Abstract
Background
This study examined the effects of atomoxetine (ATX) and OROS methylphenidate (MPH) on laboratory measures of inhibitory control and attention in youth with attention-deficit/hyperactivity disorder (ADHD). It was hypothesized that performance would be improved by both treatments, but response profiles would differ because the medications work via different mechanisms.
Methods
102 youth (77 male; mean age = 10.5 +/− 2.7 years) with ADHD received ATX (1.4 +/− 0.5 mg/kg) and MPH (52.4 +/− 16.6 mg) in a randomized, double-blind, crossover design. Medication was titrated in 4–6-week blocks separated by a 2-week placebo washout. Inhibitory control and attention measures were obtained at baseline, following washout, and at the end of each treatment using Conners’ Continuous Performance Test II (CPT-II), which provided age-adjusted T-scores for reaction time (RT), reaction time variability (RT variability), and errors. Repeated measures analyses of variance were performed, with Time (pre-medication, post-medication) and Treatment type (ATX, MPH) entered as within-subject factors. Data from the two treatment blocks were checked for order effects and combined if order effects were not present.
Clinical trial registration
Clinicaltrials.gov:NCT00183391
Results
Main effects for Time on RT (p = 0.03), RTSD (p = 0.001) and omission errors (p = 0.01) were significant. A significant Drug × Time interaction indicated that MPH improved RT, RTSD and omission errors more than ATX (p <0.05). Changes in performance with treatment did not correlate with changes in ADHD symptoms.
Conclusions
MPH has greater effects than ATX on CPT measures of sustained attention in youth with ADHD. However, the dissociation of cognitive and behavioral change with treatment indicates that CPT measures cannot be considered proxies for symptomatic improvement. Further research on the dissociation of cognitive and behavioral endpoints for ADHD is indicated.
Keywords: Attention-Deficit/Hyperactivity Disorder, Atomoxetine, Methylphenidate, Attention, Vigilance
Introduction
The robust efficacy and safety of psychostimulants for the treatment of Attention-deficit/Hyperactivity Disorder (ADHD) has been well documented (Conners, 2002). However, there is considerable variability in response, and only a subgroup of those treated achieve optimized functioning (Conners et al., 2001; Rapport, Denney, DuPaul, & Gardner, 1994). Furthermore, there are persistent and increasing concerns regarding diversion and abuse of stimulants (McCabe & West, 2013). Thus, there is considerable interest in identifying non-stimulant alternatives for ADHD. Atomoxetine (ATX), a US Food and Drug Administration (FDA) approved non-stimulant medication for ADHD, has demonstrated efficacy in reducing ADHD symptoms in numerous controlled trials (Michelson et al., 2002). Similar to stimulants, ATX is an inhibitor of the norepinephrine transporter (NET), which increases extracellular concentrations of norepinephrine diffusely and dopamine in prefrontal cortex (Bolden-Watson & Richelson, 1993; Gatley, Pan, Chen, Chaturvedi, & Ding, 1996; Newcorn et al., 2008). However, whereas stimulants enhance dopamine neurotransmission in striatum and a variety of other brain regions, the effects of ATX on dopamine are selective to prefrontal cortex (Berridge et al., 2006; Bymaster et al., 2002; Moron, Brockington, Wise, Rocha, & Hope, 2002). ATX has been shown to improve attention (Navarra et al., 2008) as well as inhibitory control and working memory in animals (Gamo, Wang, & Arnsten, 2010). The augmentation of prefrontal cortex function is likely the route by which ATX influences these aspects of cognitive functioning (Gamo et al., 2010). However, the degree to which cognitive effects follow reports of clinical improvement has yet to be established in humans.
Many studies have reported positive effects of MPH on a range of cognitive processes. A recent meta-analysis reviewing 60 studies in children and adolescents with ADHD demonstrated that MPH was superior to placebo in improving executive and non-executive memory, reaction time, reaction time variability, and response inhibition (Coghill et al., 2013). These findings were similar to those from an earlier review which reported that 63.5% of studies identified some improvement in cognitive function following treatment with immediate-release MPH in children with ADHD (Pietrzak et al., 2008).
Recent investigations examining the effects of ATX on measures of inhibitory control in healthy adults have shown mixed results, with some finding improvement (e.g., Chamberlain et al., 2008) and others showing either no effect (Nandam et al., 2010) or even a decline in performance (Graf et al., 2010). In adults with ADHD, ATX improved inhibitory control, as measured by the Stroop test (Spencer et al., 1998), faster stopping reaction times on the Stop Signal Task, and fewer commission errors on a sustained attention task (Chamberlain et al., 2007). Few studies have examined effects of ATX on cognitive functioning in children with ADHD. Improvement on a test of sustained attention was reported following acute administration of 20 mg of ATX in 50 children with ADHD (Barry et al., 2009). However, a study of youth with ADHD (n=16), reading disability (RD) (n=21), ADHD+RD (n=20) and normal controls (n=26) showed ATX-related improved inhibition only in those with ADHD+RD (de Jong et al., 2009). Finally, an open-label, dose-ranging study of 30 children with ADHD reported no effects of ATX on sustained attention as measured by Conners’ Continuous Performance Test (CPT) (Spencer et al., 2001). Importantly, sample sizes in existing child studies were limited and none of the trials were controlled.
ATX is structurally unrelated to stimulants and works via a distinct mechanism. Yet, while there is some information regarding comparative efficacy and differential response, there is little available information regarding differential cognitive effects of the two medications. In a large placebo-controlled, double-blind study, youth with ADHD received ATX, osmotic-release oral system (OROS) methylphenidate (OROS MPH), or placebo for six weeks (Newcorn et al., 2008). Both medications separated from placebo on parent ratings of behavior, as expected, but response was greater for OROS MPH than ATX, with an effect size difference of approximately 0.2 between the two treatments. However, no cognitive measures of improvement were collected. Animal studies suggest cognitive improvement from both medications (e.g., Gamo et al., 2010). In contrast, a small (n=24) randomized, double-blind, placebo-controlled crossover study in healthy men found improved inhibitory control following MPH, but not ATX (Nandam et al., 2010).
Numerous studies have utilized neuropsychological measures of attention and inhibitory control, such as the CPT, as purported objective indices of ADHD symptoms (Reinecke, Beebe, & Stein, 1999). Although the latter point has not been conclusively demonstrated, individuals with ADHD generally perform poorer than normal controls (Losier, McGrath, & Klein, 1996). However, CPT error profiles are not specific to those with ADHD (Dolan, Stone, & Briggs, 2010; Liu et al., 2011). Some studies have suggested utility of CPT indices for demonstrating medication response. For example, in 316 children from the Multimodal Treatment Study of Children with ADHD (MTA), medication treatment yielded more accurate, faster and less variable responding on CPT measures (Epstein et al., 2006). There have been no randomized and/or placebo-controlled studies with ATX, with conflicting effects on CPT performance reported from one open-label and one acute challenge study (Barry et al., 2009; Spencer et al., 2001). Recently, a small open-label study of children with ADHD randomized to receive ATX or OROS MPH (i.e., n = 15 per group) for 12 weeks demonstrated that OROS MPH was more effective than ATX in improving inhibitory control as indexed by the Stroop test (Yildiz, Sismanlar, Memik, Karakaya, & Agaoglu, 2010).
This study tested whether ATX and OROS MPH improve performance on purported laboratory measures of attention and inhibitory control as assessed by Conners’ CPT II. It was hypothesized that performance on the Conners’ CPT II would be improved by both treatments, but the nature and/or magnitude of response might differ by drug – based on data suggesting that the two medications work via somewhat different mechanisms (Schulz et al., 2012). We also hypothesized that ATX would produce smaller improvements on the Conners’ CPT II than MPH at the group level, based on the limited comparator data available to date (e.g., Yildiz et al., 2010). Additionally we examined the relationship between cognitive change and symptomatic improvement. No study to date has directly compared the effects of MPH and ATX on inhibitory control and sustained attention in a large sample of youth with ADHD.
Method
Participants
Participants, ages 6–17 years, were recruited in two urban areas (New York City and Chicago). Parents gave written consent and youth gave verbal assent to participate; the study was approved by the Institutional Review Boards at the participating institutions. The trial was registered at ClinicalTrials.gov (NCT00183391). All youth had a DSM-IV diagnosis of ADHD, any subtype, and participated in a larger crossover trial to evaluate comparative efficacy/tolerability and predictors of response to OROS MPH and ATX. Exclusionary criteria were: WISC-IV full-scale IQ below 75, non-English speaking parent or child, neurological dysfunction, systemic medical illness, uncorrected sensory impairments, and history of psychosis or bipolar disorder. Other comorbidity was permitted provided ADHD was the primary disorder and the comorbid condition did not require medication treatment. Participants may have been previously treated with ATX or MPH, but must not have been non-responders to an adequate trial and must not have experienced disabling adverse effects with either medication. Most participants were medication naïve (65%).
Participant characteristics are displayed in Table 1. The sample consisted of 102 6–17 year-old youth (mean age = 10.5 ± 2.7 years). Approximately two-thirds were male, and the majority had either Combined or Inattentive Type ADHD.
Table 1.
Participant Characteristics.
| Total Sample (n=102) | |
|---|---|
| Age (range), yr | 10.5 ± 2.7 (6–17) |
| Gender, n (% male) | 75 (74%) |
| Full-Scale IQ, n (range)* | 96.5 ± 13.0 (70–126) |
| Ethnicity, n (%) | |
| African-American | 32 (31%) |
| Asian | 1 (1%) |
| Caucasian | 37 (36%) |
| Hispanic | 20 (20%) |
| Biracial/Other | 13 (12%) |
| ADHD DSM-IV Subtype, n (%) | |
| Inattentive | 37 (37%) |
| Hyperactive/Impulsive | 3 (3%) |
| Combined | 61 (60%) |
| Baseline ADHD-RS, mean (SD) | |
| Hyperactive/Impulsive Total | 18.2 ± 7.3 |
| Inattentive Total | 22.0 ± 5.0 |
| Comorbid ODD, n (%) | 35 (34%) |
| Previously medicated (%) | 36 (35%) |
| ATX Final Dose, n (%) | |
| 0.5 mg/kg | 12% |
| 1.0 mg/kg | 22% |
| 1.4 mg/kg | 19% |
| 1.8 mg/kg | 47% |
| MPH Final Dose, n (%) | |
| 18 mg | 7% |
| 36 mg | 27% |
| 54 mg | 35% |
| 72 mg | 31% |
| Mean Final Dose, mean (SD) | |
| ATX | 1.4 (0.46) mg/kg |
| MPH | 52.4 (16.6) mg |
Inclusion criteria allowed individuals to participate if their General Ability Index was greater than 75 when, in the opinion of the psychologist or psychiatrist, their Full Scale IQ did not adequately represent their intellectual potential. ADHDRS = Attention-Deficit/Hyperactivity Disorder Rating Scale; ATX = atomoxetine; MPH = Methylphenidate; ODD = oppositional defiant disorder
Diagnostic Assessment
ADHD diagnosis was determined by K-SADS-PL interview, during which we systematically evaluated each of the 18 DSM-IV ADHD symptoms as well as contextual and impairment criteria (Kaufman, Birmaher et al. 1997). A clinician-rated ADHD-RS severity score of 1.5 SD above age and gender means for either the inattention or hyperactivity/impulsivity scales (DuPaul, Power, Anastopoulos, & Reid, 1998) was additionally required. Diagnostic impressions derived from these measures were confirmed by a child psychiatrist or psychologist based upon clinical interview and review of all assessment information.
Measures
Participants were administered Conners’ Continuous Performance Test II Version 5 (CPT II: Conners, 2000), a computer-administered task that requires participants to respond to 360 letters which appear on the monitor, one at a time, for 250 msec. Participants are instructed to press the space bar whenever any letter except “X” appears, which happens on 10% of trials. The interstimulus interval varies among one, two, or four seconds across 18 blocks of 20 trials each. T-scores for age and sex were generated for indices of sustained attention including omission errors (i.e., failure to respond to non-Xs), reaction time for correct responses (RT), and reaction time variability (RT variability), and for commission errors (i.e., responses to Xs) which were used to assess response inhibition.
Medication Titration
All participants were treated with both MPH and ATX, in a randomized, double blind, double dummy, counterbalanced, crossover design (Figure 1). Two capsules of OROS MPH or matching placebo and either two or three capsules of ATX (determined by the child’s weight) or matching placebo were administered each morning. Weekly ratings of ADHD symptoms and severity of impairment were obtained during a parent interview by blind raters who were trained research assistants, graduate students, or postdoctoral fellows using the ADHD-RS (DuPaul et al., 1998), which was used to track changes in frequency and severity of symptoms during treatment, and aid in clinical decision making. Medication was titrated using a flexible, stepped dose optimization strategy in weekly sessions, based on assessment of clinical status and adverse effects, following a pre-established algorithm to a standard of no room for improvement. There were four dose levels for each drug (OROS MPH: 18mg, 36 mg, 54 mg, 72 mg; ATX: 0.5 mg/kg, 1.0 mg/kg, 1.4 mg/kg, 1.8 mg/kg). The end point of titration for each treatment was the conclusion of two weeks on the most effective dose. CPT-II was administered pre-medication, following placebo washout (i.e., baseline for the second medication block), and at the conclusion of each treatment block (Figure 1).
Fig. 1.
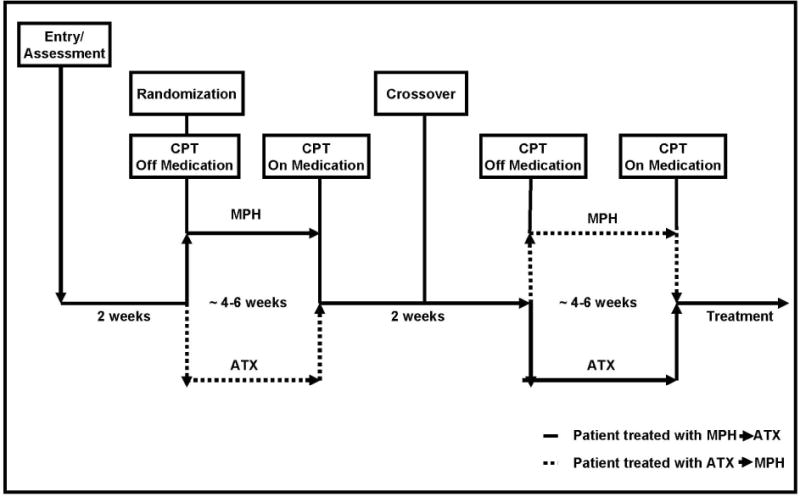
Schematic of study design.
Data Analysis
Data were included only if the CPT-II task was administered successfully at all four time points (See Figure 2). All data were checked for outliers. Tabachnik’s (1989) most conservative score changing option was selected when subjects had a T-score > 100. Each outlier score was changed to equal the next highest score in the distribution, plus one unit. Thus, the score remained as the most extreme in the distribution while at the same time minimizing the skew. This procedure was used to adjust 2.5% of data points, and was only applicable for omission error T-scores (Pre-ATX: 12/102; Post-ATX: 10/102; Pre-OROS MPH 13/102; Post-OROS MPH: 6/102). Potential differences in medication effects by testing site were examined using repeated-measures analyses of variance (ANOVA) with Drug (ATX, OROS MPH) and Time (Pre-Medication, Post-Medication) as the within-subject factors, and Site (Site 1, Site 2) as the between-subjects factor. Potential order effects were examined to make sure we could collapse data across blocks by using ANOVAs with Block (Baseline 1, Baseline 2) as the repeated factor and Treatment Order (ATX-OROS MPH, OROS MPH-ATX) as the between-subjects factor. When the Block × Treatment order interaction was not significant, repeated-measures ANOVAs were run with Drug (ATX, OROS MPH) and Time (Pre-medication, Post-medication) as within-subject factors for each dependent measure. If a significant treatment order effect occurred, only data for the first medication period were analyzed, using ANOVAs with Drug (ATX, OROS MPH) as a between-subjects factor and Time (Pre-medication, Post-medication) as the repeated within-groups factor. Potential age effects were accounted for in two ways. We used age and gender normed T-scores for all CPT II outcomes. In addition, we divided the sample into 3 age groups (Young: 7–9 year olds, Middle: 10–13 year olds, Older: 14 – 17 year olds) and the analyses were re-run with the dependent measures.
Fig. 2.
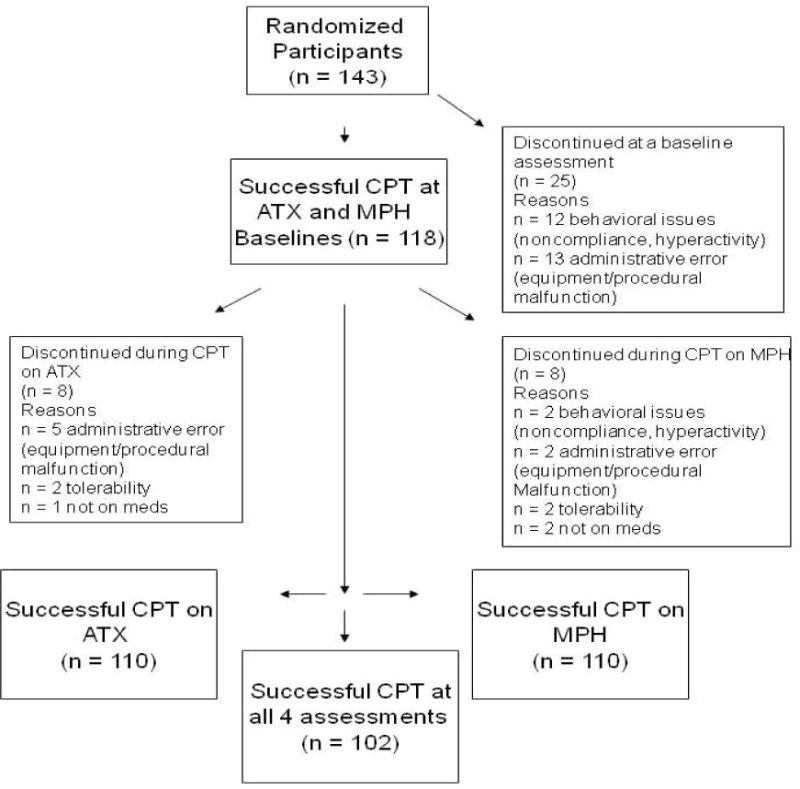
Schematic of study completers.
Pearson’s product-moment correlations were used to examine the association between changes in indices on CPT-II and changes in ADHD clinical symptom severity as a function of treatment with ATX and OROS MPH separately. ADHD symptom severity was indexed at the time of each CPT-II administration by calculating the ADHD-RS total score based on parent interview by a blind rater, as well as separate symptom domain scores for the inattention and hyperactivity/impulsivity sub-scales. Change scores for omission errors, commission errors, RT, RT variability and ADHD symptoms were computed by subtracting scores on the on-medication (end of treatment block) test day from scores on the corresponding off-medication (pre-treatment baseline, by block) test day for each drug. For all computed change scores, larger positive difference scores reflect greater positive impact of drug. Statistical significance was set at the 0.05 level and all probabilities were based on two-tailed tests.
Results
There were no significant main or interaction effects for Site (p > 0.05). Further, there were no significant effects of drug order on baseline scores for errors of commission or omission, or for RT variability. For RT, however, there was a significant main effect of Block (F1,100 = 7.58, p = 0.01, partial η2 = 0.07) and a significant Block × Treatment Order interaction (F1,100 = 5.76, p = 0.02, partial η2 = 0.05). Those who received ATX first had a slower pre-medication mean RT in the second block (59 ± 13) compared to first block (53 ± 14), whereas those receiving OROS MPH first did not (2nd Block: 55 ± 16; 1st Block: 55 ± 14). Although we considered this likely to be a random finding and not a medication carryover effect, the effects of ATX versus OROS MPH on RT were examined using data from the first treatment block only (ATX: n = 48; OROS MPH: n = 54).
Examination of potential age effects, revealed no significant age-related effects for young, middle or older children for either commission or omission errors. For RT Variability there was a significant main effect for age group such that the youngest group was more variable than the two older groups, and a significant age group × time interaction such that the younger children had a greater decrease in variability over time. There were no significant age × drug interactions. Accordingly, subsequent analyses were performed on the whole sample without stratifying by age.
Commission Errors
For commission errors there were no significant main effects of Drug (F1,101 = 0.001, p = 0.98, partial η2 < 0.001) or Time (F1,101 = 2.28, p = 0.13, partial η2 = 0.02), and the Drug × Time (F1,101 = 0.04, p = 0.85, partial η2 < 0.001) interaction was not significant. As depicted in Figure 3a, commission errors did not improve with ATX or OROS MPH treatment. However, commission errors were not elevated in this sample at baseline (T-scores~50).
Fig. 3.
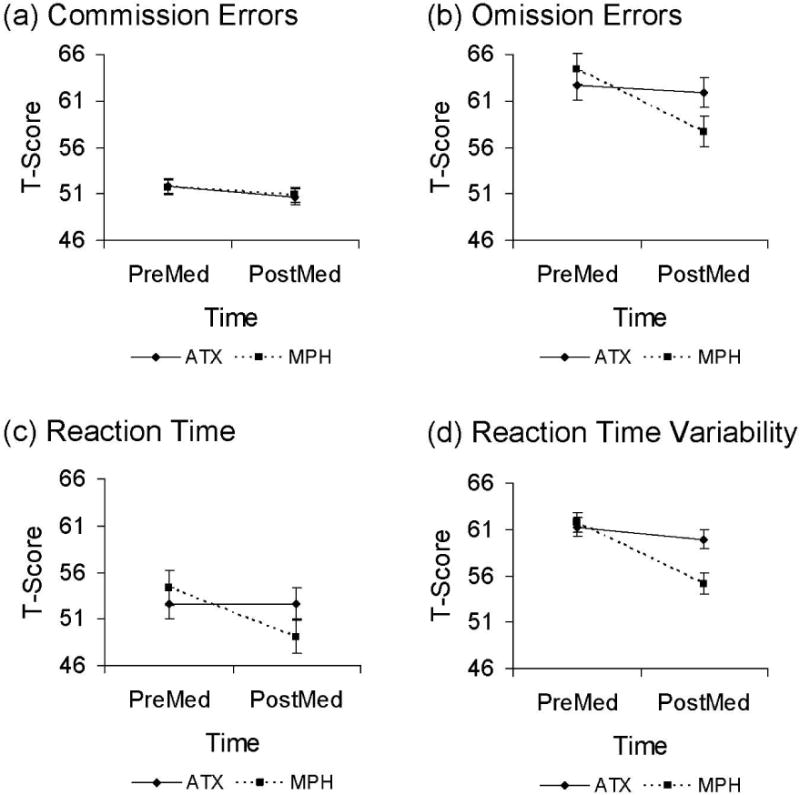
Performance on Conners CPT-II by treatment (Atomoxetine, Methylphenidate) and time (PreMed, PostMed). Conners CPT-II performance for (a) commission errors, (b) omission errors, (c) reaction time (RT), and (d) reaction time variability (RT variability).
Omission Errors
For omission errors there was a significant Drug × Time interaction (F1,101 = 4.61, p = 0.03, partial η2 = 0.04) and a significant main effect of Time (F1,101 = 6.47, p = 0.01, partial η2 = 0.06), with no main effect of Drug (F1,101 = 0.78, p = 0.38, partial η2 = 0.01). As shown in Figure 3b, treatment with OROS MPH led to larger reductions in omission errors than did treatment with ATX. Post hoc paired t-tests showed a significant reduction in omission errors following OROS MPH (p = 0.001) but not ATX (p = 0.69) treatment.
Reaction Time (RT)
There was a significant Drug × Time interaction (F1,100 = 4.92, p = 0.03, partial η2 = 0.05) such that youth treated with OROS MPH had a greater speeding of RT than those treated with ATX (see Figure 3c). There was no main effect of Drug (F1,100 = 0.015, p = 0.69, partial η2 = 0.05), but there was a main effect of Time (F1,100 = 4.90, p = 0.03, partial η2 = 0.05). A post hoc paired t-test showed no significant change in RT for ATX (p = 0.99).
RT Variability
There were main effects for Time (F1,101 = 20.07, p < 0.001, partial η2 = 0.17) and Drug (F1,101 = 4.95, p = 0.03, partial η2 = 0.05) on RT variability. There was also a significant Drug × Time interaction (F1,101 = 11.79, p = 0.001, partial η2 = 0.104). OROS MPH had a significantly larger impact than ATX (Figure 3d). A post hoc paired t-test showed no significant change in RT variability for ATX (p = 0.17).
Relationship to Changes in Behavior
Both medications produced significant improvement in ADHD symptoms on the ADHD-RS from pre- to post-treatment (ATX Pre: 36.55±11.09; ATX Post: 20.54±12.96; ATX: t1,101 = 13.61, p < 0.001; MPH Pre: 37.09±10.96; MPH Post: 17.37±11.29; MPH: t1,101 = 17.27, p < 0.001). Changes from baseline in the ADHD-RS were not significantly associated with changes in any CPT measure as a function of treatment with either ATX (commission errors r = 0.08, omission errors r = 0.02, RT r = −0.06, RT Variability r = −0.04) or MPH (commission errors r = 0.02, omission errors r = 0.14, RT r = −0.2, RT Variability r = 0.05). In addition, changes from baseline in the ADHD-RS inattention and hyperactivity/impulsivity subdomains were not significantly associated with changes in any CPT measure as a function of treatment with either medication (all p > 0.05).
Discussion
This is the first controlled study comparing the effects of non-stimulant (ATX) and stimulant (OROS MPH) medications on laboratory measures of sustained attention and inhibitory control in youth with ADHD. Our findings are buttressed by the large sample that was treated with both medications (n = 102) and the fact that drug effects were examined after treatment at optimally titrated doses rather than after single-dose challenge. Overall, treatment with medication improved measures of attention, but not inhibitory control. However, these effects were mainly carried by MPH, as MPH produced significant change in these measures while ATX did not. However, MPH CPT-II performance changes were not correlated with symptomatic improvement. The latter finding suggests that change in CPT performance with treatment may represent a qualitatively different aspect of response relative to behavioral change, and possibly attributable to a different mechanism which may overlap with but is not identical to ADHD symptom response (Coghill, Rhodes, & Matthews, 2007).
Our results are consistent with findings that have shown a positive effect of stimulants on CPT indices (Epstein et al., 2006; Riccio, Waldrop, Reynolds, & Lowe, 2001). Most importantly, this study adds to the literature on treatment effects of ATX on objective measures of ADHD symptoms in youth with ADHD – an area that has received only minimal investigation to date, with inconsistent findings. Several studies in both healthy adults and children with ADHD did not find positive effects of ATX on inhibitory control as measured by the Stop Signal task, Conners’ CPT, and a combined Eriksen flanker-Go/NoGo task (Graf et al., 2010; Nandam et al., 2010; Spencer et al., 2001). However, other studies using single dose challenge in children and healthy adults found positive effects on measures of inhibition (Stop signal task) and sustained attention (CANTAB Rapid Visual Information Processing, CPT) (Barry et al., 2009; Chamberlain et al., 2007; Chamberlain et al., 2009; Gau & Shang, 2010). It is noteworthy that the latter studies used lower doses of ATX than we used to achieve behavioral response, or single dose challenge rather than ongoing treatment; it is likewise possible that we might have found positive effects at lower doses or following single challenge doses.
Our findings that OROS MPH had a greater impact than ATX on CPT measures of attention (particularly for RT variability) in youth with ADHD are in line with previous reports from a single dose challenge study in healthy adults (Nandam et al., 2010) and small treatment studies in youth with ADHD (Kratz et al., 2012; Yildiz et al., 2010). Kratz et al. (2012) and Spencer et al. (2009) have suggested that the sensitivity of RT variability for detecting cognitive effects of MPH may be due to drug-related reduction of lapses in attention and improvement in self-regulatory processes. Our results are in agreement with the previous findings of MPH effects on RT variability. However, the lack of correlation with ratings of inattention and hyperactive/impulsive symptoms in this study raises the question that this cognitive effect of MPH may be independent of the clinical effects of treatment.
One important question raised by these results is why two medications that are each effective for improving clinical symptoms of inattention and hyperactivity/impulsivity in youth with ADHD have different effects on neurocognitive function as measured by CPT. It is possible that this finding reflects factors specific to our study – e.g., the relatively high proportion of youth with predominantly inattentive subtype, restricting the sample to only those who completed the CPT-II at all four assessment points, excluding youth who could not complete the CPT-II due to excessive hyperactivity or noncompliance from the analyses, titrating medication treatment based on severity of symptoms and tolerability but not on cognitive functioning per se, and using a dose optimization rather than a dose-response titration strategy. Nevertheless, the lack of concordance between improvement in CPT performance with MPH and parent reports of clinical improvement is consistent with findings from at least one study which also demonstrated a lack of association between the Conners’ CPT with parent and teacher ratings of inattention or hyperactivity (McGee, Clark, & Symons, 2000). One intriguing explanation for these findings is that they may indicate dissociation between cognitive and behavioral effects of stimulant medication. This hypothesis had received considerable attention and some empirical support in an influential early study examining single challenge dose effects of amphetamine (Sprague & Sleator, 1977), but this finding is generally not considered in the current approach to titrating stimulants – which is based primarily on behavior change and assumes a linear dose-response relationship. Since the end-of-treatment dose in this study was determined by changes in behavior and not cognitive performance, it is possible that we missed the optimal cognitive effects of MPH and the main cognitive effects of ATX. If such relationships are indeed present, they would be best identified using a dose-response design, which would collect both clinical ratings and CPT data at each dose level, rather than a flexible dose optimization strategy which obtained CPT data only at the beginning and end of treatment.
A possible explanation for the superiority of MPH over ATX on CPT indices relates to differences in neurobiological mechanisms of action between the two medications. Although MPH and ATX both amplify dopamine and noradrenaline signaling in prefrontal cortex (Gamo et al., 2010), key differences in the activity of the two medications in other brain regions may account for the observed differential effects on cognitive function. For example, MPH produces deactivation of the default mode network in healthy adults and in youth with ADHD, which could facilitate vigilance by filtering irrelevant stimuli (Peterson et al., 2009; Tomasi et al., 2011). Additionally, MPH increases activity in caudate (Vaidya et al., 1998) due to its effect of binding to dopamine transporters (Volkow et al., 2002), yet ATX should not have direct effects in caudate as there are few norepinephrine transporters in this region. In contrast, ATX has no direct effects on striatum and effects on the default mode network are uncertain, owing to disparate findings across studies (Marquand et al., 2011; Schulz et al., 2012). Finally, it is possible that the effects of ATX on enhancing prefrontal activity (Gamo et al., 2010) produce either qualitatively or quantitatively different effects than are seen with MPH.
Alternately, since ADHD medications are typically titrated on severity of symptoms and functional impairments, and not on neurocognitive function per se, the finding of heterogeneity in cognitive response may not reflect the behavioral effects of the medications. In other words, while CPT measures of inattention and impulsivity are often considered to be objective measures of ADHD symptoms, it is possible and even likely that this is not the case. The fact that two of the four CPT measures studied here (commission errors; RT) were within one SD of the norms for these indices prior to treatment is consistent with the suggested dissociation of ADHD symptoms and CPT measures. Further, the effect sizes for CPT-measured cognitive improvements with MPH here are in agreement with previous reviews that demonstrate smaller effects on cognitive function than on behavior (Faraone & Buitelaar, 2010; Pietrzak, Mollica, Maruff, & Snyder, 2006).
Although not the main aim of this study, this design also enabled an examination of the relationship between different aspects of cognitive performance in relation to treatment. For example, previous research has suggested aetiological separation between omission errors and reaction time variability (Kuntsi et al., 2010). In this study, we see significant covariation suggesting a shared underlying mechanism in relation to treatment response for these two different aspects of attention (r = 0.63, p < .001).
Several methodological limitations of the present study need to be considered. For example, only one measure of cognitive performance was used in this study. Although CPT tests are widely used to assess youth with ADHD, additional measures tapping different constructs may have done a better job of identifying response to ATX (Bedard, Jain, Johnson, & Tannock, 2007). Also, our sample was not impaired on the CPT-II measure of inhibitory control (i.e., commission errors); hence, there was little opportunity for improvement with either treatment. Overall the study cannot be said to provide substantive information on the relationship between measures of inhibitory control (such as CPT commission errors) and clinical response to MPH and ATX.
Conclusion
These results provide evidence for a positive effect of OROS MPH, but not ATX, on attention as measured by the Conners CPT-II in youth with ADHD. However, as MPH effects on cognition and behavior were not correlated, one cannot consider these CPT indices to reflect objective measures of ADHD symptom change. These findings suggest that titration approaches that are based on behavioral domains may not necessarily yield optimal improvement in cognitive performance, and additionally, that using CPT measures to titrate ADHD medication treatments may potentially lead to suboptimal behavioral response.
Key Points.
Clinically effective doses of MPH improved performance on CPT-II measures of sustained attention.
Clinically effective doses on ATX had no impact on CPT performance
Changes in CPT performance following medication were not related to clinical improvements
CPT performance cannot be used to titrate medication dose in youth with ADHD
Stimulants such as methylphenidate may be superior to atomoxetine for neuropsychologically defined cognitive endpoints (such as the CPT indices used in this paper). However, the optimal methylphenidate dose for cognitive and behavioral improvement may not be identical.
More research examining the possible dissociation of cognitive and behavioral outcomes for stimulants is recommended.
Acknowledgments
This project was funded in part by grants from the NIMH (R01 MH070935, R01 MH70564, DSIR 84-CTM) and the National Center for Research Resources and the National Center for Advancing Translational Sciences of the NIH (UL1TR000067), and through a Canadian Institutes of Health Research Fellowship to A.-C.V.B. Study medication was provided by Eli Lilly and Co. and Ortho-McNeil-Janssen.
No third party had any role in the design of the study or the interpretation of the results. The authors are solely responsible for the integrity of the data and A.-C.V.B is the guarantor for the study findings on behalf of all the authors.
The authors are grateful to Heather Philips, Jennifer Davidow, Jodi Uderman Lipsky, Joanne Philips, Erika Zeranski, Juan Pedraza, and Iliyan Ivanov for their assistance with data collection.
Footnotes
Conflict of interest statement: M.A.S. has received research support from Shire Pharmaceuticals, and is a consultant/advisor for Alcobra, GencoSciences, and Novartis. J.H.N. has received research support from Shire Pharmaceuticals; he is an advisor and/or consultant for Alcobra, BioBehavioral Diagnostics, Enzymotec, GencoSciences, Sunovion, and Shire Pharmaceuticals. The rest of the authors have declared that they have no potential or competing conflicts of interest.
References
- Barry RJ, Clarke AR, Hajos M, McCarthy R, Selikowitz M, Bruggemann JM. Acute atomoxetine effects on the EEG of children with attention-deficit/hyperactivity disorder. Neuropharmacology. 2009;57(7–8):702–707. doi: 10.1016/j.neuropharm.2009.08.003. doi: S0028-3908(09)00270-6 [pii] 10.1016/j.neuropharm.2009.08.003. [DOI] [PubMed] [Google Scholar]
- Bedard AC, Jain U, Johnson SH, Tannock R. Effects of methylphenidate on working memory components: influence of measurement. J Child Psychol Psychiatry. 2007;48(9):872–880. doi: 10.1111/j.1469-7610.2007.01760.x. doi: JCPP1760 [pii] 10.1111/j.1469-7610.2007.01760.x. [DOI] [PubMed] [Google Scholar]
- Berridge CW, Devilbiss DM, Andrzejewski ME, Arnsten AF, Kelley AE, Schmeichel B, Spencer RC. Methylphenidate preferentially increases catecholamine neurotransmission within the prefrontal cortex at low doses that enhance cognitive function. Biol Psychiatry. 2006;60(10):1111–1120. doi: 10.1016/j.biopsych.2006.04.022. [DOI] [PubMed] [Google Scholar]
- Bolden-Watson C, Richelson E. Blockade by newly-developed antidepressants of biogenic amine uptake into rat brain synaptosomes. Life Sci. 1993;52(12):1023–1029. doi: 10.1016/0024-3205(93)90194-8. [DOI] [PubMed] [Google Scholar]
- Bymaster FP, Katner JS, Nelson DL, Hemrick-Luecke SK, Threlkeld PG, Heiligenstein JH, Perry KW. Atomoxetine increases extracellular levels of norepinephrine and dopamine in prefrontal cortex of rat: a potential mechanism for efficacy in attention deficit/hyperactivity disorder. Neuropsychopharmacology. 2002;27(5):699–711. doi: 10.1016/S0893-133X(02)00346-9. doi: S0893133X02003469 [pii] 10.1016/S0893-133X(02)003469. [DOI] [PubMed] [Google Scholar]
- Chamberlain SR, Del Campo N, Dowson J, Muller U, Clark L, Robbins TW, Sahakian BJ. Atomoxetine improved response inhibition in adults with attention deficit/hyperactivity disorder. Biol Psychiatry. 2007;62(9):977–984. doi: 10.1016/j.biopsych.2007.03.003. doi: S0006-3223(07)00213-2 [pii] 10.1016/j.biopsych.2007.03.003. [DOI] [PubMed] [Google Scholar]
- Chamberlain SR, Hampshire A, Muller U, Rubia K, Campo ND, Craig K, Sahakian BJ. Atomoxetine Modulates Right Inferior Frontal Activation During Inhibitory Control: A Pharmacological Functional Magnetic Resonance Imaging Study. Biol Psychiatry. 2008 doi: 10.1016/j.biopsych.2008.10.014. doi: S0006-3223(08)01243-2 [pii] 10.1016/j.biopsych.2008.10.014. [DOI] [PubMed] [Google Scholar]
- Chamberlain SR, Hampshire A, Muller U, Rubia K, Del Campo N, Craig K, Sahakian BJ. Atomoxetine modulates right inferior frontal activation during inhibitory control: a pharmacological functional magnetic resonance imaging study. Biol Psychiatry. 2009;65(7):550–555. doi: 10.1016/j.biopsych.2008.10.014. doi: S0006-3223(08)01243-2 [pii] 10.1016/j.biopsych.2008.10.014. [DOI] [PubMed] [Google Scholar]
- Coghill DR, Rhodes SM, Matthews K. The neuropsychological effects of chronic methylphenidate on drug-naive boys with attention-deficit/hyperactivity disorder. Biol Psychiatry. 2007;62(9):954–962. doi: 10.1016/j.biopsych.2006.12.030. doi: S0006-3223(07)00015-7 [pii] 10.1016/j.biopsych.2006.12.030. [DOI] [PubMed] [Google Scholar]
- Conners CK. I. Multi Health Systems 2000 [Google Scholar]
- Conners CK. Forty years of methylphenidate treatment in Attention-Deficit/Hyperactivity Disorder. J Atten Disord. 2002;6(Suppl 1):S17–30. doi: 10.1177/070674370200601s04. [DOI] [PubMed] [Google Scholar]
- Conners CK, Epstein JN, March JS, Angold A, Wells KC, Klaric J, Wigal T. Multimodal treatment of ADHD in the MTA: an alternative outcome analysis. J Am Acad Child Adolesc Psychiatry. 2001;40(2):159–167. doi: 10.1097/00004583-200102000-00010. doi: S0890-8567(09)60365-8 [pii] 10.1097/00004583-200102000-00010. [DOI] [PubMed] [Google Scholar]
- de Jong CG, Van De Voorde S, Roeyers H, Raymaekers R, Allen AJ, Knijff S, Sergeant JA. Differential effects of atomoxetine on executive functioning and lexical decision in attention-deficit/hyperactivity disorder and reading disorder. J Child Adolesc Psychopharmacol. 2009;19(6):699–707. doi: 10.1089/cap.2009.0029. [DOI] [PubMed] [Google Scholar]
- Dolan GP, Stone DH, Briggs AH. A systematic review of continuous performance task research in children prenatally exposed to alcohol. Alcohol Alcohol. 2010;45(1):30–38. doi: 10.1093/alcalc/agp062. doi: agp062 [pii] 10.1093/alcalc/agp062. [DOI] [PubMed] [Google Scholar]
- DuPaul GJ, Power TJ, Anastopoulos AD, Reid R. ADHD Rating Scale – IV: Checklists, Norms, and Clinical Interpretation. New York: The Guilford Press; 1998. [Google Scholar]
- Epstein JN, Conners CK, Hervey AS, Tonev ST, Arnold LE, Abikoff HB, Wigal T. Assessing medication effects in the MTA study using neuropsychological outcomes. J Child Psychol Psychiatry. 2006;47(5):446–456. doi: 10.1111/j.1469-7610.2005.01469.x. doi: JCPP1469 [pii] 10.1111/j.1469-7610.2005.01469.x. [DOI] [PubMed] [Google Scholar]
- Faraone SV, Buitelaar J. Comparing the efficacy of stimulants for ADHD in children and adolescents using meta-analysis. Eur Child Adolesc Psychiatry. 2010;19(4):353–364. doi: 10.1007/s00787-009-0054-3. [DOI] [PubMed] [Google Scholar]
- Gamo NJ, Wang M, Arnsten AF. Methylphenidate and atomoxetine enhance prefrontal function through alpha2-adrenergic and dopamine D1 receptors. J Am Acad Child Adolesc Psychiatry. 2010;49(10):1011–1023. doi: 10.1016/j.jaac.2010.06.015. doi: S0890-8567(10)00522-8 [pii] 10.1016/j.jaac.2010.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatley SJ, Pan D, Chen R, Chaturvedi G, Ding YS. Affinities of methylphenidate derivatives for dopamine, norepinephrine and serotonin transporters. Life Sci. 1996;58(12):231–239. doi: 10.1016/0024-3205(96)00052-5. [DOI] [PubMed] [Google Scholar]
- Gau SS, Shang CY. Improvement of executive functions in boys with attention deficit hyperactivity disorder: an open-label follow-up study with once-daily atomoxetine. Int J Neuropsychopharmacol. 2010;13(2):243–256. doi: 10.1017/S1461145709990836. doi: S1461145709990836 [pii] 10.1017/S1461145709990836. [DOI] [PubMed] [Google Scholar]
- Graf H, Abler B, Freudenmann R, Beschoner P, Schaeffeler E, Spitzer M, Gron G. Neural Correlates of Error Monitoring Modulated by Atomoxetine in Healthy Volunteers. Biol Psychiatry. 2010 doi: 10.1016/j.biopsych.2010.10.018. doi: S0006-3223(10)01111-X [pii] 10.1016/j.biopsych.2010.10.018. [DOI] [PubMed] [Google Scholar]
- Kratz O, Studer P, Baack J, Malcherek S, Erbe K, Moll GH, Heinrich H. Differential effects of methylphenidate and atomoxetine on attentional processes in children with ADHD: an event-related potential study using the Attention Network Test. Prog Neuropsychopharmacol Biol Psychiatry. 2012;37(1):81–89. doi: 10.1016/j.pnpbp.2011.12.008. [DOI] [PubMed] [Google Scholar]
- Kuntsi J, Wood AC, Rijsdijk F, Johnson KA, Andreou P, Albrecht B, Asherson P. Separation of cognitive impairments in attention-deficit/hyperactivity disorder into 2 familial factors. Arch Gen Psychiatry. 2010;67(11):1159–1167. doi: 10.1001/archgenpsychiatry.2010.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu ST, Tsai FJ, Lee WT, Lee CM, Fan PC, Lin WS, Gau SS. Attentional processes and ADHD-related symptoms in pediatric patients with epilepsy. Epilepsy Res. 2011;93(1):53–65. doi: 10.1016/j.eplepsyres.2010.10.012. doi: S0920-1211(10)00302-5 [pii] 10.1016/j.eplepsyres.2010.10.012. [DOI] [PubMed] [Google Scholar]
- Losier BJ, McGrath PJ, Klein RM. Error patterns on the continuous performance test in non-medicated and medicated samples of children with and without ADHD: a meta-analytic review. J Child Psychol Psychiatry. 1996;37(8):971–987. doi: 10.1111/j.1469-7610.1996.tb01494.x. [DOI] [PubMed] [Google Scholar]
- Marquand AF, De Simoni S, O’Daly OG, Williams SC, Mourao-Miranda J, Mehta MA. Pattern classification of working memory networks reveals differential effects of methylphenidate, atomoxetine, and placebo in healthy volunteers. Neuropsychopharmacology. 2011;36(6):1237–1247. doi: 10.1038/npp.2011.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCabe SE, West BT. Medical and nonmedical use of prescription stimulants: results from a national multicohort study. J Am Acad Child Adolesc Psychiatry. 2013;52(12):1272–1280. doi: 10.1016/j.jaac.2013.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGee RA, Clark SE, Symons DK. Does the Conners’ Continuous Performance Test aid in ADHD diagnosis? J Abnorm Child Psychol. 2000;28(5):415–424. doi: 10.1023/a:1005127504982. [DOI] [PubMed] [Google Scholar]
- Michelson D, Allen AJ, Busner J, Casat C, Dunn D, Kratochvil C, Harder D. Once-daily atomoxetine treatment for children and adolescents with attention deficit hyperactivity disorder: a randomized, placebo-controlled study. Am J Psychiatry. 2002;159(11):1896–1901. doi: 10.1176/appi.ajp.159.11.1896. [DOI] [PubMed] [Google Scholar]
- Moron JA, Brockington A, Wise RA, Rocha BA, Hope BT. Dopamine uptake through the norepinephrine transporter in brain regions with low levels of the dopamine transporter: evidence from knock-out mouse lines. J Neurosci. 2002;22(2):389–395. doi: 10.1523/JNEUROSCI.22-02-00389.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nandam LS, Hester R, Wagner J, Cummins TD, Garner K, Dean AJ, Bellgrove MA. Methylphenidate But Not Atomoxetine or Citalopram Modulates Inhibitory Control and Response Time Variability. Biol Psychiatry. 2010 doi: 10.1016/j.biopsych.2010.11.014. doi: S0006-3223(10)01202-3 [pii] 10.1016/j.biopsych.2010.11.014. [DOI] [PubMed] [Google Scholar]
- Newcorn JH, Kratochvil CJ, Allen AJ, Casat CD, Ruff DD, Moore RJ, Michelson D. Atomoxetine and osmotically released methylphenidate for the treatment of attention deficit hyperactivity disorder: acute comparison and differential response. Am J Psychiatry. 2008;165(6):721–730. doi: 10.1176/appi.ajp.2007.05091676. doi: appi.ajp.2007.05091676 [pii] 10.1176/appi.ajp.2007.05091676. [DOI] [PubMed] [Google Scholar]
- Peterson BS, Potenza MN, Wang Z, Zhu H, Martin A, Marsh R, Yu S. An FMRI study of the effects of psychostimulants on default-mode processing during Stroop task performance in youths with ADHD. Am J Psychiatry. 2009;166(11):1286–1294. doi: 10.1176/appi.ajp.2009.08050724. doi: appi.ajp.2009.08050724 [pii] 10.1176/appi.ajp.2009.08050724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietrzak RH, Mollica CM, Maruff P, Snyder PJ. Cognitive effects of immediate-release methylphenidate in children with attention-deficit/hyperactivity disorder. Neurosci Biobehav Rev. 2006;30(8):1225–1245. doi: 10.1016/j.neubiorev.2006.10.002. doi: S0149-7634(06)00114-X [pii] 10.1016/j.neubiorev.2006.10.002. [DOI] [PubMed] [Google Scholar]
- Rapport MD, Denney C, DuPaul GJ, Gardner MJ. Attention deficit disorder and methylphenidate: normalization rates, clinical effectiveness, and response prediction in 76 children. J Am Acad Child Adolesc Psychiatry. 1994;33(6):882–893. doi: 10.1097/00004583-199407000-00015. doi: S0890-8567(09)64291-X [pii] 10.1097/00004583-199407000-00015. [DOI] [PubMed] [Google Scholar]
- Reinecke MA, Beebe DW, Stein MA. The third factor of the WISC-III: it’s (probably) not freedom from distractibility. J Am Acad Child Adolesc Psychiatry. 1999;38(3):322–328. doi: 10.1097/00004583-199903000-00020. [DOI] [PubMed] [Google Scholar]
- Riccio CA, Waldrop JJ, Reynolds CR, Lowe P. Effects of stimulants on the continuous performance test (CPT): implications for CPT use and interpretation. J Neuropsychiatry Clin Neurosci. 2001;13(3):326–335. doi: 10.1176/jnp.13.3.326. [DOI] [PubMed] [Google Scholar]
- Schulz KP, Fan J, Bedard AC, Clerkin SM, Ivanov I, Tang CY, Newcorn JH. Common and unique therapeutic mechanisms of stimulant and nonstimulant treatments for attention-deficit/hyperactivity disorder. Arch Gen Psychiatry. 2012;69(9):952–961. doi: 10.1001/archgenpsychiatry.2011.2053. [DOI] [PubMed] [Google Scholar]
- Spencer SV, Hawk LW, Jr, Richards JB, Shiels K, Pelham WE, Jr, Waxmonsky JG. Stimulant treatment reduces lapses in attention among children with ADHD: the effects of methylphenidate on intra-individual response time distributions. J Abnorm Child Psychol. 2009;37(6):805–816. doi: 10.1007/s10802-009-9316-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer T, Biederman J, Heiligenstein J, Wilens T, Faries D, Prince J, Zervas S. An open-label, dose-ranging study of atomoxetine in children with attention deficit hyperactivity disorder. J Child Adolesc Psychopharmacol. 2001;11(3):251–265. doi: 10.1089/10445460152595577. [DOI] [PubMed] [Google Scholar]
- Spencer T, Biederman J, Wilens T, Prince J, Hatch M, Jones J, Seidman L. Effectiveness and tolerability of tomoxetine in adults with attention deficit hyperactivity disorder. Am J Psychiatry. 1998;155(5):693–695. doi: 10.1176/ajp.155.5.693. [DOI] [PubMed] [Google Scholar]
- Sprague RL, Sleator EK. Methylphenidate in hyperkinetic children: differences in dose effects on learning and social behavior. Science. 1977;198(4323):1274–1276. doi: 10.1126/science.337493. [DOI] [PubMed] [Google Scholar]
- Tabachnik BG, Fidell LS. Using Multivariate Statistics. 2. Harper & Row; 1989. [Google Scholar]
- Tomasi D, Volkow ND, Wang GJ, Wang R, Telang F, Caparelli EC, Fowler JS. Methylphenidate enhances brain activation and deactivation responses to visual attention and working memory tasks in healthy controls. Neuroimage. 2011;54(4):3101–3110. doi: 10.1016/j.neuroimage.2010.10.060. doi: S1053-8119(10)01353-4 [pii] 10.1016/j.neuroimage.2010.10.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaidya CJ, Austin G, Kirkorian G, Ridlehuber HW, Desmond JE, Glover GH, Gabrieli JD. Selective effects of methylphenidate in attention deficit hyperactivity disorder: a functional magnetic resonance study. Proc Natl Acad Sci U S A. 1998;95(24):14494–14499. doi: 10.1073/pnas.95.24.14494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Logan J, Franceschi D, Maynard L, Swanson JM. Relationship between blockade of dopamine transporters by oral methylphenidate and the increases in extracellular dopamine: therapeutic implications. Synapse. 2002;43(3):181–187. doi: 10.1002/syn.10038. doi: 10.1002/syn.10038 [pii] 10.1002/syn.10038. [DOI] [PubMed] [Google Scholar]
- Yildiz O, Sismanlar SG, Memik NC, Karakaya I, Agaoglu B. Atomoxetine and Methylphenidate Treatment in Children with ADHD: The Efficacy, Tolerability and Effects on Executive Functions. Child Psychiatry Hum Dev. 2010 doi: 10.1007/s10578-010-0212-3. [DOI] [PubMed] [Google Scholar]


