Abstract
Objective
To investigate the incidence and pre-operative risk factors for developing pelvic pain after hysteroscopic sterilization using the Essure™ micro-inserts
Design
Retrospective cohort study (Canadian Task Force classification II-2).
Setting
University Medical Center
Patients
A total of 458 patients who underwent hysteroscopic sterilization with Essure™ between January 1, 2005 and June 30, 2012.
Results
The incidence of acute pelvic pain after hysteroscopic sterilization was 8.1%, and the incidence of persistent pain after 3 months post-procedure was 4.2%. The range of presentation with pain was 1 to 469 days, with a mean time of 56 days. Of the patients that developed chronic pelvic pain after the procedure, 75% presented within 130 days of the procedure. Patients with previous diagnoses of any chronic pain (chronic pelvic pain, chronic low back pain, chronic headache, and fibromyalgia) were more likely to report both acute pain (OR 6.81, 95% CI 2.95,15.73) and chronic pain (OR 6.15, 95% CI 2.10,18.10) after hysteroscopic sterilization.
Conclusions
Pelvic pain may develop after hysteroscopic sterilization. Patients with preexisting chronic pain diagnoses may be at increased risk of developing pelvic pain after the procedure. Fifty percent of new pelvic pain after Essure™ placement will resolve by 3 months.
Keywords: chronic pelvic pain, hysteroscopic sterilization, Essure™
Introduction
Since approval by the FDA in 2002, hysteroscopic sterilization with the Essure™ micro-insert (Bayer Healthcare Pharmaceuticals, Whippany, NJ) has become a popular, safe, and reliable choice for women who desire a permanent form of contraception. It has gained favor partly because it can be completed without incisions, in the operating room or office setting. The rate of successful placement on initial attempt ranges from 92-96% (1,2). Once successfully placed, the pregnancy rate is approximately 0.2% (3). Most women who undergo Essure™ placement are very satisfied with their choice and report no long-term complications (4). However, there have been case reports of chronic pelvic pain after Essure™ placement.
To date, there is limited long-term data regarding the incidence of new onset pelvic pain after Essure™ placement. There is no data regarding pre-operative risk factors for the development of chronic pelvic pain after this procedure. Currently available information on Essure-related complications is limited to case reports, post-procedure questionnaires, pre-market device development information, and patient or provider-initiated reporting. The Manufacturer and User Facility Device Experience (MAUDE) database contains voluntary reports of device complications by consumers and providers. Using the MAUDE database between January 2004 and January 2009, one report found 20 cases of pelvic pain after Essure™ placement (3). The causes cited for pain included malposition of device, cornual perforation, multiple micro-inserts placed in a single fallopian tube, and complications with concomitant ablation (3). Additional case reports noted three patients with pain severe enough to require implant removal (5,6).
Given the widespread use of Essure™ as a method of contraception and the current lack of information on post-Essure™ complications, we chose to investigate this further in a large University-based practice. The aim of our study was to determine the incidence of post-Essure™ pelvic pain and any related risk factors.
Materials and Methods
All patients who underwent a hysteroscopic sterilization procedure at Vanderbilt University Medical Center between January 1, 2005 and June 30, 2012 were identified using an institutional electronic medical record database. A total of 509 patients were identified for retrospective chart review, and 458 patients met criteria for inclusion in this study. Patients were included in the study if they underwent bilateral hysteroscopic sterilization using Essure™ micro-insert in the operating room or office setting. Patients were excluded from the study if they underwent hysteroscopic sterilization with a device other than Essure™, did not have successful placement of one or both Essure™ coils, or underwent conversion to laparoscopic sterilization.
Data were collected by chart review and included demographic data (age, race, body mass index), medical history (metal allergy, multiple allergies, parity, number of vaginal deliveries, previous sexually transmitted infection, previous pelvic surgery, and previous chronic pain condition including chronic pelvic pain, chronic low back pain, chronic headache, and fibromyalgia), procedure characteristics (place of surgery, experience of surgeon, type of anesthesia), and follow-up information (confirmation of bilateral tubal occlusion with hysterosalpingogram, report of pelvic pain, timing of pelvic pain).
Acute pain after hysteroscopic sterilization was defined as new pelvic pain, beginning after the procedure, and lasting up to 3 months post-operatively. Chronic pain was defined as pain lasting more than 3 months after procedure. Patient and operative characteristics were compared between those without pain and those with acute pain. The same was repeated comparing those without pain and those with chronic pain. Statistical analysis was performed using chi-square, Fisher’s exact, and independent t-tests where appropriate. Multivariate analysis was completed to adjust for confounders. Variables with p < 0.05 were considered statistically significant. All statistical analyses were conducted using STATA v. 11 (College Station, TX).
The Vanderbilt University Medical Center Institutional Review Board approved this study.
Results
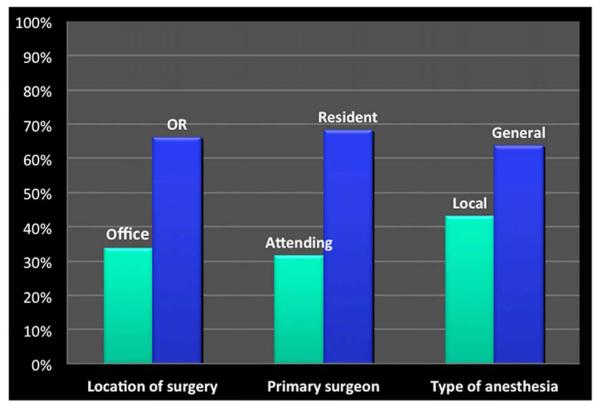
A total of 458 patients met inclusion criteria for the study. Demographics, medical history and follow up with hysterosalpingogram are listed in Table 1. Only 67.0% of patients completed a follow-up hysterosalpingogram; and of those, 93.5% confirmed bilateral occlusion of the fallopian tubes. The majority of procedures were completed by a supervised resident in the operating room under general anesthesia (Figure 1).
Table 1.
Demographics, medical history and follow up
| Variable | Mean/Median (SD) or % |
|---|---|
| Age | 32.4/32 (6.5) |
| Race | |
| White | 45.0% |
| Black | 43.7% |
| Hispanic | 2.4% |
| Asian | 3.1% |
| Other/unknown | 5.0% |
| BMI | 30.5/32.3 (8.9) |
| Parity | 2.9/3 (1.3) |
| Vaginal deliveries | 2.1/2 (0.4) |
| Previous chronic pain condition | 8.7% |
| Previous pelvic surgery | 28.0% |
| Previous sexually transmitted infection | 15.9% |
| Metal allergy | 0.9% |
| Multiple allergies | 2.2% |
| Completion of HSG | 67.0% |
| Confirmed bilateral occlusion on HSG | 93.5% |
HSG = hysterosalpingogram
Figure 1.
Classification of procedures by location, primary surgeon, and type of anesthesia used.
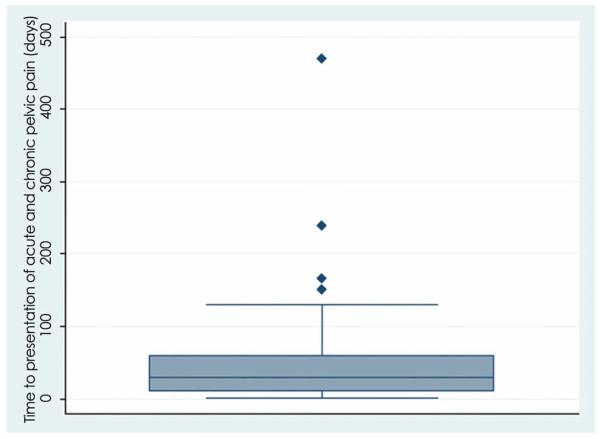
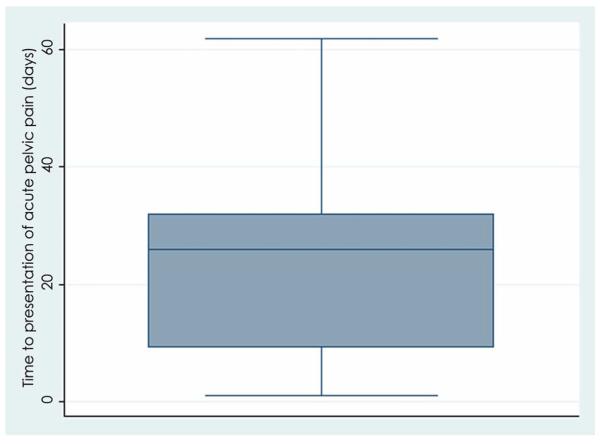
The incidence of acute pelvic pain after hysteroscopic sterilization was 8.1%, and the incidence of persistent pain 3 months or greater after hysteroscopic sterilization was 4.2%. The mean time from procedure to presentation with pain, for all subjects with new pelvic pain, was 56 days and ranged from 1 to 469 days. The majority (75%) of those with acute pain presented within 32 days of the procedure. Of those who developed chronic pelvic pain after hysteroscopic sterilization, 75% presented within 130 days of the procedure (Figures 2a and 2b).
Figure 2.
Time (in days) to presentation with pain.
Patients with a previous diagnosis of chronic pain (chronic pelvic pain, chronic low back pain, chronic headache, or fibromyalgia) were more likely to report both acute pain (OR 6.81, 95% CI 2.95,15.73) and chronic pain (OR 6.15, 95% CI 2.09,18.05) after hysteroscopic sterilization (Table 2). Having a prior sexually transmitted infection was associated with acute, but not chronic, pain (OR 2.33, 95% CI 1.01,5.39). In our cohort, a history of a metal allergy or multiple medical allergies was associated with a decreased risk of developing chronic pelvic pain after Essure™ (OR 0.08, 95% CI 0.01,0.85; OR 0.18, 95% CI 0.04,0.88). However, it should be noted that the representation in these categories is minimal (n=4, n=10).
Table 2.
Associated risk factors for development of acute and chronic pain after Essure
| Variable | Unadjusted OR (CI) acute pain |
Adjusted OR (CI) acute pain |
Unadjusted OR (CI) chronic pain |
Adjusted OR (CI) chronic pain |
|---|---|---|---|---|
| Age | 0.98 (0.93, 1.03) | NS | 0.99 (0.92, 1.06) | NS |
| Race | 0.95 (0.65, 1.40) | NS | 0.76 (0.41, 1.39) | NS |
| BMI | 1.00 (0.96, 1.04) | NS | 0.99 (0.93, 1.04) | NS |
| Parity | 1.07 (0.82, 1.40) | NS | 0.90 (0.61, 1.34) | NS |
| Previous chronic pain condition | 6.74 (3.06, 14.81) | 6.81 (2.95, 15.73) | 7.18 (2.65, 19.46) | 6.15 (2.09, 18.05) |
| Previous pelvic surgery | 0.72 (0.36, 1.44) | NS | 0.86 (0.32, 2.27) | NS |
| Previous sexually transmitted infection | 1.79 (0.81, 3.98) | 2.33 (1.01, 5.39) | 0.99 (0.28, 3.48) | NS |
| Metal allergy | 0.26 (0.03, 2.55) | NS | 0.12 (0.01, 1.25) | 0.08 (0.01, 0.85) |
| Multiple allergies | 0.19 (0.05, 0.77) | 0.41 (0.09, 1.90) | 0.09 (0.02, 0.37) | 0.18 (0.04, 0.88) |
| Location of surgery | 0.57 (0.29, 1.13) | NS | 0.87 (0.34, 2.26) | NS |
| Primary surgeon | 0.74 (0.37, 1.49) | NS | 2.55 (0.73, 8.89) | NS |
NS = not significant
A history of pelvic surgery was not associated with an increased risk of chronic pain after Essure™ placement. This category was well-represented with 28% of the cohort.
Discussion
In this retrospective cohort study, the incidence of acute pelvic pain after Essure™ sterilization was 8.1%, and 4.2% had persistent pain three or more months after the procedure. While the pivotal trial on Essure™ found that 99% of participants rated their comfort as “good to excellent” at all follow up visits up to one year after Essure™ placement, this trial excluded women with any prior pelvic disease, severe dysmenorrhea, or any chronic pain (2). This trial was also performed under “ideal” conditions, with highly trained surgeons and optimized patients.
A prospective study by Kerin, et al found 13% of patients reported dysmenorrhea and 9% reported dyspareunia in the first 3 months after hysteroscopic sterilization, but 96% reported their tolerance of the micro-inserts as “good to excellent” at three and 24 months (7). Thus, 4% of patients in the Kerin study were unable to report tolerance of the device after 3 months. This is very similar to our reported incidence of chronic pelvic pain after Essure™. Also similar to our incidence of acute pain, Sinha, et al found that 6% of participants reported new pain or discomfort when surveyed three months after hysteroscopic sterilization (4).
Our data suggests that pain can develop both immediately after the procedure and more remotely, even weeks to months later. Since the timing of pain is measured as time from procedure to time of reported pain in the medical record, it is not possible to identify the exact timing of onset of pain. This would likely represent an overestimate in the time between procedure and onset of pain. Still, the majority of patients with documented pelvic pain reported pain within 130 days of the procedure. While it cannot be definitively determined that the hysteroscopic sterilization procedure or the micro-inserts are the cause of pain, this close temporal relationship makes it probable. Pain after hysteroscopic sterilization has been reported in the setting of tubal perforation or misplacement of the devices, but it has also been reported in patients with appropriately placed devices (5-7). The majority (91%) of patients in our study with pain presented after appropriate tubal occlusion confirmed on hysterosalpingogram. This suggests that the pain may be associated with the presence of a foreign body, possibly warranting the removal of the devices. It may also suggest that a hysterosalpingogram, while radiographically identifying bilateral device placement and occlusion, may not be able to discern small perforations or malposition.
Our data also suggests that a significant percentage of new acute pain (50%) will resolve by 3 months post-procedure. This should be reassuring for patients and most practitioners, and can aid in counseling patients who may desire removal immediately after placement.
To our knowledge, this is the first study to examine risk factors for developing pain after hysteroscopic sterilization. We found a 6-fold increase in the risk of both acute and chronic pain after hysteroscopic sterilization with Essure™ in women with a previous diagnosis of chronic pain conditions. These conditions included chronic pelvic pain, chronic low back pain, chronic headache, or fibromyalgia. Multiple studies have demonstrated that patients with other chronic pain conditions are more likely to develop chronic pelvic pain in general (8-11). The timing of the development of pain and the strength of the association suggests that those with chronic pain syndromes may be predisposed to the development of pain after hysteroscopic sterilization with Essure™.
Our study population is limited to a single academic medical center, which may not be applicable to other populations. However, the cohort is large and inclusive of all patients who underwent bilateral placement of Essure™. Data is limited to retrospective chart review, which introduces recall and reporting bias and may not identify other confounding factors. Additionally, within our study population, 33% did not obtain a 3-month post-Essure HSG. It can be assumed that the majority of these patients were lost to follow-up, and their missing data may influence the results. A few of these patients did not undergo post-Essure HSG because they underwent Essure coil removal for pain prior to the 3-month time period. For the remainder, though, they were included in the analysis and assumed to be symptom-free, so as not to erroneously inflate the results. Since incidence data relies on both the patient reporting pain to their provider and the provider recording this in the medical record, the reported results are likely an underestimate rather than an overestimate of pain incidence.
For clarification, a sub-set analysis was performed in those with a follow-up HSG. The incidence of acute and chronic pelvic pain is this group was 7.5% and 4.1%, respectively. This is very similar to the reported results for the entire cohort.
Grimes and Schulz suggest that weak associations of OR less than 4 or greater than 0.25 in observational studies should not be considered credible (12). In contrast, OR greater than 4 or less than 0.25 suggest an association of potential interest that merits further investigation. Since our OR of 6 shows a strong association, it may indicate that chronic pain syndromes are a risk factor for pain after hysteroscopic sterilization. We suggest caution in performing this procedure on patients with a history of chronic pain and counseling patients that pelvic pain may develop after the procedure. Future prospective studies should focus on chronic pain as a risk factor for development of new pain after hysteroscopic sterilization.
Acknowledgments
Study utilized REDCap database with grant support from UL1 TR000445 from NCATS/NIH.
Footnotes
The authors report no conflict of interest.
Findings were presented at the 2013 International Pelvic Pain Society Annual Fall Meeting on Pelvic Pain, Orlando, Florida, October 17-20, 2013.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Levie MD, Chudnoff SG. Prospective analysis of office-based hysteroscopic sterilization. J Minim Invasive Gynecol. 2006;13:98–101. doi: 10.1016/j.jmig.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 2.Cooper JM, Carignan CS, Cher D, Kerin JF. Microinsert nonincisional hysteroscopic sterilization. Obstet Gynecol. 2003;102:59–67. doi: 10.1016/s0029-7844(03)00373-9. [DOI] [PubMed] [Google Scholar]
- 3.Connor VF. Essure: A review six years later. J of Minim Invasive Gynecol. 2009;16:282–90. doi: 10.1016/j.jmig.2009.02.009. [DOI] [PubMed] [Google Scholar]
- 4.Sinha D, Kalathy V, Gupta JK, Clark TJ. The feasibility, success and patient satisfaction associated with outpatient hysteroscopic sterilisation. BJOG. 2007;114(6):676–83. doi: 10.1111/j.1471-0528.2007.01351.x. [DOI] [PubMed] [Google Scholar]
- 5.Beckwith AW. Persistent pain after hysteroscopic sterilization with microinserts. Obstet Gynecol. 2008;111:511–2. doi: 10.1097/01.AOG.0000299878.28166.ba. [DOI] [PubMed] [Google Scholar]
- 6.Lannon BM, Lee SY. Techniques for removal of the Essure hysteroscopic tubal occlusion device. Fertil Steril. 2007;88:497.e13–4. doi: 10.1016/j.fertnstert.2006.11.072. [DOI] [PubMed] [Google Scholar]
- 7.Kerin JF, Cooper JM, Price T, Herendael BJ, Cayuela-Font E, Cher D, Carignan CS. Hysteroscopic sterilization using a micro-insert device: results of a multicentre Phase II study. Hum Reprod. 2003;18(6):1223–30. doi: 10.1093/humrep/deg256. [DOI] [PubMed] [Google Scholar]
- 8.Gordon A, Paneduro D, Pink L, Lawler V, Lay C. Evaluation of the frequency and the association of sexual pain and chronic headaches. Headache. 2014;54(1):109–15. doi: 10.1111/head.12271. [DOI] [PubMed] [Google Scholar]
- 9.Karp BI, Sinaii N, Nieman LK, Silberstein SD, Stratton P. Migraine in women with chronic pelvic pain with and without endometriosis. Fertil Steril. 2011;95(3):895–9. doi: 10.1016/j.fertnstert.2010.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van Os-Bossagh P, Pols T, Hop WC, Nelemans T, Erdmann W, Drogendijk AC, Bohnen AM. Questionnaire as diagnostic tool in chronic pelvic pain (CPP): a pilot study. Eur J Obstet Gynecol Reprod Biol. 2002;103(2):173–8. doi: 10.1016/s0301-2115(02)00041-6. [DOI] [PubMed] [Google Scholar]
- 11.Warren JW, Langenberg P, Clauw DJ. The number of existing functional somatic syndromes (FSSs) is an important risk factor for new, different FSSs. J Psychosom Res. 2013;74(1):12–7. doi: 10.1016/j.jpsychores.2012.09.002. [DOI] [PubMed] [Google Scholar]
- 12.Grimes DA, Schulz KF. False alarms and pseudo-epidemics: the limitations of observational epidemiology. Obstet Gynecol. 2012;120(4):920–7. doi: 10.1097/AOG.0b013e31826af61a. [DOI] [PubMed] [Google Scholar]