Abstract
Schistosomes are parasitic worms that can live in the bloodstream of their vertebrate hosts for many years. It has been proposed that the worms impinge on host purinergic signalling by degrading proinflammatory molecules like ATP as well as prothrombotic mediators like ADP. This capability may help explain the apparent refractoriness of the worms to both immune elimination and thrombus formation. Three distinct ectoenzymes, expressed at the host-exposed surface of the worm’s tegument, are proposed to be involved in the catabolism of ATP and ADP. These are alkaline phosphatase (SmAP), phosphodiesterase (SmNPP-5), and ATP diphosphohydrolase (SmATPDase1). It has recently been shown that only one of these enzymes—SmATPDase1—actually degrades exogenous ATP and ADP. However, a second ATP diphosphohydrolase homolog (SmATPDase2) is located in the tegument and has been reported to be released by the worms. It is possible that this enzyme too participates in the cleavage of exogenous nucleotide tri- and di-phosphates. To test this hypothesis, we employed RNA interference (RNAi) to suppress the expression of the schistosome SmATPDase1 and SmATPDase2 genes. We find that only SmATPDase1-suppressed parasites are significantly impaired in their ability to degrade exogenously added ATP or ADP. Suppression of SmATPDase2 does not appreciably affect the worms’ ability to catabolize ATP or ADP. Furthermore, we detect no evidence for the secretion or release of an ATP-hydrolyzing activity by cultured parasites. The results confirm the role of tegumental SmATPDase1, but not SmADTPDase2, in the degradation of the exogenous proinflammatory and prothrombotic nucleotides ATP and ADP by live intravascular stages of the parasite.
Keywords: Schistosoma, Trematode, Tegument, ATPase, ADPase, RNA interference
Introduction
Schistosomes are intravascular parasitic worms that cause the debilitating disease schistosomiasis. Over 200 million people are estimated to be infected with these worms globally and more than 600 million live at risk of infection [1]. Schistosomes have a complex life history. Larval forms called cercariae escape from an intermediate freshwater snail host. These free-swimming forms can infect the human definitive host. The cercaria penetrates unbroken skin and transforms into a morphologically and biochemically distinct life stage called the schistosomulum. This juvenile parasite then invades a blood vessel and migrates in the blood stream through the lungs and heart to the portal vasculature. Here, the worms mature and pair up. Females often reside in a specialized groove called the gynacophoric canal that is found on the ventral surface of adult males. From the liver, adult Schistosoma mansoni pairs migrate to the mesenteric veins where egg laying begins.
Using oral and ventral suckers, S. mansoni adult worms move extensively within the complex venous system draining the intestinal tract [2, 3]. The worms can enter blood vessels whose diameter is equivalent to their own [3]. These movements can impinge on the vascular endothelium [4] and can hamper and alter blood flow within the blood vessels [3]. Such outcomes can lead to endothelial cell stress and trigger the release by host cells of endogenous distress signals. These signals, collectively called damage-associated molecular patterns (DAMPs), indicate tissue damage to the host and can initiate primary immune responses. Extracellular nucleotides such as ATP are known to function as DAMPs by acting as endogenous tissue-derived signalling molecules that contribute to inflammation and immunity [5, 6]. Extracellular ATP can act as a proinflammatory immunomediator by acting on multiple immunological effector cell types including neutrophils, macrophages, dendritic cells, and lymphocytes (reviewed in [5, 7, 8]). It has been hypothesized that schistosome ectoenzymes can degrade exogenous ATP so as to impede host immune cell activation in the vicinity of the worms [9], and it has long been known that schistosome tegumental extracts do possess ATP and ADP hydrolyzing activity [10, 11]. In fact, the worms possess a panel of three ectoenzymes at the host/parasite interface that have been hypothesized to catabolize exogenous ATP. These are alkaline phosphatase (SmAP), phosphodiesterase (SmNPP-5), and ATP-diphosphohydrolase (SmATPDase1) [9]. By comparing the ATP-hydrolyzing abilities of worms that have had each of these genes suppressed, it has recently been shown that only the last enzyme in this list, i.e., SmATPDase1, is actually capable of cleaving exogenous ATP [12].
An additional concern for intravascular schistosomes, which can act as obstructions in the blood vessels, is the need to avoid promoting blood coagulation in their vicinity. Platelets play a central role in blood clotting and ATP can regulate platelet reactivity by way of direct action on platelet purinergic receptors [13]. In addition, ATP hydrolysis commonly leads to the generation of ADP and ADP is a major agonist of platelet recruitment and aggregation [14]. Furthermore, platelets themselves can damage schistosomes [15]. Therefore, the catabolism of exogenous ADP, in addition to ATP, would lead to the inhibition of platelet aggregation and thrombus formation around the worms. The three ectoenzymes at the host/parasite interface that were listed above have each been hypothesized to also catabolize ADP [9]. However, as for ATP cleavage, it has been shown that SmATPDase1 is the only one of the three that is actually capable of cleaving exogenous ADP [12].
While SmATPDase1 may interfere with host purinergic signalling, the enzyme might additionally (or instead) be important with regard to purine salvage. Schistosomes are unable to synthesize purines de novo [16]. The ATP catabolic pathway that is driven by SmATPDase1action in the external environment of the intravascular worms may yield purine derivatives in their vicinity that can then be easily imported from the host bloodstream [17]. In this manner, SmATPDase1 may contribute to parasite nutrition.
The ability of SmATPDase1 to hydrolyze ATP and ADP was confirmed following the functional characterization of enzymatically active, full-length recombinant SmATPDase1 in CHO-S cells [12]. The enzyme-degraded ATP and ADP in a cation-dependent manner. Optimal activity was seen at alkaline pH and the Km of the recombinant SmATPDase1 for ATP was 0.4 ± 0.02 mM and for ADP, 0.25 ± 0.02 mM. The work confirmed the ability of tegumental SmATPDase1, expressed on the tegumental surface of live intravascular life stages of the parasite, to hydrolyze exogenous ATP and ADP [12].
A second ATP-diphosphohydrolase homolog (SmATPDase2) has been described in S. mansoni [18]. Unlike SmATPDase1, which has been immunolocalized at the outer border of the tegument at the host/parasite interface [19, 20], SmATPDase 2 is found “in some internal cellular structure of the tegument syncytium” from where it has been hypothesized to be secreted into the exterior environment [18]. In this circumstance, SmATPDase2 might perform a similar function to that of SmATPDase1; by degrading exogenous ATP and ADP, this externalized enzyme too may be important in dampening host purinergic signalling pathways and/or in providing purine metabolites suitable for easy uptake as nourishment for the worms.
In this work, we classify both SmATPDase1 and SmATPDase2 as apyrases since they exhibit homology to cation-activated enzymes that catalyze the hydrolysis of ATP and ADP to generate AMP and inorganic phosphate [11, 10]. Sequence comparisons more formally position both proteins in the GDA1_CD39 superfamily (also known as the nucleotide triphosphate diphosphohydrolases, NTPDases).
In this work, we set out to determine whether degradation of the proinflammatory DAMP, ATP, as well as its prothrombotic derivative ADP—both purinergic signalling molecules—could be mediated by SmATPDase2 in addition to SmATPDase1. In this work, we employed RNA interference (RNAi) to suppress the expression of these genes in order to compare the ability of SmATPDase1-suppressed worms versus SmATPDase2-suppressed worms to cleave exogenous ATP and ADP.
Materials and methods
Parasites
Snails were provided by the Schistosome Research Reagent Resource Center for distribution by BEI Resources, NIAID, NIH: S. mansoni, strain NMRI-exposed Biomphalaria glabrata snails, strain NMRI, NR-21962. Cercariae were obtained from infected snails and isolated parasite bodies were prepared as described [21]. Parasites were cultured in complete DMEM/F12 medium supplemented with 10 % heat-inactivated fetal bovine serum, 200 U/ml penicillin and 200 μg/ml streptomycin, 0.2 μM Triiodo-L-thyronine, 1.0 μM serotonin, and 8 μg/ml human insulin. Parasites were maintained at 37 °C, in an atmosphere of 5 % CO2. Adult male and female parasites were recovered by perfusion from Swiss Webster mice that were infected with 125 cercariae, 7 weeks previously. Parasite eggs were isolated from infected mouse liver tissue, as described previously [22].
Treatment of parasites with siRNAs
Schistosomula and adult worms were treated with the following synthetic small interfering RNAs (siRNAs) targeting SmATPDase1 (GenBank accession number AY323529): 5′-GGACUUUAUGGUUGGGUAUCAGUGA-3′ or SmATPDase2 (GenBank accession number DQ868522): 5′-CUUGCACAAACAAUUGGUACAUUAG-3′. The control, irrelevant siRNA is 5′-CUUCCUCUCUUUCUCUCCCUUGUGA-3′.
To deliver the siRNAs, parasites (1,000 schistosomula or 10–12 adults/group) in 50–100 μl electroporation buffer (BioRad, CA) containing 5–10 μg siRNA, were electroporated in a 4-mm cuvette by applying a square wave with a single 20-ms impulse, at 125 V and at room temperature, as described [23]. Parasites were transferred to complete DMEM/F12 medium after electroporation. After overnight culture, medium was replaced with fresh rich medium (complete DMEM/F12).
Gene expression analysis
To assess the level of target gene suppression post-siRNA treatment, RNA was isolated from parasites and cDNA was synthesized using 1 μg of total RNA, an oligo(dT)20 primer and Superscript III RT (Invitrogen, CA). Gene expression was measured by quantitative real time PCR (qRT-PCR), using custom TaqMan gene expression systems from Applied Biosystems, CA. The procedure, involving total RNA extraction and qRT-PCR, has been described [24]. To detect SmATPDase1 expression, the following primers and probe were used: SmATPDase1 forward; 5′-CTGATGCCGTTATGAAGTTTTGCA-3′, SmATPDase1 reverse; 5′-GCAGTAAACCCTTGGTCAGATAATTTTG-3′, SmATPDase1 probe; 5′-FAM-AAAGAT GTGGCTAAAATT-3′. To detect SmATPDase2, the following primers and probe were used: SmATPDase2 forward; 5′ GGTTATGGATTCCCGGCAGATA 3′, SmATPDase2 reverse; 5′-TGAAAATAAGGCACCAAGACTCCAA-3′, SmATPDase2 probe; 5′-FAM-TTGGATTTTTTAGAAAAGTTAATTCT-3′. Alpha tubulin was used as the endogenous control gene for relative quantification, as described [25], employing the ΔΔCt method [26]. Results obtained from parasites treated with irrelevant siRNA were used for calibration. For graphical representation, the ΔΔCt values were normalized to controls and expressed as percentage difference.
SmATPDase assay: Pi determination
In this assay, the amount of Pi released following hydrolysis of ATP or ADP is determined. Both ATPase and ADPase activities were assayed in 200 μl reactions in 96-well microtiter plates at 37 °C. The standard assay buffer contains 20 mM HEPES buffer, pH 7.4, 1 % Triton X-100, 0.135 M NaCl, 5 mM KCl, and 1 mM CaCl2. Reactions were initiated by the addition of ATP or ADP nucleotide solution to a final concentration of 2 mM. Reactions were incubated at 37 °C for 30–120 min. At different time points, 10 μl aliquots were transferred to 190-μl ice-cold water and stored at −20 °C until analyzed. The amount of inorganic phosphate (Pi) released by the enzyme was determine using a Phosphate Colorimetric Assay Kit (BioVision) according to the manufacturer’s instructions. Activity was calculated by subtracting the minimal, nonspecific ATP or ADP hydrolysis that was detected in the absence of the enzyme. Nucleotide hydrolysis was linear with time under the assay conditions used.
SmATPDase assay: ATP quantification
Instead of measuring the amount of Pi generated by ATPDase action as described above, an alternative measure of enzyme activity is the amount of ATP remaining after the incubation period. For this analysis, parasites (12–15 adults), were washed three times in fresh culture medium before being incubated in medium containing 10 mM ATP at 5 % CO2 and 37 °C . The ATP concentration in the medium was determined after 55 h using an ATP chemiluminiscence assay kit (Sigma-Aldrich, MO), following the manufacturer’s instructions. Medium containing ATP and lacking parasites was incubated as above and served as a control. To look for the release of ATPase activity from cultured parasites, groups of adults (12–15) were first incubated in medium for 55 h at 5 % CO2 and 37 °C. Next, the parasites were removed and ATP (to 10 mM) was added to the medium. Finally, the ATP concentration was determined after a further 55-h incubation.
Developmental expression of SmATPDase1 and SmATPDase2 using qRT-PCR
The levels of expression of the SmATPDase1 and SmATPDase2 genes in different life cycle stages was measured by quantitative real time PCR (qRT-PCR) as described above and using the housekeeping gene triose phosphate isomerase as the endogenous control [27].
Data analysis
For statistical analysis, one-way analysis of variance (ANOVA) and Tukey as the post hoc test was used. Differences were considered significant with P values <0.05.
Results
Suppression of the SmATPDase1 and SmATPDase2 genes using RNAi
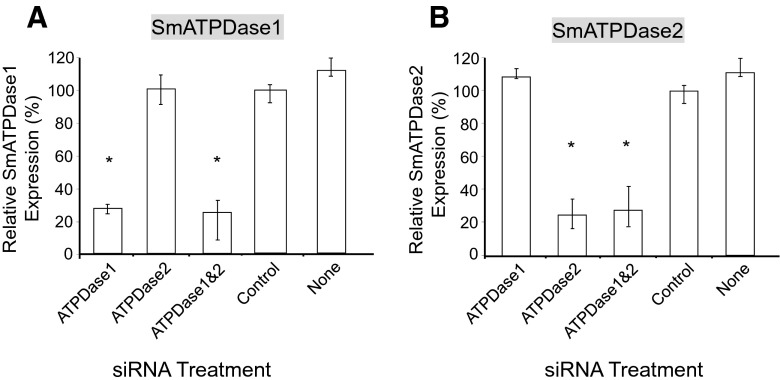
SmATPDase1 has recently been shown capable of cleaving exogenous ATP [12]. In order to determine whether SmATPDase2 is also involved in exogenous ATP catabolism, the genes encoding SmATPDase1 and SmATPDase2 were first subjected to suppression using RNAi. The genes were targeted for suppression alone or together by treatment with specific siRNAs via electroporation. Suppression was monitored by qRT-PCR, 7 days after treatment and results are shown in Fig. 1. Gene expression is depicted relative to the control group treated with an irrelevant siRNA (set at 100 %, Fig. 1). Figure 1a shows that the SmATPDase1 gene was ~75 % suppressed when targeted either alone or together with SmATPDase2. Similar findings were seen in regard to the SmATPDase2 gene, i.e., this gene was suppressed by ~75 % when it was targeted alone or when it was targeted together with SmATPDase1 (Fig. 1b). Similar levels of suppression are seen when either schistosomula (shown) or adult worms are treated with the siRNAs. Gene knockdown was specific; siRNAs targeting SmATPDase1 had no demonstrable effect on SmATPDase2 expression levels and vice versa.
Fig. 1.
SmATPDase1 (a) and SmATPDase2 (b) gene expressions (mean ± SD, n = 3) in schistosomula treated with SmATPDase1 (ATPDase1), SmATPDase2 (ATPDase2), SmATPDase1 plus SmATPDase2 (ATPDase1&2), control, or no (none) siRNA. *P < 0.05 compared to the control group. The data shown are representative of four independent experiments
SmATPDase suppression phenotype
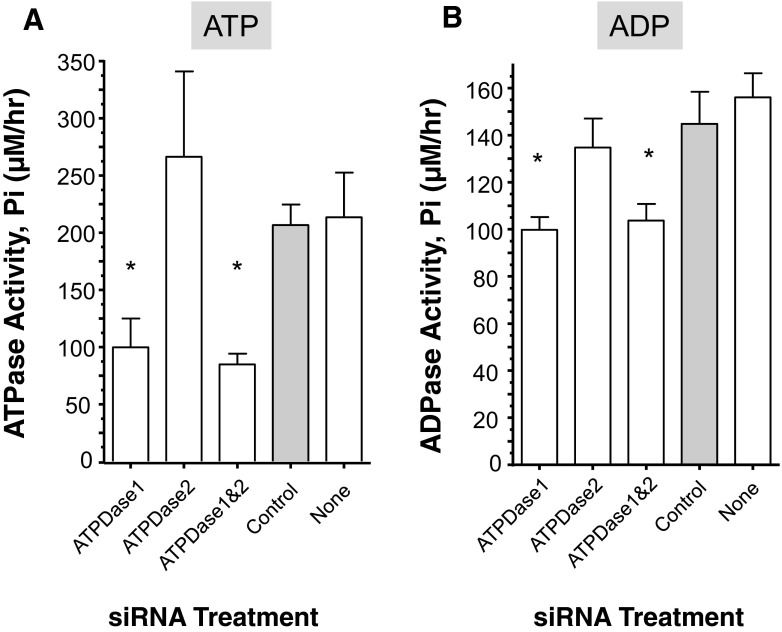
Suppression of the SmATPDase genes, alone or together, yielded no overt phenotype in cultured parasites. However, SmATPDase1-suppressed parasites were impaired in their ability to degrade exogenously added ATP to generate inorganic phosphate (Pi) in comparison to controls (P < 0.05, Fig. 2a). The SmATPDase1-suppressed parasites generated only ~50 % of the Pi that was produced by control parasites. In contrast, the SmATPDase2 suppressed parasites exhibit no such impairment in their ability to break down ATP and generate Pi. There is no significant difference between the amount of Pi generated by the SmATPDase2-suppressed parasites versus controls (Fig. 2a). In addition, doubly suppressed parasites (lane “ATPDase1&2”, Fig. 2a) show no significantly greater impairment in their ability to degrade ATP compared to parasites whose SmATPDase1 gene alone is suppressed (lane “ATPDase1”, Fig. 2a).
Fig. 2.
Enzyme activity (phosphate (Pi) release (μM/hr, mean ± SE, n = 5) from individual, living adult male schistosomes treated with SmATPDase1 (ATPDase1), SmATPDase2 (ATPDase2), SmATPDase1 plus SmATPDase2 (ATPDase1&2), control (grey bar), or no (none) siRNA and incubated for 1 h with 2-mM ATP (a) or ADP (b). *P < 0.05 compared to the control group .The data shown are representative of three independent experiments
SmATPDase1-suppressed parasites were additionally impaired in their ability to degrade exogenously added ADP when compared to controls (P < 0.05, Fig. 2b). Results are similar to those just described for ATP but are less dramatic: SmATPDase1-suppressed parasites generated ~70 % of the Pi that was produced by control parasites, when ADP is the substrate. SmATPDase2-suppressed parasites exhibit no appreciable diminution in their ability to break down ADP and generate Pi; there is no significant difference between the amount of Pi generated by the SmATPDase2-suppressed parasites versus controls (Fig. 2b). Doubly suppressed parasites (lane “ATPDase1&2”, Fig. 2b) are not significantly impaired in their ability to degrade ADP compared to parasites with only their SmATPDase1 gene suppressed (lane “ATPDase1”, Fig. 2b).
ATP degradation in conditioned medium
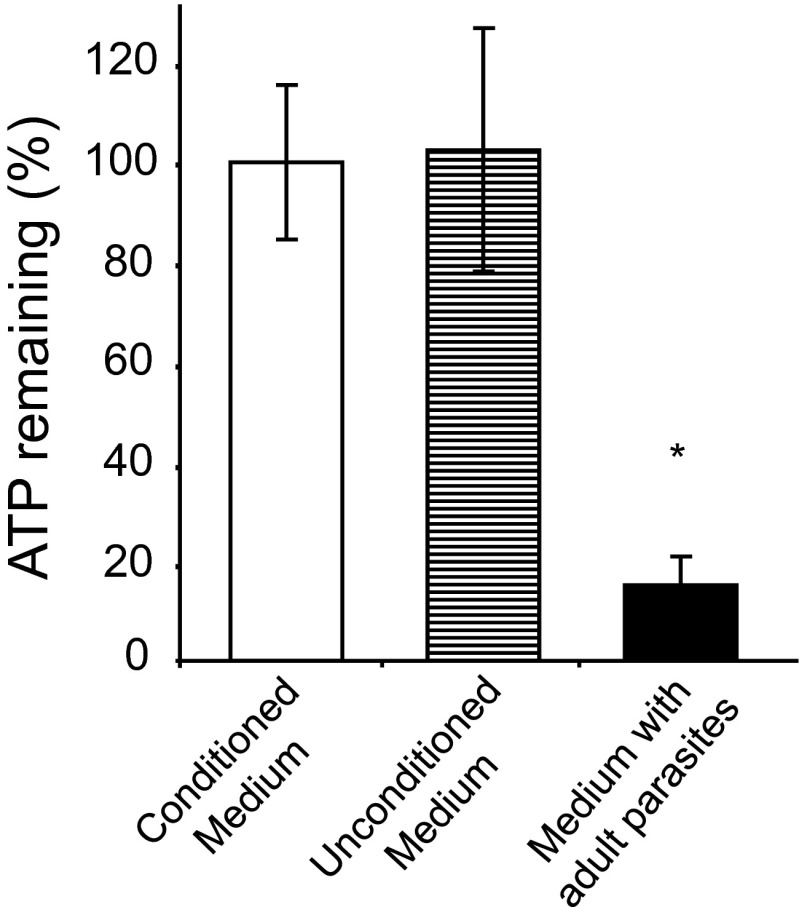
To determine if an ATP degrading molecule is secreted by the parasites, ATP breakdown was monitored in conditioned medium (i.e., medium that had previously contained 10–15 adult parasites, incubated for 55 h at 37 °C) versus in unconditioned medium (i.e., control medium that had been incubated for 55 hours at 37 °C but in the absence of parasites). ATP (10 mM) was then added to both samples which were incubated for a further 55 h. Figure 3 shows that the percentage of ATP remaining in conditioned medium (Fig. 3, white bar) is essentially the same as in unconditioned medium (Fig. 3, striped bar) after this 55-h incubation. ATP levels remain at almost 100 %, showing that there was no appreciable breakdown of ATP in either case. In contrast, worms recovered from the conditioned medium and then incubated in fresh unconditioned medium containing 10 mM ATP retain their ATP-degrading capability. Less than 20 % of the ATP remains in the medium containing worms, at the end of the experiment (Fig. 3, black bar).
Fig. 3.
ATP remaining (% ± SD, n = 3) in medium that previously contained a group of 10 adult male schistosomes for 55 h (i.e., conditioned medium, white bar) or control medium that had not contained parasites (i.e., unconditioned medium, striped bar) or fresh medium containing the 10 adult male worms (i.e., medium with adult parasites, black bar). Measurement was taken after 55 h incubation at 37 °C. *P < 0.05 compared to the conditioned medium and unconditioned medium groups. The data shown are representative of three independent experiments
SmATPDase 1 and SmATPDase2 developmental expression
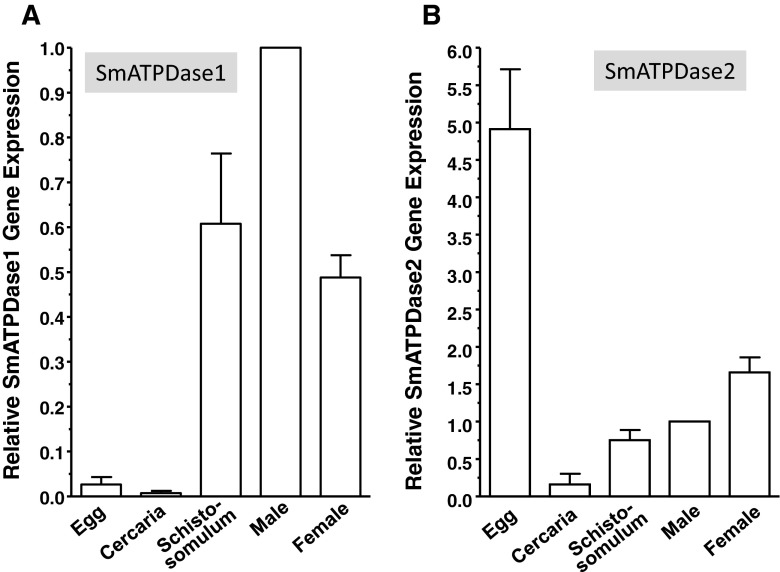
The developmental expression of SmATPDase1 and SmATPDase2 was examined in several schistosome life stages by qRT-PCR, and the results are shown in Fig. 4. The genes show quite divergent expression profiles. Of the various life stages tested, the relative gene expression of SmATPDase1 was negligible in eggs and cercariae (Fig. 4a). The highest expression was in the intravascular life stages particularly in adult males. In contrast, SmATPDase2 shows highest relative expression in eggs (Fig. 4b). Furthermore, the expression level of SmATPDase2 in eggs is >60-fold higher than the expression level of SmATPdase1 in the same life stage.
Fig. 4.
Developmental expression of SmATPDase1 (a) and SmATPDase2 (b) as determined by qRT-PCR (mean ± SD, n = 3) in the following life cycle stages: egg, cercaria, schistosomulum, and adult male and female. Data are expressed relative to adult males, set at 1. For SmATPDase1, all stages are significantly different from all other stages (P < 0.05) except egg versus cercariae and schistosomulum versus female. For SmATPDase2, all stages are significantly different from all other stages (P < 0.05) with the exception of schistosomulum v male. The data shown are representative of three independent experiments
Discussion
S. mansoni is an intravascular parasite that can survive in an infected host for years, sometimes decades. With the aid of suckers, tegumental tubercles and spines, the parasites directly impinge on the vascular endothelium as they migrate throughout the mesenteries [4]. Adult worms are large relative to the size of some mesenteric venules into which they travel and they can stress the endothelium by impeding blood flow [3]. Such direct and indirect impacts of schistosomes in the vasculature have implications for endothelial health and hemostasis. It has been hypothesized that endothelial cells stressed by schistosomes may indicate this condition with the release of metabolites that signal damage in the host [9]. One such “damage associated molecular pattern” (DAMP) is ATP. In the extracellular environment, ATP is a potent proinflammatory mediator which could promote immune cell activation and chemotaxis to the location of the worms to allow the host to debilitate the parasites and contain the infection. We hypothesize that schistosomes possess host-exposed, nucleotide metabolizing ectoenzymes on their surfaces to degrade any extraneous ATP, specifically to prevent the host from attracting and activating immune cells in their vicinity [9].
In addition, by acting as obstructions in blood vessels to impede blood flow, schistosomes may promote blood clot formation. Platelets play a key role in blood clotting and the ATP hydrolysis product ADP is a major agonist of platelet recruitment and aggregation [14]. Additionally, platelets have been shown to be directly damaging to schistosomes [15]. The surface-exposed, nucleotide metabolizing ectoenzymes that are predicted to degrade extraneous ATP, could likewise act on ADP to prevent the host from mobilizing the blood coagulation machinery to activate platelets in their vicinity [9]. Therefore, the catabolism of ATP and ADP by intravascular schistosomes may inhibit both ATP-mediated inflammation and ADP-mediated thrombosis.
It has long been known that schistosome tegumental extracts do possess ATP- and ADP-hydrolyzing capabilities and that living worms can deplete exogenous ATP and ADP [10], and we have confirmed that living parasites (both adults and schistosomula) can degrade exogenous ATP, ADP, and AMP [12].
In the case of vertebrates, ectoenzymes belonging to three different major classes are known to engage in the extracellular ATP degradation pathway [7]. Schistosomes are known to possess ectoenzymes belonging to each of these classes in their tegumental membranes, at the host/parasite interface [9]. By comparing the ATP- and ADP-hydrolyzing ability of parasites in which each of the three ectoenzyme genes was suppressed using RNAi, only one of them (the ATP diphosphohydrolase SmATPDase1) was shown to be capable of cleaving these substrates [12].
In the work just described, the ATP- and ADP- degrading abilities of the SmATPDase1-suppressed parasites was reduced by ~50 % relative to controls but was not completely abolished [12]. We attribute this to the fact that SmATPDase1 is not 100 % suppressed following RNAi treatment. Residual SmATPDase1 likely contributes to the ATP and ADP cleavage that is still observed in the SmATPDase1-suppressed group. An alternative explanation is that an additional ATP- and ADP-cleaving enzyme is present that contributed to the ATP and ADP hydrolysis observed in the SmATPDase1-suppressed group. The second reported S. mansoni tegumental ATP diphosphohydrolase homolog (designated SmATPDase2) is a likely candidate to fulfill such a role. While this second enzyme (SmATPDase2) is not a tegumental surface membrane protein (unlike SmATPDase1), it has been reported to be released by the worms [18]. In this capacity, it might act on exogenous ATP and/or ADP. To examine this possibility in this work, we employed RNAi to suppress the expression of the SmATPDase1 as well as SmATPDase2 genes (alone or in combination) and then we looked at the ATP- and ADP-degradation capabilities of the parasites. High levels of specific target gene suppression were recorded in both cases. The results of this work provided no evidence that SmATPDase2 contributes to exogenous ATP or ADP degradation. It was only in those instances in which SmATPDase1 was suppressed that there was a significant diminution in ATP or ADP cleavage levels compared with controls. SmATPDase2 suppression did not impact the ability of the parasites to degrade exogenous ATP or ADP.
To formally look for the presence of any ATP-cleaving activity released by schistosomes, conditioned medium (i.e., medium which had contained a group of schistosome adults for 55 h) was loaded with ATP (to 10 mM), and after a further 55-h incubation, the amount of ATP remaining was ascertained. This work revealed essentially no change in ATP levels at the end versus at the beginning of this experiment. The conditioned medium had no ATP degrading ability. In contrast, the worms removed from the conditioned medium and placed in ATP-containing fresh medium, retained their ability to break down ATP. This result indicates that SmATPDase1 is not released by schistosomes even after prolonged incubation, and that if SmATPDase2 is released, it is not capable of degrading ATP under the conditions used. Further evidence that SmATPDase2 is not secreted by schistosomes comes from proteomic analysis of material released by schistosomes following 96 h in culture; SmATPDase2 is not among the many proteins detected [28]. In addition, protein sequence analysis reveals that, unlike SmATPDase1, SmATPDase2 is devoid of a predicted canonical signal peptide (and any transmembrane domain) [18].
Since both SmATPDase1 and SmATPDase2 possess conserved catalytic regions, characteristic of NTPDase family members [10, 11], it seems likely that SmATPDase2 (and not just SmATPDase1) is capable of degrading nucleotide tri- and di-phosphates. Since, as shown here, SmATPDase2 has no demonstrable impact on ATP and ADP levels external to the worm, we presume that this enzyme functions in the internal tissues of the parasites, and especially in eggs (see below). In silico analysis of the SmATPDase1 protein sequence reveals that it has two transmembrane domains and a high structural similarity to the membrane-associated NTPDases of other organisms [29]. In contrast, analysis of the amino acid sequence of SmATPDase2 shows that it more closely resembles intracellular and soluble enzymes of the NTPDase 5 and 6 families [18]. Proteins in these families have been reported to support glycosylation reactions in the Golgi apparatus or the endoplasmic reticulum [30–32]. It is possible that SmATPDase2 likewise participates in Golgi and/or ER glycosylation reactions inside schistosomes.
The developmental expression pattern of SmATPDase1 is consistent with the hypothesis that the protein plays an important role in modulating nucleotide di- and tri-phosphate levels around the parasites in the blood stream, since its highest relative expression is in the intravascular life stages. Males in particular have a high relative level of SmATPDase1 expression, and it may be that one function of the schistosome male is to use tegumental surface enzymes like SmATPDase1 to help maintain an anti-inflammatory and anti-thrombotic environment around him and around any female partner within his gynacophoric canal. The developmental expression pattern of SmATPDase2 is quite different from that of SmATPDase1; its highest relative expression is in eggs. This is consistent with strong immunolocalization of SmATPDase2 throughout the S. mansoni egg [33].
An ability to cleave ATP and/or ADP in the extracellular environment has been described in several pathogens including bacteria, protozoans, and nematodes [34–40]. This conserved feature of several pathogens is also found in schistosomes, here mediated by SmATPDase1. In all cases, this conserved ability to control local ATP and ADP levels via ectoenzymes like SmATPDase1 likely impedes host purinergic signalling and helps control inflammation and thrombosis in the local environment of each pathogen. In addition, the enzyme may help to generate purine metabolites close to the worms that can be conveniently taken in as food. Further experiments will be required to test these hypotheses in order to uncover the definitive impact of SmATPDase1 on host physiology
Acknowledgments
This work was supported by grant AI-056273 from the NIH-NIAID. Infected snails were provided by BRI via the NIAID schistosomiasis resource center under NIH-NIAID Contract No. HHSN272201000005I.
Footnotes
Akram A. Da’dara and Rita Bhardwaj contributed equally to this work.
References
- 1.Vennervald BJ, Dunne DW. Morbidity in schistosomiasis: an update. Curr Opin Infect Dis. 2004;17(5):439–447. doi: 10.1097/00001432-200410000-00009. [DOI] [PubMed] [Google Scholar]
- 2.Pellegrino J, Coelho PM. Schistosoma mansoni: wandering capacity of a worm couple. J Parasitol. 1978;64(1):181–182. doi: 10.2307/3279647. [DOI] [PubMed] [Google Scholar]
- 3.Bloch EH. In vivo microscopy of schistosomiasis. II. Migration of Schistosoma mansoni in the lungs, liver, and intestine. AmJTrop Med Hyg. 1980;29(1):62–70. [PubMed] [Google Scholar]
- 4.Smith JH, von Lichtenberg F. Observations on the ultrastructure of the tegument of Schistosoma mansoni in mesenteric veins. AmJTrop Med Hyg. 1974;23(1):71–77. doi: 10.4269/ajtmh.1974.23.71. [DOI] [PubMed] [Google Scholar]
- 5.Hanley PJ, Musset B, Renigunta V, Limberg SH, Dalpke AH, Sus R, Heeg KM, Preisig-Muller R, Daut J. Extracellular ATP induces oscillations of intracellular Ca2+ and membrane potential and promotes transcription of IL-6 in macrophages. Proc Natl Acad Sci U S A. 2004;101(25):9479–9484. doi: 10.1073/pnas.0400733101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lohman AW, Billaud M, Isakson BE. Mechanisms of ATP release and signalling in the blood vessel wall. Cardiovasc Res. 2012;95(3):269–280. doi: 10.1093/cvr/cvs187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bours MJ, Swennen EL, Di Virgilio F, Cronstein BN, Dagnelie PC. Adenosine 5'-triphosphate and adenosine as endogenous signaling molecules in immunity and inflammation. Pharmacol Ther. 2006;112(2):358–404. doi: 10.1016/j.pharmthera.2005.04.013. [DOI] [PubMed] [Google Scholar]
- 8.Yegutkin GG. Nucleotide- and nucleoside-converting ectoenzymes: important modulators of purinergic signalling cascade. Biochim Biophys Acta. 2008;1783(5):673–694. doi: 10.1016/j.bbamcr.2008.01.024. [DOI] [PubMed] [Google Scholar]
- 9.Bhardwaj R, Skelly PJ. Purinergic signaling and immune modulation at the schistosome surface? Trends Parasitol. 2009;25(6):256–260. doi: 10.1016/j.pt.2009.03.004. [DOI] [PubMed] [Google Scholar]
- 10.Vasconcelos EG, Nascimento PS, Meirelles MN, Verjovski-Almeida S, Ferreira ST. Characterization and localization of an ATP-diphosphohydrolase on the external surface of the tegument of Schistosoma mansoni. Mol Biochem Parasitol. 1993;58(2):205–214. doi: 10.1016/0166-6851(93)90042-V. [DOI] [PubMed] [Google Scholar]
- 11.Vasconcelos EG, Ferreira ST, Carvalho TM, Souza W, Kettlun AM, Mancilla M, Valenzuela MA, Verjovski-Almeida S. Partial purification and immunohistochemical localization of ATP diphosphohydrolase from Schistosoma mansoni. Immunological cross-reactivities with potato apyrase and Toxoplasma gondii nucleoside triphosphate hydrolase. J Biol Chem. 1996;271(36):22139–22145. doi: 10.1074/jbc.271.36.22139. [DOI] [PubMed] [Google Scholar]
- 12.Da'dara AA, Bhardwaj R, Ali YB, Skelly PJ. Schistosome tegumental ecto-apyrase (SmATPDase1) degrades exogenous pro-inflammatory and pro-thrombotic nucleotides. Peer J. 2014;2:e316. doi: 10.7717/peerj.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mahaut-Smith MP, Ennion SJ, Rolf MG, Evans RJ. ADP is not an agonist at P2X(1) receptors: evidence for separate receptors stimulated by ATP and ADP on human platelets. Br J Pharmacol. 2000;131(1):108–114. doi: 10.1038/sj.bjp.0703517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gachet C. Regulation of platelet functions by P2 receptors. Annu Rev Pharmacol Toxicol. 2006;46:277–300. doi: 10.1146/annurev.pharmtox.46.120604.141207. [DOI] [PubMed] [Google Scholar]
- 15.Joseph M, Auriault C, Capron A, Vorng H, Viens P. A new function for platelets: IgE-dependent killing of schistosomes. Nature. 1983;303(5920):810–812. doi: 10.1038/303810a0. [DOI] [PubMed] [Google Scholar]
- 16.Levy MG, Read CP. Purine and pyrimidine transport in Schistosoma mansoni. J Parasitol. 1975;61(4):627–632. doi: 10.2307/3279455. [DOI] [PubMed] [Google Scholar]
- 17.Levy MG, Read CP. Relation of tegumentary phosphohydrolase to purine and pyrimidine transport in Schistosoma mansoni. J Parasitol. 1975;61(4):648–656. doi: 10.2307/3279457. [DOI] [PubMed] [Google Scholar]
- 18.Levano-Garcia J, Mortara RA, Verjovski-Almeida S, DeMarco R. Characterization of Schistosoma mansoni ATPDase2 gene, a novel apyrase family member. Biochem Biophys Res Commun. 2007;352(2):384–389. doi: 10.1016/j.bbrc.2006.11.023. [DOI] [PubMed] [Google Scholar]
- 19.Braschi S, Curwen RS, Ashton PD, Verjovski-Almeida S, Wilson A. The tegument surface membranes of the human blood parasite Schistosoma mansoni: a proteomic analysis after differential extraction. Proteomics. 2006;6(5):1471–1482. doi: 10.1002/pmic.200500368. [DOI] [PubMed] [Google Scholar]
- 20.Braschi S, Wilson RA. Proteins exposed at the adult schistosome surface revealed by biotinylation. Mol Cell Proteomics MCP. 2006;5(2):347–356. doi: 10.1074/mcp.M500287-MCP200. [DOI] [PubMed] [Google Scholar]
- 21.Skelly PJ, Da'dara A, Harn DA. Suppression of cathepsin B expression in Schistosoma mansoni by RNA interference. Int J Parasitol. 2003;33(4):363–369. doi: 10.1016/S0020-7519(03)00030-4. [DOI] [PubMed] [Google Scholar]
- 22.Hackett F. The culture of Schistosoma mansoni and production of life cycle stages. In: Hyde JE, editor. Protocols in Molecular Parasitology. Totowa: Humana Press Inc.; 1993. pp. 89–99. [DOI] [PubMed] [Google Scholar]
- 23.Krautz-Peterson G, Radwanska M, Ndegwa D, Shoemaker CB, Skelly PJ. Optimizing gene suppression in schistosomes using RNA interference. Mol Biochem Parasitol. 2007;153(2):194–202. doi: 10.1016/j.molbiopara.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 24.Ndegwa D, Krautz-Peterson G, Skelly PJ. Protocols for gene silencing in schistosomes. Exp Parasitol. 2007;117(3):284–291. doi: 10.1016/j.exppara.2007.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Krautz-Peterson G, Simoes M, Faghiri Z, Ndegwa D, Oliveira G, Shoemaker CB, Skelly PJ. Suppressing glucose transporter gene expression in schistosomes impairs parasite feeding and decreases survival in the mammalian host. PLoS Pathog. 2010;6(6):e1000932. doi: 10.1371/journal.ppat.1000932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCt Method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 27.Krautz-Peterson G, Radwanska M, Ndegwa D, Shoemaker CB, Skelly PJ. Optimizing gene suppression in schistosomes using RNA interference. Mol Biochem Parasitol Notes. 2007;153(2):194–202. doi: 10.1016/j.molbiopara.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 28.Hall SL, Braschi S, Truscott M, Mathieson W, Cesari IM, Wilson RA. Insights into blood feeding by schistosomes from a proteomic analysis of worm vomitus. Mol Biochem Parasitol. 2011;179(1):18–29. doi: 10.1016/j.molbiopara.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 29.DeMarco R, Kowaltowski AT, Mortara RA, Verjovski-Almeida S. Molecular characterization and immunolocalization of Schistosoma mansoni ATP-diphosphohydrolase. Biochem Biophys Res Commun. 2003;307(4):831–838. doi: 10.1016/S0006-291X(03)01268-3. [DOI] [PubMed] [Google Scholar]
- 30.Abeijon C, Yanagisawa K, Mandon EC, Hausler A, Moremen K, Hirschberg CB, Robbins PW. Guanosine diphosphatase is required for protein and sphingolipid glycosylation in the Golgi lumen of Saccharomyces cerevisiae. J Cell Biol. 1993;122(2):307–323. doi: 10.1083/jcb.122.2.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Braun N, Fengler S, Ebeling C, Servos J, Zimmermann H. Sequencing, functional expression and characterization of rat NTPDase6, a nucleoside diphosphatase and novel member of the ecto-nucleoside triphosphate diphosphohydrolase family. Biochem J. 2000;351(Pt 3):639–647. doi: 10.1042/0264-6021:3510639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Knowles AF. The single NTPase gene of Drosophila melanogaster encodes an intracellular nucleoside triphosphate diphosphohydrolase 6 (NTPDase6) Arch Biochem Biophys. 2009;484(1):70–79. doi: 10.1016/j.abb.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 33.Mendes RG, Gusmao MA, Maia AC, Detoni Mde L, Porcino GN, Soares TV, Juliano MA, Juliano L, Coelho PM, Lenzi HL, Faria-Pinto P, Vasconcelos EG. Immunostimulatory property of a synthetic peptide belonging to the soluble ATP diphosphohydrolase isoform (SmATPDase 2) and immunolocalisation of this protein in the Schistosoma mansoni egg. Mem Inst Oswaldo Cruz. 2011;106(7):808–813. doi: 10.1590/S0074-02762011000700005. [DOI] [PubMed] [Google Scholar]
- 34.Bermudes D, Peck KR, Afifi MA, Beckers CJ, Joiner KA. Tandemly repeated genes encode nucleoside triphosphate hydrolase isoforms secreted into the parasitophorous vacuole of Toxoplasma gondii. J Biol Chem. 1994;269(46):29252–29260. [PubMed] [Google Scholar]
- 35.Berredo-Pinho M, Peres-Sampaio CE, Chrispim PP, Belmont-Firpo R, Lemos AP, Martiny A, Vannier-Santos MA, Meyer-Fernandes JR. A Mg-dependent ecto-ATPase in Leishmania amazonensis and its possible role in adenosine acquisition and virulence. Arch Biochem Biophys. 2001;391(1):16–24. doi: 10.1006/abbi.2001.2384. [DOI] [PubMed] [Google Scholar]
- 36.de Jesus JB, de Sa Pinheiro AA, Lopes AH, Meyer-Fernandes JR. An ectonucleotide ATP-diphosphohydrolase activity in Trichomonas vaginalis stimulated by galactose and its possible role in virulence. Z Naturforsh C J Biosci. 2002;57(9–10):890–896. doi: 10.1515/znc-2002-9-1022. [DOI] [PubMed] [Google Scholar]
- 37.Zaborina O, Li X, Cheng G, Kapatral V, Chakrabarty AM. Secretion of ATP-utilizing enzymes, nucleoside diphosphate kinase and ATPase, by Mycobacterium bovis BCG: sequestration of ATP from macrophage P2Z receptors? Mol Microbiol. 1999;31(5):1333–1343. doi: 10.1046/j.1365-2958.1999.01240.x. [DOI] [PubMed] [Google Scholar]
- 38.Punj V, Zaborina O, Dhiman N, Falzari K, Bagdasarian M, Chakrabarty AM. Phagocytic cell killing mediated by secreted cytotoxic factors of Vibrio cholerae. Infect Immun. 2000;68(9):4930–4937. doi: 10.1128/IAI.68.9.4930-4937.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thammavongsa V, Kern JW, Missiakas DM, Schneewind O. Staphylococcus aureus synthesizes adenosine to escape host immune responses. J Exp Med. 2009;206(11):2417–2427. doi: 10.1084/jem.20090097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Melnikov A, Zaborina O, Dhiman N, Prabhakar BS, Chakrabarty AM, Hendrickson W. Clinical and environmental isolates of Burkholderia cepacia exhibit differential cytotoxicity towards macrophages and mast cells. Mol Microbiol. 2000;36(6):1481–1493. doi: 10.1046/j.1365-2958.2000.01976.x. [DOI] [PubMed] [Google Scholar]