >30 to ≤40 | 14 (9.3%) | 50 (13.4%) | |
| >40 to ≤50 | 86 (57.3%) | 183 (49%) | |
| >50 to ≤60 | 38 (25.3%) | 101 (27%) | |
| >60 | 12 (8%) | 39 (10.5%) | |
| Current Smoker | 0.368 | ||
| No | 141 (94%) | 342 (91.7%) | |
| Yes | 9 (6%) | 31 (8.3%) | |
| Mean AST ±SD (U/L) | 21.4±10.6 | 25.6±15.7 | 0.003 |
| Mean ALT ±SD (U/L) | 27±17.4 | 32.3±22.3 | 0.008 |
| Liver Steatosis | |||
| No | 17 (11.3%) | 15 (4%) | 0.002 |
| Yes | 133 (88.7%) | 358 (96%) | |
| NASH | |||
| No | 139 (92.7%) | 291 (78%) | <0.001 |
| Yes | 11 (7.3%) | 82 (22%) | |
| Liver Fibrosis | 0.009 | ||
| No | 121 (80.7%) | 259 (69.4%) | |
| Yes | 29 (19.3%) | 114 (30.6%) | |
DM: diabetes, HTN: hypertension, BMI: body mass index, AST: aspartate aminotransferase
ALT: alanine aminotransferase, NASH: nonalcoholic steatohepatitis
Table 2.
Unadjusted and Adjusted Odds Ratios for Adults without Diabetes and Hypertension undergoing Bariatric Surgery
| Unadjusted OR | Adjusted* OR | |||||
|---|---|---|---|---|---|---|
| OR | 95%CI | P value | OR | 95% CI | P value | |
| White versus Black | 1.2 | 0.7–1.9 | 0.494 | 1.9 | 1.1–3.1 | 0.013 |
| Female Sex | 2.6 | 1.5–4.4 | 0.001 | 2.1 | 1.2–3.6 | 0.014 |
| Younger Age (<45 years) | 4.2 | 2.8–6.4 | <0.001 | 4.1 | 2.6–6.3 | <0.001 |
| NASH versus not | 0.3 | 0.2–0.5 | <0.001 | 0.3 | 0.2–0.6 | 0.001 |
| Any Steatosis versus none | 0.3 | 0.3–0.7 | 0.002 | 0.4 | 0.2–0.9 | 0.025 |
Adjusted for: Race, Sex, Age, NASH and Steatosis.
NASH: nonalcoholic steatohepatitis
Figure 1.
Odds of Individuals without Diabetes and Hypertension given Potential Correlates NASH: nonalcoholic steatohepatitis, OR: odds ratio
In the second analysis where we defined MHO phenotype as obesity without diabetes, hypertension and with fasting triglycerides less than 150 mg/dl, our findings remained very similar to the first analysis (Tables 3 and 4).
Table 3.
Physical and Metabolic Characteristics of 260 Consecutive Bariatric Surgery Patients according to MHO Status defined as obesity without diabetes, hypertension and elevated fasting triglycerides
| MHO | Non-MHO | P value | |
|---|---|---|---|
| Variables | 44 (16.9%) | 216 (83.1%) | |
| Race | 0.405 | ||
| White | 31 (70.5%) | 165 (76.4%) | |
| Black | 13(29.6%) | 51 (23.6%) | |
| Sex | 0.419 | ||
| Female | 37 (84.1%) | 170 (78.7%) | |
| Male | 7 (15.9%) | 46 (21.3%) | |
| Age (years) | <0.001 | ||
| <45 | 35(79.6%) | 83 (38.4%) | |
| ≥45 | 9 (20.4%) | 133(61.6%) | |
| BMI (kg/m2) | 0.539 | ||
| >30 to ≤40 | 5 (11.4%) | 29(13.4%) | |
| >40 to ≤50 | 19 (43.2%) | 114 (52.8%) | |
| >50 to ≤60 | 14 (31.8%) | 51 (23.6%) | |
| >60 | 6 (13.6%) | 22 (10.2%) | |
| Current Smoker | 0.557 | ||
| No | 42 (95.5%) | 201 (93.1%) | |
| Yes | 2 (4.5%) | 15 (6.9%) | |
| Mean AST ±SD (U/L) | 20.9±12.7 | 25.1±15.6 | 0.974 |
| Mean ALT ±SD (U/L) | 25.5±21.8 | 32±21.8 | 0.999 |
| Liver Steatosis | |||
| No | 7 (15.9%) | 8 (3.7%) | 0.002 |
| Yes | 37 (84.1%) | 208 (96.3%) | |
| NASH | |||
| No | 42 (95.4%) | 176 (81.5%) | 0.022 |
| Yes | 2(4.6%) | 40 (18.5%) | |
| Liver Fibrosis | 0.127 | ||
| No | 37 (84.1%) | 158 (73.1%) | |
| Yes | 7 (15.9%) | 58 (26.9%) | |
MHO: metabolically healthy obese, BMI: body mass index, AST: aspartate aminotransferase
ALT: alanine aminotransferase, NASH: nonalcoholic steatohepatitis
Table 4.
Unadjusted and Adjusted Odds Ratios for Metabolically Healthy Obesity defined as obesity without diabetes, hypertension and elevated fasting triglycerides
| Unadjusted OR | Adjusted* OR | |||||
|---|---|---|---|---|---|---|
| OR | 95%CI | P value | OR | 95%CI | P value | |
| White versus Black | 0.7 | 0.4–1.5 | 0.406 | 1.2 | 0.6–32.8 | 0.609 |
| Female Sex | 1.4 | 0.6–3.4 | 0.421 | 0.9 | 0.3–2.2 | 0.743 |
| Younger Age (<45 years) | 6.2 | 2.9–13.6 | <0.001 | 6.4 | 2.8–14.3 | <0.001 |
| NASH versus not | 0.2 | 0.1–0.9 | 0.036 | 0.2 | 0.1–1.0 | 0.054 |
| Any Steatosis versus none | 0.2 | 0.1–0.6 | 0.004 | 0.2 | 0.1–0.8 | 0.019 |
Adjusted for Race, Sex, Age, NASH and Steatosis.
MHO: metabolically healthy obese, NASH: nonalcoholic steatohepatitis
DISCUSSION
In this cross-sectional study of bariatric patients from our institution, we found that the prevalence of obese individuals without diabetes and hypertension was more than 25%. Furthermore, we found significant racial and gender differences, with Whites and females being more likely to have this phenotype compared to their counterparts as demonstrated in previous studies [8–10]. These racial and gender differences exist despite the fact that the current obesity epidemic in the USA affects more African-Americans (44.1%) compared to general population (34%) and that 40% of morbidly obese individuals are men [11]. Bariatric surgery is the most effective obesity treatment leading to significant improvements in obesity-related comorbidities such as diabetes and cardiovascular diseases and reduced mortality [12,13].
Our study showed that men and Blacks were less likely to be metabolically healthy when they undergo bariatric surgery. Despite the higher rates of obesity, African-Americans are half as likely to undergo bariatric surgery compared to Whites [14]. Similarly, fewer males, in particular African-American males (15%), undergo bariatric surgery compared to either White or Hispanic males (22 and 23% respectively) [15]. Prior studies have shown that men and African-Americans were older and had greater illness severity at the time of undergoing bariatric surgery, which suggests a delay in receiving bariatric surgery in these groups [15,16]. Given significant benefits of bariatric surgery particularly on diabetes, the ongoing sex and race differences of bariatric surgery performance suggest the potential barriers such as access to care, patient preference, cultural differences as well as other factors yet to be explored that influence who seeks and gets bariatric surgery.
To our knowledge, this is the first study that examines the association between liver injury, as determined by liver biopsy, the gold standard method for the evaluation of liver disease, with MHO phenotype. In prior studies, MHO phenotype has been associated with favorable hepatic markers including lower hepatic enzymes and lower liver fat content measured by 1H-MR spectroscopy [3,4]. Our results extend these findings by twofold. First, we document a high prevalence of liver steatosis and a moderately high prevalence of liver fibrosis and inflammation among those participants characterized as MHO and therefore disagree with the notion of MHO as a truly metabolically healthy entity. Second, our results showed that liver fibrosis and NASH on liver biopsy are strong correlates of non-MHO phenotype independent of the other variables. The high prevalence of obesity-related liver disease found in our study population is consistent with a similar recent study of liver biopsy results in a large cohort of bariatric surgery patients [17].
The clinical utility of MHO phenotype remains un-established, despite the increasing number of related studies. One contributing factor is the lack of a standard definition of MHO phenotype. The task of comparing the results of various studies on MHO remains a challenge with more than 15 different methods of defining MHO in the literature based on markers of insulin resistance, pro-atherogenic lipid profile, proinflammatory state, or hypertension [5]. The definition of MHO used in this study was based on the scope of our retrospective data: we defined MHO phenotype as absence of diabetes, hypertension and hypertriglyceridemia as defined in previous studies[18,19]. Another contributing factor is the recent evidence of the association of MHO with subclinical disease such as subclinical atherosclerosis and sonographic evidence of fatty liver, which questions whether MHO phenotype is truly a healthy phenotype [20,21]. In the future, establishing a consensus on the definition of MHO phenotype will facilitate furthering our understanding of MHO phenotype and its clinical implications.
The present study has several limitations. First, this is a cross-sectional study, which does not allow us to assess causal or temporal associations between the study correlates and MHO phenotype. Second, we had incomplete data on fasting lipids and no data on serum insulin, hemoglobin A1c or inflammatory markers, which limited our ability to look at other characterizations or definitions of MHO and only one blood pressure measurement was used to identify hypertension in those with no prior diagnosis. Our second analysis of individuals with fasting triglycerides data did not alter our findings in the first analysis, suggesting that the obese individuals without diabetes and hypertension in the first analysis likely capture the characteristics of MHO reported in literature. Third, we obtained a single sample of liver biopsy per patient given its invasiveness, which can lead to intra-individual variability in interpretation [22]. Fourth, all the patients were undergoing bariatric surgery, thus limiting our findings to this population.
Several strengths of our study include a relatively large and diverse sample, and availability of liver biopsy data to allow us to examine the relationship between liver injury and MHO phenotype.
In conclusion, almost a third of patients undergoing bariatric surgery did not have diabetes and hypertension and could be probably considered “MHO” and were more likely to be white, young, female and have less liver injury. The high prevalence of liver steatosis in MHO individuals among bariatric surgery patients challenges the notion of MHO as a truly metabolically healthy entity in the setting of significant obesity. Whether MHO phenotype leads to decreased morbidity and mortality remains unclear [23–25]. Better characterization and understanding of the prognostic significance of MHO phenotype in bariatric surgery may help tailor our approaches to preventing and treating obesity.
Acknowledgments
This research was supported by Grant Number KL2 (1KL2TR001077-01) as part of the Institute for Clinical and Translational Research Grant from the NIH/NCAT.
Footnotes
Disclosure: The authors have no commercial associations that might be a conflict of interest in relation to this article.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Brochu M, Tchernof A, Dionne IJ, et al. What are the physical characteristics associated with a normal metabolic profile despite a high level of obesity in postmenopausal women? J Clin Endocrinol Metab. 2001;86:1020–5. doi: 10.1210/jcem.86.3.7365. [DOI] [PubMed] [Google Scholar]
- 2.Karelis AD, Faraj M, Bastard JP, et al. The metabolically healthy but obese individual presents a favorable inflammation profile. J Clin Endocrinol Metab. 2005;90:4145–50. doi: 10.1210/jc.2005-0482. [DOI] [PubMed] [Google Scholar]
- 3.Stefan N, Kantartzis K, Machann J, et al. Identification and characterization of metabolically benign obesity in humans. Arch Intern Med. 2008;168:1609–16. doi: 10.1001/archinte.168.15.1609. [DOI] [PubMed] [Google Scholar]
- 4.Messier V, Karelis AD, Robillard ME, et al. Metabolically healthy but obese individuals: Relationship with hepatic enzymes. Metabolism. 2010;59:20–4. doi: 10.1016/j.metabol.2009.06.020. [DOI] [PubMed] [Google Scholar]
- 5.Primeau V, Coderre L, Karelis AD, et al. Characterizing the profile of obese patients who are metabolically healthy. Int J Obes (Lond) 2011;35:971–81. doi: 10.1038/ijo.2010.216. [DOI] [PubMed] [Google Scholar]
- 6.Bouhours-Nouet N, Dufresne S, de Casson FB, et al. High birth weight and early postnatal weight gain protect obese children and adolescents from truncal adiposity and insulin resistance: Metabolically healthy but obese subjects? Diabetes Care. 2008;31:1031–6. doi: 10.2337/dc07-1647. [DOI] [PubMed] [Google Scholar]
- 7.Westerbacka J, Corner A, Kannisto K, et al. Acute in vivo effects of insulin on gene expression in adipose tissue in insulin-resistant and insulin-sensitive subjects. Diabetologia. 2006;49:132–40. doi: 10.1007/s00125-005-0075-5. [DOI] [PubMed] [Google Scholar]
- 8.Bonora E, Kiechl S, Willeit J, et al. Prevalence of insulin resistance in metabolic disorders: The bruneck study. Diabetes. 1998;47:1643–9. doi: 10.2337/diabetes.47.10.1643. [DOI] [PubMed] [Google Scholar]
- 9.Wildman RP, Muntner P, Reynolds K, et al. The obese without cardiometabolic risk factor clustering and the normal weight with cardiometabolic risk factor clustering: Prevalence and correlates of 2 phenotypes among the US population (NHANES 1999–2004) Arch Intern Med. 2008;168:1617–24. doi: 10.1001/archinte.168.15.1617. [DOI] [PubMed] [Google Scholar]
- 10.Jimenez A, Perea V, Corcelles R, Moize V, Lacy A, Vidal J. Metabolic effects of bariatric surgery in insulin-sensitive morbidly obese subjects. Obes Surg. 2013;23:494–500. doi: 10.1007/s11695-012-0817-7. [DOI] [PubMed] [Google Scholar]
- 11.Flegal KM, Kit BK, Orpana H, Graubard BI. Association of all-cause mortality with overweight and obesity using standard body mass index categories: A systematic review and meta-analysis. JAMA. 2013;309:71–82. doi: 10.1001/jama.2012.113905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sjostrom L. Bariatric surgery and reduction in morbidity and mortality: Experiences from the SOS study. Int J Obes (Lond) 2008;32 (Suppl 7):S93–7. doi: 10.1038/ijo.2008.244. [DOI] [PubMed] [Google Scholar]
- 13.Buchwald H, Estok R, Fahrbach K, et al. Weight and type 2 diabetes after bariatric surgery: Systematic review and meta-analysis. Am J Med. 2009;122:248–256. e5. doi: 10.1016/j.amjmed.2008.09.041. [DOI] [PubMed] [Google Scholar]
- 14.Martin M, Beekley A, Kjorstad R, Sebesta J. Socioeconomic disparities in eligibility and access to bariatric surgery: A national population-based analysis. Surg Obes Relat Dis. 2010;6:8–15. doi: 10.1016/j.soard.2009.07.003. [DOI] [PubMed] [Google Scholar]
- 15.Sudan R, Winegar D, Thomas S, Morton J. Influence of ethnicity on the efficacy and utilization of bariatric surgery in the USA. J Gastrointest Surg. 2014;18:130–6. doi: 10.1007/s11605-013-2368-1. [DOI] [PubMed] [Google Scholar]
- 16.Tiwari MM, Goede MR, Reynoso JF, Tsang AW, Oleynikov D, McBride CL. Differences in outcomes of laparoscopic gastric bypass. Surg Obes Relat Dis. 2011;7:277–82. doi: 10.1016/j.soard.2011.02.005. [DOI] [PubMed] [Google Scholar]
- 17.Kleiner DE, Berk PD, Hsu JY, et al. Hepatic pathology among patients without known liver disease undergoing bariatric surgery: Observations and a perspective from the longitudinal assessment of bariatric surgery (LABS) study. Semin Liver Dis. 2014;34:98–107. doi: 10.1055/s-0034-1371083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aguilar-Salinas CA, Garcia EG, Robles L, et al. High adiponectin concentrations are associated with the metabolically healthy obese phenotype. J Clin Endocrinol Metab. 2008;93:4075–9. doi: 10.1210/jc.2007-2724. [DOI] [PubMed] [Google Scholar]
- 19.Lynch LA, O’Connell JM, Kwasnik AK, Cawood TJ, O’Farrelly C, O’Shea DB. Are natural killer cells protecting the metabolically healthy obese patient? Obesity (Silver Spring) 2009;17:601–5. doi: 10.1038/oby.2008.565. [DOI] [PubMed] [Google Scholar]
- 20.Chang Y, Kim BK, Yun KE, et al. Metabolically healthy obesity and coronary artery calcification. J Am Coll Cardiol. 2014 doi: 10.1016/j.jacc.2014.03.042. [DOI] [PubMed] [Google Scholar]
- 21.Heianza Y, Arase Y, Tsuji H, et al. Metabolically healthy obesity, presence or absence of fatty liver, and risk of type 2 diabetes in japanese individuals: Toranomon hospital health management center study 20 (TOPICS 20) J Clin Endocrinol Metab. 2014:jc20134427. doi: 10.1210/jc.2013-4427. [DOI] [PubMed] [Google Scholar]
- 22.Ratziu V, Charlotte F, Heurtier A, et al. Sampling variability of liver biopsy in nonalcoholic fatty liver disease. Gastroenterology. 2005;128:1898–1906. doi: 10.1053/j.gastro.2005.03.084. [DOI] [PubMed] [Google Scholar]
- 23.Kuk JL, Ardern CI. Are metabolically normal but obese individuals at lower risk for all-cause mortality? Diabetes Care. 2009;32:2297–9. doi: 10.2337/dc09-0574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van der ADL, Nooyens AC, van Duijnhoven FJ, Verschuren WM, Boer JM. All-cause mortality risk of metabolically healthy abdominal obese individuals: The EPIC-MORGEN study. Obesity (Silver Spring) 2014;22:557–64. doi: 10.1002/oby.20480. [DOI] [PubMed] [Google Scholar]
- 25.Kramer CK, Zinman B, Retnakaran R. Are metabolically healthy overweight and obesity benign conditions?: A systematic review and meta-analysis. Ann Intern Med. 2013;159:758–69. doi: 10.7326/0003-4819-159-11-201312030-00008. [DOI] [PubMed] [Google Scholar]