Abstracts—Plenary Lectures
Plenary Lecture 1
The field of purinergic signalling is expanding in many different directions
By Geoff Burnstock
Autonomic Neuroscience Centre, University College Medical School, Rowland Hill Street, London NW3 2PF, UK; Department of Pharmacology and Therapeutics, The University of Melbourne, Australia
Aims of talk:
• To identify areas of high current interest;
• To highlight controversial areas that need resolution;
• To point out areas which are, in my opinion, very important, but are largely neglected.
The talk will start with basic science topics and then focus on the pathophysiology and therapeutic potential of purinergic signalling.
Plenary Lecture 2 “Burnstock Lecture”
Purinergic receptors in cancer and inflammation: from Rudolph Virchow to Geoff Burnstock
Francesco Di Virgilio
Department of Morphology, Surgery and Experimental Medicine, University of Ferrara, Italy
Inflammation and cancer have a long-standing association. Until recently, it was generally thought that inflammation was a protective response, or in the worst case the epiphenomenon of body reaction to cancer with no relevant consequences. This changed dramatically following the seminal observation of Francis Balkwill that TNF-ko mice were surprisingly less prone to cancer than wt [1]. Thousand reports followed ever since highlighting the cancer-promoting effects of inflammation, a finding to be honest that was well known to practicing physicians. The cancer-promoting effect of inflammation was further dissected into its mechanistic components with the discovery of those cells responsible for immunosuppression within the cancer microenvironment, the myeloid-derived suppressor cells (MDSCs). Men are often oblivious of the contributions of our ancestors. The relationship between inflammation and cancer is a paradigmatic example of this. As early as 1858 Virchow had clearly suggested that there was a causal link between chronic inflammation and cancer [2], but we have simply forgotten this crucial observation. Now, we are still struggling to fully understand the role of inflammation in cancer progression and metastatic spreading, and try to exploit it to the benefit of the patient. Then, Geoff Burnstock came to show us that purines have a much wider role to play in physiology and pathology than ever thought before [3]. Investigation of the pleiotropic functions of extracellular nucleotides (and their receptors, the P2 receptors) revealed that they are fundamental constituents of the inflammatory as well as tumor microenvironment [4]. Why? Do nucleotides support tumor growth as they support inflammation? Do they modulate responses of inflammatory cells in the tumor interstitium? And the P2 receptors? Do they participate in anti-tumor responses or rather support cancer progression? Does the tumor-bearing host benefit from P2 receptor blockade? And what we know of cancer growth in P2-KO animals? These are currently hot issues that are enormously increasing our understanding of cancer, besides of course purinergic signalling. Rudolf Virchow
Rudolf Virchow Geoff Burnstock
Geoff Burnstock
References
1. Moore et al (1999) Nat Med 5:828–831
2. Virchow R (1858) Die cellularpathologie
3. Burnstock G (1970) Br J Pharmacol 40:668–688
4. Burnstock G, Di Virgilio F (2013) Purinergic Signal 9:491–540
Plenary Lecture 3 “John Daly Lecture”
Structure-based discovery of novel ligands of GPCRs: adenosine and P2Y receptors
Kenneth A. Jacobson
Molecular Recognition Section, Laboratory of Bioorganic Chemistry, NIDDK, National Institutes of Health, Bethesda, Maryland 20892
Extracellular nucleosides and nucleotides acting at adenosine receptors (ARs) and P2Y receptors (P2YRs), respectively, both G protein-coupled receptors (GPCRs), are important signals to modulate biological processes in many organs and tissues. We establish structure activity relationships in both receptor families, in order to provide selective agents as pharmacological probes and potential therapeutic agents. We utilize detailed structural information derived from the X-ray crystallographic structures within these families to enable discovery of novel ligands, to guide modification of known agonists and antagonists and to predict of polypharmacology at off-target GPCRs associated with otherwise ‘selective’ ligands. The most recent examples include the P2Y12 receptor (P2Y12R), which is a target for anti-thrombotic drugs Plavix, Effient and Brilinta [1]. Comparison of agonist-bound and antagonist-bound forms of the P2Y12R indicates unprecedented structural plasticity in the outer portions of the transmembrane domains and the extracellular loops. Structures of the A2AAR have been effectively applied to homology modeling of closely related A1AR and A3AR subtypes. Nonphosphate-containing ligands of the P2YRs, such as the selective P2Y14R antagonist PPTN [2], are desired for bioavailability and increased stability. Among nucleoside and nucleotide ligands, conformational constraint of the normally flexible ribose moiety by synthesis of bicyclic analogues increased the selectivity. Increased conformational rigidity of A3AR agonists allows the exploration of interaction of specific regions of the nucleoside analogues with the target and off-target GPCRs, such as biogenic amine receptors [3]. Plasticity of the A3AR structure with respect to TM2 is proposed, based on molecular modeling, to accommodate highly rigidified ligands. Novel fluorescent derivatives of high affinity GPCR ligands are useful tool compounds for characterization of receptors and their oligomeric assemblies [4,5]. Fluorescent probes are useful for characterization of GPCRs in living cells by flow cytometry. Fluorescent agonists but not antagonists are highly internalized consistent with receptor internalization. Some of the numerous therapeutic concepts associated with selective modulation of ARs and P2YRs, such as selective A3AR agonists for treating neuropathic pain [6], inflammatory diseases and liver cancer [7], will be discussed. Thus, the 3D knowledge of receptor binding and activation is facilitating drug discovery for GPCRs.
References
1. Zhang K et al (2014) Nature 509:115 and 509:119
2. Barrett M et al (2013) Mol Pharmacol 84:41
3. Paoletta S, Tosh D et al (2014) PLoS One. doi: 10.1371/journal.pone.0097858
4. Jayasekara PS et al (2013) Med Chem Comm 4:1156
5. Fernández-Dueñas V, Gómez-Soler M, Jacobson KA, Kumar TS, Fuxe K, Borroto-Escuela DO, Ciruela FJ (2012) Neurochem 123:373
6. Chen Z et al (2012) FASEB J 26:1855
7. Fishman P et al (2012) Drug Disc Today 17:359
Plenary Lecture 4
Therapeutic manipulation of hypoxia-A2-adenosinergic suppression and redirection of immune response
Michail V. Sitkovsky
New England Inflammation and Tissue Protection Institute, Northeastern University; DFCI, Cancer Vaccine Center, Harvard Institutes of Medicine, Boston, MA, USA
Hypoxia-A2-adenosinergic immunosuppression and re-direction of immune response was initially recognized to be critical and non-redundant in protecting normal tissues from excessive inflammatory damage and autoimmunity. This pathway was also shown to protect cancerous tissues from the anti-tumor immune response. Conclusive implication of A2A adenosine receptor in immunoregulation was provided in genetic in vivo experiments that also served to provide genetic evidence for the immunosuppressive role of pathophysiologically-induced extracellular adenosine, a “metabokine”. The power and versatility of this pathway is confirmed by other scientists and by bacteria that evolved to hijack the A2-adenosinergic immunosuppression for their own protection.
The hypoxia-A2-adenosinergic explanation for the long-standing “Hellstrom paradox,” the peaceful coexistence of tumors and anti-tumor killer cells in the same cancer patient, suggested the novel approach of using “anti-hypoxia-A2-adenosinergic” immunological co-adjuvants. These co-adjuvants unleash the full anti-tumor or anti-pathogen potential of T and NK cells by : i) inhibiting hypoxia-HIF-1alpha signaling, ii) inhibiting generation of extracellular adenosine by CD39/CD73 ecto-enzymes, iii) degrading extracellular adenosine, and iv) antagonizing A2A and A2B adenosine receptors. Recent important studies of human tumors and pre-clinical data added to growing support to combine these co-adjuvants with cancer immunotherapies.
The discovery and medical applications of targeting the hypoxia-A2-adenosinergic pathway were made possible by earlier accomplishments of an elite group of multidisciplinary scientists who succeeded in developing the sophisticated knowledge and tools to study the role of extracellular ATP and adenosine in biological processes despite the overwhelming skepticism by the mainstream.
Plenary lecture 5
Paracrine purinergic signaling in CNS
Ditte Lovatt and Maiken Nedergaard
Center for Translational Neuromedicine, University of Rochester Medical Center, Rochester, NY, USA
Purines act as extracellular signaling molecules in the brain and are involved in a number of diverse functions, such as sleep, learning and memory, myelination and vasodilation. The diversity of functional roles carried out by ATP, ADP and adenosine questions how cross-talk among them is minimized. While it is known that the structurally complex microenvironment of the brain forms anatomical signaling niches between different cells, or cell types, less is known about the molecular composition of such signaling domains in relation to purinergic signaling. Here, we performed a molecular analysis of the cell type specific pathways involved in purinergic signaling, including the cellular sources of ATP, ADP and adenosine as ligands, the purine receptors sensing purines, and ecto-enzymes and transporters metabolizing purines and eliminating extracellular adenosine. Given the vast number of purinergic effector molecules, we established a brain transcriptome databank from FACS-purified astrocytes, microglia, oligodendrocytes, endothelial cells and pericytes from cortical brain dissociates. Surprisingly, we found that ecto-enzymatic sources of adenosine are strictly compartmentalized to oligodendrocytes, and that an ATP-to-adenosine pathway is not ubiquitously present among all cell types. We propose that cross-talk among adenosine pathways is likely minimized through the combined effect of anatomical signaling niches and cell type specific mosaic expression of purinergic activities therein.
Plenary Lecture 6
Structure and function of ectonucleotidases
Norbert Sträter1,*, Karen Knapp, Jan Pippel, Ulrike Krug, Christoph Döhler, Michel Krauss, Christa Müller2 and Matthias Zebisch3
1Institute of Bioanalytical Chemistry, University of Leipzig, Deutscher Platz 5, 04103 Leipzig, Germany;2Pharmaceutical Institute, An der Immenburg 4, 53121 Bonn, Germany;3Division of Structural Biology, University of Oxford, Roosevelt Drive, Oxford OX3 7BN, England
For most of the enzymes that metabolize extracellular nucleotides, detailed models for the structures and catalytic mechanisms have become available in recent years [1]. In my talk, I will first give an overview of these structures and chemical mechanisms in comparison. After that I will focus on the ecto-5′-nucleotidase (eN, CD73) since the other enzymes are mostly covered by the ectonucleotidase session of the meeting.
The homodimeric CD73 is attached via a GPI-anchor at the C-terminus to the cell membrane. It catalyzes the hydrolysis of AMP and is the primary source of extracellular adenosine. The effects of adenosine are often opposite to those of ATP and CD73 activity has an important regulatory role in processes such as inflammation, hypoxia and certain types of cancer.
Fig. 1 Structures of CD73 revealed an open and closed conformation for the enzyme [2,3]. The structural characterization showed that each subunit of CD73 is composed of a C- and N-terminal domain and that conformational change is achieved by a large (~100°) rotation of the N-terminal domain
Homologs of CD73 exist in bacteria and other microorganisms. These enzymes are usually monomeric and in the case of the best studied enzyme from E.coli unselective as they hydrolyze ATP, ADP and AMP with comparable efficiency. In contrast, CD73 is specific for AMP and inhibited by ADP or ATP. A comparison of the structures suggests that a structural control of the low energy domain orientations is responsible for this substrate selectivity. Spin-label EPR studies demonstrate that the bacterial enzyme is present in the open state in the absence of ligands and a mixture of open and closed forms exists in the presence of AMPCP. Based on the structures of CD73 we aim to support rational inhibitor design by determining complex structures. These structures show the interactions that increase the affinity of the non-hydrolyzable ADP-analog α,β-methylene-ADP to nanomolar affinity by modifications at the nucleotide base. Allosteric inhibitors might be developed that block the domain motion of the enzyme.
References
1. Zimmermann H, Zebisch M, Sträter N (2012) Purinergic Signal 8:437–502
2. Knapp K, Zebisch M, Pippel J, El-Tayeb A, Müller CE, Sträter N (2012) Structure 20:2161–2173
3. Heuts DPHM, Weissenborn MJ, Olkov RV, Shaw AM, Levy C, Scrutton NS (2012) Chem Bio Chem 13:2384–2391
Plenary Lecture 7
The A, B and C of structure based drug design
Fiona H. Marshall
Heptares Therapeutics Biopark, Welwyn Garden City, Hertfordshire, AL7 3AX England
A wide range of biophysical and structural techniques can now be applied to GPCRs. Such methods can be used to design small molecules modulators with improved physicochemical properties, potency and selectivity. The first step in structure based discovery is the generation of large quantities of purified protein required for biophysical and structural studies. Heptares StaR technology is used whereby GPCRs are engineered to include a small number of point mutations which greatly increase their thermostability and facilitate the purification of stable correctly folded protein capable of ligand binding and retaining the correct pharmacology.
We have solved X-ray structures of GPCRs across all the major sub-classes of the GPCR superfamily and for the first time structure based drug design can be applied across the entire GPCRome. In this talk structural features of the different classes of receptors will be compared. For Class A the adenosine A2A receptor [1] will be used as an example of insights that can be derived from new ligand-receptor complexes and to illustrate a variety of computational methods, including water energetics, which are used analyse druggability and drive design. The CRF1 receptor [2] will be used as an example of a Class B receptor structure which illustrates the problems in the discovery of small molecule modulators for this class of peptide receptors. Finally we will present our recently solved Class C structure the metabotropic glutamate receptor mGlu5 [3] which explains some of the challenges in designing negative allosteric modulators for this receptor.
References
1. Dore A et al (2014) Structure 19:1283–1293
2. Hollenstein K et al (2014) Nature 25; 499(7459):438–43
3. Dore A et al (2014) Nature, in press
Plenary Lecture 8 (Cancelled)
Microbial NTPDases and their role during infection
Elizabeth L. Hartland
Department of Microbiology and Immunology, University of Melbourne at the Peter Doherty Institute for Infection and Immunity, Victoria 3010
Legionella pneumophila is an opportunistic pathogen that replicates within alveolar macrophages resulting in the onset of severe atypical pneumonia known as Legionnaire’s Disease. Those most at risk of Legionnaire’s Disease are the elderly, particularly patients with compromised lung function. The ability of L. pneumophila to replicate in human cells depends in part on virulence proteins that share similarity with human proteins and alter host cell function through molecular mimicry. Previously we identified an ecto-nucleoside triphosphate diphosphohydrolase (NTPDase), Lpg1905, from L. pneumophila, which was required for optimal intracellular replication and virulence in a mouse lung infection model. The eukaryotic CD39/NTPDase1 family of enzymes hydrolyse extracellular nucleoside tri- and di-phosphates. More recently, we characterised the activity of a second NTPDase, Lpg0971, from L. pneumophila. We observed that recombinant, refolded Lpg0971 hydrolysed ATP only and exhibited divalent cation preference for manganese (II) ions. Similar to lpg1905, an lpg0971 mutant was impaired for replication in both human alveolar macrophages and amoebae, however, complementation with either lpg1905 or lpg0971 restored intracellular replication, suggesting some functional redundancy between the two enzymes. Unlike many eukaryotic-type proteins from L. pneumophila, neither Lpg1905 nor Lpg0971 appeared to be Dot/Icm effectors, suggesting that their activity is restricted to the Legionella-containing vacuole. In summary, the ability of L. pneumophila to replicate in eukaryotic cells relies in part on the ability of the pathogen to hydrolyse ATP within an intracellular compartment.
Abstracts—Symposium Sessions
- Thursday -
Thu 1 A: Potential clinical candidates for purine receptors
New regenerative medicine via P2Y and P2Y-like receptors: the case of GPR17, a new target for remyelination
Maria P. Abbracchio
Laboratory of Molecular and Cellular Pharmacology of Purinergic Transmission, Department of Pharmacological and Biomolecular Sciences, University of Milan, Via Balzaretti 9, 20133 Milan, Italy
P2Y receptors (P2YRs) have established roles in the cardiovascular, nervous, respiratory and immune systems [1]. Moreover, accumulating evidence suggests that the coordinated action of multiple P2YRs crucially orchestrates both acute remodelling events after tissue injury and the subsequent repair and regeneration. Some P2YRs indeed act as sensors of phagocytosis and participate to clearance of dying cells and debris after damage (e.g., P2Y6 [2]), or to the uptake and degradation of toxic endogenous substances, as shown for microglial P2Y2 toward the amyloidogenic Aβ1-42 peptide [3]. Moreover, several other P2YRs (e.g., P2Y1, P2Y12 and the P2Y-like receptor GPR17) have been implicated in the proliferation, differentiation and migration of adult multipotent stem/progenitor cells [4–6]. Globally, these findings open up new perspectives in the exploitment of P2YRs for new regenerative therapies. In this respect, GPR17 has recently emerged as a new target for demyelinating disorders. GPR17 is closely related to P2Y and CysLT receptors [7,8] and can be activated by both uracil nucleotides and cysteinyl-leukotrienes, as well as by new synthetic ligands [9,10]. Under physiological conditions, GPR17 is transiently expressed by oligodendrocyte precursor cells (OPCs) in transition to pre-immature oligodendrocytes and markedly downregulated in mature myelinating cells [11,12]. In vivo, increased levels of GPR17 at the site of brain injury, indicate a role in post-damage events [13,14]. Targeted inhibition of GPR17 markedly affected OPC differentiation in vitro, suggesting a potential role in myelin repair [11] (see also Abbracchio et al., poster at this meeting). In silico modeling and virtual screening, followed by functional and pharmacological in vitro confirmation have identified additional GPR17 ligands [9] that may represent prototypic molecules for new regenerative medicine therapies. Based on these and other findings [15], in 2012, the National Multiple Sclerosis Society USA has officially proposed GPR17 as a “model receptor” for new re-myelinating therapies in multiple sclerosis. Sponsored by Italian FISM, Grant N. 2010/R/2 and 2013/R/1 to MPA.
References
1. Abbracchio MP, Burnstock G, Boeynaems JM et al (2006) Pharmacol Rev 58:281–341
2. Koizumi S, Shigemoto-Mogami Y, Nasu-Tada K et al (2007) Nature 446:1091–1095
3. Kim HJ, Ajit D, Peterson TS et al (2012) J Neurochem 121:228–238
4. Burnstock G, Ulrich H (2011) Cell Mol Life Sci 68:1369–1394
5. Ulrich H, Abbracchio MP, Burnstock G (2012) Stem Cell Rev 8:755–767
6. Boccazzi M, Rolando C, Abbracchio MP et al (2014) Glia 62:428–439
7. Parravicini C, Abbracchio MP, Fantucci P et al (2010) BMC Struct Biol 10:8
8. Parravicini C, Ranghino G, Abbracchio MP et al (2008) BMC Bioinformatics 9:263
9. Eberini I, Daniele S, Parravicini C et al (2011) J Comput Aided Mol Des 25:743–752
10. Hennen S, Wang H, Peters L et al (2013) Sci Signal 6:ra93
11. Fumagalli M, Daniele S, Lecca D et al (2011) J Biol Chem 286:10593–10604
12. Daniele S, Trincavelli ML, Fumagalli M et al (2014) Cell Signal 26: 1310–1325
13. Ciana P, Fumagalli M, Trincavelli ML et al (2006) Embo J 25:4615–4627
14. Lecca D, Trincavelli ML, Gelosa P et al (2008) PLoS ONE 3:e3579
15. Chen Y, Wu H, Wang S et al (2009) Nat Neurosci 12:1398–1406
A medchem case study on the discovery of regadenoson
Jeff A Zablocki1,*, Elfatih Elzein1, Xiaofen Li1 and Luiz Belardinelli2
1Department of Medicinal Chemistry,2Department of Clinical Administration, Gilead Sciences, 333 Lakeside Drive, Foster City CA 94404
We started our drug discovery efforts for a novel pharmacological stress agent to replace adenosine (Ado) with the hypothesis that a short acting functionally selective A2A adenosine receptor agonist would have fewer side effects. It was known at the onset of the program that an appropriately 2-substituted adenosine analog [1] could impart selectivity for A2A AdoR selectivity over A1 AdoR, so we designed series exemplified by acetylene 1, and N-pyrazole 2 (regadenoson). Critical to the success of our program was the choice of measuring the vasodilatation induced by our A2A AdoR agonist in an isolated heart model (rat) attempting to match the duration of effect of adenosine [2]. The acetylene 1 had too long of a vasodilatory effect (23 min) that was attributed to the high affinity for the A2A AdoR. Regadenoson 2 had a modest duration of effect (11 min) more closely matching the duration of effect of adenosine (8 min), and a modest A2A AdoR affinity (1.2 uM h A2A AdoR). Regadenoson is 10 fold selective over A1 AdoR binding affinity, but more than 100 fold functionally selective for coronary vasodilation over A1 AdoR mediated negative dromotropic effects, and highly selective over peripheral vasodilatory effects (hypotension). The latter tissue selectivity may be due to the partial A2A AdoR agonism (cAMP), and the high receptor reserve of the coronary arterial circulation, hence requiring only a smaller percentage of A2A AdoR occupancy to cause near-maximal vasodilatation. The hypothesis that a short acting functionally selective A2A AdoR agonist would have fewer side effects was answered in the clinic with a decrease in the % and the severity of the side effects observed with regadenoson, fewer cases of first degree A-V block (3 versus 7 % for Ado) and second degree A-V block (0.1 vs 1 % for Ado) [3].
References
1. Cristalli G, Camaioni E, Francesco E, Di Eleuteri A, Vittori S, Volpini (1997) Nucleosides & Nucleotides 16(7–9):1379–1388
2. Gao Z, Li Z, Baker SB, Lasley RD, Meyer S, Elzein E, Palle V, Zablocki JA, Blackburn B, Belardinelli L (2001) JPET 298:209
3. Lexiscan® Package Insert, https://www.astellas.us/docs/lexiscan.pdf
Partial adenosine A1 receptor agonists
B. Albrecht-Küpper
Bayer Healtcare AG, Global Drug Discovery, D-42096 Wuppertal; Cardiology Research, Department of Heart Diseases
There are several options for new cardiovascular therapies involving adenosine A1 receptor activation in e.g. angina pectoris, control of cardiac rhythm, ischemic injury during acute coronary syndrome or heart failure. The main issue of using A1 receptor agonists in therapies is the broad spectrum of physiological A1 effects. Desired cardiovascular effects such as cardioprotection can be counter-regulated by undesired ones like pronounced bradycardia (AV-block) or decreased glomerular filtration rate and diuresis. This restriction for the use of A1 receptor agonists as therapeutic target can be overcome by tailoring compounds only to the desired pharmacological efficacy by the development of partial adenosine A1 receptor agonists.
There are now several partial A1 agonists described in the literature like GS-9667 and VCP-103 with different degrees of partiality and resulting pharmacological activity. Bayer has identified the first non-adenosine like partial A1 agonists like Capadenoson which are suitable for an oral once daily treatment of patients. Capadenoson has an EC50 of 0.1 nM on human A1 receptors and a selectivity factor of 1,800 vs. A2a, 900 vs. A2b and no activity on A3 receptors [1]. It shows an efficacy of only 75 % in comparison to the full adenosine A1 receptor agonist CPA on A1 receptors prepared from human cortex membranes. In consistency with the predicted pharmacological effects of a partial A1 receptor agonist, Capadenoson showed reduced bradycardic effects and no AV block. In preclinical models of acute myocardial infarction and heart failure the partial A1 agonist reduced infarct size and significantly improved heart function [1,2].
In clinical studies the compound did not reduce heart rate at rest in healthy subjects or patients with stable angina pectoris, but reduced heart rate at peak exercise compared with placebo [3]. A single dose of Capadenoson resulted in a significant increase of exercise time. Clinical studies with a follow-up compound are currently ongoing in patients with heart failure.
Future studies will show which benefits partial adenosine A1 receptor agonists will have in cardiovascular and other diseases.
References
1. Albrecht-Küpper B, Leineweber K, Nell P (2012) Purinergic Signal 8:S91–S99
2. Sabbah HN, Gupta RC, Wang M et al (2013) Circ Heart Failure 6:563–571
3. Tendera M, Gaszewska-Zurek E, Parma Z et al (2012) Clin Res Cardiol 101:585–591
P2X3 antagonism with AF-219: clinical potential and findings
Anthony P. Ford
Afferent Pharmaceuticals, San Mateo, CA, USA
Patients with somatosensory & visceral disorders experience chronic pain, discomfort & irritative symptoms driven by hypersensitized primary afferent neurons. Despite decades of innovation, therapeutic options remain limited, and research attention has turned to targeting excitatory channels localised preferentially to primary afferents (e.g., TRP, NaV, P2X), to suppress peripheral inputs driving sensitization. One such approach is blocking P2X3 channels (P2X3 homotrimers), expressed selectively by large proportions of neural crest derived C-fibers, deletion of which in mice led to findings consistent with attenuated sensitization [1], including urinary bladder hyporeflexia, and reduced hyperalgesia [2].
Developable “drug-like” inhibitors of P2X3 channels have been widely sought, and the first such molecule, AF-219, has successfully progressed to clinic: completed studies include four Ph 1 studies, & four Ph 2 studies in patients with a range of common clinical conditions. AF-219 is a novel (MWt. ~ 350) 2,4-diaminopyrimidine which allosterically blocks human P2X3 homotrimeric channels (IC50 ~ 30 nM) with selectivity over P2X2/3 heterotrimers & no effect on other channels studied.
Clinical experience with AF-219 reveals a favorable safety profile to date from inhibition of P2X3 & P2X2/3 receptors, with one tolerability finding of altered taste perception [anticipated given reduced taste sensibility of P2X2-, P2X3- & double-KO mice [3]] reflecting high dose inhibition of heteromeric P2X2/3 channels that dominate transduction in the placodal gustatory afferents. In the first completed patient study, a high POC dose of AF-219 given over a 2 week period, was shown to dramatically reduce cough frequency & severity in refractory patients [4]. Clinical potential and additional findings will be presented.
Fig. 1 AF-219 (600 mg BID) reduces daytime cough frequency 84 % (p < 0.001 vs. PLA) in patients with treatment refractory cough
References
1. Ford (2012) doi: 10.1007/s11302-011-9271-6
2. Cockayne et al (2000) Nature 407:1011
3. Finger et al (2005) Science 310:1495
4. Abdulqawi et al (2013) Eur Resp J 42(Suppl 57):386s
Targeting A2Areceptor to treat neurodegenerative diseases: design, synthesis and evaluation of potential antagonists
Valeria Moas-Heloire1,2, Nicolas Renault1,2, Vania L. Batalha3, Philippe Chavatte1,2, Said Yous1,2, Luc Buée1,4, David Blum1,4, Luisa V. Lopes3, Laurence Agouridas1,2 and Patricia Melnyk1,2,*
1Univ Lille Nord de France, F-59000 Lille, France;2UDSL, EA 4481, UFR Pharmacie, F-59000 Lille, France;3Institut de Médecine Moléculaire, Unit 2P1B-49, 1640-028 Lisboa, Portugal;4Alzheimer and Tauopathies, Inserm UMR-U837, Lille, France
Adenosine is a ubiquitous endogenous purine nucleoside able to regulate many physiological processes as an intercellular messenger and plays an important neuroprotective role in the central nervous system. In the brain, adenosine and its receptors are presents in high levels, and it has been shown to be involved in both normal and pathological processes including arousal, motor control, neuroprotection, mood, learning and memory. Its effects are transmitted by four distinct receptor subtypes designated A1, A2a, A2b, and A3 belonging to the G protein-coupled receptor superfamily. Adenosine A1 and A3 receptors are coupled to inhibitory G proteins, while A2a and A2b receptors are coupled to stimulatory G proteins. A2a receptors (A2aR) show a restricted distribution, being characteristic of the dopamine enriched areas, the highest concentration being in the caudate-putamen. This anatomical selection suggests a unique role in neuronal signaling with this region and potential involvement in neurologic disease of extrapyramidal origin.
In fact, A2a antagonism appeared to be a promising pharmacological target in Parkinson’s disease (PD). Furthermore, an increasing number of studies suggest that pharmacological or genetic blockade of A2aR might be of great interest in Alzheimer’s disease as it reduces β-amyloid deposits, τ-phosphorylation and neurodegeneration. Currently, only three compounds are still being tested in clinical phase for PD treatment. Even if they show good affinities for the receptor, there is still a need for improving their ADME properties by keeping their selectivity towards other adenosine receptors.
Based on the published crystalline structure of the A2A receptor complexed with the selective and high-affinity antagonist ZM241385 [1] and on a pharmacophoric model [2], we have designed new ligands using in silico docking studies starting from a preliminary hit that we recently identified in our group. Then, using original chemical pathways, three new families of compounds have been synthesized and tested for their affinity towards A2a receptor.
References
1. Jaccola V et al (2008) Science 322:1211–1217
2. Xu Z et al (2010) J Mol Model 16:1867–1876
Thu 1 B: Transport and release I: nucleosides and nucleobases
Adenosine signaling in neuron-glial interaction is essential for ethanol seeking behaviors
Doo-Sup Choi
Mayo Clinic College of Medicine, Molecular Pharmacology and Experimental Rochester, Minnesota 55905, USA
Our laboratory is studying a role of the ethanol-sensitive adenosine transporter, ENT1 (equilibrative nucleoside transporter type 1; Slc29a1), in alcohol use disorders. We are particularly interested in adenosine-regulated glutamate signaling in the striatum and cortico-striatal circuit in addictive ethanol seeking behaviors. In the nucleus accumbens (NAc), we found that ENT1 expression regulates EAAT2 (excitatory amino acid transporter type 2; Slc1a2), an astrocyte-specific glutamate transporter, which is known to be responsible for over 90 % of glutamate uptake in the striatum. Reduced EAAT2 expression in ENT1 null mice dampens glutamate uptake activity, and thereby increases synaptic glutamate levels, which constitutively activate NMDA glutamate receptors (NMDAR) in the NAc. Ceftriaxone, an antibiotic compound known to increase EAAT2 expression and function, reduced ethanol drinking in ENT1 null mice. Recently, we discovered that ceftriaxone treatment reduced ethanol withdrawal symptoms (AWS) in alcohol preferring P rats and Wistar rats. In the dorsomedial striatum (DMS), ENT1 deficiency dampens adenosine A2AR function, which promotes the transition from goal-directed to habitual behaviors. Consistently, pharmacological inhibition of DMS A2AR increases goal-directed behavior and increases both sucrose and ethanol seeking in operant self-administration experiments. Decreased A2AR signaling appears to causally decrease PKA activity in the DMS, which is consistent with the fact that inhibition of A2AR is coupled to decreased adenylyl cyclase activity and PKA activity. Moreover, ENT1 null mice showed a higher rate of initial acquisition and increased vulnerability toward habitual behavior in operant instrumental conditioning tests. Thus, hypo-A2AR function in the DMS of ENT1 null mice may lead to increased goal-oriented behavior. Using circadian locomotor activity, we found that ENT1 null mice were hyperactive compared to wild-type mice at night. Moreover, adenosine signaling regulates cellular and behavioral circadian timing and influences alcohol intake during chronodisruption. In summary, ENT1-regulated adenosine signaling plays an essential role in ethanol-seeking behaviors.
Supports: NIH, Samuel C. Johnson Foundation, Ulm Foundation, Godby Foundation, and Mayo Clinic.
Mechanism and function of transient adenosine release in the brain
B. Jill Venton*, Michael D. Nguyen and Ashley E. Ross
Department of Chemistry and Neuroscience Graduate Program, University of Virginia, Charlottesville, VA, USA
Adenosine is a neuromodulator in the brain that regulates cerebral blood flow and neurotransmission. Our lab has developed a rapid electrochemical sensing method that allows us to measure adenosine release on the subsecond time scale. We have discovered spontaneous, transient adenosine release in the prefrontal cortex and caudate-putamen that lasts for only 3 s [1]. The mechanism and function of this transient adenosine release is not known. Thus, we have performed a series of studies to understand the regulation of these adenosine transients. Pharmacological tests indicate that spontaneous adenosine release is self-regulated by A1 but not A2a receptors. Both metabolism and nucleoside transporters are responsible for clearing adenosine from the extracellular space. In brain slices, we have observed the mechanism of formation and function of transient adenosine release. Adenosine is transiently released due to mechanical stimulation, which is not due to cell death [2]. This mechanosensitive release is dependent on ATP breakdown and is calcium and tetrodotoxin dependent. The rapid adenosine can transiently modulate phasic dopamine release in brain slices. Adenosine transiently released 2–5 s before dopamine stimulation inhibits dopamine release via A1 receptors, but adenosine released over 10 s before stimulation has no effect. Thus, our research has shown that there is a rapid mode of adenosine signalling that lasts only a few seconds in the brain. This signalling may be important for transient modulation of dopamine neurotransmission.
References
1. Nguyen MD, Lee ST, Ross AE, Ryals M, Choudhry VI, Venton BJ (2014) PLoS One 9:e87165
2. Ross AE, Nguyen MD, Venton BJ (2014) J Neurochem. doi: 10.1111/jnc.12711
Novel modes of regulation of the equilibrative nucleoside transporters (ENTs)
Imogen R. Coe
Department of Chemistry and Biology, Ryerson University, Toronto, Ontario, M5B 2K3, Canada
Equilibrative Nucleoside Transporters (ENTs) comprise the SLC29 family of integral membrane proteins which are responsible for the uni- or bi-directional flux of the purine nucleoside adenosine, and other nucleosides and nucleobases, across cell membranes. Compared to other members of the purinome such as purinergic receptors and enzymes, very little is known about the role of ENTs in purinergic physiology, signaling and metabolism. In addition, the role of ENTs in determining impact and efficacy of nucleoside analog drugs is still not clearly defined. Using a variety of biochemical, physiological and pharmacological approaches, we have investigated the structure, function and regulation of ENTs. We have determined that the prototypic equilibrative nucleoside transporter, ENT1, plays an integral role in modulating the effects of adenosine in a number of physiological settings. The absence of ENT1, in knockout mouse models, leads to enhanced adenosinergic responses in the cardiovasculature and the renal system [1,2]. To better understand the role of ENT1 within the purinome and the contribution of ENT1 to the regulation of purinergic activities, we have investigated and described the post-translational modifications of ENT1 undergoes and are gradually elucidating the regulatory roles of these modifications. We have also investigated the regulatory roles of a variety of interacting proteins that appear to be involved in modulating ENT1 function. In addition, it is increasingly clear that there are multiple levels of regulation of ENT1, from gene to protein, which affect the function of this transporter [3]. Taken together, our data suggest that ENT1 is an integral member of the purinome and activity of the transporter is regulated to optimize and coordinate purinergic signaling in complex and unexpected ways.
References
1. Rose JB et al (2010) Am J Physiol 298: H771–H777
2. Grenz A et al (2012) J Clin Invest 122:693–710
3. dos Santos-Rodrigues A et al (2014) Neurochem Int 73:229–237
Adenosine release during neocortical network activity
Mark J. Wall
School of Life Sciences, University of Warwick, UK
The purine adenosine is a potent neuromodulator involved in many physiological and pathological CNS processes. Although much is known about its cellular actions less is known about the mechanisms of release and the spatial/temporal properties of adenosine signalling. There is accumulating evidence that only small numbers of actions potentials, fired at a low frequency, are sufficient to release adenosine and thus adenosine release occurs during physiological activity. The mechanism of release differs in different brain regions with release occurring from neurones and glia either directly or indirectly, as ATP. Using electrophysiology, biosensors and simulations we have begun to characterise the properties of adenosine signalling during neocortical network activity.
In the neocortex, although the frequency of population spikes is modulated by blocking A1 receptors, adenosine release cannot be directly measured with adenosine biosensors. From simulations and experimental data we have determined that released adenosine is rapidly diluted and is therefore only detectable very close to release sites. This is consistent with data from paired recordings, were the degree of A1 receptor activation differs between proximal synapses within a slice. During pathological activity, when adenosine release occurs from many release sites inosine is detected rather than adenosine. This is consistent with the local uptake of adenosine, deamination by adenosine deaminase, and efflux out of the cells.
Functions and regulation of erythrocyte equlibrative nucleoside transporter 1 (ENT1) in acute hypoxia mediated tissue injury
Anren Song1, Yujin Zhang1, Jessica Lee1, Almut Grenz2, Michael R. Blackburn1, Holger Eltzschig2, Rodney E. Kellems1 and Yang Xia1
1University of Texas-Medical School, Houston, TX, USA;2University of Colorado-Medical School, Denver, USA
Equlibrative nucleoside transporters (ENTs) on erythrocytes have been long speculated to regulate extracellular adenosine concentrations under hypoxic conditions. Thus, we hypothesize that erythrocyte ENT is likely a key molecule responsible for elevated circulating adenosine levels and protects tissues from hypoxia induced injury. To test this hypothesis, we first conducted western blot analysis to compare expression profiles of ENTs on erythrocyte. We found that ENT1 is the major ENT expressed on both mouse and human erythrocytes. Using genetic approach combined with functional adenosine uptake assay, we demonstrated that ENT1 1) is the major adenosine transporter in erythrocyte and 2) red blood cell (RBC) is the major cell type involved in regulating circulating adenosine levels. Next, we performed pharmacological studies to determine the exact role of ENT1 in erythrocyte. We found that treatment with dipyridamole or an ENT1 specific inhibitor (NBMPR) enhanced adenosine-induced elevation of 2,3-bisphosphoglycerate (2,3-BPG), an erythrocyte specific metabolite known to decrease hemoglobin O2 binding affinity, in cultured mouse RBCs. Using Hemox Analyzer, we found that co-treatment of adenosine with either dipyridamole or NBMPR resulted in a further right shift of oxygen equilibrium curve (OEC) and further increase in P50 compared to the cells treated with adenosine alone. Similar to our pharmacological studies, we found that genetic deletion of ENT1 further enhanced adenosine-induced 2,3-BPG production in cultured erythrocytes, additional right shift of OEC and increased P50. Finally, we found that, during acute hypoxia treatment, genetic ablation of erythrocyte ENT1 significantly reduced the speed of adenosine uptake and promoted 2,3-BPG production, triggered more oxygen release, and protected acute hypoxia-mediated tissue injury. Overall, our studies demonstrate that 1) ENT1 is a major adenosine transporter expressed by RBCs and RBCs are the major cell type responsible for regulating circulating adenosine. 2) Inhibition or deletion of erythrocyte ENT1 results in enhanced adenosine-mediated 2,3-BPG induction and hemoglobin deoxygenation in RBCs when hypoxia is encountered. Therefore, our findings reveal a previously unrecognized role of erythrocyte ENT1 in hypoxia-mediated tissue damage by regulating extracellular adenosine and provide new therapeutic possibility to prevent hypoxia-induced tissue damage.
Thu 1 C: ATP-mediated talk between microglia, astrocytes and neurons
Role for ATP receptors in astrocyte-neuron communications in neocortex
Yuriy Pankratov, Seyed Rasooli-Nejad and Ulyana Lalo
School of Life Sciences, University of Warwick, Coventry, UK
Communication between neuronal and glial cells is very important for brain function. Astrocytes enwrap neurons and therefore can be exposed to various neurotransmitters. In response, astrocytes can modulate synaptic signaling via vesicular release of gliotransmitters, such as D-serine, glutamate and ATP. Our recent work highlighted an important role played by ATP in bi-directional glia-neuron communications in the brain.
Firstly, we have shown that cortical astrocytes express ionotropic receptors to ATP, composed of P2X1 and P2X5 subunits. Astroglial P2X1/5 receptors mediate fast electrical and Ca2+ -signals triggered by stimulation of neuronal afferents.
Secondly, we have demonstrated that vesicular release of ATP from cortical astrocytes can be activated via various pathways including Ca2+-permeable ionotropic receptors or UV-uncaging of Ca2+. We have not observed release of ATP from astrocytes of dnSNARE mice in which SNARE proteins were selectively impaired in astroglial cells. We found out that glia-derived ATP down-regulated both synaptic and tonic inhibitory currents in the neocortical neurons; this effect was mediated by phosphorylation of GABAA receptors. Furthermore, modulation of neuronal inhibition by astrocyte-driven ATP affected the synaptic plasticity in the neocortex.
These findings demonstrate an importance of SNARE complex-dependent exocytosis of ATP for glia-neuron interaction in the neocortex. Our results also show a novel pathway of glia-neuron communication involving release of ATP and modulation of postsynaptic GABA receptors. Importantly, ATP-mediated communication between astrocytes and neurons in the neocortex can undergo remodeling during brain ageing and decrease in the ATP release from astrocytes may contribute to the age-related impairment of synaptic plasticity.
ATP-mediated communication between astrocytes and neurons at lowPO2
Alexander V. Gourine1 and Sergey Kasparov2
1University College London, London, UK;2University of Bristol, Bristol, UK
Astrocytes are the most abundant type of brain glial cells. They are closely associated with cerebral blood vessels—all penetrating and intracerebral arterioles and capillaries are enwrapped by astrocytic endfeet. By having contacts with cerebral vasculature as well as multiple neurons, astrocytes are in a position to ‘taste’ the chemical composition of the arterial blood entering the brain and integrate this information with that of brain parenchyma. Does it reflect their functional importance for the operation of brain interoceptors, which monitor key homeostatic parameters including pH, PCO2 and possibly PO2 levels? We found that astrocytes which reside within the brainstem chemoreceptor areas located near the ventral surface of the medulla oblongata are highly chemosensitive. They respond to small physiological decreases in pH or PO2 with vigorous elevations in intracellular Ca2+ and release of ATP. ATP spreads astroglial Ca2+ excitation within the neuropil, activates key respiratory neurons of the medullary rhythm-generating circuits and induces adaptive increases in breathing. During systemic hypoxia ATP is also released and acts to maintain respiratory activity in conditions when hypoxia-induced depression of respiration occurs. Mimicking astroglial pH-evoked Ca2+ responses by selective light stimulation of astrocytes expressing channelrhodopsin-2 activates chemoreceptor neurons via ATP-dependent mechanism and triggers robust respiratory and sympathetic responses in vivo. Thus, medullary astrocytes appear to be highly sensitive to physiological chemosensory challenges and have the ability to impart chemosensory information onto a modified pattern of cardiorespiratory activity via release of ATP.
Ischemic tolerance mediated by glia purinergic system
Schuichi Koizumi* and Yuri Hirayama
University of Yamanashi, Department of Neuropharmacology, Yamanashi, Japan
The use of a preceding sub-lethal ischemic insult, preconditioning, is an attractive strategy for protecting neurons by inducing ischemic tolerance in the brain. Although the underlying molecular mechanisms have been studied extensively, almost all experiments have been performed on neurons. Here, we show that activation of glial cells by sub-lethal brain ischemia is essential for induction of ischemic tolerance using a middle cerebral artery occlusion mouse model. The lethal middle cerebral artery occlusion-evoked damage was significantly reduced when mice received preconditioning 3 or 6 days earlier, i.e., induction of ischemic tolerance, whereas preconditioning 1 day prior had no effect on brain damage. Interestingly, the region where ischemic tolerance occurred correlated well with where astrocytes were activated. Fluorocitrate, a metabolic inhibitor of astrocytes, inhibited the preconditioning-induced activation of astrocytes, which was associated with the disappearance of ischemic tolerance, suggesting an indispensable role of activated astrocytes to the ischemic tolerance. Minocycline, an inhibitor of microglial activation, had no such effect. As for the mechanisms, we found that upregulation of P2X7 receptors by preconditioning was responsible for astrocyte-mediated ischemic tolerance. Using P2rx7-EGFP mice, we analyzed preconditioning-induced changes on the spatiotemporal pattern of P2X7 receptor expression. P2X7 receptors were expressed mainly in microglia in naive mice. However, upon receiving preconditioning, they were dramatically upregulated in activated astrocytes rather than in microglia. The preconditioning-induced ischemic tolerance was abolished in P2X7 receptor knockout (P2X7−/−) mice, although there was no significant difference in the activation of astrocytes between wild-type and P2X7−/− mice. Moreover, hypoxia inducible factor-1α and its target gene erythropoietin, well-known mediators of oxygen homeostasis in neurons, were upregulated in astrocytes following preconditioning in wild-type mice, but expression was suppressed in P2X7−/− mice. However, neuronal hypoxia inducible factor-1α was not affected by P2X7 deficiency. Taken together, upregulation of P2X7 receptors in preconditioning-activated astrocytes should be essential for glia-mediated ischemic tolerance.
Regulation of gliotransmission by microglial cells
Camille Philippot, Naura Chounlamountri and Olivier Pascual
Centre de Recherche en Neuroscience de Lyon (CRNL), Lyon, France
Purines are involved in cellular communication throughout the central nervous system (CNS). In particular ATP, that is an important extracellular messenger involved in the communication between astrocytes, a subtype of glial cells. Astrocytes stimulation by ATP has been found to modulate the probability of glutamate release by hippocampal neurons through a pre synaptic regulation involving the the release of glutamate by astrocytes. This ATP mediated signaling pathway is part of the gliotransmission a term that refers to the capacity of glial cells to release gliotransmitter (ATP, Glutamate, D-serine…) to modulate synaptic activity. We recently demonstrated that microglia release ATP to modulate synaptic transmission using astrocytes as intermediate. We now report that microglia regulates the astrocytic process of gliotransmission. Indeed we found that gliotransmission triggered by P2Y1 agonist is impaired in slices from transgenic mice devoid of microglia. To better understand the mechanisms involved in this process, we further studied the cellular mechanisms of gliotransmitter release. We found that the lack of microglia alter neither P2Y1 receptor expression nor intracellular calcium responses following ATP stimulation. However using TIRF imaging we found that the vesicular release of gliotransmitter was slightly different in cultures lacking microglia compared to vesicular release in mixed cultures. Indeed vesicular release kinetics appears to be faster in pure astrocyte cultures when compared to mixed cultures with more vesicles close to the membrane. The results we obtained suggest an altered anchoring of ATP containing vesicles to the membrane in absence of microglia potentially leading to a defective gliotransmission.
Role of adenosine A2Areceptors in cerebral ischemia
Felicita Pedata1,*, Alessia Melani1,2, Lucrezia Cellai1, Ilaria Dettori1 and Anna Maria Pugliese1
1Department of Neuroscience, Psychology, Drug Research and Child Health (NEUROFARBA), University of Florence, Viale Pieraccini, 6, 50139 Florence, Italy;2Fellow of the Fondazione Umberto Veronesi, Milan, Italy
The extracellular concentration of adenosine in the brain increases dramatically during ischemia due to degradation of extracellularly released ATP in the first minutes after stroke and to adenosine released per se from cells [1]. Adenosine A2A receptor is expressed in neurons and glial cells and in peripheral inflammatory cells (lymphocytes and granulocytes). Adenosine A2A receptor emerged as a potential therapeutic attractive target in ischemia. Ischemia is a multifactorial pathology characterized by different events evolving in the time. After ischemia the early massive increase of extracellular glutamate is followed by activation of resident immune cells, i.e. microglia, and production or activation of inflammation mediators. Proinflammatory cytokines, that upregulate cell adhesion molecules, exert an important role in promoting recruitment of leukocytes that promote expansion of the inflammatory response in ischemic tissue. Protracted neuroinflammation is recognized as the predominant mechanism of secondary brain injury progression. Adenosine A2A receptors present on central cells and on blood peripheral cells account for important effects depending on the time-related evolution of the pathological condition. Evidence indicate that A2A antagonists provide early protection via centrally-mediated control of excessive excitotoxicity [2], while A2A agonists provide protection by controlling massive blood cell infiltration in the hours and days after ischemia [3].
References
1. Melani A, Corti F, Stephan H, Müller CE, Donati C, Bruni P, Vannucchi MG, Pedata F (2012) Exp Neurol 233(1):193–204
2. Melani A, Cipriani S, Vannucchi MG, Nosi D, Donati C, Bruni P, Giovannini MG, Pedata F (2009) Brain 132:1480–1495
3. Melani A, Corti F, Cellai L, Vannucchi MG, Pedata F (2014) Brain Res 1551:59–72
Thu 1 D: Pancreatic purinergic signaling in health and disease—exocrine and endocrine functions
Bile acid induced Ca2+responses are mediated in part by ATP release and purinergic signalling in pancreatic exocrine cells
Justyna Magdalena Kowal*, Kristian Agmund Haanes, Nynne M. Christensen and Ivana Novak
University of Copenhagen, Department of Biology, Copenhagen, Denmark
Selected poster N 226
VNUT mediated ATP release in exocrine pancreas
Kristian A. Haanes1,2,* and Ivana Novak1
1Department of Biology, University of Copenhagen, Copenhagen, Denmark;2Clinical Experimental Research, Copenhagen University Hospital, Glostrup, Denmark
ATP is released from pancreatic acini in response to cholinergic and hormonal stimulation. The same stimuli cause exocytosis of ZG (zymogen granules) and release of digestive enzymes. Our aim was to establish the role of the vesicular nucleotide transporter (VNUT), SLC17A9, in storage and release of ATP. We determined that ZG stored ATP and our findings indicated that VNUT may be responsible for the ATP uptake into ZG [1]. We further used freshly prepared acini from mice and AR42J rat acinar cells. We illustrate that in AR42J cells, quinacrine (an ATP store marker) and Bodipy ATP (a fluorescent ATP analogue) co-localized with VNUT-mCherry to vesicles/granules. Furthermore, in acini and AR42J cells, a marker of the zymogen granule membranes, Rab3D, and VNUT co-localized. Dexamethasone treatment of AR42J cells promoted formation of acinar structures, paralleled by increased amylase and VNUT expression, and increased ATP release in response to cholinergic stimulation [2]. In conclusion ATP is stored together with digestive enzymes in ZG, where it is taken up by VNUT. VNUT-dependent ATP release pathway is associated with agonist-induced secretion process. We propose that co-released ATP would regulate P2 receptors in pancreatic ducts and, thus, ductal secretion, and this would aid delivery of enzymes to the duodenum.
References
1. Haanes KA, Novak I (2010) Biochem J doi: 10.1042/BJ20091337
2. Haanes KA et al (2014) Purinergic Signal doi: 10.1007/s11302-014-9406-7
Functional role of vesicular nucleotide transporter in pancreatic β cells on insulin secretion
Jessica Geisler1, Hongxia Chal1, Peilin Chen1, David Castle2 and Chien Li1,*
1Department of Pharmacology,2Department of Cell Biology, University of Virginia Health System, Charlottesville, Virginia, USA
Secretion of insulin in response to glucose stimulation requires the participation of an array of factors in glucose sensing, metabolism-secretion coupling and insulin granule exocytosis. The secretory response to glucose stimulation begins with a first phase of rapid insulin release and is typically followed by a second phase of slower release that is sustained until stimulation ceases. Intracellular ATP elevated in response to glucose is a key mediator of insulin secretion through closure of ATP-sensitive potassium (KATP) channels and consequent depolarization- and calcium induced granule exocytosis. We have now uncovered a new molecular mechanism that regulates insulin secretion by controlling ATP release from pancreatic β cells. Vesicular Nucleotide Transporter (VNUT) is a vesicular membrane protein that mediates ATP uptake into secretory vesicles. We found that VNUT is expressed in pancreatic β cells [1], where VNUT is found in both insulin secretory granules and plasma membrane. In the secretory granules VNUT regulates granule ATP uptake and consequently its release by β cells. In addition to ATP secretion [1], VNUT regulates insulin secretion as suppression of VNUT expression in both mouse and human islets significantly attenuates glucose stimulated insulin secretion (GSIS) [1]. Specifically, we found that the first phase of GSIS was significantly decreased in islets from islet-specific VNUT knockout mice. The effect of VNUT on insulin secretion is mediated by extracellular ATP as exogenous ATP treatment recovers impaired insulin secretion induced by abrogating VNUT function. Taken together, VNUT is expressed in pancreatic β cells and plays a critical role in regulating glucose-induced insulin secretion.
Reference
1. Geisler JC, Corbin KL,Li Q, Feranchak AP, Nunemaker CS, Li C (2013) Endocrinology 154:675–684
Paracrine activation of P2Y13: a partial mediator of glycolipotoxic effects
Chanyuan Tan1,*, Ulrikke Voss2, Siv Svensson1, Bernard Robaye3, Jean-Marie Boeynaems4, David Erlinge1 and Björn Olde1
1Department of Cardiology, Lund University, 22185, Lund, Sweden;2Department of Experimental Medical Science, Lund University, #22185, Lund, Sweden;3Institute of Interdisciplinary Research, IRIBHM, Université Libre de Bruxelles, Gosselies, Belgium;4Department of Laboratory Medicine, Erasme Hospital, Brussels, Belgium
High levels of glucose and saturated fatty acids are known to have detrimental effects on the function and survival on several cell types of the body. In a previous study [1], we found that ADP regulates beta cell apoptosis. Using MIN6c4 cells as a model system, we investigated if autocrine/paracrine mechanisms of ADP and purinergic receptors are involved in this process. High glucose (16.7 mmol/l) and palmitate (100 μmol/l), but not mannitol, rapidly and potently elevated ATP release. Tolbutamide and diazoxide were both without effect, while the calcium channel blocker nifedipine, the Cl− channel/ VRAC inhibitor NPPB, and the pannexin inhibitor carbenoxolone could inhibit both effects. Similarly, silencing the MDR1 gene also blocked nutrient-generated ATP release. These results indicate that calcium channels and VRACs might be involved in the ATP release mechanism. Furthermore, high glucose and palmitate inhibited cAMP production, reduced cell proliferation in MIN6c4 and increased activated Caspase-3 cells in mouse islets and in MIN6c4 cells. The P2Y13-specific antagonist MRS2211 antagonized all these effects. Further studies showed that blocking the P2Y13 receptor resulted in enhanced CREB, Bad and IRS-1 phosphorylation.
Preliminary results, using HFD fed P2Y13 KO mice, indicate a tendency to reduced insulin production in pancreas and a significant protective effect on HFD stimulated enteric neural-loss.
Reference
1. Tan C, Salehi A, Svensson S, Olde B, Erlinge D (2009) Cell Mol Life Sci 67(3): 445–453
Beneficial effects of P2Y6receptor agonists on insulin release and survival of pancreatic β-islet cells and in target tissue: involvement of 5′-AMP-activated protein kinase (AMPK)
Kenneth A. Jacobson1,*, Bernard Robaye2, Jean-Marie Boeynaems3 and Ramachandran Balasubramanian1
1Molecular Recognition Section, National Institute of Diabetes and Digestive and Kidney Diseases, NIH, Bethesda, MD, USA;2Institute of Interdisciplinary Research, IRIBHM, Université Libre de Bruxelles, Gosselies, Belgium;3Université Libre de Bruxelles, Brussels, Belgium
P2Y6 receptor (P2Y6R) activation protects pancreatic islet cells from apoptosis, stimulates glucose-dependent insulin release and increases glucose uptake in target tissues. These actions involve the enzyme 5′-AMP-activated protein kinase (AMPK), a metabolic regulator that is a target for treatment of type 2 diabetes [1]. In MIN6 β-islet cells, treatment with a potent and selective P2Y6R dinucleotide agonist MRS2957 (500 nM) activated AMPK, which was blocked by P2Y6R-selective antagonist MRS2578. Also, MRS2957 induced phosphorylation of acetylcoenzyme A carboxylase (ACC), a marker of AMPK activity. Calcium chelator BAPTA-AM, calmodulin-dependent protein kinase kinase (CaMKK) inhibitor STO-069 and IP3 receptor antagonist 2-APB attenuated P2Y6R-mediated AMPK phosphorylation revealing involvement of intracellular Ca2+ pathways. P2Y6R agonist induced insulin secretion at high glucose, which was reduced by AMPK siRNA. In target cells (C2C12 skeletal muscle cells and 3T3-L1 adipocytes), MRS2957 significantly increased glucose uptake compared to control, which was antagonized by MRS2578. MRS2957-treatment resulted in significant phosphorylation of AMPK in both cell lines, which was abolished by pre-incubation with MRS2578. Also, MRS2957 (30 min incubation) increased glucose transporter GLUT4 recruitment to the cell membrane, which was blocked by MRS2578 or AMPK inhibitor (Compound C). Glucose uptake in primary mouse adipocytes from WT but not P2Y6R KO mice was stimulated by P2Y6R activation. Our results indicate that the P2Y6R is involved in controlling glucose metabolism at multiple levels, and this may be mediated through AMPK signaling.
Reference
1. Balasubramanian R, Maruoka H, Jayasekara PS, Gao ZG, Jacobson KA (2013) Biochem Pharmacol 85: 991–998
Thu 2 A: Roundtable discussion: challenges for purinergic drugs
“Purine-based drug development strategy: what lessons do we learn and what are the challenges”
Organized and chaired by Maria P. Abbracchio (Milan, Italy) and Jiang-Fan Chen (Boston, USA)
Presentation: Over the last decade, increasing numbers of clinical trials testing novel purine-based drugs in a variety of indications have been initiated, including several largest clinical phase III trials of the A2A receptor antagonists istradeffyline and preladenant for Parkinson’s disease, the A1 receptor antagonist rolofylline for heart failure, antithombotic agents targeting the platelet P2Y12 receptor and agents acting via P2Y2 receptors for cystic fibrosis and dry eye disease. However, very few drugs have actually made a clinical impact yet.
Aims of the Round-Table: In this Round-Table discussion, we call on preclinical and clinical investigators as well as pharmaceutical industries and private no-profit research and Patients’ Foundations to: (i) identify major hurdles for the development of pharmacological compounds that target purine signaling, (ii) envisage new effective operational drug development models to best synergize basic/clinical researchers, pharmaceutics and Foundations.
We anticipate that this Round-Table will stimulate the discussion on some critical issues in this topic:
i) What are the greatest challenge in developing purine ligands for specific clinical applications?
ii) What are general lessons we learned from these late stages of clinical trials?
iii) How can we dissect out the complexity of purinergic signaling to reduce debilitating side effects?
iv) Are there specific/unique factors that should be taken into consideration for clinical trials design for purine-based therapy?
New operational models in drug discovery and development: the role of patients’ foundation
Paola Zaratin* and Mario Alberto Battaglia
Italian MS Society Foundation, Genoa, Italy
Discovering and developing new disease-modifying therapies for neurodegenerative diseases hinges on innovative research and new operational models promoting multidisciplinary and integrated efforts of all stakeholders involved. Traditional Industry or Biotech-based models do no longer always represent the best possible option to keep science moving forward the development of innovative therapies. Given the challenges presented, for the benefit of people with Multiple Sclerosis, the Italian Multiple Sclerosis Society Foundation is committed to play a role in contributing to filling this translational gap, often also referred to as the ‘Valley of Death’ [1]. During the past years, as other Patients’ Foundations, we have been taking on the role of ‘venture philanthropists’ by contributing to bridge the gap between promising discoveries and the commercial expertise to move them forward. We will present the case of the GPR17 research project that aims at developing new re-myelinating therapies. However, it is only the shared responsibility of the stakeholders involved in every stage of drug discovery and development—at any phase, in the public, non-profit and private sector—to contribute to filling the translational gap [2]. It is urgent to conceive new collaborative operational models to create a bioinnovation ecosystem that fosters collegial interactions among all the relevant stakeholders on non-competitive research. We will present our experience towards expediting the discovery and development of therapies for Progressive Multiple Sclerosis (MS) [3] for the benefit of patients of the estimated 1 million people worldwide who have Progressive MS. Despite great progress in relapsing MS, much work is needed to achieve similar successes for the neurodegenerative progressive forms of MS.
References
1. Finkbeiner S (2010) Nature Med 16:1227–1232
2. Sherer TB (2013) Nature Med 19:127
3. Fox RJ, Thompson A, Baker D, Baneke P, Brown D, Browne P, Chandraratna D, Ciccarelli O, Coetzee T, Comi G, Feinstein 747 A, Kapoor R, Lee K, Salvetti M, Sharrock K, Toosy A, Zaratin P, Zuidwijk K (2012) Mult Scler 18(11):1534–1540
Thu 2 B: Transport and release II: nucleotides
Opening remarks and introduction to vesicular nucleotide transporter (VNUT)
Yoshinori Moriyama
Department of Membrane Biochemistry, Okayama University Graduate School of Medicine, Dentistry and Pharmaceutical Sciences, Okayama, Japan
Release of ATP from cells triggers purinergic response among various purinoceptor-expressing cells. In spite of well-understood features on the signaling cascade after stimulation of the purinoreceptors, the mechanism of how ATP is released from the purinergic cells is less characterized. ATP is released through at least three distinct pathways: vesicular secretion, channel-mediated release and simply cell breakdown. Recent studies have revealed two membrane proteins which play an essential role in these pathways: vesicular nucleotide transporter (VNUT) that is responsible for vesicular storage of ATP, and pannexin 1 that is responsible for ATP release through plasma membrane. In this symposium, we focus on VNUT and pannexin 1, and discuss the significance of the two membrane proteins in purinergic chemical transmission. After introducing the overall feature of mechanism of ATP release, three young Japanese researchers will talk about frontier of VNUT study: Dr. Hiasa is going to talk the role of VNUT in platelet, Dr. Sakamoto will talk about the effect of VNUT knock out on the neuroendocrine functions, and Dr. Nakagomi will talk about the effect of VNUT knock out on physiological and pathological aspects of bladder epithelium. Then, Dr. Isakson will talk about role of pannexin 1 in vascular ATP release. Finally Dr. Lazarowski will overview of progress of ATP release and talk about recent work on nucleotide release from airway epithelial cells as a good model of coordinated participation of both VNUT and pannexin 1.
Vesicular nucleotide transporter (VNUT) in platelets
Miki Hiasa
Department of Membrane Biochemistry, Okayama University Graduate School of Medicine, Dentistry and Pharmaceutical Sciences, Okayama, Japan
Extracellular nucleotides function as intercellular messengers and cause various physiological or pathological responses upon binding to purinoceptors on the target cells. In platelets, nucleotides are stored in the dense granules and their release facilitates platelet aggregation through purinoceptors and that in consequence, platelets play an essential role in hemostasis and thrombosis. However, the mechanism of how platelets store nucleotides in the granules is far less understood.
In 2008, Dr. Moriyama and colleagues identified Vesicular nucleotide transporter (VNUT, SLC17A9 protein) that is responsible for the vesicular storage and subsequent exocytosis of ATP [1]. VNUT transports nucleotides such as ATP and ADP using the membrane potential established by vacuolar proton ATPases. The ATP uptake requires low concentrations of Cl− and inhibited by Evans Blue, DIDS and keton bodies. In analogy to other vesicular neurotransmitter transporters, VNUT is also a potential molecular probe for identifying the sites of vesicular ATP storage and secretion.
In the present study, we investigated the possible involvement of VNUT in the vesicular storage and release of nucleotides in platelets. We demonstrated that VNUT was expressed in human platelets and associated with dense granules and detected VNUT transport activity in platelet membrane vesicles. RNA interference suppressed both VNUT expression and Ca2+-dependent release of nucleotides in clonal human megakaryoblastic cells. Furthermore, identification of glyoxylate as a reversible inhibitor of VNUT provided a clue for development of selective modulator of VNUT activity to control nucleotides release in vivo [2].
References
1. Sawada K et al (2008) Proc Natl Acad Sci USA 105:5683-5686
2. Hiasa M et al (2014) Physiol Rep. electric version
Vesicular nucleotide transporter (Vnut) regulates glucose metabolism.
Shohei Sakamoto1,*, Takaaki Miyaji2, Miki Hiasa3, Reiko Ichikawa4, Akira Uematsu4, Ken Iwatsuki4, Atsushi Shibata1, Hisayuki Uneyama4, Ryoichi Takayanagi1, Akitsugu Yamamoto5, Hiroshi Omote3, Masatoshi Nomura1 and Yoshinori Moriyama2,3
1Department of Medicine and Bioregulatory Science, Graduate School of Medical Sciences, Kyushu University, Fukuoka 812-8582, Japan;2Advanced Research Science Center, Okayama University, Okayama 700-8530, Japan;3Department of Membrane Biochemistry, Okayama University Graduate School of Medicine, Dentistry and Pharmaceutical Sciences, Okayama 700-8530, Japan;4Institute for Innovation, Ajinomoto Co., Inc. Kawasaki 210-5893, Japan;5Faculty of Bioscience, Nagahama Institute of Bio-science and Technology, Nagahama 526-0829, Japan
Neuroendocrine cells store ATP in secretory granules and release it along with hormones that may trigger a variety of cellular responses in a process called purinergic chemical transmission. Although the vesicular nucleotide transporter (VNUT) has been shown to be involved in vesicular storage and release of ATP, its physiological relevance in vivo is far less well understood. In Vnut knockout (Vnut−/−) mice, we found that the loss of functional VNUT in adrenal chromaffin granules and insulin granules in the islets of Langerhans led to several significant effects. Vesicular ATP accumulation and depolarization-dependent ATP release were absent in the chromaffin granules of Vnut−/− mice and the synthesis and exocytosis of adrenaline and noradrenaline were significantly decreased. Glucose-responsive ATP release was also absent in pancreatic β-cells in Vnut−/− mice, while glucose-responsive insulin secretion was enhanced to a greater extent than that in wild-type tissue. Vnut−/− mice exhibited improved glucose tolerance and low blood glucose upon fasting due to increased insulin sensitivity. These results demonstrated an essential role of VNUT in vesicular storage and release of ATP in neuroendocrine cells in vivo and suggest that vesicular ATP and/or its degradation products act as feedback regulators in catecholamine and insulin secretion, thereby regulating blood glucose homeostasis.
The role of VNUT in bladder epithelium
Hiroshi Nakagomi1,*, Tsutomu Mochizuki1, Mitsuharu Yoshiyama1 Youichi Shinozaki2, Keisuke Shibata2, Tatsuya Miyamoto1, Masayuki Takeda1, Yoshinori Moriyama3 and Schuichi Koizumi2
1Department of Urology, Interdisciplinary Graduate School of Medicine and Engineering, University of Yamanashi, Yamanashi, Japan;2Department of Neuropharmacology, Interdisciplinary Graduate School of Medicine and Engineering, University of Yamanashi, Yamanashi, Japan;3Department of Membrane Biochemistry, Graduate School of Medicine, Dentistry and Pharmaceutical Sciences, Okayama University, Okayama, Japan
The urothelium of urinary bladder functions not only a passive barrier against ions and infections, but also functions as a sensor responding to various types of stimuli including physical stimulation by bladder filling. Urothelial cells release ATP as well as other chemical mediators in response to stretch-stimulation. Among these, ATP plays a central role because it activates P2 receptors on primary afferent fibers to transmit sensation of micturition to the CNS. However, the mechanisms underlying ATP release in response to various stretch stimuli remain largely unknown. Here we show that the urothelial cells exocytose ATP via a VNUT-dependent mechanism. Immunostaining studies showed that VNUT signals were highly expressed in all urothelial cell layers in mouse bladder and cultured urothelial cells. The VNUT signals labeled by RFP were co-located with the fluorescent ATP analogue mant-ATP and quinacrine. We visualized the real-time dynamics of ATP release using quinacrine, and found that they were exocytosed. The stretch-evoked ATP release was significantly reduced by the treatment of VNUT siRNA or several inhibitors of vesicular exocytosis. These findings suggest that VNUT plays an important role in stretch-elicited ATP release from urothelium. Recently we have succeeded in generating VNUT-KO mice. We have started to analyze phenotypes of these mice both in vivo and in vitro experiments, including the stretch-evoked ATP release from urothelium, bladder functions and urination behaviors. I will also present these results obtained from VNUT-KO mice in this symposium.
Pannexin 1 channels on smooth muscle cell regulates α1-adrenergic vasoconstriction and blood pressure
Brant E. Isakson
Robert M. Berne Cardiovascular Research Center, University of Virginia School of Medicine, Charlottesville Virginia USA
We recently demonstrated a key role for pannexin (Panx1) in α1-adrenergic receptor (α1-AR) stimulation of resistance arteries (Billaud et al., Circ Res 2012). However, the participation of Panx1 in other contractile pathways has not been investigated. Therefore, we stimulated resistance arteries with endothelin-1, serotonin, AngII, ATP, noradrenaline and phenylephrine (PE). Only noradrenaline and PE responses were altered by pharmacological pannexin inhibitors. In addition, release of ATP and Panx 1 currents could only be detected when PE or noradrenaline was used. These results were verified using proximity ligation assays demonstrating α1-AR/Panx1 interaction, but no Panx1 interactions with any of the other vasoconstrictor receptors. Using a heterologous expression system expressing α1-AR and Panx1, we created mutations in the Panx1 intracellular loop and found tyrosine 198 conversion to alanine inhibited ATP release and channel current after PE or noradrenaline. Custom antibodies were created which demonstrated specific phosphorylation of Panx1 at tyrosine 198 only after noradrenaline or phenylephrine stimulation of resistance arteries. To further evaluate the potential role for Panx1 in smooth muscle, a novel transgenic mouse that had specific deletion of Panx1 in the smooth muscle (Panx1fl/fl/Cre+) cells under the control of tamoxifen was created. Functionally, these mice after tamoxfien injection had significantly decreased responses to noradrenaline and phenylephrine, but the vasoconstrictions to endothelin-1 or serotonin were unchanged. Importantly, Panx1fl/fl/Cre+ +tamoxifen mice were significantly hypotensive. Our results show that Panx1 has an exclusive interaction with α1-AR in smooth muscle of resistance arteries that together can regulate vascular tone and blood pressure.
Pannexin 1 and vesicular nucleotide transporter (VNUT) contribute to airway epithelial nucleotide release
Juliana I. Sesma, Barbara R. Grubb, Catharina van Hesden, Silvia M. Kreda and Eduardo R. Lazarowski*
Cystic Fibrosis/Pulmonary Research & Treatment Center University of North Carolina, Chapel Hill, USA
Extracellular ATP and its metabolic product adenosine regulate ion channel/fluid secretion activities necessary for mucin hydration via activation of airway epithelial P2Y2 and A2B receptors. In spite of the pathophysiological relevance of the responses triggered by extracellular nucleotides in the lung, the mechanisms of airway epithelial nucleotide release in normal and inflamed airways has only recently begun to be addressed. Insights into these mechanisms emerged from the identification of (i) pannexin 1 as a plasma membrane ATP channel and (ii) solute carrier (SLC) transporters that control the uptake and storage of nucleotides in secretory vesicles/granules. Our recent studies suggest that, in the airways, pannexin 1 mediates ATP release from ciliated cells-dominated airway epithelia, whereas VNUT controls nucleotide levels within mucin granules and contributes to the release of ATP from mucin secreting cells. Notably, primary cultures of human bronchial epithelial (HBE) cells exposed to inflammatory factors from cystic fibrosis (CF) airways exhibited enhanced hypotonicity-evoked ATP release, relative to non-inflamed cultures. ATP release from inflamed cells was sensitive to inhibitors of the secretory pathway, but was not accompanied by mucin secretion. Thus, vesicular mechanisms, additional or alternative to mucin granules, contribute to the release of ATP from CF-like inflamed airway epithelia. Current studies in our lab are addressing the contribution of VNUT and pannexin 1 to mucociliary clearance activities and inflammation in mouse models of obstructed lung diseases.
Thu 2 C: Purinergic control of synaptic transmission
Purinergic signalling in neuronal-glial networks
Alexei Verkhratsky
Faculty of Life Sciences, The University of Manchester, Manchester, UK
Purinergic transmission is one of the most ancient and widespread extracellular signalling systems. In the brain, purinergic signalling plays a unique role in integrating neuronal and glial cellular circuits, as virtually every type of glial cell possesses receptors to purines and pyrimidines. These receptors, represented by metabotropic P1 adenosine receptors, metabotropic P2Y purinoceptors and ionotropic P2X purinoceptors, control numerous physiological functions of glial cells and are intimately involved in virtually every form of neuropathology.
Key words: P2X receptors, NMDA receptors, Ca2+ signalling; Na+ signalling
Modulation of GABAergic inhibition in the hippocampus by adenosine
Ana M. Sebastião1,*, Diogo M. Rombo1, Karri P. Lamsa2 and Joaquim A. Ribeiro1
1Institute of Pharmacology and Neurosciences, Faculty of Medicine and Unit of Neurosciences, Institute of Molecular Medicine, Lisbon, Portugal;2Department of Pharmacology, University of Oxford, Oxford, UK
Adenosine is an endogenous antiepileptic substance, with well known modulatory actions upon excitatory transmission and plasticity in the hippocampus [1]. However, in spite of the relevance of GABAergic inhibitory transmission to control seizures, little is known about the role of adenosine on this system. We recently reported that adenosine can interfere with the life-span of GABA at synapses, by regulating the activity of GABA transporters. At nerve endings only A2A receptors (R) are involved [2], but in astrocytes A1R and A2AR act as tetramers that signal via two different G proteins, Gs and Gi/0, to fine-tune GABA uptake [3]. We now focused upon the action of adenosine at inhibitory synapses, distinguishing between modulation of inhibitory inputs to excitatory neurons and modulation of inhibitory inputs to inhibitory neurons.
We recorded GABAAR-mediated currents in pyramidal neurons and in interneurons of the CA1 area in hippocampal slices, and found that activation of A1R inhibits GABAAR currents both in pyramidal cells and in specific interneurons expressing cannabinoid receptor type 1. Interestingly, the A1R-mediated suppression was only detected in GABAergic currents generated at perisynaptic/extrasynaptic site. Regarding adenosine A2ARs, we found, in contrast, that they operate presynaptically at parvalbumin expressing neurons to facilitate GABAergic inputs to other inhibitory neurons.
We conclude that cell type-specific localization and function of A1R and A2AR control phasic (A2AR) and tonic (A1R) GABAergic transmission in the hippocampus. In addition, the finding that adenosine modulation of inhibition is synapse-specific indicates that it operates at key points of the hippocampus to control network excitability.
References
1. Dias RB, Rombo DM, Ribeiro JA, Henley JM, Sebastião AM (2013) Trends in Neuroscience 36:248–257
2. Cristóvão-Ferreira S, Vaz SH, Ribeiro JA, Sebastião AM (2009) J Neurochem 109:336–347
3. Cristóvão-Ferreira S, Navarro G, Brugarolas M, Pérez-Capote K, Vaz SH, Fattorini G, Conti F, Lluis C, Ribeiro JA, Mccormick PJ, Casadó V, Franco R, Sebastião AM (2013) Purinergic Signal 9:433–449
Interaction between purinergic and glutamatergic synaptic inputs in the neocortex
Yuriy Pankratov1,*, Alexej Verkhratsky2 and Ulyana Lalo1
1School of Life Sciences, University of Warwick, Coventry, UK;2Faculty of Life Science, The University of Manchester, Manchester, UK
Studies performed during last two decades highlighted important role for purinoreceptors in modulation of synaptic transmission and plasticity. Our recent work was focused on the interaction of postsynaptic P2X and NMDA receptors.
Interaction between P2X and NMDA receptors was studied in the pyramidal neurons of neocortical slices and in the individual synapses. Advanced technique of acute cell isolation allowed cells to retain the proximal dendrites with attached synaptic boutons. The presence of functional synapses was confirmed by activity-dependent staining with FM1-43 and recording of miniature spontaneous mEPSCs resembling those recorded in slices. This preparation enabled to elicit synaptic currents in single bouton by stimulation with external electric field applied via micropipette. Excitatory synaptic currents, recorded both in the individual synapses and in neurons in slice, exhibited two components: glutamatergic and purinergic, mediated by P2X receptors. The co-localization of P2X and glutamate receptors was verified by immunolabeling of living cells with P2X and GluN antibodies.
We found out the ATP can be co-released with glutamate and activate P2X receptors causing a decrease in the NMDA receptor-mediated synaptic currents via Ca2+-dependent de-phosphorylation. P2X-activated down-regulation of NMDA receptors was abolished in the mutant mice, lacking the PDZ-domains of PSD-95 complex. Abolishing of purinergic regulation of NMDA receptors either by knock-out of P2X receptor or by PDZ mutation or pharmacologically lead to substantial increase in the long-term potentiation induced by weaker tetanic stimulation. Combined, these results suggest an important physiological role for postsynaptic P2X receptors in central synapses.
Synaptic effects of adenosine A2ARs: interactions with other receptors and possible implications for CNS diseases
Patrizia Popoli
Department of Therapeutic Research and Medicines Evaluation, Istituto Superiore di Sanità, Viale Regina Elena 299, 00161 Roma, Italy
Adenosine A2A receptors (A2ARs) are highly expressed in the striatum and, thanks to their ability to establish functional and molecular interactions with many other receptors, they play a pivotal role in the modulation and integration of striatal neurotransmission.
Besides the recently reported regulatory role of striatal A2ARs on the synaptic effects of BDNF and cocaine [1,2], their interaction with cannabinoid CB1 receptors seems to be particularly intriguing, since A2ARs can exert both a permissive and an inhibitory influence on CB1-dependent effects [3]. To further investigate such a complex interaction, we studied, by extracellular field potential recordings in corticostriatal slices, the synaptic effects of the CB1R agonist WIN 55212,2 (WIN) in wild type (WT) and transgenic rats overexpressing A2ARs, TGR(NSEhA2A) [4]. While WIN (2 μM) induced a marked depression of synaptic transmission in both genotypes, it was significantly less effective in transgenic (−40 % of basal amplitude) than in WT rats (−70 % of basal, P < 0.001). Such an impairment of CB1-mediated effects could depend on the overexpression of pre-synaptic A2ARs, since the inhibition exerted by WIN on K+-evoked glutamate efflux was significantly lower (P < 0.05) in striatal synaptosomes from TGR(NSEhA2A) than WT rats. Furthermore, TGR(NSEhA2A) rats showed a significantly reduced hypolocomotion in response to WIN (5 mg/kg i.p., P < 0.05). These data confirm the strong modulatory role exterted by A2ARs on CB1-mediated effects. The different role of pre- vs. postsynaptic A2ARs, their interaction with other receptors, the role of A2ARs-CB1 heteromers, and the potential relevance of the A2AR-CB1R interaction to neurodegenerative diseases will be further evaluated.
References
1. Martire A, Pepponi R, Domenici MR, Ferrante A, Chiodi V, Popoli P (2013) J Neurochem 125:225–235
2. Chiodi V, Mallozzi C, Ferrante A, Chen JF, Lombroso PJ, Di Stasi AM, Popoli P, Domenici MR (2014) Neuropsychopharmacology 39:569–578
3. Tebano MT, Martire A, Popoli P (2012) Brain Res 1476: 108–118
4. Giménez-Llort L, Schiffmann SN, Shmidt T, Canela L, Camón L, Wassholm M, Canals M, Terasmaa A, Fernández-Teruel A, Tobeña A, Popova E, Ferré S, Agnati L, Ciruela F, Martínez E, Scheel-Kruger J, Lluis C, Franco R, Fuxe K, Bader M (2007) Neurobiol Learn Mem 87:42–56
Fine tuning of neurotransmitter output and excitability in peripheral neurons by P2Y receptors
Arsalan Yousuf, Giri Chandaka, Klaus Schicker, Hend Gafar and Stefan Boehm*
Department of Neurophysiology and Neuropharmacology, Centre of Physiology and Pharmacology, Medical Univ. Vienna, Vienna, Austria
While the roles of P2X receptors in autonomic and sensory neurons have been elucidated to a great extent, the functions of P2Y receptors in the peripheral nervous system remained largely controversial. In dorsal root ganglion neurons, nucleotides increased excitability simultaneously via P2Y1 and P2Y2 receptors. The underlying mechanisms involved an inhibition of Kv7 channels and a facilitation of TRPV1 channels. Both effects relied on activation of phospholipase C and increases in intracellular Ca2+. The facilitation of TRPV1, but not the inhibition of Kv7 channels, required protein kinase C. In the presence of blockers of Kv7 and TRPV1 channels, nucleotides did not affect the excitability of sensory neurons [1]. In postganglionic sympathetic neurons, nucleotides co-released together with noradrenaline mediate feedback regulation of transmitter output: through presynaptic P2Y12 receptors, nucleotides caused an inhibition of transmitter release via pertussis toxin-sensitive G proteins; through presynaptic P2Y1 receptors, nucleotides caused an enhancement via phospholipase C. Activation of P2Y1 receptors also led to an inhibition of Kv7 channels in sympathetic neurons, as did the activation of P2Y6 [2]. However, neither activators nor inhibitors of Kv7 channels did affect transmitter release. Phorbol esters and diacyl glycerol analogues occluded facilitatory effects of nucleotides, but PKC inhibitors had no effect. Long lasting phorbol ester treatment down regulated munc13 and prevented facilitatory effects of nucleotides. Thus, the facilitation of sympathetic transmitter release via P2Y1 receptors appears to be mediated by munc13 isoforms. In summary, in peripheral autonomic and sensory neurons various P2Y receptors cooperate to tightly control excitability and neurotransmitter output.
This study was supported by the Austrian Science Fund (FWF).
References
1. Yousuf A, Klinger F, Schicker K, Boehm S (2011) Pain 152:1899–1908
2. Chandaka GK, Salzer I, Drobny H, Boehm S, Schicker KW (2011) Br J Pharmacol 164:1522–1533
Thu 2 D: Purines in wound healing and fibrosis
Effect of adenosine receptors on keratinocyte function
M. Carmen Montesinos*, Rosa M. Andrés, Jorge Arasa and M. Carmen Terencio
Center of Molecular Recognition and Technological Development. Department of Pharmacology, University of Valencia, Ave. Vicent Andrès Estellès s/n, 46100 Burjassot, Valencia, Spain
Wound re-epithelialization is the ultimate step of the reestablishment of the epithelial barrier after injury. Topical application of selective adenosine A2A receptor agonists promotes wound closure in both healthy and diabetic animals1. Despite the poor granulation tissue formation observed in A2A receptors deficient mice, re-epithelialization was not compromised2. Examination of adenosine receptor expression in foreskin keratinocytes confirmed that the A2B subtype is most prominently expressed, followed by the A2A receptor, while subtypes A1 and A3 were undetectable. Both the non-selective adenosine receptor agonist 5′-(N-ethylcarboxamido)adenosine (NECA) and the selective A2B agonist BAY 60-6583 decreased keratinocyte proliferation and increased cAMP production and intracellular calcium levels. In contrast, the selective A2A agonist CGS-21680 promoted cell proliferation and failed to increase cAMP, but dose-dependently inhibit TNFα production by stimulated keratinocytes. Pretreatment with CGS-21680 (5 μg per site) prevented the epidermal hyperplasia and inflammatory response induced by topical application of 12-O-tetradecanoylphorbol-13-acetate (TPA, 2 nmol/site) for three consecutive days, while promoted dermal fibroblasts proliferation and collagen deposition in dermis. Interestingly, in epidermis from psoriatic patients we found decreased A2B receptors expression and increased A2A receptor expression. We could reproduce this pattern by treating keratinocytes from healthy donors with the cytokine IFNγ. Additionally, TNFα and IL-1β also increased A2A receptor expression, whereas IFNα and TPA decreased A2B receptor expression. Our results suggest that adenosine plays an important role regulating epidermal inflammation and keratinocyte function, and thus may constitute an interesting therapeutic strategy in inflammatory hyperproliferative skin diseases such as psoriasis.
References
1. Valls MD, Cronstein BN, Montesinos MC (2009) Biochem Pharmacol 77:1117–1124
2. Montesinos MC, Desai A, Chen JF et al (2002) Am J Pathol 160:2009–2018
Adenosine A2Areceptor (A2AR) stimulates bone regeneration and healing
Aránzazu Mediero
Division of Translational Medicine, Department of Medicine, NYU Langone Medical Center. New York, NY, USA
Bone is a dynamic organ that undergoes continuous remodeling whilst maintaining a balance between bone formation and resorption. Various types of orthopedic procedures, including spinal fusion and repair of bone defects due to trauma, infection or metastatic disease, require formation of new bone. We have previously reported that A2AR stimulation inhibits osteoclast differentiation but only A2BR stimulation affects osteoblast differentiation or function. Here we report on the role of A2AR in promoting bone regeneration and healing both in vitro and in vivo and explore the mechanisms by which A2AR stimulates bone regeneration.
Adenosine receptors and tissue fibrosis
Bruce N. Cronstein1, Miguel Perez Aso2 and Jessica Feig1
1NYU School of Medicine, New York, NY, USA; 2Barcelona, Spain
Recent studies have clearly demonstrated the role of adenosine and its receptors in promoting wound healing and tissue repair. Indeed, overabundant tissue repair and fibrosis are consequences of adenosine generation and receptor ligation in the skin and such organs as the liver, lungs and peritoneum [1]. In our recent studies we have found that adenosine A2A receptor ligation plays a critical role in diffuse dermal fibrosis, hepatic fibrosis, scars in the skin and, more recently, radiation dermatitis. Here we will review recent studies demonstrating the central role of adenosine, generated as a result of extracellular nucleotide hydrolysis, and adenosine A2A receptors in promoting dermal and hepatic fibrosis. Moreover, we have recently found that agents that diminish extracellular adenosine levels inhibit fibrosis in mice and there is evidence from clinical studies that some of these agents may be useful for the treatment of hepatic fibrosis. Adenosine receptor blockade and diminished adenosine generation may be useful approaches to the treatment of fibrosing diseases, currently an unmet medical need.
Reference
1. Cronstein BN (2011) Adenosine receptors and fibrosis: a translational review. F1000 biology reports 3:21
Purinergic signalling and sensing of renal tubular flow
Jens Leipziger
Department of Biomedicine, Physiology, Aarhus University, Aarhus, Denmark
During the last 10 years the renal research community has set the primary cilium into the lime light. From being viewed as a possible evolutionary rudiment, today the primary cilium has achieved the noble status of a physiologically relevant cellular structure. Its prime function in renal epithelium appears to be its ability to sense urinary flow. Much is still lacking to understand how the primary cilium senses flow. Transducer proteins, such as specific mechano-sensory ion channels, have been identified and are necessary for flow-dependent increases of epithelial [Ca2+]i. A flow-induced increase of [Ca2+]i has been observed in all renal and other ciliated epithelial cells. Work over the last 5 years has addressed the mechanism underlying the flow-induced increase of [Ca2+]i. It has become apparent that an initial Ca2+ influx triggers a global increase of epithelial [Ca2+]i. Eventually, it is also clear that mechanical stimulation of the epithelial cells triggers the release of ATP. Intriguingly, ATP is an auto-and paracrine signaling molecule that regulates electrolyte and water transport in the nephron by binding to apical and basolateral purinergic receptors. ATP inhibits transport at almost all sites from the proximal to the distal tubule and thus elicits a diuretic response. In this perspective, the primary cilium is a sensory structure and the adequate stimulus is the mechanical deflection. The output signal is the released ATP, a paracrine factor that ultimately modulates the main function of the kidney, i.e. the enormous task of absorbing some 180 l of filtrate every day.
Thu 3 A: New technologies in purine receptor research
Single molecule detection at adenosine receptors
Stephen J. Hill
Cell Signalling Research Group, School of Life Sciences, University of Nottingham, UK
Our previous work, using fluorescent adenosine receptor agonists and antagonists, has provided novel insights into the allosteric regulation of adenosine A3 and A1 receptors by allosteric ligands and homodimerisation [1,2]. We have also used fluorescence correlation spectroscopy (FCS) to investigate ligand binding to A1 and A3 receptors in small microdomains of single living cells [3,4]. FCS studies with a fluorescent A3-agonist has enabled high affinity labeling of the active conformation (R*) of the receptor. We have now used a fluorescent adenosine A3-antagonist (CA200645) to study the binding characteristics of antagonist-occupied receptor conformations (R) in membrane microdomains of individual cells. FCS analysis of CA200645-occupied A3-receptors revealed two species (τD2 and τD3) that diffused at 2.29 ± 0.35 and 0.09 ± 0.03 μm2/s, respectively. FCS analysis of a GFP-tagged A3-receptor exhibited a single diffusing species (0.087 μm2/s) that was not altered by pre-treatment with A3-agonists or A3-antagonists. The binding of CA200645 to τD3 was antagonized by nanomolar concentrations of the A3-antagonist MRS 1220, but not by NECA (up to 300 nM) consistent with labeling of the inactive conformation (R) of the receptor. CA200645 normally dissociated slowly from the A3-receptor but inclusion of the allosteric ligand VUF 5455, or the orthosteric ligand xanthine amine congener, during washout markedly accelerated the reduction in the number of particles exhibiting τD3 characteristics consistent with an allosteric effect. The potential for FCS analysis of ligand-occupied receptors to provide a powerful and unique means to monitor ligand residence times, and the impact of allosteric regulators on them, will be discussed.
References
1. May LT, Self TJ, Briddon SJ, Hill SJ (2010) Mol Pharmacol 78:511–523
2. May LT, Bridge LJ, Stoddart LA, Briddon SJ, Hill SJ (2011) FASEB J 25:3465–3476
3. Cordeaux Y, Briddon SJ, Alexander SPH, Kellam B, Hill SJ (2008) FASEB J 22 850–860
4. Briddon SJ, Middleton RJ, Cordeaux Y, Flavin FM, Weinstein JA, George MW, Kellam B, Hill SJ (2004) Proc Natl Acad Sci USA 101:4673–4678
Optogenetic activation of intracellular adenosine A2Areceptor signaling in hippocampus is sufficient to impair memory through CREB phosphorylation
Jiang-Fan Chen1,2,*, Ji-Hoon Yoo1, Wei Li1, Xiangtian Zhou2, Yumei Wang1, Gerard van Westen3, Marie-Pierre Payen1, Elisabete Augusto 1,4, Zhihui Li2, Zhongnan Wu2, Xianhua Hou1, Rodrigo Cunha1,4, Yuanguo Zhou5, Ad IJzerman3, Edward Boyden6 and Jia Qu2
1Boston University School of Medicine, Neurology, Boston, Portugal;2Wenzhou Medical University, School of Optometry and Ophthalmology, Wenzhou, China, China;3Leiden University, Leiden Academic Centre for Drug Research, Leiden, Netherlands;4University of Coimbra, Center for Neurosciences and Cell Biology, Coimbra, Portugal;5Third Military Medical University, Research Institute of Surgery, Chongqing, China;6Massachusetts Institute of Technology, MIT McGovern Institute, Cambridge, USA
Human and animal studies have converged to suggest that caffeine consumption prevents memory deficits in aging and Alzheimer’s disease through the antagonism of adenosine A2A receptors (A2AR). To address the central question whether A2AR activation in hippocampus is sufficient to impair memory function and to begin elucidating the intracellular pathways operated by A2AR, we have developed a chimeric rhodopsin-A2AR protein (optoA2AR), which retains the extracellular and transmembrane domains of rhodopsin (conferring light responsiveness and eliminating adenosine binding pockets) fused to the intracellular loop of A2AR to confer specific A2AR signaling. The specificity of the optoA2AR signaling was confirmed by light-induced selective enhancement of cAMP and phospho-MAPK (but not cGMP) levels in HEK293 cells, which was abolished by a point-mutation at the C-terminal of A2AR. Supporting its physiological relevance, optoA2AR and the A2AR agonist CGS21680 produced comparable and additive activation of cAMP and phospho-MAPK signaling in HEK293 cells and of c-Fos in the mouse brain. Remarkably, optoA2AR and CGS21680 triggered a preferential phosho-CREB signaling in hippocampusor phospho-MAPK signaling in nucleus accumbens. Importantly, light optoA2AR activation of CREB signaling in the hippocampus impaired spatial memory performance while optoA2AR activation of MAPK signaling in the nucleus accumbens modulated locomotor activity. This shows that the recruitment of intracellular A2AR signaling in hippocampus is sufficient to trigger memory dysfunction. Furthermore, the demonstration of the intracellular control of biased A2AR signaling and behaviors prompts the possibility of targeting the intracellular A2AR interacting partners to selectively control different neuropsychiatric behaviors.
Screening in academia. A case for P2X7 allosteric modulators
Michael Schaefer*, Christoph Hempel, Melanie Kaiser, Tanja Plötz, Helga Sobottka, Wolfgang Fischer and Wolfgang Nörenberg
Rudolf-Boehm-Institute of Pharmacology and Toxicology, University of Leipzig, Haertelstr. 16-18, 04107 Leipzig, Germany
P2X receptors are plasma membrane-resident, ligand-gated cation channels that feature an extracellularly exposed ATP binding pocket that may be addressed by orthosteric ligands. In addition, the complex structure of P2X receptors offers many opportunities to bind small drug-like molecules that may affect the ligand binding or gating properties in an allosteric manner. Applying libraries of known biologically active molecules and an academic-scale screening assay, we found that human P2X7 (hP2X7) is modulated by a plethora of chemically diverse molecules, including perazine-type tricyclic antipsychotics, first generation antihistamines, an unexpected modulation by the otherwise P2X4-specific ivermectin, natural compounds and semisynthetic drugs. Mechanistically, all novel modulators that we have identified act in an allosteric fashion, but display distinct properties with regard to the positive or negative mode of P2X7 modulation, as well as the underlying biophysical mechanisms. Clemastine stabilizes the open state of the channel and increases the potency of ATP. The perazines PCP and TFP change the channel’s activation and deactivation times, causing a suppression of P2X7 currents and Ca2+ fluxes. Ivermectin causes higher maximal current amplitudes without affecting the ligand binding potency. Tanshinone IIA sulfonate inhibits hP2X7 in a voltage-dependent manner, presumably by binding to the intracellular moiety of the channel protein. Even more intriguingly, the majority of allosteric P2X7 modulators were not only isotype-selective, but also discriminated between species variants of P2X7. In agreement with recent work of Anton D. Michels group, we found that exchanging few amino acid residues in a region that corresponds to the top of the outer vestibule in P2X4 dramatically changes the impact of allosteric modulators on the ATP-induced P2X7 gating. We conclude that the unique susceptibility of P2X7 to allosteric modulation is an isotype- and species-specific phenomenon.
The value of label-free biosensors for deciphering signaling and function of orphan P2Y-like purinoreceptors
Evi Kostenis
Institute of Pharmaceutical Biology, University of Bonn, Nussallee 6, 53115 Bonn, Germany
For a number of GPCRs, primary messengers are known and these have often aided in understanding their biological function. Orphan GPCRs, on the contrary, are receptors which are targets for undiscovered transmitters and this lack of knowledge often precludes assignment of a precise biological role. Nevertheless, orphan receptors may create excitement in academia and the drug discovery industry, if, for example, knockout mouse models reveal an intriguing phenotype.
GPR17 is such an orphan receptor. It is a member of the rhodopsin-family of GPCRs phylogenetically related to receptors of the purinergic cluster. In line with this notion, uracil-nucleotides and cysteinyl-leukotrienes have been proposed as endogenous ligands [1]. However, we and several other laboratories failed to confirm activation of GPR17 by the proposed natural ligands [2–6]. In this talk I will initially illustrate the efforts we made to recapitulate activation of GPR17 by the proposed endogenous modulators and then present the strategy we applied to identify a surrogate agonist for GPR17 taking advantage of the cutting edge label-free technology platforms based on dynamic mass redistribution and bio-impedance, respectively, which resolve cellular signaling irrespective of the receptor’s primary signaling pathway. I will then exemplify how we utilized the novel ligand to decipher the biological role of orphan GPR17 and its therapeutic potential [6].
References
1. Ciana P, Fumagalli M, Trincavelli ML, Verderio C, Rosa P, Lecca D, Ferrario S, Parravicini C, Capra V, Gelosa P, Guerrini U, Belcredito S, Cimino M, Sironi L, Tremoli E, Rovati GE, Martini C, Abbracchio MP (2006) EMBO J 25(19):4615–4627
2. Heise CE, O’Dowd BF, Figueroa DJ, Sawyer N, Nguyen T, Im DS, Stocco R, Bellefeuille JN, Abramovitz M, Cheng R, Williams DL Jr, Zeng Z, Liu Q, Ma L, Clements MK, Coulombe N, Liu Y, Austin CP, George SR, O’Neill GP, Metters M, Lynch KR, Evans JF (2000) J Biol Chem 275(39):30531–30536
3. Maekawa A, Balestrieri B, Austen KF, Kanaoka Y (2009) Proc Natl Acad Sci USA 106(28):11685–11690
4. Benned-Jensen T, Rosenkilde MM (2010) Br J Pharmacol 159(5):1092–1105
5. Qi, AD; Harden, TK; Nicholas, RA (2013) J Pharmacol Exp Ther 347(1):38–46
6. Hennen S, Wang H, Peters L, Merten N, Simon K, Spinrath A, Blättermann S, Akkari R, Schrage R, Schröder R , Schulz D, Vermeiren C, Zimmermann K, Kehraus S, Drewke C, Pfeifer A, König GM, Mohr K, Gillard M, Müller CE, Lu QR, Gomeza J, Kostenis E (2013) Sci Signal 6(298):ra93
Molecular basis of ligand dissociation from GPCRs—a molecular dynamics and mutagenesis study on the adenosine A2Areceptor
Laura H. Heitman*, Dong Guo, Tamara A. M. Mocking and Ad P. IJzerman
Department of Medicinal Chemistry, Leiden Academic Centre for Drug Research (LACDR), Leiden University, The Netherlands
The molecular mechanism of how ligands dissociate from their receptors remains to be determined. We therefore performed long-timescale molecular dynamics simulations of a prototypic GPCR, the adenosine A2A receptor, to identify amino acid residues that influence the dissociation of the antagonist ZM241385 from the receptor. We selected 12 amino acids that were mutated to alanine and experimentally determined ZM241385’s affinity and binding kinetics. Several mutants significantly affected the kinetics of the antagonist ZM241385, despite minimally influencing its binding affinity. These mutants include E1695.30Q, H2646.66A and T2566.58A accelerating ZM241385’s egress from the receptors and I662.64A, S672.65A, K153ECL2A and L2677.32A retarding the process. We conclude that ZM241385 follows a multi-step dissociation pathway, consecutively interacting with topographically distinct domains in the receptor. We speculate that this multi-step dissociation process may be common to other GPCRs as well.
Thu 3 B: Purinergic signaling in the cardiovascular system
Novel protective role of endogenous cardiac myocyte P2X4 receptors in heart failure
Tiehong Yang1, Jian-Bing Shen1, Ronghua Yang1, John Redden1, Kimberley Dodge-Kafka1, James Grady1, Kenneth A Jacobson and Bruce T. Liang1,*
1University of Connecticut School of Medicine, Farmington, CT, USA;2National Institutes of Health, Bethesda, MD, USA
Background: Heart failure (HF), despite continuing progress, remains a leading cause of mortality and morbidity. P2X4 receptors (P2X4R) have emerged as potentially important molecules in regulating cardiac function and as potential targets for HF therapy. Transgenic (Tg) P2X4R overexpression can protect against HF, but this does not explain the role of native cardiac P2X4R.
Objective: Our goal is to define the physiological role of endogenous cardiac myocyte P2X4R under basal conditions and during HF induced by myocardial infarction or pressure overload.
Methods and Results: Mice established with conditional cardiac-specific P2X4R knockout (KO) were subjected to left coronary ligation-induced post-infarct or transverse aorta constriction-induced pressure overload HF. KO cardiac myocytes did not show P2X4R by immunoblotting or by any response to the P2X4R-specific allosteric enhancer ivermectin. KO hearts showed normal basal cardiac function but depressed contractile performance in post-infarct and pressure overload models of HF by in vivo echocardiography and ex vivo isolated working heart parameters. P2X4R co-immunoprecipitated and co-localized with nitric oxide synthase 3 (eNOS) in wild type cardiac myocytes. Mice with cardiac-specific P2X4R overexpression had increased S-nitrosylation, cGMP, NO formation, and were protected from post-infarct and pressure overload HF. Inhibitor of eNOS L-NIO blocked the salutary effect of cardiac P2X4R overexpression in post-infarct and pressure overload HF as did eNOS knockout.
Conclusions: This study establishes a new protective role for endogenous cardiac myocyte P2X4R in HF and is the first to demonstrate a physical interaction between the myocyte receptor and eNOS, a mediator of HF protection.
Extracellular adenosine production in human coronary arteries
A. Deussen1, P. Dieterich1, C. Mehnert1, C. von Klitzing1, V. Thom1 and K. Matschke2
1Institut für Physiologie, Medizinische Fakultät der TU Dresden, Fetscherstr. 74, 01307 Dresden;2Herzzentrum Dresden, Universitätsklinik an der TU Dresden Fetscherstr. 76, 01307 Dresden; Institut für Physiologie, Medizinische Fakultät der TU Dresden, Fetscherstr. 74, 01307 Dresden
Extracellular adenine nucleotide and adenosine concentrations are effectively controlled by ecto-ATPases. In particular, ecto-5′-nucleotidase (CD73) has attracted much notice in the past as its activity is typically rate limiting for the conversion from ATP to adenosine. However, in addition to CD73 many tissues exhibit expression and activity of alkaline phosphatase which also hydrolyses 5′-AMP to adenosine. In previous cell studies (endothelial cells, vascular smooth muscle cells) we could not find evidence for significant alkaline phosphatase activity. Similarly, isolated guinea pig heart did not show evidence of alkaline phosphatase activity. However, more recent experiments conducted on isolated mouse hearts exhibited alkaline phosphatase activity in addition to CD73 activity. This prompted us to address the question whether alkaline phosphatase activity is also active in human vessels. For this purpose we studied human internal mammary arteries and coronary vessels obtained from tissues removed during cardiac surgery. In addition to expression, immunohistochemistry and enzyme activity measurements (etheno(ε)-AMP conversion to ε-adenosine) in a flow-through vessel preparation we performed a mathematical model analysis based on the activity measurements. Whereas isolated human umbilical vein endothelial cells did not exhibit alkaline phosphatase activity, isolated human arteries exhibited expression of both, alkaline phosphatase and CD73. In addition, similar enzymatic activities of alkaline phosphatase and CD73 were found. While CD73 expression was confined to the endothelial cells layer, alkaline phosphatase was attributed to the vessel media (immunostaining). When the endothelial layer had been removed from vessels, AOPCP was ineffective to inhibit AMP conversion to adenosine whereas levamisole was still active. The experiments reveal that in human arterial vessels alkaline phosphatase activity needs to be taken into account. The mathematical model analysis allows quantifying adenosine formation through both pathways. The model results show that the flux through the alkaline phosphatase pathway is quantitatively similar to that catalyzed by CD73.
Role of A2Badenosine receptors in differentiation of infiltrating mononuclear leukocytes during myocardial infarction
Igor Feoktistov and Sergey Ryzhov
Vanderbilt University, Medicine, Nashville, TN, USA
Adenosine is a part of pathological microenvironment in ischemic myocardium and as such may participate in the regulation of various stages of events triggered by myocardial infarction (MI). Our previous studies have demonstrated an important role of A2B adenosine receptors (A2BR) in upregulation of paracrine factors in cardiac stem cell antigen-1-positive cells and promoting repair after MI. These data were in agreement with the current view of adenosine as a cardioprotective agent. We have now discovered that A2BR signaling also profoundly affects the response of mononuclear phagocyte system early after MI. We found that MI induced an acute accumulation of Ly6Chi monocytes in the injured myocardium of wild-type (WT) mice, with high and relatively stable numbers seen on days 3–5 post-MI. These myocardium-infiltrating monocytes eventually differentiated into mature myeloid mononuclear cells (MMCs) with a peak by day 7. In A2BR knockout (KO) mice, MI also induced accumulation of monocytes by day 3 in numbers comparable to those seen in WT hearts. However, the lack of A2BR resulted in accelerated differentiation of monocytes into MMCs, which numbers were greater and peaked earlier (by day 5) compared with WT animals. Analysis of the expression of pro-inflammatory (iNOS) and pro-angiogenic (VEGF) markers on day 5 post-MI revealed that Ly6Chi monocytes express high levels of iNOS but not VEGF. In contrast, MMCs expressed VEGF but significantly lower levels of iNOS. These data suggest that, whereas myocardium-infiltrating monocytes have a pro-inflammatory phenotype, their differentiation into MMCs may promote cardiac repair. To study the role of A2BR signaling in these cells in vitro, we generated Ly6Chi monocytes from bone marrow hematopoietic progenitor cells and incubated in a differentiating medium for 2 days in the absence or presence of the stable adenosine analog NECA. NECA significantly decreased the proportion of differentiated MMCs. In contrast, NECA had no effect on differentiation of HPC-derived monocytes obtained from A2BR KO mice. Importantly, populations of HPC-derived monocytes and MMCs recapitulated also the expression pattern of iNOS and VEGF seen in their in vivo counterparts. To determine if Gαs- or Gαq-linked pathways could contribute to A2BR-dependent regulation of monocyte differentiation, we compared the effects of their selective activators forskolin and Pasterela multocida toxin (PMT) to those of NECA. We found that, similar to stimulation of A2BR with NECA, direct stimulation of Gαq with PMT inhibited generation of MMCs, whereas stimulation of Gαs with forskolin had no effect. In summary, our new data suggest a surprisingly important role for A2BR-Gαq-mediated regulation of monocyte differentiation in the myocardium that represents a novel mechanism, by which these receptors may in fact worsen cardiac injury and delay reparative phase of MI.
Role of CD73-derived adenosine and A2A/A2Breceptors on T-cells in cardiac remodeling after ischemia/reperfusion
Nadine Borg1,*, Florian Bönner2, Christoph Jacoby2, Nicole Görldt1, Zaoping Ding1, Daniela Friebe1, Sebastian Temme1, Ulrich Flögel1 and Jürgen Schrader1
1Institute of Molecular Cardiology, Heinrich-Heine-University of Düsseldorf, Universitätsstr. 1, 40225 Düsseldorf, Germany;2Department of Cardiology, Pneumology and Angiology, Heinrich-Heine-University of Düsseldorf, Moorenstr. 5, 40225 Düsseldorf, Germany
Myocardial infarction leads to a massive release of pro-inflammatory nucleotides, the extracellular degradation of which is controlled by a cascade of ectoenzymes. The final conversion to adenosine, a potent anti-inflammatory local autacoid, is catalyzed by Ecto-5′-nucleotidase (CD73). We recently found, that global deficiency of CD73 results in impaired cardiac function after severe ischemia and subsequent reperfusion (I/R), accompanied by a prolonged cardiac inflammatory response, enhanced fibrosis, and immature scar formation. Experiments with chimeric mice demonstrate that CD73 present on cardiac immune cells can fully account for the observed phenotype. Since CD73 expression and activity was upregulated on infiltrating T-lymphocytes we explored the functional role of this ecto-nucleotidase on T-cells in the process of cardiac healing after I/R by using T-cell specific CD73−/− mice. Surprisingly, ventricular impairment and immature scar formation was found to be the same in the global and T-cell-specific CD73−/− mice as determined with cine 1H MRI and histology. To gain further mechanistic insights, we isolated T-cells from control and T-cell specific CD73−/− mice hearts and found that lack of CD73-derived adenosine triggers the formation of pro-inflammatory and pro-fibrotic cytokines, which may serve as instructive signals for the observed changes in phenotype. Gene expression analysis revealed that cardiac T-cells express both A2A and A2B adenosine receptors after I/R, while the A2B receptor was not expressed on respective blood cell controls.
In summary our findings provide first evidence, that adenosine formed by CD73 on T-cells plays a central role in the healing process after myocardial infarction.
Effects of adenosine A2Breceptor blockade on atherosclerosis
Joel Linden1,*, R. Chris Harmon2, Robert A. Figler2, Carmen Klein Herenbrink3, Hong Pei1, Runpei Wu1, Ad IJzerman3 and Catherine C. Hedrick1
1La Jolla Institute for Allergy and Immunology, La Jolla, USA;2University of Virginia, Charlottesville, USA;3Leiden/Amsterdam Center for Drug Research, The Netherlands
Diabetes is a major risk factor for atherosclerosis. We found that IP injection in mice of Bay 60-6583, a selective agonist of the adenosine A2B receptor (A2BR), produces acute hyperglycemia that is absent in A2BR-/- animals, is not dependent on A2BRs on bone marrow-derived cells, and is attenuated by prior IP injection of the A2BR antagonist, ATL-801. Atherosclerosis was induced by feeding a 42 % fat 0.2 % cholesterol Western diet ± ATL-801 (ingested at 10 mg/kg/day) to atherosclerosis-prone mice lacking either ApoE (Fig. 1) or LDLR for 16 weeks. In both models, the inclusion of ATL-801 in the western diet produced a 30 % significant reduction in aortic lesions without affecting plasma lipids. A2BR blockade also reduced plasma IL-6 and IL-6 mRNA in the aorta. A separate cohort of mice were fed a diet-induced obesity (DIO) diet consisting of 55 % fat for 10 weeks to create hepatosteatosis and insulin resistance. In mice fed DIO, inclusion of ATL-801 in the diet caused a marked reduction in hepatosteatosis and visceral fat mass, a reduction in blood glucose in fed animals, and improved glucose tolerance in IPGTT tests in vivo in fasted animals. When added to peritoneal macrophages in vitro, the A2BR agonist Bay 60-6580 produced acute anti-inflammatory effects, including rapid induction of NR4A transcription factors that are known to be repressors of NK-κB. However, A2BR activation also stimulated the induction in macrophages of atherogenic factors including IL-6, Vegf and IL-1b. We conclude that inclusion of an A2BR antagonist in high fat diets has beneficial effects on glucose metabolism that may reduce atherosclerosis and hepatosteatosis. It is of interest that these pharmacological effects of ATL-801 may not be replicated by global A2BR deletion [1], which may influence hepatic and APC [2] development.
Fig. 1 Inclusion of ATL-801, an adenosine A2B receptor blocker, in aWestern diet of ApoE−/− mice reduces athero-sclerosis. 1322 (left) En face analysis of Oil Red O lesion area in ApoE−/− mice fed a western diet ± ATL-801 for 16 weeks. (right) Data from 1323 groups of ten mice
References
1. Koupenova M et al (2012) A2b adenosine receptor regulates hyperlipidemia and atherosclerosis. Circulation 125:354–363. doi:10.1325/1161/CIRCULATIONAHA.111.057596
2. Novitskiy SV et al (2008) Adenosine receptors in regulation of dendritic cell differentiation and function. Blood 112:1822–1831,1327 doi:10.1182/blood-2008-02-136325
Thu 3 C: Medicinal chemistry and drug development I: P1-receptor ligands
Utilizing the A3 adenosine receptor agonist CF101 for the treatment of autoimmune inflammatory indications: data from advanced clinical studies
Pnina Fishman*, Sari, Fishma*, Shira Cohen*, Motti Farbstein, Zivit Harpaz and Michael Silverman
Can Fite BioPharma, Petach-Tikva, Israel
The A3 adenosine receptor (A3AR) belongs to the family of the Gi-protein coupled receptors (GPCR). Much evidence including pre-clinical and clinical studies has been accumulated showing the anti-inflammatory effect mediated via the A3AR. A3AR agonists induce anti-inflammatory effect in both murine and rat models of autoimmune arthritis, via down regulation of nuclear factor kappa B (NF-kB) and related proteins as well as tumor necrosis factor-α (TNFα) (4), resulting in the inhibition of inflammatory cytokines. A3AR is over-expressed in cells from inflammatory tissues whereas normal cells have low expression of the receptor. Furthermore, A3AR was found to be up-regulated in the peripheral blood mononuclear cells (PBMCs) of patients with autoimmune inflammatory diseases. PBMCs drawn from Rheumatoid Arthritis (RA), Psoriasis and Crohn’s Disease (CD) patients, showed A3AR up-regulation compared to that of healthy subjects, suggesting that the high A3AR expression levels in the inflammatory tissues is reflected in the PBMCs.
A3AR agonists such as CF101 (IB-MECA) and CF102 (Cl-IB-MECA) were investigated in several phase II clinical studies including RA and Psoriasis showing clear evidence of efficacy and excellent safety profile, demonstrating the validity of the A3AR as a therapeutic target. Data from these studies will be presented and discussed.
Ligand discovery from adenosine receptor crystal structures and homology models
David Rodriguez1, Anirudh Ranganathan1, Steven M. Moss2, Zhan-Guo Gao2, Leigh A. Stoddart3, Stephen J. Hill3, Kenneth A. Jacobson2 and Jens Carlsson1,*
1Science for Life Laboratory; Center for Biomembrane Research; Department of Biochemistry and Biophysics, Stockholm University, Stockholm, Sweden;2Molecular Recognition Section, Laboratory of Bioorganic Chemistry, National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, Bethesda, Maryland 20892;3Institute of Cell Signalling, School of Biomedical Science, Queen’s Medical Centre, University of Nottingham, Nottingham NG7 2UH, UK Email: jens.carlsson@dbb.su.se
Selected poster D 059
Search for adenosine receptors ligands among annelated xanthine derivatives
Anna Drabczyńska1, Tadeusz Karcz 1, Ewa Szymańska1, Christa Müller2, Meryem Köse2, Małgorzata Zygmunt3, Jacek Sapa3, Gniewomir Latacz1, Jakub Mazurkiewicz1, Katarzyna Stanuch1, Michał Załuski1, Agnieszka Olejarz1 and Katarzyna Kieć-Kononowicz1,*
1Department of Technology and Biotechnology of Drugs, Jagiellonian University Medical College, Faculty of Pharmacy, Medyczna 9, PL 30-688 Kraków, Poland;2PharmaCenter Bonn, Pharmaceutical Institute, University of Bonn, An der Immenburg 4, D-53121 Bonn, Germany;3Department of Pharmacological Screening, Jagiellonian University Medical College, Medyczna 9, PL 30-688 Kraków, Poland
To date four adenosine receptor (AR) subtypes designated A1, A2A, A2B and A3 have been cloned and pharmacologically characterized. A1 and A2A ligands are currently being developed as promising therapeutic agents for CNS disorders (especially A2A antagonists utility in Parkinson’s disease), cardiac failure, depression and addictions. Use of adenosine A2B antagonists in the therapy of pain, asthma or related pulmonary diseases and diabetes has been proposed [1,2].
Our studies are focused on the search for ligands of adenosine receptors A1, A2A, A2B in the group of tricyclic derivatives of xanthines of the type of: imidazo-, pyrimido- and diazepinopurine-2,6-diones [3,4]. Compounds with (non)selective activity at these three types of receptors have been found using in vitro tests on as well rat as human receptors expressed in CHO cells (as radioligands [3H]CCPA, [3H]MSX-2 and [3H]PSB-603 were used for A1, A2A and A2B receptors respectively). Their antioxidative properties were estimated. Chosen compounds were then evaluated at different in vivo tests on the analgesic and antiparkinsonian activity [5]. Several compounds were in silico and experimentally examined for their druglikness in the tests evaluating their cytotoxic properties and influence on CYP3A4 cytochrome activity. Molecular modelling and docking methods have been applied to predict possible modes of the obtained derivatives binding inside the pocket of the human A2A and A2B receptors.
Partly supported by Polish National Science Center DEC-2012/04/M/NZ4/00219, supported by the DAAD (Deutsche Akademische Austauschdienst, PPP project to K.K.-K and C.E.M), GLISTEN CMST Action 1207
References
1. Müller CE, Jacobson KA (2011) Recent developments in adenosine receptor ligands and their potential as novel drugs. Biochim Biophys Acta 1808:1290–1308
2. Müller CE, Jacobson KA (2011) Xanthines as adenosine receptor antagonists, Handbook of Experimental Pharmacology 200:151–199
3. Drabczyńska A et al. (2006) Synthesis and biological activity of tricyclic aryloimidazo-, pyrimido-, and diazepinopurinediones. Bioorg Med Chem 14:7258–7281
4. Drabczyńska A et al. (2013) Synthesis, biological activity andmolecular modelling studies of tricyclic alkylimidazo-, pyrimido- and diazepinopurinediones. Purinergic Signal 9:395–414
5. Drabczyńska A et al (2011) Antiparkinsonian effects of novel adenosine A2A receptor antagonists. Arch Pharm Chem Life Sci 1:20–27
Looking for new chemical entities to target adenosine receptors
Fernanda Borges
CIUP/Department of Chemistry and Biochemistry, Faculty of Sciences, University of Porto
Despite the increasing investments in R&D, the number of new drugs reaching the market has been decreasing, namely by a faced marked decline in innovation. Low productivity, rising R&D costs, dissipating proprietary products and dwindling pipelines are driving the pharmaceutical industry to unprecedented challenges and scrutiny.
Innovation has always been the backbone and underlying strength of the pharmaceutical industry. The life cycle management opportunities comprising for instance the discovery of new chemical entities and new drug delivery options are becoming an important part of the strategies to maximize reward. As a result today drug discovery is often best-served by collaborations, among companies, with the bio industry, and with academia.
The identification of high-quality hits and lead compounds is crucial in the drug discovery process as well as the understanding of structure–activity relationships. In addition, it is therefore essential to obtain high-quality data on affinity, kinetic, mechanistic and thermodynamic aspects of the interaction between potential candidates and their targets.
Over the past years the privileged structure concept has emerged as a stimulus to accelerate drug discovery and development processes. The decoration of the privileged scaffolds using diversity-oriented synthesis has been a fast-track instrument in the discovery of new active small molecules. In this context our group have been shown that chromones (non-flavonoid type) are a valid scaffold for the design of selective adenosine receptor (AR) ligands, and in that way can supplement the pipeline of this area. The data obtained so far in the discovery of potent and selective AR ligands will be presented.
Reference
1. Gaspar A, Matos MJ, Garrido J, Uriarte E, Borges F (2014) Chromone: a valid scaffold in medicinal chemistry. Chem Rev 114(9):4960–4992
[1,2,4]triazolo[1,5-c]pyrimidines as A3adenosine receptor antagonists
Stephanie Federico1,*, Sara Redenti1, Antonella Ciancetta2, Barbara Cacciari3, Karl-Norbert Klotz4, Stefano Moro2 and Giampiero Spalluto1
1Università degli Studi di Trieste, Dipartimento di Scienze Chimiche e Farmaceutiche, Trieste, Italy;2Università degli Studi di Padova, Molecular Modeling Section (MMS), Dipartimento di Scienze del Farmaco, Padova, Italy;3Università degli Studi di Ferrara, Dipartimento di Scienze Farmaceutiche, Ferrara, Italy;4Universität of Würzburg, Institut für Pharmakologie, Würzburg, Germany
Selected poster D 060
Thu 3 D: Roles of purines in gastrointestinal physiology and disease
Purinergic communication between enteric neurons and glia in health and disease
Brian D. Gulbransen
Neuroscience Program and Department of Physiology, Michigan State University, 567 Wilson Rd., East Lansing, Michigan, 48864, USA
Reflex behaviours of the intestine such as peristalsis are orchestrated by the enteric nervous system (ENS); a complex neural network embedded in the gut wall. Like the brain, the ENS is composed of neurons that are surrounded by glial cells. Enteric glia are a unique type of peripheral glia that are similar to astrocytes of the central nervous system. Enteric glia were previously considered to function as passive support cells for neurons. Yet, our recent findings challenge this view by demonstrating that enteric glia detect, and in turn, influence neuron activity. Purines have emerged as the primary mediators of intercellular communication between enteric neurons and glia. Neuronal release of ATP recruits intracellular Ca2+ responses in the surrounding enteric glial cells and downstream pathways lead to glial release of ATP through hemichannels. Our data show that, in the healthy intestine, purinergic neuron-glia communication is required for the maintenance of normal intestinal reflexes. Specifically, impairing the purinergic signalling pathway in glial cells impairs colonic motility. Our new findings indicate that, under pathological conditions, excessive stimulation of these same purine signalling pathways in glial cells leads to the death of enteric neurons by activation of neuronal P2X7 purine receptors. Thus, purinergic communication between enteric neurons and glia has paradoxical effects in the intestine; both contributing to functional circuits in health and potentially driving network dysfunction in disease.
P2Y1receptors mediate neuromuscular transmission in the gastrointestinal tract: functional implications
Marcel Jimenez
Department of Cell Biology, Physiology and Immunology and Neuroscience Institute, Universitat Autónoma de Barcelona, Bellaterra, Spain; Centro de Investigación Biomédica en Red de enfermedades hepáticas i Digestivas (CIBERehd), Instituto de Salud Carlos III, Spain
Purinergic neurotransmission mediates the fast component of the inhibitory junction potential (IJPf) [1]. Nitric oxide (NO) is co-transmitted with ATP or a related purine and leads a sustained IJP (IJPs). Both neurotransmitters mediate smooth muscle relaxation. Recently, the P2Y1 receptor has been identified as the purinergic receptor responsible for purinergic neuromuscular transmission: 1- P2Y1 antagonists (MRS2179, MRS2279 and MRS2500) concentration dependently inhibit the IJPf [2] and purinergic relaxation both in laboratory animals and human tissues and 2- P2Y1 KO mice lack IJPf and purinergic relaxation [3,4]. According to these experimental evidences we have found that the mechanisms involved in purinergic and nitrergic neurotransmission are complementary: 1-Spontaneous IJP often recorded in electrophysiological recordings are MRS2500 sensitive and absent in P2Y1 KO mice. 2-The nitric oxide blocker L-NNA (but not MRS2500) depolarises smooth muscle cells and increases contractility 3-Increase in frequency of electrical stimulation exponentially attenuates purinergic responses and exponentially increases nitrergic hyperpolarization and 4-The co-transmission process is organ and regional dependent i.e. in some regions of the colon purinergic neurotransmission dominates whereas in other areas nitrergic responses are dominant. These experimental data allow us to conclude that ATP and NO are co-transmitters with different functions. 1—Low frequency firing of inhibitory neurons causes purinergic sudden smooth muscle hyperpolarization and relaxation that cannot be sustained. We speculate that this mechanism participates in propulsive movements. In contrast 2—High frequency firing of inhibitory neurons causes nitrergic long lasting hyperpolarizations and relaxations possibly responsible for gastrointestinal functions such as accommodation and storage.
References
1. Burnstock G, Campbell G, Satchell D, Smythe A (1970) Br J Pharmacol 40(4):668–688
2. Gallego D, Hernández P, Clavé P, Jiménez M (2006) Am J Physiol 291(4):G584–G594
3. Gallego D, Gil V, Martínez-Cutillas M, Mañé N, Martín MT, Jiménez M (2012) J Physiol 15:1943–1956
4. Hwang SJ, Blair PJ, Durnin L, Mutafova-Yambolieva V, Sanders KM, Ward SM (2012) J Physiol 590:1957–1972
Role of adenosine receptors in the control of enteric neuromuscular functions under normal conditions and in the presence of bowel inflammation
Corrado Blandizzi1,*, Matteo Fornai1, Rocchina Colucci1, Carolina Pellegrini1, Deborah Sacco1, Erika Tirotta1, Valentina Caput2, Maria Cecilia Giron and Luca Antonioli1
1Division of Pharmacology, Department of Clinical and Experimental Medicine, University of Pisa, Pisa, Italy,2Department of Pharmacology and Anesthesiology, University of Padova, Padova, Italy
Under normal conditions, all adenosine receptors are expressed in the colonic neuromuscular layer, and they are involved in the tonic inhibitory control of cholinergic motor activity, acting both at neuronal level (A1, A2A and A3 receptors) and through a direct modulation of smooth muscle (A2B receptors). In the presence of bowel inflammation, adenosine receptors undergo dynamic changes in their expression and/or function, with up-regulation of A2A and A3 receptor. However, this receptor rearrangement correlates only in part with the patterns of evoked contractions recorded in functional experiments. Indeed, the enhancing effects resulting from the blockade of A1, A2B or A3 receptors, on cholinergic contractions, were no longer evident in the presence of colitis, while the potentiating effect obtained by A2A receptor blockade was more pronounced as compared to normal conditions. In experiments where the levels of endogenous extracellular adenosine were decreased, the pharmacological stimulation of all adenosine receptors inhibited the electrically induced contractions, and the efficacy of the inhibitory effects mediated by A2A or A3 receptors was higher than that observed in normal tissues. This discrepancy, between the effects recorded under normal conditions and those in the presence of colitis, suggests that, during bowel inflammation, all adenosine receptors are present in the neuromuscular compartment and are available for pharmacological recruitment, even if only A2A receptors appear to be preferentially and tonically recruited by endogenous adenosine. Our findings might represent a promising basis for the development of novel pharmacological tools potentially useful for therapeutic management of enteric dysmotility associated with bowel inflammation.
New insights into nucleotide metabolism and intestinal inflammation
Sean P. Colgan1,*, Valerie F. Curtis1, Stefan F. Ehrentraut1,2, Eric L. Campbell1, Louise E. Glover1, Caleb J. Kelly1 and Douglas J. Kominsky1
1Mucosal Inflammation Program, Department of Medicine, University of Colorado School of Medicine, Aurora, Colorado, USA;2Department of Anaesthesiology and Intensive Care Medicine, University Hospital Bonn, Bonn, Germany
There is currently significant interest in understanding post-translational modifications of proteins in the establishment, progression and resolution of inflammatory disease. One such modification is neddylation, the conjugation of the ubiquitin-like molecule Nedd8 to promote protein stabilization. Cullins are a major family of Nedd8 targets important in the induction of the NFκB (via Cullin-1) and stabilization of hypoxia-inducible factor (HIF, via Cullin-2). Our past studies have revealed that human epithelial cells exposed to adenosine drives the deneddylation of Cullin-1 and concomitantly, decreases NFκB transactivation. Here, we elucidate the role of the human deneddylase-1 (also called SENP8) in inflammatory responses in vitro / in vivo and define mechanisms for targeting SENP8 in models of mucosal inflammation. Given that epithelial HIF provides significant protection in inflammatory models, in the current studies we examined the contribution of SENP8 to HIF stabilization. Pharmacological targeting of SENP8 activity with MLN4924 stabilized HIF-1α protein, activated HIF promoter activity and induced a number of HIF-target genes in intestinal epithelial cells. These responses were nearly completely lost in cells lacking SENP8 (via lentiviral knock-down). In parallel studies in vivo, MLN4924 abrogated disease severity resulting from DSS colitis, including weight loss, colon length and histological scoring. We conclude that SENP8 is a proximal regulator of Cullin neddylation and fine-tunes the inflammatory response in vitro and in vivo. Pharmacological inhibition of Cullin neddylation may provide a viable therapeutic opportunity in mucosal inflammatory disease.
Specialized interstitial cells mediate purine post-junctional neuroeffector responses in the gastrointestinal tract
Sean M. Ward
Department of Physiology & Cell Biology, University of Nevada School of Medicine, Anderson Medical Sciences Building, Reno, NV 89557, USA
Control of gastrointestinal (GI) muscles by enteric motoneurons is critical for orderly processing of food, absorption of nutrients and elimination of waste products. Work over the past several years has suggested that motor neurotransmission is more complicated than simple release of transmitter from nerve terminals and binding of receptors on smooth muscle cells. Activation of enteric inhibitory motor neurons produces inhibitory junctional potentials (IJPs) and muscle relaxation in GI muscles. IJPs in many GI muscles are bi-phasic with a fast initial hyperpolarization (fIJP) due to release of a purine neurotransmitter and a slower hyperpolarization component (sIJP) due to release of nitric oxide (NO). It has been demonstrated that NO-dependent responses are mediated via interstitial cells of Cajal. However, there is a growing body of evidence that a specialized population of interstitial cells, termed fibroblast-like cells, which are positive for platelet-derived growth factor receptor α (PDGFRα+), participate in purine mediated inhibitory neurotransmission in the GI tract. P2Y1R is highly expressed in PDGFRα+ cells compared to smooth muscle cells and stimulation of enteric motor nerves or exogenous application of purines causes robust calcium signals in PDGFRα+ cells and activation of small conductance SK3 potassium channels. SK3 activation caused robust outward currents in PDGFRα+ cells (FLCs), but currents of far less density were evoked in smooth muscle cells under the same conditions. fIJP was completely absent in the colons of a murine model with genetic deactivation of P2ry1. Video imaging revealed that transit of fecal pellets was significantly delayed in the colons from P2ry1 (−/−) mice. Data demonstrating the role of specialized PDGFRα+ cells in purinergic neuroeffector transmission via P2Y1 receptors will be discussed.
Thu 4 A: Regulation of the immune system by purines
Multi-inhibitory effects of an A2Aadenosine receptor agonist on neutrophil adhesion signaling pathways under flow
Tadayuki Yago1, Hiroki Tsukamoto1,2, Ying Wang1, Rodger P. McEver1 and Linda F. Thompson1
1Oklahoma Medical Research Foundation, Oklahoma City, USA;2Tohoku University, Sendai, Japan
Signaling through the A2A adenosine receptor increases intracellular cAMP, activates protein kinase A, and negatively regulates inflammatory responses. Recently, the A2A adenosine receptor agonist, ATL313, has been widely used as an anti-inflammatory reagent in a variety of disease models. We examined ATL313 functional effects on neutrophil adhesion under physiological flow in vitro and in vivo. In in vitro flow chamber studies using human neutrophils or murine bone marrow leukocytes, ATL313 treatment abolished β2 integrin-dependent slow rolling induced by P-selectin/PSGL-1 signaling, and inhibited neutrophil arrest on ICAM-1 mediated by IL-8 or CXCL1 chemokine signaling. Flow cytometry data with ATL313-treated human neutrophils revealed reduced binding of mAb KIM127 suggesting inhibition of β2 integrin extension which supports integrin-dependent slow rolling. “Swing out” of the hybrid domain associated with high affinity for β2 integrin ligands and recognized by mAb MEM148 was also impaired. Furthermore, ATL313 treatment reduced the spreading of cells induced by integrin outside-in signaling mediated by engagement by F(ab′)2 fragments of an anti-β2 integrin mAb. We found that ATL313 treatment suppressed activation of SFK/p38 MAPK involved in selectin signaling, Rap1 involved in chemokine signaling, and SFK/Vav involved in integrin outside-in signaling. In addition, intravital microscopy revealed impairment of leukocyte adhesion to blood vessel walls after treatment with ATL313. Our findings provide new insight about the anti-inflammatory action of ATL313 and its potential clinical utility in a variety of inflammatory diseases.
A2Badenosine receptors prevent adipose tissue inflammation and insulin resistance by maintaining alternative macrophage activation
György Haskó
Rutgers New Jersey medical School, Newark, USA
Insulin resistance is a major metabolic abnormality in the great majority of patients with type 2 diabetes. It is now recognized that chronic adipose tissue inflammation is an important cause of insulin resistance. Macrophages are central regulators of inflammation in the adipose tissue. While macrophages in lean adipose tissue are important for the maintenance of insulin sensitivity, it is their transition from this protective anti-inflammatory or alternatively activated macrophage phenotype to inflammatory or classically activated macrophage during adipose tissue expansion that leads to the development of inflammation and insulin resistance. We have discovered that adenosine augments alternative macrophage activation and suppresses classical macrophage activation by activating A2B adenosine receptors both in vitro and in the adipose tissue. This beneficial switch in macrophage phenotype caused by A2B adenosine receptor activation led to the maintenance of an anti-inflammatory, insulin-sensitive state in the adipose tissue. We propose that modulating adipose tissue inflammation by A2B adenosine receptor stimulation has great potential for improved treatment of type 2 diabetes.
Adenosine agonists for the management of graft versus host disease
Elizabeth Kang*, Karlie Sharma, Kyu Lee Han, Harry Malech
National Institutes of Allergy and Infectious Disease/National Institutes of Health, Bethesda MD, USA
Allogeneic Transplantation is curative for a number of disorders including many congenital immunodeficiencies; however it is still associated with significant morbidity and mortality. This is primarily due to graft versus host disease (GvHD), an immune mediated reaction generated by alloreactive T-cells in the acute phase. Various transplant regimens use immunosuppressants to prevent the development of GvHD, but these are not always successful. Corticosteroids are the first line of treatment for acute GvHD but are effective only 50–70 % of the time and are associated with significant adverse effects as well. Novel prevention and/or treatment strategies are of significant interest and various approaches are being studied. We have been using adenosine agonists, specifically A2a specific receptor agonists as they have been shown to reduce inflammation in various models including ischemia and sickle cell. Using HLA major and minor mismatched murine transplants, we have shown that the administration of an A2a specific receptor agonist will reduce the incidence of GvHD and improve overall survival. The mechanism is unclear but we have observed elevated numbers of T-regulatory cells in both the peripheral blood and in the target organs of the treated animals [1]. We are currently developing a humanized mice model of GvHD to confirm these results as well as working to develop a clinical compound to use in phase 1 and 2 studies.
Reference
1. Han et al (2013) J Immunol 190(1):458–468
Renal ischemia reperfusion injury is reduced by the adenosine receptor agonist VCP746
Benjamin Seibt1,2,*, Bo Lu1, Veena Roberts1,2, Doreen Fang1,2, Peter Cowan1,2and Karen Dwyer1,2
1St. Vincent’s Hospital, Immunology Research Centre, Fitzroy, Australia;2University of Melbourne, Department of Medicine, Melbourne, Australia
Background: The activation of the adenosine A2B receptor (A2BR) is protective in renal ischemia reperfusion injury (IRI). Clinically the use of adenosine/adenosine analogues is limited by unwanted systemic effects. VCP746 is a novel adenosinergic compound which is a hybrid molecule comprising of adenosine linked to a positive allosteric modulator specifically to engender preferential signalling at the A1R/A2BR.
Aim: To characterise VCP746 in vitro and assess its efficacy in a mouse model of renal IRI.
Methods: Calcium mobilisation studies were performed in stably transfected CHO-A2BR cells. C57Bl/6 wild type mice (n = 8–10) underwent right nephrectomy and 22.0 min left renal ischemia or no IRI (Sham) at 370C. Mice received 100 μg/kg VCP746 (10 % DMSO/90 % Saline) or Vehicle (10 % DMSO/90 % Saline) intravenously 5 min before IRI. Twenty-four hours post IRI mice were sacrificed; kidney harvested for histological analysis and serum creatinine (SeCr) assayed.
Results: VCP746 activated the A2BR with similar potency to adenosine (EC50 1,290 vs. 813 nM, ns). Severe renal injury was induced following 22 min ischemia (SeCr 177 ± 20 μmol/L) which was significantly attenuated with VCP746 treatment (SeCr 116 ± 15 μmol/L). No overt unwanted effects were observed with VCP746 pre-treatment.
Conclusion: VCP746 is a novel adenosinergic compound with A2BR activity which is well tolerated and attenuates renal IRI.
Adenosine signaling through the NR4A subfamily of orphan nuclear receptors attenuates pro-inflammatory gene expression in monocytic cells
Daniel Crean and Evelyn P. Murphy*
UCD Veterinary Sciences Centre and Conway Insitute for Biomolecular and Biomedical Research, University College Dublin, Belfield, Dublin 4, Ireland
Adenosine receptor mediated regulation of monocyte/macrophage inflammatory responses is critical in the maintenance of tissue homeostasis. In this study, we reveal that adenosine potently modulates the expression of NR4A1, 2 and 3 orphan nuclear receptors in myeloid cells and this modulation is primarily through the adenosine A2a receptor subtype. We demonstrate that A2a receptor activation of NR4A1-3 receptor synthesis is further enhanced in Toll-like receptor (TLR)-stimulated macrophages. Following TLR stimulation, NR4A receptor depleted macrophage cells produce increased inflammatory cytokine and chemokine secretion rendering the cells more pro-inflammatory. Blockade of NF-κB signaling fully abrogates TLR-dependent production of pro-inflammatory mediators in these cells. Exposure of adenosine analogues to TLR-stimulated NR4A-depleted macrophage cells generates a hyper-inflammatory response suggesting that NR4A receptor activity is required to limit these transcriptional effects. Comparative modulation of NF-κB-mediated downstream targets, including MIP3a and IL23/p19, are also observed in TNFα-treated cells. Furthermore we establish that nuclear levels of NF-κB/p65 are significantly increased in TLR/adenosine-stimulated NR4A2 depleted cells. We show that, following TLR/adenosine receptor stimulation, NR4A2 depletion promotes significant binding of NF-κB/p65 to a κB consensus binding motif within the MIP-3α proximal promoter leading to increased protein secretion. Finally, the importance of NF-κB activity in mediating A2a receptor/TLR/TNF modulation of NR4A expression confirms a pivotal role for NF-κB activity in controlling gene expression outcomes in response to these mediators. Thus, these data support the observation that during an inflammatory response, adenosine modulation of NR4A receptor activity acts to limit NF-κB-mediated effects and that loss of NR4A2 expression leads to enhanced NF-κB activity and pro-inflammatory responses in myeloid cells.
Thu 4 B: Role of purines in embryonic development and in adult stem cell growth and differentiation
P2X7 receptors in neural differentiation of pluripotent stem cells
Henning Ulrich
Department of Biochemistry, Institute of Chemistry, University of São Paulo, Av. Prof. Lineu Prestes 748, São Paulo, Brazil
Purinergic receptors participate in developmental functions and stem cell biology. Recent data suggest that the P2X7 receptor (P2X7R) promotes proliferation and regulates neural differentiation. Here, we use mouse embryonic stem cells (ESC) for further studying P2X7R functions in proliferation and neural differentiation. P2X7R expression together with the pluripotency marker Oct-4 was highest in undifferentiated ESC. In undifferentiated cells, the P2X7R agonist Bz-ATP accelerated cell cycle entry, which was blocked by the P2X7R blocker KN-62. ESC, induced to neural differentiation by retinoic acid, reduced Oct-4 together with P2X7R expression. In agreement with differential P2X7R expression patterns, receptor-promoted intracellular calcium fluxes were obtained at lower Bz-ATP ligand concentrations in undifferentiated than in differentiated cells. The presence of KN-62 led to increased numbers of cells expressing SSEA-1, Dcx and β3-tubulin and cells positive for SSEA-1 and β3-tubulin immunostaining confirming that onset of neuroectodermal differentiation and neuronal fate determination depends on suppression of P2X7R activity. Moreover, an increase in the number of Ki-67 positive cells in conditions of P2X7R inhibition indicates rescue of progenitors into the cell cycle, increasing the neuroblast population and consequently promoting neurogenesis. In agreement with the proliferation-stimulating effect mediated in ESC, down-regulation of P2X7R expression resulted in diminished proliferation of murine P19 embryonal carcinoma cells and reduction of gliogenesis. In summary, P2X7R expression and activity is upregulated in embryonic cells for maintenance of pluripotency and proliferation. Down regulation of P2XR expression and activity favors neurogenesis, while gliogenesis rates are decreased under these conditions.
NTPDase2 controls progenitor cell proliferation in the adult rodent neurogenic niches
Kristine Gampe1,*, Jennifer Stefani1, Klaus Hammer1, Peter Brendel1, Alexandra Pötzsch1, Grigori Enikolopov2, Keiichi Enjyoji3, Amparo Acker-Palmer1, Simon C. Robson3 and Herbert Zimmermann1
1Institute of Cell Biology and Neuroscience, Goethe-University, Frankfurt, Germany;2Cold Spring Harbor Laboratory, Cold Spring Harbor, NY 11724, USA; Beth Israel Deaconess3Medical Center, Division of Gastroenterology, Harvard Medical School, Boston, MA, USA
Two major neurogenic niches, the subventricular zone (SVZ) and the hippocampal dentate gyrus retain the capacity to generate mature neurones from stem cell populations in the adult mammal. We have shown that stem and progenitor cell populations in both neurogenic regions highly express the membrane-bound nucleoside triphosphate diphosphohydrolase 2 (NTPDase2) hydrolysing nucleoside tri- and diphosphates. The localisation of high NTPDase2 expression in neurogenic regions prompted the question whether NTPDase2 influences the impact of extracellular nucleotides on adult neurogenesis. Its deletion would increase extracellular nucleoside triphosphate concentrations in the neurogenic microenvironment thereby possibly revealing roles of purinergic signalling in neurogenesis. Using mice lacking NTPDase2 expression, we demonstrate that NTPDase2 is the major ectonucleotidase in both neurogenic niches. Its absence does not result in any upregulation of other ectonucleotidases. Entpd2 null mice showed augmented progenitor cell proliferation in both the SVZ and the dentate gyrus without changes in long-term survival. The hippocampal stem cell population and the intermediate progenitor type-2 cell population were expanded. Quantifications further revealed that this surplus of cells was lost at the type-3 cell stage and was accompanied by a strong decrease of CREB phosphorylation in Doublecortin-positive cells in NTPDase2 depleted mice. We propose that NTPDase2 reduces mitogenic extracellular nucleotide concentration in the neurogenic microenvironment thereby regulating not only nucleotide-mediated neural progenitor cell proliferation but also its expansion.
Differentiation of hematopoietic stem cell and leukemia stem cell by ATP
Antonio Carlos Ribeiro Filho, Christiano Marcello Vaz Barbosa, Alice Teixeira Ferreira and Edgar Julian Paredes-Gamero*
Department of Biochemistry, Universidade Federal de São Paulo, R. Pedro de Toleto 669, São Paulo, SP, Brazil
Extracellular nucleotides are emerging as key regulators of inflammation, and cell proliferation in a variety of tissues, including the hematopoietic system. In this study, the differentiate ability of ATP was tested in hematopoietic stem cells (HSC/Lin−Sca-1+c-Kit+Thy1.1lowFLK2−) and leukemia stem cells (LSC/CD34+CD38−Linlow/−) that are emerged such as new target in leukemia. ATP was able to reduce the percentage of HSC and common myeloid progenitors and granulocyte-macrophage progenitors. Additionally, in vivo administration of ATP to mice reduced the number of progenitors, but increased the number of mature myeloid cells (Gr-1+Mac-1+) myeloid cells. ATP also induced an increased proliferation rate and reduced Notch expression in HSCs and impaired HSC-mediated bone marrow reconstitution in sub-lethally irradiated mice. Human leukemia lineages K562 and KG1 cells were used and the LSC in these lineages were identified by flow cytometry. ATP promotes a reduction of LSC after 3 days of treatment in a concentration-dependent manner. Furthermore, ATP leads to a decrease in the clonogenic capacity and also in the total number of K562 and KG1 cells. Thus, our data suggest that ATP induces the differentiation of murine HSCs and human LSC decreasing their undifferentiated state.
Effects of the activation of adenosine A1receptors on the adipogenic differentiation of stem cells from human adipose tissue
Renata Ciccarelli1,*, Catia Lambertucci2, Patrizia Di Iorio1, Rosaria Volpini2 and Francesco Caciagli1
1Department of Experimental and Clinical Sciences, Section of Pharmacology, University of Chieti-Pescara, Via dei Vestini, 31, I-66100 Chieti, Italy;2School of Pharmacy, Medicinal Chemistry Unit, University of Camerino, Via S. Agostino 1, I-62032 Camerino, MC, Italy
Adenosine A1 receptors (A1R) are abundantly expressed in adipose tissue, where their activation, inhibiting the adenylate cyclase activity, stimulates lipogenesis and decrease insulin resistance. These effects could be useful to manage the metabolic syndrome, often present in obese subjects. Since the use of full A1R agonists is hindered by unpleasant side effects, especially on the heart where A1R are present, research is now focusing on partial A1R agonists, which might have better handling. Recently, two derivatives of the full agonist 2-Chloro-N6-cyclopentyladenosine (CCPA), named C1 and C2, were found to behave as partial agonist in a model of spontaneous contraction of mouse ileum. Thus, we investigated the influence of C1 and C2 in comparison with CCPA on the adipogenic process. To this aim, we used stem cells, derived from human subcutaneous adipose tissue (S-ASCs) of normal and obese subjects, as they more largely than that from visceral tissue contribute to the increase of the fat mass in obesity. S-ASCs showed multi-potency and expressed A1R. Cell exposure to CCPA or to the partial agonists did not affect their proliferation whereas, interestingly, in cells induced towards an adipogenic phenotype, the partial agonist C2 significantly reduced the formation of cytosolic lipid vacuoles, the activity of the enzyme glycerol-3-phosphate dehydrogenase (GPDH) and the expression of PPARγ and FABP-4, all adipogenic markers. These effects were mediated by an interference with the phosphatidylinositol-3-kinase/Akt pathway. These results encourage us to continue the study on this new compound, the effects of which could be exploited in experimental therapy of obesity.
Purinergic signalling in mesenchymal stem cell proliferation and differentiation
Yu Zhang, Constanze Kaebisch, Dorothee Schipper, Patrick Babczyk, Dilek Gueneri and Edda Tobiasch*
Department of Natural Sciences, University of Applied Science Bonn-Rhine-Sieg, von-Liebig –Str. 20, 53359 Rheinbach, Germany
With the exception of P2X1 and P2X2, all P2 receptors are expressed in human mesenchymal stem cells (hMSCs). However, the activation or repression of only very few purinergic receptors defines hMSC fate during the differentiation towards the adipogenic, osteogenic, smooth muscle and endothelial cell lineage. The application of artificial agonists or antagonists leads to a strong increase or loss of the respective differentiations as defined by specific markers and staining. In detail, up-regulation of P2Y4 and P2Y14 favors the differentiation towards the vascular lineages, namely to endothelial and smooth muscle cells whereas the down-regulation of the same receptors supports the differentiation towards the adipogenic and osteogenic lineage. The second step of branching of the differentiation pathways is accompanied by the regulation of one receptor only. The up- vs down-regulation of P2Y1 defines the division of the two vascular differentiation pathways, whereas the up- vs down-regulation of P2X6 is linked to the forking of the adipogenic and osteogenic lineages. In addition, our recent studies support a similar defined pattern for P1 receptors. Here the up-regulation of P1A3 favors the differentiation towards the osteo- and adipogenic lineages whereas this receptor is absent in the vascular lineages. The second step of separation is linked to the up- vs. down-regulation of the P1A1 receptor in the two vascular lineages as well as in the other two lineages. Here we show for the first time that a unique pattern of both, P2 and P1 receptor expression defines major differentiation pathways of human mesenchymal stem cells.
Thu 4 C: Medicinal chemistry and drug development II: P2-receptor ligands and inhibitors of NPPs and NTPDases
Design, synthesis and SAR studies of novel P2X receptor antagonists
Jin-Hee Park, Ga-Eun Lee, Joong-Heui Cho, Kwan-Young Jung, Younghwan Jung, Hyojin Ko, Chul-Seung Park and Yong-Chul Kim*
School of Life Sciences, Gwangju Institute of Science and Technology (GIST), 123 Cheomdan-gwagiro, Buk-gu, Gwangju, Republic of Korea
The P2X1-P2X7 receptors belonging to P2 purinergic receptor family are nonselective cation channels gated by extracellular ATP. Particularly, P2X3 and P2X7 receptor subtypes have shown a close relationship with several pathological conditions, such as chronic pain and inflammaton in nervous and immune systems, respectively. The P2X3 receptor has been identified as a drug target for chronic pain, due to its selective expression at high levels in nociceptive primary sensory neurons in trigeminal, nodose, and dorsal root ganglia. In the case of the P2X7 receptor, several new drug development programs for rhematoid arthritis and other autoimmune diseases have been attempted, based on its unique function of trigerring IL-1β processing in various conditions, such as nerve injury and joint inflammation. In this presentation, we report the discovery and biological evaluation of novel pyridine based P2X3 and hydantoin based P2X7 receptor antagonists designed from PPADS and KN-62, respsectively. The optimized P2X3 receptor antagonist in this study showed an IC50 value of 60 nM at hP2X3 receptor in the two-electrode voltage clamp assay using Xenopus oocytes with marginal antagonistic activities of 10 μM at mP2X1 and hP2X7. The newly developed hP2X7 receptor antagonist displayed in-vitro biological profiles with IC50 values of 54 nM in the ethidium uptake assay and 9 nM in an IL-1β ELISA assay, and effective in-vivo pharmacological activity in inflammatory animal models. Furthermore, using an ex-vivo assay system, we found that both of the P2X3 and P2X7 receptor antagonists more efficiently inhibited the pain signaling in rat dorsal horn than pregabalin.
Fig. 1 Novel pyridine based P2X3 and hydantoin based P2X7 receptor antagonists
References
1. Joong-Heui Cho et al (2013) Eur J Med Chem 70:811–830
2. Jin-Hee Park et al (2014) J Med Chem (in revision)
Blockers of the P2Y12receptor permeable to the blood–brain barrier
Stefano Costanzi1,2,*, Samiye Yaman1 and Brian Williams1,2
1Department of Chemistry, American University, Washington, DC, USA;2Center for Behavioral Neuroscience, American University, Washington, DC, USA
The P2Y12 receptor is a G protein-coupled receptor (GPCR) naturally activated by adenosine diphosphate (ADP) and coupled to the inhibition of adenylyl cyclase through Gi. In humans, it is found in platelets and the central nervous system (CNS), primarily microglial cells, and it acts as a pathology sensor that mediates cellular response after injury. Damaged or injured cells release ATP, which is then degraded to ADP by extracellular nucleotidases [1]. In turn, ADP activates the P2Y12 receptor, which in platelets triggers aggregation, while in the CNS it triggers the activation of microglial cells. Notably, the P2Y12-mediated activation of microglial cells has been shown to be a critical component of the etiology of neuropathic pain [2]. A wealth of P2Y12 blockers was developed to target the P2Y12 receptor in platelets. Conversely, P2Y12 blockers permeable to the blood–brain barrier (BBB) are lacking.
Through this presentation, we report the identification of P2Y12 blockers that, according to our PAMPA (parallel artificial membrane permeability assays) analysis, are permeable to the BBB. Moreover, we discuss the putative interactions of these blockers with the P2Y12 receptor, derived from molecular docking studies based on the recently published structures of the P2Y12 receptor [3,4].
References
1. Kettenmann H (2006) Nat Neurosci 9:1463–1464
2. Ando RD et al (2010) Br J Pharmacol 159:1106–1117
3. Zhang K et al (2014) Nature 509:115–118
4. Zhang J et al (2014) Nature 509:119–122
Highly efficient biocompatible neuroprotectants with dual-activity as antioxidants and P2Y-receptor agonists
Bilha Fischer1,*, Sagit Azran1, Ortal Shimon1, Daniel Forster2, Yael Nadel1 and Georg Reiser2
1Department of Chemistry, Bar-Ilan University, Ramat-Gan 52900, Israel;2Neurobiochemistry Institute, Otto von Guericke University, Leipziger Str. 44, 39120, Magdeburg, Germany
A novel therapeutic approach suggests the activation of P2Y-receptors, widely expressed in the nervous system and involved in neuroprotection, for the treatment of oxidative damage. Here, we developed nucleotide-based neuroprotectants acting dually as antioxidants and P2Y-R agonists. We designed a series of nucleotide analogues based on boranophosphate- and thiophosphate-ADP scaffold. These two series of analogues proved potent P2Y1-R agonists, EC50 7 and 2.6 nM, respectively, vs. 2-SMe-ADP, EC50 13 nM, thus making them the most potent P2Y1-R agonists currently known. Furthermore, they were P2Y1-R selective and showed poor or no activity at the P2Y11-R. Both boranophosphate- and thiophosphate- nucleotide analogues were found to be good Fenton reaction inhibitors/antioxidants. The most potent borano- and thio-phosphate based P2Y1-R agonists, inhibited Fenton reaction with IC50 values of 175 and 37 μM, respectively. The thiophosphate analogues were better radical scavengers than the boranophosphate analogues (IC50 ca. 12 vs. 24 μM, respectively, vs. 18 μM for Trolox). These compounds protected PC12 cells under oxidizing conditions (IC50 40 vs. 80 nM, for boranophosphate- and thiophosphate- compounds, respectively, vs. 21,000 nM for ADP). Blocking P2Y12-R activation in PC12-cells under oxidizing conditions by 2-MeS-AMP resulted in decrease of up to 60–70 % in the antioxidant activity of the analogues, suggesting that the antioxidant activity of the latters also involves the activation of P2Y12-R. Boranophosphate- and thiophosphate- compounds also rescued primary neurons subjected to FeSO4 oxidation (EC50 170 and 40 nM, respectively, vs. ADP, 19,000 nM), and were stable in human blood serum with t1/2 of 7 h (boranophosphate-) and 15 h (phosphorothioate-) nucleotides vs. 1.5 h for ADP. Moreover, the formers resisted hydrolysis by ectonucleotidases NTPDase1,8 and NPP1,3 (0–5 % hydrolysis). In conclusion, the most promising compounds here, exhibited dual activity as both P2Y1/12-Rs agonists and antioxidants. Antioxidant activity involved mostly Fe(II)-chelation, and also radical scavenging by thiophosphate analogues. Hence, we propose these nucleotide analogues as highly promising neuroprotectants.
New ligands for the purinergic P2 and P2Y-like receptors
Rosaria Volpini
School of Pharmacy, Medicinal Chemistry Unit, University of Camerino, Via S. Agostino 1, 62032 Camerino, Italy
A number of purinergic P2 receptor ligands have been obtained by structural modification of ATP. In particular, the 2-phenylethynylATP has been reported to antagonize the P2Y1-mediated platelet aggregation induced by ADP [1]. Moreover, it behaves as strong agonist (EC50 = 0.036 nM) of the P2Y-like receptor GPR17, a dual receptor that responds to two unrelated families of endogenous ligands: extracellular nucleotides and cysteinyl-leukotrienes. On the contrary, N6-methylATP and some bisphosphate analogues resulted GPR17 antagonists [2]. Based on these observations and in the search of new ligands, 2-phenylethynylATP nucleotide derivatives, including stable analogues, have been designed and synthesized. The new compounds were tested on GPR17 transfected cells, by using an innovative and non-radioactive functional cAMP assay [3], where they proved to be potent ligands of the receptor. Functional experiments at some P2Y receptors are in progress. Furthermore, the biological characterization of a dual ligand, able to activate both the purinergic and cysteinyl-leukotriene sites of GPR 17 will be presented.
References
1. Cristalli G, Podda GM, Costanzi S, Lambertucci C, Lecchi A, Vittori S, Volpini R, Zighetti ML, Cattaneo M (2005) J Med Chem 48:2763–2766
2. Calleri E, Ceruti S, Cristalli G, Martini C, Temporini C, Parravicini C, Volpini R, Daniele S, Caccialanza G, Lecca D, Lambertucci C, Trincavelli ML, Marucci G, Wainer IW, Ranghino G, Fantucci P, Abbracchio MP, Massolini G (2010) J Med Chem 53:3489–3501
3. Buccioni M, Marucci G, Dal Ben D, Giacobbe D, Lambertucci C, Soverchia L, Thomas A, Volpini R, Cristalli G (2011) Purinergic Signal 7:463–468
Bicyclic thioacetamide derivatives and analogs as potent inhibitors of nucleotide pyrophosphatase/phosphodiesterase 1 (NPP1)
Sang-Yong Lee1,*, Lei Chang2,3, Piotr Leonczak2,3, Jef Rozenski2, Frauke Löhr1, Steven De Jonghe2,3, Theodor Hanck1, Piet Herdewijn2,3 and Christa E. Müller1
1University of Bonn, PharmaCenter Bonn, Pharmaceutical Institute, Pharmaceutical Chemistry I, An der Immenburg 4, Bonn, Germany;2Katholieke Universiteit Leuven, Interface Valorisation Platform, Kapucijnenvoer 33, Leuven, Belgium;3Katholieke Universiteit Leuven, Rega Institute for Medical Research, Laboratory of Medicinal Chemistry, Minderbroedersstraat 10, Leuven, Belgium
Nucleotide pyrophosphatase/phosphodiesterase 1 (NPP1, EC 3.1.4.1) is a membrane-associated or secreted glycoprotein, which mainly hydrolyzes extracellular ATP yielding AMP and diphosphate (pyrophosphate, PPi) [1]. The enzyme is involved in the regulation of bone mineralization and soft-tissue calcification [2]. NPP1 has also been reported to down-regulate insulin signaling independent of its catalytic activity by inhibiting tyrosine kinase activity of insulin receptors [3]. Recently, NPP1 was detected to be highly expressed in human astrocytic brain tumors and its level was found to be correlated with the tumor grade [4]. Therefore, NPP1 inhibitors might be useful to treat brain cancers.
In order to identify novel starting points for the development of NPP1 inhibitors with drug-like properties, we screened a commercial compound library of small molecules utilizing a colorimetric assay employing the artificial substrate p-nitrophenyl 5′-thymidine monophosphate (p-Nph-5′-TMP) and human soluble NPP1. This led to the discovery of 2-((3H-imidazo[4,5-b]pyridin-2-yl)thio)-N-(3,4-dimethoxyphenyl) acetamide (1) as a potent NPP1 inhibitor (Ki 217 nM). We subsequently performed extensive structure-activity relationship studies to improve its potency. The most potent NPP1 inhibitors were imidazopyridine 2 (Ki 29.6 nM) and adenine derivative 3 (Ki 5.00 nM). Surprisingly, these compounds displayed significantly lower NPP1 inhibitory activity when tested against the natural substrate ATP in a capillary electrophoresis-based assay (Ki in the low micromolar range). The thioacetamide derivatives were found to be competitive inhibitors of NPP1 versus both, the artificial and natural substrate. The most potent inhibitor against ATP of the present series was approximately 13-fold selective versus other NPP isoenzymes, human NPP2 and NPP3.
References
1. Bollen M, Gijsbers R, Ceulemans H, Stalmans W, Stefan C (2000) Crit Rev Biochem Mol Biol 35:393–432
2. Mackenzie NCW, Huesa C, Rutsch F, MacRae VE (2012) Bone 51:961–968
3. Zimmermann H, Zebisch M, Sträter N (2012) Purinergic Signal 5:437–502
4. Aerts I, Martin JJ, De Deyn PP, Van Ginniken C, Van Ostade X, Kockx M, Dua G, Slegers H (2011) Clin Neurol Neurosurg 13:224–229
Thu 4 D: Purine receptors and platelet function, and P2Y12 receptors in brain
Bleeding manifestations of congenital and drug-induced P2Y12defects
Marco Cattaneo
Unità di Medicina 3—Ospedale San Paolo; Dipartimento di Scienze della Salute. Università degli Studi di Milano, Milan, Italy
P2Y12, one of the two platelet receptors for adenosine diphosphate (ADP), plays a central role in platelet function. Defects of P2Y12 should be suspected when ADP, even at high concentrations (≥10 μM), is unable to induce full, irreversible platelet aggregation. Patients with congenital P2Y12 defects display a mild-to-moderate bleeding diathesis of variable severity, characterized by mucocutaneous bleeding and excessive post-surgical and post-traumatic blood loss. Drugs that inhibit P2Y12 are potent antithrombotic drugs, attesting the central role played by P2Y12 in platelet thrombus formation. Clopidogrel, the most widely used drug that inhibits P2Y12, is effective both in monotherapy and in combination with acetylsalicylic acid (ASA). Its most important drawback is the inability to inhibit adequately P2Y12-dependent platelet function in about 1/3 of patients, at the recommended therapeutic doses. The incidence of bleeding events is similar in ASA-treated and clopidogrel-treated patients; however, the combination of ASA and clopidogrel causes more bleeding than each drug in monotherapy. Compared to clopidogrel, new drugs inhibiting P2Y12, such as prasugrel and ticagrelor, decrease the risk of cardiovascular events and increase the risk of bleeding complications, because they adequately inhibit P2Y12-dependent platelet function in the vast majority of treated patients.
Platelet inhibition by prasugrel treatment
Joseph A. Jakubowski
Lilly Research Laboratories, Indianapolis, IN, USA
Activation of platelet P2Y12 ADP receptors at sites of atherosclerotic plaque rupture promotes platelet activation, aggregation and occlusive thrombus formation. P2Y12 is the target of the thienopyridine class of oral antiplatelet agents that includes ticlopidine and clopidogrel. Despite providing important clinical benefits, clopidogrel’s platelet inhibitory action is relatively slow and the extent of inhibition is highly variable. Prasugrel is a 3rd generation thienopyridyl P2Y12 antagonist which, like clopidogrel, is a prodrug requiring metabolism in vivo to an active metabolite (AM). Prasugrel’s distinct chemical structure permits rapid and efficient conversion to this AM which inhibits a wide range of ADP-mediated platelet activities. Clinical data show more effective platelet inhibition with prasugrel compared to clopidogrel. Pharmacokinetic studies have shown that prasugrel generates substantially more of its AM compared to clopidogrel. Genetic studies have demonstrated that variations in CYP2C19 have little impact on prasugrel’s metabolism but result in reduced exposure to clopidogrel’s AM and diminished platelet inhibition. Taken together, these data provide a mechanistic basis for the distinct profile of more consistent platelet inhibition observed with prasugrel. TRITON-TIMI 38 demonstrated that compared to clopidogrel prasugrel resulted in a 19 % relative reduction in the risk of ischemic events in ACS patients undergoing PCI and stent placement. Notably there was a 52 % relative reduction in the risk of stent thrombosis in the prasugrel treated patients compared to those on clopidogrel. In the overall TRITON population, prasugrel was associated with 32 % increase in the relative risk of major bleeding compared to patients treated with clopidogrel.
Reference
1. Jakubowski et al (2010) J Cardio Pharm 56:29–37
Learning from evolution—the use of P2Y12ortholog data
Doreen Thor
Institute of Biochemistry, Johannisallee 30, Leipzig, Germany
During the last decades sequencing has undergone much progress and a growing number of whole genome sequences became available. The Genome 10K project, for example, aims to assemble a “genomic zoo” by collecting sequences of 10,000 vertebrate species. While evaluating the functional relevance of gene mutations usually requires experimental testing and appropriate functional assays, which are time- and cost-intensive, information hidden in many genome sequences from vertebrates as well as invertebrates is already available. But the question is if this information is suitable to predict functional characteristics of proteins.
As a proof of principle a model protein (G protein-coupled receptor for ADP P2Y12) was chosen and comparative sequence data from orthologs were analysed and compared to functional in vitro testing. Comparison with sequence analysis data of over 70 P2Y12 vertebrate orthologs revealed that the amino acid variability assuring proper receptor function in vivo highly correlates (over 90 %) between in vitro experimental and ortholog sequence data. The combined information from natural variation in P2Y12 together with high-throughput saturation mutagenesis, in vitro pharmacological testing, and bioinformatic analysis was used to generate a predictive and dynamic receptor model.
The results demonstrate that ortholog sequence data are sufficient to predict the functional relevance of individual positions and mutations in P2Y12 and very likely in other receptors and proteins as well.
P2Y12receptors in control of pain and neuroinflammation
Beata Sperlagh1,*, Gergely Horváth1, Katinka Bekő1, Bálint Botz2, Christa E. Müller3, Ivar Von Kügelgen4 and Zsuzsanna Helyes2
1Laboratory of Molecular Pharmacology, Institute of Experimental Medicine, Hungarian Academy of Sciences, Budapest, Hungary;2Department of Pharmacology and Pharmacotherapy, University of Pécs, School of Medicine, Pécs, Hungary;3Department of Pharmacology and Toxicology, University of Bonn, D-53105 Bonn, Germany;4PharmaCenter Bonn, Pharmaceutical Institute, University of Bonn, D-53119, Germany
P2Y12 receptor (P2Y12R) antagonists are widely used as safe drugs for the prevention of myocardial infarction and stroke by their action to inhibit the platelet ADP-receptor. However, P2Y12Rs are also expressed in the brain, in particular in microglia and previous studies indicate their role in CNS pathology. In our studies the participation of P2Y12Rs were explored in different rodent models of inflammatory and neuropathic pain and in acute thermal nociception. A variety of P2Y12R antagonists (clopidogrel, ticlopidine, cangrelor, MRS2395, reactive blue 2, PSB-0739), and the P2Y12R deficient mouse line (P2ry12−/−) were used to probe P2Y12Rs.
In parallel with their activity to block the recombinant human P2Y12R, P2Y12R antagonists dose-dependently alleviated mechanical hyperalgesia in both acute and chronic CFA induced inflammatory pain models. Similar findings were obtained in a neuropathic pain model and in the hot plate test, where they caused an increase in thermal nociceptive threshold.
P2Y12R mRNA and proinflammatory cytokines were time-dependently overexpressed in the rat hindpaw and lumbar spinal cord following intraplantar CFA injection, which was accompanied by the induction of myeloperoxidase activity. The selective P2Y12R antagonist PSB-0739 (0.3 mg/kg i.t.) attenuated CFA-induced expression of cytokines in the hindpaw and the spinal cord. Subdiaphragmatic vagotomy and the α7 nicotinic acetylcholine receptor antagonist MLA occluded the effect of PSB-0739 on pain behavior and peripheral cytokine induction. Genetic deletion of the P2Y12R reproduced the effect of P2Y12R antagonists.
In conclusion our data argues for the profound involvement of both central and peripheral P2Y12Rs in acute and chronic pain and inflammation.
Molecular mode of action of ticagrelor at human P2Y12-receptors
Kristina Hoffmann*, Dominique A. Lutz, Jens Straßburger, Ivar von Kügelgen
Universtity of Bonn, Pharma Center Bonn, Department of Pharmacology and Toxicology, Sigmund-Freud-Strasse 25, 53127 Bonn, Germany
The P2Y12-receptor plays an important role in ADP-induced platelet aggregation. In 2010, ticagrelor was licensed as the first reversibly binding and perorally available P2Y12-receptor antagonist for pharmacotherapy of cardiovascular events. A recent study provided evidence for a non-competitive mode of action of ticagrelor at ADP-activated P2Y12-receptors [1]. In the present study, we therefore analyzed the mode of action of ticagrelor at recombinant wild-type and mutant hP2Y12-receptors stably expressed in CHO Flp-In cells. Receptor function was assessed by a [3H]cAMP radioaffinity assay or a cAMP response element (CRE)-driven luciferase reporter-gene assay. The natural agonist ADP as well as the synthetic agonist 2-methylthio-ADP (2-MeSADP) decreased forskolin-induced cAMP accumulation in a concentration-dependent manner with 2-MeSADP (EC50 concentration about 1 nM) being more potent than ADP (EC50 concentration about 200 nM). Addition of ticagrelor at increasing concentrations (3 to 10 nM) led to increasing rightward shifts of the concentration-response curves of ADP and 2-MeSADP, respectively. An analysis according to Arunlakshana and Schild [2] revealed a pA2 value of 8.79 at ADP-activated receptors and a pA2 value of 8.69 at 2-MeSADP-activated receptors. The slope of the respective regression lines did not differ from unity, indicating a competitive mode of interaction of ticagrelor with both agonists at the receptor protein. At several mutant receptor constructs, the antagonistic potency of ticagrelor was maintained, whereas potency was clearly reduced at cells expressing the C194A-mutant construct. Thus, the amino acid residue C194 (transmembrane region 5) or a nearby residue is part of the binding site of ticagrelor at the P2Y12-receptor protein.
References
1. van Giezen JJJ, Nilsson L, Berntsson P, Wissing BM, Giordanetto F, Tomlinson W, Greasley PJ (2009) J Thromb Haemost 7:1556–65
2. Arunlakshana O, Schild HO (1959) BR J Pharmacol 14:48–58
Abstracts—Symposium sessions
- Friday -
Fri 1 A: X-ray structures and molecular modeling I
Structure and functional implications of purinoceptor 12
Qiang Zhao1,*, Kaihua Zhang1, Jin Zhang1, Zhan-Guo Gao3, Hualiang Jiang1, Christa E. Müller2, Vadim Cherezov4, Vsevolod Katritch4, Raymond Stevens4, Kenneth A. Jacobson3 and Beili Wu
1Shanghai Institute of Materia Medica, 555 Zuchongzhi Road, Shanghai, China;2Pharma-Center Bonn, University of Bonn, An der Immenburg 4, Bonn, Germany;3National Institute of Diabetes and Digestive and Kidney Diseases, MD, USA;4The Scripps Research Institute, 10550 North Torrey Pines Road, La Jolla, USA
Purinoceptor 12 (P2Y12R) is a major clinical target, which regulates platelet activation and thrombus formation. Several antithrombotic drugs targeting P2Y12R, including covalently binding prodrugs clopidogrel (Plavix) and prasugrel (Effient), and directly acting nucleotide analogue ticagrelor (Brilinta), have been approved for the prevention of stroke and myocardial infarction. However, clinical experience has revealed certain limitations of these drugs (e.g., a very long half-life action of Plavix and a characteristic adverse effect profile of Brilinta), suggesting an unfulfilled medical need in developing a new generation of P2Y12R inhibitors. Here we report the 2.7 Å resolution crystal structure of human P2Y12R in complex with its non-nucleotide reversible antagonist AZD1283. The structure reveals a distinct straight conformation of helix V, which sets P2Y12R apart from other known class A G protein-coupled receptor (GPCR) structures. The highly conserved disulfide bridge between helix III and extracellular loop 2 (ECL2) is absent in the structure. The missing disulfide is supported by our biochemical analysis indicating that these two cysteines likely exist as free thiols in the native receptor. The structure reveals details of AZD1283 interactions with the receptor, and points to the existence of at least two non-overlapping ligand binding pockets at its extracellular interface. The structure provides essential insights and a solid 3D platform for the development of improved P2Y12R ligands and allosteric modulators as drug candidates.
Tweaking the P2X7 ion channel with nanobodies
Carolina Pinto1,*, Welbeck Danquah1, Björn Rissiek1, Miriam Amadi1, Joana Assunção3, Wendy Rotthier3, Tim Magnus2, Eva Tolosa1, Friedrich Haag1, Toon Laeremans3, Catelijne Stortelers2 and Friedrich Koch-Nolte1
1Institute of Immunology, University Medical Center, Hamburg, Germany;2Department of Neurology, University Medical Center, Hamburg, Germany;3Ablynx nv, Zwijnaarde, Belgium
The ATP-gated ion channel P2X7 senses extracellular ATP released from cells and plays a pivotal role in health and disease [1]. Since elucidation of the crystal structure of the closely related P2X4 from zebrafish, structural modeling has provided insight into the mechanistic underpinnings of P2X7 function [2]. We recently generated novel biological antagonists—single domain nanobodies derived from camelid heavy chain antibodies—that target human P2X7. One nanobody demonstrated complete blockade of P2X7, a second one showed partial blockade similar to that observed with the previously reported mouse mAb L4. In order to understand whether this disparity might be related to different binding epitopes, we studied binding of these antibodies to chimeric human-mouse P2X7 variants and mutants generated on the basis of comparative analyses of the primary sequences and model structures of the human and mouse P2X7 orthologs. Interestingly, the epitope of the nanobody with the highest blocking potency mapped in the vicinity of the ATP-binding pocket, whereas the binding sites for mAb L4 and the weaker nanobody mapped to overlapping epitopes aloof from this site. Our results indicate that the blocking potencies of the antibodies, indeed, may depend on the targeted P2X7 epitope. Owing to their ability to bind to cryptic epitopes, nanobodies may stabilize the structure of membrane proteins and thus provide potential chaperones to aid crystallization of P2X7 [4].
References
1. Khakh BS, North RA (2006) Nature 442:527–532
2. Jiang LH et al (2013) Front Pharmacol 4:55
3. Buell G et al (1998) Blood 92:3521–3528
4. Pardon E et al (2014) Nat Protoc 9:674–693
Inspecting receptor-ligand interaction using molecular dynamics simulations: new insights from Adenosiland
Stefano Moro*, Davide Sabbadin, Alberto Cuzzolin and Antonella Ciancetta
Molecular Modeling Section (MMS), Department of Pharmaceutical and Pharmacological Sciences, University of Padova, Via Marzolo 5, 35131 Padova (Italy)
One of the most challenging issues for the future of drug discovery is the capability to understand the GPCR–ligand recognition pathway with the aim to facilitate the development of drug candidates with more favorable phamacodynamic profiles.
Unfortunately, the recognition process between a ligand and its receptor is a very rare event to describe at the molecular level, and even with the recent GPU-based computing resources, it is necessary to carry out classical molecular dynamics (MD) experiments in a long microsecond time scale. In order to overcome this limiting factor, we have implemented an alternative MD approach, named supervised molecular dynamics (SuMD), that enables us to follow GPCR–ligand approaching process within a time scale reduced up to three orders of magnitude compared to classical MD [1]. SuMD enables the investigation of ligand–receptor binding events independently from the starting position, chemical structure of the ligand, and also from its receptor binding affinity (Fig. 1).
We selected as a key study the human A2A adenosine receptor (hA2AAR) that has been recently crystallized with different ligands, both agonists and antagonists, characterized by different receptor binding affinities. We are able to accurately completely explore the receptor–ligand event in a nanosecond time scale. This approach is also very useful to analyze both orthosteric and allosteric binding events broadening our perspectives in several scientific areas from molecular pharmacology to drug discovery.
Fig. 1 Overview of the adenosine receptor antagonist ZM241385–human A2A adenosine receptor recognition mechanism using supervised molecular dynamics (SuMD)
Reference
1. Sabbadin D, Moro S (2014) J Chem Inf Model 54:372–376
Molecular modeling studies on A2Badenosine receptor ligands
Diego Dal Ben
School of Pharmacy, Medicinal Chemistry Unit, University of Camerino, Via S. Agostino 1, 62032 Camerino, Italy
A2B Adenosine Receptor (A2BAR) agonists are studied as possible therapeutic tools for a variety of pathological conditions. Unfortunately, medicinal chemistry efforts have led to the development of a limited number of potent agonists of this receptor, in most cases with a low or no selectivity versus the other adenosine receptor subtypes. Among the developed molecules, two structural families of compounds have been identified based on nucleoside and non-nucleoside (pyridine) scaffolds [1,3].
To analyse the binding mode of these molecules, 3D models of the human A2BAR were developed by using two recently published crystal structures of the human A2AAR in complex with two different agonists. The developed models were employed as targets for molecular docking studies of nucleoside and non-nucleoside agonists. Results suggest a set of common interaction points between the two structural families of agonists and the receptor binding site. This conserved pattern of interaction between the A2BAR and its agonists could provide useful data for the design and the development of A2BAR agonists belonging to nucleoside or non-nucleoside structural families [4].
Fig. 1 Human A2B adenosine receptor in complex with the non-nucleoside agonist BAY 60–6583
References
1. Baraldi PG, Tabrizi MA, Fruttarolo F, Romagnoli R, Preti D (2009) Purinergic Signal 5:3–19
2. Beukers MW, Chang LC, von Frijtag Drabbe Kunzel JK, Mulder-Krieger T, Spanjersberg RF, Brussee J, IJzerman AP (2004) J Med Chem 47:3707–3709
3. Eckle T, Krahn T, Grenz A, Köhler D, Mittelbronn M, Ledent C, Jacobson MA, Osswald H, Thompson LF, Unertl K, Eltzschig HK (2007) Circulation 115:1581–1590
4. Dal Ben D, Buccioni M, Lambertucci C, Thomas A, Volpini R (2013) In Silico Pharmacol 1:24
Fri 1 B: Adenosine deaminase and intracellular purine metabolizing enzymes
Caffeine blocks LDL endocytosis in neurons
Xuesong Chen, Shanshan Li, Liang Hui, Nicholas H. Geiger and Jonathan D. Geiger*
Department of Basic Biomedical Sciences, School of Medicine & Health Science, University of North Dakota, Grand Forks, ND 58202, USA
Elevated circulating LDL-cholesterol, as an extrinsic factor, has been robustly linked to the pathogenesis of sporadic Alzheimer’s disease (AD), the major form (95 %) of AD for which no effective treatment is available. Our recent in vivo and in vitro studies demonstrated that circulating LDL-cholesterol enters brain parenchyma via a leaky blood–brain barrier and is internalized by neurons, and such a process contributes directly to the pathogenesis of sporadic AD by promoting amyloid beta precursor protein (AβPP) internalization and disrupting neuronal endolysosome function, both of which contribute to overproduction of amyloid beta (Aβ), one of the major pathological factors in AD. Thus, blocking neuronal uptake of LDL-cholesterol could provide a novel therapeutic strategy against AD. Recent epidemiological and experimental studies suggest that caffeine is protective against AD, but with poorly understood underlying mechanisms. Here, we tested the hypothesis that caffeine blocks neuronal uptake of LDL-cholesterol and LDL-cholesterol induced overproduction of Aβ in primary cultured neurons using receptor-mediated endocytosis assays, immunoblotting, immunostaining, ELISA, and RNA interference methods. We demonstrated that caffeine pretreatment blocked LDL-cholesterol induced elevations of Aβ. Mechanistically, we found that caffeine concentration-dependently blocked LDL-cholesterol internalization. Using a pharmacological approach, we demonstrated that an adenosine A3 receptor antagonist mimicked caffeine’s effects on neuronal uptake of LDL-cholesterol. Furthermore, knocking down adenosine A3 receptors with siRNA technology blocked LDL-cholesterol internalization. Moreover, we demonstrated that caffeine pretreatment increased surface protein levels of both LDL receptors and AβPP. Our findings suggest that caffeine exerts its protective effects against AD by blocking neuronal uptake of LDL-cholesterol, thus attenuating AβPP internalization and decreasing Aβ levels.
(Supported by 2P20RR0017699, P30GM103329 and R21AG043338)
The 5′-nucleotidases cN-I and cN-II as regulators of purine nucleotide metabolism
Maria Grazia Tozzi
Department of Biology University of Pisa, via san Zeno, 51 56127 Pisa, Italy
Among the members of the 5′-nucleotidase family, there is only one membrane-bound ectosolic isoenzyme [1]. This esterase prefers AMP as substrate but can hydrolyse a number of purine and pyrimidine phosphorylated compounds, indicating that no evolutive pressure to develop a more restricted specificity was exerted on this enzyme [1]. On the contrary, five cytosolic isoforms have been described, probably originated by convergent evolution, showing different and restricted substrate specificity [2]. The different isoforms have different level of expression and distribution in organs of vertebrates [3]. The cytosolic nucleotidase specific for AMP (cN-I) and the cytosolic nucleotidase specific for IMP and GMP (cN-II), are allosterically regulated enzymes, which possibly can be responsible for the regulation of AMP intracellular concentration and adenosine production [4]. While cN-I is expressed mainly in the skeletal muscle and heart, cN-II is a structurally strongly conserved protein expressed at a low but constant level in all organs and tissues in vertebrates [4]. As far as we know, alteration of cN-II expression is limited to pathological conditions. I will report the results of the modulation of cN-I and cN-II specific activity exerted by silencing or hyperexpression in different cell types, in the attempt to better understand their role and implications in pathology and therapy.
References
1. Zimmermann H (2002) Biochem J 285:345–365
2. Itoh R (2013) Curr Med Chem 20:4260–4284
3. Bianchi V, Spychala J (2011) J Biol Chem 278:46195–4619
4. Tozzi MG (2013) Curr Med Chem 20:4285–4291
Intracellular enzymes regulating the availability of extracellular adenosine: The Yin and Yang of creatine kinase and the purine salvage pathway
Bruno G. Frenguelli
School of Life Sciences, University of Warwick, Coventry, UK
ATP is the primary cellular energy source and the main reservoir of adenosine, a compound with important extracellular signalling properties. Depletion of the intracellular pool of ATP, as occurs during metabolic stress, results in an immediate increase in adenosine levels which provides a neuroprotective influence in the traumatised brain. However, the release of ATP metabolites such as adenosine, inosine and hypoxanthine, comes at a cost: their loss from the brain into the blood. Such a loss deprives the brain of the substrates needed for the purine salvage pathway, the major route by which the brain makes adenine nucleotides, and likely contributes to the prolonged depression of ATP seen after brain injury.
Brain slices provide a convenient model for the injured brain due to their lower levels of ATP. We have shown that the provision of ribose (1 mM) and adenine (50 μM; “RibAde”) restored brain slice ATP levels to values observed in vivo. Moreover, the larger reservoir of ATP translated into greater release of adenosine in response to both physiological [1] and pathological [2] stimulation, and greater inhibition of synaptic plasticity and transmission, respectively. The main purine salvage enzymes, APRT and HPRT, likely mediate the conversion of RibAde into ATP. In contrast, the provision of creatine (1 mM) had no effect on ATP levels but reduced the quantity of adenosine released, likely via creatine kinase-mediated buffering of ATP metabolism.
Given that both ribose and adenine are safe and well tolerated by humans, RibAde may represent a means by which to improve ATP levels and foster recovery of function in the injured human brain.
Fig. 1 Schematic diagram for the actions of RibAde and creatine on adenosine release [2]
References
1. zur Nedden S, Hawley S, Pentland N, Hardie DG, Doney AS, Frenguelli BG (2011) J Neurosci 31:6221–6334
2. zur Nedden S, Doney AS, Frenguelli BG (2014) J Neurochem 128:111–124
Adenosine kinase: exploitation for therapeutic gain
Detlev Boison
Robert Stone Dow Neurobiology Laboratories, Legacy Research Institute, 1225 NE 2nd Ave, Portland, OR 97232, USA
Adenosine kinase (ADK; EC 2.7.1.20) is an evolutionarily conserved phosphotransferase, which converts adenosine into 5′-adenosine-monophosphate. This enzymatic reaction plays a fundamental role in determining the tone of adenosine, which acts as homeostatic and metabolic regulator in all living systems. Adenosine not only activates specific signalling pathways by activation of adenosine receptors, but it is also a primordial metabolite and regulator of biochemical enzyme reactions that couple to bioenergetic and epigenetic functions. By regulating adenosine, ADK can thus be identified as upstream regulator of complex homeostatic and metabolic networks. Not surprisingly, ADK dysfunction is involved in several pathologies including diabetes, epilepsy, and cancer. Consequently, ADK emerges as a rational therapeutic target, and adenosine regulating drugs have been tested extensively. In recent attempts to improve specificity of treatment for epilepsy, focal therapies have been developed to augment adenosine signalling at sites of injury or pathology; those approaches include transplantation of stem cells with deletions of ADK, or the use of gene therapy vectors to down-regulate ADK expression. More recently, the first human mutations in ADK have been described and novel findings suggest an unexpected role of ADK in a wider range of pathologies. ADK-regulating strategies represent innovative therapeutic opportunities to reconstruct network homeostasis in a multitude of conditions. New findings suggest that ADK plays a role in regulating the status of DNA methylation. Therefore, ADK emerges as a promising target for epigenetic therapies, which might influence chronic disease processes such as epileptogenesis. This lecture will focus on ADK-related pathologies and therapeutic interventions.
Adenosine kinase and cell proliferation in the brain
Ursula S. Sandau1,*, Letisha Wyatt1, Rebecca L. Williams-Karnesky2 and Detlev Boison1,2
1Robert Stone Dow Neurobiology Laboratories, Legacy Research Institute, 1225 NE 2nd Ave, Portland, OR 97232, USA;2Department of Neurology, Oregon Health and Sciences University, 3181 S.W. Sam Jackson Park Rd, Portland, OR 97239, USA
Spontaneous neurogenesis in the adult brain occurs within two discrete regions—the subgranular zone (SGZ) of the dentate gyrus and the subventricular zone of the lateral ventricles. The rate of adult SGZ neurogenesis is activity dependent that increases in response to many stimuli including hippocampal dependent learning and memory tasks. In addition, seizures cause an aberrant increase in SGZ cell proliferation, which has been controversially implicated in promoting epileptogenesis and cognitive impairments in epilepsy. This seizure induced neurogenesis is partially dependent on active DNA demethylation [1].
Adenosine is an obligatory end-product of the transmethylation pathway, whereby its continuous removal by adenosine kinase (ADK) is required for maintaining the DNA methylation status [2]. Consequently, adenosine and ADK emerge as candidates to regulate activity induced neurogenesis. In support of this hypothesis, we observe a correlation between decreased ADK expression in the nuclei of dentate granular neurons (DGN) and increased SGZ proliferation following kainic acid induced seizures. To investigate the causal relationship between ADK expression and neurogenesis, we created a transgenic mouse line with a conditional ADK deletion in mature DGN neurons (ADKDGN-KO). Following kainic acid induced status epilepticus we found a 2 fold increase in SGZ proliferation in ADKDGN-KO mice compared to wild-type littermates; whereas, spontaneous neurogenesis and acute responses to the chemical convulsants pilocarpine, kainic acid and pentylenetetrazole, were normal under baseline conditions. Further understanding of the underlying mechanisms by which the nuclear isoform of ADK regulates neurogenesis may lead to novel epigenetic therapies for the treatment of epilepsy.
References
1. Naegele J (2009) Epilepsy Curr 6:166–169
2. Williams-Karnesky RL, Sandau US, Lusardi TA, Lytle NK, Farrell JM, Pritchard EM, Kaplan DL, Boison DB (2013) J Clin Invest 123:3552–3563
Fri 1 C: Adenosine, ATP, and sleep
Pumping sodium: adenosine release in the basal forebrain
Nicholas Dale
School of Life Sciences, University of Warwick, Coventry, UK
Adenosine, acting in the basal forebrain, is a key mediator of sleep homeostasis. Extracellular adenosine concentrations increase during wakefulness and result in increased sleep pressure and, following prolonged waking, sleep rebound. We have used adenosine-sensitive biosensors in slices of the basal forebrain (BFB) from rats and mice to study both glutamate receptor-evoked adenosine release and the steady-state adenosine tone [1]. Evoked and basal adenosine release in the BFB in vitro exhibited three key features: the magnitude of each varied systematically with the diurnal time at which the animal was sacrificed; sleep deprivation prior to sacrifice greatly increased both evoked adenosine release and the basal tone; and the enhancement of evoked adenosine release and basal tone resulting from sleep deprivation was reversed by the inducible nitric oxide synthase inhibitor, 1,400 W.
The mechanisms that link adenosine release to neural activity are only incompletely understood. We have tested whether the Na+ influxes resulting from neuronal signalling activate the Na+-K+ ATPase which, by consuming ATP, generates intracellular adenosine, which is then released via transporters [2]. AMPA-receptor evoked adenosine release in basal forebrain and cortex depends on extracellular Na+. Simultaneous imaging of intracellular Na+ and adenosine biosensor measurements revealed that accumulation of intracellular Na+ during AMPA receptor activation preceded adenosine release. We have used ouabain to test the connection between activation of the Na+-K+ ATPase and adenosine release, in the absence of extracellular Ca2+. Under conditions which caused a Na+ influx, brief applications of ouabain increased the accumulation of intracellular Na+ but conversely rapidly reduced extracellular adenosine levels. In addition, ouabain greatly reduced the amount of adenosine released during application of AMPA. These data suggest that activation of the Na+-K+ ATPase is directly linked to the efflux of adenosine. This provides a universal mechanism to couple adenosine release with neuronal activity, which is likely to be relevant to the accumulation of adenosine during wakefulness.
References
1. Sims R0, Wu HHT, Dale N (2013) PLoS One 8:e53814
2. Sims RE, Dale N (2014) PLoS One 9:e87481
An adenosine-mediated, glial-neuronal circuit for sleep need
Theresa E. Bjorness1, Ayako Suzuki1,2, Nicholas Dale3, Gabriel Mettlach1, Allen A. Fienberg4, Masashi Yanagisawa5,3, James A. Bibb1,6 and Robert W. Greene1,2,7
1Department of Psychiatry, UTSW, Dallas, TX, USA;2International Institute for Integrative Sleep Medicine (WPI-IIIS), University of Tsukuba, Japan; 3University of Warwick, Coventry, UK;4Intra-Cellular Therapies, NYC, NY, USA;5Department of Molecular Genetics, UTSW, Dallas, TX, USA;6Department of Neurology and Neurotherapeutics, UTSW, Dallas, TX, USA;7Department of Neuroscience, UTSW, Dallas, TX, USA
Homeostatic sleep need increases during waking and decreases during sleep. We show that encephalographic slow wave activity, an index of sleep need, exhibits a single exponential decay during a single slow wave sleep episode in mice. The decay rate constant slows significantly and in correlation with the amount of sleep deprivation. Conditional knockout of neuronal adenosine A1 receptors demonstrate their necessity for this decay as it is absent in the mutant. Furthermore, reduced expression of the glial adenosine kinase, an adenosine-metabolizing enzyme, is sufficient to slow the decay rate constant to a degree comparable with that induced by 6-h sleep deprivation. Consolidation of slow wave sleep, another index for sleep need, is also decreased with neuronal A1 receptor deletion, and increased with glial adenosine kinase deficiency. These results demonstrate a glial-neuronal circuit mediated by intercellular adenosine, controlling the expression of sleep need. The high affinity, low capacity adenosine kinase is regulated by glial metabolic state, which may then influence the neuronal expression of sleep need as it resolves during sleep.
Adenosine, sleep and depression
Tarja Porkka-Heiskanen* and Olena Ventskovska
Institute of Biomedicine, University of Helsinki, PO Box 16 00014 University of Helsinki, Finland
The majority of depressed patients suffer from sleep problems, including increase in REM sleep and decrease in slow wave sleep. Adenosine is one of the main regulators of sleep homeostasis and has also been indicated in induction of depression. We hypothesized that adenosine may be one of the common molecules that regulate both sleep and depression. We have used two animal models of depression (clomipramine treatment of pups and change of pups between mothers) to study changes in brain levels of extracellular adenosine in treated vs. non-treated animals. We have detected that in the clomipramine-treated animals the adenosine levels in the basal forebrain are lower in the treated animals, and do not have the normal response to sleep deprivation, indicating that the adenosine-regulated sleep responses are affected in depression. Preliminary results in pup change-animals show also changes in the adenosine responses. In a population-based human cohort (Health 2000, N = 1,423) we identified an association between one single nucleotide polymorphism in the adenosine transporter SLC29A3 (rs 12256138) and depression with sleep problems, indicating that adenosine transport may be disturbed in depressive patients. These results, combined with results from other laboratories, suggest that disturbances in adenosine metabolism and transport are inflicted in depression, and may contribute to the duration/seriousness of the condition.
CaV2.1 calcium channels as effectors of adenosine’s somnogenic action
Tom Deboer
Laboratory for Neurophysiology, Department of Molecular Cell Biology, Leiden University Medical Center, P.O. Box 9600, 2300 RC, Leiden, The Netherlands
e-mail: Tom.de_Boer@lumc.nl
Adenosine modulates sleep via A1 and A2A adenosine receptors. As the A1 receptor influences CaV2.1 calcium channel functioning via G-protein inhibition, there is a possible role for the CaV2.1 channel in sleep regulation. We therefore investigated sleep and sleep regulation in transgenic Cacna1a R192Q mutant mice that express mutant CaV2.1 channels, which are less susceptible to G-protein inhibition. We hypothesized that Cacna1a R192Q mice may show reduced susceptibility to adenosine, which could result in a sleep phenotype.
To investigate this, we subjected R192Q mutant and wild-type control mice to a 6-h sleep deprivation. In two further experiments mice were treated with caffeine (15 mg/kg, a non-specific adenosine receptor antagonist, which induces waking), and cyclopentyladenosine (CPA, 1 mg/kg, an A1 receptor agonist which induces a sleep like state) i.p injections.
Compared to wildtype, Cacna1a R192Q mice were more awake with longer waking episodes and less non-rapid eye movement sleep in the dark period, but similar amounts of rapid eye movement sleep. After treatment with caffeine, R192Q mice initiated sleep 0.5 h earlier than wild-type. After CPA treatment, R192Q mice woke up 4.3 h earlier than wild-type.
Together, these results indicate that Cacna1a R192Q mice are less susceptible to adenosinergic signaling. This may explain the reduced amount of NREM sleep under baseline conditions. We here show that sleep, and responses to caffeine and CPA, are modified in the R192Q mutant, consistent with decreased susceptibility to adenosinergic inhibition [1]. The data suggest that the CaV2.1 channel is an effector of adenosine’s somnogenic action.
Reference
1. Deboer T, van Diepen HC, Ferrari MD, Van den Maagdenberg AMJM, Meijer JH (2013) Sleep 36:127–136
Deciphering the mechanisms of sleep homeostasis: electrophysiological analysis of the effects of adenosine and ATP on cortically-projecting basal forebrain cholinergic and GABAergic neurons
Chun Yang1, Radhika Basheer2 and Ritchie E. Brown1,*
1In Vitro Electrophysiology Section, Laboratory of Neuroscience, VA BHS & Harvard Medical School, 940 Belmont Street, Brockton MA 02301 USA;2Molecular Biology Section, Laboratory of Neuroscience, VA BHS & Harvard Medical School, 1400 V.F.W. Parkway, West Roxbury, MA 02132, USA
The basal forebrain (BF) region of the brain plays a crucial role in sleep homeostasis: the process by which we become sleepier the longer we stay awake [1]. Extracellular BF levels of the purine, adenosine, in the BF, correlate with time awake and infusion of adenosine causes sleep, implicating adenosine as a homeostatic sleep factor [1,2]. One possible source of adenosine is adenosine triphosphate (ATP), released from glia or via neurotransmission, and broken down to adenosine by the action of extracellular ectonucleotidases. Here we studied the effect of adenosine and ATP on identified cortically projecting cholinergic and GABAergic neurons.
Coronal brain slices were prepared from 13 to 22 days GAD67-GFP knock-in mice [3]. Whole-cell recordings were made from GFP-positive GABAergic neurons and GFP-negative cholinergic neurons identified by their distinctive intrinsic membrane properties (confirmed by posthoc immunohistochemistry for ChAT).
A brief (2–3 min) bath application of adenosine (100 μM) reduced the frequency of spontaneous excitatory postsynaptic currents (sEPSC) in both cholinergic and GABAergic neurons [4]. Similarly, bath application of ATP (100 μM) reduced sEPSC frequency in cholinergic (n = 8) and two types of cortically-projecting GABAergic neurons (n = 6 for each). The effect of both AD and ATP was blocked by a selective AD A1 receptor antagonist, CPT (1 μM). The effect of ATP was also blocked by a selective blocker of the ecto-5′-nucleotidase, AOPCP (100 μM).
Thus, increases in extracellular ATP during prolonged waking may cause sleepiness and deficits in attention via breakdown to adenosine, activation of A1 receptors and an inhibition of cortically projecting cholinergic and GABAergic neurons.
Support: Supported by VA &, NIMH R01 MH039683 & R21 MH094803
References
1. Brown RE, Basheer R,McKenna JT, Strecker RE, McCarley RW (2012) Control of sleep and wakefulness. Physiol Rev 92:1087–1187
2. Basheer R, Strecker RE, Thakkar MM, McCarley RW (2004) Adenosine and sleep-wake regulation. Progr Neurobiol 73(6):379–396
3. McKenna JT, Yang C, Franciosi S,Winston S, Abarr KK, RigbyMS, Yanagawa Y, McCarley RW, Brown RE (2013) Distribution and intrinsic membrane properties of basal forebrain GABAergic and parvalbumin neurons in themouse. J Comp Neurol 521:1225–1250
4. Yang C, Franciosi S, Brown RE. Adenosine inhibits the excitatory synaptic inputs to basal forebrain cholinergic, GABAergic, and parvalbumin neurons in mice. Front Neurol 4:77
Fri 1 D: Purines in host-pathogen interactions
Nonlinear dNTP pool modulation dynamics by SAMHD1 protein in monocyte-derived macrophages
Joseph A. Hollenbaugh1, Sijia Tao1, Gina M. Lenzi1, Sulryung Ryu3, Dong-Hyun Kim3, Raymond F. Schinazi1,2 and Baek Kim1,3,*
1Center for Drug Discovery, Emory Center for AIDS Research, Laboratory of Biochemical Pharmacology, Department of Pediatrics, Emory University School of Medicine, Atlanta, Georgia;2Veterans Affairs Medical Center, Decatur, Georgia 30033, USA;3College of Pharmacy, Kyung-Hee University, Seoul, South Korea
SAMHD1 degrades deoxyribonucleotides (dNTPs), suppressing viral DNA synthesis in macrophages. Recently, viral protein X (Vpx) of HIV-2/SIVsm was shown to target SAMHD1 for proteosomal degradation and led to elevation of dNTP levels, which in turn accelerated proviral DNA synthesis of lentiviruses in macrophages. We investigated both time-dependent and quantitative interplays between SAMHD1 level and dNTP concentrations during multiple exposures of Vpx in macrophages. We observed the following. First, SAMHD1 level, which is rapidly reduced by Vpx to less than 3 % of the normal macrophage level, remained low until day 6 post Vpx treatment, and SAMHD1 level only recovered to about 30 % at day 14. However, dNTP levels peaked at day 1 post Vpx treatment and rapidly decreased back to normal levels by day 7, while still having less than 3 % SAMHD1 protein detected. Second, when Vpx pretreated macrophages were re-exposed to a second Vpx treatment at day 7, we observed a greater dNTP elevation than the first Vpx treatment. Third, we performed a short kinetic analysis of the second Vpx treatment and found dNTP levels peaked at 2–8 h post secondary VLP treatment, which was faster than the dNTP increase by the first Vpx treatment. Lastly, HIV-1 replication kinetics were faster in macrophages treated after the secondary Vpx treatments as compared to the initial single Vpx treatment. This study reveals that a very low level of SAMHD1 sufficiently modulates the normally low dNTP levels in macrophages and proposes potential diverse mechanisms of Vpx-mediated dNTP regulation in macrophages.
Purinergic signaling in Leishmania × host interaction
Luís C. C. Afonso
Department of Biological Sciences, ICEB, Federal University of Ouro Preto, Brazil
Leishmania are intracellular protozoan that infect macrophage as well as other immune cells. In order to escape the host immune response, these parasites use several pathways to down modulate the pro-inflammatory response. Our laboratory has been investigating the role of purinergic signaling during the infection as a new mechanism by which these parasites are able to inhibit the activation of host cells. We have previously shown that upon infection of dendritic cells by Leishmania amazonensis an upregulation of the expression of CD39 and CD73 is observed and is associated with the down modulation of activation markers such as MHCII and CD86. In addition, we showed that inhibition of DC activation by L. amazonensis is dependent on the activation of the A2b adenosine receptor. In recent experiments, we demonstrate that infection of resident macrophages by L. amazonensis also upregulates CD73 expression. The inhibition of adenosine production by CD73 decreased parasite survival in infected macrophages. These results were corroborated by the observation that inhibition of adenosine receptors also decreased parasitism in these cells. Interestingly, the decrease in parasite survival was not associated with alterations in cytokine or nitric oxide production, suggesting that other mechanisms such as inhibition of ROS production may be involved in the regulation of the macrophage microbicidal activity mediated by infection-induced adenosine production.
Financial Support: CNPq, FAPEMIG, CAPES
The role of ATP in control ofToxoplasma gondiiinfection
Robson Coutinho-Silva*, Aline Cristina A. M. Souza, Gladys Correa, Carolina Lindemberg, Christina M. Takiya and Rossiane C. Vommaro
Institute of Biophysics Carlos Chagas Filho, Federal University of Rio de Janeiro, Rio de Janeiro, Brasil
Extracellular nucleotides are danger signals involved with natural resistance to infection with intracellular parasites. They activate P2 receptors responsible for a variety of physiological response such as cell death, pro-inflammatory cytokine secretion, production of lipid mediators, reactive oxygen species, and control of intracellular parasites. Extracellular ATP after activation of P2X7 receptors mediates responses against intracellular bacteria in macrophages through induction of vacuole fusion, and phagolysossome formation, as shown for Chlamydia and mycobacteria, two intracellular bacteria that evade the host by avoiding vesicle fusion.
Toxoplasma gondii is an obligate intracellular protozoan parasite that infects 1/3 of the world population and can cause toxoplasmosis in immunossuppressed individuals. Severe toxoplasmosis can cause damage to the brain, eyes, or other organs. P2X7 receptors participate in immune response against infection with this intracellular parasite. Infected P2X7 −/− mice have shown a shift in the survival curve when compared with wild type animals. The histopathological analysis showed strong differences between these groups. P2X7−/− mice showed the greatest destruction of shock organs such as liver, spleen and mesenteric lymph nodes. Also, the number of T. gondii cysts from the brain was higher in P2X7−/− mice. In vitro, activation of P2X7 receptor confers resistance to infection via ROS production, induction of fusion between lysosomes and the parasitophorous vacuole, with consequent parasite elimination in murine and human macrophages. Therefore, the knowledge of the role of purinergic signaling against intracellular invaders could be an important tool to the development of new strategies against infection with intracellular parasites. Funds: CNPq, FAPERJ.
Involvement of purinergic signaling inTrichomonas vaginalis-host interaction
Tiana Tasca
Pharmacy Faculty, Federal University of Rio Grande do Sul, Avenida Ipiranga, 2752, Porto Alegre, RS, Brazil
Trichomonas vaginalis causes trichomonosis, the most prevalent non-viral sexually transmitted disease worldwide. Most patients are asymptomatic leading to silence infection with serious consequences, such as cervical and prostate cancer, and problems during pregnancy. Importantly, the disease is also a co-factor for HIV/AIDS. Metronidazole and tinidazole are the only two drugs for the treatment of trichomonosis. However, drug-resistant isolates of T. vaginalis have been reported and therapeutic alternatives are not available. ATP can act as damage-associated molecular patterns (DAMPs) performing a proinflammatory effect; in the other hand, adenosine exerts mainly immunosuppressive modulation. The regulation of this cell signaling can be attributed to NTPDase and ecto-5′-nucleotidase enzymes. Pathogens exploit the purinergic system as mechanism of escape of host immune response. Moreover, extracellular adenosine must be uptake by the parasites due to lack of the ability to synthesize the purine ring de novo. NTPDase, ecto-5′-nucleotidase, and adenosine deaminase activities have been characterized in T. vaginalis by our group. We have shown the role of NTPDase and ecto-5′-nucleotidase for the parasite, since the enzymes provide the nucleoside necessary for the parasite growth [1]. In addition, ATP, ADP, and ADP were not decisive for NO release by human T. vaginalis-stimulated neutrophils. Unlike ATP, adenosine and inosine decreased significantly the NO levels, revealing the immunosuppressive effect of adenosine promoted by A2A activation [2]. In this context, the enzymes NTPDase and ecto-5′-nucleotidase may be considered pathogenic markers in the identification of T. vaginalis isolates as well as possible interesting targets of new alternatives for the treatment of trichomonosis.
References
1. Frasson AP, De Carli GA, Bonan CD, Tasca T (2012) Purinergic Signal 8:1–9
2. Frasson AP, Charão MF, Rosemberg DB, de Souza AP, Garcia SC, Bonorino C, Bogo MR, De Carli GA, Tasca T (2012) Memórias do Instituto Oswaldo Cruz 107:170–177
Surprising roles for nucleoside triphosphate diphosphohydrolases (NTPDases) in the pathogenesis of leishmaniasis
Fiona M. Sansom1,2,*, Julie E. Ralton1, M. Fleur Sernee1, David J. Hooker2, Alice M. Cohen1,2, Elizabeth L. Hartland3, Thomas Naderer1 and Malcolm J. McConville1
1Department of Biochemistry and Molecular Biology, Bio21 Institute of Molecular Science and Biotechnology, University of Melbourne, Flemington Rd, Parkville, VIC 3010, Australia;2Faculty of Veterinary Science, University of Melbourne, Parkville, 3010, VIC, Australia;3Department of Microbiology and Immunology, University of Melbourne, VIC, 3010, Australia
Nucleoside triphosphate diphosphohydrolases (NTPDases) hydrolyze a broad range of nucleoside tri- and diphosphates, and in mammals are involved in various functions including inflammation and immunity. Although secreted NTPDases are known virulence factors of Legionella bacteria, the functional significance of NTPDases in parasitic protozoa is less well-defined. Leishmania protozoa are responsible for a number of important clinical syndromes, known collectively as leishmaniasis. We characterized the two putative NTPDases encoded on the genome of Leishmania major, demonstrating that L. major possesses a secreted NTPDase (LmNTPDase2) and, somewhat unexpectedly, a Golgi-targeted NTPDase (LmNTPDase1). An L. major LmNTPDase1 null mutant exhibited a lag in lesion development when infections in susceptible BALB/c were initiated with promastigotes, but not when the obligate intracellular amastigote stage was used. This phenotype is similar to that of L. major strains lacking lipophosphoglycan (LPG), which is the major promastigote surface glycoconjugate. Biochemical studies showed that the L. major NTPDase1 null mutant synthesized normal levels of LPG, but that the LPG had shorter phosphoglycan chains. This change in structure also resulted in functional changes, including increased sensitivity of the LmNTPDase1 null mutant to complement-mediated serum lysis. These data suggest that the Golgi-localized LmNTPase1 is involved in regulating the normal sugar-nucleotide dependent elongation of LPG. In contrast, deletion of the gene encoding LmNTPDase2 had no apparent effect on parasite virulence in BALB/c mice. Overall, our data suggest that Leishmania NTPDases may have important roles in the insect stage, but play only a transient or non-major role in pathogenesis in the mammalian host.
Fri 2 A: X-ray structures and molecular modeling II
Opening of a P2X ion channel with light
Thomas Grutter
Laboratoire de Chimie et Neurobiologie Moléculaire, UMR 7199 CNRS, Faculté de Pharmacie, 74 route du Rhin, Illkirch, France
P2X receptors are ligand-gated ion channels activated by extracellular ATP. There are seven mammalian P2X subtypes that assemble to form functional homo- or hetero-trimeric cation-selective channels. P2X receptors contribute to many important physiological and pathological roles, including the modulation of neurotransmitter release, male fertility, the response to inflammation, pain sensation, and neuropathic pain. They are thus recognized as important therapeutic targets. Recent X-ray structures of a P2X receptor in closed and open-channel states have greatly advanced our understanding of agonist binding and channel gating. However, despite these significant biophysical advances, a lack of powerful pharmacological tools has considerably impaired our knowledge of the precise contribution of P2X receptors in cell signaling. Recent developments of optogenetics offer great opportunities to control selectively channel function by optical methods with high temporal and spatial resolution. Inspired by these achievements, we have recently developed a new and versatile approach, called optogating [1], in which the gating machinery of the P2X2 receptor was reprogrammed to respond to light. We demonstrated photocontrol of neuronal activity by a light-gated P2X receptor, in which the natural sensitivity to ATP was genetically removed. These light-gated purinergic receptors represent valuable tools for investigating P2X signaling in native tissues.
Reference
1. Lemoine D, Habermacher C, Martz A, Méry PF, Bouquier N, Diverchy F, Taly A, Rassendren F, Specht A, Grutter T (2013) Proc Natl Acad Sci USA 110:20813–20818
P2X7 receptor sensitization by a protein acyltransferase
Toshi Kawate
Department of Molecular Medicine, Cornell University 930 Campus Rd, Ithaca, New York, USA
Activation of P2X7 receptors has been implicated in many devastating pathological conditions such as chronic pain and Alzheimer’s disease. It remains controversial, however, whether P2X7 receptors can be activated under physiological conditions, as the effective concentration of ATP for these receptors are much higher than for the other subtypes in heterologous systems. In this talk, I will present that a P2X7 receptor is sensitized when co-expressed with a protein acyltransferase in HEK293 cells. Biochemical and electrophysiological experiments suggest that the acyltransferase directly interacts with the P2X7 receptor to increase its ATP sensitivity. Mathematical modeling postulates that P2X7 sensitization by acyltransferase is different from the well-documented sensitization triggered by prolonged ATP application. Given that these two proteins co-exist in multiple areas in the brain, this type of P2X7 sensitization could be a mechanism by which P2X7 channels open under physiological conditions.
Contribution of salt-bridge switching within in the ATP binding pocket to the gating of the P2X2 receptor
Ralf Hausmann1, Daniel Kuhlmann1, Achim Kless2, Fritz Markwardt3 and Günther Schmalzing1,*
1Molecular Pharmacology, RWTH Aachen University, Aachen, Germany;2Grünenthal GmbH, Global Drug Discovery, Aachen, Germany;3Julius-Bernstein-Institut für Physiologie, Martin-Luther University Halle-Wittenberg, Halle, Germany
A homology model of the closed-state of the rat P2X2 receptor based on the X-ray structure of the apo closed-state of the zebrafish P2X4 receptor shows that the side chains of the residues Glu167/Arg290 and Arg290/Asp82, Glu84 or Glu85 are in close spatial vicinity within the ATP-binding pocket. Functional analysis of mutants resulting in charge reversal, charge swapping and charge neutralization suggests that at least two of the possible four oppositely charged ion pairs are electrostatically coupled, namely Glu84/Arg290 and Glu167/Arg290. A triple mutant cycle analysis of Glu167, Arg290 and Glu84 indicated energetic coupling between Glu167/Arg290 and Arg290/Glu84 and thus cooperative interaction in a larger salt bridge network. A comparison of the closed-state and open-state model of the rat P2X2 receptor revealed that the residues Glu84/Arg290 and Glu167/Arg290 markedly relocate and move apart during the closed-to-open transition into positions that impair the electrostatic interactions. Indeed, disulfide trapping confirmed that the side chains of the pairs Glu84/Arg290 and Glu167/Arg290 are in close proximity when the channel is in the apo closed state, but in weakly cross-linkable and thus more distant positions in the ATP-bound open state. A driving force for the breaking of the salt bridges Glu84/Arg290 and Glu167/Arg290 seems to be the γ-phosphate oxygen of ATP, which undergoes a strong ionic interaction with Arg290 of the P2X2 receptor. Our data are in line with the concept that this newly formed electrostatic interaction of Arg290 with ATP competitively releases Glu84 and Glu167 from their strong electrostatic coupling to Arg290 and thus initiates a destabilization of the closed-state that favours channel opening.
This work was supported by Deutsche Forschungsgemeinschaft Grants Schm536/8-2 and HA6095/1-1.
3D structure of a P2X antagonist binding domain
Philippe Séguéla
Montreal Neurological Institute and Alan Edwards Centre for Research on Pain, Department of Neurology and Neurosurgery, McGill University, Montreal, Quebec, Canada
Knowledge on the role of P2X4 receptor channels in the pathophysiology of inflammation and chronic pain mainly relies on knockout or knockdown strategies due to the lack of selective and potent ligands. In search of a P2X4 antagonist, we conducted the high-throughput screening of a library of small organic compounds using calcium uptake readout on a human P2X4-expressing stable cell line. We discovered the phenylurea compound BX430 endowed with interesting pharmacological properties on P2X4 activity. Based on electrophysiology, calcium imaging and YOPRO-1 uptake, we showed that BX430 has potent inhibitory effects on recombinant and native human P2X4 (IC50 = 0.8 μM) as well as ideal subtype selectivity, i.e. negligible blockade of the other P2X subtypes (P2X1, P2X2, P2X3, P2X5 and P2X7) when applied at 10 μM. Non-competitive BX430 acts extracellularly and displays an unique species selectivity of action: it does not inhibit rat or mouse P2X4 while it inhibits effectively P2X4 orthologs from several other vertebrate species. Alignment of the primary sequences of P2X4 orthologs tested revealed a small set of extracellular residues involved in differential sensitivity to BX430. With site-directed mutagenesis, we identified a single critical residue that accounts for effective blockade by this novel antagonist. In agreement with current structural models of ATP binding and gating in P2X receptor channels, we propose a 3D model for the binding domain of BX430 on P2X4 that explains both its species and subtype selectivity, with strong potential for the rational design of other P2X antagonists.
Supported by CIHR and NSERC.
Expression of mammalian P2X receptors for structural studies
Leanne M. Grimes, Wynand van der Goes van Naters and Mark T Young*
School of Biosciences, Cardiff University, Museum Avenue, Cardiff, CF10 3AX, UK
2X receptors are eukaryotic ATP-gated cation channels which function in nerve transmission, control of vascular tone, pain sensation and inflammation. At present, to understand the structure-function relationships of mammalian P2X receptors, we depend on homology models derived from the apo- and ATP-bound crystal structures of modified zebrafish P2X4 receptors, which display substantially altered ion channel function. To determine the roles in channel function and signalling of the intracellular N- and C-termini, which were not present in the reported structures, and for structure-aided drug design it is clear that 3D-structures of mammalian P2X receptors are needed. Structural studies of membrane proteins are highly challenging, mainly due to problems with poor protein yield and stability. Success with zebrafish P2X4 required use of the Sf9 insect-cell expression system coupled with GFP-fusion, to enable assessment of protein yield and quality during purification. Drawing upon this success, we expressed C-terminally GFP-tagged rat P2X2 in Drosophila melanogaster, an insect amenable to genetic manipulation and large-scale culture. In addition, the photoreceptor cells of the Drosophila eye form specialised membrane stacks (rhabdomeres) adapted for high-density expression of rhodopsin. For protein production, we used the eye-specific GMR (glass multiple reporter) driver, and confirmed expression by fluorescence microscopy and Western blotting. For functional assay, we used the pan-neural C155 driver and demonstrated ATP-induced action potential firing using electrophysiological recordings of taste neurons. In summary, we present Drosophila melanogaster as both a novel expression system for mammalian P2X receptors, and a medium-throughput functional assay for potential P2X receptor agonists and antagonists.
Fri 2 B: Purine nucleotides and their metabolizing enzymes in inflammation and immunology
Role of extracellular adenosine in shaping the leukemic niche: the example of CD73 expression in chronic lymphocytic leukemia
Sara Serra1,2, Roberta Buonincontri1,2, Valentina Audrito1,2, Tiziana Vaisitti1,2, Davide Brusa1,2, Simon Robson3 and Silvia Deaglio1,2,*
1Human Genetics Foundation (HuGeF), Via Nizza 52, Turin, Italy;2Department of Medical Sciences, University of Turin, Via Nizza 52, Turin, Italy;3Division of Gastroenterology, Beth Israel Deaconess Medical Center, Harvard Medical School, Boston, MA, USA
Extracellular adenosine generated from ATP/ADP through the concerted action of the ectoenzymes CD39 and CD73 elicits potent cytoprotective and immunosuppressive effects mediated by type-1 purinergic receptors.
Chronic lymphocytic leukemia (CLL) patients expressing the ectoenzymes CD39 and CD73 can actively produce adenosine. This condition activates an autocrine adenosinergic axis that supports engraftment of leukemic cells in a growth-favorable environment. These effects are mediated by the A2A adenosine receptor, which inhibits chemotaxis and limits spontaneous and drug-induced apoptosis of CLL cells.
Following the reported cross-talk between hypoxia and adenosine, we tested the hypothesis of a functional interplay between the adenosinergic axis and hypoxic signals in the CLL microenvironment.
Results confirm that the CLL cells robustly increase HIF-1α expression when cultured under low oxygen tension. Under these conditions a significant increase in the mRNA and protein levels of CD73, CD26 and of A2A was observed. An HPLC assay confirmed that hypoxic CLL cell cultures are characterized by higher extracellular adenosine levels, further improved upon inhibition of adenosine deaminase and nucleoside transporters.
Attention was then concentrated on the stromal compartment, which is critical to the formation and maintenance of the leukemic niche. Here, hypoxia enhanced differentiation of circulating monocytes into nurse-like cells, macrophages of the M2 type, which play an essential role in nurturing leukemic cells. During hypoxic culture, differentiating monocytes up-regulated A2A and A3. Both receptors were overexpressed by NLC under hypoxic conditions. Furthermore, they were functional, as determined by the finding of increased AKT and ERK1/2 phosphorylation following pharmacological activation of the receptors. The enhancement of NLC differentiation under hypoxic conditions relied, at least in part, on the activation of A2A and A3: their engagement using agonists enhanced NLC differentiation in normoxia, with overexpression of IDO, CD163 and CD206. Furthermore, activation of A2A and A3 favored secretion of immunomodulatory cytokines such as IL-10 and IL-6. On the contrary, their pharmacological blockade under hypoxia prevented NLC differentiation.
Together, these results indicate that the adenosinergic and hypoxic axes synergize in shaping the CLL niche, suggesting that the pharmacological inhibition of adenosinergic signals may counteract some of the effects mediated by an hypoxic microenvironment.
Regulation and trafficking of CD73 in health and disease
Michel Fausther1,2,*, Elise G. Lavoie1,2, Jessica R. Goree1,2, Giulia Baldini3 and Jonathan A. Dranoff1,2
1Division of Gastroenterology and Hepatology, University of Arkansas for Medical Sciences, Little Rock, AR 72205, USA;2Research Service, Central Arkansas VA Healthcare System, Little Rock, AR 72205, USA;3Department of Biochemistry and Molecular Biology, University of Arkansas for Medical Sciences, Little Rock, AR 72205, USA
Ecto-5′-nucleotidase/CD73/NT5E, the product of the NT5E gene, is the dominant enzyme in the generation of adenosine from degradation of AMP in the extracellular milieu. Nonsense (c.662C → A, p.S221X designated F1, c.1609dupA, p.V537fsX7 designated F3) and missense (c.1073G → A, p.C358Y designated F2) NT5E gene mutations have been recently identified and linked to human arterial calcification disease [1]. However, the cellular fate of mutant NT5E proteins has yet to be investigated. We hypothesized that human NT5E gene mutations cause mistrafficking of the defective proteins within cells. To test this hypothesis, we generated and used plasmids encoding cDNAs of wild type and mutant human NT5E fused with fluorescent probe DsRed for transient transfection and heterologous expression in immortalized monkey COS-7 kidney cells lacking native functional NT5E protein. Biochemical activities of wild type and mutant fusion NT5E proteins were assessed by enzyme histochemistry and Malachite green assay. Subcellular trafficking of fusion NT5E proteins was monitored by confocal microscopy and immunoblot analysis of fractionated cell constituents. All 3 (F1, F2 and F3) mutations resulted in absent cell-surface NT5E enzymatic activity, and proteins with impaired trafficking to the plasma membrane and reduced ER retention, as compared to wild type protein. Confocal immunofluorescence demonstrates vesicles containing mutant DsRed-tagged NT5E proteins (F1, F2 and F3) in the cell synthetic apparatus. In conclusion, three familial NT5E mutations result in novel trafficking defects associated with human disease. These novel genetic causes of human disease suggest that the syndrome of premature arterial calcification due to NT5E mutations may also involve a novel “trafficking-opathy”.
Reference
1. St Hilaire C, Ziegler SG, Markello TC, Brusco A, Groden C, Gill F, Carlson-Donohoe H, Lederman RJ, Chen MY, Yang D, Siegenthaler MP, Arduino C, Mancini C, Freudenthal B, Stanescu HC, Zdebik AA, Chaganti RK, Nussbaum RL, Kleta R, Gahl WA, Boehm M (2011) N Engl J Med 364(5):432–442
Characterization of P2X7 receptor secretome during inflammation
Carlos de Torre and Pablo Pelegrin*
Murcia BioHealth Research Institute, Murcia, Spain
Inflammatory diseases affect over 80 million people worldwide and accompany many diseases of industrialized countries, being the majority of them infection-free conditions. We now know that innate immunity is the main coordinator and driver of inflammation through the secretion of cytokines and other signaling proteins upon innate immune cell activation by pathogen associated molecular patterns. The activation of purinergic P2X7 receptors (P2X7R) in immune cells is a novel and increasingly validated “sterile” pathway to initiate inflammation through the activation of the NLRP3 inflammasome and the release of IL-1beta and IL-18 cytokines. Extracellular ATP, the physiological P2X7R agonist, is a crucial danger signal released by injured cells, and one of the most important mediators of infection-free inflammation. P2X7R signaling goes beyond the NLRP3 inflammasome, and includes the release of proteases or inflammatory lipids. Here, we used mass spectrometry and cytokine arrays to detect protein secretion from macrophages upon P2X7R activation. We found that P2X7R controls the release of 34 proteins in macrophages. Secretome to transcriptome comparisons revealed the transcriptionally decoupled release of several proteins and the identification of different release pathways activated by P2X7R. From macrophage polarization models we found that P2X7R differentially regulates protein secretory profiles that promoted the release of pro- or anti-inflammatory proteins in M1 or M2 macrophages. Therefore, P2X7R could be an important mediator for the resolution of inflammation and the development of P2X7R blockade therapy should be tailored not to affect the resolution phase of inflammation.
This work was supported by grants from PN I+D+I 2008–2011-Instituto Salud Carlos III-FEDER (EMER07/049, PI09/0120, PI13/00174), Fundación Séneca (11922/PI/09).
Purinergic signaling pathways during HIV-1 infection
Jean-Luc Perfettini
Cell death and Aging team, Institut Gustave Roussy, 114 rue Edouard Vaillant, F-94805 Villejuif, France;Laboratory of Molecular Radiotherapy, INSERM U1030, Institut Gustave Roussy, 114 rue Edouard Vaillant, F-94805 Villejuif, France;Institut Gustave Roussy, 114 rue Edouard Vaillant, F-94805 Villejuif, France;Université Paris Sud—Paris 11, 114 rue Edouard Vaillant, F-94805 Villejuif, France
Extracellular nucleotides and purinergic receptors participate in numerous cellular processes during viral infection. We demonstrated that upon interaction between the HIV-1 envelope protein and host coreceptors, ATP is released from the target cell through pannexin-1 channels. Once released, ATP acts on purinergic receptors, in particular P2Y2 and stimulates a signal transduction pathway that involves Pyk2 kinase, which in turn stimulates the fusion between Env-expressing membranes and membranes containing CD4 plus appropriate chemokine co-receptors. Inhibition of each of the protein constituents of this cascade impairs the replication of HIV-1 mutants that are resistant to conventional antiretroviral agents. Altogether our results reveal the involvement of purinergic signaling pathways in early steps of HIV-1 infection and offers ample opportunities for the development of new therapeutic approaches.
Role of CD39 in inflammatory hepatic and gastrointestinal diseases
Simon C. Robson
Division of Gastroenterology and Liver Clinic, Department of Medicine, Beth Israel Deaconess Medical Center, Harvard Medical School, Boston, USA
Inflammatory illnesses impacting the liver, pancreas and gut are important health problems resulting in hepatobiliary-pancreatic and gastrointestinal damage with progressive fibrosis, end-organ failure and cancer. Novel and non-toxic therapies are urgently needed to address these devastating diseases. In the liver, pancreas and gut, several resident cell populations, most prominently innate and adaptive immune cells, macrophages, endothelium and myofibroblasts express high levels of CD39 and CD39L (ike)-1 that generate the immune suppressant adenosine and/or other nucleotides from extracellular nucleotides. Extracellular ATP, if unopposed, will promote macrophage polarization towards an inflammatory phenotype, while provoking procoagulant responses by the endothelium and myofibroblastic proliferation. Comparable aberrant purinergic responses noted in the setting of genetic inactivation of CD39 (as well as CD39L1) further modulate immune or toxic metabolic mediated liver, pancreatic and biliary injury, culminating in heightened levels of disease and altered scarring in several experimental models. Furthermore, genetic deletion of CD39 results in aberrant hepatocyte regeneration and development of spontaneous liver cancer. In a similar manner, inflammatory bowel disease (IBD) provokes abnormal immune responses involving the gut, and occasionally the hepatobiliary system, in genetically susceptible individuals. Genetic polymorphisms of CD39 can be linked to both Crohn’s disease and to aberrant immunological phenotypes. Decreased levels of expression of CD39 predisposes to heightened inflammatory and innate responses resulting in adaptive immune deviation away from T cell regulation towards pathogenic T helper 17 cells, with loss of immunological self-tolerance. These deleterious changes can be addressed by pharmacological and genetic strategies, as will be demonstrated in this presentation. In conclusion, purinergic signaling pathways might be effectively targeted to develop safe and effective immune and antifibrotic treatments to prevent, halt or even reverse progression of common liver, pancreatic and gastrointestinal inflammatory diseases.
Role of P2X7 receptor in inflammatory mechanisms of amyotrophic lateral sclerosis
Savina Apolloni1, Susanna Amadio2, Chiara Parisi1, Cinzia Montilli2, Alessandra Matteucci3, Rosa L. Potenza3, Monica Armida3, Patrizia Popoli3, Nadia D’Ambrosi2 and Cinzia Volonté1,2,*
1Cellular Biology and Neurobiology Institute, CNR, Via Del Fosso di Fiorano 65, 00143 Rome, Italy;2Fondazione Santa Lucia, Rome, Italy;3Department of Therapeutic Research and Medicines Evaluation, ISS, Rome, Italy
A pathological hallmark of Amyotrophic Lateral Sclerosis (ALS) is neuroinflammation and evidence obtained in animal models of the disease indicates a non-cell-autonomous motor neuron death enhanced by nonneuronal cells via an inflammatory response that accelerates disease progression [1]. Consistently, analysis of tissues from ALS patients reveals a marked microglial-dominated neuroinflammation. Purine/pyrimidine nucleotides are prominent triggers of microglia activation and play a well-established pro-inflammatory role, by binding to ionotropic P2X and metabotropic P2Y receptors. Compelling evidence is emerging on purinergic receptor involvement in ALS and particularly P2X7 receptor was recognized as a major effector in exacerbating the neurotoxic action of ALS microglia [2]. In addition, Brilliant Blue G used to antagonize P2X7 reduces neuroinflammation in traumatic brain injury, cerebral ischemia/reperfusion, neuropathic pain and experimental autoimmune encephalitis [3]. In our work, by genetic and pharmacological manipulation we inhibited P2X7 in SOD1-G93A mouse model of ALS. We demonstrated that Brilliant Blue G treatment started at late pre-onset reduces microgliosis but not astrocytosis, modulates inflammatory genes enhancing motor neuron survival in lumbar spinal cord. This is accompanied by a slightly delayed onset with modest improvement of general conditions and motor performance. However, in SOD1-G93A mice with constitutive deletion of P2X7, ALS pathogenesis is aggravated: the clinical onset is significantly anticipated and the disease progression worsened, with concomitant increase of astrogliosis, microgliosis, motor neuron loss and induction of pro-inflammatory markers. This study highlights the complex dual role of P2X7 receptor in ALS and strengthens the importance of a precise time window of therapeutic intervention in contrasting the disease.
References
1. Ilieva HH, Polymenidou M, Cleveland DW (2009) J Cell Biol 187(6):761–772
2. Volonté C, Apolloni S, Carrì MT, D’Ambrosi N (2011) Pharmacol Ther 132(1):111–122
3. Chrovian CC, Rech JC, Bhattacharya A, Letavic MA (2014) Prog Med Chem 53:65–100
Fri 2 C: Purine receptors in neuroinflammation and neurodegeneration
P2X receptors and glial ATP regulate AMPAR trafficking and synaptic plasticity in hippocampus
Eric Boué-Grabot
Univ. de Bordeaux, Institut des Maladies Neurodégénératives, CNRS UMR 5293, Bordeaux, France
P2X receptors are ATP-gated cation channels widely expressed in the brain where they mediate action of extracellular ATP released by neurons or glia. Although purinergic signaling has multiple effects on synaptic transmission and plasticity, the function of P2X receptors at brain synapses remains to be established. NMDAR and AMPAR have critical roles in excitatory synaptic transmission and plasticity in the CNS. Synaptic strength is thought to be determined in part through activity-dependent insertion and endocytosis as well as lateral mobility of AMPAR which regulate long-term potentiation (LTP) and long-term depression (LTD), that is widely recognized to underlie cognitive functions such as learning and memory. We have addressed the role of postsynaptic P2X receptors on AMPAR and excitatory glutamatergic synaptic transmission in hippocampal neurons. We show that activation of postsynaptic P2X receptors by exogenous ATP or noradrenaline-dependent glial release of endogenous ATP decreases the amplitude of miniature excitatory postsynaptic currents and AMPA-evoked currents in cultured hippocampal neurons. We also observed a P2X-mediated depression of field potentials recorded in CA1 region from brain slices. Using a combination of biochemical, electrophysiological and super resolution imaging techniques, we demonstrated that P2X2 or P2X4 trigger dynamin-dependent internalization of AMPAR leading to reduced surface AMPAR in dendrites and at synapses. AMPAR alteration required calcium influx through opened ATP-gated channels and phosphatase or CamKII activities.
Our main finding provide a mechanism by which postsynaptic P2X receptors can directly modulate excitatory synaptic transmission and further expand the role of glial-derived ATP at brain synapses.
Synaptic and glia involvement in the purinergic control of neurodegenerative diseases
Paula M. Canas1, Daniel Rial1, Ana Patrícia Simões1, João Pedro Lopes1, Henrique B. Silva1, Nuno J. Machado1, Francisco Queiroz1, Nélio Gonçalves1, Lisiane O. Porciúncula2, Geanne Matos3, Jiang-Fan Chen4, Catarina V. Gomes1, Ricardo J. Rodrigues1, Paula M. Agostinho1,5 and Rodrigo A. Cunha1,5,*
1CNC-Center for Neuroscience and Cell Biology, University of Coimbra, Portugal;2Instituto de Ciências Básicas da Saúde, Departamento de Bioquímica, Universidade Federal Rio Grande do Sul, Porto Alegre/RS, Brazil;3Department of Physiology and Pharmacology, Faculty of Medicine, Federal University of Ceará, Brazil;4Department of Neurology and Pharmacology, Boston University School of Medicine, Boston, MA, USA;5Faculty of Medicine, University of Coimbra, Portugal
Adenosine assists encoding information salience through a combined activation of inhibitory A1 and facilitatory A2A receptors (A2AR). This brain modulation system is imbalanced in stressful conditions, with increased A2AR density and decreased A1R density. Neuroprotection is afforded by A2AR blockade and by repeated caffeine consumption (adenosine receptor antagonist), which prophylactically prevents depression, suicide ideation and memory deficits, namely in Alzheimer’s disease (AD). In animal models of AD, A2AR blockade prevents memory deficits and synaptic plasticity in hippocampal circuits and this neuronal A2AR activation depends on ATP-derived adenosine. Apart from adenosine, ATP concomitantly controls synaptic plasticity, AD features and brain damage through P2Y1 receptors. Neuronal A2AR also control memory and mood deficits upon chronic stress and A2AR blockade can actually therapeutically revert these installed aberrant phenotypes. The mood/memory normalizing impact of neuronal A2AR blockade is further highlighted by the prevention of fear memory and amygdalar synaptic plasticity. Glial A2AR also play a role in the imbalance of synaptic dysfunction and behavioral maladaptive changes upon brain disorders: thus, astrocytic A2AR control astrocytic glutamate uptake and drive synaptic changes controlling both synaptic plasticity and adaptive behavior. In parallel, microglia A2AR control proliferation and pro-inflammatory phenotype of microglia contributing for spreading and evolution of brain damage. Overall these findings unveil a dual role of A2AR controlling glial-neuronal communication as well as synaptic transmission to impact on dysfunction and damage of brain circuits, while the source of the adenosine activating A2AR (ATP) plays a parallel role as a danger signal.
(Supported by DARPA, FCT, QREN, NARSAD, CAPES, CNPq-Ciência sem Fronteiras)
Adenosine A2Areceptors and stress—impact on cognition
Luísa Vaqueiro Lopes
Instituto de Medicina Molecular, Lisbon
e-mail: lvlopes@fm.ul.pt
Aging is associated with cognitive decline in both humans and animals and of all brain regions, the hippocampus appears to be particularly vulnerable to senescence. Stress is believed to contribute to the variability of the ageing process and to the development of age-related neuro- and psychopathologies We have previously described that long-term therapy with an adenosine A2A receptor blockade would revert aging-like memory deficits by restoring corticosterone circadian oscilation and hippocampal inhibitory stress feedback to the hypothalamus (Batalha et al., 2013, Mol Psychiatry). We now found that human brains of both aged individuals and Alzheimer’s disease (AD) patients exhibit a striking overexpression of adenosine A2A receptors (A2AR) in cortical areas, which is associated to a downregulation of glucococorticoid receptor (GR). We are now focused on unraveling the molecular mechanisms, and adenosine A2A receptor overactivation is clearly contributing to this age and age-related hippocampal deficits. We will discuss recent evidence showing that activation A2A receptors increases glucocorticoid translocation to the nucleus and hence its promoter transcriptional activity. These effects are associated with the reestablishment of the HPA-axis function, since both the plasma corticosterone levels and glucocorticoid expression pattern returned to physiological-like status after the treatment. We also show that A2AR activation modulates glucocorticoid-induced deficits in hippocampal synaptic plasticity, increasing susceptibility to GR activation. Finally, we dissected a new molecular mechanism whereby A2AR blockade prevents GR transcriptional activity and nuclear translocation and modulates histone H3 association with GR gene.
These findings are not only relevant in psychopathologies but also in age-related diseases exhibiting glucocorticoid response impairment. The expansion of this interaction to the immune response, cell proliferation, tumor response and other cellular functions that imply GR or corticosteroids use in therapeutics, could have an enormous clinical impact.
Microglial P2X4 receptors in status epilepticus
Lauriane Ulmann1,2,*, Françoise Levavasseur3,4, Elena Avignone3,4,5,6, Ronan Peyroutou1,2, Hélène Hirbec1,2, Etienne Audinat3,4 and François Rassendren1,2
1Institut de Génomique Fonctionnelle, Labex ICST, Centre National de la Recherche Scientifique, Unité Mixte de Recherche 5203, Université Montpellier, Montpellier, France;2The Institut National de la Santé et de la Recherche Médicale Unité 661, Montpellier, France;3Inserm, U603, Paris, France;4Université Paris Descartes, UMR-S603, CNRS UMR 8154, Paris, France;5University Bordeaux, IINS, UMR 5297, F-33000 Bordeaux, France;6CNRS, IINS, UMR 5297, F-33000 Bordeaux, France
Within the CNS, ATP-gated channel P2X4 (P2X4R) are expressed in subset of neurons and in activated microglia. P2X4R functions in brain are still poorly understood, yet their activation in both neurons and microglia coincide with high or pathological neuronal activities. Activity-dependence of P2X4R activation suggest that these receptors might be involved in excitotoxic process associated with different pathologies. We thus investigated the potential involvement of P2X4R in a model of kainate (KA)-induced status epilepticus (SE), in which both neurons and microglia activities contribute to excitotoxicity.
Whereas P2X4R are expressed in hippocampal pyramidal neurons in basal conditions, the induction of SE was associated with lasting P2X4R upregulation localized in activated microglia. Shortly after induction of SE, a transient decrease of the expression of the transporter KCC2 was observed in wild-type mice, but not in P2X4R-deficient mice. Because the down regulation of KCC2 was observed prior the induction of the microglial P2X4R expression, these results suggest the involvement of neuronal P2X4R.
Forty-eight hours after induction of the SE, several features of microglial activation, such as cell recruitment, upregulation of potassium channels and transcriptional regulation of pro-inflammatory genes, were impaired in P2X4R-deficient mice. Consistent with the role of P2X4R in activity-dependent degenerative processes, CA1 area was partially protected from neuronal death in P2X4R-deficient mice compared to wild-type animals. These observations demonstrate that both neuronal and microglial P2X4R contribute to excitotoxic damages associated with SE. They also suggest the existence of a cross-talk between neuronal and microglial P2X4R in hippocampal remodeling associated with SE.
Role of the purinergic receptor P2X7 in Alzheimer’s disease
Cécile Delarasse
Alzheimer disease (AD) is a neurodegenerative disease characterized by two main lesions: senile plaques and neurofibrillary tangles. The exact processes that cause the disease are still poorly understood. Senile plaques are composed of extracellular aggregates of amyloid beta (Abeta) peptides and might act as an essential trigger in the disease to initiate a cascade of lesions leading to clinical dementia. The purinergic P2X7 receptors (P2X7R) have been linked to the physiopathology of AD as their expression is significantly increased in glial cells populations surrounding amyloid plaques in human brain tissues and also in tissues from mice models of AD. We have recently demonstrated that in vitro stimulation of P2X7R increases levels of sAPPalpha precluding the formation of neurotoxic Abeta peptides and promoting neurotrophic effects. In addition, P2X7R plays a role in the non-cytolytic release of inflammatory cytokines. In particular, P2X7R is a key component of inflammasome activation which contributes to the AD pathological processes. These pleiotropic actions of P2X7R lead us to hypothesize that P2X7R stimulation may have a dual effect in AD depending upon the stage of the disease. P2X7R could first sense ATP as a danger signal and in response trigger the non amyloidogenic pathway which is neuroprotective. Then, at more advanced stages P2X7R could have a deleterious effects through uncontrolled microglial activation. APPxPS1 transgenic mice modeling AD brain lesions were crossed with P2X7RKO mice to generate AD transgenics with or without functional P2X7R. Aged animals (>10 months) with full-blown pathology were behaviorally assessed in different cognitive paradigms before being sacrificed. Histological, biochemical and flow cytometry analysis were subsequently performed to assess amyloid and inflammatory processes. We first evidenced that invalidation of P2X7R induced a significant recovery of function in APPxPS1 mice trained in a cognitive paradigm. In parallel we showed, in the same animals, a drastic reduction of amyloid loads in APPxPS1xP2X7KO as compared to APPxPS1 transgenics. While we previously showed a neuroprotective effect of P2X7R stimulation, the present data indicate that, at late stages of the disease, P2X7 exerts deleterious functions and potentiates AD-related lesions and symptoms. This strengthens the status of P2X7R as a target for future pharmacological intervention.
Fri 2 D: Purinergic signaling in the muscosceletal system
Overview of P2 receptors in bone metabolism and disease
Alison Gartland
The Mellanby Centre for Bone Research, The University of Sheffield, Sheffield, UK
The field of purinergic signalling in the musculoskeletal system was first recognised in the early 1990s and has been rapidly expanding ever since. All of the seven P2X ion channel receptor subtypes (P2X1-7) and eight P2Y G protein-coupled receptor subtypes (P2Y1, P2Y2, P2Y4, P2Y6, P2Y11, P2Y12, P2Y13, and P2Y14) are known to be expressed in bone and cartilage cells. Extracellular nucleotides bind to these cell surface P2 receptors, trigger the intracellular calcium signalling cascades, direct the fate of bone or cartilage cells, and ultimately control the homeostasis of the skeleton. The availability of P2R knockout mice has further enhanced the field of P2 receptors in musculoskeletal system. This talk will highlight recent progress and the potential pharmacological benefits of targeting P2 receptors signalling in treating various musculoskeletal diseases.
The regulation of skeletal and soft tissue mineralization by ATP and NPP1
Isabel R. Orriss1, Mark OR Hajjawi2 and Timothy R Arnett2
1Department of Comparative Biomedical Sciences, Royal Veterinary College, London, UK;2Department of Cell and Developmental Biology, University College London, London, UK
Extracellular nucleotides, signalling through P2 receptors, play a significant role in bone biology modulating osteoblast and osteoclast function. Osteoblasts, the bone forming cells, express multiple P2X and P2Y receptors. We have shown that ATP and UTP (≥1 μM), acting via the P2Y2 receptor, strongly inhibit the mineralisation of the collagenous matrix. Selective P2X receptor agonists (≥1 μM α,β-meATP, β,γ-meATP, BzATP) also block bone mineralisation; pharmacological studies showed this inhibition was mediated via the P2X1 and P2X7 receptors. Under normal conditions, osteoblasts constitutively release ATP in the nanomolar range. Osteoblasts express many ecto-nucleotidases, which rapidly hydrolyse extracellular nucleotides. The hydrolysis of ATP and other NTPs by NPP1 (ecto-nucleotide pyrophosphatase/phosphodiesterase 1) is particularly important in bone because the product, PPi, is the key, local physicochemical inhibitor of mineralisation. NPP1 knockout mice (Enpp1−/−) display significant changes in the trabecular and cortical bone including hypermineralisation of the osteocyte lacunae. We found that osteoblastic NPP activity can generate significant amounts of PPi in vitro. Thus, NTPs can exert a dual inhibitory action on bone mineralisation via both P2-receptor-mediated signalling and a receptor independent mechanism (hydrolysis by NPP1 to produce PPi). Extracellular nucleotides and NPP1 are also emerging as important regulators of calcification in soft tissues. Recently, we demonstrated that Enpp1−/− mice display mineralisation of whisker follicles, trachea and ear pinna and that ATP/UTP are potent inhibitors of vascular calcification. Together, these data highlight the key role of extracellular nucleotides, P2 receptors and NPP1 in the regulating calcification of both skeletal and soft tissues.
Purinergic signalling in cartilage (cancelled)
James A. Gallagher*, Jane P. Dillon, Giulia Vindigni, Craig M. Keenan, Damiano Piselli and Peter J. Wilson
Department of Musculoskeletal Biology, University of Liverpool, Ashton Street Liverpool, UK
There is much evidence indicating that extracellular ATP signalling through P2 purinoceptors plays a major role in the regulation of bone remodelling, particularly in mechanotransduction. In contrast the role of extracellular ATP signalling in cartilage, which is an avascular and aneural tissue, is still largely unexplored. We investigated expression of P2 receptors in cartilage by RT PCR on mRNA extracted from a.) primary human chondrocytes cultured in monolayer or alginate beads b.) the C20 human chondrocyte cell line and c.) samples of cartilage obtained at joint replacement surgery. We also undertook immunohistochemistry on sections of human and mouse joint tissues. We found that chondrocytes express a range of P2 receptors in particular P2Y2. P2X7 receptor was found to have a focal distribution. Cultured chondrocytes were observed to release ATP constitutively and the release was stimulated by fluid shear force. When we investigated the functional responses of chondrocytes to purinergic stimulation, we found that low concentrations of extracellular ATP have a trophic effect on cell growth and survival. However when extracellular ATP was elevated to concentrations above 10−4 M, there was a massive ATP-induced release of ATP from the cells into conditioned medium. Initially we suspected that this release was mediated via P2X7 receptors but the effect could not be fully replicated by BzATP. The release of ATP was followed by acidification of the culture medium through release of metabolic acid. These results indicated that P2 signalling is likely to play a significant role in regulating cartilage growth, maintenance and mineralisation.
Adenosine in the musculoskeletal system
Bronwen A. J. Evans
Institute of Molecular and Experimental Medicine, School of Medicine, Cardiff University, Cardiff, UK
There has been increased interest recently in the role of purinergic signalling in musculoskeletal tissue physiology and pathophysiology, with most of the published work on the role of purinergic signalling pathways in bone. Whereas the role of ATP in bone has been extensively studied and revealed, comparatively little is known about the role of adenosine in this tissue.
Osteocytes are the most abundant cells in bone and are dispersed throughout the bone mineralised matrix. They form a communication network via dendritic processes which generally radiate towards the bone surface and the blood supply. Evidence is accumulating that these cells are the mechano-sensitive cells in bone and that they thus respond to mechanical loading.
This presentation will provide an overview of published work on the role of adenosine in musculoskeletal tissues. Our recent unpublished work on the involvement of adenosine signalling pathways in the response of osteocytes to mechanical loading will also be presented. To undertake these studies we have firstly developed a novel method of maintaining osteocytes in 3-dimensional, collagen gel, in vitro cultures which mimic their natural environment. Secondly, we have developed methods of applying appropriate mechanical load to these cultures, and used these novel techniques to study the involvement of adenosine signalling pathways in the osteocyte’s response to loading.
The work presented will highlight the complexities of the role of adenosine in musculoskeletal tissues. Hopefully, future work will lead to a better understanding of the details of biology and signalling pathways involved.
The role of P2X7R and DMT-1 in cellular uptake of metal ions by human osteoblasts and osteoclasts
Karan M. Shah, J. Mark Wilkinson and Alison Gartland
The Mellanby Centre for Bone Research, The University of Sheffield, Sheffield, UK
Bone related adverse events including femoral neck fracture, failure of osseointegration, femoral neck narrowing and pain occur more frequently following after metal-on-metal hip resurfacing (MOMHR) versus conventional total hip arthroplasty (THA) due to elevated levels of local and systemic concentrations of cobalt (Co) and chromium (Cr). We have previously shown that Co and Cr ions (Co2+, Cr3+ and Cr6+) impair osteoblast and osteoclast survival and function at clinically relevant concentrations [1]. In a subsequent study, we confirmed the entry of Co and Cr ions in these cells and described their intracellular distribution and speciation [2]. However, the mechanism of cellular entry of these ions remains unclear although roles for several putative transporters and receptors have been implicated. These include the divalent metal transporter-1 (DMT-1), a widely expressed transmembrane protein that is responsible for the transport of ions such as Fe2+, Ni2+ and Co2+, and P2X7R an ATP-gated ion channel capable of forming non-selective pores with sustained stimulation. In this study we investigated the role of DMT-1 and P2X7R in real-time uptake of Co2+ at different clinically relevant concentrations.
Human osteoblast-like (SaOS-2) and primary human osteoclasts cells were loaded with 0.25 μM Calcein-AM, a fluorescent compound which is quenched in presence of Co2+. The cells were treated with Co2+(0, 5, 50, 500, 5,000 and 50,000 μg/L) with or without DMT-1 specific antagonist (NSC306711) and P2X7R specific antagonist (A740003) and imaged using Leica AF6000 Time-Lapse imaging system for 1 h The mean fluorescence of cells was measured at each time-point using ImageJ, with 10–15 cells being measured for each condition. The data from three experimental repeats was analysed with One-way ANOVA and Students t-test using Prism®5, GraphPad Software, Inc. San Diego, USA.
A significant change in fluorescence was observed with increasing concentrations of Co2+ for both osteoblasts and osteoclasts suggesting intracellular entry. P2X7R antagonist significantly decreased the cellular uptake of Co2+ in osteoblasts at concentrations of 500 μg/L (p < 0.0001) and greater, equivalent to those observed in patient hip aspirate. There was no significant change in Co2+ related fluorescence in osteoclasts treated with P2X7R antagonists. DMT-1 antagonism did not significantly change the fluorescence in neither osteoblasts nor osteoclasts.
This study highlights different mechanisms of cellular entry in bone cells with P2X7R having a role in osteoblasts but not in osteoclasts. The study offers a novel approach to investigating metal toxicity in periprosthetic tissues by visualizing Co2+ uptake in real-time and establishes the role of two implicated mechanisms. The results also highlight an osteoblast specific therapeutic target that can alter the balance of bone-remodelling in the periprosthetic environment.
References
1. Andrews RL et al (2011) Bone 49:717–723
2. Shah KM et al (2012) Osteoporosis International 23:S592
Fri 3 A: Structures and functions of ectonucleotidases
Small molecule inhibitors of Ca-activated and NTPDase classes of nucleotidases
Terence L. Kirley1,*, William L. Seibel2 and Matthew D. Wortman3
1Department of Pharmacology and Cell Biophysics, University of Cincinnati 231 Albert Sabin Way, Cincinnati, Ohio, USA;2Division of Oncology, Cincinnati Children’s Hospital Medical Center 3333 Burnet Ave, Cincinnati, OH 45229;3Department of Internal Medicine, Division of Endocrinology, University of Cincinnati 231 Albert Sabin Way, Cincinnati, Ohio, USA
Soluble Ca-Activated Nucleotidase (SCAN, aka CANT1) was identified as one of 46 “druggable” cancer protein targets in a recent analysis.1 In order to identify SCAN inhibitors, a virtual representation of the University of Cincinnati’s compound library comprised of more than 350,000 small molecules was screened in silico for ligand binding within the vicinity of the substrate binding site observed in crystal structures of SCAN. The top 2,808 “hits” were examined and ranked on the basis of various calculated energetics of binding. From that group, the top 467 compounds were obtained and each compound (20 μM) was tested in vitro for inhibition of SCAN activity using GDP (50 μM) as substrate. In silico similarity searches using the eight best in vitro inhibitors led to the in vitro screening of 88 additional related compounds. Dose–response measurements of the top 10 inhibitors identified two low micromolar IC50 SCAN inhibitors.
In other studies designed to identify Nucleoside TriPhosphate Diphosphohydrolase (NTPDase) nucleotidase inhibitors, the same 467 compounds (20 μM) were screened in vitro for NTPDase3 ATPase (100 μM) inhibition. The 34 most potent inhibitory compounds were re-screened at 2 μM for both NTPDase3 and NTPDase1 inhibition. The nine best inhibitors were characterized by dose–response curves, and evaluated for nucleotidase (GDPase, 50 μM) inhibition selectivity by measurements utilizing NTPDase5, NTPDase6, and SCAN. Several compounds were found to be very selective for NTPDase1/3 inhibition. Both classes of inhibitors identified in this study (SCAN and NTPDase1/3) may be useful for developing novel modulators of cancer and purinergic signaling.
Reference
1. Patel MN, Halling-Brown MD, Tym JE, Workman P, Al Lazikani B (2013) Nature Reviews/Drug Discovery 12:35–50
Targeting Autotaxin: structure, function, inhibitors, interactions
Willem-Jan Keune1, Jens Hausmann1, Wouter Moolenaar2 and Anastassis Perrakis1,*
1Department of Biochemistry, Netherlands Cancer Institute, Amsterdam, The Netherlands; 2Department of Cell Biology, Netherlands Cancer Institute, Amsterdam, The Netherlands
Autotaxin is a multi-domain protein of the ENPP family of extracellular phosphodiesterases, which mediate signalling from the outside to the inside of the cell. Autotaxin acts as a phospholipase D, mainly hydrolysing lysophosphatidylcholine (LPC), to produce the signalling phospholipid lysophosphatidic acid (LPA) that acts through a family of seven GPCRs to elicit a variety of signals [1]. Autotaxin and LPA are linked with angiogenesis, fibrosis, neuropathic pain, inflammatory disorders and cancer.
I will present the crystal structure of Autotaxin [2], which allowed us to understand how, unlike other ENPP family members, it accommodates lipids in a deep hydrophobic pocket. This pocket is entirely absent in the ENPP1 structure [3] we also determined. High throughput screening for Autotaxin chemical inhibitors in vitro allowed us to develop a new potent (sub-nanomolar range) inhibitor of Autotaxin, capable of reducing LPA levels in mice. The structure of the bound inhibitor served as the first model for further pharmacological developments [4], and new generation inhibitors are now in pre-clinical testing.
We have also discovered that Autotaxin bind to cell surface integrins [2] and heparin [5], in an isoform dependent manner. That data raises many questions about the importance of localized LPA production for signalling. Next generation RNA sequencing data are now providing important clues for the importance of localization in Autotaxin signalling, and might lead to a next generation of inhibitors targeting Autotaxin interactions.
Fig. 1 A graphical overview of Autotaxin-mediated signalling
References
1. Moolenaar WH, Perrakis A (2011) Nat Rev Mol Cell Biol 12(10):674–679
2. Hausmann J et al (2011) Nat Struct Mol Biol 18(2): 198–204
3. Jansen S et al (2012) Structure 20(11):1948–1959
4. Albers HM et al (2011) J Med Chem 54:4619–4626
5. Houben AJ et al (2013) J Biol Chem 288(1):510–519
Alkaline phosphatase as a physiological regulator of the phosphate/pyrophosphate and ATP/adenosine ratios
José Luis Millán
Sanford Children’s Heath Research Center, Sanford-Burnham Medical Research Institute, La Jolla, CA 92037
Alkaline phosphatases are extracellular hydrolases with wide substrate specificity at high pH in vitro. However, their functions in vivo are quite specific. Much of what we know comes from studies of mice and patients suffering from hypophosphatasia (HPP), caused by mutations in the gene encoding the tissue-nonspecific alkaline phosphatase (TNAP) isozyme. TNAP has a fundamental role in skeletal and dental tissues where it regulates the Pi/PPi ratio in the extracellular matrix allowing for propagation of hydroxyapatite deposition in mineralizing tissues, acting both as a potent pyrophosphatase and an ATPase. NPP1 can partially compensate for the lack of TNAP in both of those functions. Initiation of matrix vesicle mineralization is done by an apparent dual mechanism: Pi production via PHOSPHO1 action and phosphate transporter-mediated incorporation of Pi produced peri-vesicularly by the actions of TNAP and/or NPP1 [1]. Other phenotypic abnormalities in HPP mice and patients, such as hyperalgia, allodynia, inflammation, muscle weakness may be explained at least in part by the ability of TNAP to release Pi from ATP, ADP, and AMP ultimately leading to adenosine production. Thus, TNAP action is important for the establishment of the ATP/adenosine ratio that affects purinergic signaling in systems such as in pain receptors in the spinal cord ganglia [2] or circulating immune cells [3], where adenosine levels play an anti-inflammatory role. These mechanisms need to be explored in detail in HPP subjects.
Fig. Mechanisms of action of tissue-nonspecific alkaline phosphatase (TNAP). Left panel: its role in regulating the Pi/PPi ratio in skeletal tissues. Right panel: Its role in regulating the ATP/adenosine ratio in noniception and signaling of inflammatory cells in blood and tissues
References
1. Millán JL (2013) Calcif Tissue Int 93:299–306
2. Street SE et al (2013) J Neurosci 33:11314–11322
3. Pettengill M et al (2013) J Biol Chem 288:27315–27326
Structural insight into NTPDase function of parasitic microbes
Matthias Zebisch1, Ulrike Krug2, Michel Krauss3 and Norbert Sträter3
1,3Division of Structural Biology, University of Oxford, Roosevelt Drive, Oxford OX3 7BN, England;2,3University of Leipzig, Härtelstr. 16-18, 04107 Leipzig, Germany;3Institute of Bioanalytical Chemistry, Center for Biotechnology and Biomedicine, University of Leipzig, Deutscher Platz 5, 04103 Leipzig, Germany
In vertebrates, surface-expressed membrane-bound ecto-nucleoside triphosphate diphosphohydrolases (NTPDases) are responsible for signal conversion and termination in purinergic signalling by extracellular nucleotides [1].
NTPDases have also been found to be expressed by numerous human parasites including the protozoan Toxoplasma gondii (Tg) and the bacterium Legionella pneumophila (Lp). Both parasites multiply intracellularly and the expression of NTPDases has been associated with their virulence [2, 3]. In contrast to mammalian NTPDases, TgNTPDases and LpNTPDases are soluble proteins secreted into the parasitophorous resp. replicative vacuole.
We solved the structure of the LpNTPDase1 in multiple conformational states and in complex with a wide range of nucleotide ligands including substrate analogs, transition state mimics and products. This allowed us to follow the enzyme closely along the reaction pathway and for example to describe a substrate-induced 17° domain motion with high accuracy [4, 5].
The protozoan TgNTPDases are highly enriched in the dense granules within the vacuolar space as an inactive—dormant—protein [6]. In vitro activation can be achieved by treatment with thiols to reduce a regulatory disulfide bridge. Our structures of TgNTPDase1 and -3 revealed for the first time their rectangular, tetrameric architecture. We could identify the regulatory disulfide bridge to reside in a loop between the two structural domains of each monomer. Comparison of the dormant state to the structure of an engineered permanently active mutant allowed us to describe the structural rearrangements following reduction of the regulatory cysteine bridge in stunning detail. The analysis shows that the activation mechanism is strictly dependent on the tetrameric architecture [7-9].
References
1. Zimmermann H, Zebisch M, Sträter N (2012) Cellular function and molecular structure of ecto-nucleotidases. Purinergic Signal 8:437–502
2. Sansom FM, Newton HJ, Crikis S, Cianciotto NP, Cowan PJ, d’Apice AJ, Hartland EL (2007) A bacterial ecto-triphosphate diphosphohydrolase similar to human CD39 is essential for intracellular multiplication of Legionella pneumophila. Cell Microbiol 9:1922–1935
3. Asai T, Miura S, Sibley LD, Okabayashi H, Takeuchi T (1995) Biochemical and molecular characterization of nucleoside triphosphate hydrolase isozymes from the parasitic protozoan Toxoplasma gondii. J Biol Chem 270:11391–11397
4. Zebisch M, Krauss M, Schäfer P, Lauble P, Sträter N (2013) Crystallographic snapshots along the reaction pathway of nucleoside triphosphate diphosphohydrolases. Structure 21:1460–1475
5. Zebisch M, Schäfer P, Lauble P, Sträter N (2013) New crystal forms of NTPDase1 from the bacterium Legionella pneumophila. Acta Crystallographica. Section. F. 69:257–262
6. Sibley LD, Niesman IR, Asai T, Takeuchi T (1994) Toxoplasma gondii: secretion of a potent nucleoside triphosphate hydrolase into the parasitophorous vacuole. Exp Parasitol 79:301–311
7. Krug U, Totzauer R, Sträter N (2013) The crystal structure of Toxoplasma gondii nucleoside triphosphate diphosphohydrolase 1 represents a conformational intermediate in the reductive activation mechanism of the tetrameric enzyme. Proteins 81(7):1271-1276
8. Krug U, Totzauer R, Zebisch M, Sträter N (2013) The ATP/ADP substrate specificity switch between Toxoplasma gondii NTPDase1 and NTPDase3 is caused by an altered mode of binding of the substrate base. Chembiochem 14:2292–2300
9. Krug U, Zebisch M, Krauss M, Sträter N (2012) Structural insight into activation mechanism of Toxoplasma gondii nucleoside triphosphate diphosphohydrolases by disulfide reduction. J Biol Chem 287:3051–3066
Functions of NPP7 in the gut and its implications in colonic tumorigenesis
Rui-Dong Duan
Gastroenterology and Nutrition Lab, Institution of Clinical Sciences, University of Lund, Lund Sweden
NPP7 is the newest member in NPP family. Due to the structural modifications, NPP7 has no activity against nucleotides but cleaves phosphocholine from several phospholipids including sphingomyelin (SM), platelet activating factor (PAF), and lysophosphatidylcholine. Previous studies showed that NPP7 may have anticancer effects by generating ceramide from SM to inhibit cell proliferation, inactivating PAF to suppress PAF-induced inflammation, and by competing with NPP2 to reduce the formation of lysophosphatidic acid. NPP7 activity progressively decreases in patients with chronic colitis and colon cancer. Inactive forms of NPP7 are also found in colon and liver cancer cells. We recently generated NPP7 knockout (KO) mice and found that hydrolyses of both endogenous and exogenous SM were decreased by 75–90 %, resulting in reduced ceramide formation and cholesterol absorption in the gut of the KO mice. Spontaneous tumors were not found but signs of hypertrophy were identified. When the mice were treated with carcinogen azoxymethane plus dextran sulfate to induce tumor, the tumor incidence, tumor number per mouse, and tumor size in the colon were all markedly increased compared to wild type mice. While all tumors in wild type mice were adenomas, 32 % of tumors in the KO mice were adenocarcinomas. The enhanced susceptibility of colonic tumorigenesis was associated with decreased ceramide, increased PAF, increased sphingosine-1-phosphate and increased expression and nuclear transformation of beta-catenin. Our studies indicate that NPP7 is a physiological factor in the gut to hydrolysis SM, regulate cholesterol absorption and protect the mucosa from carcinogenesis induced by carcinogens and inflammation.
Fri 3 B: Purine signaling in pain
ATP in peripheral sensory ganglia
Tanja Bele and Elsa Fabbretti*
University of Nova Gorica, Center for biomedical sciences and engineering, Nova Gorica, Slovenia
ATP modulates neuronal function, and, in particular, neuronal and non-neuronal cells crosstalk, neuronal sensitization and neuronal firing [1]. Among other subtypes, P2X3 receptors expressed on sensory neurons open to nanomolar concentrations of extracellular ATP, and are therefore important sensors of extracellular environment changes, occurring in neuropathic pain or injury. P2X3 receptor function is highly sensitive to soluble factors like neuropeptides and neurotrophins, and is controlled by transduction mechanisms, protein-protein interactions and discrete membrane compartmentalization. More recent findings have demonstrated that P2X3 receptors interact with several intracellular signalling elements and surface scaffolds, such as the Calcium/calmodulin-dependent serine protein kinase (CASK) in a state dependent fashion [2,3], conferring important consequences for neuronal plasticity and abnormal neuronal processing [4].
References
1. Fabbretti E (2013) ATP P2X3 receptors and neuronal sensitization. Front Cell Neurosci 7:236
2. Gnanasekaran A, Bele T, Hullugundi S, van den Maagdenberg Arn MJM, Nistri A, Fabbretti E (2013) Cav2.1 P/Q channels regulate CASK/P2X3 complex in trigeminal sensory neurons. Molecular Pain 9:62
3. Gnanasekaran A, Sundukova M, Hullugundi S, Birsa N, Bianchini G, Hsueh YP, Nistri A, Fabbretti E (2013) CASK is a new intracellular modulator of P2X3 receptors. J Neurochem 126(1):102–112
4. Volonté C, Burnstock G (2013) P2X3 receptor: a novel ‘CASKade’ of signaling? J Neurochem 126(1):1–3
Microglial purinoceptors and chronic pain
Makoto Tsuda*, Takahiro Masuda and Kazuhide Inoue
Kyushu University, Department of Molecular and System Pharmacology, Fukuoka, Japan
Neuropathic pain, a debilitating chronic pain condition, is a common consequence of damage to the nervous system that can be induced by cancer, diabetes mellitus, infection, autoimmune diseases, or traumatic injury. The underlying mechanisms remain unclear, and currently available treatments are frequently ineffective. Thus, effective treatment of neuropathic pain is a major clinical challenge. A growing body of evidence indicates that peripheral nerve injury (PNI) converts spinal microglia into reactive cells that are required for the development and maintenance of neuropathic pain. Results of our laboratory have demonstrated that following PNI, P2X4 receptors (P2X4Rs) are upregulated in spinal microglia and are necessary for the pathogenesis of neuropathic pain. Thus, these results indicate that the upregulation of P2X4Rs in microglia is an important process. We have further found that the transcription factor interferon regulatory factor 8 (IRF8) is expressed in microglia after PNI and that IRF8 activates the expression of a variety of genes associated with the activated processes of microglia, including P2X4R. Mice lacking IRF8 exhibited a reduction in PNI-induced pain hypersensitivity. More recently, we have identified IRF5 as a target of IRF8 and as being required for upregulation of P2X4R expression through its binding to the promoter region of the P2rx4 gene. These results indicate a crucial role of the IRF8-IRF5 transcriptional axis in shifting spinal microglia toward a P2X4R-expressing reactive state after PNI and provide a new mechanism for neuropathic pain.
P2X3 and P2X7 receptor-signaling and pain sensation
Christoph Ficker1, Katalin Rozmer1, Thomas Riedel1, Heike Franke1, Andó Rómeó2, Beáta Sperlágh2 and Peter Illes1,*
1Rudolf-Boehm Institute of Pharmacology and Toxicology, University of Leipzig, Leipzig, Germany;2Institute of Experimental Medicine, Hungarian Academy of Sciences, Budapest, Hungary
P2X3 receptors (R) are located at the terminals of primary afferent C fibers projecting to layer II (substantia gelatinosa; SG) or the superficical layer I of the spinal cord dorsal horn. They potentiate the action potential-induced release of glutamate from these terminals onto glutamatergic or glycinergic/GABAergig interneurons in Layer II or directly onto ascending output neurons in Layer I. P2X3Rs thereby enforce pain transmission. In addition, during neuropathic pain, astrocytic P2X7Rs and microglial P2X4/P2X7Rs lead to a release of inflammatory mediators causing hyperalgesia and allodynia. Whole-cell patch clamp recordings from SG spinal cord slices of rats documented that in a low Ca2+/no Mg2+ (low X2+) external medium ATP and dibenzoyl-ATP (Bz-ATP) caused inward current responses, much larger in amplitude than those recorded in a normal X2+-containing bath medium. The effect of Bz-ATP was antagonized by the selective P2X7R antagonist A-438079. Neuronal but not astrocytic Bz-ATP currents were strongly inhibited by a combination of the ionotropic glutamate receptor antagonists AP-5 and CNQX. The reactive oxygen species (ROS) H2O2 potentiated the effect of Bz-ATP at neurons but not at astrocytes. A Bz-ATP-dependent and A-438079-antagonizable ROS production in SG slices was proved by a microelectrode biosensor. Bz-ATP elicited current responses in visually identified astrocytes of transgenic Tg(GFAP/mRFP1) mice. Nevertheless immunohistochemical investigations showed the co-localization of P2X7-immunoractivity with microglial but not astrocytic or neuronal markers. It is concluded that SG astrocytes possess P2X7Rs; their activation leads to the release of glutamate, which via NMDA- and AMPAR stimulation induce cationic current in the neighbouring neurons. We assume that P2X7Rs have a very low density under resting conditions but become functionally up-regulated under pathological conditions.
Involvement of purines in acupuncture-induced analgesia
Yong Tang
Chengdu University of Traditional Chinese Medicine, 37 Shi-er Qiao Road, Chengdu, China
Acupuncture has been used in China from ancient times since more than 2,000 years ago. A variety of disorders can be treated effectively by inserting long, fine needles into specific acupuncture points (acupoints) on the skin of the patient’s body. Since acupuncture was proposed by National Institutes of Health (NIH) consensus in 1997 as a therapeutic intervention of complementary medicine, acupuncture efficacy has become more and more accepted in the Western world. Among acupuncture therapies, the acupuncture-induced analgesic effect has been used widely to alleviate diverse types of pain, particularly chronic pain. To date, acupuncture analgesia has drawn the attention of many investigators and become an important research subject of international interest around the world. Numerous studies have also demonstrated that acupuncture analgesia has physiological, anatomical and neurochemical basis despite that there is still an ongoing debate about the mechanism by which acupuncture alleviates pain.
Since Professor Geoffrey Burnstock proposed that purinergic signalling, rather than a mystical subepidermal energy, may explain how acupuncture works in an article in Medical Hypotheses in 2009, the role of purinergic signalling in acupuncture research has gained much attention. So far, more scientists have got started to study the role of purinergic signalling in acupuncture-induced analgesia. In my talk, the work have been done by our group and other scientists will be summarized and where we are going and how we are going to get there in this amazing field will be described.
P2Y receptors in trigeminal pain
Giulia Magni1,2, Davide Merli1, Claudia Verderio3, Maria P. Abbracchio1 and Stefania Ceruti1,*
1Department of Pharmacological and Biomolecular Sciences—Università degli Studi di Milano—via Balzaretti, 9—20133 Milan—Italy;2Department of Drug Discovery and Development—Italian Institute of Technology (IIT)—via Morego, 30—16163 Genoa, Italy;3CNR Institute of Neuroscience—via Vanvitelli, 32—20129 Milan, Italy
Trigeminal (TG) pain often lacks a satisfactory pharmacological control. Innovative targets to be exploited for the development of more effective analgesics could emerge from a better understanding of the molecular cross-talk between TG neurons and surrounding satellite glial cells (SGCs). We have previously demonstrated that G protein-coupled P2Y receptors (P2YR) expressed by TG SGCs are upregulated by pro-algogenic molecules in vitro, through the release of neuronal mediators [1,2]. We have now identified the specific P2YR subtypes involved (i.e., the ADP-sensitive P2Y1R and the UTP-responsive P2Y2R subtypes), and demonstrated the contribution of prostaglandins to their upregulation. Next, we have translated our data to an in vivo model of TG pain (namely, the injection of Complete Freund’s adjuvant in the temporomandibular joint of rats) [3], and demonstrated activation of SGCs, and upregulation of P2Y1R and P2Y2R expression in the ipsi-lateral TG. To unequivocally link P2YRs to the development of facial allodynia, we treated animals with PPADS (non-selective P2XR/P2YR antagonist), MRS2179 (selective P2Y1 antagonist), AR-C118925 (selective P2Y2 antagonist), or the anti-inflammatory drug acetylsalicylic acid (ASA). The AR-C118925 compound completely inhibited SGCs activation, exerted a potent anti-allodynic effect that lasted over time, and was still effective when its administration started 6 days post induction of allodynia, i.e. under sub-chronic pain conditions. Conversely, MRS2179 had no effect on facial allodynia. Similarly to ASA, PPADS was only partially effective, and completely lost its activity under sub-chronic conditions. Taken together, our results highlight the P2Y2R subtype as a new potential “druggable” target for the successful management of TG-related pain.
References
1. Ceruti S, Fumagalli M, Villa G, Verderio C, Abbracchio MP (2008) Cell Calcium 43:576–590
2. Ceruti S, Villa G, Fumagalli M, Colombo L, Magni G, Zanardelli M, Fabbretti E, Verderio C, van den Maagdenberg AMJM, Nistri A, Abbracchio MP (2011) J Neurosci 31:3638–3649
3. Villa G, Ceruti S, Zanardelli M, Magni G, Jasmin L, Ohara PT, Abbracchio MP (2010) Mol Pain 6:89
Fri 3 C: Intracellular purine receptors
Intracellular P2X receptors as calcium release channels
Samuel J. Fountain
School of Biological Sciences, University of East Anglia, Norwich Research Park, UK
P2X receptors are calcium-permeable ion channels activated by ATP. Their role as cell surface receptors for ATP released physiologically by mammalian cells is well established. However, some P2X receptors reside on the membranes of organelles such as lysosomes [1]. It is not clear whether intracellular P2X receptors are functional or by what mechanism they could be activated. We identified weakly related but functional P2X receptors in the amoeba Dictyostelium discoideum [2]. P2X receptors of Dictyostelium are exclusively intracellular and located on the contractile vacuole (CV), an acidic calcium store required for osmoregulation [2,3]. Knockout of P2XA receptor in Dictyostelium produces a hypotonic phenotype suggesting an involvement in CV function [2,4]. Within the vacuole, P2X receptors are orientated with the ectodomain (ATP binding domain) facing towards the lumen [3]. In biochemical assays using highly purified and intact CVs, we demonstrated a mechanism, of unknown molecular basis, capable of translocating ATP and accumulating it within the CV lumen [3]. ATP accumulation triggers release of stored calcium. ATP translocation, CV calcium release and inhibition of osmoregulation share common pharmacology suggesting the processes are linked. Furthermore, sequential knockout of P2X receptors reduced the magnitude of ATP-evoked calcium release from purified CVs, with the response ablated in cells lacking all P2X receptors (P2XA – P2XE) [3,4]. These data reveal a role for intracellular P2X receptors as calcium release channels akin to other calcium release channels activated by intracellular nucleotides (e.g. NAADP, cyclic ADP-ribose). Further work is required to determine if intracellular P2X receptors in mammalian cells (e.g. P2X4) can function similarly.
References
1. Qureshi OS, Paramasivam A, Yu JC, Murrell-Lagnado RD (2007) J Cell Sci 120:3838–3849
2. Fountain SJ, Parkinson K, Young MT, Cao L, Thompson CRL, North RA (2007) Nature 448:200–203
3. Sivaramakrishnan V, Fountain SJ (2012) J Biol Chem 287:28315–28326
4. Sivaramakrishnan V, Fountain SJ (2013) Channels 7:43–46
Vesicular P2X4in lung epithelia
Manfred Frick
Institute of General Physiology, Ulm, Germany
Two fundamental mechanisms within epithelia of the airways and alveoli are essential for lung function—regulated fluid transport and secretion of surfactants and mucus. Several P2X receptors are expressed within lung epithelia and have been implicated in regulation of exocrine secretion and transepithelial fluid transport. Yet, cell-type specific expression and (sub)cellular localisation of P2X receptor isoforms is mainly elusive. We identified specific, intracellular expression of P2X4 receptors in alveolar type II epithelial (ATII) cells [1]. P2X4 receptors are located on lamellar bodies (LBs), large lysosome-related organelles storing pulmonary surfactant. Upon exocytosis of LBs these vesicular P2X4 receptors are selectively incorporated into the apical plasma membrane. Subsequent activation of newly inserted P2X receptors results in a local “fusion-activated cation entry” (FACE) at the site of vesicle fusion. This leads to a localised elevation of the cytoplasmic Ca2+ concentration around the fused vesicle promoting Ca2+-dependent fusion pore dilation, which in turn facilitates surfactant secretion. FACE also results in transepithelial ion transport across ATII cells driving fluid resorption from lung alveoli. This causes temporary thinning of the alveolar hypophase that facilitates insertion of newly secreted surfactant into the air-liquid interphase. Together our data reveal that selective translocation and activation of “vesicular” P2X4 in ATII cells is essential for surfactant release and “activation” of surfactant [1,2]. In addition to this role for vesicular P2X4 receptors in ATII cells we have new evidence that vesicular P2X4 receptors are also expressed in secretory cells of the airways and play a role in maintaining airway homeostasis.
References
1. Miklavc P, Mair N, Wittekindt OH, Haller T, Dietl P, Felder E, Timmler M, Frick M (2011) PNAS 108(35):14503–14508
2. Thompson KE, Korbmacher JP, Hecht E, Hobi N, Wittekindt OH, Dietl P, Kranz C, Frick M (2013) FASEB J 27:1772–1783
Calcium dependent regulation of Rab activation and vesicle fusion by an intracellular P2X ion channel
Nicole Gruenheit1, Katie Parkinson1, Abigail E. Baines1, Thomas Keller1, Laricia Bragg2, R. Alan North1,2 and Christopher R.L. Thompson1,*
1Faculty of Life Sciences and2Faculty of Medical and Human Sciences, University of Manchester, Michael Smith Building, Oxford Road, Manchester M13 9PT, UK
Rab GTPases play key roles in the delivery, docking and fusion of different intracellular vesicles. However, the mechanism by which spatial and temporal regulation of Rab GTPase activity is controlled is poorly understood. Here we describe a novel mechanism by which localized calcium release through a vesicular ion channel controls Rab GTPase activity. We show that activation of P2XA, an intracellular ion channel localized to the Dictyostelium discoideum contractile vacuole system, results in calcium efflux required for downregulation of Rab11a activity and efficient vacuole fusion. Furthermore, we show that vacuole fusion and Rab11a downregulation require the activity of CnrF, a novel EF hand containing Rab GAP protein found in a complex with Rab11a and P2XA. The Rab GAP activity of CnrF is specific to Rab11a and greatly enhanced by the presence of calcium and the EF-hand domain in vivo and in vitro. These findings suggest that P2XA activation results in vacuolar calcium release, which triggers activation of CnrF Rab GAP activity and subsequent downregulation of Rab11a to allow vacuole fusion. Given that P2X channels and this novel class of calcium dependent Rab GAPs are widely conserved, this work provides fundamental insights into Rab GTPase regulation in vesicular trafficking.
References
1. Parkinson K, Baines AE, Keller T, Gruenheit N, Bragg L, North RA, Thompson CR (2014) Calcium-dependent regulation of Rab activation and vesicle fusion by an intracellular P2X ion channel. Nat Cell Biol 16(1):87–98
2. Baines A, Parkinson K, Sim JA, Bragg L, Thompson CR, North RA (2013) Functional properties of five Dictyostelium discoideum P2X receptors. J Biol Chem 288(29):20992–21000
Functional properties of the lysosome-targeted P2X4 receptor
Ruth D. Murrell-Lagnado
Department of Pharmacology, University of Cambridge, Tennis Court Road, Cambridge CB2 1PD, UK
P2X4 receptors are widely expressed and function as ATP-gated cations channels at the plasma membrane. They are however predominantly targeted to endolysosomes and the extent to which they traffic between these compartments and the cell surface varies depending upon the cell type and environmental factors. In macrophages P2X4 receptors stably reside within the lysosomes whereas in activated microglia, they rapidly and constitutively cycle via a dynamin and clathrin-dependent mechanism. Inhibiting this cycling alters the functional properties of receptors at the cell surface; they no longer undergo pore dilation and show slower recovery from desensitization. Within lysosomes, P2X4 receptors resist degradation because of N-linked glycosylation and thus the turnover of the receptors is extremely slow compared to other receptors. Stimulating lysosome fusion up-regulates the expression of functional P2X4 receptors at the plasma membrane, showing that receptors within the lysosomes retain their functional integrity. Endolysosomes contain several different ion channels that play important roles in pH regulation and in traffic through the late endocytic pathway. Homotypic and heterotypic fusion events require Ca2+ release from the lumen of endolysosomes. P2X4 receptors are highly pH sensitive and plasma membrane receptors are inhibited at the acidic pH that occurs within the lumen of the lysosome. This inhibition can, however, be overcome by increasing the concentration of ATP to millimolar levels. We have used wild type and mutant P2X4 receptors to investigate their properties within the endocytic pathway.
Cardioprotective mechanisms of intracellular adenosine receptors
Thomas Krieg
Department of Medicine, University of Cambridge, UK
Ischemic preconditioning (IPC) triggers powerful endogenous signalling that ultimately makes the heart resistant to infarction from ischaemia. A key step in preconditioning’s signal transduction pathway is activation of adenosine A2B receptors (A2BAR) early in reperfusion. Analogously the highly selective A2B agonist BAY60-6583 given at reperfusion reduces infarct size following prolonged myocardial ischaemia. A2BAR communicate through both Gαs and Gαq, which in turn lead to increased cytosolic cAMP, inositol trisphosphate and Ca2+. There is agreement that coronary endothelial cells contain A2BAR, but there is discrepant information about the expression of A2BAR on cardiomyocytes, the cells that IPC must ultimately protect.
We were able to detect A2BAR mRNA transcripts using quantitative RT-PCR in individually selected cardiomyocytes uncontaminated with other cell types. Furthermore, western blot analysis of both heart biopsy and isolated cardiomyocyte lysate showed a strong A2BAR band. Since the A2BAR is a GPCR, we assumed that immunofluorescence would reveal plasma membrane staining. To our surprise, the A2BAR antibody did not bind to the cardiomyocyte sarcolemma as expected, but rather caused structures at intracellular sites to fluoresce, co-localizing with mitochondria (Fig. 1) [1]. There is growing evidence that protection afforded by pre- and postconditioning is related to kinase-dependent inhibition of mitochondrial transition pores upon reperfusion and that A2BAR control those kinases. It therefore seems quite conceivable that A2BAR localized on mitochondria could be involved in this signalling cascade.
Fig. 1 Isolated rat cardiomyocytes were stained with A2BAR antibody coupled to a fluorochrome (green). Additionally, nuclei, Z bands, mitochondria and Na+/K+-ATPase were detected using DAPI, α-actinin antibody, MitoTracker Red and Na+/K+-ATPase antibody, respectively
Reference
1. Grube et al (2011) Basic Res Cardiol 106:385–96
Fri 3 D: Nucleotide signaling in plants
Apyrases (NTPDases) and extracellular nucleotides regulate plant responses to biotic and abiotic stresses
Min Hui Lim, Jian Wu, Greg Clark and Stanley J. Roux*
Department of Molecular Biosciences, University of Texas, Austin, TX, USA
Plant cells release ATP and other nucleotides into their extracellular matrix when they are wounded, undergo mechanical stress, or are actively secreting. These extracellular nucleotides activate receptors and induce signaling changes similar to those induced in animal cells, including rapid increases in [Ca2+]cyt and production of reactive oxygen species and nitric oxide. Plants, like animals, use ecto-phosphatases to lower the concentration of extracellular ATP [eATP], and two apyrases (NTPDases) in the model plant Arabidopsis, APY1 and APY2, appear to have [eATP] control as one of their functions. The expression of these two apyrases is highest in cells that are actively releasing ATP, and is induced by wound stimuli that increase the [eATP]. When their expression is suppressed by an inducible RNAi construct, the [eATP] rises, cell growth is inhibited, and the gene expression changes that occur are characteristic of those that happen when plants experience biotic or abiotic stress. These changes include enhanced expression of genes encoding wall enzymes that lignify and stiffen cell walls. Mechanical stimuli and pathogen attack both can transiently increase [eATP] and induce signaling changes that increase plant pathogen resistance. Apyrase activity found in the saliva of herbivore insects, which can decrease [eATP], suppresses the expression of plant defense genes. These data are consistent with the hypothesis that eATP and apyrases play key roles in the signaling steps that link biotic and abiotic stresses to plants’ increased resistance to these stresses. Grants from NSF, NASA, and CottonGen, Inc. supported this research.
Identification of a plant receptor for extracellular ATP
Kiwamu Tanaka1, Jeongmin Choi1, Yangrong Cao1, Yan Liang1, Yue Qi2, Jing Qiu2 and Gary Stacey1,3,*
1Plant Sciences, University of Missouri, Columbia, MO, USA;2Department of Statistics, University of Missouri, Columbia, MO, USA;3Division of Biochemistry, University of Missouri, Columbia, MO, USA
ATP is a cellular energy source but also acts as a signaling molecule when secreted outside cells, where it is referred to as extracellular ATP. Extracellular ATP has been extensively studied in mammalian systems due to its essential roles in a broad range of physiology, including neurotransmission, muscle contraction, inflammation, cell growth and death. This signaling molecule is perceived by plasma membrane-localized P2 receptors. In plants, although a number of studies have demonstrated the involvement of extracellular ATP in plant growth, development and stress responses, there is no information as to how plants perceive ATP. The net result is that there remains some skepticism as to whether extracellular ATP is important in plants. We identified an ATP-insensitive Arabidopsis mutant, dorn1 (Does not Respond to Nucleotides 1), defective in a lectin receptor kinase [1]. The DORN1 protein directly binds ATP with high affinity, and is required for ATP-induced calcium response, MAPK activation, and gene expression. Overexpression of the DORN1 gene increased the plant response to physical wounding. We propose that DORN1 is indispensable for perception of extracellular ATP and that ATP acts as a damage associated molecular pattern (DAMP) during the plant wound response.
Reference
1. Choi J, Tanaka K, Cao Y, Xi Y, Qiu J, Liang Y, Sang YL, Stacey G (2014) Science 343:290–294
Evidence for a Golgi function of NTPDases in plants
Iris Steinebrunner
Biology Department, Technische Universität Dresden, 01062 Dresden, Germany
The two highly similar NTPDases AtAPY1 and AtAPY2 (87 % amino acid identity) from the model plant Arabidopsis thaliana each contain five apyrase-conserved regions and one transmembrane (TM) domain near the N-terminus. Genetic studies revealed that knocking out one of the two NTPDase genes had no obvious phenotypic effect, but disrupting both caused fatal developmental and growth defects [1]. External application of antibodies against AtAPY1 and AtAPY2 inhibited extracellular ATP hydrolysis which suppressed growth [2]. This pointed to a role of the enzymes in purinergic signaling by breaking down a growth inhibitory ATP signal. Surprisingly, confocal and electron microscopy, as well as Golgi proteome analysis, localized AtAPY1 and AtAPY2 in the Golgi [3,4,5]. We expressed AtAPY1 in human embryonic kidney (HEK) cells and purified it for biochemical analyses. The nucleotides UDP, GDP and IDP were identified as substrates of AtAPY1, and ATP was not hydrolyzed. The substrate specificity and the predicted membrane topography (the TM domain anchoring the enzyme in the membrane and the catalytic domain protruding into the Golgi lumen) are reminiscent of the mammalian NTPDase6. Since NTPDase6 additionally exists in a soluble form, AtAPY1 was also expressed without the TM domain, which did not change the substrate specificity.
We propose that AtAPY1 serves as a regulator of glycosylation reactions in the Golgi. The transfer reactions of glycosyl groups produce NDPs as by-products which inhibit glycosyltransferases in a negative feedback mechanism [6]. Therefore, removal of the NDPs by AtAPY1 would promote glycosylation reactions and allow the Golgi to provide the building blocks for growth.
References
1. Wolf C, Hennig M, Romanovicz D, Steinebrunner I (2007) Plant Mol Biol 64:657–672
2. Wu J, Steinebrunner I, Sun Y, Butterfield T, Torres J, Arnold D, Gonzalez A, Jacob F, Reichler S, Roux SJ (2007) Plant Physiol 144:961–975
3. Schiller M, Massalski C, Kurth T, Steinebrunner I (2012) BMC Plant Biol 12:123
4. Chiu TY, Christiansen K, Moreno I, Lao J, Loqué D, Orellana A, Heazlewood JL, Clark G, Roux SJ (2012) Plant Cell Physiol 53:1913–1925
5. Parsons HT, Christiansen K, Knierim B, Carroll A, Ito J, Batth TS, Smith-Moritz AM, Morrison S, McInerney P, Hadi MZ et al (2012) Plant Physiol 159:12–26
6. Hirschberg CB, Robbins PW, Abeijon C (1998) Annu Rev Biochem 67:49–69
Calcium transport changes induced by extracellular nucleotides in plants
Julia M. Davies
Department of Plant Sciences, University of Cambridge, Cambridge CB2 3EA, UK
Although higher plant genomes apparently lack equivalents of purinoceptors, both extracellular ATP and ADP can elicit a transient increase in plant cytosolic free calcium as a possible second messenger. In roots of the model plant Arabidopsis thaliana, the increase in calcium can be inhibited by cation channel blockers, indicating calcium influx across the plasma membrane. This is consistent with the depolarization of plasma membrane voltage caused by exogenous ATP or ADP. The calcium increase can still be effected in root epidermal protoplasts, indicating that the cell wall can be redundant. Applying patch clamp electrophysiology to such protoplasts has revealed a plasma membrane calcium- and potassium-permeable conductance that activates rapidly in response to extracellular ADP [1]. This manifests as net calcium influx and potassium efflux in intact roots. In longer term patch clamp recordings, calcium currents evoked by extracellular ATP are abolished by reducing conditions at the extracellular face of the plasma membrane or loss of NADPH oxidase function [2]. In root epidermal plasma membrane, oxidation of the extracellular face activates a calcium influx that is dependent on annexin1 [3]. Accordingly, the extracellular ATP-induced current was abolished in the annexin1 knockout mutant. The ADP-dependent current was unaffected by loss of annexin1, suggesting that discrete channel activation pathways are in operation.
References
1. Demidchik et al (2011) Plant Physiology 156:1375–1385
2. Demidchik et al (2009) Plant Journal 58:903–913
3. Laohavisit et al (2012) Plant Cell 24:1522–1533
Extracellular ATP signaling is mediated by hydrogen peroxide and cytosolic Ca2+in plants
Shaoliang Chen1,*, Jian Sun2 and Xuan Zhang1
1College of Biological Sciences and Technology (Box 162), Beijing Forestry University, Beijing 100083, PR China;2College of Life Science, Jiangsu Normal University, Xuzhou 221116, Jiangsu Province, PR China
Extracellular ATP (eATP) has been implicated in mediating plant growth, antioxidant defense, and programmed cell death [1]. However, it is largely unknown whether eATP might mediate salinity tolerance. We used confocal microscopy, a non-invasive vibrating ion-selective microelectrode, and quantitative real time PCR analysis to evaluate the physiological significance of eATP in the salt resistance of cell cultures derived from a salt-tolerant woody species, Populus euphratica. Application of NaCl (200 mM) shock induced a transient elevation in eATP. We investigated the effects of eATP by blocking P2 receptors with suramin and PPADS and applying an ATP trap system of hexokinase-glucose. We found that eATP regulated a wide range of cellular processes required for salt adaptation, including vacuolar Na+ compartmentation, Na+/H+ exchange across the plasma membrane (PM), K+ homeostasis, reactive oxygen species regulation, and salt-responsive expression of genes related to K+/Na+ homeostasis and PM repair. Furthermore, we found that the eATP signaling was mediated by H2O2 and cytosolic Ca2+ released in response to high salt in P. euphratica cells. We concluded that salt-induced eATP was sensed by purinoceptors in the PM, and this led to the induction of downstream signals, like H2O2 and cytosolic Ca2+, which are required for the up-regulation of genes linked to K+/Na+ homeostasis and PM repair. Consequently, the viability of P. euphratica cells was maintained during a prolonged period of salt stress.
Fig. 1 Schematic model shows proposed eATP signals that mediate the NaCl stress response in Populus euphratica cells
Reference
1. Sun J, Zhang C, Deng S, Lu C, Shen X, Zhou X, Zheng X, Hu Z, Chen S (2012) Plant, Cell & Environment 35:893–916
Abstracts—Symposium sessions
- Saturday -
Sat 1 A: Role of ectonucleotidases in nucleotide signaling
Regulation of P2 signaling by NTPDase1 coordinates vascular and non-vascular smooth muscle contraction: implication for male fertility
Jean Sévigny1,2,*, Julie Pelletier1, Catherine Vial3, Élise G. Lavoie1,2, Mireia Martín-Satué1,2,4, Filip Kukulski1,2 and Gilles Kauffenstein1,2,5
1Centre de recherche du CHU de Québec, Québec, QC, Canada;2Département de microbiologie-infectiologie et d’immunologie, Faculté de Médecine, Université Laval, Québec, QC, Canada;3Department of Cell Physiology and Pharmacology, University of Leicester, Leicester, UK;4Departament de Patologia i Terapèutica Experimental, Facultat de Medicina, Universitat de Barcelona, Spain;5CNRS UMR 6214, INSERM U1083, l’UNAM, CHU d’Angers, Angers France
Entpd1−/− mice, deficient for the ectonucleotidase nucleoside triphosphate diphosphohydrolase-1 (NTPDase1), produce smaller litters (27 % reduction) compared to wild type C57BL6 animals. This deficit is linked to reduced in vivo oocytes fertilization by Entpd1−/− males (61 ± 11 % vs. 88 ± 7 % for Entpd1+/+). Normal epididymal sperm count, spermatozoa morphology, capacitation and motility, associated to reduced ejaculated sperm number (2.4 ± 0.5 vs. 3.7 ± 0.4 million for Entpd1+/+) pointed on a vas deferens dysfunction. NTPDase1 was immunolocalized in the tunica muscularis of vas deferens. Its absence resulted in a major ATP hydrolysis deficiency in situ as well as in primary smooth muscle cell (SMC) cultures and homogenates. Entpd1−/− vas deferens displayed an exacerbated contraction to ATP but a diminished response to its non-hydrolysable analogue αβMeATP, suggesting an altered P2X1 receptor function with a propensity to desensitize. This functional alteration was accompanied with a dramatic decrease in P2X1 protein expression with no variation in mRNA levels. Accordingly, an optimal amount of nucleotidase activity (~1 IU/mL) was required to preserve P2X1 receptor activation by ATP in vitro.
As NTPDase1 is also expressed in SMC in general we questioned whether other Entpd1−/− SMC, namely from the bladder and the aorta would also contract differently. Interestingly in the later 2 tissues constriction was due to P2Y6 receptor that did not desensitize. Therefore, the absence of NTPDase1 resulted in a stronger constriction. In summary NTPDase1 is the major ectoATPase in SMC in general and it affects nucleotide dependent constriction by regulating the activation of different P2 receptors depending of the tissue.
Ectonucleotidase-bearing plasma microparticles as possible biomarkers with therapeutical implications in liver diseases
Moritz Schmelzle
Department of Surgery, University Hospital Leipzig, Germany; Translational Centre for Regenerative Medicine (TRM), University of Leipzig, Germany
Activation of cells due to physical or chemical stress and the response to pro-inflammatory cytokines, e.g. TNF-α and IL-1β, leads to membrane destabilization with distinct modifications of the plasma membrane. Inflammation and apoptosis lead to an externalization of phosphatidylserine and bleb formation, resulting in the release of submicron fragments, so called microparticles (MP).
MP can be isolated by ultracentrifugation and detected by flow cytometry, as they are characterized not only by cell-specific cytosol, but further by distinct surface antigens. CD39 is incorporated in plasma MP and has been shown to phosphohydrolyze extracellular ATP. Plasma MP exhibit CD39-dependent paracrine effects and were demonstrated to modulate endothelial inflammation.
CD39 is involved in the modulation of numerous acute and chronic liver diseases and CD39+ MP are differentially noted in mice and patients with liver injury. We tested ectonucleotidase bearing immune and stem cell MP as potential biomarkers in pharmacological acute liver failure, acute rejection after liver transplantation, in xeno-liver transplantation and in obese patients with fatty liver disease. Mechanistic studies in vitro indicate that MP might further represent important regulators of intercellular communication.
Our results suggest CD39+ MP subpopulations to have future potential to distinguish between mild and severe acute and chronic liver injury, respectively, and define patients at high risk for acute rejection after organ transplantation. Further research is needed to determine which MP subsets represent robust biomarkers of clinical significance and whether MP fluxes are secondary to pathophysiologic insults to the liver or might reflect compensatory responses.
TNAP controls the neuronal differentiation through regulating P2X receptors presents on axonal growth cone. Involvement on neurodegenerative disorder
Miguel Diaz-Hernandez1,*, Juan Ignacio Diaz-Hernandez1, Maria Diez-Zaera1, Alvaro Sebastian Serrano1, Laura De-Diego Garcia1, Carlos Martinez-Frailes1, Rosa Gomez-Villafuertes1, Jesus Avila2, Herbert Zimmermann3 and Maria Teresa Miras-Portugal1
1Department of Biochemistry and Molecular Biology, Veterinary Faculty, Complutense university of Madrid, 28040 Madrid, Spain;2Center of Molecular Biology “Severo Ochoa”, CSIC/UAM, Madrid Spain; 3Institute of Cell Biology and Neuroscience, Biocenter, J. W. Goethe-University, Frankfurt 60438, Germany
New evidences have been reported that point to the ecto-enzyme, tissue-nonspecific alkaline phosphatase (TNAP), as a key element at the origin of two opposite phenomena, neuronal differentiation and neuronal degeneration. During brain development, TNAP plays an essential role for establishing neuronal circuits. The pro-neuritic effect induced by TNAP, which results in axonal length increase, is due to its enzymatic hydrolysis of extracellular ATP at the surrounding area of the axonal growth cone. In this way, the activation of ionotropic P2X7 receptor is prevented and as a consequence there is no inhibition of axonal growth. The existence of a close functional interrelation between both purinergic elements is finally supported by the fact that both elements may control, in a reciprocal, way the expression level of the other [1].
On the opposite stage, recent solid evidences indicate that TNAP plays a key role in spreading the neurotoxicity effect induced by extracellular hyperphosphorylated tau protein, the main component of intracellular neurofibrillary tangles present in the brain of Alzheimer disease (AD) patients. TNAP exhibits a broad substrate specificity and in addition to nucleotides it is able to dephosphorylate extracellular proteins, such as the hyperphosphorylated tau protein once it is released to the extracellular medium upon neuronal death. Dephosphorylated tau protein behaves as an agonist of muscarinic M1 and M3 receptors, provoking a robust and sustained intracellular calcium increase that finally triggering neuronal death [2]. Besides, activation of muscarinic receptors by dephosphorylated tau increases the expression of TNAP, which could explain the increase in TNAP activity and protein levels detected in AD.
References
1. Diez-Zaera et al (2011) Mol Biol Cell 22:1014–1024
2. Diaz-Hernandez et al (2010) J Biol Chem 285:32539–32548
Interrelationship between NPP1 and P2Y2receptor: implications for ectopic mineralization
P. Mathieu
Gene knockout of NPP1 is associated with ectopic mineralization. However, NPP1 is overexpressed in mineralized atherosclerotic plaques and mineralized heart valves. Moreover, overexpression of NPP1 increases the mineralization of vascular and valve interstitial cells. The underlying mechanism behind this U-shape response has recently been identified. The well-known association between an invalidation of the NPP1 gene and ectopic mineralization is explained by a lack of pyrophosphate, a potent inhibitor of ectopic mineralization. On the other hand, when NPP1 is highly expressed it contributes to deplete the pericellular pool of nucleotides and thus decreases purinergic signalling considerably. Specifically, a decrease signalling through PI3K/Akt downstream of P2Y2 receptor promotes apoptosis-mediated mineralization when NPP1 is overexpressed. This finding is exemplified by the increase mineralization of valve interstitial cells following a knockdown of P2Y2 receptor. Hence, in the cardiovascular structures a delicate balance exists between the expression of NPP1 and purinergic signalling through P2Y2 receptor. When disrupted the survival signal mediated by nucleotides is lost and mediates ectopic vascular/valvular mineralization.
The role of CD39-CD73 axis in control of angiogenesis and tumor growth
Gennady G. Yegutkin
Medicity Research Laboratory, University of Turku, Turku, Finland
Extracellular ATP and other purines are important signaling molecules mediating diverse effects in virtually all organs and tissues. Most models of purinergic signaling depend on functional interactions between distinct processes, including (i) release of endogenous ATP; (ii) triggering of signaling events via a series of ligand-gated P2X and metabotropic P2Y receptors; (iii) ectoenzymatic inactivation of nucleotides; (iii) binding of generated adenosine to its own receptors; and finally, (iv) metabolism and re-uptake of adenosine and other nucleosides by the cells [1] (Fig. 1). The last decade has seen a great increase in publications concerning nucleoside triphosphate diphosphohydrolase-1 (NTPDase1, otherwise known as CD39) and ecto-5′-nucleotidase/CD73. These key nucleotidases have been extensively investigated with regard to their molecular structures and cellular functions, as well as implication in such (patho)physiological states as inflammation, host defense during microbial infection, tumor growth and metastasis [1–3]. Our recent findings provide evidence for the important role of CD39-CD73 axis in the maintenance of balanced equilibrium between pro-inflammatory ATP and its counteracting anti-inflammatory metabolite adenosine. Particularly, disordered nucleotide homeostasis was shown to enhance the permeability and sprouting of vascular endothelial cells both in vitro and in vivo, and in addition, contributes to the pathogenesis of sight-threatening forms of diabetic retinopathy and also affects the invasion and migration of breast and prostate cancer cells.
Fig. 1 Purinergic signalling and metabolic pathways on the cell surface
References
1. Yegutkin GG (2008) Biochim Biophys Acta 1783:673–694
2. Zimmermann H, Zebisch M, Sträter N (2012) Purin Signal 8:437–502
3. Antonioli L, Pacher P, Vizi ES, Hasko G (2013) Trends Mol Med 19:355–367
Sat 1 B: Purines and neuroinflammation
P2X receptors in neuron-glia crosstalk in pain
Michael W. Salter
Neurosciences & Mental Health Program, Hospital for Sick Children, and Department of Physiology, University of Toronto, Toronto, Ontario, Canada
Pain and discomfort serve a vital function as warning of tissue damage, injury or infection, which is particularly important for highly vulnerable structures such as the eye. Acute pain, which is protective, is a reflection of adaptive, physiological functioning of the normal nervous system. However, pain and discomfort may arise with minimal tissue damage and may persist after healing is complete. Such persistent pain is maladaptive, serving no known protective function, and is a reflection of dysfunction of a nervous system pathologically altered by neuroplasticity mechanisms. Pathological neuroplasticity may occur in either the peripheral nervous system or the central nervous system but the end result is sensitization of the nociceptive (‘pain’) system such that innocuous stimuli, such as gentle mechanical stimulation, cooling or warming, are perceived as uncomfortable or frankly painful. Maladaptive pain may also occur spontaneously, in the absence of overt stimulation. The underlying neuroplasticity mechanisms, which are increasingly well understood, involve neuron-neuron and neuron-immune/glia signaling. Signaling molecules such as the purinergic receptors, P2X4 and P2X7, brain-derived neurotrophic factor (BDNF) and the NMDA receptor subtype of glutamate receptor have been identified as playing critical roles in maladaptive pain neuroplasticity. Susceptibility to and severity of maladaptive pain is highly variable between individuals, and much of that variability is heritable. We, and others, have found that in several chronic pain conditions specific genetic variations, for example in P2X7 receptors, are linked to increased pain after injury or surgery.
Understanding the cells, proteins and genes mediating the pathological pain alterations is providing unanticipated new avenues to the diagnosis and management of chronic pain.
Acknowledgments: CIHR, HHMI, CRC Program, Krembil Foundation, ORF Research Excellence Program.
Transmitter receptors in microglia
Helmut Kettenmann
Max Delbrueck Center for Molecular Medicine Berlin
In the last years we have characterized a number of neurotransmitter receptors on microglial cells in cell culture and in acute brain slices. Using the patch clamp technique we have identified purinergic, GABAB, adrenergic, dopaminergic, and serotonergic receptors. Activation of these receptors triggers a change in membrane currents. Since many metabotropic receptors are linked to calcium signaling, we have transduced a calcium sensor protein via a retrovirus into microglia in vivo and recorded activity in freshly isolated slices. We recorded responses to ATP, endothelin-1, substance P, histamine and serotonin. We also found a variety of receptors linked to calcium signaling in freshly isolated microglial cells from adult tissue, namely receptors for endothelin, histamine, substance P, serotonin, galanin, somatostatin, angiotensin II, vasopressin, neurotensin, dopamine or nicotine. We also noted that microglia do not constitute a homogeneous cell population but vary with respect to their functional neurotransmitter receptor expression. We found a similar heterogeneity in cell culture from neonatal and adult tissue. These receptors are linked to different microglial functions including cytokine release, migration, phagocytosis activity and the ability of microglial cells to respond to a local injury by process extension. Serotonin for instance controls this process motility while muscarinic acetylcholine receptors control the phagocytosis activity.
P2X7-dependent, but differentially regulated release of IL-6, CCL2 and TNFα in cultured mouse microglia
Chu-Hsin Shieh1, 2, Annette Heinrich1, Tsvetan Serchov1, Dietrich van Calker1 and Knut Biber 1,3,*
1Department of Psychiatry and Psychotherapy, University Hospital of Freiburg, Germany;2Faculty of Biology, University of Freiburg, Germany;3Department of Neuroscience, University Medical Center Groningen, The Netherlands
ATP is an important regulator of microglia and its effects on microglial cytokine release are currently discussed as important contributors in a variety of brain diseases. We here analysed the effects of ATP on the production of six inflammatory mediators (IL-6, IL-10, CCL2, IFN-γ, TNFα, IL-12p70) in cultured mouse primary microglia. Stimulation of P2X7 receptor by ATP (1 mM) or BzATP (500 μM) evoked the mRNA expression and release of pro-inflammatory cytokines IL-6, TNFα and the chemokine CCL2 in WT cells but not in P2X7−/− cells. The effects of ATP and BzATP were inhibited by the non-selective P2 receptor antagonists PPADs and suramin. Various selective P2X7 receptor antagonists blocked the P2X7-dependent release of IL-6 and CCL2, but, surprisingly, had no effect on BzATP-induced release of TNFα in microglia. Calcium measurements confirmed that P2X7 is the main purine receptor activated by BzATP in microglia and showed that all P2X7 antagonists were functional. It is also presented that pannexin-1 hemichannel function and potential P2X4/P2X7 heterodimers are not involved in P2X7-dependent release of IL-6, CCL2 and TNFα in microglia. How P2X7-specific antagonists only affect P2X7-dependent IL-6 and CCL2 release, but not TNFα release is at the moment unclear, but indicates that the P2X7-dependent release of cytokines in microglia is differentially regulated [1].
Reference
1. Shie CH, Heinrich A, Serchov T, van Calker D, Biber K (2014) Glia 62:592–607
Adenosine-chemokine cross talk in neuroprotection
Cristina Limatola*, Clotilde Lauro, Maria Rosito and Flavia Trettel
Department of Physiology and Pharmacology, University of Rome “Sapienza”, Rome, Italy
Adenosine is a well known modulator of synaptic transmission and has protective roles in the nervous system, both effects being mediated by activation of its specific G-protein coupled receptors. We have recently demonstrated that the transmembrane chemokines CXCL16 and CX3CL1 are neuroprotective in different neurotoxicity models. To exert their protective activity, both chemokines requires the simultaneous co-activation of adenosine receptors, with different mechanisms. We now demonstrate that CX3CL1 and CXCL16 synergistically drive cross-talk between neurons, microglia and astrocytes to promote neuroprotective mechanisms that counteract neuronal cell death due to ischemic and excitotoxic insults. In in vivo model of pMCAO we found that exogenous administration of CXCL16 reduced ischemic volume and, in CXCR6−/− mice, lacking the unique CXCL16 receptor, pMCAO induction reduced brain ischemic volume. We also demonstrated that CX3CL1, acting on microglia, elicits CXCL16 release from glial cells and this is important to induce neuroprotection since hampering CXCL16 signaling reduced CX3CL1 neuroprotection both in vitro and in vivo models. Moreover the activity of adenosine receptor A3R and the release of CCL2 from astrocytes also contribute to the neuroprotective effects of CX3CL1 and CXCL16, since their inactivation reduces chemokine-induced neuroprotection.
Sat 1 C: Transgenic models for the investigation of purinergic signaling
P2RX7—a susceptibility gene for mood disorders?
Fernando Aprile-Garcia2, Michael W. Metzger1, Nina Dedic1, Sandra M. Walser1, Vladimira Jakubcakova1, Darina Czamara1, Mišo Mitkovski3, Bertram Mueller-Myhsok1, Mayumi Kimura1, Wolfgang Wurst, Walter Stühmer1, Florian Holsboer1, Eduardo Arzt2 and Jan M. Deussing1,*
1Max Planck Institute of Psychiatry, Munich, Germany;2Instituto de Investigación en Biomedicina de Buenos Aires (IBioBA)-CONICET- Partner Institute of the Max Planck Society, Bueons Aires, Argentina;3Max Planck Institute of Experimental Medicine, 37075 Göttingen, Germany;4Helmholtz Zentrum München, German Research Center for Environmental Health, Institute of Developmental Genetics, Neuherberg, Germany
A single-nucleotide polymorphism (rs2230912) in the purinergic P2X7 receptor (P2X7R) gene leading to a glutamine (Gln) by arginine (Arg) substitution at codon 460 (Gln460Arg) has been associated with mood disorders. We could demonstrate at molecular and cellular level that hetero-oligomerization between human wild-type P2X7R (hP2X7R-WT) and hP2X7R-Gln460Arg impairs normal receptor function, which depends on direct physical interaction of the two P2X7R isoforms.
To further investigate the functional significance in vivo, we generated mice expressing hP2X7R-WT and hP2X7R-Gln460Arg, respectively. Interestingly, mice heterozygous for both variants showed signs of a pre-symptomatic disease stage. Along these lines heterozygote hP2X7R mice revealed an increased vulnerability to develop mood disorder-related endophenotypes in response to chronic stress. These results suggest that heterozygosity of wild-type P2X7R with P2X7R-Gln460Arg alters receptor function and thereby impacts the stress vulnerability. Taken together, these humanized mouse lines provide functional evidence supporting P2RX7 as a susceptibility gene of mood disorders.
Optical and genetic approaches to explore P2X4 receptor function, expression and regulation
Ji Xu1, Hua Chai1, Konstantin Ehinger2, Xiaohong Lu3, Terrance M. Egan4, Rahul Srinivasan1, Xiangdong W. Yang3, Manfred Frick2 and Baljit S. Khakh1,*
1Department of Physiology, David Geffen School of Medicine, University of California Los Angeles, CA 90095 USA;2Institute of General Physiology, University of Ulm, 89081 Ulm, Germany;3Center for Neurobehavioral Genetics, Semel Institute for Neuroscience and Human Behavior, David Geffen School of Medicine, University of California Los Angeles, Los Angeles, CA 90095, USA;4Department of Pharmacological and Physiological Science and The Center for Excellence in Neuroscience, Saint Louis University School of Medicine, St. Louis, Missouri, 63130, USA
P2X4 receptors (P2X4Rs) are ATP-gated cation channels that are widely expressed in the central nervous system. However, our understanding of their function, expression and regulation is still in its infancy. Progress has been hindered because it is not possible to identify and record from the sparse population of P2X4R expressing cells in tissue slices and because there have been no approaches available to track P2X4R trafficking in real time. In this talk, we will present two new optical tools developed in our lab to address these issues. The first tool is the development of BAC transgenic mice expressing the bright orange/red fluorescent protein tdTomato from the endogenous P2X4 locus (P2X4-tdTomato reporter mice). These mice revealed P2X4 expressing cells in fixed and live brain slices, which is not possible with in situ hybridization of P2X4 mRNA or immunostaining. Cellular Tdtomato expression was also observed in live brain slices using standard epifluorescence and confocal microscopes, allowing us to directly make fluorescence guided electrophysiological recordings from P2X4 expressing cells. The second tool is the development of an engineered P2X4R carrying pH sensitive superecliptic pHluorin at an innocuous location in the extracellular domain (P2X4-pHluorin123); this functions like wild type P2X4Rs. P2X4-pHluorin123 permitted the measurement of the subcellular proportions of P2X4Rs in HEK-293 cells, hippocampal neurons, C8-B4 microglia and alveolar type II (ATII) cells and allowed for the direct study of P2X4R secretion onto plasma membrane during lamellar body exocytosis in ATII cells. During the talk, we will present detailed data on both optical tools that can now be used to explore P2X4R physiology using electrophysiology and imaging.
Purinergic control of inflammation and thrombosis: role of P2X1 ion channels
Céline Delierneux, Christelle Lecut, Alexandre Hego, Richard J Evans and Cécile Oury
1GIGA-Research, Human Genetics Unit, Laboratory of Thrombosis and Hemostasis, University of Liège, Liège, Belgium;2Department of Cell Physiology and Pharmacology, University of Leicester, Leicester, UK
Inflammation shifts the hemostatic mechanisms in favor of thrombosis. Upon tissue damage or infection, a sudden increase of extracellular ATP occurs, that might contribute to the crosstalk between thrombosis and inflammation. On platelets, the ATP-gated P2X1 ion channel acts to amplify platelet activation and aggregation induced by other platelet agonists. Platelet P2X1 ion channels critically contribute to thrombus stability in small arteries. Besides platelets, studies by our group indicate that these ion channels facilitate neutrophil chemotaxis, both in vitro and in vivo. These recent data suggest that P2X1 ion channels may be involved in the interplay between platelets and innate immune cells. To address this issue, we used P2X1−/− mice in two distinct mouse models of acute inflammation associated with disturbed hemostasis.
In a model of sepsis, endotoxin-treated P2X1−/− mice exhibited exaggerated neutrophil sequestration in the lungs and aggravated oxidative tissue damage, along with exacerbated thrombocytopenia and increased activation of coagulation, which translated into higher susceptibility to septic shock. In a model of acute colitis, after a 7-day treatment with 5 % dextran sulfate sodium (DSS), P2X1−/− mice showed significantly higher disease activity index, colonic crypt erosion, as compared to wild type mice. Though DSS accelerates extraintestinal thrombosis, intravital microscopy experiments revealed defective platelet accumulation in cremaster muscle arterioles of colitic P2X1−/− versus wild type mice upon laser-induced endothelial injury.
We propose that activation of P2X1 ion channels by ATP on neutrophils and platelets represents a new mechanism that may regulate acute inflammatory responses and associated disturbance of hemostasis.
BAC transgenic reporter mice to analyze the P2X3 and P2Y1 receptor expression in health and disease
Ralf Hausmann1,*, Michaela Schumacher1, Janka Günther1, Franziska Barthel2, Henning Hermanns2, Marcus Grohmann3, Heike Franke3 and Günther Schmalzing1
1Department of Molecular Pharmacology, RWTH Aachen University, Aachen, Germany;2Department of Anesthesiology, Heinrich-Heine-University of Düsseldorf, Germany;3Rudolf-Boehm-Institute of Pharmacology and Toxicology, University of Leipzig, Germany
P2X3 and P2Y1 receptors are known to be crucially involved in sensory neurotransmission and pain sensation. Accordingly, information about the precise distribution of P2X3 and P2Y1 receptors on neurons in health and disease is of significant interest. For instance, although the crucial importance of P2X3 and P2Y1 receptors in neuropathic and chronic inflammatory pain is well established, changes of the expression pattern in the spinal cord in these pain states is still unclear. To facilitate the morphological and functional identification of neurons expressing P2X3 or P2Y1 receptors, we have generated C57BL/6J BAC-transgenic reporter mice expressing eGFP or TagRFP as reporter protein for the gene expression of the P2X3 or P2Y1 receptor, respectively.
In P2X3 eGFP reporter mice native fluorescence was detected only in cultured dorsal root ganglia (DRGs) neurons. Immunostaining of tissue sections with a GFP antibody revealed that the P2X3 receptor gene was also expressed in situ in neurons of DRGs, the dorsal horn of the spinal cord and the trigeminal nucleus. In P2Y1 TagRFP-reporter mice native fluorescence was detected in hippocampal neurons and platelets. Immunostaining with a TagRFP monoclonal antibody revealed in situ gene expression of the P2Y1 receptor in neurons of the dorsal horn of the spinal cord, cerebellar Purkinje neurons, the hippocampus, dopaminergic and non-dopaminergic cells of the ventral tegmental area and in hepatocytes. P2X3 receptor gene expression was analyzed in the chronic constriction injury (CCI) model of neuropathic pain. Mice that underwent unilateral loose ligation of the sciatic nerve developed ipsilateral mechanical allodynia and thermal hyperalgesia at day 10 after surgery as compared to the sham group. Immunohistochemistry of the DRGs and the spinal cord using a GFP or P2X3 receptor antibody was performed to analyze the GFP or P2X3 expression in ipsi- and contralateral DRGs and the dorsal horn of the spinal cord. Preliminary evaluation of the tissue sections provided no evidence for a marked difference in the number of P2X3 expressing neurons between ipsi- and contralateral sides. Whether the P2X3 receptor expression level per neuron has changed on the affected ispilateral side is under study.
A BAC transgenic mouse model to study localization, protein interactions, and pathophysiological functions of P2X7 receptors
Anika Saul1,*, Karina Kaczmarek-Hájek1, Stefanie Schuster2, Volker Eulenburg2 and Annette Nicke1
1Max-Planck-Institute of Experimental Medicine, Herrmann-Rhein-Str. 3, Göttingen, Germany;2Institute of Biochemistry, Friedrich-Alexander University, Fahrstraße 17, Erlangen, Germany
The P2X7 receptor (P2X7R) plays a central role in cytokine production and inflammation and its up-regulation was shown in several neurodegenerative diseases. Their activation has been shown to result in diverse cellular events such as the formation of large membrane pores, plasma membrane blebbing and cytokine release. Despite its importance as drug target, its precise localization and its molecular and physiological functions, signaling, and regulation under physiological and pathophysiological conditions remain poorly understood. In particular, the location and function of P2X7Rs in neurons and their interactions with other proteins remain a matter of ongoing debate.
To determine the cellular and subcellular distribution of P2X7Rs and to investigate their physiological and pathophysiological roles in vivo, a transgenic mouse model (X7EGFP) was generated that overexpresses an EGFP-tagged murine P2X7R under the control of the endogenous promoter. Following pronuclei microinjection, four transgenic lines were obtained that harbor different amounts of inserted transgene copies and expression levels, but show identical expression patterns in the brain with predominant expression in the cerebellar molecular layer.
Costaining with different cellular markers confirmed the presence of P2X7-EGFP in microglia (Iba1), oligodendocytes (NG2), and subpopulations of astrocytes (GFAP) in the brain as well as the spinal cord. Mice from the strain with the highest transgene expression (X7EGFP-61) develop significant motor deficits already at a very young age suggesting a pathophysiological effect of P2X7R overexpression in motor coordination.
In conclusion, the novel X7EGFP mouse model provides a novel promising tool to study the localization of P2X7Rs in specific cell types and to investigate its role in the central nervous system.
Sat 1 D: ATP and P2 receptors in the regulation of renal transport and blood pressure
The primary cilium and mechanically induced ATP release from the renal epithelium
Helle Praetorius
Deptartment of Biomedicine—Physiology, Aarhus University, Denmark
The primary cilium is s sensory organelle able to detect subtle chemical or mechanory stimulation. In certain situations the delicate signals that arise from the cilium can be amplified by local pararcrine mediators, such as ATP. The principle is that the cilium from a single cell, via its antenna function makes the ciliated cell susceptible to more subtle stimuli than it would otherwise not be able to detect. For these signals to transmit into a coordinated tissue function, in for example epithelia, the stimulus would have to be substantial enough to stimulate the majority of the cells simultaneously. There is evidence that suggest that the subtle stimuli detected by a few of the cells in an epithelium may spread and create an integrated epithelial response through release of ATP and purinergic signalling upon cilium activation. In this perspective, it is interesting to consider paracrine signalling—not as the primary event but rather as the tissue function coordinator. The presentation will focus on this perspective through examples from various organ systems but with focus on renal epithelia.
Role of P2Y receptors in vasopressin resistance: insights from lithium-induced NDI
Bellamkonda K. Kishore
University of Utah and VA SLC Health Care System, Salt Lake City, Utah, USA
Arginine vasopressin (AVP) through the V2 receptor-cAMP-AQP2 pathway plays a critical role in the maintenance of body water homeostasis by the kidney. However, there are clinical conditions where the kidneys are non-responsive to AVP, such as the acquired nephrogenic diabetes insipidus (NDI). Recent reports showed that purinergic signaling mediated by P2Y receptors opposes the action of AVP on renal collecting duct (CD) by decreasing the cellular cAMP and thus the AQP2 protein levels. Studies conducted by us and others revealed the complex interactions among the AVP, purinergic and prostanoid systems in the CD in relation to renal water handling in health and disease [1]. We also showed the potential involvement of the ATP/UTP-activated P2Y2 receptor in lithium-induced NDI in rats [2]. This was further confirmed in P2Y2 receptor knockout mice [3,4]. Interestingly, resistance to lithium-induced NDI in the knockout mice was due to altered prostanoid receptor signaling, but not decreased production of renal PGE2. Extension of these studies revealed that the ADP-activated P2Y12 receptor is expressed in the kidney, and its blockade by the administration of clopidogrel bisulfate (Plavix®) ameliorates lithium-induced NDI in rodents [5]. In vitro studies showed that P2Y12 receptor blockade sensitizes CD to the action of AVP [6], and may re-sensitize the CD in the presence of lithium. Thus, the studies on lithium-induced NDI unraveled the potential beneficial effects of targeting P2Y2 or P2Y12 receptors to relieve AVP resistance in the clinic. (in collaboration with: Raoul D. Nelson, Noel G. Carlson, Donald E. Kohan, Janos Peti-Peterdi and Christa E. Müller)
References
1. Kishore BK et al (2009) Purinergic Signal 5:491–499
2. Zhang Yet al (2009) Am J Physiol Renal Physiol 296:F1194–F1201
3. Zhang Yet al (2012) Am J Physiol Renal Physiol 302:F70–F77
4. Zhang Yet al (2013) Am J Physiol Renal Physiol 305:F407–F416
5. Zhang Yet al (2011) J Am Soc Nephrol 22:50A (abstract)
6. Zhang Yet al (2013) J Am Soc Nephrol 24:138A (abstract)
Renal epithelial ATP release by connexin30 and pannexin1 and their relevance to blood pressure regulation
Janos Peti-Peterdi
Deptartment of Physiology and Biophysics, University of Southern California, Los Angeles, CA, USA
A local purinergic system intrinsic to the distal nephron-collecting ducts (CD) provides a powerful control of renal epithelial sodium and water excretion. Over the past years our laboratory characterized several mechanisms by which ATP, a ligand of purinoceptors, is released from renal epithelial cells into the tubular lumen. Connexin (Cx) 30 hemichannels are expressed in distinct, continuous pattern in the intercalated cell’s luminal plasma membrane. An ATP biosensor approach demonstrated functional Cx30 hemichannels mediating luminal ATP release in the intact, microperfused CD and their control of CD salt and water transport by paracrine purinergic signaling. Cx30 hemichannel opening required mechanical stimulation by increased tubular fluid flow rate. Genetic deletion of Cx30 markedly reduced flow-induced luminal ATP release and impaired salt and water excretion associated with pressure natriuresis, an important mechanism that maintains body fluid and electrolyte balance and blood pressure. Cx30−/− mice express a salt retention phenotype and salt-sensitive hypertension due to the hyperactive Cx30-expressing CD and the lack of this dietary salt-sensitive negative feedback mechanism for the control of renal salt excretion via ATP release from intercalated cells, and its paracrine purinergic actions on principal cells targeting ENaC. In addition, the recently identified plasma membrane ATP channel pannexin 1 (Panx1) showed strong expression in the apical membrane of the CD system and in the renal vasculature. Panx1−/− mice showed reduced urinary ATP content consistent with Panx1 functioning as an important ATP releasing channel in renal epithelia. Intravital calcium imaging confirmed the in vivo relevance and (patho)physiological role of these ATP channels in the intact kidney.
P2Y2receptor activation lowers blood pressure by vascular and renal tubular actions
Timo Rieg
Nucleotides, such as ATP and UTP, are important paracrine regulators of vascular tone. Importantly, we previously demonstrated that in vivo activation of P2Y2 receptors causes an acute NO-independent decrease in blood pressure (BP), indicating this signaling pathway requires an endothelial-derived hyperpolarization (EDH) response. To define the mechanisms by which in vivo activation of P2Y2 receptors initiates EDH and vasodilation, we studied connexin 37 and 40 (Cx37, Cx40), both part of the myoendothelial gap junction, as well as big-conductance (KCa1.1, expressed in smooth muscle cells) and intermediate-conductance K channels (KCa3.1, expressed in endothelial cells), all hypothesized to be part of the EDH response. We compared the effects of the P2Y2/4 receptor agonist, INS45973, in wild-type (WT) mice and in mice lacking Cx37, Cx40, KCa1.1, or KCa3.1 (n = 4−5/genotype) under anesthesia, while monitoring intra-arterial BP. Acute application of INS45973 (0.1, 0.3, 1, or 3 mg/kg bw i.v. in 0.5 μl/g bw over 45 s) dose-dependently and rapidly (within 15 s of starting infusion) decreased BP in WT (max response % of baseline: −38 ± 1 %, EC50: 0.8 ± 0.2 mg/kg) for 2–3 min. Whereas the responses in Cx40−/− and KCa1.1−/− mice were comparable to WT (−41 ± 2 %, 0.3 ± 0.1 mg/kg and −44 ± 3 %, 0.3 ± 0.1, respectively), responses in Cx37−/− and KCa3.1−/− mice were impaired (−27 ± 1 %, 1.2 ± 0.3 mg/kg and −13 ± 5 %, 1.6 ± 0.8, respectively). Heart rate was not affected in either genotype. Our data indicate that Cx37 and KCa3.1 are required, at least in part, for the P2Y2 receptor-initiated EDH response and subsequent vasodilation.
P2X receptors in the regulation of renal transport and blood pressure
Robert Unwin
University College London
Purinergic P2X receptor signaling has been described in several forms of experimental and essential hypertension. Several P2X receptor variants have been implicated in cardiovascular disease. In the vasculature, P2X1 and P2X4 remain the best described, but there is also growing evidence for a role for P2X7.
P2X1 receptors are expressed throughout the smooth muscle of vascular beds and contribute to the myogenic response. P2X4 receptors are generally localized to the vascular endothelium where they mediate calcium-dependent release of vasodilators, such as nitric oxide, but they are also present in the renal tubular epithelium, where they may affect sodium transport. In the kidney, the role of these P2X receptors (and others) in blood pressure homeostasis is becoming clearer.
In this short lecture I will present and review some of the evidence suggesting a role for P2X4 and P2X7 receptor subtypes in hypertension, linking their distribution to renal tubular and vasculature function.
Sat 2 A: Purinergic regulation of tumor growth and metastasis I
The many faces of adenosinergic signaling in cancer
John Stagg
University of Montreal Hospital Research Center, Faculty of Pharmacy, Montreal Cancer Institute, Quebec, Canada
The generation of immunosuppressive adenosine by CD73 in the hypoxic tumor microenvironment causes dysregulation of immune cell infiltrates, resulting in tumor progression, metastases and poor disease outcomes. In a large meta-analysis of CD73 gene expression in breast cancer patients, we found that CD73 is significantly associated with poor prognosis in triple negative breast cancer (TNBC). CD73 also correlated with an increased resistance to anthracycline chemotherapy due to A2A adenosine receptor-mediated suppression of anti-tumor immunity. In addition to its immunosuppressive effects, CD73derived adenosine promotes tumor cell migration in vitro and metastasis in vivo via A2B adenosine receptor. Our studies converge to suggest an important role for the CD73-A2B axis in metastatic dissemination, independently of immunosuppression. CD73 also promotes cellular adhesion, particularly lymphocyte interactions with the endothelium, and tumor angiogenesis. Recently, monotherapies with anti-CTLA-4 and anti-PD-1 antibodies have shown success in enhancing patient survival in a growing number of cancer types. Nevertheless, tumors can deploy multiple mechanisms to avoid immune-surveillance. We recently demonstrated that targeted blockade of the adenosinergic pathway significantly enhances the therapeutic activity of anti-CTLA-4 and anti-PD-1 antibodies. While CD73 inhibitors are still in clinical development, the clinical studies of A2A adenosine receptor antagonists are indicative of their potential safety for rapid translation. In particular, preclinical evidence suggests that A2A antagonists may potentiate chemotherapy and immunotherapeutic strategies. In conclusion, the development of therapies that modulate adenosine receptor signaling in the cancer setting must be taken forward cautiously, but are likely to improve treatment efficacy.
Role of P2X7 receptor in tumor-host interaction
Elena Adinolfi
Department of Morphology, Surgery and Experimental Medicine, Section of Experimental Pathology, Oncology and Biology, University of Ferrara, Via L. Borsari, 46, 44121, Ferrara, Italy
The P2X7 receptor is well known to regulate immune responses and has been recently identified as promoter of tumor engraftment, growth and vascularization (1). However, there is limited evidence on the role of P2X7 in host response to tumor and, in general, on P2X7-activated biochemical pathways in oncogenesis. To further investigate this aspects we induced cancer formation in P2X7 null mice or systemically administered P2X7 antagonists to tumor bearing mice. B16 melanoma injected-P2X7 null mice developed tumors almost completely lacking immune cell infiltration. However, systemic administration of P2X7 antagonists, in experimental models of melanoma and neuroblastoma, had a potent-anti tumor activity, causing reduction of cancer growth and VEGF tumor content. Interestingly, P2X7 receptor activation was central in VEGF secretion both from host and cancer cells. In the neuroblastoma models tested, the signalling pathway responsible for P2X7-dependent VEGF secretion was that of PI3K and Akt. In fact, in ACN neuroblastoma cells, P2X7-activated PI3K/Akt axis leads to HIF1α expression causing increased VEGF secretion. In the same model, P2X7 causes inactivation of the Akt-controlled oncosuppressive kinase GSK3β. In two experimental neuroblastoma models, obtained by injection of either ACN or Neuro2A cells respectively in nude/nude or Albino/J mice, treatment with P2X7 antagonists was efficacious in blocking the Akt/GSK3β/VEGF pathway. Taken together our data identify P2X7 antagonist administration as a good therapeutic strategy to reduce tumor growth, angiogenesis and progression by targeting the PI3K/Akt/GSK3β/HIF1α/VEGF signalling network.
Reference
1. Adinolfi E, Raffaghello L, Giuliani AL, Cavazzini L, Capece M, Chiozzi P, Bianchi G, Kroemer G, Pistoia V, Di Virgilio F (2012) Cancer Research 72:2957–2969
Opportunities and challenges for anti-CD73 cancer therapy
Siqi Chen1, Long Wang2, Jie Fan1, Donye Dominguez1, Lei Qin1, Yi Zhang3 and Bin Zhang1,2,*
1Robert H. Lurie Comprehensive Cancer Center, Department of Medicine-Division of Hematology/Oncology, Northwestern University Feinberg School of Medicine, Chicago, IL 60611, USA;2Cancer Therapy & Research Center, Department of Medicine, University of Texas Health Science Center, San Antonio, TX 78229, USA;3Biotherapy Center, The First Affiliated Hospital of Zhengzhou University, Zhengzhou, 450052, Henan, China
Tumor-induced immunosuppression is one of the main causes of tumor escape and failure of immunotherapy. Therefore, understanding immunosuppressive mechanisms is critical for optimization of cancer immunotherapeutic benefit. CD73-mediated adenosinergic effects can now be viewed as among the most important immunosuppressive regulatory pathways in the tumor microenvironment. From a translational perspective, small-molecule inhibitors and mAb against CD73 are effective to treat cancer in multiple mouse tumor models and are well tolerated in mice. Moreover, the importance of the CD73-mediated adenosinergic pathway in the process of cell growth and invasion of human cancer cells and Treg-induced immunosuppression in various human cancers has been independently confirmed. We are faced with a good opportunity to develop CD73-targeting immunotherapies for treating solid cancers and leukemia based on the knowledge obtained from these recent studies. The development and clinical applications of CD73 blockade using clinical-grade antihuman CD73 mAb and small-molecule inhibitors are thus warranted. However, these are merely potential therapeutics, and we still have many challenges when translating this therapeutic approach into cancer patients. By reinforcing our investigation on further characterizing CD73 expression and function in different human cancers in addition to understanding both ecto-5′-nucleotidase enzymatic activity-dependent and -independent mechanisms of CD73, it might be possible to develop effective CD73-targeted therapies to conquer tumor-induced immunosuppression in combination with other T-cell-directed approaches or even conventional therapeutic regimens such as chemotherapy and radiation therapy [1–3].
References
1. Zhang B (2010) Cancer Research 70:6407–6411
2. Zhang B (2012) OncoImmunology 1:67–70
3. Zhang B (2012) Immunotherapy 4:861–865
The role of ecto-nucleotidases in bladder cancer progression
Liliana Rockenbach1, Elizandra Braganhol2, Fabrícia Dietrich1, Fabrício Figueiró1, Maria I. A. Edelweiss3, Fernanda B. Morrone4, Jean Sévigny5 and Ana M. O. Battastini1
1Departamento de Bioquímica, ICBS, UFRGS, Porto Alegre, Brasil;2PPG: Bioquímica e Bioprospecção, Centro de Ciências Químicas, Farmacêuticas e de Alimentos, UFPEL, Pelotas, Brasil;3Departamento de Patologia HCPA, UFRGS, Porto Alegre, Brasil;4Faculdade de Farmácia, PUCRS, Porto Alegre, Brasil;5Département de microbiologie-infectiologie et d’immunologie, Faculté de Médecine, Université Laval and Centre de recherche du CHU de Québec, Québec, QC, Canada
Studies from our laboratory have shown the differential pattern of ectonucleotidases in malignant bladder cancer cell lines. We have also shown that quercetin was able to increase ADP hydrolysis and inhibit the ecto-5′-nucleotidase in bladder cancer cells. In addition, the treatment with APCP (an ecto-5′-nucleotidase inhibitor) led to a significant reduction in cell proliferation and AMP had an antiproliferative effect on these cells, adding some evidence to the importance of the ecto-5′-nucleotidase in this malignancy. It was also shown that urinary bladder exhibits a cell-specific expression of ectonucleotidases [1]. Considering these data, we have invetigated the expression of ecto-nucleotidases in a mouse bladder cancer induced by N-butyl-N-(hydroxyl-butyl)-nitrosamine (BBN).
Bladder cancer was induced in mice by BBN in the drinking water. After 4, 8, 12, 18 and 24 weeks histopathological and immunofluorescence analyses were done. The bladder of animals, which received BBN, had mainly inflammation, after 4–12 weeks of tumor induction. After 18 weeks, mice’s bladder has developed histological alterations similar to human transitional cell carcinoma. The cancerous urothelium after BBN for 18 and 24 weeks presented a weak immunofluorescence to NTPDase3 and a strong expression of ecto-5′-nucleotidase, which contrast with the normal epithelium that shows a strong NTPDase3 expression and a low ecto-5′-nucleotidase expression. Although additional studies are needed, the results of this set of studies already suggest the participation of ectonucleotidases in the development of bladder cancer and points to the ecto-5′-nucleotidase as a promising therapeutic target for this malignancy.Support: CNPq, FIPE-HCPA—Brazil and CIHR—Canada.
Reference
1. Yu et al PloS ONE 6(4):e18704
Ecto-5′-CD73 inhibition as a therapeutic strategy for glioblastoma multiforme treatment
Fernanda C. Teixeira1, Fernanda Bruxel2, Juliana H. Azambuja1, Priscila T. Ramos1, Alexandre M. Berenguer3, Iscia Lopes-Cendes3, Marco A. Stefani4, Jean Sevigny5, Roselia M. Spanevello1, Helder Teixeira6, Ana M. O. Battastini7 and Elizandra Braganhol1,*
1Programa de Pós-Graduação em Bioquímica e Bioprospecção, CCQFA, UFPEL, Pelotas, RS, Brasil;2Grupo de Pesquisa em Nanobiotecnologia e Nanotoxicologia, UNIPAMPA, Uruguaiana, RS, Brasil;3Departamento de Genética Médica, Escola de Medicina, UNICAMP, Campinas, SP, Brasil;4Serviço de Neurologia do Hospital de Clínicas, Porto Alegre, RS, Brasil;5Département de microbiologie-infectiologie et d’immunologie, Faculté de Médecine, Université Laval; Centre de recherche du CHU de Québec, Québec, QC, G1V 4G2 Canada;6Programa de Pós-Graduação em Ciências Farmacêuticas, UFRGS, Porto Alegre, RS, Brasil;7Departamento de Bioquímica, ICBS, UFRGS, Porto Alegre, RS, Brasil
Glioblastoma multiforme (GBM) is the worst and most common brain tumor characterized by poor prognosis and limited therapeutic options. Ecto-5′-NT/CD73 overexpression has been reported in many solid tumors, including GBM. By increasing extracellular adenosine, ecto-5′-NT/CD73 contributes to cancer angiogenesis and immunessupression. Recently, siRNA approach has been considered as newer strategy to cancer treatment. However, the lack of effective delivery methods reduces the potential effectiveness of siRNA-based therapies. An alternative to overcome these drawbacks is the employment of nanocarriers which are advantageous for cancer therapy due to their high delivery specificity. Here we evaluated the potential of ecto-5′-NT/CD73 knockdown as strategy to GBM treatment. To this end, specific siRNA-CD73 sequences were designed and its functionality/specificity was evaluated by transfecting C6 glioma cells using nanoemulsion (NE) as delivery system. Toxicity of NE was determined in astrocyte and C6 glioma cultures by the MTT method. Physicochemical properties of NE/siRNA-CD73 complexes were characterized by particle size, zeta potential, polydispersity and TEM. Ecto-5′NT/CD73 protein expression, enzyme activity and glioma cell viability following NE/siRNA-CD73 transfection were evaluated by immunocitochemistry, AMP hydrolysis, and MTT assay, respectively. Our results indicated that siRNA-CD73 sequences were efficiently loaded to NE particles and the NE/siRNA-CD73 complexes did not promote astrocyte toxicity. Moreover, the formulation was effective to decrease the glioma ecto-5′-NT/CD73 expression at protein and enzyme activity levels. Finally, ecto-5′-NT/CD73 knockdown decreased the glioma viability, indicating the effectiveness of NE/siRNA-CD73 complexes to reduce glioma progression. The results shown here give new insights into how CD73 knockdown may contribute to antiglioma therapy.
Sat 2 B: Purines and neurodegeneration
Purine targets in Parkinson’s trials: from adenosine to urate
Michael A. Schwarzschild
MassGeneral Institute for Neurodegenerative Disease, Massachusetts General Hospital, Boston, USA
Purines are well represented among the targets of recent clinical trials for Parkinson’s disease (PD), a neurodegenerative disease characterized by progressive loss of dopaminergic neurons and consequently the loss of normal movement. The neurobiology of purines ranging from adenosine to urate has been pursued to phase III trials in PD and other CNS disorders. Adenosine A2A receptor antagonists offer a leading example of efforts to develop non-dopaminergic antiparkinsonian treatments to improve the symptoms of PD without incurring adverse effects of mainstay dopaminergic drugs. And in contrast to other non-dopaminergc receptors, the A2A receptor offers the advantage of relatively restricted expression within the brain to the region of motor dysfunction in PD. In addition to alleviating PD motor deficits, A2A receptor antagonists hold promise as disease-modifying therapy. The latter is based both on the neuroprotective properties of A2A antagonists and on the epidemiological link between caffeine (a non-specific adenosine antagonist) and reduced risk of PD. Urate, the enzymatic end product of purines in humans, has similarly been found to be an inverse risk factor for PD. In addition, higher serum or cerebrospinal fluid levels have been found to be predictive of a slower rate of clinical progression of the disease. Such epidemiological links together with the well-known antioxidant and neuroprotective actions of urate have been rapidly translated into therapeutic strategies to elevate urate in PD, stroke and multiple sclerosis. Thus convergent evidence from multiple disciplines supports the targeting of purines to improve our treatment of Parkinson’s and related diseases.
P2X7 inhibition reduces amyloid plaques in Alzheimer’s disease through GSK3β and secretases “in vivo”
Maria T. Miras-Portugal*, Juan I. Diaz-Hernandez, Rosa Gomez-Villafuertes, Javier Gualix and Miguel Diaz-Hernandez
Department of Biochemistry and Molecular Biology, Complutense University of Madrid, Madrid, Spain
Amyloid precursor protein (APP) processing produces β-Amyloid, the Aβ42 peptide being at the origin of the senile plaques characteristic of Alzheimer’s disease (AD). However, extracellular signals maintaining the balance between nonpathogenic and pathologic forms of APP processing, mediated by α-secretase and β-secretase respectively, remain poorly understood. In Neuro-2a neuroblastome cell line, activation of the P2X7 receptor leads to reduction of α-secretase activity, the opposite effect being obtained by P2Y2R activation. These promising results were reproduced in other cellular models, confirming that inhibition of either native or overexpressed P2X7R increased α-secretase activity. These data suggest that P2X7R and P2Y2R could be novel therapeutic targets in AD [1].
The in vivo approach to AD was possible working with transgenic J20 mice model, expressing two human APP mutant proteins. This animal exhibits prominent amyloid plaques by the 6 months of life. In vivo inhibition of the P2X7R in J20 mice induced a significant decrease in the number of hippocampal amyloid plaques (Fig. 1). This reduction is mediated by increasing the proteolytic processing of APP through α-secretase activity and correlates with an increase in the GSK-3 phosphorylated form that is a less active enzyme. The in vivo findings presented here demonstrate for the first time the therapeutic potential of P2X7R antagonism in the treatment of familiar Alzheimer’s disease (FAD) [2]. Besides, the therapeutic potential of P2Y2R receptor in FAD, is still waiting for pharmacological agonists or allosteric effectors with good bioavailability acting on this receptor.
Fig. 1 BBG-treatment reduces the number and size of amyloid plaques in the J20 mouse hippocampus modifying APP processing. Detection of Aβ plaques are made with monoclonal antibody against Aβ peptide, clone WO-2
References
1. Leon-Otegui M, Gomez-Villafuertes R, Diaz-Hernandez JI, Diaz-Hernandez M, Miras-Portugal MT, Gualix J (2011) FEBS Letters 585:2255–2262
2. Diaz-Hernández JI, Gomez-Villafuertes R, Leon-Otegui M, Hontecillas-Prieto L, del Puerto A, Trejo JL, Lucas JJ, Garrido JJ, Gualix J, Miras-Portugal MT, Diaz-Hernandez M (2012) Neurobiology of Aging 33:1816–1828
Regulatory mechanisms and functional significance of P2X7R in epilepsy
Eva M. Jimenez Mateos1, Amaya Sanz-Rodriguez1, Marina Arribas Blazquez2, Elena Langa1, Alba Jimenez Pacheco1, Cristina Ruedell Reshke1, Maria Teresa Portugal2, Antonio R. Artalejo2, Luis A Olivos-Ore2, Miguel Diaz-Hernandez2, David C. Henshall1 and Tobias Engel1
1Royal College of Surgeons in Ireland, Dublin, Ireland;2Faculty of Veterinary Medicine, Complutense University of Madrid
Epilepsy is a common, chronic neurological disorder characterized by recurring unprovoked seizures affecting ~50 million people worldwide with a prevalence of about 0.9 %. There are over 20 anti-epileptic drugs in clinical use; however the proportion of pharmacoresistant epilepsy patients has changed relatively little, remaining at ~30 %.
The P2X7 receptor has been proposed as promising novel drug target and P2X7 receptor antagonists show strong anticonvulsive and neuroprotective properties after prolonged seizures in animal models of epilepsy [1–3].
Recent data, investigating P2X7 receptor regulation after seizures show now a specific down-regulation of P2X7 receptor protein and function in cell death protected brain areas. MicroRNA arrays, bioinformatics and the usage of microRNA inhibitors and activators identified a specific microRNA targeting the P2X7 receptor after seizures in the brain controlling its expression into protein. Sustained suppression of this microRNA led to increased P2X7 receptor expression and function after seizures and an exacerbated epileptic phenotype with mice showing increased brain inflammation which was mediated by the P2X7 receptor. Experiments showed that microRNA expression is partly induced by the P2X7 receptor-inducing transcription factor SP1 (specificity protein 1) (negative feed-back loop) and can be blocked by the P2X7 receptor antagonist BzATP (positive feed-back loop).
In summary, our results show a novel pathway regulating P2X7 receptor expression and the resulting inflammation in the brain after seizures and add further evidence towards a role of P2X7 receptors in the pathogenesis of epilepsy.
References
1. Engel et al (2012) FASEB J 26(4):1616–1628
2. Jimenez Pacheco A et al (2013) 54(9):1551–1561
3. Mesuret G et al (2014) CNS Neurosci Ther 26(7):237
Microglial purinergic receptors in Alzheimer’s disease
Matthias Brückner1, Mareike Schnaars1, Younis Baqi2, Christa E. Müller2 and Annett Halle1
1Max-Planck Research Group “Neuroimmunology”, Research center caesar, Bonn, Germany;2PharmaCenter Bonn, Pharmaceutical Institute, Pharmaceutical Chemistry I, University of Bonn, Germany
Microglia, the resident immune cells of the CNS, get recruited to amyloid-β plaques in Alzheimer’s disease (AD) and it has been shown that microglia become functionally impaired in Alzheimer’s disease mouse models [1]. Purinergic receptors have been described as mediators of microglial chemotaxis and phagocytosis [2, 3]. However, their role in AD remains elusive. The aim of this study was to investigate the role of microglial purinergic receptors in AD, with focus on functional changes of microglia during AD.
To this end, we assessed microglial phagocytic capacity in acute cerebral slices from an AD mouse model, which revealed a striking difference between microglial cells associated with plaques and plaque-distant microglia. Phagocytic capacity of microglia was impaired to a similar degree when P2 receptor antagonists were added to acute slices of non-transgenic mice, suggesting that activation of these receptors promotes microglial phagocytosis. Furthermore, ADP, a P2 purinergic receptor agonist, reversed phagocytic impairment of plaque-associated microglia.
Our data suggest that P2 microglial purinergic receptors are involved in regulating microglial phagocytosis in AD. Thus, they may serve as targets for pharmacological treatment given that enhanced phagocytic capacity of microglia could counteract deposition of amyloid plaques in AD.
References
1. Krabbe G et al (2013) Functional impairment of microglia coincides with beta-amyloid deposition in mice with Alzheimer-like pathology. PLoS One 8(4):e60921
2. Koizumi S et al (2007) UDP acting at P2Y6 receptors is a mediator of microglial phagocytosis. Nature 446(7139):1091–1095
3. Haynes SE et al (2006) The P2Y12 receptor regulates microglial activation by extracellular nucleotides. Nat Neurosci 9(12):1512–1519
Sat 2 C: Purines in tissue fibrosis
The role of purinergic signaling in pancreatic fibrosis
Ivana Novak, Kristian A. Haanes and Nynne M. Christensen
Department of Biology, University of Copenhagen, Universitetsparken 13, Copenhagen, Denmark
The pancreatic stellate cells (PSCs) have complex roles in pancreas—in tissue repair, inflammation and fibrosis [1]. PSCs play a key role in several pancreatic diseases including pancreatitis, cystic fibrosis and pancreatic cancer. Relatively little is known about regulation of PSCs functions and fibrosis. Since PSCs surround ATP releasing exocrine cells [2], we address the question whether purinergic signaling, in particular the P2X7 receptor, could regulate PSC functions. For this purpose we used isolated murine PSCs and immortalized human PSCs (RLT-PSC). Our first study shows that the number PSCs isolated from Pfizer P2X7 receptor KO mice was about 50 % lower that from WT mice. The P2X7 receptor protein and mRNA of all known isoforms were expressed in murine WT PSCs, while KO PSCs only expressed truncated versions of the receptor [3]. Exogenous ATP had dual effect on cultured murine and human cells. Low concentration (μM) sensitized P2 receptor mediated Ca2+ signaling and increased cell proliferation, which could be inhibited by AZ10606120. High ATP (mM) concentrations killed PSCs, though this could be partially prevented by pre-treatment with A438079. Also PSCs from P2X7R KO mice were protected from ATP-induced cell death. We propose that ATP and P2X7 receptors are important regulators of PSC proliferation and death, and therefore might be potential targets for treatments of pancreatic fibrosis, especially in pancreatic adenocarcinoma.
References
1. Erkan M et al (2012) Gut. doi: 10.1136/gutjnl-2011-301220
2. Haanes KA, Novak I (2010) Biochem J. doi: 10.1042/BJ20091337
3. Haanes KA, Schwab A, Novak I (2012) PLoS One. doi: 10.1371/journal.pone.0051164
Adenosine receptors and pulmonary fibrosis: role of the ADORA2B on pulmonary macrophages
Michael R. Blackburn
Department of Biochemistry and Molecular Biology, University of Texas Medical School at Houston, 6431 Fannin, Houston Texas, USA
Extracellular adenosine is generated following hypoxia and/or cellular stress, and serves to orchestrate tissue responses to injury by engaging cell surface adenosine receptors. This pathway has beneficial functions on features of acute lung injury; however, it appears to contribute to chronic lung injury by promoting fibrosis and the development of pulmonary hypertension secondary to fibrosis. Our major hypothesis is that activation of adenosine signaling through its receptors contributes to the progression of pulmonary fibrosis and pulmonary hypertension by promoting excessive remodeling processes in the lung. This presentation will focus on our efforts to test this hypothesis by examining adenosine signaling through the A2B adenosine receptor (ADORA2B) and its role in the recruitment and activation of alternatively activated macrophages and how this process contributes to fibroproliferation and vascular remodeling in the lung. Data will be shown demonstrating that the ADORA2B is elevated in patients with pulmonary fibrosis and pulmonary hypertension and that genetic removal of this receptor from pulmonary macrophages is associated with attenuated pulmonary fibrosis and hypertension in animal models. In this setting, ADORA2B is responsible for the production of pro-fibrotic mediators from alternatively activated macrophages. Interestingly, high levels of this receptor on this cell type appears only during chronic stages of lung disease suggesting that the emergence of this cell type is important for the pro-fibrotic effects of the ADORA2B seen in chronic stages of disease.
ATP Release, hydrolysis and signaling in cardiac fibroblasts
David Lu and Paul A. Insel*
Department of Pharmacology, UCSD, La Jolla, CA 92093 USA
Cardiovascular disease (in particular myocardial infarction and heart failure), the leading cause of death in developed countries, is associated with cardiac damage and repair and the activation of cardiac fibroblasts (CFs) to pro-fibrogenic myofibroblasts. We sought to define the contribution of ATP release, hydrolysis (ultimately to adenosine) and signaling by ATP and adenosine in the regulation of adult rat and mouse CFs. We found that CFs release ATP both constitutively and in response to physical stimuli via connexin hemi-channels and that released ATP is an autocrine/paracrine signal that predominantly activates P2Y2 receptors, which is among the most highly expressed G-protein-coupled receptors of CFs. P2Y2 activation stimulates collagen production and expression of α-smooth muscle actin and other pro-fibrotic markers as well as increasing CF proliferation, migration, contraction. CFs also express multiple ectonucleoside triphosphate diphosphohydrolases (ENTPDs). ENTPDs hydrolyze ATP and diminish ATP-P2Y2 signaling and lead to the generation of adenosine, which preferentially activates A2B receptors in CFs; such activation attenuates pro-fibrotic ATP-P2Y2 signaling. The release and hydrolysis of ATP, in part regulated by ENTPDs—which both decrease the pro-fibrotic action of ATP and increase anti-fibrotic activity via adenosine—help establish the set-point of fibrotic activity by CFs. Taken together, our results define an autocrine/paracrine mechanism that is initiated by cellular ATP release and modulated by ENTPDs, whereby the phenotype and activity of CFs represent the integration of counterbalancing pro-fibrotic ATP-P2Y2 and anti-fibrotic adenosine-A2B signaling. The results suggest several targets in purinergic pathways for the treatment of common cardiac disorders.
Purines in tissue inflammation and fibrosis
Marco Idzko
Department of Pneumoglogy, University Hospital Freiburg
Chronic inflammatory lung disease such as Asthma, chronic obstructive pulmonary disease (COPD) or interstitial lung diseases are associated with remodelling the airways. The exact mediators responsible for the airway remodelling and/or development of fibrosis in these chronic lung diseases are still unknown.
Increasing evidences point out to an important role of extracellular nucleotides and their P2-receptors in the modulation of immune responses in the lung. Indeed previously we demonstrated that BALF ATP-levels are increased after allergen challenge in humans and mice and that neutralizing intrapulmonary ATP-levels or the application of unselective P2-receptor antagonists can abrogate all cardinal features of experimental asthma in mice. Interestingly also in the airways of patient with COPD and mice with smoke-induced lung inflammation increased ATP-levels could be detected, which were correlating with of BALF-neutrophila and air flow limitation. Furthermore also in patients with interstitial lung diseases such as UIP or NSIP display pulmonary ATP-levels, and in in vitro experiments as well as a translation mouse model for lung fibrosis point of the caridinal role of ATP/P2R axis in airway remodelling and fibrogenesis. By using specific P2R-subtype antagonist, knockout animals and bone marrow-chimeras, we could demonstrate that P2Y2R- and P2X7R-signalling on haematopoietic system (HS) and P2Y6R-signalling on the non-haematopoietic system (NHS) contributes to airway remodelling, destruction and fibrosis in these diseases (2, 3, 4, 5, 6, 7). In summary our data suggest that targeting of P2Y2, P2Y6 and P2X7R might be a new therapeutic option for the treatment of airway remodeling and/or prevention of airway destruction and lung fibrosis.
Sat 2 D: Purines in sensory systems
Purinergic signaling in the cochlea
Gary D. Housley1,*, Jennie M.E. Cederholm1, Matthias Klugmann1 and Allen F. Ryan2
1Translational Neuroscience Facility and Department of Physiology, UNSW Australia Sydney, NSW 2052, Australia;2Departments of Surgery & Neuroscience, University of California, San Diego, and VA Medical Center, La Jolla, CA, USA
Purinergic signaling has a broad base within the hearing organ. Both P1 and P2 receptors are expressed across sensory and supporting cells, and the auditory neurons. With regard to the P2 receptors, the majority of subtypes of P2X and P2Y (G protein-coupled) receptors have been identified, at least transiently, during the development and maturation of the cochlea. Within this group, the P2X2 receptor subunit dominates hearing physiology. These findings were established across a range of in vitro and in vivo experiments. The studies point to two complementary processes within the cochlea utilising P2X2-type ATP-gated ion channels. The first concerns electrochemical homeostasis: P2X2 expression is evident in the sensory hair cells and the cells lining the endolymphatic compartment. Electrophysiological characteristics of the ATP-activated conductance in these cells is consistent with a P2X2 homomeric configuration. In P2rx2 null mice, the ATP-gated conductance is absent1. Given that noise exposure stimulates release of ATP from cochlear tissues, this likely drives an electrical shunt that causes a decrease in the driving force for sound transduction. This represents an adaptive oto-protective signaling mechanism2. A second process involves post-synaptic P2X2 receptors of the spiral ganglion neuron—hair cell synapse that modulates glutamatergic transmission3. These processes are integrated with longer-term transcriptional responses which we have studied using genome-wide microarray analysis of the cochlea. This has been established using sustained elevated sound levels that produce P2X2 receptor-selective hearing adaptation. The results indicate a major contribution from the MAP kinase signaling pathways.
References
1. Housley GD, Morton-Jones R, Vlajkovic SM, Telang RS, Paramananthasivam V, Tadros SF, Wong ACY, Froud KE, Cederholm JME, Sivakumaran Y, Snguanwongchai P, Khakh BS, Cockayne DA, Thorne PR, Ryan AF (2013) Proc Natl Acad Sci USA 110:7494–7499
2. Housley GD, Bringmann A, Reichenbach A (2009) Trends Neurosci 32:128–141
3. Weisz C, Glowatzki E, Fuchs P (2009) Nature 461:1126–1129
Purinergic modulation of developing central auditory circuits
Ivan Milenkovic1,*, Saša Jovanovic2, Tamara Radulovic1,2 and Rudolf Rübsamen2
1Carl Ludwig Institute for Physiology, Faculty of Medicine, University of Leipzig, Germany;2Institute of Biology, Faculty of Biosciences, Pharmacy and Psychology, University of Leipzig, Germany
In the developing auditory system, the endogenous ATP release within the cochlea and the cochlear nucleus, the first central station along the afferent auditory pathway, enhances the action potential firing that arises independently of experience or any systemic input. Such neuronal activity during the critical period of postnatal development is considered important for establishment and refinement of topographic maps.
Our studies investigate the contribution of purinergic signaling to development of functional brainstem circuits involved in processing of acoustic cues. To address this question, we use extracellular in vivo recordings with iontophoretic drug applications and whole cell recordings combined with imaging in acute brainstem slices. We showed that purinergic modulation in developing auditory brainstem circuits of altricial rodents is cell-type specific, follows a defined time course, and a topographic pattern [1]. The release of ATP enhances glutamate-driven firing of bushy cells in the cochlear nucleus via P2X2/3R and a mechanism engaging the activation of the protein kinase C through an increase of cytosolic calcium concentration [2]. This modulatory action is apparently important for development of tuning properties of bushy cells. The experiments performed in P2X2/3RDbl−/− mice revealed impaired frequency selectivity of bushy cells and significantly lower acoustically evoked firing rates compared to wild type controls.
In summary, our studies suggest that the specific pattern of bushy cell modulation mediated by P2X2/3R might be required for a functional stimulus coding in high sound intensity environments.
References
1. Dietz B, Jovanović S, Wielsch B, Nerlich J, Rübsamen R, Milenkovic I (2012) J Neurosci 32:10699–10721
2. Milenkovic I, Rinke I, Witte M, Dietz B, Rübsamen R (2009) J Neurophysiol 102:1821–1833
Purinergic signaling in taste buds
Sue C. Kinnamon1,* and Thomas E. Finger2
1Departments of Otolaryngology and2Cell and Developmental Biology, University of Colorado Medical School, Aurora, CO, 80045, USA
ATP meets all the requirements for a taste transmitter: (1) presence in taste cells, (2) release upon taste stimulation, (3) activation of purinergic receptors P2X2 and P2X3 on afferent nerve fibers, and (4) enzymatic degradation by NTPDase2, expressed by the glial-like taste cells. Evidence for purinergic neurotransmission in taste is based primarily on the finding that mice lacking both P2X2 and P2X3 lack responses to all taste stimuli [1]. These data suggest that for all taste qualities, ATP release is required to activate afferent nerve fibers. However, subsequent studies have measured ATP release only from taste cells that respond to bitter, sweet, and umami taste; no release has been detected from taste cells that transmit sour and salty taste information [2,3]. To further investigate the role of ATP as a taste transmitter, we obtained mice genetically lacking NTPDase2, the predominant ectoATPase of taste buds. These mice show elevated levels of extracellular ATP in taste buds. Remarkably, responses to all taste qualities were depressed in these knockouts, presumably due to desensitization of the P2X3-containing subunits of the afferent fibers [4]. Finally, we used a membrane-permeant inhibitor of P2X3-containing subunits, AF-353, to pharmacologically inhibit the P2X receptors on the afferent nerves. Applied either directly to the tongue or injected i.p., AF-353 blocks all taste evoked responses in a dose-dependent fashion. These data strongly suggest that ATP is required for transmission of all taste qualities to the nervous system. Further studies are required to determine the cellular source of ATP for sour and salty tastes.
References
1. Finger TE et al (2005). Science 310:1495–1499
2. Huang YJ et al (2007) Proc Natl Acad Sci U S A 104:6436–6441
3. Romanov RA et al (2007) Embo J 26:657–667
4. Vandenbeuch A et al (2013) Proc Natl Acad Sci USA 110:14789–14794
From the front to the back of the eye: new functions for P2 receptors
Maria Jesus Perez de Lara1, Patricia Loma1, Carmen Dominguez-Godinez1, Alba Martin-Gil1, Gonzalo Carracedo2, Ana Guzman-Aranguez1 and Jesus Pintor1,*
1Faculty of Optometry, Universidad Complutense, Biochemistry, Madrid, Spain;2Faculty of Optometry, Universidad Complutense, Optica II, Madrid, Spain
There are an increasing number of actions performed by the nucleotides and dinucleotides through P2 purinergic receptors in the control of ocular physiology. In addition to regulation of relevant processes such as lacrimation or intraocular pressure, we have discovered that P2 receptors also play an important role in facilitating the delivery of drugs into the eye as well as a substantial role in retinal degeneration associated with pathology called glaucoma. In recent years we have seen that the existence of P2Y2 purinergic receptors in corneal epithelial cells, the outermost part of our eye, not only promotes healing of superficial wounds but can also open “the door” for facilitating the entrance of drugs into the eyeball. This fact undoubtedly favors a more efficient manner the treatment of ocular diseases. Our results demonstrate that P2Y2 agonists are able to temporarily change the permeability of the cornea and thereby allow access of drugs to the intraocular environment so, with smaller doses of therapeutic agents greater effects are obtained. This discovery will permit the combined formulation of P2Y2 agonists with different types of drugs for a great variety of intraocular diseases. Since the ocular surface is continuously bathed by the tear, very often the active amount of compound that can be delivered inside the eye is small due to the tear drainage. We have started to use contact lenses as systems for drug delivery, loading them with nucleotides and studying their release as well as their physiological effects.
We have also checked in the disease known as glaucoma, which causes irreversible blindness, the presence of P2X7 receptors in the retinal cells. In glaucoma there is a gradual cell death and therefore the presence of this receptor could be, at least in part, involved in retinal apoptotic processes. In particular, the retinas of glaucomatous animals have abnormally high levels of extracellular ATP as the disease progresses. In parallel, there is also an increase of the P2X7 receptor, which in combination with elevated levels will ATP justify the death of cells of the retina.
In summary, new aspects related to P2 purinergic receptors are appearing in the eye, that span the importance of nucleotides and dinucleotides as molecules for the treatment of coular pathologies.
Purinergic signalling in the olfactory bulb
Christian Lohr
Division of Neurophysiology, University of Hamburg, Martin-Luther-King-Pl. 3, 20146 Hamburg, Germany
In the olfactory bulb, ATP is released as a co-transmitter together with glutamate from axon terminals of sensory neurons. Brief and rapid application of ATP excites mitral cells, the principle neurons in the olfactory bulb, projecting to higher brain centres such as the piriform (olfactory) cortex. Sustained application of ATP, in contrast, results in a hyperpolarisation and hence inhibition of mitral cells. This hyperpolarisation can be mimicked by adenosine, suggesting that adenosine derived from ATP degradation inhibits mitral cells (Fig. 1A). A1 receptor-deficient mice lack the inhibitory effect of adenosine, and adenosine fails to hyperpolarize mitral cells in the presence of the A1 antagonist DPCPX, indicating that the adenosine-dependent inhibition is mediated by A1 receptors. Adenosine increases the K+ conductance of the mitral cell membrane in a G-protein-dependent manner. G-protein-dependent inwardly rectifying K+ (GIRK) channels are often modulated by A1 receptors. However, the GIRK channel blocker barium, as well as various other canonical K+ channel blockers, failed to inhibit the adenosine-mediated hyperpolarisation, leaving the identity of the A1-activated channel unknown. To analyse the effect of A1 receptor-mediated inhibition of mitral cells on the activity of the neuronal network, we measured spontaneous synaptic currents in voltage-clamped mitral cells, which receive synaptic input from, and give synaptic input into most if not all types of neurons in the olfactory bulb. Adenosine significantly reduced the frequency of synaptic events, indicating that the inhibition of mitral cells reduces the activity of the entire network (Fig. 1B). The direct synaptic input from sensory neurons to mitral cells, in contrast, was not affected by adenosine. The unchanged sensory input in conjunction with the reduced background network activity results in an increased signal-to-noise ratio upon stimulation of sensory neurons in the presence of adenosine. This renders adenosine as a good candidate to modulate odour information processing in the olfactory bulb.
Fig. 1 A) Effect of 100 μM adenosine on the membrane potential of a mitral cell. B) Adenosine reduces the activity of the olfactory bulb neuronal network as measured by spontaneous synaptic currents in a mitral cell
Sat 3 A: Purine-based therapeutic approaches in cancer therapy
A2AAR antagonists: the next generation of checkpoint blockade
Jonathan D. Powell
Sidney Kimmel Comprehensive Cancer Center, Johns Hopkins University, Baltimore, MD, USA
The last several years have witnessed exciting progress in the development of immunotherapy for the treatment of cancer. In great part this has been due to the development of so-called checkpoint blockade. That is antibodies that block inhibitory receptors such as CTLA-4 and PD-1 and thus unleash antigen specific immune responses against tumors. It is clear that tumors evade the immune response by usurping pathways that play a role in negatively regulating normal immune responses. In this regard, adenosine in the immune microenvironment leading to the activation of the A2a receptor has been shown to represent one such negative feedback loop. Indeed, the tumor microenvironment has relatively high concentrations of adenosine. To this end blocking A2a receptor activation has the ability to markedly enhance anti-tumor immunity in mouse models. Data will be presented demonstrating the ability of A2A receptor blockade to enhance tumor vaccines, checkpoint blockade and anti-tumor antibody mediated responses. Taken together these studies support the development of A2a receptor antagonists (some of which have already been tested in phase 3 clinical trials for Parkinson’s disease) as novel modalities in the immunotherapy armamentarium.
Adenosine effects on T cell homeostasis in tumors
Caglar Cekic* and Joel Linden
La Jolla Institute for Allergy and Immunology, La Jolla, CA, USA
Extracellular adenosine generated in tumor microenvironments contributes to immune escape orchestrated by tumor cells and T regulatory cells through the expression of exonucleases such as CD39 and CD73, which convert extracellular ATP to adenosine. Adenosine A2A receptors (A2AR) on innate and adaptive immune cells are high affinity targets for adenosine and regulate many physiological processes such as heart rate, wakefulness, inflammation, T cell development and maintenance1. In this study we demonstrated that myeloid-deletion of the adenosine A2A receptor gene (adora2a) strongly inhibits solid tumor growth and metastasis. This was associated with increased antigen presentation by macrophages and increased numbers and activation of tumor-associated T cells. Surprisingly, global and lymphoid deletion of adora2a produced modest and substantial increases, respectively, in B16F10 melanoma growth and decreased the numbers of tumor associated effector/memory T cells. In mice with ovalbumin-expressing tumors, adoptively co-transferred adora2a−/− OT-I T cells upregulated the activation marker CD25, but these cells were not maintained and failed to differentiate into effector/memory populations as compared to adora2a+/+ OT-I T cells, suggesting that T cell inhibitory A2AR signaling plays an important role in T cell homeostasis in solid tumors. Mechanistic studies revealed that T cell adora2a deletion significantly reduced anti-apoptotic CD127 (IL-7Rα) expression while increasing apoptosis. In summary, myeloid vs. T cell signaling of A2AR signaling differentially affects homeostasis of tumor associated T cells (Fig. 1). The results of this study have important implications for the development cell-based therapies targeting adenosine receptors.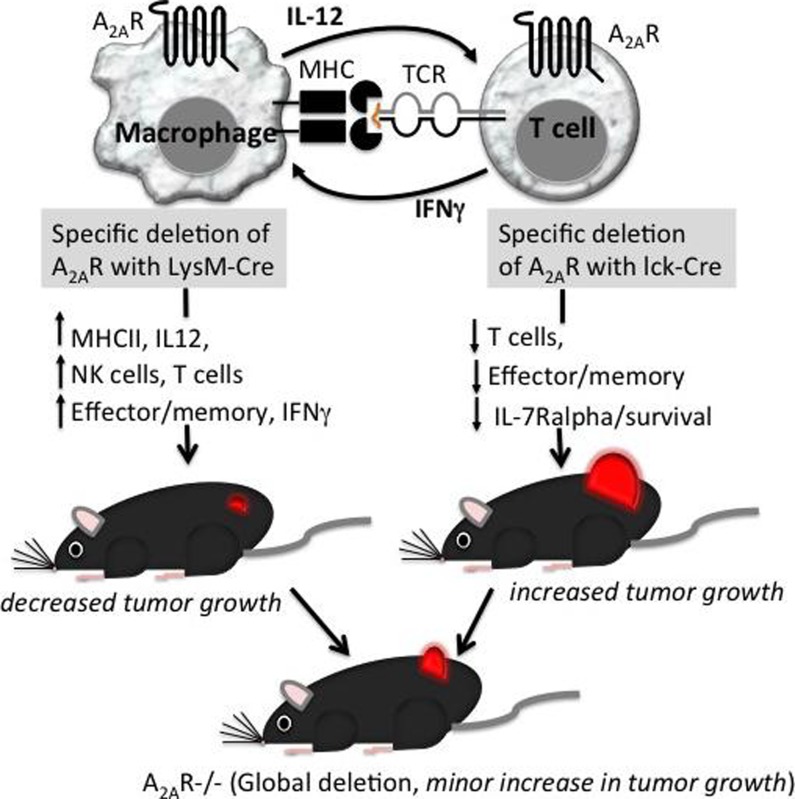
Fig. 1 Summary of findings
Reference
1. Cekic C, Sag D, Day YJ, Linden J (2013) Extracellular adenosine regulates naive T cell development and peripheral maintenance. J Exp Med 210:2693
The P2X7 receptor enhances sensitivity to chemotherapeutic drugs in lymphoma cells
Vera Labitzky, Janne Becher, Welbeck Danquah, Friedrich Koch-Nolte and Friedrich Haag*
Institute of Immunology, University Medical Center, Hamburg, Germany
Extracellular ATP (eATP) concentrations are elevated in many tumors. eATP and its metabolites exert multiple effects on tumors, including the transmission of survival signals and regulation of the anti-tumor immune response. Among other receptors, eATP acts on the P2X7 receptor, which is expressed by immune cells and many tumors. Accumulation of eATP not only results from tissue damage, but also from regulated secretion of ATP. We have recently observed that gating of P2X7 itself is a potent stimulus for ATP secretion. Low-level gating of P2X7 has been shown to benefit tumor growth by stimulating ATP production [1]. However, when Yac-1 cells were treated with the cytostatic drug doxorubicine (DXR), stimulation of P2X7 with ATP synergistically enhanced its cytotoxic effects. Intriguingly, incubation with ATP and DXR for 1 h was sufficient to kill the majority of cells under conditions where neither agent alone showed significant cytotoxicity. Co-incubation with ATP also increased DXR-mediated surface exposure of calreticulin, a hallmark of the immunogenic cell death and unfolded protein response (UPR) pathways [2]. Indeed, co-incubation with ATP also amplified cell death caused by other inducers of the UPR, such as the proteasome inhibitor bortezomib and the reducing agent DTT. In conclusion, our studies show that gating of P2X7 induces Yac-1 lymphoma cells to actively secrete substantial amounts of ATP into the extracellular environment. Under “steady-state” conditions, this may be beneficial for the tumor. In the context of chemotherapy, however, P2X7 signalling may amplify drug effects by increasing drug uptake and synergistically amplifying drug-induced cell death pathways.
References
1. Adinolfi E, Raffaghello L, Giuliani AL, Cavazzini L, Capece M, Chiozzi P, Bianchi G, Kroemer G, Pistoia V, Di Virgilio F (2012) Cancer Res 72:2957–2969
2. Kroemer G, Galluzzi L, Kepp O, Zitvogel L (2013) Ann Rev Immunol 31:51–72
Platelet-derived nucleotides promote tumor-cell transendothelial migration and metastasis via P2Y2 receptor
Dagmar Schumacher1,3, Boris Strilic1,3, Kishor Kumar Sivaraj1, Nina Wettschureck1,2 and Stefan Offermanns1,2,*
1Max-Planck-Institute for Heart and Lung Research, Department of Pharmacology, Ludwigstr. 43, 61231 Bad Nauheim, Germany;2Medical Faculty, J.W. Goethe University Frankfurt, Theodor-Stern-Kai 7, 60590 Frankfurt, Germany
3These authors contributed equally to this work.
Tumor cells can activate platelets which in turn facilitate tumor cell survival and dissemination. The exact mechanisms by which platelets promote metastasis have remained unclear. We show that adenine nucleotides released from tumor cell-activated platelets induce opening of the endothelial barrier to allow transendothelial migration of tumor cells and thereby promote cancer cell extravasation. We identified the endothelial P2Y2 receptor, which is activated by ATP, as the primary mediator of this effect. Mice lacking ATP-secretion from platelets and mice with endothelial-specific P2Y2 deficiency show strongly reduced tumor cell metastasis. These findings demonstrate a novel mechanism by which platelets promote cancer cell metastasis and suggest the P2Y2 receptor and its endothelial downstream signaling mechanisms as a target for anti-metastatic therapies.
Genetic and pharmacological targeting of A2A adenosine receptor unleashes anti-tumor effector functions of CD8+ T cells and enables immunological memory
Stephen Hatfield, Jorgen Kjaergaard, and Michail Sitkovsky
Northeastern University, Biology, Boston, USA
The immunosuppression of tumor-reactive T cells in the tumor microenvironment (TME) involves many functionally distinct mechanisms including suppressor cells and soluble immunosuppressive factors. Tumor hypoxia-driven accumulation of extracellular adenosine has been suggested to be instrumental in aiding the process of tumor evasion by engaging the intracellular cAMP-elevating A2 adenosine receptors on tumor-reactive effector T cells.
Here, we report and review the effects of targeting the hypoxia-driven and CD39/CD73 mediated immunosuppression in the TME caused by extracellular adenosine. This suppression is governed by cAMP-elevating A2A adenosine receptors on the surface of endogenously developed or adoptively transferred T cells. The role of anti-hypoxia A2A and A2B adenosinergic coadjuvants is discussed in the context of recent successes of promising cancer immunotherapies. It is emphasized that even after the removal of all immunological negative regulators of the immune response, tumors may still be protected by hypoxia-adenosinergic immunosuppression.
Sat 3 B: Neuroprotective functions for purinergic receptors in the CNS
Mechanisms of neuroprotection for nucleotides involving P2Y receptor activation and control of reactive oxygen
Georg Reiser*, Michael Haas and Daniel Förster
Medizinische Fakultät der Otto-von-Guericke Universität Magdeburg, Institut für Neurobiochemie, Leipziger Str. 44, 39120 Magdeburg
Genetic polymorphisms of the human P2Y11 receptor lead to the development of neurodegenerative disease. 3′UTR-SNP of the P2RY11 gene is associated with narcolepsy/cataplexy, an auto-immune disorder resulting in the loss of hypocretin-producing neurons in the hypothalamus. This SNP results in threefold P2Y11 receptor expression reduction in CD8+T-lymphocytes and natural killer cells. Our study of the Ala-87-Thr mutation of the P2Y11 receptor revealed a detrimental effect on P2Y11 receptor function in cells co-expressing the P2Y1 receptor. Both receptors form hetero-oligomers. The Ala-87-Thr SNP is associated with increased risk for acute myocardial infarction. This P2Y11 receptor mutation was detrimental to intracellular signaling. Furthermore, nucleotide-induced receptor internalization was abolished. Importantly, calcium response resensitization after prolonged ATP treatment was improved. Thus, genetic polymorphisms of the P2Y11 receptor cause neurodegenerative disorders by altering long-term activity [1]. We aim to mediate neuroprotection through regulation of the human P2Y11 receptor activity. Additionally, we developed novel potent and specific P2Y11 receptor agonists. Furthermore, we explore the physiological consequences of the P2Y11 receptor activity in cells. Oxidative stress is important for pathogenesis of many neurodegenerative diseases. P2Y receptors are targets for protection by attenuating oxidative stress. We show that treatment of hydrogen peroxide-challenged primary rat astrocytes with P2Y1 receptor-specific agonist 2MeSADP and with P2Y11 receptor-specific agonist NF546 increased viability, reduced reactive oxygen species production, and cell death. Also increased levels of GPX and SOD3 were shown after preincubation with P2Y1 and P2Y11 receptor agonists. These results complete previously published data (Azran et al. 2013; J Med Chem. 2013; 56:4938–52), which showed that primary cortical neurons from rats are protected by nucleotide analogues against iron-induced oxidative stress. We address the concept of nucleotides being dual neuroprotectants with P2Y receptor ligands and antioxidants. Further, we consider receptor agonists in animal models of neurodegeneration.
Reference
1. Haas M, Shaaban A, Reiser G (2014) J Neurochem 129:602–613
Activation of cytokine responses by the P2X7 receptor in chronic neurodegenerative diseases; the good, the bad and the unexpected
Claire H. Mitchell
University of Pennsylvania, Philadelphia, PA 19104
The P2X7 receptor is expressed throughout the nervous system, and while originally thought of as pathological, newer findings suggest beneficial roles. Previous work showed over-stimulation of the P2X7R kills retinal ganglion cells, but dephosphorylation of released ATP into adenosine protects via actions at A1 and A3 receptors, implying ecto-ATPases regulate neuronal survival. However, recent findings show P2X7R stimulation triggers cytokine release, and some of these released cytokines may be protective. Transient stretch of retinal cells in vivo following elevation of intraocular pressure (IOP) upregulated mRNA and protein for cytokine IL-6; this rise was blocked by P2X7R antagonist BBG. Cytokine and pannexin upregulation also occurred in a chronic model of IOP elevation, implying a sustained response. Isolated retinal astrocytes and neurons were stretched separately in vitro to identify key cell types. In astrocytes, stretch led to pannexin-mediated release of ATP and autostimulation of P2X7Rs, with sustained stretch triggering an upregulation of pannexin message. Both stretch and P2X7R agonist BzATP triggered release of IL-6 from astrocytes. Stretch of isolated retinal ganglion cells led to release of IL-3; release was blocked by P2X7R antagonists, emulated by P2X7R agonists and required extracellular calcium. Interestingly, IL-3 was neuroprotective. In summary, in both astrocytes and neurons, stretch leads to ATP release from pannexin channels and autostimulation of P2X7Rs, leading to cytokine release and, in some cases, protection. The sustained upregulation of pannexins with prolonged insults suggest possible protective roles for the pannexin/P2X7/cytokine axis in chronic neural diseases.
P2Y2receptors mediate neuroprotective responses: a potential role in retarding Alzheimer’s disease progression
Gary A. Weisman1,3,*, Deepa Ajit1, Lucas T. Woods1, Jean M. Camden1, Laurie Erb1,3, Douglas C. Miller2 and Grace Y. Sun1, 3
1Department of Biochemistry,2Department of Pathology & Anatomical Sciences and3Interdisciplinary Neurosciences Program, University of Missouri, Columbia, MO, USA
Alzheimer’s disease (AD) is a neurodegenerative disorder associated with chronic neuroinflammation. Activation of microglia leads to release of cytokines, including IL-1β, that induce the expression of G protein-coupled P2Y2 nucleotide receptors (P2Y2Rs) in neurons. Subsequent activation of P2Y2Rs by adenosine 5′-triphosphate (ATP) or uridine 5′-triphosphate (UTP) promotes neurite extension and non-amyloidogenic processing of amyloid precursor protein (APP) [1,2]. Furthermore, cytotoxic, soluble Aβ1–42 exposure induces upregulation of microglial P2Y2Rs whose activation promotes microglial cell migration and the uptake and degradation of Aβ1–42 [3]. Therefore, we investigated the role of the P2Y2R in an AD mouse model (TgCRND8) that expresses the Swedish and Indiana mutations in APP. Results indicate that the P2Y2R is upregulated in TgCRND8 mice at 10 weeks of age. Heterozygous P2Y2R deletion in TgCRND8 mice leads to increases in Aβ plaque load and soluble Aβ1–42 levels, a decrease in CD11b+ and CD45+ microglia and neurological deficits within 10 weeks of age, as compared to age-matched TgCRND8 mice [4]. The survival of TgCRND8 mice after homozygous or heterozygous P2Y2R deletion is drastically reduced with few mice surviving past 85 days, a time point when TgCRND8 mice are viable. These results suggest that the P2Y2R regulates neuroprotective mechanisms in vivo that when lost accelerate an AD-like phenotype and cause premature death in the TgCRND8 mouse model of AD.
References
1. Peterson TS, Thebeau CN, Ajit D, Camden JM, Woods LT, Petris MJ, Wood WG, Sun GY, Weisman GA (2013) J Neurochem 125:885–896
2. Kong Q, Peterson TS, Baker OJ, Stanley E, Camden JM, Seye CI, Erb L, Wood WG, Sun GY, Weisman GA (2009) J Neurochem 109:1300–1310
3. Kim HJ, Ajit D, Peterson TS, Wang Y, Camden JM, Wood WG, Sun GY, Erb LE, Petris M, Weisman GA (2011) J Neurochem 121:228–238
4. Ajit D, Woods LT, Camden JM, Thebeau CN, Greeson GW, Erb L, Petris MJ, Miller DC, Sun GY, Weisman GA (2014) Mol Neurobiol 49:1031–1042
A2AR, a double-edge sword for neuroprotection in the central nervous system
Ya-Lei Ning and Yuan-Guo Zhou*
The Molecular Biology Center, State Key Laboratory of Trauma, Burn and Combined Injury, Research Institute of Surgery and Daping Hospital, Third Military Medical University, Chongqing, Chinae-mail: ygzhou@tmmu.edu.cn
The A2A receptor (A2AR) is one of four adenosine receptors, which belongs to the family of the G-protein-coupled receptor. It has been emerged as an attractive therapeutic target for modulating brain insult in various animal models of acute and chronic neurological disorders. Here we report that: (i) available evidences had suggested that either activation or inactivation of A2AR confers neuroprotection against a broad spectrum of brain insults, indicating the bidirectional effect of A2AR in the central nervous system (CNS) diseases and injuries; (ii) the local glutamate level in the brain injury is a crucial factor for the bidirectional effect of A2AR effect on neuroinflammation and brain injury outcome, increased extracellular glutamate level switches the effect of A2AR activation from antiinflammatory effect and neuroprotection to proinflammatory effect and aggravation. (iii) elevated plasma glutamate after severe traumatic brain injury (TBI) also mediated the proinflammatory effect of A2AR activation in neurogenic acute lung injury (ALI) mouse model and patients, further confirm the key role of local glutamate level in bidirectional regulation of A2AR effects on CNS. These developments offer new insight into the complexity of A2AR–glutamate interactions and suggest a novel strategy for controlling brain injury by regulating A2AR activity according to local glutamate levels or by combining A2AR modulators (agonist or antagonist) with glutamate inhibitors. These developments also confirm that A2AR ligands have many promising characteristics that encourage the pursuit of their full therapeutic potential.
Neuroprotection mediated by P2Y13nucleotide receptors in neurons
Raquel Pérez-Sen1,* Verónica Morente1, Mª José Queipo1, Felipe Ortega2, Esmerilda G. Delicado1 and Mª Teresa Miras-Portugal1
1Department of Biochemistry, Veterinary Faculty, Complutense University of Madrid, Spain;2Institute of Physiological Chemistry, University Medical Center of the Johannes Gutenberg-Universität Mainz, Germany
The ADP specific P2Y13 receptor behaves as key player in granule cell physiology, in terms of signalling events and surviving promoting actions. P2Y13 receptors were specifically coupled to the main survival promoting PI3K/Akt-cascade in neurons targeting the phosphorylation of glycogen synthase kinase-3 (GSK3). The inhibition of GSK3 catalytic activity led to nuclear translocation and accumulation of the transcriptional factors, β-catenin and Nrf-2, which regulate a set of survival genes. The activation of Nrf-2 master antioxidant regulator promoted the expression of heme oxygenase-1 (HO-1) and contributed to the neuroprotection elicited by P2Y13 receptors against apoptosis induced by oxidative stress [1]. In addition, MAPK signalling was also regulated by P2Y13 receptors. The activation of ERK1/2-dependent transcription factor, CREB, underlined the antiapoptotic action against glutamate excitotoxicity [2]. Beyond direct action on transducing kinases, a novel signalling mechanism was found for P2Y13 receptors in granule neurons that accounted for regulation of MAP kinase phosphatases, named MKPs, which function as dual-specificity phosphatases (DUSPs) and mediate recovery of basal levels of MAPKs activated after several kinds of stress stimuli. Among this family, one of the main targets was the nuclear inducible phosphatase DUSP2. DUSP2 expression in granule neurons was dependent on PI3K/ERK1/2 canonical signalling displayed by P2Y13 receptors. Restoring DUSP2 expression levels prevented p38 long-lasting activation induced by genotoxic damaging conditions and promoted neuron survival (Fig. 1) [3]. Overall these data demonstrate the pivotal role of P2Y13 receptors to cope with different types of damaging stimuli that compromise neural cell viability.
Fig. 1 P2Y13 receptors induce DUSP2 expression and regulate MAPK signalling recovery
References
1. Espada S, Ortega F, Molina-Jijón E, Rojo AI, Pérez-Sen R et al (2010) Free Radic Biol Med 49:416–426
2. Ortega F, Pérez-Sen R, Delicado EG, Miras-Portugal MT (2011) Neuropharmacology 61:1210–1221
3. Morente V, Pérez-Sen R, Ortega F, Huertas-Cepa J, Delicado EG et al (2014) BBA (second revision)
Sat 3 C: Regulation of lipid and glucose metabolism by purines / purinergic regulation of tumor growth and metastasis II
Energy homeostasis in mice lacking adenosine receptors
Yingqing Wang1, Vijay Urmalia1, Marie Björnholm2, Max Winerdal3, Mattias Carlström1 and Bertil B. Fredholm1,*
1Department of Physiology and Pharmacology,2Department of Molecular Medicine and Surgery,3Department of Woman and Child Health, Karolinska Institutet, Stockholm, Sweden
It is well known that adenosine levels are regulated by metabolism and that adenosine can influence many aspects of metabolism. We have examined how the overall metabolism of mice is affected by knocking out one or more of the adenosine receptors. We used metabolic cages from Columbus Instruments and SOMEDIC and examined glucose tolerance and temperature sensitivity.
Both male and female A2AR knockout (ko) mice gained weight compared to wild-type (Wt) mice. This was not due to altered food intake, which was in fact if anything decreased (especially after a short-term fasting), or increased heat production. Instead it could be ascribed to decreased motor activity. A1R ko mice did not differ much in overall body weight compared to Wt mice, but their body composition was very different with increased lean mass and decreased body fat. This tended to be more marked in old compared to young mice and the explanation is likely that A1R are very important in blocking lipolysis and activating lipogenesis [1]. Motor activity was not markedly affected, but food intake tended to increase, especially after fasting, in A1R ko mice. There were no clear differences in body temperature over a range of temperature from 12 to 35 degrees centigrade between Wt and A1R or A2AR ko mice, and no differences in heat production even when induced by lower ambient temperature. A1A2AR double ko mice tended to behave as A2AR ko mice. A2BR ko mice had essentially normal body weight, motor activity, food intake and body composition. A3R ko mice showed a decreased activity and heat production, and increased food intake, but an unaltered body weight [2]. Glucose tolerance tended to increase in A1R ko mice and decrease in male, but not female, A2AR and A2BR ko mice. The present results thus show that the different adenosine receptors influence overall energy balance in quite different ways.
References
1. Johansson S, Lindgren E, Yang J-N, Herling AW, Fredholm BB (2008) Eur J Pharmacol 597:92–101
2. Yang J, Wang Y, Garcia-Roves P, Björnholm M, Fredholm BB (2010) Acta Physiologica 199:221–230
An adenosine receptor-Krüppel-like factor 4 axis in control of adipogenesis
Anna Eisenstein, Shannon H. Carroll, Hillary Johnston-Cox, Melissa Farb, Noyan Gokce and Katya Ravid*
Departments of Medicine and Biochemistry, Whitaker Cardiovascular Institute, Boston University School of Medicine, Boston, MA 02118, USA
It has log been suggested that young and old fat cells have different metabolic output, which further underscores the importance of understanding the control of fat cell biogenesis within a tissue. Adenosine, an extracellular nucleotide signaling molecule, found in adipose tissue depots acts on adenosine receptors. Here, we report that among these receptors, the A2b adenosine receptor (A2bAR) is highly expressed in mouse and human adipocyte progenitors, and this receptor expression is downregulated upon fat cell differentiation. Activation of the A2bAR with a specific ligand potently inhibits differentiation of mouse stromal-vascular cells (SVCs) into adipocytes, while A2bAR knockdown stimulates adipogenesis, as marked by an array of adipose specific transcription factors. The A2bAR inhibits differentiation through a novel signaling cascade partly involving cAMP, leading to sustained expression of Krüppel-like factor 4 (KLF4), a regulator of stem cell maintenance. Knockout or knockdown of KLF4 ablates the ability of A2bAR to inhibit differentiation. A2bAR activation also inhibits adipogenesis in a human primary preadipocyte culture system. We analyzed the A2bAR-KLF4 axis in adipose tissue of obese individuals, and intriguingly found a strong correlation between A2bAR and KLF4 expression in both subcutaneous and visceral human fat. Hence, our study points to the A2bAR-KLF4 axis as a potentially significant regulator of adipose biology.
Adenosine receptor signaling in brown fat
Alexander Pfeifer
Institute of Pharmacology and Toxicology, Sigmund-Freud-Str. 25, D-53105 Bonn, Germany
Brown adipose tissue (BAT) is specialized in energy expenditure whereas white fat (WAT) is the biggest storage for energy. An imbalance between energy intake and consumption leads to obesity, the abnormal increase in white fat. Brown adipocytes contain a large number of mitochondria and express the uncoupling protein 1 (UCP1), which uncouples mitochondrial ATP production and is essential for generation of heat. BAT plays an important role for non-shivering thermogenesis in newborn humans. Importantly, recent studies from several labs have clearly shown the presence of metabolically active BAT in human adults [1–3] and BAT activity and abundance inversely correlates with body-mass-index [4,5].
Here I summarize the effects of adenosine on BAT activation.
Previous studies have shown that adenosine via adenosine receptor A1 inhibits lipolysis in WAT [6-8]. Additionally, adenosine is critical for the regulation of glucose homeostasis and insulin signaling in mice [9–11]. Importantly, similar to findings in WAT, adenosine inhibits lipolysis of hamster and rat brown adipocytes and reduces the sensitivity to catecholamines [12–16]. Besides adipocytes function, there is additional evidence that adenosine receptors regulate adipogenic differentiation [17]. Furthermore, the talk will be about our recent data on murine BAT.
References
1. Virtanen KA et al (2009) Functional brown adipose tissue in healthy adults. N Engl J Med 360:1518–1525
2. van Marken Lichtenbelt WD et al (2009) Cold-activated brown adipose tissue in healthy men. N Engl J Med 360:1500–1508
3. Cypess AM et al (2009) Identification and importance of brown adipose tissue in adult humans. N Engl J Med 360:1509–1517
4. Pfannenberg C et al (2013) Impact of age on the relationships of brown adipose tissue with sex and adiposity in humans. Diabetes 59:1789–1793
5. Vijgen GH et al (2011) Brown adipose tissue in morbidly obese subjects. PLoS One 6:e17247
6. Johansson SM, Lindgren E, Yang JN, Herling AW, Fredholm BB (2008) Adenosine A1 receptors regulate lipolysis and lipogenesis in mouse adipose tissue-interactions with insulin. Eur J Pharmacol 597:92–101
7. Johansson SM et al (2007) A1 receptor deficiency causes increased insulin and glucagon secretion in mice. Biochem Pharmacol 74:1628–1635
8. Dhalla AK, Santikul M, Chisholm JW, Belardinelli L, Reaven GM (2009) Comparison of the antilipolytic effects of an A1 adenosine receptor partial agonist in normal and diabetic rats. Diabetes Obes Metab 11:95–101
9. Faulhaber-Walter R et al (2011) Impaired glucose tolerance in the absence of adenosine A1 receptor signaling. Diabetes 60:2578–2587
10. Figler RA et al (2009) Links between insulin resistance, adenosine A2B receptors, and inflammatory markers in mice and humans. Diabetes 60:669–679
11. Johnston-Cox H et al (2012) The A2b adenosine receptor modulates glucose homeostasis and obesity. PLoS One 7:e40584
12. Schimmel RJ, McCarthy L (1984) Role of adenosine as an endogenous regulator of respiration in hamster brown adipocytes. Am J Physiol 246:C301–307
13. Szillat D, Bukowiecki LJ (1983) Control of brown adipose tissue lipolysis and respiration by adenosine. Am J Physiol 245:E555–559
14. Woodward JA, Saggerson ED (1986) Effect of adenosine deaminase, N6-phenylisopropyladenosine and hypothyroidism on the responsiveness of rat brown adipocytes to noradrenaline. Biochem J 238:95–403
15. McMahon KK, Schimmel RJ (1982) Apparent absence of alpha-2 adrenergic receptors from hamster brown adipocytes. Life Sci 30:1185–1192
16. Unelius L, Mohell N, Nedergaard J (1990) Cold acclimation induces desensitization to adenosine in brown fat cells without changing receptor binding. Am J Physiol 258:C818–826
17. Gharibi B, Abraham AA, Ham J, Evans BA (2011) Contrasting effects of A1 and A2b adenosine receptors on adipogenesis. Int J Obes (Lond) 36(3):397–440
Unsuspected role of guanylate metabolism in tumor invasion
Anna Bianchi-Smiraglia, Joseph Wawrzyniak, Sudha Mannava, and Mikhail Nikiforov*
Roswell Park Cancer Institute, Cell Stress Biology, Buffalo, USA
Question: Invasion is a prerequisites for metastasis which is the most detrimental feature of virtually all types of cancers. Often acquisition of invasive phenotypes by tumor cells occurs already at early stages of tumor progression. One of the major requirements for the invasion of any malignant cell is the ability to degrade the extra-cellular matrix (ECM) and the underlying basement membrane in order to escape the primary site of growth. Many factors can influence tumor cell invasion, including formation of invadopodia, specialized subcellular actin-rich structures that recruit proteolytic enzymes to the areas of cell-ECM contact. In many types of cancers, including melanoma, invasion and the ability to form invadopodia have been strongly associated with the activity of small GTPases, in particular those of the RHO-GTPase family. The question of whether tumor cells possess intrinsic ability to regulate activity of the above GTPases and subsequently invasion by manipulating intracellular GTP pools has never been addressed.
Methods: Cell imaging, immunoblotting, immunohistochemisry, matrigel-based invasion assay, combined gelatin degradation assay, active GTPase pull-down assay, quantitative real time PCR, HPLC-based quantifiaciton of nucleotides.
Results: Here, we demonstrate that a purine nucleotide metabolism enzyme guanosine monophosphate reductase (GMPR) suppresses the ability of highly invasive melanoma cells to invade in vitro and grow as tumor xenografts in vivo. Mechanistically, GMPR partially depleted intracellular GTP pools and reduced the amounts of several GTP-bound (active) RHO-GTPases. shRNA-mediated inhibition of GMPR or addition of exogenous guanosine increased activity of the same RHO-GTPases and up-regulated invasion in melanoma cells and cells from different types of cancers. Ectopic expression of constitutively active RAC1G12V, insensitive to GTP depletion, substantially reverted GMPR-dependent phenotypes. Moreover, the functional role of intracellular GTP levels in tumor cell invasion was confirmed by inhibiting activity of other enzymes involved in the de novo GMP biosynthesis. Importantly, GMPR expression was down-regulated in invasive stages of human melanoma progression.
Conclusions: Our data identified a previously unrecognized ability of cancer cells to increase the activity of RHO-GTPases via up-regulation of GTP pools, and established a novel and fundamental connection between tumor cell invasion and metabolism of guanylates.
Identification of a murine monoclonal antibody specific for human CD73 and inhibiting extracellular adenosine production generated by the CD38/CD203a/CD73 ectoenzymatic pathway
Antonella Chillemi1,*, Alberto L. Horenstein1,2, Valeria Quarona1, Fabio Morandi3, Gianluca Zaccarello1, Andrea Zito1, Valentina Mariani1, Vito Pistoia3 and Fabio Malavasi1,2,4
1University of Torino, Medical Sciences, Torino, Italy;2University of Torino, Centro di Ricerca in Medicina Sperimentale (CeRMS), Torino, Italy;3Istituto Giannina Gaslini, Department of Experimental and Laboratory Medicine, Genova, Italy;4“Città della Salute e della Scienza” Hospital, Transplantation Immunology Service, Torino, Italy
Selected poster K 168
Sat 3 D: Novel purine (-like) receptors
Expression and function of adenine receptors in the rodent kidney
Bellamkonda K. Kishore1,*, Yue Zhang1, János Peti-Peterdi,2 Haykanush Gevorgyan2, Donald E. Kohan1, Anke C. Schiedel3 and Christa E. Müller3
1University of Utah and VA SLC Health Care System, Salt Lake City, Utah, USA;2University of Southern California, Los Angeles, USA;3University of Bonn, Bonn, Germany
Cyclic AMP is an important mediator of kidney functions, such as regulation of water and sodium absorption in response to arginine vasopressin. Binding of adenine to the Gi-coupled adenine receptor (AdeR) reduces cellular cAMP levels. Circulating adenine levels are markedly increased in chronic kidney disease (CKD) patients, and positively correlate with the duration and severity of the disease. Thus increased adenine levels may potentially contribute to pathophysiology of CKD. Hence, we studied the expression and function of AdeR in rat and mouse kidney. One adenine receptor has been described in the rat (rAdeR), while in mouse two receptors have been reported (mAde1R & mAde2R), all coupled to Gi. Using a peptide-derived polyclonal antibody that recognizes rat and mouse AdeR we detected the expression in vasculature, collecting ducts (CD) and other structures of the kidney. In the CD, AdeR protein is expressed in AQP2 positive cells, with co-localization at the apical domain. Functionally, in freshly isolated rat inner medullary collecting ducts (IMCD), adenine significantly decreased the desmopressin (dDAVP)-induced increase in cAMP production. This was blocked by PSB-08162, a selective and potent antagonist of AdeR [1]. In contrast, in freshly isolated mouse IMCD, adenine did not decrease dDAVP-induced cAMP production. On the contrary, addition of adenine to primary cultures of mouse IMCD cells significantly augmented dDAVP-induced increase in AQP2 protein, and this effect was blocked by PSB-08162. Thus, these data suggest that in addition to the two Gi-coupled mAdeRs, a novel and perhaps more potent Gs-coupled AdeR may exist in the mouse kidney.
Reference
1. Kishore BK et al (2013) Am J Physiol Renal Physiol 305:F1298–F1305
The rat adenine receptor: mode of action and signalling pathway
Marcus Bloßfeld* and Karen Nieber
Pharmacology for Natural Sciences, University of Leipzig, Leipzig, Germany
Adenine has recently been identified as the endogenous ligand of a novel G-protein coupled receptor in rats. Radioligand binding studies using membrane preparations provide evidence for the expression of adenine receptors in rat neuronal tissue which could be coupled to a Gi-protein [1,2]. The functionality and relevance of this new receptor is still unknown. The aim of the present study was to characterize the activation of the adenine receptor by its endogenous ligand on rat neuroblastoma cells (B104) using cytotoxicity assays and calcium imaging. Intracellular recordings on cortical pyramidal cells in rat brain slices under normoxic and hypoxic conditions were done to establish the influence on synaptic transmission. Additionally experiments were carried out to determine a possible signalling pathway.
In B104 cells adenine (10 nM to 1 mM) concentration-dependently induced cell death, determined by release of lactatdehydrogenase. Adenine did not influence the basal intracellular calcium concentration but inhibited the ATP-induced increase of intracellular Ca2+ concentration in B104 cells. In rat brain slices adenine depressed concentration-dependently the electrically evoked postsynaptic potentials. The decrease of synaptic transmission during hypoxia was attenuated by adenine (1 mM), due to an interaction with the adenosine A1 receptor. The Gi protein inhibitor N-ethylmaleimide did not alter the effects of adenine in any of the methods. In summary our findings contribute to a possible mode of action of adenine receptor activation and provide evidence for the existence of different adenine receptor subtypes in rat neuronal tissue.
References
1. Bender et al (2002) PNAS 99(13):8573–8578
2. Gorzalka et al (2005) Mol Pharmacol 67:955–964
How does extracellular guanosine regulate some of its biological effects?
Shucui Jiang 1,2,*, Caixin Su1, Cai Jiang1, Mariachiara Zuccarini3, Renata Ciccarelli3, Patrizia Di Iorio3, Francesco Caciagli3 Patrizia Ballerini4 and Michel P Rathbone2
1Department of Surgery (Neurosurgery, Neurobiology),2Department of Medicine (Neurology, Neuroscience) and Hamilton NeuroRestorative Group (NRG), McMaster University, Health Sciences Centre, 1280 Main street west, Hamilton, ON, Canada L8S 4K1;3Department of Experimental and Clinical Sciences, Section of Pharmacology, University of Chieti-Pescara, Via dei Vestini, 31, I-66100 Chieti, Italy;4Department of Psychological Sciences, University of Chieti-Pescara, Via dei Vestini, 29, I-66013, Chieti, Italy
Extracellular guanosine, like adenosine, has been shown to have a plurality of physiological effects both in vitro and in vivo, affecting the growth, differentiation and survival of various cells and stimulating a number of intracellular signaling pathways typically activated by G-protein coupled receptors. We also reported preliminary evidence for the existence of a high-affinity binding site, specific for guanosine in rat brain membranes. However, the identification of a selective receptor for guanosine is still lacking. In the present study, we first identified an orphan G-protein coupled receptor (GPCR) as a possible guanosine receptor (G1 receptor) using bioinformatics approaches. Then we verified that guanosine specifically bound to the G1R when transfected into Drosophila Schneider 2 (S2) cells that normally do not express this receptor. A single binding site for [3H]-guanosine was shown. Furthermore, guanosine made functional binding to G1R. In G1R-transfected S2 cells it significantly inhibited apoptosis, whereas it did not in non-transfected wild type S2 cells. We also used biochemical, pharmacological and physiological techniques to characterize intracellular signalling pathways regulated by guanosine through G1R binding in G1R-transfected S2 and 1321N1 human astrocytoma cells, which latter also do not express G1R, as well as in primary astrocytes compared to those in which the G1R was silences by siRNA. Our data substantially support that the G1R orphan GPCR is a specific receptor for guanosine through which extracellular guanosine plays its biological role in regulating cell functions and opens the avenue to further research in this direction.
Putative involvement of novel receptors in the effects produced by guanine and its derivatives at the central nervous system
Francesco Caciagli1,2,*, Patrizia Di Iorio1, Renata Ciccarelli1, Daniele F. Condorelli2, Natale Belluardo3, Giuseppina Mudò4, Shucui Jiang5 and Michel P. Rathbone6
1Department of Experimental and Clinical Sciences, Section of Pharmacology, University of Chieti-Pescara, Via dei Vestini, 31, 66100 Chieti, Italy;2Department of Biomedical Sciences, University of Catania, V.le A. Doria 6. 95125 Catania. Italy;3Department of Experimental Biomedicine and Clinical Neuroscience, University of Palermo, Corso Tukory 129, 90134 Palermo, Italy; 5Department of Surgery (Neurosurgery, Neurobiology)6Department of Medicine (Neurology, Neuroscience), and Hamilton NeuroRestorative Group (NRG), McMaster University, Health Sciences Centre, 1280 Main street west, Hamilton, ON, Canada L8S 4K1
Non adenine- based purines are detectable in the intercellular milieu as well as the adenine-based counterparts. Indeed, GTP, like ATP, is released from cells and it is extracellularly metabolized into guanosine by the same enzyme pattern deputed to adenine-based nucleotide breakdown. Additionally, our group showed that cells release in the growth medium a pool of enzymes such as purine nucleoside phosphorylase (PNP) and guanase, that in turn are devoted to metabolize the adenine- and non adenine-based nucleosides into the corresponding nucleobases which tend to accumulate in the culture medium. Our recent studies show that guanine (GUA), hypoxanthine (HYPO) and xanthine (XAN) influence, for instance, the proliferation rate or the redox state of different cell lines through a combination of intra- and extra-cellular activities. In an attempt to identify a putative receptor for these nucleobases, we performed a bio-informatic research that allowed to individuate a G-protein coupled orphan receptor, belonging to the superfamily of the rhodopsin receptors, which seems to recognize GUA as the main putative agonist. The stable transfection of this orphan receptor in human astrocytoma cells enhanced by 3–5 fold the GUA effects whereas silencing this receptor, either in wild type cells or in stably transfected cell clones, significantly counteracted the effect of GUA. These results suggest that this receptor has suitable characteristics to become the first possible receptor for guanine nucleobases.
Development of [3H]PSB-13253—a powerful pharmacological tool to explore the P2Y receptor-like orphan G protein-coupled receptor GPR35
Dominik Thimm*, Mario Funke, Anne Meyer, Anke C. Schiedel and Christa E. Müller
PharmaCenter Bonn, Pharmaceutical Institute, Pharmaceutical Chemistry I, An der Immenburg 4, 53121 Bonn, Germany
The P2Y receptor-like GPR35 belongs to the group of G protein-coupled receptors (GPCRs) whose endogenous agonist is still unknown or speculative; therefore they are designated orphan receptors [1]. GPR35 appears to be a promising target for therapeutic intervention e. g. for the treatment of inflammatory diseases and pain. However, we are only at the beginning of understanding its physiological role. Currently available GPR35 agonists have in most cases only moderate potency, and often interact with additional targets in an equipotent manner. Therefore, there is an urgent need for potent and selective GPR35 agonists. We identified 8-amidochromen-4-one-2-carboxylic acids as a novel class of agonists for GPR35, which showed high selectivity versus the most closely related orphan receptor GPR55. The compounds were characterized in β-arrestin recruitment assays and successively optimized to obtain agonists with nanomolar potency [2]. The most potent compound of the series, 6-bromo-8-(4-methoxybenzamido)-4-oxo-4H-chromene-2-carboxylic acid (PSB-13253, EC50 12.1 nM) was selected for radiolabeling, yielding [3H]PSB-13253 with a specific activity of 36 Ci (1.33TBq)/mmol. The new radioligand labeled the human GPR35 with high affinity (KD 5.27 nM). Binding was saturable (Bmax 12.6 pmol/mg protein) and reversible [3]. Based on binding data of a large series of benzamidochromenone derivatives structure-activity relationship analysis allowed the design of new, improved derivatives. The developed fluorine-substituted 6-bromo-8-benzamidochromen-4-one-2-carboxylic acids represent the most potent GPR35 agonists known to date. 6-Bromo-8-(2,6-difluoro-4-methoxybenzamido)-4-oxo-4H-chromene-2-carboxylic acid (Ki 0.589 nM) showed the highest affinity with a subnanomolar Ki value [3]. The radioligand [3H]PSB-13253 and the new potent and selective GPR35 agonists may serve as powerful pharmacological tools to further elucidate the receptor’s (patho)physiological roles and its potential as a future drug target.
References
1. Milligan G (2011) Trends Pharmacol Sci 32:317–325
2. Funke M, Thimm D, Schiedel AC, Müller CE (2013) J Med Chem 56:5182–5197
3. Thimm D, Funke M, Meyer A, Müller CE (2013) J Med Chem 56:7084–7099
Sat 4 A: Purinergic receptor heteromers: from identification to structure and function
Methods to study adenosine heteroreceptor complexes in cellular models and in brain
Dasiel O. Borroto-Escuela1,*, Michael Di Palma1, Ismel Brito2, Manuel Narváez3, David Rodríguez4, Luigi F. Agnati1, Rafael Franco5, Jens Carlsson4 and Kjell Fuxe1
1Department of Neuroscience, Karolinska Institutet, Stockholm, Sweden; 2Artificial Intelligence Research Institute (IIIA-CSIC), Barcelona, Spain;3Department of Physiology, School of Medicine, University of Malaga, Málaga, Spain;4Department of Biochemistry and Biophysics, Stockholm University, Stockholm, Sweden;5Department of Biochemistry, University of Barcelona, Barcelona, Spain
Adenosine receptors play critical roles in cellular processes and signaling and have been shown to form heteromers with diverse biochemical and/or pharmacological activities that are different from those of the corresponding homomers(1,2). However, despite extensive experimental results supporting the formation of adenosine heteromers in heterologous systems, the existence of such heteroreceptor complexes in the brain remains largely unknown, mainly because of the lack of appropriate methodology. Also no systematic study was carried out on heteromers form by adenosine receptor subtypes alone. In this study, we used several experimental approaches(3) to investigate whether adenosine receptor subtypes can form heteromers among themselves and if this has an effect on receptor function. In situ PLA clearly demonstrated that adenosine receptors (A1-A2A, A2A-A2A, A2A-A2B, A2A-A3) exist as heteroreceptor complexes in rat brain. Furthermore, bioluminescence resonance energy transfer analysis of the four possible binary combinations of adenosine A2A receptors established that the they can physically interact in HEK293T27 cells, as both homomers and heteromers. Subsequent screenings of the dynamic agonist modulation of the A2A heteroreceptor complexes and their recruitment of β-arrestin also showed the influence of A2A heterodimerization on internalization and receptor pharmacology. In addition, static/non-dynamical human GPCR data derived from this and other interaction studies were integrated in a large scale graph, called the GPCR heterodimer network (http://www.iiia.csic.es/~ismel/GPCR-Nets/index.html), which provides global insight into adenosine heteromer connectivity, topology and organization in the context of the adenosine receptor subfamily and the GPCR network as a whole.
References
1. Borroto-Escuela DO, Romero-Fernandez W, Tarakanov AO, Ciruela F, Agnati LF, Fuxe K (2011) J Mol Biol 406(5):687–699
2. Cristóvão-Ferreira S, Navarro G, Brugarolas M, Pérez-Capote K, Vaz SH, Fattorini G, Conti F, Lluis C, Ribeiro JA, McCormick PJ, Casadó V, Franco R, Sebastião AM (2013) Purinergic Signal 9(3):433–449
3. Borroto-Escuela DO, Romero-Fernandez W, Garriga P, Ciruela F, Narvaez M, Tarakanov AO, Palkovits M, Agnati LF, Fuxe K (2013) Methods Enzymol 521:281–294
A2A-D2heteroreceptor complexes. Relevance for neurology and psychiatry
Kjell Fuxe1, Karolina Wydra2, Krystyna Gołembiowska2, Bartosz Pomierny3, Agata Suder2, Malgorzata Frankowska2 Wilber Romero Fernandez1, Luigi F. Agnati1, Michael Bader4, Pierre Trifilieff 5,6, Jonathan Javitch5, Malgorzata Filip2,2 and Dasiel Borroto-Escuela1
1Department of Neuroscience, Karolinska Institutet, Stockholm, Sweden;2Laboratory of Drug Addiction Pharmacology and Department of Pharmacology, Institute of Pharmacology, Polish Academy of Sciences, Kraków, Poland;3Department of Toxicology, Faculty of Medicine, Jagiellonian University, Kraków, Poland;4Max-Delbrück-Center for Molecular Medicine, Berlin, Germany;5Department of Neuroscience, Columbia University, New York, NY, USA;6University Bordeaux 2 INRA, Bordeaux, France
Together with Dr. Ungerstedt we found that caffeine and theophyllamine enhanced the action of L-DOPA and DA receptor agonists in the rat hemiparkinsonian model. Evidence for the existence of antagonistic receptor-receptor in A2A-D2 heteroreceptor complexes were subsequently found in collaboration with Prof. Franco [1]. There is support for the view that their A2A protomers are one of the targets for the antiparkinsonian effects of A2A receptor antagonists [2]. We also introduced the hypothesis that cocaine actions on such complexes in the striatum can lead to substance use disorder [3]. More specifically the agonist activation of the A2A receptor protomer of the heteromer inhibits the cocaine activation of D2 protomer signaling (nM range) by restoring the antagonistic A2A-D2 receptor-receptor interactions. A combination of behavioral studies with systemic or local drug injections and in vivo neurochemical analysis was used to investigate A2A receptors in cocaine-induced reward and seeking behaviors. Our findings support a role for A2A receptor activity involving the nucleus accumbens in counteracting the reinforcing and/or motivational properties of cocaine or food rewards and to block cocaine relapse. In cellular models constitutive sigma1R enhanced the cocaine binding to 3H-raclopride bound D2R already at 10 nM (competition), made possible an increase by cocaine (1 nM) of the Bmax values of 3H-raclopride and an enhancement by cocaine (100 nM) of D2 agonist induced signaling and counteracted the D2 agonist induced internalization of D2-YFP. Thus, sigma1 receptor may participate in A2A-D2 heteroreceptor complexes where its enhancing actions on D2 recognition and signaling may be blocked by agonist activation of A2A protomers.
References
1. Fuxe K, Ferré S, Canals M, Torvinen M, Terasmaa A, Marcellino D, Goldberg SR Staines W, Jacobsen KX, Lluis C, Woods AS, Agnati LF, Franco R (2005) J Mol Neurosci 26(2–3): 209–220
2. Fuxe K, Borroto-Escuela DO, Romero-Fernandez W, Palkovits M, Tarakanov AO, Ciruela F, Agnati LF (2014) Neuropsychopharmacology 39(1):131–155
3. Filip M, Zaniewska M, Frankowska M, Wydra K, Fuxe K (2012) Curr Med Chem 19(3):317–355
Evidence for adenosine A1-A2Areceptor heteromers in neurons and astrocytes
Gemma Navarro1,2,3,* Arnau Cordomí2, Monika Zelman-Femiak4,6, Marc Brugarolas1,2,3, David Aguinaga1,2,3, Mireia Medrano1,2,3, Estefania Moreno1,2,3, Antoni Cortés1,2,3, Vicent Casadó1,2,3, Josefa Mallol1,2,3, Enric I. Canela1,2,3, Carme Lluís1,2,3, Leonardo Pardo3, Ana J. García-Saez5,6, Peter J. McCormick1,2,3 and Rafael Franco2,3
1Centro de Investigación Biomédica en Red sobre Enfermedades Neurodegenerativas;2Institute of Biomedicine of the University of Barcelona (IBUB);3Department of Biochemistry and Molecular Biology, Faculty of Biology, University of Barcelona, Barcelona, 08028 Spain;4Laboratori de Medicina Computacional, Unitat de Bioestadística, Facultat de Medicina, Universitat Autònoma de Barcelona, 08193 Bellaterra, Spain;5Max Planck Institute for Intelligent Systems, Heisenbergstr. 3, 70569 Stuttgart;6German Cancer Research Center, Bioquant, Im Neuenheimer Feld 267, 69120 Heidelberg
G-protein-coupled receptor (GPCR) heteromers serve as unique protein complexes that allow cells to sense the environment in a variety of ways. The unexpected heteromerization of adenosine A1 and A2A receptors found in heterologous systems was confirmed in glutamatergic terminals innervating the striatum [1] and, more recently, in primary cultures of astrocytes [2]. The heteromer in nerve cells serves as a sensor that mediates both depression and enhancement of glutamate release depending on the adenosine concentration. Similarly the heteromer in astrocytes allows both positive and negative regulation of GABA uptake, achieved by intermolecular cross-talk within the heteromer. As A2A receptors are coupled to Gs and A1 receptors are coupled to Gi, it was hypothesized that the heteromer may couple to both Gs and Gi. Biophysical energy transfer techniques provided evidence for a tetrameric model formed by two protomers of A1 and two protomers of A2A receptors that are functionally altered by both cholera, which affects Gs, and pertussis, which affects Gi, toxins. The results suggest that the A1-A2A receptor heteromer is a tetramer coupled to both Gs and Gi proteins (see next –R Franco- presentation).
References
1. Ciruela F, Casadó V, Rodrigues RJ, Luján R, Burgueño J, Canals M, Borycz J, Rebola N, Goldberg SR,Mallol J, Cortés A, Canela EI, López-Giménez JF, Milligan G, Lluis C, Cunha RA, Ferré S and Franco R (2006) J Neurosci 26(7):2080–2087
2. Cristóvão-Ferreira S, Navarro G, Brugarolas M, Pérez-Capote K, Vaz SH, Fattorini G, Conti F, Lluis C, Ribeiro JA, McCormick PJ, Casadó V, Franco R and Sebastião AM 2013 Purinergic Signal 9(3):433–449
Allosteric communication between Gi and Gs in the adenosine A1-A2Areceptor tetrameric complex
Rafael Franco2,3,*, Arnau Cordomí3, Monika Zelman-Femiak4,6, Marc Brugarolas1,2,3, Estefania Moreno1,2,3, Antoni Cortés1,2,3, Vicent Casadó1,2,3, Josefa Mallol1,2,3, Enric I. Canela1,2,3, Carme Lluís1,2,3, Leonardo Pardo3, Ana J. García-Saez5,6, Peter J. McCormick1,2,3 and Gemma Navarro1,2,3
1Centro de Investigación Biomédica en Red sobre Enfermedades Neurodegenerativas;2Institute of Biomedicine of the University of Barcelona (IBUB);3Department of Biochemistry and Molecular Biology, Faculty of Biology, University of Barcelona, Barcelona, 08028 Spain;4Laboratori de Medicina Computacional, Unitat de Bioestadística, Facultat de Medicina, Universitat Autònoma de Barcelona, 08193 Bellaterra, Spain;5Max Planck Institute for Intelligent Systems, Heisenbergstr. 3, 70569 Stuttgart;6German Cancer Research Center, Bioquant, Im Neuenheimer Feld 267, 69120 Heidelberg
Adenosine receptors have been paradigmatic to demonstrate the occurrence of G-protein-coupled receptor (GPCR) heteromers. Heteromerization of adenosine A1 and A2A receptors was the answer to reports showing expression of Gs-coupled and Gi-coupled GPCRs in the same cell. Instead of counterbalancing cAMP levels, activation of A1 and/or A2A receptors in the A1-A2A heteromer may result in signaling either via Gs or Gi. In fact, the evidence given in the previous presentation (by G. Navarro) suggests that A1-A2A GPCR heteromers are constituted by tetramers coupled to both Gs and Gi proteins. Using single particle tracking, molecular modeling and functional studies, insight into the structural characteristics and dynamics of heteromers are provided. The A1-A2A receptor heteromeric complex is stable and constituted by two transmembrane helix-4-interacting A1 and A2A homodimers bound together via transmembrane helix 5. The resulting non-square (rombus shaped) heterotetramer forms a complementary interface where one Gi and one Gs protein. Functional studies in the absence and presence of cholera or pertussis toxins indicated that not only both Gi and Gs proteins are present in the A1-A2A GPCR complex, but that they are also are required to signal through A1R or through A2AR.
P2X2 and PX5 subunits define a new heteromeric receptor with P2X7 like properties
Francois Rassendren
Institut de Génomique Fonctionnelle, CNRS UMR5203, INSERM U661, Université de Montpellier, Montpellier, France
Ligand-gated ion channels are prototypic oligomeric membrane proteins whose stoichiometry determines their functional properties and subcellular localization. Deciphering the quaternary structure of such protein complexes is an arduous task and usually requires the combination of multiple approaches. ATP-gated P2X receptors are formed by the association of three subunits, but the quaternary arrangement of the seven P2X subunits at the plasma membrane remains poorly characterized. Based on co-expression of recombinant subunits, 13 potential heteromeric P2X receptors have been proposed. However, to date, P2X2/3 and P2X1/5 receptors are the only unambiguously characterized heteromeric P2X receptors in native tissue, and their stoichiometry remains undefined. By combining bioluminescence resonance energy transfer, bifunctional fluorescence complementation and protein biochemistry, we developed an experimental approach that allows precise determination of rat P2X receptor quaternary assembly. We found that P2X5 subunits associate with P2X1, P2X2 and P2X4 subunits. We demonstrate that P2X5 and P2X2 subunits interact to form as yet uncharacterized heteromeric receptors with alternate stoichiometries, both present at the plasma membrane. P2X2/5 receptors display functional properties such as pore dilatation, membrane blebbing and phosphatidylserine exposure that were previously thought to be characteristic hallmarks of the P2X7 receptor. In mouse, P2X2 and P2X5 subunits co-localize and physically interact in specific neuronal populations suggesting that other P2X receptors might contribute to cellular responses typically attributed to P2X7 receptor.
Sat 4 B: Adenosine receptor antagonism and Parkinson’s and Alzheimer’s disease
Antiparkinsonian actions and interactions of adenosine antagonists
Micaela Morelli1,*, Giulia Costa1 and Annalisa Pinna2
1University, Biomedical Sciences, Cagliari, Italy;2CNR, Institute of Neuroscience, Cagliari, Italy
Modulation of dopamine-mediated responses in Parkinson’s disease (PD) is pursued by several studies aimed at contrasting parkinsonian motor deficits. In experimental models of PD, adenosine A2A receptor antagonists have been shown to improve motor function and to act as disease modifying drugs, whereas in clinical trials, A2A receptor antagonists have been shown to reduce “off” time in dyskinetic PD patients with no exacerbation of dyskinesia. At the same time, 5-HT transmission has been shown to play an important role in expression of dyskinetic movements induced by chronic L-DOPA administration. However, the 5-HT1A/B receptor agonist, eltoprazine, produces suppression of dyskinetic-like movements (AIMs), in rats and monkeys, reducing the therapeutic effecacy of L-DOPA. In light of these evidence, we studied the effect of the combined administration of eltoprazine with an A2A receptors antagonists (preladenant or istradefylline) on the suppression of L-DOPA-induced dyskinesia in unilateral 6-hydroxydopamine (6-OHDA) lesioned rats. Results showed that 6-OHDA-lesioned rats, made dyskinetic by chronic L-DOPA, show a dose-dependent reduction of AIMs with eltoprazine, associated to a reduction of L-DOPA-induced rotational behavior (index of motor activation). However, administration of L-DOPA plus eltoprazine combined with preladenant or istradefylline, reduced AIMs compared to L-DOPA alone, or L-DOPA plus the A2A antagonists, without reducing L-DOPA-induced rotational behavior. Chronic administration of L-DOPA in association with eltoprazine plus preladenant prevented the development of AIMs without reducing motor activation. Biochemical striatal markers of dyskinesia, such as zif-268 or dynorphin were not increased by the chronic treatment of L-DOPA plus eltoprazine and preladenant. Administration of L-DOPA in the presence of an 5-HT1A/B receptor agonist plus an A2A receptor antagonist contrast AIMs without affecting L-DOPA-induced motor stimulation. This drug association might be the first therapy contrasting AIMs without reducing the therapeutic efficacy of L-DOPA.
Adenosine A2Aantagonists in rodent models related to motor and motivational dysfunction
John D. Salamone1,* and Merce Correa2
1Department of Psychology, University of Connecticut, Storrs, CT, USA;2Area of Psychobiology, University of Jaume I, Castello, Spain
Drugs that are selective antagonists for adenosine A2A receptors have been studied for their effects on motor and motivational function. Preclinical studies demonstrated that adenosine A2A antagonists exert antiparkinsonian effects in animal models, and some of these compounds have demonstrated therapeutic efficacy in human studies. Furthermore, adenosine A2A antagonists are being investigated for their potential antidepressant effects. Previous research has demonstrated that adenosine A2A antagonists such as istradefylline, MSX-3 and MSX-4 can reverse the catalepsy and locomotor suppression induced by antagonism of dopamine (DA) D2 receptors. Furthermore, adenosine A2A antagonists show consistent antitremor effects in studies employing the tremulous jaw movement model, reducing the oral tremor in rats induced by various tremorogenic agents, and enhancing the ability of subthalamic deep brain stimulation to suppress tremor. Recent studies have focused on the ability of MSX-3 to attenuate the motor and motivational effects of the catecholamine depleting agent tetrabenazine (TBZ). TBZ induces oral tremor and catalepsy in rats, and also suppresses locomotion; these effects are reversed by co-administration of MSX-3. MSX-3 also suppressed oral tremor in mice, and adenosine A2A knockout mice show less oral tremor than wildtype controls. In animal models related to the motivational symptoms of depression, TBZ reduces selection of high effort activities, and these effects also were attenuated by MSX-3. MSX-3 also reversed the striatal signal transduction effects (cFos or DARPP-32 expression) of TBZ. These studies highlight the potential clinical utility of adenosine A2A antagonists for the treatment of motor and motivational dysfunctions in humans.
Adenosine and cannabinoid receptor heteromer complexes within basal ganglia output neurons in macaques. Changes following experimental parkinsonism
José L. Lanciego1,2,*, Salvador Sierra San Nicolás1,2, Iria G. Dopeso-Reyes1,2, Alberto J. Rico1,2, Elvira Roda1,2, María Lanz1, Diego Pignataro1,2 and Diego Sucunza1
1Department of Neurosciences, Center for Applied Medical Research, University of Navarra, Pamplona, Spain;2CiberNed, Pamplona, Spain
Here we have demonstrated that transcripts for adenosine 2A (A2A) and cannabinoid receptors types 1 and 2 (CB1 and CB2) are co-expressed within identified pallidothalamic-projecting neurons in control, MPTP-treated and dyskinetic monkeys. Although A2A mRNA expression levels remained unchanged across all experimental groups, there is a clear downregulation of CB1 and CB2 mRNAs in dyskinetic monkeys when compared to control and MPTP-treated animals. Moreover, the use of an in situ proximity ligation assay revealed the presence of A2A-CB1 and A2A-CB2 receptor heteromers in basal ganglia output neurons in all animal groups. However, a marked reduction in the number of heteromeric complexes was observed in dyskinetic animals, mimicking the observed reduction in CB1 and CB2 mRNA expression levels, therefore suggesting that the reduction in cannabinoid receptors is the limiting factor in the formation of these heteromers since A2A mRNA levels were not affected in the dyskinetic state. Furthermore, a significant number of A2A-CB1 and A2A-CB2 heteromers was found in subcellular locations other than in the plasma membrane. Finally, it is worth noting that A2A-CB1 and A2A-CB2 heteromers were only found in somatodendritic locations of pallidothalamic neurons (e.g., not found in axon terminals). Determining the precise function of heteromeric complexes made of adenosinergic and cannabinoid receptors will pave the way for the discovery of specific drugs that may either reduce basal ganglia output increased activity of provide a better management of levodopa-induced dyskinesia.
Fig. 1 Presence of A2A-CB2 receptor heteromers (red channel) within a pallidothalamic-projecting neuron (retrogradely-labeled with CTB, green channel) of a MPTP-treated monkey
Caffeine and A2Areceptors: what interest for the TAU side of Alzheimer’s disease?
David Blum
INSERM Institut national de la santé et de la recherche médicale, Lille, France
Alzheimer’s disease (AD) is characterized by extracellular amyloid deposits and intraneuronal neurofibrillary tangles, made of aggregated hyper- and abnormally phosphorylated Tau proteins. The latter, referred to as “Tau pathology”, contribute to synaptic impairments leading to memory deficits in AD patients. Consumption of caffeine, a non-selective adenosine A2A receptor (A2AR) antagonist, reduces the risk of developing Alzheimer’s Disease (AD) in Human and mitigates both amyloid and Tau burden in transgenic mouse models. However, the impact of selective A2AR blockade on the progressive development of AD-related lesions and associated memory impairment has not been investigated. In the present study, we explored the outcome of A2AR gene deletion in the THY-Tau22 transgenic mice, which progressively develop hippocampal Tau pathology and spatial memory defects. We removed the gene encoding the A2A receptor from the THY-Tau22 mouse model of AD-like Tau pathology and analyzed the subsequent effects on both pathological (Tau phosphorylation and aggregation, neuro-inflammation) and functional impairments (spatial learning and memory, hippocampal plasticity, neurotransmitters profile). We found that deleting A2AR protects from Tau pathology-induced deficits in terms of spatial memory and hippocampal long-term depression. These effects were concomitant with a normalization of the hippocampal glutamate/GABA ratio, together with a global reduction in neuro-inflammatory markers and a decrease in Tau hyperphosphorylation. By showing that A2AR knockout improves the pathological phenotype in a Tau transgenic mouse, the present data highlight A2A receptors as important molecular targets to consider in Alzheimer’s Disease and Tauopathies.
Sat 4 C: Purinergic receptor polymorphisms and their pathological implications
P2X and P2Y receptor polymorphisms in bone health and disease
Niklas R. Jørgensen
Research Center for Ageing and Osteoporosis, Departments of Diagnostics and Medicine, Glostrup Hospital, University of Copenhagen, Glostrup, Denmark
Over the last decade, our understanding of the role of P2 receptors and ATP mediated signalling in normal bone biology as well as in osteoporosis patho-physiology has increased significantly. Not only can P2 receptor modulation influence bone formation and bone resorption in vitro and in vivo, but genetic mutations and polymorphisms affect both P2 receptor function and the quality of the skeleton and thus the ability to resist fractures. Several studies have demonstrated the presence of polymorphisms affecting P2 receptor function, with the P2X7 receptor being the most polymorphic with several loss-of-function polymorphisms identified. Recently, a number of studies have shown an association between both P2Y2 [1], P2X4 [2] and P2X7 [3-6] receptor polymorphisms and the development of osteoporosis and risk of fractures.
Very recently, it was demonstrated that P2X7 receptor polymorphisms are also associated with the risk of multiple myeloma7. Individuals carrying the variant allele of the 151+1g>t polymorphism or belonging to a high risk group defined from the number of alleles from loss-of-function polymorphisms in the P2X7 receptor gene had significantly higher OR of multiple myeloma than individuals not carrying these variant alleles. In contrast, myeloma patients carrying alleles conferring gain-of-function of the P2X7 receptor had significantly higher risk of vertebral fractures than individuals belonging to the intermediate and high risk groups (normal and low P2X7 receptor function) as defined above. Thus, P2X7 receptor polymorphisms are associated with risk of multiple myeloma and development of vertebral fractures in these patients, indicating that P2X7 receptors are involved in the diseases mechanisms.
References
1. Wesselius A et al (2013) Purinergic Signal 9:41–49
2. Wesselius A et al (2013) Purinergic Signal 9:123–130
3. Wesselius A et al (2013) Osteopor Int 24:1235–1246
4. Jørgensen NR et al (2012) Eur J Human Genetics 20:675–681
5. Gartland A et al (2012) Eur J Human Genetics 20:559–564
6. Husted LB et al (2013) Osteopor Int 24:949–959
7. Vangsted A et al (2014) Eur J Haematol (Epub ahead of print)
Polymorphisms in the P2X7 receptor and their genetic associations with multiple sclerosis and macular degeneration
Ben J. Gu
Multiple sclerosis is a chronic inflammatory disease of the central nervous system characterized by oligodendrocyte damage, demyelination and neuronal death. Genetic association studies have shown a two-fold or greater prevalence of the HLA DRB1*1501 allele in the MS population compared with normal Caucasians. In two large cohorts of patients with multiple sclerosis (total 2,636 patients and 1,794 controls) we examined the associations of twelve functional polymorphisms of P2X7, a microglial/macrophage receptor with proinflammatory effects which is activated by extracellular ATP. In initial and replication cohorts the SNP coding for Arg307Gln (rs 28360457) was associated with MS and combined analysis showed a two-fold lower minor allele frequency compared with controls (1.14 % for MS and 2.14 % for controls, p = 0.00021). Analysis showed this association was independent of adjoining functional SNPs and was significant after Bonferroni correction. Arg307Gln confers a major loss of function on the P2X7 receptor but four other SNPs conferring major loss of function on the P2X7 receptor showed no association with MS. Modelling based on the homologous zP2X4 receptor showed Arg307 was located in a region rich in basic residues which may represent a second ligand binding site. Analysis showed Arg307Gln was associated with all three clinical subtypes of MS. Analysis according to DRB1*1501 status showed significant association of Arg307Gln with patients who were DRB1*1501 negative (OR 0.53, p = 0.010) but no association was seen with patients who were DRB1*1501 positive (OR 0.69, p = 0.30). Our data show the protective effect of a rare genetic variant and thus implicates the microglial/macrophage P2X7 receptor in the neuroinflammation of MS.
Cardiovascular genetics of P2-receptors
David Erlinge
Department of Cardiology, Lund University, Lund, Sweden
This is a review-presentation about the most clinically important genetic polymorphisms in P2-receptors and purinergic genes.
A common loss-of-function SNP of the P2Y11 receptor is overrepresentated in patients with myocardial infarction. The probable mechanism of action is a proinflammatory effect, because CRP was elevated. The SNP confers partial loss-of-function, which is reflected in a decrease in the T-helper cell population in carriers, likely due to attenuation of the anti-apoptotic effects of the receptor.
Another loss-of-function SNP in the ATP stimulated P2X7 receptor, protects against myocardial infarction in smokers and against stroke in the whole population. This indicates that an antagonist against P2X7 could be a developed to a medical agent against atherosclerotic disease.
A loss-of-function polymorphism in the human P2X4 receptor is associated with increased pulse pressure. Electrophysiological studies showed that it reduced the peak amplitude of the ATP-induced inward current by 81 %. There was significant association with pulse pressure where 1 minor allele increased pulse pressure by 2.84 mmHg. Presented at this meeting by Sathanoori, transient transfection of static HUVEC cultures with the P2X4 a Tyr315>Cys mutation construct, blocks ATP-mediated KLF2 and NOS3 expression. Collectively, these results suggest that shear stress-induced KLF2 is dependent on ATP released by endothelial cells acting via the P2X4 receptor and has clinical implications for pulse pressure.
ATP acts as a survival signal and prevents the mineralization of aortic valve. A genetic polymorphism in the intron 9 of the ENPP1 gene is associated with calcific aortic valve disease.
The important platelet P2Y12 and P2Y1 receptors do not have any polymorphisms of clinical importance. However, the clopidogrel prodrug enzyme CYP2C19-polymorphisms determine responders to antiplatelet therapy.
In conclusion, polymorphisms in purinergic genes reveal new mechanisms for cardiovascular disease. P2Y11 and P2X7 are involved in development of myocardial infarction and P2X7 in stroke. P2X4 is important for shear stress and pulse pressuree and ENPP1 is involved in aortic calcification.
Origin, structure and function of frequent coding polymorphisms in P2X7
Carolina Pinto1, Welbeck Danquah1, Jan Knop1, Friedrich Haag1, James S. Wiley2 and Friedrich Koch-Nolte1,*
1Institute of Immunology, University Medical Center, Hamburg, Germany;2Florey Neuroscience Institutes, University of Melbourne, Victoria, Australia
Several single nucleotide polymorphisms have been identified in the human P2RX7 gene [1]. In contrast to other members of the P2X family, coding polymorphisms in P2X7 are quite common [2]. Three prominent polymorphisms in the head, lower body, and tail domains of P2X7 occur at allele frequencies >20 % (figure 1): 155 Y/H, 270 R/H, and 348 T/A. Comparison of the P2X7 orthologues of human and other great apes indicates that the ancestral allele is Y - R - T (at 155–270–348). Interestingly, each single amino acid variant displays lower ATP-sensitivity than the ancestral allele. The originally published reference sequence of human P2X7 [3] is often referred to as “wildtype”. Remarkably, this variant H - H - A differs from the ancestral allele at all three positions. The 1,000 genome project [2] revealed striking differences in the frequencies of the ancestral vs. variant P2X7 alleles in different human populations (e.g. 25–59 % for Y155, 59–77 % for R270, and 13–47 % for T348). BLAST analyses of ancient human genome sequences uncovered several homozygous carriers of variant P2X7 alleles, possibly reflecting a high degree of inbreeding, e.g. H - R - T for a 50,000 year old Neanderthal, H - R - A for a 24,000 year old Siberian, and Y - R - A for a 7,000 year old mesolithic European [4]. In contrast, most present day human individuals co-expresses two copies of P2X7 that differ in one or more amino acids at positions 155, 270 and 348.
Fig. 1 The high frequency coding polymorphisms of human P2X7: Y155H, R270H, T348A (7,000 year old mesolithic human P2X7 variant Y - R - A modeled on P2X4 pdb 4dw1)
References
1. Stokes L et al (2010) FASEB J 24:2916–2927
2. The 1,000 genome consortium (2010) Nature 491:56–65
3. Rassendren F et al (1997) JBC 272:5482–5486
4. Prüfer K et al (2014) Nature 505:43–49
5. Olade I et al (2014) Nature 507:225–228
Polymorphism of NPP1 and its impact on purinergic signalling
Marie-Chloé Boulanger* and Patrick Mathieu
Quebec Heart and Lung Institute, Laval University, 2725, chemin Ste-Foy, Quebec, Canada
We have recently identified that single nucleotide polymorphisms (SNPs) of NPP1 were associated with ectopic mineralization in patients with cardiovascular disorders. One of the SNPs located in intron 9 was associated with a higher level of NPP1. In-depth functional studies provided evidence that NPP1 modulates the signalling through P2Y2 receptor (P2Y2R) and in doing so promotes the mineralization of vascular and valvular cells. When, highly expressed NPP1 contributes to deplete the pericellular pool of nucleotides and decreases PI3K/Akt signalling downstream of P2Y2R, whereby apoptotic mediated mineralization is triggered. Hence, there is a critical balance in vascular and valvular cells between purinergic signalling and the expression of NPP1. Hence, SNPs of NPP1 gene may be functionally related to ectopic mineralization by modifying purinergic signalling.
Sat 4 D: Biomarkers and imaging of purine receptors
Purine nucleosides: biomarkers and neuroprotectants in ischemic brain damage
Bettina Thauerer, Stephanie zur Nedden and Gabriele Baier-Bitterlich*
CCB Biocenter, Division of Neurobiochemistry, Medical University of Innsbruck, Innrain 80-82, A-6020 Innsbruck, Austria
The blockade of oxygen flow in brain may lead to inhibition of oxidative phosphorylation and depletion of cellular ATP, resulting in profound deficiencies in cellular functions. Following ischemia, purine nucleosides are released from dying, injured, and hypoxic cells and remain elevated for days after the insult. Growing evidence suggests that purine nucleosides are not only biomarkers of ischemic brain damage but might act as trophic factors in the CNS and PNS [ref in (1)].
Multiple endogenous signaling pathways regulate the critical balance between cell death and survival. Our group has focused on purine nucleoside-mediated regulation of modalities involved in O2 sensing, such as MAPK- and protein kinase C-related kinase-pathways and the control of essential amino acids phenylalanine and tyrosine [ref in (1-4)]. An in vitro hypoxia model was developed that allows the cultivation of neurons in a reduced oxygen atmosphere. Results, also discussed in the presentation, demonstrate, that purine nucleosides potentiate endogenous protective mechanisms and corroborate current efforts to propagate purine nucleosides as potential neuroprotective and neuroregenerative substances.
References
1. Thauerer B, zur Nedden S, Baier-Bitterlich G (2008) J Neurochem 121:329–342
2. Thauerer B, zur Nedden S, Baier-Bitterlich G (2010) J Neurochem 113:432–346
3. zur Nedden S, Thauerer B, Baier-Bitterlich G (2008) J Neurochem 105:1901–1914
4. Thauerer B, Geisler S, Fuchs D, Baier-Bitterlich G (2013) Pteridines 24:245–250
PET imaging to measure therapy-related occupancy and disease-induced changes of expression of adenosine A1 receptors
Soumen Paul1, Philip H Elsinga1, Shivashankar Khanapur1, Kiichi Ishiwata2, Peter Meerlo3 Rudi A Dierckx1 and Aren van Waarde1,*
1University of Groningen, University Medical Center Groningen, Department of Nuclear Medicine and Molecular Imaging, Hanzeplein 1, 9713 GZ Groningen, The Netherlands;2Tokyo Metropolitan Institute of Gerontology, Research Team for Neuroimaging, 35-2 Sakae-Cho, Itabashi-Ku, Tokyo 173-0015, Japan;3University of Groningen, Center for Behaviour and Neurosciences, Nijenborgh 7, 9747 AG Groningen, The Netherlands
Adenosine A1 receptors (A1R) in the brain of experimental animals and humans can be visualized using a selective radioligand and positron emission tomography (PET). PET imaging may allow assessment of the occupancy of A1R by non-radioactive drugs and changes of A1R expression as a consequence of disease. We have explored these possibilities by performing preclinical pilot studies in isoflurane-anesthetized rodents, using the xanthine antagonist [11C]MPDX and microPET [1-3]. Rats were pretreated with the A1R antagonists caffeine and DPCPX, or the centrally active A1R agonist N6-cyclopentyladenosine (CPA). Other animals received the adenosine kinase inhibitor ABT-702 combined with ethanol in order to raise levels of endogenous adenosine in their brains. Herpes simplex virus-induced encephalitis was employed as a disease model. Saline-treated or sham-infected rats served as controls. MicroPET studies with arterial blood sampling were made in all groups. Dose-dependent occupancy of A1R by antagonists could be assessed with microPET. A caffeine dose of 4 mg/kg resulted in 66 % occupancy and a caffeine dose of 40 mg/kg or a DPCPX dose of 3 mg/kg in virtually complete (>98 %) occupancy. However, intraperitoneal administration of an agonist (CPA) or raising the levels of endogenous adenosine both resulted in an increase rather than a decrease of tracer binding in the brain. Thus, A1R occupancy by agonists could not be assessed with PET. Encephalitis was associated with increases of tracer binding potential in hippocampus, cerebellum and medulla. Immunohistochemistry confirmed increased expression of A1R. This upregulation may limit the release of excitatory neurotransmitters and may reduce neuronal excitability.
References
1. Paul S, Khanapur S, Rybczynska AA, Kwizera C, Sijbesma JWA, Ishiwata K, Willemsen ATM, Elsinga PH, Dierckx RAJO, van Waarde A (2011) J Nucl Med 52:1293–1300
2. Paul S, Khanapur S, Sijbesma JWA, Ishiwata K, Elsinga PH, Meerlo P, Dierckx RAJO, vanWaarde A (2014) J Nucl Med 55:315–320
3. Paul S, Khanapur S, Boersma W, Sijbesma JWA, Ishiwata K, Elsinga PH, Meerlo P, Doorduin J, Dierckx RAJO, van Waarde A (2014) Neuroimage 92C:83–89
A human pharmacogenetic and brain imaging perspective on sleep homeostasis and neuronal plasticity
Hans-Peter Landolt
Institute of Pharmacology and Toxicology, University of Zürich, Zürich, Switzerland;Zürich Center for interdisciplinary Sleep Research (ZiS), University of Zürich, Zürich, Switzerland;Zürich Center for Integrative Human Physiology (ZIHP), University of Zürich, Zürich, Switzerland;Zürich Center for Neuroscience (ZNZ), University and ETH Zürich, Zürich, Switzerland
Electroencephalographic (EEG) slow-wave activity (SWA; also known as EEG delta activity) in non-rapid-eye-movement (NREM) sleep provides the best established biomarker of sleep homeostasis. The homeostatic facet of NREM sleep may be the most important aspect of sleep-wake regulation in elucidating the unknown biological functions of sleep. SWA reflects sleep intensity and sleep need, and is tightly regulated by the prior history of wakefulness and sleep. Studies in animals demonstrate that SWA is determined by genetic factors that play important roles in activity-dependent synaptic plasticity and long-term potentiation. In humans, the genetic and molecular mechanisms underlying sleep homeostasis remain elusive. In this presentation, recent studies will be discussed that aimed at identifying molecular mechanisms determining trait-like, individual differences in the rebound of SWA after sleep deprivation. The findings provide convergent neurophysiological, genetic, pharmacological, and molecular brain imaging data that support the occurrence of plastic synaptic processes across the sleep-wake cycle in humans. More specifically, functional genetic variation of genes regulating activity-dependent synaptic plasticity, such as brain-derived neurotrophic factors (BDNF) [1], adenosine deaminase (ADA) [2] and adenosine A2A receptors (ADORA2A) [3,4], and fragile X mental retardation protein/subtype 5 metabotropic glutamate receptors (FMR1/mGluR5) [5], profoundly impact on SWA in NREM sleep. Taken together, the data are consistent with the concept that sleep homeostasis may reflect the need of the brain to counteract overstimulation and ‘excitotoxicity’ associated with (prolonged) wakefulness.
Research supported by Swiss National Science Foundation, ZiS, ZIHP, and ZNZ.
References
1. Bachmann V, Klein C, Bodenmann S, Schäfer N, Berger W, Brugger P, Landolt HP (2012) The BDNF Val66Met polymorphism modulates sleep intensity: EEG frequency- and state-specificity. Sleep 35:335–344
2. Bachmann V, Klaus F, Bodenmann S, Schäfer N, Brugger P, Huber S, Berger W, and Landolt HP (2012) Functional ADA polymorphism increases sleep depth and reduces vigilant attention in humans. Cereb Cortex 22:962–970
3. Bodenmann S, Hohoff C, Freitag C, Deckert J, Rétey JV, Bachmann V, Landolt HP (2012) Polymorphisms of ADORA2A modulate psychomotor vigilance and the effects of caffeine on neurobehavioral performance and sleep EEG after sleep deprivation. Br J Pharmacol 165:1904–1913
4. Landolt HP (2012) “No Thanks, Coffee Keeps Me Awake”: Individual Caffeine Sensitivity Depends on ADORA2A Genotype. Sleep 35:899–900
5. Hefti K, Holst SC, Sovago J, Bachmann V, Buck A, Ametamey SM, Scheidegger M, Bethold T, Gomez-Mancilla B, Seifritz E, Landolt HP (2013) Increased metabotropic glutamate receptor subtype 5 availability in human brain after one night without sleep. Biol Psychiatry 73:161–168
Imaging of adenosine receptors
David Elmenhorst1,*, Tina Kroll1, Andreas Matusch1 and Andreas Bauer1,2
1Forschungszentrum Jülich, Institute of Neuroscience and Medicine 2, Jülich, Germany;2Heinrich Heine University Düsseldorf, Medical Faculty, Neurological Department, Düsseldorf, Germany
Over the last decades adenosine receptor ligands, agonists as well as antagonists, have been developed. The requirements for compounds suitable for non-invasive in vivo imaging of adenosine receptors (radiopharmaceuticals, radiotracers) with positron emission tomography (PET) are in several aspects different from those for therapeutic drugs. This difference will be elucidated for radiotracers involved in human neurotransmission research.
In humans the A1 adenosine receptor (A1AR) shows the most abundant distribution and highest concentrations in brain cortical and subcortical areas, whereas the A2A adenosine receptor (A2AAR) can be found in selected regions like striatum, nucleus accumbens, olfactory tubercle. A2B adenosine receptors (A2BAR) and A3 adenosine receptors (A3ARs) are expressed in low levels in the brain. Most of the imaging probes therefore target the A1AR and A2AAR. The talk will give an overview of currently used imaging probes and applications. The neuroreceptor imaging technique has been used for example to investigate physiological mechanisms of the sleep wake regulation or pathophysiological conditions like cerebral ischemia, ethanol intoxication, epilepsy or Alzheimer’s disease in humans and animal models. Pharmacokinetic analysis of PET experiments allow additionally to investigate drug action in the human brain, like for example the impact of caffeine on A1AR availability.
Human brain imaging of Adenosine A1and A2Areceptors
Masahiro Mishina
Department of Neurological Science, Graduate School of Medicine, Nippon Medical School, Tokyo, Japan
Positron emission tomography (PET) is a nuclear medicine imaging technique that allows in vivo imaging of regional receptor binding capacity.
We successfully visualized adenosine A1 receptors (A1Rs) in living humans with 11C-8-dicyclopropylmethyl-1-methyl-3-propylxanthine (11C-MPDX) PET [1]. The density of A1Rs for normals was large in the striatum and thalamus, and small in the cerebellum in the PET image [2]. In Alzheimer’s disease, low density of A1Rs was observed in the temporal cortex and thalamus [3].
We also developed PET ligands for mapping adenosine A2A receptors (A2ARs) in the human brain, 11C-(E)-8-(3,4,5-trimethoxystyryl)-1,3,7-trimethylxanthine (11C-TMSX) [4,5]. The density of A2ARs is largest in the putamen, but is low in the cerebral cortex [5]. A1Rs in the human striatum decrease with age but not A2ARs [6]. In the Parkinson’s disease (PD), the putaminal density of A2ARs was increased in the PD patients with dyskinesia to compere with normals (Fig. 1) [7]. In de-novo patients, we found that A2ARs were asymmetrically down-regulated in the putamen (Figure 1B). We also found that the density of A2ARs was increased in the putamen after antiparkinsonian therapy in the de-novo patients.
Fig. 111C-TMSX PET images for a healthy man (A), a de-novo patient with Parkinson’s disease (B) and a patient with dyskinesia (C)
References
1. Ishiwata K et al (2002) Ann Nucl Med 16:377–382
2. Fukumitsu N et al (2005) J Nucl Med 46:32–37
3. Fukumitsu N et al (2008) Ann Nucl Med 22:841–847
4. Ishiwata K et al (2000) J Nucl Med 41:345–354
5. Mishina M et al (2007) Synapse 61:778–784
6. Mishina M et al (2012) Synapse 66:832–839
7. Mishina M et al (2011) PLoS One 6:e17338
Abstracts—Posters
A: Structure of purine receptors and ectonucleotidases
A 001
Structural studies on ectonucleotidases involved in purinergic signaling
Christoph Döhler1,*, Matthias Zebisch1,2 and Norbert Sträter1
1Universität Leipzig, Center for Biotechnology and Biomedicine, Leipzig, Germany;2University of Oxford, Wellcome Trust Centre for Human Genetics, Oxford, Germany
Nucleotide pyrophosphatses/phosphodiesterases (NPPs) are a family of ectophosphodiesterases comprising seven members in vertebrates. NPPs are glycoproteins and able to hydrolyze a wide range of molecules involved in different signaling pathways (e.g. in purinergic signaling). Whereas NPP1 and 3 are specific for nucleotides and dinucleotides, the natural substrates of NPP2, NPP5 and NPP7 are phospholipids. NPP1-3 include besides the catalytic domain a nuclease-like domain, which has no catalytic activity. Furthermore at the N-terminus of NPP1-3 two consecutive cysteinerich somatomedin B (SMB) like domains are located, which are involved in substrate binding (NPP2) and membrane anchoring (NPP1 and 3). NPP4-7 are only contain the catalytic domain. Apart from NPP2 all NPP family members are membrane associated [1]. Based ontheir involvement in many physiological functions and diseases NPPs are regarded as attractive drug targets. We aim to determine crystal structures of these proteins to characterize the structural basis of substrate specificity and the catalytic mechanisms. Structures of NPP1 and NPP2 from vertebrates revealed first insights in domain arrangement and ligand binding [2-3]. Neverthelessfor further investigations of the catalytic function of NPP enzymes high resolution structures in complex with substrates or substrate analogs are needed.
References
1. Stefan C, Jansen S, Bollen M (2006) Modulation of purinergic signalling by NPP-type ectophosphodiesterases. Purinergic Signal 2:361–370
2. Nishimasu H, Okudaira S, Hama K, Mihara E, Dohmae N, Inoue A, Ishitani R, Takagi J, Aoki J, Nureki O (2011) Crystal structure of autotaxin and insight into GPCR activation by lipid mediators. Nat Struct Mol Biol 18:205–212
3. Katoa K, Nishimasua H, Okudairab S, Miharac E, Ishitania R, Takagic J, Aokib J, Nureki O (2012) Crystal structure of Enpp1, an extracellular glycoprotein involved in bone mineralization and insulin signalling. PNAS 109:16876–16881
A 002
A2A/A2Badenosine receptor subtype-selectivity is defined by the extracellular loop 2
Elisabetta De Filippo*, Benjamin F. Seibt, Anke C. Schiedel and Christa E. Müller
PharmaCenter Bonn, Pharmaceutical Institute, Pharmaceutical Chemistry I, University of Bonn, An der Immenburg 4, D-53121 Bonn, Germany
G protein-coupled receptors show a large diversity in the size, structure and sequence of theirsecond extracellular loop (ECL2). Even in closely related receptors the ECL2is not well conserved. As evidenced by a number of crystal structures the ECL2 participates in ligand recognition and may contribute to receptor selectivity [1,2]. Sequence analysis of the human adenosine A2A and A2B adenosine receptor (AR) subtypes shows an overall identity of 58 % and a similarity of 73 %, whereas the ECL2 is less conserved exhibiting only 34 % identity and 46 % similarity. The A2BAR possesses the longest ECL2 of all four adenosine receptor subtypes, four amino acids longer than that of the A2AR; it also contains the most cysteine residues, namely four, of which three are homologous to the three found in the A2AAR. Despite the high overall sequence conservation, the endogenous ligand adenosine and its derivatives show considerably higher affinity for the A2AAR than for the A2BAR. Using mutagenesis studies, it was shown that the ECL2 contributes to subtype selectivity of A2A and A2B ARs [3]: The complete ECL2 of the human A2BAR was exchanged for the corresponding loop in the human A2AAR subtype resulting in the loop mutant receptor A2B(ECL2-A2A). This loop exchange had significant effects on ligand binding and receptor function. The A2A-selective agonist CGS21680 was able to activate the A2B(ECL2-A2A) mutant, but did not show any activation of the wildtype (wt) A2BAR. Moreover, the maximal effect of all agonists tested in cAMP accumulation assays of the Gs-coupled receptor was significantly increased in the loop exchange mutant as compared to the wt receptor. These results showed that the ECL2 does not only contributeto ligand selectivity but, in addition, appears to stabilizeagonist-bound active conformations of the receptor.
We have now generated and characterizedthe complementary chimeraof the human A2AAR, inwhich the ECL2 was exchanged for that of the human A2BAR (A2A(ECL2-A2B)). While the A2B(ECL2-A2A) chimera had shown a higher efficacy than the wt A2BAR for all investigated agonists, we found that the A2A(ECL2-A2B) chimera exhibited decreased efficacy compared to the wt A2AAR. This further corroborates the hypothesis that the ECL2 definesnot only subtype-selectivityof AR subtypesbut also determines receptor efficacy.
References
1. de Graaf C, Foata N, Engkvist O, Rognan D (2008) Proteins 71:599–620
2. Peeters MC, van Westen GJ, Li Q, IJzerman AP (2011) Trends Pharmacol Sci32:35–42
3. Seibt BF, Schiedel AC, Thimm D, Hinz S, Sherbiny FF, Müller CE (2013) Biochem Pharmacol 85:1317–1329
A 003
Using fruit fly eyes as membrane protein factories: Expression of rat P2X2 and pannexin-1 inDrosophila melanogaster
Leanne Grimes*, Mark Young and Wynand van der Goes van Naters
Cardiff University, School of Biosciences, Cardiff, UK
Structural studies of membrane proteins require the production of a sufficient quantity of high quality protein. Here we explore the use of the visual system of the fruit fly Drosophila melanogaster as a production platform. The photoreceptive membrane in the visual system of Drosophila is organised into a densely packed brush of microvilli, the rhabdomere, which expresses rhodopsin at high levels. To exploit the membrane protein production capacity of this system we have generated transgenic flies that drive expression of rat P2X2 under the control of eye-specific promoters via the UAS-GAL4 system. We have also generated flies that express a different membrane protein, rat pannexin-1, in the eyes. Both P2X2 and pannexin-1 had C-terminal GFP-(His)8 tags. GFP-tagging allows the monitoring of expression levels throughout fly development, and protein quality during purification.
The successful expression of P2X2 and pannexin-1 in the eyes was confirmed using western blotting; the GFP-tagged proteins can also be visualised in the eyes under UV light. Furthermore, deglycosylation assays were used to show P2X2 undergoes high mannose glycosylation in the Drosophila system. We have successfully purified P2X2 and pannexin-1 protein via their histags using nickel bead purification on a small scale. We plan to extend this to large scale purification of protein for structural studies.
To test whether our rat P2X2 construct produces functional protein in Drosophila we expressed it under control of a pan-neural driver and tested whether taste neurons in the fly, which in wild-type do not respond to ATP, acquire sensitivity to ATP application. Using the tip recording technique, we found that rat P2X2 could mediate responses to ATP in the Drosophila taste neurons.
We are currently optimising the protocols by which we extract and purify rat P2X2 from flies, and aim to develop the Drosophila taste neurons as a medium-throughput system to screen for agonists for P2X receptors.
A 004
P2X2 concatemers can form unanticipated cross-assembly products
Richard Hume*, Sukanya Punthambaker, Shlomo Dellal and Matthew Brown
University of Michigan, Molecular, Cellular and Developmental Biology, Ann Arbor, USA
In P2X receptors, the ATP binding sites as well as the binding sites for a number of allosteric modulators lie at the interface between subunits. Because the receptors are trimers, when a point mutation is made to a binding site residue, three mutant binding sites are present. In principle, the effect of a mutation at only one or two binding sites can be explored by making trimeric concatamers with different mutations in each repeat. However, for results to be readily interpretable, these concatamers must fold autonomously. We have identified a number of trimeric concatemers of rat P2X2 where this is not the case.
All experiments were conducted on Xenopus oocytes. The first indication that folding might not always be autonomous was the observation that when the key ATP binding residues K69 and K308 were both mutated to alanine in a single repeat of trimeric constructs, a response to ATP was usually undetectable if the mutations were in the first repeat, but large ATP responses with concentration-response relations similar to wild type P2X2 were present if the mutations were in the second or third repeat. A plausible explanation for these results is that in the latter two concatemers, the first repeat of three different concatemers came together to form a cross-assembled wild type subunit. To further test if subunits from one concatemer can cross-assemble with subunits from a neighboring concatemer, we made concatemers in which critical histidine residues that are required to bind zinc and cause potentiation (H120 and H213) were replaced with either alanine (which prevents zinc potentiation) or cysteine (which allows both zinc potentiation and the formation of a disulfide bond when an appropriate partner is nearby). When expressed alone, concatemers with one cysteine and one alanine in each repeat (denoted as CA-CA-CA and AC-AC-AC, where the first and second letter represents positions 120 and 213 respectively) were zinc insensitive and DTT insensitive, as expected since previous work had demonstrated that the zinc binding site was at the interface between subunits. However, when these two concatemers were co-expressed, there was small but significant potentiation by both zinc and DTT, indicating that some of the subunit interfaces must have had the C120 from one concatamer and the C213 from another. Western blot experiments indicated that the proportion of cross assembled products is small. None-the-less, when mutations drastically alter the physiological properties of receptors, the cross-assembly products may be the dominant contributor to the currents that are measured.
A 005
The open and closed states of the P2X2 receptor are differently stabilized by salt bridges in the ATP binding site
Daniel Kuhlmann1,*, Achim Kless2, Günther Schmalzing1 and Ralf Hausmann1
1Molecular Pharmacology, RWTH Aachen University, Aachen, Germany;2Grünenthal GmbH, Global Drug Discovery, Aachen, Germany
P2X receptors are trimeric ATP-gated cation channels involved in the fast signal transduction in many cell types. We have previously shown that residues Glu167 and Arg290 interact electrostatically within the ATP-binding site of the rat P2X2 receptor when the channel is in the apo closed-state, but not in the liganded open state. Both homology modeling and experimental data supported the view that the ionic coordination of ATP in the ATP-binding pocket by promoting a salt bridge switching from Arg290/Glu167 to Arg290/ATP destabilizes the closed state of the P2X2 receptor [1].
Our rP2X2 homology model further predicts close proximities between Lys308/Glu167 and Arg290/Asp82, Glu84 and Glu85 that we could all experimentally verify by disulfide trapping, suggesting that additional ionic interactions may exist. Indeed, functional analysis of charge reversal, charge swap and charge neutralization mutants revealed that Glu84/Arg290 and Glu167/Lys308 are electrostatically coupled. Functional analysis after chemical oxidation and reduction revealed further that disulfide bond formation significantly decreased the ATP potency and efficacy of the E84C/R290C and the E167C/K308C double mutants. We conclude from these results that salt bridges between Glu84/Arg290 and Glu167/Lys308 both serve to stabilize the closed state of the P2X2 receptor. Whether a coordinated rearrangement of the salt bridge network formed by Glu84/Glu167/Arg290/Lys308 within the ATP-binding pocket is crucial to allow for the opening of the channel has to be determined.
This work was supported by German Research Council Grants Schm536/8-2 and HA6095/1-1.
Reference
1. Hausmann R, Gunther J, Kless A, Kuhlmann D, Kassack MU, Bahrenberg G, Markwardt F, Schmalzing G (2013) Mol Pharmacol 83:73–84
A 006
Structural analysis of the P2X4 receptor reveals insights of the mechanism of allosteric regulation by alfaxolone
J. Pablo Huidobro-Toro*, Natalia Alveal, Camilo Navarrete and Nelson Barrera1
Universidad de Santiago de Chile, Quimica y Biologia, Santiago, Chile
The crystallization of the Danio rerio P2X4 receptor (zfP2X4R) in the apo and holo state (with 3 ATP molecules in the orthosteric site) offered the possibility of understanding at the structural/atomic level the function of this receptor channel, activated mainly by ATP and structurally related adenine triphosphates. Our laboratory has characterized pharmacologically aspects of the mechanism of the positive allosteric modulation of the Rattus norvegicus P2X4 receptor (rP2X4R) by alfaxolone (Codocedo et al., 2009), a synthetic steroid with pharmacological anesthetic activity. We described that micromolar concentrations of the steroid interact with a site localized within the transmembrane (TM) domain of the rP2X4R, not present in the rP2X2R; chimeras of the P2X4/P2X2R indicate that the steroid site occurs most likely in the TM domain. Moreover, larger steroid concentrations elicit per se an ionic current, indicating that opening of the receptor pore in the absence of ATP. Based on the current knowledge of the topology of the P2X4R, this work aims at characterizing the steroid binding site and provides information regarding the structural conformational changes elicited by alfaxolone on the rP2X4R. Based on the crystallized zfP2X4R in the apo and holo states, corresponding rP2X4R models of the extracellular and the TM domains for both states were built, including a modelling of the N and C-terminus cytoplasmic tails (which are absent in the crystallized zfP2X4R). The rP2X4R model was embedded in an artificial lipid membrane environment, and all atom molecular dynamics (MD) simulations with three docked alfaxolone molecules was developed for the rP2X4R in the apo and holo states during 100 ns each. MD calculations have been used to identify the alfaxolone binding sites in the TM domain of rP2X4R. This strategy not only allowed the identification of the steroid binding site in the TM domain, but also the possible conformational alternations elicited by steroid binding. Results show that in the apo state, 3 alfaxolone molecules interact with the TM domain throughout the simulation; however, it was clearly observed that only one of the steroid molecules is capable of forming hydrogen bonds across the subunit interface of the rP2X4R, in contrast to the other two steroids which only bind to the TM domain of its binding subunit. Surprisingly, and in full agreement with Codocedo et al., (2009), alfaxolone binding may open the rP2X4R pore, as evidenced by the rupture of interactions between TM2 helices in the pore-lining, a feature also observed in the ATP-bound structures of zfP2X4R and rP2X4R. Future studies will extend these findings to include the interaction of the rP2X4R with pregnanolone, a brain neurosteroid which elicits a negative allosteric modulation of the rP2X4R. In sum, structural analysis based on MD reveals relevant information on the mechanism of allosteric steroid modulation of the rP2X4R.
Funded by Millennium Science Initiative, grant P10-035F and Fondecyts 1141132 and 1120169.
A 007
Identification of amino acid residues in the inter-subunit interface that contribute to gating and agonist specificity of the P2X4 receptor
Vendula Tvrdonova1,2,*, Milos Rokic1,2 and Hana Zemkova1
1Institute of Physiology AS CR, Cellular and Molecular Neuroendocrinology, Prague, Czech Republic;2Charles university, Faculty of science, Prague, Serbia
Purinergic P2X receptors (P2XRs) respond to the binding of extracellular nucleotides by activation and desensitization in a receptor- and ligand-specific manner, but the structural bases of their channel kinetics and ligand preferences are still not well understood. In this study we asked if any of residues in two low-conserved ectodomain regions that form interface between two P2X4R subunits, D280-N293 (“left flipper”) and R203-L214 (“dorsal fin”), is crucial for receptor function or receptor-specific properties. We substituted one by one amino acids in both regions with alanine and used whole cell patch-clamp recording of WT and mutated receptors expressed in HEK293T cells. The results of our investigation showed that R203A, N204A, I205A, L214A, D280A, R282A and P290A mutants exhibited significantly reduced maximum current amplitude that could be fully rescued with ivermectin, positive allosteric modulator of P2X4. All these mutations favored the closed state of the channel because ATP became a less effective agonist and the deactivation time was shortened. The data indicating these residues play a role in the signal transduction pathway that regulate channel activity and, indirectly, the affinity of agonists for binding domain, too. N293A also displayed significantly reduce maximum current amplitude but it was not fully recovered with ivermectin confirming that N293 is ATP binding residue. Receptors T210A, H286A and G291A exhibited a normal maximum current amplitude but six to ten-fold lower sensitivity to agonists. In all alanine mutants the rates of receptors desensitization negatively correlated with EC50 values indicating that changes in desensitization occurred as a consequence of reduced receptor sensitivity to agonists. Substitution of T210 with aspartate (T210D) recovered receptor function, indicating that polarity of this residue could play an important role, apparently in coordination of ATP position in binding cleft. The efficacy of BzATP was increased and the efficacy of α,β-meATP was decreased for G291A mutant compared with the WT receptor, suggesting that G291 might play a role as a hinge to control the size of the ATP binding pocket. We also suggest two pairs of interacting residues that might be engaged in inter-subunit interactions based on our functional study and homology modeling. H286 might form an important pair with R203 because both residues are distant in closed state but could interact in channel open state. Similar interaction is possible for R282 and W194 residues. The results of our work provide structural and mechanistic insights for understanding the P2X4R gating, agonist selectivity and signal transduction pathway.
This study was supported by the Internal Grant Agency of Academy of Sciences (IAA500110910), Grant Agency of the Czech Republic (P304/12/G069) and the Grant Agency of Charles University in Prague (3446/2011).
A 008
Investigation of the allosteric binding site(s) for P2X4 receptor antagonists
Stephanie Weinhausen*, Theodor Hanck, Vigneshwaran Namasivayam, Aliaa Abdelrahman, Victor Hernandez-Olmos, Maoqun Tian and Christa E. Müller
PharmaCenter Bonn, Pharmaceutical Institute, Pharmaceutical Chemistry I, University of Bonn, An der Immenburg 4, 53121 Bonn, Germany
P2X receptors are ligand-gated ion channels, which belong to the family of purinergic P2 receptors. They are activated by ATP and play a role in a number of (patho)physiological processes. Therefore, P2X receptors are of interest as novel drug targets, in particular in the field of inflammation and pain. Blockade of P2X4 receptors has been proposed as a strategy to treat neuropathic pain and other inflammatory diseases, e.g. diabetic nephropathy, joint inflammation, arthritis, and spinal cord injury [1,2]. Therefore our efforts have been directed towards identifying and developing potent and selective P2X4 receptor antagonists. We recently identified N-substituted phenoxazines and acridones as well as carbamazepine derivatives as allosteric antagonists of P2X4 receptors [1,3]. The goal of the present study was to get insight into the mechanism of action of these and other allosteric P2X4 antagonists and to identify the allosteric binding site(s) on the human P2X4 receptor. Since the investigated negative allosteric modulators were inactive at the human P2X2 receptor, we prepared chimeras of the human P2X4 receptor, in which certain parts were exchanged for sequences of the human P2X2 receptor. 1321N1 astrocytoma cells stably transfected with the chimeric receptors were then obtained. We subsequently performed ATP-induced calcium influx assays in the presence or absence of allosteric antagonist, and results were compared to those obtained with cells expressing the wildtype P2X4 receptor. In chimera P2X4(V71-I83P2X2) the maximal effect of ATP was decreased whereas its EC50-value remained unaltered. Several other chimeras showed significant differences in antagonist effects. These preliminary results may contribute to the identification of the specific binding sites of allosteric antagonists at the human P2X4 receptor.
References
1. Tian M, Abdelrahman A, Weinhausen S, Hinz S, Weyer S, Dosa S, El-Tayeb A, Müller CE (2014) Bioorg Med Chem 22:1077–1088
2. lnoue K, Tsuda M (2012) Exp Neurol 234:293–301
3. Hernadez-Olmos V, Abdelrahman A, El-Tayeb A, Freudendahl D, Weinhausen S, Müller CE (2012) J Med Chem 55:9576–9588
A 009
Involvement of the second transmembrane domain in permeation and gating of human P2X7 receptors
Anja Pippel1,*, Michaela Stolz2, Achim Kless3, Günther Schmalzing2 and Fritz Markwardt1
1Julius-Bernstein-Institute for Physiology, Martin-Luther-University Halle-Wittenberg, Halle/Saale, Germany;2RWTH University, Molecular Pharmacology, Aachen, Germany;3Grünenthal GmbH, Global Drug Discovery, Departments of Molecular Pharmacology and Discovery Informatics, Aachen, Germany
Immune cells like B-cells and macrophages express P2X7-receptors at the cell membrane. These receptors are ATP-dependent cation channels which are involved in modulation of immune and inflammatory processes. P2X7 receptors are homotrimers of subunits with intracellularly located N- and C-termini, two transmembrane domains and a large extracellular domain comprising the ATP-binding site.
We performed single cysteine scanning mutagenesis of amino acids in the second transmembrane domain (TM2) of human P2X7 receptors (hP2X7), a region which is supposed to be involved in pore opening and ion permeation of other P2X receptor channels. Xenopus laevis oocytes were injected with cRNAs of the different hP2X7 constructs and ATP-dependent currents were measured using two microelectrode voltage clamp and patch clamp techniques.
At the putative extracellular end of the hP2X7R TM2 domain, we found cysteine mutated constructs which could be labeled but application of MTS reagents were without functional effect, i.e. the ATP-induced currents were not affected by MTS administration. This indicates that the corresponding amino acids were extracellularly accessible but are neither involved in gating nor in permeation of the ion channel.
Only the hP2X7 receptor construct S342C displayed the anticipated sustained reduction of the ATP-induced current after application of the sulfhydryl group modifying cationic reagent MTSEA, probably by blocking the ion channel pore.
Six other cysteine mutants between I331C and G345C were found to irreversibly increase the ATP-dependent ion current after MTSEA application. Single channel recording revealed that the stimulatory effect of MTSEA was due to an increase of the open probability.
We conclude that the TM2 region between I331C and G345C is simultaneously involved in permeation and gating of P2X7 receptor channels.
The structure-function relationship of the hP2X7R TM2 domain is discussed using a hP2X7R model based on the published zebra fish P2X4 structure.
A 010
Tridimensional structure of a fragment belonging to the M2 domain of the P2X7 receptor solved by nuclear magnetic resonance
Dinarte Neto Moreira Ferreira1,*, Rafael Ferreira Soares1, Pedro Celso Nogueira Teixeira1, Cristina Alves Magalhães de Souza1, Luiz Anastacio Alves1 and Mônica Santos de Freitas2
1Oswaldo Cruz Foundation, Rio de Janeiro, Brazil;2Federal University of Rio de Janeiro, Institute of Medical Biochemistry Leopoldo de Meis, Rio de Janeiro, Brazil
P2X7 receptor is an integral plasmatic membrane protein, which functions as an ionotropic receptor, belonging to the family of P2X receptors. It is composed of 595 amino acids in length and can be found as a trimer. Its structural domains are highlighted as an ectodomain that presents an ATP binding site, two transmembrane domains and a cytoplasmatic region. The mechanism involved in signal transduction mediated by P2X7 receptor has been extensively studied; however, many questions still remain unanswered. Nevertheless, several studies in inflammatory diseases have indicated that P2X7 is a potential pharmacological target. As such, a better structural knowledge about P2X7 structure might help to design new and more effective antagonists. In this work Nuclear Magnetic Resonance (NMR) was used to investigate the structure of P2X7, using the method of divide and conquer, which basically studies segments of a protein to establish the whole structure. It is important to mention that this method has been successful in identifying the structure of other such membrane proteins, which are difficult to obtain for structural determination by NMR or X-ray crystallography. The peptide comprising of residues 313–336 (ADSEG peptide) belonging to the M2 domain of the P2X7 receptor. In addition, the ADSEG peptide was previously published by our group to form cation selective channels in planar bilayers. The peptide was synthesized and solubilized in 100 % of deuterated DMSO. The atomic structure presented a hairpin configuration and with the application of molecular dynamics using NMR restraints refinement was possible to obtain a better structural model. The acidic amino acids were pointed to the center of a proposed peptide channel pore, suggesting an ionic driving force for cation transportation throughout the channel.
A 011
The purinergic P2X7 receptor stimulates collagen production in cardiac fibroblasts from chronic heart failure patients: a new therapeutic target for adverse cardiac remodelling?
D. Gentile1, S. Zimbone1, P. E. Lazzerini1, A. Gamberucci2, P. L. Capecchi1, M. Natale1, M. Cabiati3, S. Del Ry3, M. A. Morales3, F. Diciolla4 and F. Laghi-Pasini1
1Department of Medical Sciences, Surgical and Neuroscience; 2Department of Molecular and Developmental Medicine; 3CNR, Institute of Clinical Physiology, Pisaand4Department of Cardiovascular Desease, University of Siena, Siena, Italy
Question: Chronic heart failure (HF) is the final result of a wide range of cardiovascular diseases. The progressive worsening of cardiac function is related to a complex sequence of structural changes of the heart, collectively known as cardiac remodeling. This process, consisting in chamber dilatation, hyperthrophy and cardiac fibrosis, involves the cardiac fibroblast (cF) as one of the mainstay actors, whose functional activation is deeply influenced by several cytokines, mainly TGF-β, IL-6 and IL-1β.
P2X7 receptor (P2X7R) is a nucleotide-gated ion channel, chiefly involved in the inflammatory response triggered by passive release of ATP from damaged cells. It is largely expressed in monocytes and plays a key role in promoting the release of IL-1β and IL-6. As recent evidence suggests that P2X7R is also expressed in fibroblasts thus possibly having a role in promoting tissue fibrosis in different body districts, we hypothesized that the P2X7R may be involved in cardiac remodelling.
Aim of this study was to evaluate the expression and function of P2X7R in cultured human cardiac fibroblasts from HF patients in comparison with human atrial cardiac fibroblasts from the NHCF-A cell line.
Methods: Human cFs were obtained from human atrial fragments from 13 HF patients undergoing cardiac surgical intervention or heart transplantation. A human atrial cF cell line (NHCF-A) was used as a control.
P2X7R expression was evaluated by flow cytometry and real-time PCR, while the receptor function was assessed by the analysis of collagen production (ELISA), Ca2+ flux (single-cell fluorescent microscopy), and cytokines (IL-1-β, IL-6, TGF-β) release (ELISA) after stimulation with the selective receptor agonist BzATP. Moreover, in order to explore the putative intracellular mechanisms involved, the level of ERK-1/2 phosphorylation (p-ERK-1/2) was also studied (western blot analysis) in these cells.
Results: When compared to NHCF-A cells, HF cardiac fibroblasts displayed (i) a higher P2X7R surface expression, that was further increased by TNF-α incubation, and (ii) an enhanced P2X7R function in terms of both Ca2+ flux and collagen production. On the contrary, no significant changes in supernatant levels of TGF-β, IL-1β and IL-6 were observed. Moreover, p-ERK-1/2 levels were higher in HF than control cells, with a strict correlation between the extent of ERK activation and collagen production.
Conclusions: Our results provide evidence that in cFs from HF patients both the expression and function of the P2X7R are increased as the possible result, at least in part, of a positive modulating effect of TNF-α on the receptor. In particular, by enhancing collagen production from HF cardiac fibroblasts, P2X7R may promote the cardiac remodeling process associated with the disease. These findings increase our knowledge on the pathophysiology of HF, also suggesting a role for the P2X7R as a new attractive target for pharmacological modulation.
B: Polymorphisms of receptors and ectonucleotidases
B 012
Dissecting the function of P2RX7 as a susceptibility marker for mood disorders using a conditional humanized mouse model
Michael Metzger1,*, Sandra Walser1, Nina Dedic1, Mathias Schmidt1, Wolfgang Wurst2 and Jan Deussing1
1Max Planck Institute of Psychiatry, Department of Stress Neurobiology and Neurogenetics, Munich, Germany;2Helmholtzzentrum, Institute of Developmental Genetics, Munich, Germany
Recent linkage and association studies suggest P2RX7 as a novel susceptibility gene for major depressive disorder and bipolar disorder. In particular the association of a single nucleotide polymorphisms in the P2RX7 gene, leading to an amino-acid substitution at position 460 (Gln->Arg) has been reported by several studies lately. To study the function of the P2X7 receptor (P2X7R) and its possible relevance in mood disorders in an appropriate in vivo model, we generated mice in which the murine P2rx7 is substituted by the human P2RX7 gene using a knock-in approach. Due to integration of loxP sites, this humanized P2RX7 allele is accessible to Cre recombinase mediated inactivation. Breeding specific Cre driver lines to the humanized P2RX7 mouse line allows for spatially and/or temporally controlled disruption of P2X7R function.
By in situ hybridisation (ISH) using a human-specific probe, we could demonstrate that the expression of P2RX7 in humanized animals fully resembles the expression pattern of murine P2rx7 animals. The typical expression pattern was absent on brain sections of knockout animals. ISH analysis on animals bread with different tissue specific Cre lines revealed that e.g. the strong signal in the CA3 of the hippocampus is mainly based on P2RX7 expression of glutamatergic neurons. Cell type specific primary cultures, allowed us to further narrow down P2RX7 expression in the brain by qRT-PCR. We found that P2RX7 is expressed highest in microglia followed by neurons and astrocytes. One of the major drawbacks of most so far published P2rx7 knockout lines is the fact that they are not deficient for all splice variants of P2rx7 known by now. For our knockout approach we could proof the absence of any functional transcript of the receptor.
To also address the functional properties of the receptor in humanized and knockout animals we compared cytokine secretion levels upon stimulation of the receptor. Similar to peritoneal macrophages obtained from wildtype mice, those obtained from humanized animals were fully capable to induce Interleukin-1β (IL-1β) release upon LPS/BzATP stimulation. In contrast knockout animals did not show IL-1β release. These results were supported by the finding that calcium influx upon stimulation with BzATP was completely abolished in primary hippocampal cultures obtained from knockout mice.
Behavioral characterization of these animals showed a slight increase in anxiety related behavior in humanized P2XR7 animals compared to wildtype littermates. However, the knockout of P2RX7 did not reveal significant behavioral alterations.
Taken together, this humanized mouse line is significantly contributing to functionally validate the human association data in vivo. Furthermore it is a powerful tool to more deeply investigate the function of the P2X7 receptor in the context of anxiety and mood disorders.
B 013
A common polymorphism encoding Arg270>His in the human P2X7 receptor alters the effect of zinc
Phuong Dao-Ung1, Brett Cromer2, HanShen Tae2 and Leanne Stokes1,2,*
1University of Sydney, Nepean Clinical School, Penrith, Australia;2RMIT University, Health Innovations Research Institute, Bundoora, Australia
P2X7 is a ligand-gated ion channel involved in pro-inflammatory signalling pathways in immune cells. This study investigated the effect of two physiologically relevant divalent metal cations, zinc and copper on human P2X7 responses and the effect of polymorphisms altering amino acids in the large ectodomain region. A common polymorphism, rs7958311, alters amino acid 270 from arginine (R) to histidine (H), and is present in the Caucasian population with a minor allele frequency of 0.255. We show that ATP-induced dye uptake in HEK-293 cells expressing recombinant human P2X7 haplotypes containing H270 were potentiated by micromolar concentrations of zinc whereas haplotypes containing R270 were not. The inhibitory effect of copper on human P2X7 responses were not affected by altering residue 270. While ATP-induced responses at the wild-type human P2X7 were potentiated by zinc, BzATP-induced responses were inhibited by zinc. The degree of inhibition of BzATP-induced responses by zinc was also significantly affected by the polymorphism altering amino acid 270. Homology modelling of human P2X7 using the zebrafish P2X4 crystal structure suggests that H270 could potentially form a zinc binding pocket with two histidine residues at 267 and 268.
B 014
A missense variant leads to loss of function of the canine P2X7 receptor
Ronald Sluyter1,*, Mari Spildrejorde1, Rachael Bartlett1, Iman Jalilian1, Michelle Peranec1, Vanessa Sluyter1, Belinda Curtis2, Kristen Skarratt3, Amanda Skora1, Tahani Bakhsh1, Aine Seavers4, Jason McArthur1, Mark Dowton1 and Leanne Stokes5
1University of Wollongong, Wollongong, Australia;2Albion Park Veterinary Hospital, Albion Park, Australia;3University of Sydney, Penrith, Australia;4Oak Flats Veterinary Clinic, Oak Flats, Australia;5RMIT University, Bundoora, Australia
The relative function of the P2X7 receptor varies between humans due to polymorphisms in the P2RX7 gene. This study aimed to assess the functional impact of P2X7 variation in a random sample of the canine population. Blood and genomic DNA were obtained from 68 dogs selected as representatives of a cross-section of different breeds. P2X7 function was determined by flow cytometric measurements of dye uptake and patch-clamp measurements of inward currents. P2X7 expression was determined by immunoblotting and immunocytochemistry. Sequencing was used to identify P2RX7 gene polymorphisms. P2X7 was cloned from an English Springer Spaniel and point mutations were introduced into this receptor by site-directed mutagenesis. The relative function of P2X7 on monocytes varied between individual dogs. The canine P2RX7 gene encoded four missense polymorphisms: F103L and P452S, found in heterozygous and homozygous dosage; R270C and R365Q, found only in heterozygous dosage. Moreover, R270C and R365Q were associated with the Cocker Spaniel and Labrador Retriever, respectively. F103L, R270C and R365Q but not P452S corresponded to decreased P2X7 function in monocytes, but did not explain the majority of differences in P2X7 function between dogs, indicating that other factors contribute to this variability. Recombinant English Springer Spaniel P2X7 expressed heterologously in HEK-293 cells was activated by ATP, 2′(3′)-O-(4-benzoyl)benzoyl ATP and adenosine 5′-O-(3-thio)triphosphate, and was completely impaired by A438079, AZ10606120, AZ11645373, Brilliant Blue G and KN-62. Heterologous expression of site-directed mutants of canine P2X7 in HEK-293 cells indicated that the R270C mutant was non-functional, the F103L and R365Q mutants had partly reduced function, and the P452S mutant functioned normally. Taken together, these data highlight that the R270C variant has major functional impact on canine P2X7.
B 015
Single-nucleotide polymorphism in the P2Y2receptor gene is associated with bone mineral density in a cohort of Swedish elderly men
Maria Ellegaard1,*, Magnus Karlsson2, Mattias Lorentzon3, Claes Ohlsson3, Dan Mellström4, Östen Ljunggren5, Peter Schwarz1,6 and Niklas Rye Jørgensen1,6
1Copenhagen University Hospital Glostrup, Glostrup, Denmark;2Lund University, Malmö, Sweden;3University of Gothenburg, Gothenburg, Sweden;4Sahlgrenska University Hospital, Gothenburg, Sweden;5Uppsala University Hospital, Uppsala, Sweden;6University of Copenhagen, Copenhagen, Denmark
Background and aim: The P2Y2 receptor is a G-protein-coupled receptor, which is activated by adenosine 5′-triphosphate and involved in signaling between bone cells. The P2Y2 receptor single-nucleotide polymorphism (Leu46Pro) has been shown to be associated with high BMD in women. As gender-specific effects of P2 receptors have been suggested, the aim of this study was to investigate whether P2Y2 receptor polymorphisms are associated with BMD and fracture prevalence in elderly men. Methods: A total of 3,014 men (age 69–81 years) from the MrOS Sweden study were genotyped for three P2Y2 receptor polymorphisms (Leu46Pro, Ser359Pro and Arg312Ser). Genotyping was performed using Sequenom MassARRAY platform. BMD of lumbar spine, total hip and femoral neck was measured by DXA. Vertebral fractures were analyzed by spinal x-ray in 1,428 of the participants. Furthermore, previous sustained fractures (any type) were recorded from questionnaires. Differences in BMD and fracture prevalence in wildtype allele carriers, heterozygous and homozygous variant allele carriers were assessed using analysis of covariance (covariates: BMI and age) and Chi Square test. Data are presented as mean (SD). Results: At baseline the study population presented with an age of 75.4 (3.2) years; BMI of 26.4 (3.6) kg/m2; spine BMD of 1,142 (201) mg/cm2; femoral neck BMD of 831 (132) mg/cm2 and total hip BMD of 936 (144) mg/cm2. Fifteen percent (of the 1,428) had experienced one or more vertebral fractures. Age and BMI was compared within genotypes of each polymorphism and no significant differences were found between the genotypes (p > 0.05). BMD of the femoral neck was significantly different between genotypes for the Leu46Pro polymorphism, with hetero- and homozygous variant allele carriers showing higher BMD compared to wildtype allele carriers (wildtype: 827 (132) mg/cm2; heterozygous: 850 (131) mg/cm2; homozygous: 848 (95) mg/cm2; p = 0.018). The Arg312Ser and Ser359Pro polymorphisms showed no significant difference in BMD at any site between the different genotypes (p > 0.05). None of the polymorphisms were significantly associated with prevalent vertebral fractures or all reported fractures (p > 0.05). Conclusion: The Leu46Pro polymorphism of the human P2Y2 receptor was in our cohort associated with BMD of the femoral neck in elderly men. Further experimental studies are required in order to establish the effect of the polymorphism on receptor and bone cell function.
C: Pharmacology, biochemistry and physiology of purinergic receptors
C 016
Development of an ITC based enzyme assay for nucleoside triphosphate diphosphohydrolases (NTPDases)
Michel Krauss1, Matthias Zebisch2 and Norbert Sträter1
1University of Leipzig, Institute of Bioanalytical Chemistry, Leipzig, Germany;2University of Oxford, Wellcome Trust Centre of Human Genetics, Oxford, UK
For the regulation of the extracellular levels of nucleotides membrane-bound ectonucleotidases such as nucleoside triphosphate diphosphohydrolases play an important physiological role. CD39 or NTPDase1, the prototype member of the eukaryotic NTPDase family is the dominant NTPDase of the vasculature. Based on the hydrolysis of proinflammatory ATP and platelet-activating ADP to AMP the platelet-aggregation is blocked and blood flow is supported. Thus, an understanding of the structure and dynamics of CD39 is vitally important. Here, we present the crystal structure of a variant of soluble NTPDase1 lacking a putative membrane interaction loop (MIL). This loop was identified between the two lobes of the catalytic domain. Measurements of ATPase and ADPase activities where performed using a newly established kinetic assay via isothermal titration calorimetry (ITC). In addition, a comparison of the domain orientations of the four independent proteins in the crystal asymmetric unit provides first direct experimental evidence for a domain motion of NTPDases. An interdomain rotation angle of up to 7.4° affects the active site cleft between the two lobes of the protein. Comparison with a previously solved bacterial NTPDase structure indicates that the domains may undergo relative rotational movements of more than 20°. Our data support the idea that the influence o f transmembrane helix dynamics on activity is achieved by coupling to a domain motion.
C 017
Establishment of novel fluorescence polarization immunoassay- (FPIA-) based NTPDase assays for high-throughput inhibitor screening
Amelie Fiene1,*, Younis Baqi1,2, Joanna Lecka3, Jean Sévigny3 and Christa E. Müller1
1PharmaCenter Bonn, Pharmaceutical Institute, Pharmaceutical Chemistry I, University of Bonn, An der Immenburg 4, D-53121 Bonn, Germany;2Department of Chemistry, Faculty of Science, Sultan Qaboos University, PO Box 36, Postal Code 123, Muscat, Oman;3Centre de Recherche du CHU de Québec and Université Laval, 2705 Laurier Blvd., Québec, QC, Canada G1V 4G2
Nucleosides and nucleotides play important roles as extracellular signaling molecules by activating P1 and P2 receptors, respectively [1,2]. Their levels are tightly regulated by different members of the ecto-nucleotidase family. Nucleoside 5′-tri- and di-phosphates are hydrolyzed by members of the ecto-nucleoside triphosphate diphosphohydrolase (E-NTPDase) family forming ADP and/or AMP. These enzymes may be future targets for novel drugs acting as site- and event-specific modulators of nucleoside and nucleotide signalling. In order to dissect the (patho)physiological roles of NTPDases and their potential as drug targets, potent and subtype-selective inhibitors of NTPDases are required [1–3]. So far, most inhibitors are non-selective or only moderately potent [4].
Therefore, we decided to set up a screening campaign in order to identify new lead structures for subsequent optimization. Previously described screening assays for the identification of NTPDase inhibitors all suffer from certain drawbacks, e.g., high background (upon measurement of the released phosphate), the need for fluorescently labeled artificial substrates, the potential for interference with coupling enzymes, problems due to radioactive disposal, or the requirement of time consuming procedures [5]. Therefore, we decided to develop and establish a new assay procedure based on fluorescence polarization, which allows the use of the natural substrates (ATP, ADP) and the direct detection of the enzymatic reaction products in a 384-well format. [5,6]
During the enzymatic reaction the generated ADP or AMP displaces a fluorescent tracer nucleotide from an antibody, which leads to a change in its fluorescent properties [5,6]. The assays are highly sensitive, and therefore low substrate concentrations below the KM values of NTPDases may be used, which simplifies the identification of novel competitive inhibitors. Validation of the assays resulted in Z′-factors of >0.70 for substrate conversions of <10 %, which demonstrates their suitability for high-throughput screening campaigns under initial velocity conditions.
References
1. Zimmermann H, Zebisch M, Sträter N (2012) Purinergic Signal 8:437–502
2. Baqi Y, Weyler S, Iqbal J, Zimmermann H, Müller CE (2009) Purinergic Signal 5:91–106
3. Yegutkin GG (2008) Biochim Biophys Acta 1783:673–694
4. Lecka J, Gillerman I, Fausther M, Salem M, Munkonda MN, Brosseau J-P, Cadot C, Martín-Satué M, d’Orléans-Juste P, Rousseau É, Poirier D, Künzli B, Fischer B, Sévigny J (2013) Br J Pharmacol 169:179–196
5. Lowery RG, Kleman-Leyer K (2006) Expert Opin Ther Targets 10:179–190
6. Staeben M, Kleman-Leyer KM, Kopp AL, Westermeyer TA, Lowery RG (2010) Assay Drug Dev Technol 8:344–355
C 018
A new, sensitive ecto-5′-nucleotidase assay for compound screening
Marianne Freundlieb1,*, Herbert Zimmermann2 and Christa E. Müller1
1PharmaCenter Bonn, Pharmaceutical Institute, Pharmaceutical Chemistry I, University of Bonn, An der Immenburg 4, 53121 Bonn, Germany;2Institute of Cell Biology and Neuroscience, Molecular Neurobiology, Goethe University, 60438 Frankfurt am Main, Germany
The membrane-bound extracellular enzyme ecto-5′-nucleotidase (eN) catalyzes the dephosphorylation of nucleoside-5′-monophosphates. The main substrate is AMP, which is hydrolyzed to adenosine and inorganic phosphate. The balance between concentrations of extracellular AMP and the extracellular signalling molecule adenosine plays an important role in physiological and pathophysiological processes. For example, adenosine is immunosuppressive protecting cancer cells from attack by T-lymphocytes, and it promotes angiogenesis. Therefore eN has been proposed as a novel drug target for anti-tumour therapy. Up to date, only few classes of inhibitors for eN have been described. The most potent inhibitors known so far are nucleotide analogues, the standard inhibitor being α,β-methylene-ADP (AMPCP, AOPCP). Other described inhibitors, including anthraquinones, methylxanthines, flavonoids and sulphonamides, are only moderately potent with IC50- or Ki values in the micromolar range [1–5]. In order to find new classes and identify novel lead structures for the development of more potent eN-inhibitors, we developed a new sensitive radiometric assay using a lanthanum chloride precipitation method to separate the substrate [3H]AMP from the product [3H]adenosine with subsequent filtration through glass fiber filters [6]. We optimized the new assay for rat as well as for human eN preparations and subsequently performed a screening campaign.
References
1. Zimmermann H (1992) Biochem J 285:345–365
2. Knapp K, Zebisch M, Pippel J, El-Tayeb A, Müller CE, Sträter N (2012) Structure 20:2161–2173
3. Iqbal J, Jirovsky D, Lee S, Zimmermann H, Müller CE (2008) Anal Biochem 373:129–140
4. Baqi Y, Lee SY, Iqbal J, Lehr A, Scheiff AB, Zimmermann H, Bajorath J, Müller CE (2010) J Med Chem 53:2076–2086
5. Ripphausen P, Freundlieb M, Brunschweiger A, Zimmermann H, Müller CE, Bajorath J (2012) J Med Chem 55:6576–6581
6. Freundlieb M, Zimmermann H, Müller CE (2014) Anal Biochem 446:53–58
C 019
Generation of specific antibodies to NTPDases8 by cDNA immunization—be careful of the commercial antibodies
Mabrouka Salem1,2,*, Julie Pelletier2, Joanna Lecka1,2, Patrick Luyindula1,2, Michel Fausther1,2, Francois Bigonnesse2 and Jean Sévigny1,2
1Laval University, Microbiology-Immunology, Quebec, Canada;2Centre de recherche du CHU de Québec, Rhumatology-Immunology, Quebec, Canada
Background: NTPDase8 is the last member of the ecto-nucleoside triphosphate diphosphohydrolase (E-NTPDase) family which hydrolyse nucleotides at the cell surface. Western blot and mRNA expression was found in a very few tissues including liver, kidney, jejunum and cochlea. We recently demonstrated that this enzyme was located in liver canaliculi. The function of the enzyme is still not known. Specific antibodies represent an important tool to study the enzyme. In this work, we report the generation of antibodies against mouse and human NTPDase8. Commercial antibodies were also tested.
Methods: For the production of antibodies against mouse NTPDase8 several techniques were used which involved mainly conjugated peptides and the cDNA immunization in several hosts. Monoclonal antibodies against human NTPDase8 were obtained with the cDNA immunization technique followed by an injection of transfected HEK 293 cells expressing human NTPDase8 proteins. Antibody specificity was assessed by Western blot, immunocytochemistry, immunohistochemistry and flow cytometry.
Results: Mouse NTPDase8 appeared poorly immunogenic as most techniques were unsuccessful to produce antibodies. Fortunately the cDNA immunization technique generated potent and specific antibodies. These polyclonal antibodies as well as the monoclonal antibodies to human NTPDase8 were efficient and specific in all techniques tested. In our hands, none of the commercial antibodies to NTPDase8 tested, gave a specific signal at the right molecular weight by Western blot.
Conclusion: Specific antibodies to mouse and human NTPDase8 that are efficient in Western blot, immunocytochemistry, immunohistochemistry and flow cytometry have been produced. These antibodies can be obtained via http://ectonucleotidases-ab.com/ Web site.
C 020
Generation of a monoclonal antibody that specifically inhibit human NTPDase2
Mabrouka Salem1,2,*, Julie Pelletier2, Michel Fausther1,2, Joanna Lecka1,2 and Jean Sévigny1,2
1Laval University, Microbiology-Immunology, Quebec, Canada;2Centre de recherche du CHU de Québec, Rhumatology-Immunology, Quebec, Canada
Background: Nucleoside triphosphate diphosphohydrolase-2 (NTPDase2) is an ectonucleotidase which hydrolyses preferentially nucleoside triphosphates. NTPDase2 is expressed by adventitial cells in blood vessels and in different types of glial cells in both central and peripheral nervous systems. Specific tools to NTPDases such as antibodies and inhibitors are requested to study purinergic signalling as NTPDases are major enzymes that terminate these processes. In the present work, we generated four mouse monoclonal antibodies specific to human NTPDase2 in which one of those inhibited human NTPDase2.
Methods: The antibodies were generated by the cDNA immunization technique followed by an injection of transfected HEK 293 cells expressing human NTPDase2 proteins. The produced antibodies were validated and characterized by Western blot, immunocytochemistry and flow cytometry. Inhibition assays were performed with cell lysates of recombinant human NTPDase2 expressed in COS-7 cells and the detection of Pi produced by the enzymatic reaction was measured by the Malachite green assay.
Results: These antibodies were efficient in Western blot, flow cytometry and immunocytochemistry. The four monoclonal antibodies do not cross-react with rat NTPDase2 isoform in Western blot. One of these four antibodies inhibited the ATPase activity of recombinant human NTPDase2 by about 50 %.
Conclusion: We obtained four hybridomas that produce at least two different types of monoclonal antibodies which are specific to human NTPDase2. In addition, one of them is a specific inhibitor of human NTPDase2.
C 021
Mutational analysis of the A1compared to the A2Areceptor dynamics via FRET measurement
Anette D. Stumpf1,2,*, Nicole Ziegler1,2, Tu Dang1, Saskia Schmitt1,2, Ulrike Zabel1, Martin J. Lohse1,2 and Carsten Hoffmann1,2
1University, Pharmacology, Würzburg, Germany;2University, Rudolf Virchow Center, Bio Imaging Center, Würzburg, Germany
The crystal structure of the adenosine A2A receptor (A2AR) in the active and inactive state published in 2008 and 2011 gave insight into the endpoints of the conformational changes that a receptor undergoes when a ligand binds. To learn more about these receptor dynamics of different adenosine receptor subtypes we used fluorescence resonance energy transfer (FRET) measurements of a modified A1 receptor (A1R) and A2AR construct. Therefore, the cyan fluorescent protein (CFP) was fused to the C-terminus and the fluorescein arsenical hairpin binder (FlAsH) binding motif was inserted into the third intracellular loop of each of the two receptors. First investigations were done on the A2AR where ten optical probes including individual mutations based on the ligand binding pocket delineated from the crystal structure were created. The corresponding mutants for the A1R were identified via sequence alignment of the A1R and the A2AR. To compare A1R and A2AR ligand binding dynamics, HEK293 cell lines stably expressing these optical probes were established. FRET measurements were done in living cells where the signal amplitude and the receptor activation dynamics occurring when the ligand binds to the receptor were investigated. Measurements were done with the physiological agonist adenosine, adenosine derivatives and the antagonist theophylline. Different effects concerning the mutations could be observed. The first class causes problems in plasmamembrane localization for the A1R but not for the A2AR. The second group is more crucial for ligand binding in the A1R than in the A2AR. Mutation of the amino acids of the second group made FRET- measureable ligand binding impossible for the A1R but leads only to an affinity loss of the ligands for the A2AR. Thus, our study provides evidence that amino acids serve different functions within the A1R and A2AR ligand binding pocket. In summary the different signal amplitudes and different activation dynamic behavior are indicative for a difference in the activation process of the A1R compared to the A2AR. The data from the receptor mutants support these findings and give new insight into the A1R- structure and function.
References
1. Hoffmann C et al (2005) Nature Methods 2:171–176
2. Klotz K-N et al (1998) Naunyn-Schmiedeberg’s Arch Pharmacol 357:1–9
3. Jaakola VP et al (2008) Science 322(5905):1211–1217
4. Xu F et al (2011) Science 332(6027):322–327
5. Lane JR et al (2012) Mol Pharmacol 81:475–487
C 022
Monitoring ligand-binding to A3AR in living cells using fluorescent ligands: Application to high throughput screening of chemical libraries.
Maria Augusta Arruda1,2,*, Leigh A. Stoddart1, Stephen J. Briddon1, Barrie Kellam3 and Stephen J. Hill3
1University of Nottingham, Cell Signalling Research Group, School of Life Sciences, Nottingham, UK;2Fundacao Oswaldo Cruz, Brazilian Ministry of Health, Farmanguinhos, Rio de Janeiro, Brazil, UK;3University of Nottingham, School of Pharmacy, Centre for Biomolecular Sciences, Nottingham, UK
A number of imaging techniques using fluorescent BODIPY-labelled ligands have been used to study various GPCRs, with a focus on the adenosine receptors. This has led to the study of the adenosine receptors at the single-cell level, in both surrogate and native systems, using state of the art microscopy analysis [1,2]. Recently a competitive binding assay for the human adenosine A3 (A3AR) and A1AR receptors in live cells has been developed, using a high content screening (HCS) platform which can be used to determine the affinity of unlabelled ligands [3]. However, for hit discovery and lead optimization, a high throughput screening (HTS) approach is highly desirable, but only if the assay can preserve the fidelity of the data. In this work, we have tested the suitability of this fluorescent competition binding assay for HTS, comparing curves obtained using HCS and HTS platforms, and screening a commercially available library of pharmacological active compounds (LOPAC) at the A3AR.
It was found, that the competitive fluorescent binding assay on un-modified human A3AR using three fluorescent antagonists can be performed using a HTS platform (BMG Pherastar FS), yielding pKi values comparable to those obtained using a HCS platform (R2 = 0.99). Importantly, the assay could be performed with fluorescent ligands that emit in both the red (CA200645 and AV039) and green (AV051) range. To confirm the suitability of the assay for HTS, the LOPAC library which contains various adenosine receptor ligands was utilised and screened against the A3AR. It was initially screened at a single concentration and all of the medium-to-high affinity ligands for A3AR (pKi > 6) were detected. It was found that an additional 67 compounds inhibited CA200645 binding in line with that of a positive control and 16 were selected for further investigation. Of these 16, 4 compounds had sub-micromolar affinity at the A3AR and have not been previously described as interacting with this receptor. To investigate their mode of binding, molecular docking studies were performed for these 4 compounds and it suggested that these molecules interact with A3AR orthosteric site.
Taken together, our work demonstrates the suitability of this fluorescent ligand binding assay for GPCR Drug Discovery using the Pherastar FS as HTS platform, which has led to the characterization of 4 new A3AR ligands.
References
1. Briddon SJ et al (2004) Proc Natl Acad Sci USA 101:4673–4678
2. Vernall AJ, Hill SJ, Kellam B (2014) Br J Pharmacol 171:1073–1084
3. Stoddart LA et al (2012) Chem Biol 19:1105–1115
C 023
Characterization of A2Badenosine receptor gene knock-out rats
Shraddha Nayak1,*, Md. Abdul Khan1, Tina Wan1, Howard Jacob 2, Aron Geurts 2, John Imig1 and John Auchampach1
1Medical College of Wisconsin, Pharmacology and Toxicology, Milwaukee, UK;2Medical College of Wisconsin, Physiology, Milwaukee, USA
The A2B adenosine receptor (A2BAR) has emerged as an important and unique member of the AR family. Recent studies suggest that the A2BAR mediates many of the protective and reparative actions of adenosine during acute tissue injury in multiple different organs. In contrast, it also mediates destructive actions during chronic diseases associated with persistent adenosine production by promoting overactive healing responses that result in tissue fibrosis. Nearly all previous work investigating the A2BAR has been conducted in mouse models due to the accessibility of A2BAR gene (Adora2b)-ablated mice, and limited information is available in other species. Here, we provide a preliminary report on the characterization of a new line of Adora2b disrupted rats created using the zinc finger nuclease approach. The rat line was developed on the Dahl salt-sensitive (SS) genetic background, which develops hypertension, renal injury, and cardiovascular remodeling with high salt feeding. This strategy yielded a rat strain (SS-Adora2bem2Mcwi) with a 162 bp deletion of Adora2b that included the start codon. Functional deletion of the A2BAR in the SS-Adora2bem2Mcwi rat line was confirmed by loss of BAY 60,6583 (BAY)-induced cAMP elevation and interleukin-6 release from isolated pulmonary and dermal fibroblasts. In initial studies, SS-Adora2bem2Mcwi rats were utilized to study the importance of the A2BAR in glucose regulation during low salt feeding. Although there were no differences at baseline after a 6-h fast, we observed a dose-dependent increase in the blood glucose concentration following administration of BAY in SS rats (from 98 ± 9 to 242 ± 14 mg/dL 80 min after administration of 3 mg/kg of BAY), which was completely absent in SS-Adora2bem2Mcwi rats. In glucose tolerance tests, glucose disposal was delayed in the SS-Adora2bem2Mcwi rat line (2,744 ± 133 mg/dL·min vs. 4,311 ± 320 mg/dL·min, p < 0.05). We next examined the role of the A2BAR during experimental hypertension. Male SS and SS-Adora2bem2Mcwi rats were treated with an 8 % NaCl diet for 6 weeks beginning at 11 weeks of age. We observed no difference between the genotypes in the extent of hypertension, renal injury, or cardiovascular function/remodeling at any time during the experimental protocol. We have successfully created a novel and exciting new tool to study the biology of the A2BAR. These preliminary results confirm an important role of the A2BAR in glucose handling with implications relating to diabetes and other metabolic disorders.
C 024
Species differences and molecular mechanism of action of A3adenosine receptor allosteric modulators
Lili Du1, Zhan-Guo Gao2, Silvia Paoletta2, Tina Wan1, Jacobus van Veldhoven3, Adriaan IJzerman3, Kenneth Jacobson2 and John Auchampach1,*
1Medical College of Wisconsin, Pharmacology, Milwaukee, USA;2National Institutes of Health, Molecular Recognition Section, Bethesda, USA;3Leiden University, Division of Medicinal Chemistry, Leiden, Netherlands
Allosteric modulators for the human A3 adenosine receptor (AR) have recently been discovered including the imidazoquinoline LUF6000 and the 2,4-di-substituted quinoline LUF6096. These ligands and their derivatives function as A3AR enhancers by increasing signaling efficacy of agonists, although they also have the tendency to reduce potency to varying degrees. It remains unclear whether effects of these agents on potency are the result of direct competition at the orthosteric binding site or to allosteric interactions. In this study, enhancement by LUF6000 and LUF6096 was screened at A3ARs from species commonly used pre-clinically, i.e., dog, rabbit, and mouse. In addition, orthosteric effects of the modulators were explored in radioligand binding assays with [125I]I-AB-MECA. Functional modulation was assessed with [35S]GTPγS binding that detects receptor-induced G protein activation using membranes from HEK 293 cells stably expressing recombinant A3ARs. Both LUF6000 and LUF6096 exerted substantial enhancing activity with human, dog, and rabbit, but not with rat (limited studies) or mouse A3ARs. In responsive species, both modulators increased the maximal efficacy of the A3AR agonist Cl-IB-MECA as well as adenosine >2-fold, while slightly reducing potency in all species except rabbit. The potency reduction was uniquely explained by an allosterically induced slowing in orthosteric agonist binding kinetics, based on results from association and dissociation kinetic [125I]I-AB-MECA binding assays. Sequence comparison of A3ARs from the different species revealed variability in the first extracellular loop (EL1) region between responding and non-responding species. Mutation of four amino acid residues of the human A3AR to the murine sequence identified the EL1 region as being important in selectively controlling the negative allosteric actions of LUF6096 on orthosteric binding kinetics. Homology modeling suggested interaction between species-variable EL1 and EL2 that makes ligand contact. These results indicate that A3AR allostery is species-dependent and provide mechanistic insights into this therapeutically promising class of agents.
C 025
Heteromerization of adenosine A2Aand A2Breceptors
Sonja Hinz1,*, Benjamin F. Seibt1, Anke C. Schiedel1, Rafael Franco2 and Christa E. Müller1
1PharmaCenter Bonn, Pharmaceutical Institute, Pharmaceutical Chemistry I, University of Bonn, 53121 Bonn, Germany;2Department of Biochemistry and Molecular Biology, Faculty of Biology, University of Barcelona, Barcelona, Spain
It is now well accepted that G protein-coupled receptors are able to form dimers or oligomers in intact cells, which may consist of identical receptor proteins (“homomeric”) or of different receptors (“heteromeric”). Because of the unique pharmacological properties of these complexes, in particular in the case of heteromers, they represent novel targets for drug development. Different biophysical techniques such as resonance energy transfer (bioluminescence or fluorescence energy transfer, BRET or FRET) or fluorescence complementation techniques have been used to identify these complexes in living cells. It is of great interest to analyze their structure, including different possible interfaces that might be involved in receptor dimerization/oligomerization [1]. In the present study we examined potential heteromer formation between the adenosine A2A and A2B receptor subtypes, which play important (patho)physiological roles and are co-expressed in various cell types and tissues, e.g. in heart.2 Radioligand receptor-binding assays at membranes of stably co-transfected CHO cells were performed to examine pharmacological properties of potential heteromers. Receptor function was investigated by measuring agonist-induced cAMP production of the Gs-coupled receptors. Furthermore, we used confocal laser scanning microscopy to show that the ECFP and EYFP tagged receptor subtypes were co-localized at the plasma membrane and in the ER/Golgi apparatus in stably co-transfected CHO cells. Moreover with FRET studies in transiently transfected CHO cells expressing both, A2A and A2B receptor subtypes, we were able to show that A2A/A2B receptor heteromers were formed. Further studies using a C-terminal deletion mutant of the A2A receptor showed that the C-terminus only plays a negligible role for interaction of the receptors within heterodi- or oligomers. We report here for the first time, that human A2A and A2BARs form heterodimers with altered receptor pharmacology.
Fluorescence resonance energy transfer studies (FRET) in transiently transfected CHO cells expressing adenosine A2Aand A2Breceptors.
References
1. Ferré et al (2014) Pharmacol Rev 66:413–434
2. Zhan et al (2011) Am J Physiol Heart Circ Physiol 301:H1183–1189
C 026
A yeast screening method to decipher the interaction between the adenosine A2Breceptor and the C-terminus of different G protein α-subunits
Rongfang Liu*, Nick Groenewoud, Miriam Peeters, Eelke Lenselink and Ad IJzerman
LACDR, Leiden, Netherlands
The expression of human G protein-coupled receptors (GPCRs) in Saccharomyces cerevisiae containing chimeric yeast/mammalian Gα subunits provides a useful tool for the study of GPCR activation.
In this study we used a one-GPCR-one-G protein yeast screening method in combination with molecular modeling and mutagenesis studies to decipher the interaction between GPCRs and the C-terminus of different α-subunits of G proteins. We chose the human adenosine A2B receptor (hA2BR) as a paradigm, a typical class A GPCR that shows promiscuous behavior in G protein coupling in this yeast system.
The wild type hA2BR and five mutant receptors were expressed in eight yeast strains with different humanized G proteins, covering the four major classes: Gαi, Gαs, Gαq and Gα12. Our experiments showed that a tyrosine residue (Y) at the C-terminus of the Gα subunit plays an important role in controlling the activation of GPCRs. Receptor residues R1033.50 and I1073.54 are vital too in G protein-coupling and the activation of the hA2BR, whereas L213IL3 is more important in G protein inactivation. Substitution of S2356.36 to alanine provided the most divergent G protein coupling profile. Finally, L2366.37 substitution decreased receptor activation in all G protein pathways, although to a different extent.
In conclusion, our findings shed light on the selectivity of receptor-G protein coupling, which may help in further understanding GPCR signaling.
C 027
Channel opening of the human P2X3 receptor requires spontaneous movement of the ligand binding jaw
Anke Dopychai1,*, Maria Kowalski2,*, Ralf Hausmann1, Peter Illes2, Thomas Riedel2 and Günther Schmalzing1
1Department of Molecular Pharmacology, RWTH Aachen University, Aachen, Germany;2Rudolf-Boehm-Institute of Pharmacology and Toxicology, University of Leipzig, Leipzig, Germany
*These authors contributed equally.
Binding of ATP to P2X receptors induces a closing movement of the ATP binding jaw in the extracellular part of adjacent subunits [1–3]. Subsequent conformational changes throughout the protein lead to channel opening and allow cations to move through the channel pore. Using a hP2X3 homology model based on the zfP2X4 crystal structure [1,4], we identified amino acid residues of the hP2X3 receptor located at the lips of the binding jaw, which are potentially involved in the movement of the binding jaw. Single and double cysteine substitution mutants of these amino acid residues were expressed in HEK293 cells and Xenopus laevis oocytes and analyzed by electrophysiology with regard to current amplitudes and agonist potencies. We found that cysteine double mutations of particular residues such as K113C/R198C or K113C/K201C located at the lips of the binding jaw resulted in spontaneous disulfide bond formation, which significantly impaired agonist induced receptor activation. We excluded that cysteine substitution or disulfide formation diminished receptor assembly or cell surface expression. Also, the ligand binding ability of the K113C/K201C double mutant was not impaired as revealed by the wild-type level of binding of the fluorescent ATP-derivative Bodipy-TR ATP to intact oocytes. Altogether, our data suggest that the disulfide cross-linking of K113C and K201C of the hP2X3 receptor hinders the movement of the ATP binding jaw and thus the ATP-induced channel gating.
References
1. Hattori M, Gouaux E (2012) Nature 485:207–212
2. Jiang R, Taly A, Lemoine D, Martz A, Cunrath O, Grutter T (2012) EMBO J 31:2134–2143
3. Jiang R, Taly A, Grutter T (2013) Trends Biochem Sci 38:20–29
4. Kawate T, Michel JC, Birdsong WT, Gouaux E (2009) Nature 460:592–598
C 028
Identification of threonine and tyrosine residues important for human P2X4 receptor activity by site-directed mutagenesis
Arquimedes Cheffer and Henning Ulrich
University of São Paulo, Biochemistry, São Paulo, Brazil
The P2X4 receptor is an ATP-gated cation channel that is composed of three subunits. Each subunit has two transmembrane domains linked by a large extracellular loop and intracellularly located N- and C-termini. The receptors have been implicated in the modulation of membrane excitability, calcium signaling, neurotransmitter and hormone release, and pain physiology. We have studied the participation of highly conserved aminoacids residues located intracellularly in the N and C temini of P2X4 subunits as a critical determinant for receptor activation by using site-directed mutagenesis and electrophysiological characterization of recombinant human P2X4 receptors transiently expressed in HEK-293T cells. We have found that the mutant receptors P2X4E14A, and D16A exhibited properties not different from wild-type P2X4 receptors. However, in contrast, substitution of alanine for Tyr15, and Thr17 produced non-functional receptors expressed at the plasma membrane. Flow cytometry analysis in the presence of an antibody against phosphotyrosine residues indicated that, among the cells that express the P2X4 receptor, the percentage of phosphotyrosine-positive cells was the same for Y372A (86 ± 10 %) and Y378A (79 ± 6.9 %) mutants, however, substantially lower for Y15A (35 ± 12 %), Y367A (48 ± 6.4 %) and Y372F (31 ± 1.7 %) mutants when compared with cells expressing the wild-type receptor (76 ± 5.6 %). Western blot assays revealed that the T17A mutant was phosphorylated at threonine residues, suggesting that the human P2X4 receptor also contains further phosphorylation sites. However, no phosphotyrosine-antibody signal was detected in the wild-type receptor and mutants in which tyrosine residues were replaced by alanine or phenylalanine. The present work indicates that tyrosine phosphorylation of intermediate signaling proteins regulates P2X4 receptor activity.
C 029
Pharmacological identification of P2X4 receptor-mediated calcium influx in human macrophages
Janice Layhadi1,*, Jeremy Turner2 and Samuel Fountain1
1University of East Anglia, School of Biological Sciences, Norwich, UK;2University of East Anglia, Norwich Medical School, Norwich, UK
Macrophages express a repertoire of cell surface P2 receptors for ATP, a damage-associated molecular pattern molecule (DAMP), that are capable of raising cytoplasmic calcium when activated; either through direct permeation (ionotropic P2X receptors) or by mobilising intracellular calcium stores (metabotropic P2Y receptors). Here, the contribution of P2X4 receptor activation in ATP-evoked calcium responses in human macrophages is investigated. THP-1 monocytes were differentiated into macrophages by treatment with 320nM PMA over 48 h. Intracellular Ca2+ measurements were performed on Fura-2-loaded macrophages and quantified on Flex Station III microplate reader. ATP evoked concentration-dependent calcium responses (EC50 = 6.25 × 10−6 M, N = 6) in macrophages. Maximal response to ATP (100 μM) was strongly inhibited by the SERCA inhibitor thapsigargin (Tg, 5 μM; 74 ± 2 % inhibition, N = 6). The Tg-resistant component of the ATP response was abolished in the absence of extracellular calcium, suggesting a total dependency on calcium influx. Ivermectin (IVM) potentiated the magnitude of the Tg-resistant component by 2.3-fold and slowed the response decay kinetics (τ = 14 ± 1 s control vs 50 ± 4 s IVM; N = 4, p < 0.001). The Tg-resistant component was antagonised by P2X4 receptor antagonists 5-BDBD (10 μM; 53 ± 2 %, N = 3; p < 0.01) and PSB-12062 (10 μM; 79 ± 3 %, N = 3; p < 0.001) but not by the P2X7 receptor antagonist A438079 (10 μM). Inhibition of endocytosis with dynasore (80 μM, 30 min) caused a reduction (46.8 ± 2.97 % vs control) in the peak of the Tg-resistant component but substantially slowed decay kinetics (τ = 14 ± 1 s control vs 82 ± 7 s dynasore; N = 3, p < 0.001). Inhibition of calcium-dependent exocytosis with Vacuolin-1 (1 μM) had no effect on the Tg-resistant component. These pharmacological data suggest that P2X4 receptor activation contributes significantly to the ionotropic calcium response of human macrophages to ATP.
C 030
Identification and characterization of P2X4 receptor agonists and antagonists by radioligand binding and calcium assays
Aliaa Abdelrahman1,*, Anke C. Schiedel1, Meryem Köse1, Sonja Hinz1, Maggie Burton2, Michel Gillard2, Marc de Ryck2 and Christa E. Müller1
1PharmaCenter Bonn, Pharmaceutical Institute, Pharmaceutical Chemistry I, University of Bonn, An der Immenburg 4, D-53121 Bonn, Germany;2UCB Pharma, Chemin du Foriest, B-1420 Braine-L’Alleud, Belgium
The P2X4 receptor (P2X4R) is highly expressed in the central nervous system (CNS) and on immune cells [1]. The pharmacological blockade of P2X4R has been postulated as a novel strategy for the treatment for chronic neuropathic pain and in neuroprotection. Recent evidence has shown that P2X4 antagonists may also be useful for the treatment of various inflammatory diseases including diabetic nephropathy, [2] joint inflammation and arthritis, [3] spinal cord injury, [4] and the prevention of excitotoxic damages after epileptic seizures. With the goal to develop potent, selective P2X4 antagonists as tool compounds and potential drugs, we stably expressed the human, rat and mouse P2X4 receptor in 1321N1 astrocytoma cells by retroviral transfection, and established monoclonal cell lines. Calcium flux assay conditions were optimized for high-throughput screening resulting in a Z′-factor of >0.7. The application of ready-to-use frozen cells did not negatively affect the results of the calcium assays, which is of great advantage for the screening large compound libraries. Membrane preparations of the cell lines showed high levels of specific [35S]ATPγS binding, while non-transfected cells were devoid of specific binding sites for the radioligand. Conditions were employed which allowed binding studies to be performed at room temperature. Standard P2X4R agonists and antagonists were investigated in calcium studies as well as in radioligand binding assays at all three species. The non-nucleotidic antagonists paroxetine and 5-(3-bromophenyl)-1,3-dihydro-2H-benzofuro[3,2-e]-1,4-diazepin-2-one (BDBD) were clearly shown not to interact with the ATP binding site, but to act as negative allosteric modulators (NAMs) [5]. The established assays were successfully used to identify and develop novel classes of allosteric P2X4 antagonists, including phenoxazine [6] and carbamazepine derivatives [7].
This study was supported by the BMBF (German Federal Ministry for Education and Research) within the BioPharma initiative ‘Neuroallianz’.
References
1. Coddou et al (2011) Pharmacol Rev 63:641–683
2. Chen et al (2013) Cell Biol 45:932–943
3. Li et al (2014) Inflammation 37:146–153
4. Lu et al (2013) Neurosci Res 91: 694–705
5. Ulmann et al (2013) Glia 61:1306–1319
6. Hernandez-Olmos et al (2012) J Med Chem 55:9576–9588
7. Tian et al Bioorg (2014) Med Chem 22:1077–1088
C 031
The role of intracellular receptor regions of the P2X7 receptor in time-course regulation
Rebecca Allsopp and Richard Evans
University of Leicester, Cell Physiology and Pharmacology, Leicester, UK
The P2X7 receptor has both structural and functional features that distinguish it from other members of the P2X receptor family; (i) there is an inclusion of a cysteine rich region after the TM2 domain and a long C-terminal tail and (ii) unlike other P2X receptors, P2X7 receptor channel activation requires milli molar concentrations of ATP with repeat applications to fully facilitate the response.
Determination of the crystal structure of zebrafish P2X4 has allowed previous biochemical and mutagenic P2X studies to be interpreted in a structural context. However, the crystal structure does not include the intracellular receptor domains, which were largely removed to aid the crystallisation process. Work in our lab using P2X receptor chimeras has highlighted significant roles of these regions in P2X receptor time-course regulation.
We have previously shown a role of the pre TM1 region in the time-course regulation of P2X1/2. This study extends our findings to the P2X7 receptor and in addition determines a role of variant C-terminal residues in time-course regulation of P2X7.
Studies on the rapidly desensitising P2X1 receptor versus the non-desensitising P2X2 receptor demonstrated the ability of the N-terminus of P2X2 to slow desensitisation of P2X1 (P2X1-2N). Reciprocally, the N-terminus of P2X1 speeded desensitisation of P2X2 (P2X2-1N). This effect was narrowed down to variant amino acids close to the TM1 domain (termed β-region). Replacing the β-region (amino acid residues 13–25) of P2X7 with the corresponding amino acids of either P2X1 (P2X7-1Nβ) or P2X2 (P2X7-2Nβ) resulted in fully facilitated channel response with rapid (P2X1/P2X2 - like) rise time to initial ATP application. Replacing the pre-TM1 β-region of either P2X1 or P2X2 with that of P2X7 also showed effects on receptor time-course. The P2X1-7Nβ chimera showed rapid desensitisation and P2X2-7Nβ chimera showed biphasic desensitization with greatly prolonged time to 50 % decay on washout of ATP.
To assess whether these time-course regulatory effects are confined to the N-terminus or whether with the C-terminus also plays a contributory role we have generated mutant receptors to determine the contribution of P2X7 specific amino acids (residues 362–379) found just after TM2.
C 032
Genetic analysis of the P2X7 receptor: insights into its structure and function
Marie Brunet1,*, Evelina Gabasova2 and Ruth Murrell-Lagnado1
1Cambridge University, Pharmacology, Cambridge, UK;2Cambridge University, MRC Biostatistics Unit, Cambridge, UK
We have used genetic and mutational analyses to investigate structure-function relationships for the P2X7 receptor. Our focus has been on its uniquely long C-terminal tail, which is known to be an important determinant of receptor trafficking and function (Costa et al., 2011). Unlike the transmembrane and extracellular domains, we currently have no detailed structural information about the cytoplasmic regions. We compared the coding sequences of P2X7 receptors from 59 species, ranging from coelacanth to human, and identified the C-terminus as the most divergent region of the receptor throughout its evolutionary history. With a Bayesian analysis, individual amino acids within this 240 amino acid region were identified as under positive (diversifying) selection. This can occur as a consequence of these residues being involved in interactions either within the receptor complex or with other regulatory factors. Focusing on the possibility of intrasubunit interactions, we next used a covariance analysis and mutual information algorithm to map where interactions are likely to take place between residues within the P2X7 receptor C-terminus. Interactions were identified both between clusters of amino acids close together in sequence and thus likely to represent a structural interaction, and those far apart which are more likely to represent a functional interaction. We also identified pairs of residues that co-evolved and thus are likely to share a common structural or functional role. To investigate this further we mutated these residues individually and together within the rat P2X7 receptor, expressed the receptors in TsA201 cells and initially compared two properties; first, the rate of ATP-evoked ethidium uptake as a measure of the rate of pore dilation, second, cell survival following serum deprivation. An example of an interacting pair is the E496 and P594 residues; E496A being one of the six loss-of-function SNP in the human receptor. Across taxa, the P594 is changed to a glutamic acid when the E496 is changed to a glutamine. We observed a significantly decreased rate of dye uptake with the E496Q mutant, and this loss of function was retrieved with the double mutant. Similarly, the P594E mutant showed greater survival after serum starvation, an effect that was lost with the double mutant.
We also identified three amino acids within the rat C-terminus, namely I409, W410 and L494, which co-evolved with residues within the ATP binding pocket (E77, V190, N195, R294). The human P2X7 receptor has an arginine instead of a tryptophan at the 410 position and the W410R mutation in rat P2X7 dramatically slows ATP-evoked ethidium uptake to a rate similar to the human isoform.
Finally, we are investigating the role of a highly hydrophobic domain within the distal C-terminus of rat P2X7 in determining the association of this region with the plasma membrane, a feature that distinguishes it from other isoforms of P2X7.
C 033
Effects of carbenoxolone, niflumic acid, NPPB and probenecid on P2X7 mediated YOPRO-1 and lucifer yellow influx in RAW 264.7 and mouse P2X7 transfected HEK 293 cells
Kemal Sayar1,*, Serife Cankurtaran-Sayar2 and Mehmet Ugur2
1Ankara University Faculty of Medicine, Medical Pharmacology, Ankara, Turkey;2Ankara University Faculty of Medicine, Biophysics, Ankara, Turkey
Introduction: Activation of P2X7 receptors result in permeabilisation of cell membrane to large molecules (Molecular weight ~900 Da). Other membrane proteins (for example Pannexin-1) have been implicated in this process. In this study, we examined the effects of carbenoxolone, niflumic acid, NPPB and probenecid on P2X7 mediated YOPRO-1 and lucifer yellow influx in RAW 264.7 and mouse P2X7 transfected HEK 293 cells. In the literature, carbenoxolone, NPPB and probenecid have been used as an inhibitor of pannexin currents; niflumic acid and NPPB have been used as an inhibitor of anion channels and probenecid has been used as an inhibitor of organic anion transporters.
Materials and methods: Cell lines: RAW 264.7 cells which endogeneously express mouse P2X7 receptors and mouse P2X7 transfected HEK 293 cells. Solution: Nominally calcium free KCl solution (5 mM NaCl, 150 mM KCl, 10 mM HEPES pH: 7.4).
Fluorescent measurements: YOPRO-1 influx has been measured by 2104 EnVision Multilabel Plate Reader. Lucifer yellow influx has been measured by Leica confocal microscope.
Results: In mP2X7 transfected HEK 293 cells, carbenoxolone, niflumic acid, NPPB and probenecid did not show any inhibitory or potentiating effect on P2X7 mediated YOPRO-1 influx. In RAW 264.7 cells, these agents did not show any inhibitory effect, instead at the higher concentrations (carbenoxolone 100 uM, niflumic acid 100 uM, NPPB 100 uM and probenecid 10 mM) these agents potentiated YOPRO-1 influx. When used at higher concentration these agents also showed a potentiating effect on ATP induced lucifer yellow 2
Conclusions: Agents reported to be pannexin inhibitors (carbenoxolone, NPPB and probenecid) did not show any inhibitory effect on mouse P2X7 induced YOPRO-1 influx, neither in mP2X7 transfected HEK 293 nor in RAW 264.7 cells at concentrations that were reported to block pannexin currents effectively. However in RAW 264.7 cells these agents and also niflumic acid potentiated mP2X7 induced lucifer yellow and YOPRO-1 influxes at higher concentrations. This potentiation effect complicates the interpretation of the effectiveness of these inhibitors on the P2X7 induced dye influx in RAW 264.7 cells.
Acknowledgements This study was supported by TUBITAK research grant SBAG 112s615.
C 034
Effect of POM-1 on P2X7 receptor in macrophages
Gabriela Pimenta*, Pedro Persechini and Julieta Schachter
Universidade Federal do Rio de Janeiro, Instituto de Biofísica Carlos Chagas Filho, Rio de Janeiro, Brazil
Text: Extracellular nucleotides and nucleosides are important regulators of the immune response, with pro-inflammatory and anti-inflammatory effects.In the extracellular space, the interplay between nucleotides and their receptors is influenced by the activity of ectonucleotidases, which hydrolyze nucleotides, participating with the maintenance of the extracellular homeostasis of these molecules. Several drugs are currently used to inhibit receptors for nucleotides and ectonucleotidases but possible cross reactivity is usually not investigated. Both ectoenzymes and P receptors are found inmacrophages, which have an important function in the regulation of inflammation. We investigate the effects of POM-1, a drug described as selective CD39 inhibitor on phenomena activated by P2X7 receptor in mice macrophages.
Methods and results: Using intraperitoneal murine macrophages, we evaluated the activity of CD39 and CD73 by malachite green method. We concluded that activity of CD39 is present in macrophages and is inhibited by POM-1 with an IC50 of 50 ± 0.3 μM. However, we did not detect CD73 activity. Next, we evaluated intracellular calcium signaling in response to ATP using FURA-2 fluorescence microscopy. We observed that POM-1 100 μM inhibits the increase of free cytoplasmatic calcium concentration in response to high concentrations of ATP, suggesting that it inhibits P2X7 receptor. We also investigated ATP-induced P2X7-associated dye uptake using a fluorometer. POM-1 inhibited ATPe-induced uptake of anionic dyes, but not cationic dyes. We previously characterized the selective uptake of cationic dyes as a P2X7-dependent calcium-independent transport mechanism present in macrophages.
Conclusion: Taken together, our results demonstrated that POM-1modulates P2X7 function and provides new evidences for the existence of at least two transport mechanisms associated with this receptor: one for cations and another for anions. The extent of P2X7 inhibition by POM-1 will be further investigate byelectrophysiology and by functional assays such as the induction of cell death and secretion IL-1β and TNF-α.
C 035
Plasma membrane cholesterol regulates human and rodent P2X7 receptor activation and sensitization
Lucy Robinson*, Phil Smith, Mitesh Shridhar and Ruth Murrell-Lagnado
University of Cambridge, Department of Pharmacology, Cambridge, UK
P2X7 receptors are cation channels gated by high extracellular ATP but with sustained activation receptor sensitization occurs, whereby the intrinsic pore dilates making the cell permeable to large organic cations, leading to cell death. P2X7 is highly expressed in immune cells where this ability to cause cell death contributes to the innate immune response, but in chronic inflammation P2X7 is upregulated in other cells, where P2X7-mediated cell death contributes to pathology.
We investigated plasma membrane cholesterol as a potential regulator of P2X7 properties for several reasons. First, P2X7 receptors are present in cholesterol-rich microdomains in the plasma membrane known as lipid rafts. Second, lipid signalling pathways are activated downstream of P2X7, the products of which can displace cholesterol from lipid rafts. Third, lipid rafts may be disrupted in chronic inflammatory diseases.
We show that pore dilation of human and rodent P2X7 receptors is sensitive to cholesterol levels. Acute depletion of cholesterol with 5 mM methyl-β-cyclodextrin (MCD) caused a substantial increase in the rate of agonist-evoked pore dilation, as measured by the uptake of ethidium dye, while loading cells with cholesterol reduced the rate of pore dilation. This cholesterol sensitivity was more pronounced in the human P2X7, which shows a slower rate of sensitization than mouse P2X7. We also observed this cholesterol sensitivity in primary mouse bone marrow derived macrophages. Macrophages express high levels of P2X7 and their plasma membrane cholesterol levels change in disease states such as atherosclerosis, thus this mode of regulation may be important in pathology.
Patch clamp analysis of P2X7 receptor currents carried by Na+ and NMDG+ showed enhanced activation and current facilitation following cholesterol depletion. This contrasts with the inhibitory effect of MCD reported for other P2X subtypes.
Using site-directed mutagenesis, we probed the molecular basis of this cholesterol sensitivity. Mutations were made in the human P2X7R at cholesterol binding motifs, and within the proximal C-terminal region at hydrophobic residues and putative palmitoylation sites. Some of these mutations disrupted receptor function, but they did not selectively inhibit sensitivity to cholesterol. Mutating residues within the N-terminal region (K17R, V18M), at the same position as residues shown to be important for P2X1R sensitivity to cholesterol, did disrupt the potentiating effects of MCD.
We conclude that cholesterol is a negative regulator of P2X7 receptor pore formation: it slows receptor sensitization. Thus, targeting of P2X7 to lipid rafts may protect cells from P2X7-mediated cell death, while disruption of lipid rafts, which occurs early in chronic inflammatory diseases, may enhance P2X7 function so that it contributes to the development of chronic inflammation.
C 036
Paroxetine and trifluoperazine suppress recombinant human P2X7 responses and ATP-induced interleukin-1β secretion from human monocytes
Phuong Dao-Ung1, Kristen Skarratt1, Stephen Fuller1 and Leanne Stokes1,2,*
1University of Sydney, Nepean Clinical School, Penrith, Australia;2RMIT University, Health Innovations Research Institute, Bundoora, Australia
Genetic variants of the human P2X7 receptor (P2X7) have been linked with major depression and bipolar disorders and the P2X7 knockout mouse has been shown to exhibit anti-depressive like behaviour. P2X7 is a major regulator of the pro-inflammatory cytokine interleukin 1b (IL-1β) secretion from monocytes and microglia. Levels of pro-inflammatory cytokines can be increased in patients with depression. We determined whether common psychoactive drugs could affect recombinant and native human P2X7 responses in vitro. Common antidepressants demonstrated opposing effects on human P2X7-mediated responses; paroxetine inhibited while fluoxetine and clomipramine mildly potentiated ATP-induced dye uptake in HEK-293 cells stably expressing recombinant human P2X7. Paroxetine inhibited human P2X7 in a concentration-dependent manner with an IC50 value of 24 μM. The anti-psychotic agent trifluoperazine hydrochloride also suppressed human P2X7 responses with an IC50 value of 6.4 μM while carbamazepine and lithium chloride displayed no effect. Both paroxetine and trifluoperazine did not inhibit rodent P2X7 responses and mutation of a known antagonist binding residue (P95L) did not alter the effect of either drug suggesting neither drug binds at this site. Reduction of P2X7-induced IL-1β secretion from LPS-primed human CD14+ monocytes was observed with trifluoperazine and paroxetine. This information may be beneficial in the future treatment of mood disorders.
C 037
Ginsenosides from Panax ginseng are novel positive allosteric modulators of P2X7
Ray Helliwell, Charlene Ong ShiokHuey, Kshitija Dhuna, Charile Xue and Leanne Stokes*
RMIT University, Health Innovations Research Institute, Bundoora, Australia
Several natural products have been shown to modulate P2X7 responses. We focused on testing compounds present in the root of Panax ginseng which is well known for its medicinal properties in traditional chinese medicine. We observed that a standard formulation of ginsenosides known as G115 containing 4 % ginsenosides in total, had a potentiating effect on P2X7-induced dye uptake responses in HEK-293 cells stably expressing human P2X7. We then screened the purified ginsenosides Rb1, Rb2, Rc, Rd, Re, Rf, Rg1, Rg2, Rg3, Rh1, Rh2, PPD, PPT and the known intestinal metabolite compound K (CK) for their effect on human P2X7 responses. We found a large potentiation (10-fold) of 200 μM ATP-induced YOPRO uptake with Rh1, Rb1, Rd and CK at concentrations of 10 μM. We focused on the smallest compound to have a significant effect, CK, to investigate the mechanism and species dependence of the potentiation effect on P2X7 further. CK potentiated human, rat and mouse P2X7 receptor responses to both ATP and BzATP. CK could also potentiate ATP-induced dye uptake in J774 macrophages endogenously expressing P2X7. Patch clamp studies revealed that CK potentiated ATP-induced inward currents at concentrations as low as 0.1 μM. Effects were rapid in onset and readily reversible upon washout. The effect of CK was dependent on extracellular application since intracellular CK did not affect ATP-induced responses. Finally, we determined whether CK could increase the apoptotic effects of ATP in HEK-293 cells stably expressing human P2X7 using an MTS assay. A sub-threshold concentration of ATP (200 μM) was used which did not affect cell viability alone after 24 h incubation, but in the presence of 10 μM CK, cell viability was significantly reduced compared to vehicle control treated cells.
C 038
Immunohistochemical characterization of purinoceptor expression during spermatogenesis
Nadine Mundt1,*, Patricia A. Machado1,*, David Fleck1, Sophie Veitinger2, Corinna Engelhardt1, Susanne Lipartowski1, Jennifer Spehr1 and Marc Spehr1
1RWTH Aachen University, Institute for Biology II, Chemosensation, Aachen, Germany;2Ruhr-University Bochum, Department for Cellular Physiology, Bochum, Germany
Spermatogenesis is one of the most intricate processes of sequential cell proliferation and differentiation in postnatal life. Initially, spermatogonial stem cells divide mitotically into different types of spermatogonia, which finally give rise to haploid spermatozoa arising from successive mitotic, meiotic and postmeiotic divisions within the seminiferous tubules of the testis. Thus, a fundamental regulatory aspect of spermatogenesis is cell-to-cell communication both between developing germ cells as well as germ and Sertoli cells. As previously shown, a likely mediator of such communication is ATP. In both previous and on-going studies, electrophysiological characterization revealed the functional expression of P2X2 receptors in Sertoli cells as well as P2X4 and P2X7 receptors in spermatogonia. Here, we use molecular and immunochemical methods to obtain a more detailed profile of purinoceptor expression in murine seminiferous tubules at different stages of development. RT-PCR, western blotting and immunohistochemistry confirmed the presence of P2X2, P2X4, and P2X7 as well as BKca channels in murine testes. Focusing on the cell type-specific distribution of purinergic receptors, we performed co-localization studies with cell-specific markers. Together, our studies provide new insight into the complex field of purinergic cell-cell communication during spermatogenesis.
C 039
Optogating a powerful approach to control an ion-channel gate
Damien Lemoine1,*, Chloé Habermacher1, Adeline Martz1, Pierre-François Méry2, Nathalie Bouquier2, Fanny Diverchy1, Antoine Taly3, François Rassendren2, Alexandre Specht1 and Thomas Grutter1
1CNRS, UMR 7199, université de Strasbourg, Chhimie et Neurobiologie Moléculaire, Illkirch, France;2CNRS, UMR 5203, université de Montpellier, Institut de Génomique Fonctionnelle, Montpellier, France;3CNRS, université Paris Diderot, Laboratoire de Biochimie Théorique, Paris, France
The ATP-gated P2X receptors (P2XR) are trimeric ion channels selective to cations. These ion channels are involved in various physiological processes such as nociception and neuromodulation. The study of P2XR physiology suffers from a lack of selective pharmacological molecules. Optogenetic pharmacology could solve this problem. Here we developed a unique and versatile method, in which the gating machinery of the P2X2 receptor was reprogrammed to respond to light. We found that channels covalently modified by azobenzene-containing reagents at the transmembrane segments could be reversibly turned on and off by light, without the need of the natural ligand. We demonstrated photocontrol of neuronal activity by a light-gated P2X receptor, in which the natural sensitivity to ATP was genetically abolished. These light-gated P2X receptors represent valuable tools for investigating the physiological functions of P2XR.
C 040
Functional characterization of purinergic ion channels in single spermatogonia in vitro and in situ
David Fleck1,*, Sophie Veitinger2, Thomas Veitinger1, Patricia Almeida Machado1, Susanne Lipartowski1, Corinna Engelhardt1, Jennifer Spehr1 and Marc Spehr1
1RWTH Aachen University, Chemosensorik, Aachen, Germany;2Ruhr-University Bochum, Department for Cellular Physiology, Bochum, Germany
Spermatogenesis is a fundamental and highly complex biological process that ensures male fertility. Spermatogonia are the precursors of all male germ cell stages. Their differentiation assures the lifelong production of mature sperm. However, few physiological details are known about testicular cell communication during spermatogenesis. Since we and others have previously shown that Sertoli cells are able to communicate via ATP, we hypothesize a general role for purinergic signaling in the testis.
Using wildtype C57BL/6 mouse pups, we first developed a coculture of Sertoli cells and spermatogonia. Next, we investigated ATP-dependent signaling by whole-cell patch-clamp recordings from cultured spermatogonia. Pharmacological profiling and gene expression knockdown allowed identification of involved ion channels.
Here, we report that cultured spermatogonia respond to extracellular ATP (1–100 μM). ATP-induced currents show fast activation and moderate desensitization. The current–voltage relationship reveals strong inward rectification. Current potentiation by ivermectin and inhibition by an acidic extracellular pH (6.3) and extracellular copper (100 μM) indicate a functional role of P2X4 receptors. Accordingly, knockdown of P2X4R expression by RNA interference significantly reduced currents activated by ATP concentrations ≤ 300 μM. Interestingly, an increased ATP concentration (>300 μM) activated an additional current with different kinetics. A similar current could be activated by 300 μM 3′-O-(4-Benzoyl)benzoyl ATP (BzATP). This current was blocked by the P2X7-Antagonist A-438079. Knockdown of P2X7R expression decreased the current activated by higher ATP concentrations (>300 μM). Combined with molecular evidence, our results indicate that at least two different of P2X receptor subunits (P2X7R and P2X4R) are functionally expressed in spermatogonia of young prepubescent mice. Downstream of P2X receptor activation, we found a slowly activating calcium-dependent potassium current functionally antagonizing the depolarizing P2XR-mediated current.
To confirm these results in situ, we established a new experimental approach. Using acute tissue slices of prepubescent mouse testis we electrophysiologically analyzed spermatogonia and found ATP-induced currents with similar characteristics.
Together, these data represent a first important step towards a deeper understanding of cellular purinergic communication during spermatogenesis.
C 041
Identifying ligand induced conformational changes of human P2X1 receptors with voltage clamp fluorometry
Alistair Fryatt* and Richard Evans
University of Leicester, Cell Physiology and Pharmacology, Leicester, UK
P2X1 receptors are part of the family of ATP-gated ion channels involved in a wide array of physiological processes, such as regulating cardiovascular tone and neuropathic pain states. Recent advances have been made to understanding the molecular structure and function of these ion channels, including the generation of crystal structures of zebrafish P2X4 in both ATP-free and ATP-bound states. However, these structures only give snapshots of the conformation of the receptor and do not address the time-course of conformational changes following agonist binding, channel opening, desensitization or agonist unbinding. To address these questions we used voltage clamp fluorometry to study human P2X1 receptors by introducing single cysteine point mutations along the subunit interface at residues around the cysteine rich head region (K138C, E181C), adjacent to the agonist binding site(N284C, K190C) and linking the binding site to the transmembrane portions (D320C, P196C, I62C).
Voltage clamp fluorometry combines the simultaneous measurement of agonist induced channel current and the fluorescence output of an environmentally sensitive probe covalently bonded to an introduced mutant cysteine residue. This allows real time measurement of any conformational changes by detecting any changes in the fluorescence output. This technique correlates conformational changes with the generated current and movements after current flow stops or “electrically silent” events, such as desensitization or ligand unbinding.
In this study cysteine mutant human P2X1 receptors were expressed in Xenopus oocytes and labelled with 2-((5(6)-Tetramethyl-rhodamine)carboxylamino)ethyl Methanethiosulfonate (MTS TAMRA). When purified P2X1 receptors were separated by SDS-PAGE electrophoresis, MTS TAMRA labelling was seen for each of the single cysteine point mutants above but not for wild type P2X1 receptors. Additionally labelling with MTS TAMRA had no effect on agonist evoked currents, showing that fluorescent probe binding did not inhibit channel function. ATP application had little or no effect on fluorescence at D320C, P196C, I62C and E181C mutants however decreases in fluorescence were recorded at K138C, N284C and K190C mutants (approx. 5 %, 10 % and 15 % decrease respectively) which was sustained during application despite current desensitization. These decreases in fluorescence returned to baseline levels following ATP washout. At the K190C mutant, short ATP applications had limited current desensitization on agonist washout and faster initial rates of fluorescence recovery. In addition the rates of fluorescence and current recovery from desensitization were not the same. This suggests that there are temporally distinct and state dependent conformational changes of the receptor.
C 042
Optical dissection of gating in P2X receptors
Chloé Habermacher*, Damien Lemoine, Adeline Martz, Alexandre Specht and Thomas Grutter
CNRS, UMR7199, Université de Strasbourg, Chimie et Neurobiologie Moléculaire, Illkirch, France
P2X receptors are ligand-gated ion channels activated by extracellular ATP. They form trimeric pores selective to cations and are involved in different physiological and pathological functions, including neuromodulation, neuropathic pain and vascular remodeling (1). Crystal structures were solved recently (2) but they give little insight into the dynamic motions involved in channel gating. Complementary approaches have thus to be developed to address the issue. Here we present a new method, which uses azobenzene-containing derivatives as photoisomerizable crosslinkers to investigate channel gating. We have engineered a P2X receptor to obtain an optical control of the channel: it can be opened by irradiation at 525 nm and closed at 365 nm in the absence of its native ligand. The cis-trans isomerization of the covalently tethered bis-maleimide azobenzene-containing derivatives between substituted cysteine residues induces movements of the transmembrane helices that is similar to that induced by ATP. With these results we confirm the X-ray predicted expansion of the extracellular part of the channel during opening. Therefore this approach could be used to explore molecular motions of distant regions relevant to activation, and can be extended to any membrane-embedded proteins.
References
1. Lemoine D et al (2012) Ligand-gated ion channels: new insights into neurological disorders and ligand recognition. Chem Rev 112(12):6285–6318
2. Hattori M, Gouaux E (2012) Molecular mechanism of ATP binding and ion channel activation in P2X receptors. Nature 485:207–212
C 043
The homodimeric anoctamin-1, but not the homodimeric anoctamin-6, is activated by calcium rises mediated by P2Y1and P2X7 receptors
Michaela Stolz1, Manuela Klapperstück1,*, Thomas Kendzierski2, Silvia Detro-Dassen1, Anna Panning1, Günther Schmalzing1 and Fritz Markwardt2
1RWTH Aachen University, Molecular Pharmacology, Aachen, Germany;2Martin-Luther-Universität Halle-Wittenberg, Medizinische Fakultät, Julius-Bernstein-Institut für Physiologie, Halle, Germany
The P2X7 receptor (P2X7R) is a ligand-gated ion channel that conducts Na+, K+, and Ca2+ when activated by extracellular ATP. In various cell types, such as secretory epithelia, the P2X7R is co-expressed with Ca2+-dependent Cl− channels of the TMEM16/anoctamin family. Here, we studied whether the P2X7R and TMEM16A/anoctamin-1 or TMEM16F/anoctamin-6 interact functionally and physically, using Xenopus laevis oocytes and Ambystoma mexicanum (Axolotl) oocytes for heterologous expression. As a control, we co-expressed anoctamin-1 with the P2Y1 receptor (P2Y1R), which induces the release of Ca2+ from intracellular stores via activating phospholipase C through coupling to Gαq. We found that co-expression of anoctamin-1 with the P2Y1R resulted in a small transient increase in Cl− conductance in response to ATP. Co-expression of anoctamin-1 with the P2X7R resulted in a large sustained increase in Cl− conductance via Ca2+ influx through the ATP-opened P2X7R in Xenopus oocytes, and also in Axolotl oocytes, which lack endogenous Ca2+-dependent Cl− channels. No such increase in Cl− conductance was observed in either Xenopus oocytes or Axolotl oocytes co-expressing Ano6 and the P2X7 receptor, despite the verified presence of Ano6 as a non-covalently assembled homodimer in the plasma membrane. The P2Y1R- or P2X7R-mediated stimulation of Ano1 is merely functional, as demonstrated by the absence of a physically stable interaction between Ano1 and the P2X7R. The P2X7R-mediated sustained activation of Ano1 may be physiologically relevant to the time course of stimulus-secretion coupling in secretory epithelia.
C 044
Increased intrinsic activities of ADP and ATP at the human P2Y12-receptor fused to ECFP
Sami Khaznadar1,*, Kristina Hoffmann1, Jens Straßburger1, Ali El-Tayeb2, Christa E. Müller2 and Ivar von Kügelgen1
Pharma Center Bonn,1Department of Pharmacology, and2Pharmaceutical Chemistry I, University of Bonn, Bonn, Germany
P2Y12-receptors play an important role in platelet aggregation. A human P2Y12-receptor construct fused at its C-terminus to enhanced cyan fluorescent protein (ECFP) showed marked expression at the cell membrane when expressed in CHO cells, 1321N1 astrocytoma cells or PC12 cells. P2Y12-receptor agonists reduced the forskolin-induced cAMP accumulation in CHO Flp-In cells expressing the P2Y12-ECFP-fusion protein. Interestingly, the respective concentration-response curves were shifted to the left (ADP EC50 3 nM; 2-methylthio-ADP EC50 7 pM) when compared to those obtained in cells expressing the wild-type P2Y12-receptor (ADP EC50 200 nM; 2-methylthio-ADP EC50 1.5 nM). In addition, ATP caused an inhibition of forskolin-induced cAMP production (EC50 707 nM) in cells expressing the P2Y12-ECFP-fusion protein. Analysis by LC-ESI-MS (liquid chromatography coupled to electrospray ionization mass spectrometry) and NMR (1H, 13C, and 31P-nuclear magnetic resonance) spectroscopy indicated the absence of ADP in the batch of ATP tested. In contrast to ADP and ATP, AMP caused at best a slight response. Increases in agonistic potencies were also observed in a cAMP response element-directed reporter gene assay. The P2Y12-receptor antagonist cangrelor (0.1 nM) shifted the concentration-response curves of 2-methylthio-ADP to the right in cells expressing wild-type P2Y12-receptors (pKB 8.7) or P2Y12-ECFP-fusion proteins (pKB 8.5). In cells expressing the K280A-mutant of the P2Y12-ECFP-fusion protein, 2-methylthio-ADP inhibited forskolin-induced responses only at high concentrations (EC50 400 nM) in agreement with the involvement of K280 in agonist recognition. The data also indicate a role of the C-terminus of the human P2Y12-receptor in control of receptor expression and intrinsic activities of agonists.
C 045
The G protein-coupled receptor heterodimer network (GPCR-HetNet) and its hub components
Michael Di Palma1,*, Dasiel O. Borroto-Escuela1, Ismel Brito2, Wilber Romero-Fernandez1, Julia Oflijan3, Kamila Skieterska 4, Jolien Duchou 4, Kathleen Van Craenenbroeck 4, Diana Suárez-Boomgaard 5, Alicia Rivera 5, Diego Guidolin6, Luigi F. Agnati7 and Kjell Fuxe1
1Karolinska Institutet, Department of Neuroscience, Stockholm, Sweden;2IIIA-CSIC, Barcelona, Spain3University of Tartu, Department of Physiology, Tartu, Estonia;4Ghent University, Laboratory of Eukaryotic Gene Expression and Signal Transduction, Ghent, Belgium;5University of Málaga, Department of Cell Biology, Málaga, Spain;6University of Padova, Department of Molecular Medicine, Padova, Italy;7IRCCS, Venice, Italy
G protein-coupled receptors (GPCRs) oligomerization has emerged as a vital characteristic of receptor structure. Substantial experimental evidence supports the existence of GPCR-GPCR interactions in a coordinated and cooperative manner. However, despite the current development of experimental techniques for large-scale detection of GPCR heteromers, to understand their connectivity it is necessary to develop novel tools to study the global heteroreceptor networks. To provide insight into the overall topology of the GPCR heteromers and identify key players, a collective interaction network was constructed. Experimental interaction data for each of the individual human GPCR protomers was obtained manually from the STRING and SCOPUS databases. The interaction data were used to build and analyze the network using Cytoscape software. The network was treated as undirected throughout the study. It is comprised of 156 nodes, 260 edges and has a scale-free topology. Connectivity analysis reveals a significant dominance of intrafamily versus interfamily connections. Most of the receptors within the network are linked to every other by a small number of edges. DRD2, OPRM, ADRB2, AA2AR, AA1R, OPRK, OPRD and GHSR are identified as hubs. In a network representation also ten modules/clusters appear as a highly interconnected group of nodes. Information on this GPCR network can improve our understanding of molecular integration. GPCR-HetNet has been implemented in Java and is freely available at http://www.iiia.csic.es/~ismel/GPCR-Nets/index.html
C 046
GPR17: still an orphan GPCR?
Katharina Simon1,*, Stephanie Hennen1, Nicole Merten1, Ralf Schröder1, Lucas Peters1, Ramona Schrage 2, Celine Vermeiren4, Klaus Mohr2, Christa E. Müller3, Michel Gillard4, Jesús Gomeza1 and Evi Kostenis1
1Institute of Pharmaceutical Biology, University of Bonn, Nussallee 6, 53115 Bonn, Germany;2Pharmacology and Toxicology, University of Bonn, Gerhard-Domagk 3, 53121 Bonn, Germany;3Pharmaceutical Chemistry I, University of Bonn, An der Immenburg 4, 53121 Bonn, Germany;4CNS Research, UCB Pharma sprl, 1420 Braine l’Alleud, Belgium
GPR17 is a G protein coupled receptor (GPCR), which is predominantly expressed in the oligodendrocyte-lineage cells of the central nervous system. Recently inhibition of this receptor has been proposed as a novel and promising therapeutic strategy for the treatment of demyelinating diseases such as multiple sclerosis.
In spite of its attractivity as novel target to foster repair of demyelinated neurons, the nature of its true endogenous ligands is still under debate. While uracil nucleotides and cysteinyl-leukotrienes have been proposed as endogenous activators [1], a number of independent studies did not confirm the original deorphaning report [2-6]. We further investigated whether GPR17 represents the elusive orphan receptor indeed responding to two ligand classes or whether it may still require pairing with a yet unknown endogenous modulator. To this end we employed a number of different functional assay platforms based on label-free dynamic mass redistribution and label-free bioimpedance, but also classical assays capturing defined intracellular events such as Gαi, Gαq, and Gαs activation, β- arrestin recruitment, activation of extracellular signal-regulated kinases 1 and 2, and binding of [35S]GTPγS to Gα proteins. Notably, we were unable to demonstrate activation of GPR17 by any of the purported endogenous ligands. Yet, robust activation was obtained across all assays when the receptor was stimulated with the small molecule MDL29,951.
Based on these data and the current literature, which does not support the notion that GPR17 has been successfully matched with its correct endogenous partners, we propose that GPR17 must still be regarded as orphan, awaiting the identification of the true native ligands.
References
1. Ciana P, Fumagalli M, Trincavelli ML, Verderio C, Rosa P, Lecca D, Ferrario S, Parravicini C, Capra V, Gelosa P, Guerrini U, Belcredito S, Cimino M, Sironi L, Tremoli E, Rovati GE, Martini C, Abbracchio MP (2006) EMBO J 25(19):4615–4627
2. Heise CE, O’Dowd BF, Figueroa DJ, Sawyer N, Nguyen T, Im DS, Stocco R, Bellefeuille JN Abramovitz M Cheng R, Williams DL Jr, Zeng Z, Liu Q, Ma L, Clements MK, Coulombe N, Liu Y, Austin CP, George SR, O’Neill GP, Metters KM, Lynch KR, Evans JF (2000) J Biol Chem 275(39):30531-30536
3. Maekawa A, Balestrieri B, Austen KF, Kanaoka Y (2009) Proc Natl Acad Sci USA 106(28):11685–11690
4. Benned-Jensen T, Rosenkilde MM (2010) Br J Pharmacol 159(5):1092–1105
5. Qi AD, Harden TK, Nicholas RA (2013) J Pharmacol Exp Ther 347(1):38–46
6. Hennen S, Wang H, Peters L, Merten N, Simon K, Spinrath A, Blättermann S, Akkari R, Schrage R, Schröder R, Schulz D, Vermeiren C, Zimmermann K, Kehraus S, Drewke C, Pfeifer A, König GM, Mohr K, Gillard M, Müller CE, Lu QR, Gomeza J, Kostenis E (2013) Sci Signal 6(298):ra93
D: Medicinal chemistry and drug development
D 047
Design, synthesis and structure-activity relationship of anthraquinone derivatives as NTPDase3 inhibitors
Enas M. Malik1,*, Younis Baqi1,2, Amelie Fiene1, Joanna Lecka3,4, Jean Sévigny3,4 and Christa E. Müller1
1PharmaCenter Bonn, Pharmaceutical Institute, Pharmaceutical Chemistry I, University of Bonn, An der Immenburg 4, D-53121 Bonn, Germany;2Department of Chemistry, Faculty of Science, Sultan Qaboos University, PO Box 36, Postal Code 123, Muscat, Oman;3Département de microbiologie-infectiologie et d’immunologie, Faculté de Médecine, Université Laval, Québec, QC, Canada;4Centre de Recherche du CHU de Québec, Québec, QC, Canada
NTPDase3 is a member of the ecto-nucleoside triphosphate diphosphohydrolase (E-NTPDase) family, one prominent family of ectonucleotidases. The other E-NTPDase homologs are NTPDase1, −2, and −8. These membrane-bound enzymes catalyze the hydrolysis of nucleoside tri-and diphosphates, the physiological agonists of P2 purinergic receptors, into smaller nucleotides (e.g. ATP and ADP are hydrolyzed to AMP). The latter is then subject to hydrolysis by ecto-5′-nucleotidase (e5NT) to nucleosides (e.g. AMP to adenosine) [1]. While ATP is known for its cytotoxic and proinflammatory effects, adenosine promotes angiogenesis and immunesuppression. Since tumor cells show both, high concentration of ATP and overexpression of NTPDases and e5NT, inhibitors of these enzymes have potential utility in the treatment of cancer and immunodeficiency disorders [2,3]. In this context, a series of anthraquinone derivatives structurally related to the anthraquinone-chlorotriazinyl dye Reactive Blue 2 (see structure), has been synthesized and evaluated for their inhibitory activity at the different human NTPDase subtypes using the malachite green assay. Synthesis of the compounds was possible by using a Cu0-catalyzed microwave-assisted Ullmann coupling reaction of bromaminic acid with different aryl- and alkylamines in phosphate buffer [4]. Variation of the nature of the substituent at position 4 was found to be crucial for conferring both, potency and subtype-selectivity. The focus will be on the NTPDase3 enzyme subtype where acidic functionalities were essential for activity. Analysis of the structure-activity relationship regarding this particular subtype will be discussed.
References
1. Zimmermann H, Zebisch M, Sträter N (2012) Purinergic Signal 8:437–502
2. Wang L, Tang S, Wang Y, Xu S, Yu J, Zhi X, Ou Z, Yang J, Zhou P, Shao Z (2013) Clin Exp Metastasis 30:671–680
3. Stagg J, Smyth MJ (2010) Oncogene 29:5346–5358
4. Baqi Y, Müller CE (2010) Nat Protoc 5:945–953
D 048
Synthesis and structure-activity relationships of AOPCP derivatives: potent, metabolically stable and selectiveecto-5′-nucleotidase inhibitors
Sanjay Bhattarai1,*, Marianne Freundlieb1, Ali El-Tayeb1, Herbert Zimmermann2 and Christa E. Müller1
1PharmaCenter Bonn, Pharmaceutical Institute, Pharmaceutical Chemistry I, University of Bonn, An der Immenburg 4, D-53121 Bonn, Germany;2Institute of Cell Biology and Neuroscience, Goethe-University, Frankfurt am Main, Germany
Ecto-5′-nucleotidase (eN, CD73) belongs to a group of enzymes, the ecto-nucleotidases, which dephosphorylate extracellular nucleotides. It catalyzes the dephosphorylation of nucleoside monophosphates, mainly of AMP. Other membrane-bound ecto-nucleotidases include the nucleoside triphosphate diphosphohydrolases (NTPDases; subtypes 1, 2, 3 and 8), the nucleotide pyrophosphatases/phosphodiesterases (NPP1-4) and the alkaline phosphatases (tissue non-specific, intestinal, placental and germ cell APs) [1]. eN is often co-localized with adenosine receptors, and eN inhibitors reduce extracellular adenosine levels, which results in an indirect blockade of adenosine (P1) receptor activation. Thus, they possess potential as novel drugs, e.g. for cancer therapy or for the treatment of neurodegenerative diseases. AOPCP (AMPCP, Ki = 197 nM, rat eN), an analogue of ADP, is currently one of the most potent competitive inhibitors of eN [2]. In the present study, a series of 6- and 8-substituted derivatives of AOPCP was synthesized and evaluated as rat eN inhibitors using a radiometric assay employing [3H]AMP as a substrate [2]. 6-(Ar)alkyl-substituted AOPCP derivatives were found to display improved potency as compared to the parent compound. Mono-substitution was superior to symmetrical di-substitution. The most potent inhibitors were N6-(4-chlorobenzyl)adenosine-5′-methylenebis(phosphonic acid) (I, Ki = 7.23 nM), and O6-benzyladenosine-5′-methylenebis(phosphonic acid) (II, Ki = 9.20nM). N6-disubstituted and O6-substituted derivatives cannot be hydrolyzed to produce adenosine derivatives that might activate adenosine receptors. Compounds I and II are the most potent inhibitors of eN known to date and might serve as valuable pharmacological tools to further elucidate the enzyme’s (patho)physiological roles.
References
1. Zimmermann H, Zebisch M, Sträter N (2012) Purinergic Signal 8:437–502
2. Freundlieb M, Zimmermann H, Müller CE (2014) Anal Biochem 446:53–58
D 049
Peptidonucleosides as inhibitors of ecto-5′-nucleotidase—a promising target for anti-cancer-drugs
Wenjin Li1,*, Andreas Brunschweiger1, Herbert Zimmermann2 and Christa E. Müller1
1Pharma-Zentrum Bonn, Pharmazeutische Chemie I, Universität Bonn, An der Immenburg 4, 53121 Bonn, Germany;2Institute of Cell Biology and Neuroscience, J.W. Goethe-Universität, Max-von-Laue-Str. 13, 60438 Frankfurt am Main, Germany
Ecto-5′-nucleotidase (eN, CD73) catalyzes the hydrolysis of the phosphoric ester bond of nucleoside monophosphates, mainly AMP, yielding the corresponding nucleoside (mainly adenosine) and inorganic phosphate. The enzyme has been found to be overexpressed on many cancer cells, and eN-generated adenosine prevents tumour destruction by inhibiting antitumor immunity. Therefore treatment with eN inhibitors may be a promising novel strategy for cancer therapy. In the present study we synthesized nucleotide mimetics which consist of a nucleoside scaffold substituted in the 5′-position with a dipeptide moiety. The compounds were investigated at rat eN using a capillary electrophoresis-based assay; identified inhibitors at rat eN were further investigated at human eN. Our test results showed that the inhibitory potency of the compounds appeared to be pH-dependent: when the buffer pH was decreased, the potency of the compounds increased. Since tumor tissues typically show low extracellular pH values, the new inhibitors might act as tumor-selective eN inhibitors without affecting physiologically important functions of eN, such as the production of adenosine in blood vessels, which mediates vasodilatory effects.
D 050
Biological evaluation of novel potent competitive CD73 inhibitors
Anne Meyer1,2,*, Sanjay Bhattarai2, Ali El-Tayeb2, Sirpa Jalkanen1, Christa E. Müller2 and Gennady G. Yegutkin1
1Medicity Research Laboratory, University of Turku, Turku, Finland;2PharmaCenter Bonn, Pharmaceutical Institute, University of Bonn, Germany
Ecto-5′-nucleotidase (eNT)/CD73-mediated hydrolysis of extracellular AMP to adenosine and subsequent activation of adenosine receptors is known to be involved in several pathophysiological processes such as immunity, inflammation, neurotransmission and tumorigenesis [1,2]. Recent development of extremely potent, metabolically stable inhibitors of eNT gives us the possibility to further investigate the role of this enzyme in vitro and in vivo. Inhibitory potencies of several small molecule inhibitors were measured using thin-layer chromatographic assay with [3H]AMP substrate, and further compared with the widely employed CD73 inhibitor α,β-methylene-ADP (AMPCP). All compounds progressively inhibited the activity of purified human eNT with the following rank order of potency (IC50, nM): PSB12489 (0.65) > PSB12497 (1.6) > PSB12555 (3.9) ≥ PSB12437 (4.6) ≥ PSB12379 (4.8) > PSB12553 (27) >> AMPCP (175). Interestingly, the inhibitory potencies of all tested compounds were clearly lower in studies with soluble serum eNT: PSB12489 (3.4) > PSB12437 (12) ≥ PSB12555 (16) ≥ PSB12497 (18) > PSB12379 (30) > PSB12553 (74) >> AMPCP (480). The ability of the most potent inhibitor, PSB12489, to block AMP hydrolysis was further ascertained in enzymatic assays with human MDA-MB-231 and murine 4T1 breast cancer cells, and in lead nitrate-based histochemical analysis of ecto-5′-nucleotidase/AMPase distribution in human tonsil and mouse spleen sections. Collectively, this study provides comprehensive analysis of novel highly potent, competitive CD73 inhibitors, further underlining the importance of employment of different biological systems for characterization of inhibitors, and in addition, provides sufficient background for future studies on the role of CD73 in control of tumor growth and metastasis.
References
1. Zimmermann H Zebisch M, Sträter N (2012) Purinergic Signal 8:437–502
2. Yegutkin GG (2008) Biochim Biophys Acta 1783:673–694
D 051
Rational design of inhibitors for ecto-5′-nucleotidase (CD73)
Jan Pippel1,*, Karen Knapp1, Matthias Zebisch1,2, Ali El-Tayeb3, Christa E. Müller3 and Norbert Sträter1
1University of Leipzig, Institute of Bioanalytical Chemistry, Leipzig, Germany;2University of Oxford, Division of Structural Biology, Oxford, Germany;3University of Bonn, Pharma Center Bonn, Pharmaceutical Institute, Pharmaceutical Chemistry I, Bonn, Germany
The eukaryotic ecto-5′-nucleotidase (CD73 or e5NT) is an extracellular enzyme attached to the cell membrane. It constitutes part of the purinergic signalling pathway in which purine nucleotides and its derivatives act as extracellular signalling molecules. The important regulatory role of e5NT evolves from its ability to switch on P1 receptor signalling by generation of adenosine via hydrolysis of AMP. With such a crucial function e5NT has become an appealing drug target for the treatment of inflammation or various types of cancer.
Structures of e5NT revealed an open and closed conformation for the dimeric enzyme. The subunits of each monomer are composed of a C- and N-terminal domain with the conformational change being achieved by a large rotation (~100°) of the N-terminal domain. Although several inhibitors for e5NT have already been published, complex structures for elucidation of detailed molecular interactions with corresponding compounds are rare. By using our expression and crystallization strategies, we were able to determine co-crystal structures for several commercial as well as newly identified compounds. Those results were then in cooperation with Prof Christa Müller in Bonn used for rational modifications. Until now, a derivative of the substrate-mimicking compound AMPCP showed the most promising results with pi-stacking interaction being the base for improvement of Ki value up to the low nM range. Further modifications could include extension into a large pocket which is, in the apo form, occupied by water molecules.
D 052
Photoswitch kinase inhibitors
Ruben Ferreira1,*, Jesper Nilsson1, Carlos Solano2, Joakim Andreasson1 and Morten Grøtli2
1Department of Chemical and Biological Engineering Physical Chemistry, Chalmers University of Technology, Göteborg, Sweden;2Department of Chemistry and Molecular Biology, University of Gothenburg, Göteborg, Sweden
After the discovery of kinase-mediated signaling pathways, designing small-molecule inhibitors of kinases has emerged as an potentially important way to target many dysregulated pathways, particularly in cancer progression and metastasis.
However, target selectivity remains a formidable challenge in drug development because almost all approved kinase inhibitor drugs work by competing with ATP for the ATP binding site of the enzyme.
Light is a convenient and powerful trigger to control the activity of biomolecules. Cells environments benefit not only from the noninvasive and noninterfering nature of light, but also from its spatial and temporal resolution. The artificial introduction of photosensitivity in biomolecules relies in most of the cases on the covalent functionalization with small photoactive molecules as spiropyranes or azobenzenes.
Here we describe the synthesis of photochromic RET inhibitors obtained from 3-substituted pyrazolopyrimidine-based compounds by incorporating an aza-bridge. The compounds demonstrated excellent switching properties and stability. The best compound gave good inhibitory effect both in cell-free as well as in whole cell assays with a significant difference in inhibitory activity between its two photoisomeric forms. Further developments could ultimately lead to a photoswitchable compound suitable for studying RET signalling in a novel manner, with activity addressable by light and with precise spatiotemporal control over events at the molecular level.
D 053
Design, synthesis, and pharmacological characterization of 2,N6-disubstituted adenosine analogues as P1 receptor ligands
Ajiroghene Thomas, Michela Buccioni, Catia Lambertucci, Diego Dal Ben, Gabriella Marucci, Claudia Santinelli, Andrea Spinaci and Rosaria Volpini
School of Pharmacy, Medicinal Chemistry Unit, University of Camerino, Via S. Agostino 1, 62032 Camerino, Italy
The available crystallographic structures of the A2A adenosine receptor (A2AAR) present structural features typical of G protein-coupled receptors besides very important information about the interaction of the receptor with the co-crystallized ligands [1]. In particular, the residues Asn253 and Glu169 form polar interactions with the ligand, while Phe168 forms π-stacking bonding with the aromatic scaffold of the bound molecule (Fig. 1). Furthermore, water molecules are distributed in a network of reciprocal interaction and they play a role as a “bridge” in the ligand-receptor contact [2]. Comparative sequence analysis between the A2A and A3 AR subtypes shows that, among the residues mentioned above, only Phe168 and Asn253 are conserved in these receptors. In contrast, the Glu169 of A2AAR is replaced by Val169 in the A3AR [3].
Fig. 1 Receptor-ligand interactions for A2A receptor bound to the agonists NECA and adenosine [2]
Based on these observations, known A2A ligands modified through the introduction of an N6-amino or N6-alkylamino group were designed and synthesized. These analogues were tested in binding and functional studies at human A1, A2A, A2B, and A3 ARs cloned and transfected in CHO cells. Preliminary results show that the synthesized derivatives present pronounced affinity and potency at the A3AR subtype and reduced interaction with the A2AAR, contrary to what had been expected. Surprisingly, these compounds behave as A2AAR agonists but show an antagonist profile at the A3AR.
References
1. Jacobson KA (2013) In Silico Pharm 1:22
2. Lebon G, Warne T, Edwards PC, Bennett K, Langmead CJ, Leslie AGW, Tate CG (2011) Nature 474:521–525
3. Dal Ben D, Lambertucci C, Marucci G, Volpini R, Cristalli G (2010) Curr Top Med Chem 10:993–1018
D 054
Selectivity is species-dependent: characterization of standard antagonists at human, rat and mouse adenosine receptors
Mohamad Wessam Alnouri*, Stephan Jepards, Alessandro Casari and Christa E. Müller
PharmaCenter Bonn, Pharmaceutical Institute, Pharmaceutical Chemistry I, University of Bonn, An der Immenburg 4, 53121 Bonn, Germany
Adenosine receptors (ARs) are considered as novel drug targets with great potential for the treatment of a variety of diseases including cardiovascular and brain disorders, inflammation and pain, and cancer [1]. For hit identification, lead generation and optimization, as well as further initial steps of drug development, assays employing recombinant human receptors are typically used. However, preclinical in vivo evaluation of novel drugs is performed in animal models. Since rat and mouse are the most widely employed animals in preclinical studies, it is extremely important to know and to consider potential differences in potency and selectivity of the employed compounds.
In the present study, a set of 16 widely used standard antagonists was selected and their potency and selectivity were investigated in radioligand binding assays at the four AR subtypes of the three species, human, rat and mouse. Our goal was to provide a recommendation regarding the best commercially available antagonist for each receptor subtype. The A1 antagonist PSB-36 (1), with a potency in the nanomolar range and a selectivity of at least 400-fold versus other AR subtypes, represents a suitable tool for studying the A1AR in all three species. The non-xanthine A2A antagonist SCH240814 (2) displayed the highest potency and selectivity for that subtype with nanomolar affinity and at least ca. 1,000-fold selectivity versus all other AR subtypes in the three investigated species.
The high affinity and selectivity and of PSB-603 (3) for human and rat A2BARs could be clearly confirmed. Interestingly, PSB-603 showed an affinity in the higher nanomolar range for the mouse A1AR. Despite this fact, PSB-603 can still be considered as one of the most suitable antagonists for studying A2BARs due to its outstanding affinity and high selectivity (at least 170-fold) in all three species. The A3AR antagonist MRS-1523 (4) proved to be the best currently available compound to address the issue of the low similarity between human and rodent A3AR. However, its affinity for rat and especially for mouse A3ARs is significantly lower than that for the human A3AR. The compound is selective vs. A2AAR only in humans but not in rat or mouse.
Reference
1. Müller CE, Jacobson KA (2011) Biochim Biophys Acta 1808:1290–1308
D 055
Targeting A2Areceptor to treat neurodegenerative diseases: design, synthesis and evaluation of potential antagonists
Valeria Moas-Heloire1,2, Nicolas Renault1,2, Vania L. Batalha3, Philippe Chavatte1,2, Luc Buée1,4, David Blum1,4, Luisa V. Lopes3, Laurence Agouridas1,2 and Patricia Melnyk1,2,*
1Univ Lille Nord de France, F-59000 Lille, France;2UDSL, EA 4481, UFR Pharmacie, F-59000 Lille, France;3Institut de Médecine Moléculaire, Unit #P1B-49, 1640-028 Lisboa, Portugal;4Alzheimer and Tauopathies, Inserm UMR-U837, Lille, France
Adenosine is a ubiquitous endogenous purine nucleoside able to regulate many physiological processes as an intercellular messenger and plays an important neuroprotective role in the central nervous system. In the brain, adenosine and its receptors are presents in high levels, and it has been shown to be involved in both normal and pathological processes including arousal, motor control, neuroprotection, mood, learning and memory. Its effects are transmitted by four distinct receptor subtypes designated A1, A2a, A2b, and A3 belonging to the G protein-coupled receptor superfamily. Adenosine A1 and A3 receptors are coupled to inhibitory G proteins, while A2a and A2b receptors are coupled to stimulatory G proteins. A2a receptors (A2aR) show a restricted distribution, being characteristic of the dopamine enriched areas, the highest concentration being in the caudate-putamen. This anatomical selection suggests a unique role in neuronal signaling with this region and potential involvement in neurologic disease of extrapyramidal origin.
In fact, A2a antagonism appeared to be a promising pharmacological target in Parkinson’s disease (PD). Furthermore, an increasing number of studies suggest that pharmacological or genetic blockade of A2aR might be of great interest in Alzheimer’s disease as it reduces β-amyloid deposits, τ-phosphorylation and neurodegeneration. Currently, only three compounds are still being tested in clinical phase for PD treatment. Even if they show good affinities for the receptor, there is still a need for improving their ADME properties by keeping their selectivity towards other adenosine receptors.
Based on the recently published crystalline structure of the A2A receptor complexed with the selective and high-affinity antagonist triazine [1] and on a pharmacophoric model [2], we have designed new ligands using in silico docking studies starting from antagonists that we recently identified in our group. Then, using originals chemicals pathways, three new families of compounds have been synthesized and tested for their affinity towards A2a receptor.
References
1. Congreve M et al (2008) J Med Chem 55:1898–1903
2. Xu Z et al (2010) J Mol Model 16:1867–1876
D 056
In silico design of adenosine A2Aantagonists
Nicolas Renault1,2, Valeria Moas-Heloire1,2, Delphine LeBroc3, Christophe Furman3, Luc Buée1,4, David Blum1,4, Philippe Chavatte1,2, Laurence Agouridas1,2 and Patricia Melnyk1,2,*
1Univ Lille Nord de France, F-59000 Lille, France;2UDSL, EA 4481, UFR Pharmacie, F-59000 Lille, France;3UDSL, EA 4483, UFR Pharmacie, F-59000 Lille, France;4Alzheimer and Tauopathies, Inserm UMR-U837, Lille, France
Human A2A adenosine receptor belongs to the superfamily of G protein coupled receptors and its blockade is highly relevant for therapeutics of Parkinson and Alzheimer diseases. Co-crystallization of A2A receptor with selective antagonists was further analyzed from in silico studies to highlight polar and aromatic inter-molecular hotspots for design of novel more efficient and soluble antagonists. A virtual screening protocol has been trained by docking from a library training set including 35 known A2A antagonists as positive controls and 19,000 decoy ligands as negative controls. This protocol has been applied to screen academic chemical libraries and de novo combinatorial libraries.
D 057
Development of prediction models for the purinergic receptor compounds in different local SAR environments using emerging chemical patterns
Vigneshwaran Namasivayam1,*, Disha Gupta-Ostermann2, Jenny Balfer2 and Jürgen Bajorath2
1PharmaCenter Bonn, Pharmaceutical Institute, Pharmaceutical Chemistry II, Rheinische Friedrich-Wilhelms-Universität Bonn, An der Immenburg 4, 53121 Bonn, Germany;2Department of Life Science Informatics, B-IT, Rheinische Friedrich-Wilhelms-Universität Bonn, Dahlmannstr. 2, 53113 Bonn, Germany.
Purinergic receptors [1] are cell-surface receptors activated by signaling molecules, purines and pyrimidines. These receptors have diverse functions including neurotransmission, inflammation and modulation of cardiac effects. The purinergic receptors are mainly classified into P1 and P2 receptors that recognize extracellular nucleoside and nucleotides, respectively. The receptor subtypes of P1 receptor or adenosine receptors (A1, A2A, A2B, A3), P2X receptors (P2X1–7), and P2Y receptors (P2Y1,2,4,6,11,12,13,14) have been identified as potential therapeutic targets. The understanding of purinergic signaling has grown steadily and novel compounds are emerging for the subtypes of P1 and P2 receptors. Although there has been progress in the development of inhibitors for these subtypes, the number of potential compounds is still limited.
The classification of active compounds according to SAR discontinuity assists in identifying local SAR environments. Recently, we have reported an Emerging Chemical Pattern (ECP) approach to predict compounds active against these receptors in different local SAR environments. The approach has yielded accurate predictions on the basis of much smaller training sets of 3, 5 and 10 active compounds than other machine learning approaches such as support vector machine (SVM) and random forest (RF) [2]. Hence, ECP can be applied for classification with limited numbers of active compounds in different subtypes of purinergic receptors. For our analysis, purinergic receptor ligands available in ChEMBL were assembled. The compounds in these data sets were evenly distributed over the three different SAR discontinuity categories representing low, intermediate, and high SAR discontinuity. The predictions of compounds in highly discontinuous local SAR environments might ultimately yield highly active compounds to further progress optimization efforts.
References
1. Ralevic V, Burnstock G (1998) Pharmacol Rev 50:413–492
2. Namasivayam V, Gupta-Ostermann D, Balfer J, Heikamp K, Bajorath J (2014) J Chem Inf Model 54:1301–1310
D 058
Different efficacy of adenosine and NECA derivatives at the human A3adenosine receptor: molecular modeling analysis and insight into the receptor activation switch
Diego Dal Ben1,*, Michela Buccioni1, Catia Lambertucci1, Gabriella Marucci1, Andrea Spinaci1, Ajiroghene Thomas1, Karl-Norbert Klotz2 and Rosaria Volpini1
1School of Pharmacy, Medicinal Chemistry Unit, University of Camerino, Via S. Agostino 1, 62032 Camerino, Italy;2Institut für Pharmakologie und Toxikologie, Universität of Würzburg, Versbacher Str. 9 D-97078 Würzburg, Germany
A3 Adenosine Receptor (A3AR) regulates a variety of key physiological processes such as release of inflammatory mediators and inhibition of tumour necrosis factor-α production. The design and synthesis of potent and selective A3AR agonists could provide tools for further characterization of the role of this receptor and for the development of new drugs having anti-inflammatory, anticancer, and cardioprotective effects [1,2].
A series of adenosine derivatives with 2-(ar)-alkynyl chains, with high affinity and different degrees of selectivity for human A3AR was tested for the ability to inhibit forskolin-stimulated adenylyl cyclase. All these derivatives are partial agonists at A3AR; further modification of these analogues with introduction small alkyl groups in N6-position does not change their partial agonist profile. In contrast, the adenosine-5′-N-ethyluronamide (NECA) analogues of 2-(ar)-alkynyladenosine derivatives are full A3AR agonists [3].
Molecular modeling studies were performed analyzing both the conformational behavior of the ligands and the impact of 2- and 5′-substituents on ligand-receptor interaction. The results suggest an explanation for the different agonistic behavior of adenosine and NECA derivatives, with a sub-pocket of the binding site identified as a crucial interaction domain for receptor activation [4].
Fig. 1 Different interaction of adenosine and NECA derivatives with the human A3 adenosine receptor binding site
References
1. Cristalli G, Volpini R (2003) Curr Top Med Chem 3:355–469
2. Jacobson KA, Gao ZG (2006) Nat Rev Drug Dis 5:247–264
3. Volpini R, Costanzi S, Lambertucci C, Taffi S, Vittori S, Klotz K-N, Cristalli (2002) J Med Chem 45:3271–3279
4. Dal Ben D, Buccioni M, Lambertucci C, Kachler S, Falgner N, Marucci G, Thomas A, Cristalli G, Volpini R, Klotz K-N (2014) Biochem Pharmacol 87:321–331
D 059
Ligand discovery from adenosine receptor crystal structures and homology models
Jens Carlsson1,*, David Rodriguez1, Anirudh Ranganathan1, Steven M. Moss2, Zhan-Guo Gao2, Leigh A. Stoddart3, Stephen J. Hill3 and Kenneth A. Jacobson2
1Stockholm University, Biochemistry and Biophysics, Stockholm, Sweden;2National Institute of Diabetes and Digestive and Kidney Diseases, NIH, Molecular Recognition Section, Laboratory of Bioorganic Chemistry, Bethesda, USA;3University of Nottingham, Institute of Cell Signalling, School of Biomedical Science, Nottingham, UK
G protein-coupled receptors (GPCRs) are intensely studied as drug targets and for their role in signaling. With the determination of the first crystal structures for GPCRs, interest in structure-based ligand discovery has increased. Several high-resolution structures of the A2A adenosine receptor (A2AAR), which is a target for development of drugs against Parkinson’s disease, have now been determined. Examples of structure-based ligand discovery based on crystal structures of the A2AAR receptor and homology models of other adenosine receptor subtypes will be presented.
We have identified several novel ligands of the A2A adenosine receptor based on screens of large chemical libraries against antagonist-bound crystal structures. All discovered ligands have been A2AAR antagonists, in agreement with the efficacy of the co-crystallized ligand and the inactive conformational state of the receptor structure. A2AAR agonists have several potential therapeutic applications, e.g. as drugs for inflammation, but unfortunately there are very few available ligand scaffolds that have been shown to activate adenosine receptors. Recently, several agonist-bound structures of the A2AAR have been determined and efforts to identify novel compounds with the ability to activate adenosine receptors will be presented.
Unfortunately, structures for three adenosine receptors subtypes are still unknown. In these cases, structure-based screens are forced to rely on homology models. Access to high-resolution crystal structures of the A2AAR enables generation of models for the three closely related A1, A3, and A2B receptor subtypes, which could enable structure-based discovery of new lead compounds with specific selectivity profiles. However, it is not entirely clear if homology models can be used in ligand discovery. We have explored the use of biophysical and in silico structure-based screening to identify fragment ligands of several adenosine receptor subtypes. These screens have enabled us to carry out comparisons of ligand discovery using modeled structures versus crystal structures. Our results from these docking screens highlight opportunities and limitations of the use of crystal structures and homology models in drug discovery for this pharmaceutically interesting group of receptors.
D 060
[1,2,4]triazolo[1,5-c]pyrimidines as A3adenosine receptor antagonists
Stephanie Federico1,*, Sara Redenti1, Antonella Ciancetta2, Barbara Cacciari3, Karl-Norbert Klotz4, Stefano Moro2 and Giampiero Spalluto1
1Università degli Studi di Trieste, Dipartimento di Scienze Chimiche e Farmaceutiche, Trieste, Italy;2Università degli Studi di Padova, Molecular Modeling Section (MMS), Dipartimento di Scienze del Farmaco, Padova, Italy;3Università degli Studi di Ferrara, Dipartimento di Scienze Farmaceutiche, Ferrara, Italy;4Universität of Würzburg, Institut für Pharmakologie, Würzburg, Germany
Question: [1,2,4]Triazolo[1,5-c]pyrimidine (TP) derivatives are reported in literature as A2A adenosine receptor (AR) antagonists useful for the treatment of Parkinson’s disease, senile dementia and depression (1) [1]. The Schering compound, preladenant (2), a pyrazolo[4,3-e]1,2,4-triazolo[1,5-c]pyrimidine (PTP) A2A antagonist, possesses a lateral substituent similar to those of the TP compound 1 [2,3]. Although these two compounds are both potent A2A antagonist, the TP nucleus possesses a simpler structure and less nitrogen atoms than PTPs, thus it may be a scaffold with promising pharmacokinetics properties. So, starting from the structural similarity between TP and PTP A2A AR antagonists, and our experience on PTP nucleus as A3 AR antagonists, we decided to explore the TP scaffold in order to obtain potent antagonists towards A3 AR.
Methods: All compounds were synthesized according to literature [1] and have been evaluated for potency at all four human ARs.
Results: Initially, we introduced at the 5 position of the TP scaffold the same substituents which gave affinity and selectivity at the A3 AR at the 5 position in the PTP nucleus. On the basis of obtained results, the optimization of substitutions at the 5, 8 and 2 positions, which led to 147 [1,2,4]triazolo[1,5-c]pyrimidine derivatives, allowed us to reach subnanomolar Ki values at the A3 AR and good levels of selectivity versus the other adenosine receptor subtypes.
Conclusions: A novel promising class of potent and selective A3 adenosine receptor antagonist with a [1,2,4]triazolo[1,5-c]pyrimidine nucleus was discovered.
References
1. Matasi JJ, Caldwell JP, Zhang H, Fawzi A, Higgins GA, Cohen-Williams ME, Varty GB, Tulshian DB (2005) 2-(2-Furanyl)-7-phenyl[1,2,4]triazolo[1,5-c]pyrimidin-5-amine analogs as adenosine A2A antagonists: The successful reduction of hERG activity. Bioorg Med Chem Lett 15:3675–3678
2. Muller CE, Jacobson KA (2011) Recent developments in adenosine receptor ligands and their potential as novel drugs. Biochim Biophys Acta 1808:1290–1308
3. Neustadt BR, Hao J, Lindo N, Greenlee WJ, Stamford AW, Tulshian D, Ongini E, Hunter J, Monopoli A, Bertorelli R, Foster C, Arik L, Lachowicz J, Nga K, Feng KI (2007) Potent, selective, and orally active adenosine A2A receptor 6984 antagonists: arylpiperazine derivatives of pyrazolo[4,3-e][1,2,4]triazolo[1,5-c]pyrimidines. Bioorg Med Chem Lett 17:1376–1380
D 061
Binding kinetics of ZM241385 derivatives at the human adenosine A2Areceptor
Dong Guo*, Lizi Xia, Jacobus van Veldhoven, Marc Hazeu, Tamara Mocking, Johannes Brussee, Adriaan IJzerman and Laura Heitman
Leiden University, Leiden, Netherlands
Classical drug design and development rely mostly on affinity- or potency-driven structure-activity relationships (SAR). So far a compound’s binding kinetics has been largely ignored, which importance, however, is now increasingly recognized.
In the present study we performed an extensive structure-kinetics relationship (SKR) study in addition to a traditional SAR analysis at the adenosine A2A receptor (A2AR).
The ensemble of 24 A2AR compounds, all triazolotriazine derivatives resembling the prototypic antagonist ZM241385, displayed only minor differences in affinity, while they varied substantially in their dissociation rates from the receptor.
We believe that such a combination of SKR and SAR analysis as on the A2AR will have general importance for the superfamily of G protein-coupled receptors, since it can serve as a new strategy to tailor the interaction between ligand and receptor.
D 062
An optimized synthesis of paraxanthine
Sarah Katzemich*, Diana Horn and Christa E. Müller*
PharmaCenter Bonn, Pharmaceutical Institute, Pharmaceutical Chemistry I, University of Bonn, An der Immenburg 4, D-53121 Bonn, Germany
Paraxanthine, the main metabolite of caffeine in humans, displays a number of pharmacological activities. Like caffeine, it acts as a central nervous stimulant [1] and increases lipolysis [2]. Furthermore, paraxanthine has been found to protect from dopaminergic cell death [3]. Paraxanthine acts as an adenosine receptor antagonist, but displays higher potency for the A2B receptor subtype than caffeine [4]. Compared to caffeine, paraxanthine exhibits lower toxicity and less anxiogenic effects. The compound induces higher locomotor activation than caffeine in rats, which may be due to the blockade of cGMP-preferring PDEs [5]. This profile makes paraxanthine an interesting candidate for the treatment of neurological disorders like Parkinson’s disease or narcolepsy. Clinical studies are warranted to further investigate the therapeutic potential of paraxanthine. Previously reported syntheses of paraxanthine are not well suitable for obtaining large amounts required for extended preclinical and clinical studies due to low yields and the requirement for time-consuming purification procedures. We have now developed a straightforward procedure for the synthesis of paraxanthine which allows the preparation of large quantities of the compound. The 2,4-dimethoxybenzyl group was used for protecting the unsubstituted nitrogen atom N3. It was cleaved off by treatment with trifluoroacetic acid in the last step. The individual reaction steps were carried out in satisfactory to high yields and required little effort for purification.
Fig. 1 Synthesis overview
References
1. Okuro M, Fujiki N, Kotorii N, Ishimaru Y, Sokoloff P, Nishino S (2010) Sleep 33:930–942
2. Hetzler RK, Knowlton RG, Somani SM, Brown DD, Perkins RM (1990) J Physiol 68:44–47
3. Xu K, Xu YH, Chen JF, Schwarzschild MA (2010) Neuroscience 167:475–481
4. Fredholm BB, ed (2011) Methylxanthines, Springer-Verlag: Berlin Heidelberg
5. Orru M, Guitart X, Karcz-Kubicha M, Solinas M, Justinova Z, Barodia SK, Zanoveli J, Cortes A, Lluis C, Casado V, Moeller FG, Ferre S (2013) Neuropharmacol 67:476–484
D 063
Drug-likeness properties of the most active A1/A2Aadenosine receptor ligands among N-substituted tricyclic xanthine derivatives
Anna Drabczyńska1, Jadwiga Handzlik1,*, Meryem Köse2, Tadeusz Karcz1, Christa E. Müller2, Gniewomir Latacz1 and Katarzyna Kieć-Kononowicz1
1Jagiellonian University-Medical College, Department of Technology and Biotechnology of Drugs, Kraków, Qatar;2University of Bonn, PharmaCenter Bonn, Pharmaceutical Institute, Bonn, Germany
Adenosine receptors (ARs) are divided into four cloned and pharmacologically characterized subtypes: A1, A2A, A2B and A3. Adenosine A1 receptors are widespread expressed in the central nervous system (CNS), especially in the brain with high levels being expressed in many regions, whereas the A2A ones are wide-ranging but restricted, including lymphocytes, platelets, brain striatum, vascular smooth muscle and endothelium. The A1 adenosine receptor selective antagonists are being developed as cognition enhancers, including dementias, such as Alzheimer’s disease. The A2A adenosine receptor antagonists are able to prevent neurodegenerative diseases such as Parkinson’s and Alzheimer’s disease, ischemic brain damage and recently epilepsy and sensorimotor. Within our previous studies, a library of selective xanthine derived ligands with various affinities and selectivity for A2A/ A1was obtained. Although derivatives of xanthine are the richest and most promising chemical group of adenosine receptors ligands, especially tricyclic xanthine analogues, their potential therapeutic usage is frequently limited by physicochemical properties that do not satisfy requirements of ADME-Tox processes. Thus, the present study is concentrated on the “drug-likeness” properties of selected most active agents, coming from our xanthine library [1]. A series of tricyclic xanthine derivatives (fig. 1) with significant affinity at A1 or A2A (Ki < 1.5 μM) were investigated in silico on their “drugability”, including physicochemical properties (clogP, logS, TPSA), toxic effects (service OSIRIS) and blood–brain barrier permission (models of Zhao, Norinder-Haeberlein or Lipinski) [2]. To study the potential cytotoxicity, the antiproliferative effect of selected compounds against HEK-293 cell line was tested in vitro. Additionally, to predict the potential drug-drug interactions, their influence on CYP3A4 cytochrome activity was examined. The most active R2-alkyl compounds (Ki < 300 nM) showed also profitable drug-likeness properties with significant drug-score values (>0.75). Partly supported by DEC-2012/04/M/NZ4/00219.
Fig. 1 Structures of tricyclic xanthine derivatives
References
1. Drabczyńska A et al (2013) Purinergic Signal 9:395–414
2. Broccatelli F et al (2012) Adv Drug Deliv Rev 64:95–109
D 064
Upside-down purine scaffold for the design and synthesis of new antagonists for A2Aadenosine receptors
Catia Lambertucci1,*, Michela Buccioni1, Diego Dal Ben1, Karl-Norbert Klotz2, Gabriella Marucci1, Claudia Santinelli1, Andrea Spinaci1, Ajiroghene Thomas1 and Rosaria Volpini1
1School of Pharmacy, Medicinal Chemistry Unit, University of Camerino, Via S. Agostino 1, I-62032 Camerino, MC, Italy;2Institut für Pharmakologie und Toxikologie, Universität of Würzburg, Versbacher Str. 9, 97078 Würzburg, Germany
The relevance of A2A adenosine receptors (A2AARs) for the regulation of the dopaminergic system in the striatum and the targeting of A2AAR subtype for the design of new therapy for the treatment of Parkinson’s disease are well known [1].
Hence, a number of A2AAR antagonists are under study for this disorder and, among them, there are adenine derivatives showing different A2AAR antagonist potency and selectivity degree depending on substitutions of the purine core. In particular, the adenine derivative ST-1535 is currently in phase I clinical trials.
The publication of several crystal structures of the human A2AAR in complex with agonist or antagonist ligands provided useful information for the analysis of ligand-target interaction and for the design of new ligands. A molecular modeling strategy consisting in a combination of molecular docking and molecular superimposition steps led to the development of a pharmacophore model that was employed to analyze possible different binding modes of the purine core within the receptor cavity. Among these poses, an orientation of the purine core resulting upside-down with respect to the binding mode observed i.e. for the same moiety in A2AAR-adenosine complex was selected for further exploration. The introduction of an amine function in 2-position and different in N6- and 8-substituents led to the development of the so-called upside-down purine derivatives.
The synthesized compounds were tested at the human A2AR stably transfected in CHO cells using a fluorimetric functional assay. Biological data showed that they behave as A2AAR antagonists with sub-micromolar potency and could be a starting point for the design of new potent A2AAR antagonists.
References
1. Morelli M, Carta AR, Jenner P (2009) Handb Exp Pharmacol 193:589–615
2. Volpini R, Dal Ben D, Lambertucci C, Marucci G, Mishra RM, Ramadori AT, Klotz K-N, Trincavelli ML, Martini C, Cristalli G (2009) Chem Med Chem 4:1010–1019
D 065
4′-tetrazolyl-alkyl-N6-substituted adenosine derivatives as highly potent dual acting A1adenosine receptor agonists and A3adenosine receptor antagonists
Riccardo Petrelli1, Ilaria Torquati1, Livio Luongo2, Francesca Guida2, Serena Boccella2, Antonio Lavecchia3, Karl-Norbert Klotz4, Sonja Kachler4, Palmarisa Franchetti1, Mario Grifantini1, Sabatino Maione2 and Loredana Cappellacci1,*
1University of Camerino, School of Pharmacy, Medicinal Chemistry Unit, Camerino, Italy;2II University of Naples, Section of Pharmacology “L. Donatelli”, Naples, Italy;3University of Naples “Federico II”, Department of Pharmacy, “Drug Discovery” Laboratory, Naples, Italy;4University of Wurzburg, Institut fur Pharmakologie and Toxicologie, Wurzburg, Germany
Adenosine is an endogenous purine nucleoside that modulates a variety of physiological functions as a result of its activation of specific G protein-coupled receptors defined as A1, A2A, A2B, and A3 adenosine receptors (ARs). Selective A1AR agonists mediate neuro- and cardioprotective effects, and reduce lipolysis in adipose tissue and elevated intraocular pressure (IOP), the most widely recognized risk factor for the onset and progression of glaucoma. Selective A3AR agonists have been demonstrated to be both cardio- and cerebroprotective. Moreover, it has been reported that A3 agonists increase, whereas A3 antagonists decrease IOP in mice. A selective A3 antagonist, OT-7999 is being studied for the treatment of glaucoma, whereas an A1 agonist, INO-8875/PJ-875, is in Phase I/II for the treatment of patients with ocular hypertension or primary open angle glaucoma (POAG).
Recent findings indicated that some compounds might activate two different subtypes of a certain receptor family, both leading to beneficial effects. The dual-acting ligands of ARs may have considerable promise as novel approaches to treat pathological conditions e.g. ischemic conditions, asthma, inflammatory diseases and glaucoma.
In our previous work we reported that N6-substitution in 5′-chloro-5′-deoxy-adenosine derivatives with cycloalkyl- or bicycloalkyl groups led to very potent and selective A1 agonists [1]. 5′Cl5′d-(±)-ENBA was the most potent and selective A1 agonist of the series and was endowed with analgesic activity in SNI mice [2,3], a model of neuropathic pain. Functional assays showed that 5′Cl5′d-(±)-ENBA was a full A1 agonist and a weak A3 antagonist, confirming that the lack of a group hydrogen bonding donor in the A3AR binding site alters the ability to induce the conformational change essential for receptor activation.
As the proof of the concept and in order to find dual acting A1AR agonists and A3AR antagonists, we designed and synthesized a new series of N6-substituted adenosine and 2-chloro-adenosine derivatives in which the 4′-hydroxymethyl group was replaced by a 2-ethyl-2H-tetrazol-5-yl moiety. The novel compounds displayed a subnanomolar affinity at both A1 and A3ARs and acted as A1 full agonists and A3 antagonists. These compounds represent the first example of highly potent dual acting A1AR agonists and A3AR antagonists useful for the treatment of glaucoma and other diseases.
References
1. Franchetti P, Cappellacci L et al (2009) J Med Chem 52:2393–2406
2. Luongo L, Cappellacci L et al (2012) Molecules 17:13712–13726
3. Luongo L, Cappellacci L et al (2014) Glia 62:122–132
D 066
Synthesis and structure activity relationships of 2- and 6-sulfohydrazinyl-substituted purine-β-D-ribofuranoside derivatives as adenosine receptor agonists
Ali El-Tayeb, Michael Budzisz, Anis Talbalaghi, Markus Kuschak and Christa E. Müller
PharmaCenter Bonn, Pharmaceutical Institute, Pharmaceutical Chemistry I, University of Bonn, An der Immenburg 4, D-53121 Bonn, Germany
Four adenosine receptor (AR) subtypes are known to exist, designated A1, A2A, A2B and A3. The A1 and A3 subtypes are Gi-coupled receptors, activation of which leads to inhibition of adenylate cyclase, while activation of A2A and A2B receptors results in an activation of adenylate cyclase through Gs protein [1]. In order to study the (patho)physiological roles of the individual AR subtypes, agonists with high selectivity for each subtype are required. Moreover, due to the wide distribution of ARs, selective AR agonists are needed for the treatment of a variety of pathological conditions [1-3]. However, truly selective adenosine-derived A2B AR agonists have not been developed so far. The non-nucleosidic A2B AR agonist BAY-60-6583 shows high selectivity, at least in humans, however we recently found that BAY-60–6583 exhibits only partial agonistic activity and acts as an antagonist in the presence of high concentrations of adenosine. In the present study we selected (1-deoxy-1-{6-[N′-(furan-2-carbonyl)hydrazino]-9H-purin-9-yl}-N-ethyl-β-D-ribofuranuronamide (I) as a lead structure, which had been described as a relatively potent A2B AR agonist [4]. We synthesized series of novel adenosine derivatives (structures II and III) substituted in the 2- or 6-position, or in both positions, to study their structure-activity relationships (SAR), and to optimize them with regard to high affinity and AR subtype-selectivity.
The synthesized adenosine derivatives did not bind to the A2B AR. However, many of the new nucleoside derivatives showed high A1 and/or A3 affinity and some of them were subtype-selective. Further optimizations are underway to obtain A2B AR agonists e.g. by introducing an N-ethylcarboxamide moiety at the 5′-position of the ribose in structures II and III.
References
1. Fredholm BB, IJzerman AP, Jacobson KA, Linden J, Müller CE (2007) Pharmacol Rev 63:1
2. Müller CE, Jacobson KA (2011) Biochim Biophys Acta 1808:1290
3. Hinz S, Lacher SK, Seibt B, Müller CE (2014) J Pharmacol Exp Ther 349:427
4. Baraldi, PG, Tabrizi MA, Fruttarolo F, Romagnoli R, Preti D (2008) Purinergic Signal 4:287
D 067
Chromone as a privileged structure for lead optimization of new adenosine receptors ligands
Alexandra Gaspar1,*, Fernando Cagide1, Joana Reis1, Eugenio Uriarte2, Stefano Alcaro3, Stefano Moro4, Karl-Norbert Klotz5 and Fernanda Borges1
1CIQ/Faculty of Sciences University of Porto, Porto, Portugal;2Faculty of Pharmacy, Organic Chemistry, Santiago Compostela, Spain;3Facoltà di Farmacia, Università “Magna Græcia”, Dipartimento di Scienze Farmacobiologiche, Catanzaro, Italy;4Dipartimento di Scienze Farmaceutiche, Università di Padova, Molecular Modeling Section (MMS), Padova, Italy;5Universität Würzburg, Institut für Pharmakologie und Toxikologie, Würzburg, Germany
The extensive medicinal chemistry artwork dedicated to the physiological and pathological role of extracellular adenosine, allowed the linkage between the cellular signal promoted by the adenosine receptors (ARs) and several types of pathologies including neurological, cardiovascular and inflammatory diseases as well as cancer. In fact, the adenosine receptors, in particular the A2A and A3 subtypes are being recognized as promising drug targets to design and develop of new drug candidates for cancer and neurodegenerative diseases [1]. Accordingly, in the pursuit of new potent and selective ARs ligands, our research group as being focused into the validation of the chromone core as potential ligands for the adenosine receptors [2].
Chromones are a group of natural occurring compounds, with a high degree of chemical diversity, ubiquitous in nature, and a broad spectrum of pharmacological activities has been attributed to this chemical core. These features allied to some structural similarities with flavones, a family of compounds already described as putative adenosine ligands, highlighted the use of the chromone skeleton as a privileged structure for the development of novel adenosine receptor ligands. The work herein described involves the pursuing of a new lead(s) compound(s) for AR ligands based on a chromone scaffold. The ongoing research regards the design and synthesis of a small library of compounds that were evaluated for their binding affinity towards AR receptors. The acquired results represented a precious income for the structure activity relationship (SAR) studies and consequently for the lead optimization of chromone carboxamides. Additionally the studies were complemented with molecular modeling studies. The overall data will be presented in this communication.
References
1. Fredholm BB, AP IJ, Jacobson KA, Linden J, Muller CE (2011) Pharmacol Rev 63(1):1–34
2. Gaspar A, Reis J, Kachler S, Paoletta S, Uriarte E, Klotz KN, Moro S, Borges F (2012) Biochem Pharmacol 84(1):21–29
D 068
Novel fluorescent probes for studying adenosine receptors
Sabrina Gollos*, Ali El-Tayeb, Fabian Heisig, Andrea Behrenswerth, Sven Jan Freudenthal and Christa E. Müller
Pharma-Center Bonn, Pharmaceutical Chemistry I, University of Bonn, D-53121 Bonn, Germany.
Adenosine receptors are of considerable interest as novel drug targets [1,2]. In the present study we developed fluorescent adenosine receptor ligands containing 4,4-difluoro-1,3,5,7-tetramethyl-4-bora-3a,4a-s-indacene (BODIPY) as a fluorescent dye. Such fluorophores are useful tools to image processes in living cells due to their high photochemical stability, their exceptional spectral properties and their small size [3]. Hence they are highly suitable for biological investigations [4]. In the present study we synthesized new, functionalized BODIPY derivatives, which fluoresce at approximately 500 nm and therefore do not interfere with biological fluorophores. Various functional groups (Br, I, NH2, SH, OH) were attached via alkyl spacers of different lengths to the fluorophore in order to allow for its attachment to receptor ligands, or its integration into the pharmacophore of adenosine receptor ligands. All new, functionalized BODIPY derivatives showed high fluorescence quantum yields and absorption and emission wavelengths near 500 nm. In a subsequent step we coupled the functionalized BODIPY dyes with different alkyl spacer lengths to 2-thioadenosine. The affinities of the obtained derivatives were determined in radioligand binding studies at A1, A2A, A2B and A3 receptors. Additionally, functional assays (cAMP accumulation studies) were performed.
Some of the fluorescent-labeled 2-thioadenosine derivatives showed high affinities at A1, A2A and/or A3 receptors (Ki values in the nanomolar range). The derivative with a short linker (n = 1) showed receptor subtype-selectivity for A3, while a longer alkyl chain (n = 9) led to a preference for A1 receptors. The new fluorescent ligands will be useful tools for studying adenosine receptors.
References
1. Fredholm BB, IJzerman AP, Jacobson KA, Linden J, Müller CE (2011) International union of basic and clinical pharmacology. LXXXI. Nomenclature and classification of adenosine receptors—an update. Pharmacol Rev 63:1–34
2. Müller CE, Jacobson AJ (2011) Recent developments in adenosine receptor ligands and their potential as novel drugs. Biochim Biophys Acta - Biomembranes 1808:1290–1308
3. Gollos S, Heisig F, Freudenthal, El-Tayeb A, SJ, Iqbal J, Müller CE (2013) Synthesis of BODIPY derivatives substituted with various bioconjugatable linker groups: A construction kit for fluorescent labeling of receptor ligands. J Fluoresc 24:213–230
4. Middleton RJ, Briddon SJ, Cordeaux Y, Yates AS, Dale CL, George MW, Baker JG, Hill SJ, Kellam B (2007) New Fluorescent Adenosine A1-Receptor Agonists That Allow Quantification of Ligand-Receptor Interactions in Microdomains of Single Living Cells. J Med Chem 50:782–793
D 069
Development of structural models of purinergic P2X receptors and analysis of interaction with agonists and antagonists
Diego Dal Ben*, Michela Buccioni, Catia Lambertucci, Gabriella Marucci, Andrea Spinaci, Ajiroghene Thomas, Rosaria Volpini
School of Pharmacy, Medicinal Chemistry Unit, University of Camerino, Via S. Agostino 1, 62032 Camerino, Italy
The purinergic P2X receptors are ligand-gated cation channels activated by the endogenous ligand ATP and assembled as homo- or heterotrimers from seven cloned subtypes (P2X1-7). All trimer subunits share a common topology consisting in intracellular N- and C- termini, two transmembrane domains and a large, glycosylated and disulphide-rich extracellular domain. P2X receptors are present in virtually all mammalian tissues and regulate a large variety of responses. The development of ligands that selectively activate or block specific P2X receptor subtypes represents a promising strategy to obtain novel pharmacological tools for the treatment of pain, cancer, inflammation, and neurological, cardiovascular, and endocrine diseases [1].
The publication of the crystal structures of P2X4 receptor in apo and ATP-bound forms represents a key step for the analysis of the receptor structure, the interpretation of mutagenesis data, and the depiction of ligand binding and receptor activation mechanism [2,3]. In addition, the availability of ATP-competitive ligands presenting selectivity for P2X receptor subtypes provides useful information for the design of new potent and selective ligands with possibly improved pharmacokinetic profiles, with the final aim to obtain new drugs.
Molecular modeling studies were performed to develop structural models of the human and rat P2X receptors in apo and ATP-bound forms. These models allowed to analyse the role of some non-conserved residues at ATP binding site and to study the receptor interaction with some non-specific or subtype selective agonists and antagonists [4].
Fig. 1 ATP interaction with zebrafish P2X4, with the receptor binding site represented as molecular surface
References
1. Coddou C, Yan Z, Obsil T, Huidobro-Toro JP, Stojilkovic SS (2011) Pharmacol Rev 63:641–683
2. Kawate T, Michel JC, Birdsong WT, Gouaux E (2009) Nature 460:592–598
3. Hattori M, Gouaux E (2012) Nature 485:207–212
4. Dal Ben D, Buccioni M, Lambertucci C, Marucci G, Thomas A, Volpini R (manuscript in preparation)
D 070
Structure-activity relationships of anthraquinone derivatives as modulators of P2X3 receptors
Claudia Spanier1,*, Younis Baqi1,2, Enas M. Malik1, Ralf Hausmann3 and Christa E. Müller1
1PharmaCenter Bonn, Pharmaceutical Institute, Pharmaceutical Chemistry I, University of Bonn, 53121 Bonn, Germany;2Department of Chemistry, Faculty of Science, Sultan Qaboos University, PO Box 36, Postal Code 123, Muscat, Oman;3Department of Molecular Pharmacology, Medical Faculty of the RWTH Aachen University, Wendlingweg 2, 52074 Aachen, Germany
P2X3 is one of 7 known subunits of purinergic P2X receptors, which can assemble as homotrimeric receptors, or as heterotrimeric receptors together with P2X2 subunits. The homomeric P2X3 receptor undergoes rapid desensitization, less than 100 milliseconds after activation with its physiological agonist ATP, which complicates its pharmacological characterization. Site-directed mutagenesis of the rat P2X3 receptor led to the discovery that the S15V mutation reduced desensitization of the receptor (S15VrP2X3). Since the mutation is localized right before the first transmembrane domain of the protein, ATP-binding is not affected [1]. A 1321N1 astrocytoma cell line permanently expressing the non-desensitizing P2X3 mutant has now been used for compound screening and characterization of P2X3 receptor modulators. The anthraquinone dye Reactive Blue 2 (RB-2) had previously been identified as a weak, non-selective antagonist at nucleotide receptors. Based on RB-2 as a lead structure, a library of 165 anthraquinone derivatives was synthesized [2-6] and tested in ATP-induced calcium influx assays using the fluorescent calcium-chelating dyes Calcium-4 and Calcium-5, respectively. Anthraquinone derivatives were found to be moderately potent inhibitors of the S15VrP2X3 receptor. The most potent inhibitor was 1, which displayed an IC50 value of 2.39 μM, and was found to be selective towards other P2X receptor subtypes except P2X2 receptor (IC50 3.32 μM). Several anthraquinone derivatives were identified as potent allosteric enhancers of the P2X3 receptor. They led to an increase in the ATP signal (ATP was used at its EC50 concentration, 50 nM) by 24–111 %. For example, compound 2 showed a 95 % increase in the ATP effect and displayed an EC50 value of 2.09 μM. The compound appeared not to be selective, since similar IC50-values could be determined for the other receptor subtypes, where it acted as an inhibitor (IC50 3.25–10.22 μM).
Fig. 1 Structure of two active anthraquinone derivatives at the S15VrP2X3 receptor
References
1. Hausmann R, Bahrenberg G, Schmalzing G (2014) Neuropharm 79:603–615
2. Baqi Y, Müller CE (2010) Nat Protoc 5:945–953
3. Baqi Y, Hausmann R, Rosefort C, Rettinger J, Schmalzing G, Müller CE (2011) J. Med. Chem. 54:817–830
4. Baqi Y, Atzler K, Köse M, Glänzel M, Müller CE (2009) J Med Chem 52:3784–3793
5. Baqi Y, Lee S, Müller CE (2010) J Med Chem 53:2076–2086
6. Weyler S, Baqi Y, Müller CE (2008) Bioorg Med Chem Lett 18:223–227
D 071
Polyoxometalates as potent inhibitors of P2X receptors
Claudia Spanier1,*, Holger Stephan2, Ulrich Kortz3, Ralf Hausmann4, Aliaa Abdelrahman1 and Christa E. Müller1
1PharmaCenter Bonn, Pharmaceutical Institute, Pharmaceutical Chemistry I, University of Bonn, 53121 Bonn, Germany;2Institute of Radiopharmaceutical Cancer Research, Helmholtz-Zentrum Dresden Rossendorf, P.O. Box 510119, 01314 Dresden, Germany;3School of Engineering and Science, Jacobs University, P.O. Box 750561, 28725 Bremen, Germany;4Department of Molecular Pharmacology, Medical Faculty of the RWTH Aachen University, Wendlingweg 2, 52074 Aachen, Germany
P2X receptors are trimeric ion channels that are activated by ATP and are permeable for the cations Na+, K+ and Ca2+. Seven different subunits exist, which are assembled as homo- or heterotrimers of various stoichiometry [1,2]. Polyoxometalates (POMs) are discrete, polynuclear metal-oxo anions of early transition metals in high oxidation states (e. g. W6+, Mo6+, V5+), comprising edge- and corner-shared MO6 octahedra. They exhibit enormous flexibility with respect to shape, size, composition and charge [3]. POMs are relatively large molecules (>1 nm) and bear several negative charges. In this respect they bear similarity to ATP, which binds to P2X2 and P2X4 in its tetraanionic form (ATP4−) and to P2X1 and P2X3 possibly also in its dianionic state as a Mg2+ complex (MgATP2−) [4]. We previously found that certain POMs can inhibit alkaline phosphatase [5] and ectonucleotidases, [6,7] enzymes that are capable of hydrolyzing nucleotides such as ATP and ADP. In the present study we investigated whether POMs can interact with P2X receptors. A series of POMs was investigated for their effects to inhibit ATP-induced calcium influx in recombinant 1321N1 astrocytoma cells stably transfected with P2X1, P2X2, P2X3, P2X4 or P2X7 receptors. Several POMs were found to be highly potent inhibitors of P2X receptors with potency in the low nanomolar range. The compounds were found to be non-cytotoxic at pharmacologically active concentrations, whereas some POMs showed cytotoxic effects in an MTT assay at concentrations typically higher than 1 μM.
References
1. Young MT (2010) Trends Biochem Sci 35:83–90
2. Torres GE, Egan TM, Voigt MM (1999) J Biol Chem 274:6653–6659
3. Hasenknopf B (2005) Front Biosci 10:275–28
4. Li M, Silberberg SD, Swartz KJ (2013) Proc Natl Acad Sci USA 110:E3455–E3463
5. Raza R, Matin A, Sarwar S, Barsukova-Stuckart M, Ibrahim M, Kortz U, Iqbal J (2012) Dalton Trans 41:14329–14336
6. Müller CE, Iqbal J, Baqi Y, Zimmermann H, Röllich A, Stephan H (2006) Bioorg Med Chem Lett 16:5943–5947
7. Stephan H, Kubeil M, Emmerling F, Müller CE (2013) Eur J Inorg Chem 1585–1594
D 072
Synthesis of the tetra-heterocyclic systems containing the pyrazolopyridine derivative with expected the antiviral activity
Farag El-Essawy
Menoufia University, Chemistry, Shebin El-Koam, Egypt;Menoufia University, Organic, Shebin El-Koam, Egypt
Where the pyrazolopyridines constitute a very interesting class of compounds of thei significant and versatile biological and pharmacological activities. Pyridopyrazolopyrimidine derivatives were synthesized by cyclocondensation of aminopyrazolopyrimidines with malonate derivatives and also with the butyrolactone. Dichloro-, and diazido-derivatives were obtained. The tetracyclic systems were also obtained. The antiviral activity now for these compounds under investigation. Also, the tetrahetrocyclic isolated with pyrazolopyridine can be obtained to see the difference in biological activities between the fused and isolated systems.
References
1. Farag A, El-Essawy J (2010) Heterocylic Chem 47:318
2. Farag A, El-Essawy J (2010) Synth Commun 40:877
3. Farag A, El-Essawy J, Abdallah Sh El-Ertawy J (2014) Heterocylic Chem (in production)
D 073
Synthesis of some new organo-complexes containing new pyrazolopyridine derivatives with expected biological activity
Yasser K. Abdelmoneam, Farag A. EL-Essawy, Saeda A. Abou El-Enein, Mona M. El-Sheikh-Amer and Yasser Abdel-Moneam*
Menoufia University, chemistry, shebin el-kom, Egypt
Due to the high biological properties of many pyrazolopyridine derivatives as drugs for treatment of wide range of diseases, we aim to synthesize new derivatives of these heterocyclic compounds as well as their acyclic nucleosides analogues. The biological activity of these compounds is studied by DPPH assay which provided an easy and rapid way to determine the antioxidant activity of the substances tested in this study.
Also the coordination behavior of ligand 2-(3-Amino-4,6-dimethyl-1H-pyrazolo[3,4-b] pyridin-1-yl)acetohydrazide (HL) towards some metals is investigated, on the basis of: elemental analysis, spectral measurements (UV–Vis, IR), 1HNMR, mass spectra, molar electrical conductivity, magnetic susceptibility and thermal analysis.
References
1. Bondock S, Fadaly W, Metwally MA (2009) Eur J Med Chem 44:4813
2. Hawkins MJ, Soon-Shiong P, Desai N (2008) Adv Drug Delivery Rev 60:876
3. Primik MF, Muehlgassner G, Jakupec MA, Zava O, Dyson PJ, Arion VB, Keppler BK (2010) Inorg Chem 49:302
4. Emam SM, El-Saied FA, AbouEl-Enein SA, El-Shater HA (2009) Spectrochim Acta A 72:291
D 074
Design, synthesis and characterization of high affinity fluorescent agonist and antagonist ligands of G protein-coupled P2Y receptors
Kenneth A. Jacobson1,*, Evgeny Kiselev1, P. Suresh Jayasekara1, Matthew O. Barrett2, Vsevolod Katritch4, Silvia Paoletta1, Clarissa Weitzer2, Eva Hammes1, Ramachandran Balasubramanian1, Zhan-Guo Gao1, Qiang Zhao3, Raymond C. Stevens4 and T. Kendall Harden2
1Molecular Recognition Section, Laboratory of Bioorganic Chemistry, National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, Bethesda, MD 20892 USA;2Department of Pharmacology, University of North Carolina, School of Medicine, Chapel Hill, North Carolina 27599 USA;3CAS Key Laboratory of Receptor Research, Shanghai Institute of Materia Medica, Chinese Academy of Sciences, 555 Zuchongzhi Road, Pudong, Shanghai, China 201203;4Department of Integrative Structural and Computational Biology, The Scripps Research Institute, 10550 North Torrey Pines Road, La Jolla, CA 92037, USA
Extracellular nucleotides acting at Gq- and Gi-coupled P2Y receptors (P2YRs) modulate biological processes in many organs and tissues. We explore novel P2YR agonists and antagonists, to identify selective agents as pharmacological probes and potential therapeutic agents. We designed fluorescent conjugates of functionalized congeners that display high P2YR affinity, for characterization of these GPCRs in living cells by flow cytometry and in cell membranes. Fluorescent agonists are mostly internalized consistent with agonist-induced receptor internalization, and this labeling is attenuated by specific P2YR ligands. Examples are MRS4129 and MRS4162 [1], which are fluorescent pyrimidine nucleotides, respectively, selective for the P2Y6R (EC50 9 nM, phospholipase C activation) and high affinity pan-agonist at P2Y2R, P2Y4R and P2Y6R (expressed in astrocytoma cells). We synthesized P2Y14R fluorescent antagonists based on potent and highly selective 2-naphthoic acid derivative PPTN. We modeled the hP2Y14R based on recent hP2Y12R X-ray structures and simulated docking, suggesting that a piperidine of PPTN is accessible for tethering fluorophores. Click chemistry was used to conjugate functionalized PPTN alkyne derivatives and azide-bearing fluorophores. Flow cytometry showed high specific P2Y14R binding of AlexaFluor488 derivative MRS4174 (Ki 80 pM, cAMP inhibition in P2Y14R-expressing CHO cells). Known P2Y ligands inhibited cell labeling consistently with affinity order. Thus, the 3D knowledge of ligand recognition in GPCRs promises to enable drug discovery through design of fluorescent molecular probes for P2YRs. This approach demonstrates the predictive power of GPCR homology modeling and the value of applying newly determined X-ray structures to the medicinal chemistry of GPCRs.
Reference
1. Jayasekara PS, Jayasekara PS, Barrett MO, Ball CB, Brown KA, Hammes E, Balasubramanian R, Harden TK, Jacobson KA (2014) J Med Chem 57:3874–3883
D 075
Antagonistic action of farnesyl pyrophosphate at the human platelet P2Y12-receptor
Kristina Hoffmann*, Dominique A. Lutz, Jens Straßburger and Ivar von Kügelgen
Universtity of Bonn, Pharma Center Bonn, Department of Pharmacology and Toxicology, Sigmund-Freud-Strasse 25, 53127 Bonn, Germany
The nucleotide P2Y12-receptor plays a prominent role in ADP-induced platelet aggregation. It is the pharmacological target of the active metabolites of the thienopyridines clopidogrel and prasugrel and of ticagrelor, which is the first perorally active and reversible inhibitor of platelet aggregation. Recently, an antagonistic action of farnesyl pyrophosphate (FPP), an intermediate product in the HMG-CoA reductase pathway, at the platelet P2Y12-receptor was described [1]. In the present study we analyzed the interaction of FPP with recombinant wild-type and mutant human P2Y12-receptors, stably expressed in CHO Flp-In cells. Receptor function was assessed by quantification of cellular cAMP with a [3H]cAMP-radioaffinity assay. The synthetic ADP-analogue 2-methylthio-ADP (2-MeSADP) inhibited forskolin-induced cAMP accumulation in a concentration-dependent manner at all tested P2Y12-receptor constructs. Addition of FPP [30 μM] shifted the concentration-response curve of the agonist to the right in a surmountable manner at the wild-type receptor with an apparent pKB value of 5.2. Similar results were obtained at R2566.55A-mutant P2Y12-receptors and at wild-type receptors fused to enhanced cyan fluorescent protein (ECFP). At K2807.35A-mutant P2Y12-receptors fused to ECFP, a smaller rightward shift of the concentration-response curve of 2-MeSADP by FPP and a decreased maximal effect of the agonist were observed. In contrast, at C194A-mutant receptors, the antagonistic potency of FPP was significantly increased (pKB 6.3). Thus, FPP acts as an antagonist at the P2Y12-receptor, suggesting a possible interaction of the HMG-CoA reductase pathway with platelet P2Y12-receptor function. The amino acid residues C1945.43 and K2807.35 may be involved in the interaction of FPP with the receptor protein.
Reference
1. Högberg C, Gidlöf O, Deflorian F, Jacobson KA, Abdelrahman A, Müller CE, Olde B, Erlinge D (2012) Thromb Haemost 108:119–132
D 076
Synthesis, characterisation, in vitro evaluation, and receptor docking of the selective P2Y2receptor antagonist AR-C118925
Muhammad Rafehi*, Joachim C. Burbiel, Vigneshwaran Namasivayam, Michael Wiese and Christa E. Müller
PharmaCenter Bonn, Pharmaceutical Institute, Pharmaceutical Sciences Bonn (PSB), Pharmaceutical Chemistry, University of Bonn, Bonn, Germany
The nucleotide receptor family P2Y comprises G protein-coupled receptors that are of considerable interest, owing to their potential to function as drug targets in novel therapeutic schemes for a range of different disorders. Targeting P2Y2 is particularly promising, as it could be beneficial against tumour cell metastasis, excessive inflammatory reactions, atherosclerosis, neurodegenerative disorders, and cystic fibrosis. The precise role of the P2Y2 receptor in these conditions is often not clear, which can be partially attributed to the limited availability of potent and selective inhibitors that could be used for pharmacological studies. One of the most selective antagonists to date is AR-C118925 that has been developed by AstraZeneca. However, this compound is not commercially available and, as a result, is very vaguely described in the literature. We, therefore, synthesised AR-C118925 as well as two derivatives and assessed their inhibitory potential on P2Y2, further subtypes of the P2Y receptor family, P2X ion channels, adenosine receptors, as well as a range of other potential targets that complement the work of Kemp et al [1]. Furthermore, we created a model of the ligand binding site based on the crystal structure of the P2Y12 receptor [2]. The results obtained from this study constitute a significant contribution towards a comprehensive characterisation of this promising pharmacological tool and the synthesis scheme described here will facilitate access to this compound by other research groups.
References
1. Kemp PA, Sugar RA, Jackson AD (2004) Nucleotide-mediated mucin secretion from differentiated human bronchial epithelial cells. Am J Respir Cell Mol Biol 31:446–455
2. Zhang K, Zhang J, Gao Z-G, Zhang D, Zhu L, Han GW, Moss SM, Paoletta S, Kiselev E, Lu W, Fenalti G, Zhang W, Müller CE, Yang H, Jiang H, Cherezov V, Katritch V, Jacobson K, Stevens RC, Wu B, Zhao Q (2014) Structure of the human P2Y12 receptor in complex with an antithrombotic drug. Nature [Online early access]. DOI: 10.1038/nature13083. Published Online: March 23, 2014. http://www.ncbi.nlm.nih.gov/pubmed (accessed April 7, 2014)
D 077
Anthraquinone derivatives as potent and selective P2Y4receptor antagonists
Muhammad Rafehi1,*, Younis Baqi2, Enas M. Malik1 and Christa E. Müller1
1PharmaCenter Bonn, Pharmaceutical Institute, Pharmaceutical Sciences Bonn (PSB), Pharmaceutical Chemistry I, University of Bonn, Bonn, Germany;2Department of Chemistry, Faculty of Science, Sultan Qaboos University, P.O. Box 36, Postal Code 123, Muscat, Oman
The P2Y family of G protein-coupled receptors has become a major research focus in recent years and constitutes prospective novel therapeutic targets for a range of different indications [1]. Despite the strong interest in this receptor family, relatively little is known regarding the P2Y4 subtype. It is of significant clinical interest though: experimental observations suggest a role in neurodegenerative disorders [2]. Its involvement in epithelial Cl−-secretion could make it a new target for the treatment of cystic fibrosis and diarrhoea [3]. One reason for the limited insights on this receptor is the lack of subtype-selective ligands, which can be used for pharmacological studies. Therefore, the focus of this study is to specifically design and develop potent and selective antagonists for the P2Y4 receptor. To achieve this, a library of anthraquinone derivatives structurally related to reactive blue 2 (one of the most potent, albeit non-selective, antagonists so far) was tested for inhibitory potency using fluorescence-based Ca2+-mobilisation assays in recombinant, human P2Y4 receptor-expressing 1321N1 astrocytoma cells.
Core structure of the test compounds.
This data was the basis for subsequent structure-activity-relationship analyses aimed at enhancing our knowledge with respect to P2Y4 receptor-ligand preferences. The findings are currently implemented in the design of novel synthetic compounds to develop more potent and P2Y receptor subtype-selective inhibitors of the P2Y4 receptor, which are required as pharmacological tools and lead compounds for drug development.
References
1. Abbracchio MP, Burnstock G, Boeynaems J-P, Barnard EA, Boyer JL, Kennedy C, Knight GE, Fumagalli M, Gachet C, Jacobson KA, Weisman GA (2006) International Union of Pharmacology LVIII: Update on the P2Y G protein-coupled nucleotide receptors: From molecular mechanisms and pathophysiology to therapy. Pharmacol Rev 58:281–341
2. Tran MD (2011) P2 receptor stimulation induces amyloid precursor protein production and secretion in rat cortical astrocytes. Neurosci Lett 492:155–159
3. Robaye B, Ghanem E, Wilkin F, Fokan D, Van Driessche W, Schurmans S, Boeynaems J-M, Beauwens R (2003) Loss of nucleotide regulation of epithelial chloride transport in the jejunum of P2Y4-null mice. Mol Pharmacol 63:777–783
D 078
Synthesis and structure-activity relationship of uracil nucleotide derivatives as antagonists of human P2Y6receptor
Diana Meltzer1, Ofir Ben Yaacov1, Guillaume Arguin2, Yael Nadel1, Fernand-Pierre Gendron2 and Bilha Fischer1,*
1Bar Ilan University, Chemistry, Ramat Gan, Israel;2University de Sherbrooke, Anatomy and cellular biology, Sherbrooke, Canada
Pharmacological modulation of P2Y6-R has been proposed to be useful in treatment of numerous diseases. For instance, a P2Y6-R antagonist offers new opportunities for the treatment of inflammatory bowel diseases (IBDs) and the treatment of vasospasm. To date, there are no P2Y6-R antagonists based on UDP scaffold. With a view to identify the first nucleotide-based reversible P2Y6-R antagonists, we designed and synthesized several series of UDP derivatives and evaluated them at human P2Y6-R (hP2Y6-R). We explored the effect of various modifications at the uracil ring, ribose moiety, as well as, phosphate chain of UDP and established the structure-activity relationship at hP2Y6-R. 5-OMe-uridine-5′-α,β-methylenediphosphonate, analogue 4, was found to inhibit hP2Y6-R by 70 and 90 % at 30 and 100 μM, respectively. However, compound 4 had also some agonist activity (ca. 1.6-fold less than UDP) at P2Y6-R as well. Other modifications such as, 3-NMe and C5-alkyl/aryl, were not tolerated by hP2Y6-R, while, 2′-OH was found to be crucial for both agonist and antagonist activity at the receptor. Uridylyl phosphosulfate, compound 14, displayed only weak P2Y6-R activation and caused 90 % inhibition at 100 μM.
Fig. 1 Uracil nucleotide analogues designed as potential P2Y6-R antagonists
D 079
Development of [3H]2-carboxy-4,6-dichloro-1H-indole-3-propionic acid ([3H]PSB-12150): a useful tool for studying GPR17
Meryem Köse1,*, Kirsten Ritter1, Katharina Sylvester1, Michel Gillard2, Evi Kostenis3 and Christa E. Müller1
1PharmaCenter Bonn, Pharmaceutical Institute, Pharmaceutical Chemistry I, University of Bonn, Bonn, Germany;2UCB Pharma S.A., CNS Research, Chemin du Foriest, B - 1420 Braine-l’Alleud, Belgium;3PharmaCenter Bonn, Institute of Pharmaceutical Biology, Section of Molecular-, Cellular-, and Pharmacobiology, University of Bonn, Bonn, Germany
The G protein-coupled receptor GPR17 is an orphan, rhodopsin-like receptor, which is phylogenetically related to nucleotide P2Y and cysteinylleukotriene receptors (Cys-LTR) [1]. It is predominantly expressed in the central nervous system (CNS), particularly in differentiating oligodendrocyte precursor cells [2-4]. GPR17 was identified as a key player in the modulation of CNS myelination and has recently been shown to negatively regulate the oligodendrocyte differentiation process.2,5 Hence, inhibition of GPR17 emerges as a promising therapeutic approach for the treatment of demyelinating diseases such as multiple sclerosis (MS), for the treatment of brain and spinal cord injury and neurodegenerative diseases. Recently Hennen et al [5]. identified 2-carboxy-4,6-dichloro-1H-indole-3-propionic acid (MDL29,951, 1) and 3-[(E)-3-(anilino)-3-oxoprop-1-enyl]-4,6-dichloro-1H-indole-2-carboxylic acid (GV150526A) as synthetic, small molecule GPR17 agonists and characterized them in different functional assays. Compound 1 was found to selectively activate GPR17, but not P2Y or Cys-LT receptors. So far, no radioligand is available to determine GPR17 ligand binding affinities. Especially in view of the controversies in the field regarding GPR17 agonists and antagonists [6], such a tool would be highly useful to directly study receptor-ligand interaction. Thus, we decided to prepare the potent and selective GPR17 agonist 1 in tritium-labeled form. The radioligand [3H]PSB-12150 was obtained by catalytic hydrogenation of a precursor, which contained an exocyclic double bond, using tritium gas. The product was obtained with a specific activity of 17 Ci/mmol (629 GBq/mmol). It showed specific and saturable binding to a single binding site in membrane preparations from Chinese hamster ovary cells recombinantly expressing the human GPR17. A competition assay procedure was established which allows the determination of ligand binding affinities. In the newly developed radioligand binding studies we could confirm that the Cys-LTR antagonists pranlukast and montelukast bind to GPR17, although with moderate affinities. However, we did not observe any binding affinity or modulation of radioligand binding by the nucleotides UDP and UDP-glucose, which were previously postulated to be GPR17 agonists. The current data are therefore not supporting an interaction of nucleotides and nucleotide-sugars with GPR17.
In conclusion, we developed and characterized [3H]PSB-12150 as the first radioligand for the pathophysiologically relevant orphan receptor GPR17. The new radioligand has allowed us to investigate the binding affinities of potential GPR17 agonists and antagonists.
References
1. Ciana P et al (2006) EMBO J 25:4615–4627
2. Chen Yet al (2009) Nat Neurosci 12:1398–1406
3. Lecca D et al (2008) PLoS One 3:e3579
4. Fumagalli M et al (2011) J Biol Chem 286:10593–10604
5. Hennen S et al (2013) Sci Signal 6, ra93. doi: 10.1126/scisignal.2004350
6. Qi AD, Harden TK, Nicholas RA (2013) J Pharmacol Exp Ther. doi: 10.1124/jpet.113.207647
D 080
Design, synthesis and structure-activity relationship studies of 4/6-substituted 2-carboxy-1H-indole-3-propionic acid derivatives as GPR17 agonists
Thanigaimalai Pillaiyar1,* Younis Baqi1,2 Samer Alshaibani1, Aliaa Abdelrahman1, Vigneshwaran Namasivayam1, Evi Kostenis3 and Christa E. Müller1
1PharmaCenter Bonn, Pharmaceutical Institute, Pharmaceutical Chemistry I, University of Bonn, An der Immenburg 4, D-53121 Bonn, Germany;2Department of Chemistry, Faculty of Science, Sultan Qaboos University, PO Box 36, Postal Code 123, Muscat, Oman;3Institute of Pharmaceutical Biology, Section Molecular-, Cellular-, and Pharmacobiology, University of Bonn, Bonn, Germany
The orphan G protein-coupled receptor 17 (GPR17) belongs to the rhodopsin-like class A of G protein-coupled receptors (GPCRs). Phylogenetically it is located between nucleotide P2Y and cysteinylleukotriene (CysLT) receptors [1]. Similar to P2Y12,13,14 and CysLT1 receptors, GPR17 is coupled to inhibition of adenylate cyclase via Gi proteins resulting in decreased intracellular cAMP levels; in addition, it is coupled to Gq proteins which activate phospholipase C leading to IP3-mediated intracellular calcium release. GPR17 is predominantly expressed in the central nervous system (CNS), particularly in oligodendrocyte precursor cells. GPR17 was recently proposed to be a key player in the modulation of myelination of neurons in the CNS. To study the (patho)physiological roles of GPR17 selective ligands, agonists and antagonists, are required.
A series of compounds has been reported to activate GPR17, including nucleotides (UDP) and nucleotide-sugars (UDP-glucose and UDP-galactose) as well as cysteinylleukotrienes (CysLTC4 and CysLTD4). However, several groups, including ours, were unable to confirm the described activity. Recently, Hennen et al., identified 3-(2-carboxy-4,6-dichloro-indol-3yl)-propionic acid (1) as a synthetic agonist for GPR17 [2]. Compound 1 showed moderate potency at the human GPR17 (EC50 = 0.332 μM in our assay system measuring calcium mobilization in recombinant 1321N1 astrocytoma cells). Nevertheless, 1 could be successfully applied in tritium-labelled form as a radioligand for labeling GPR17 (KD = 1,256 nM) [3]. Preliminary structure-activity relationships were performed indicating that the size of the substituents at 4 and/or 6-position on indole in 1 is important for receptor interaction. The dibromo-substituted indole 2a (EC50 = 0.332 μM), for instance, showed slightly higher potency compared to 1. However, large substituents were less tolerated and reduced the potency (2b: EC50 = 4.790 μM) [4].
In order to improve the agonistic potency of the GPR17 agonists we subsequently synthesized a large series of indole derivatives derived from compound 1 and extensively analyzed their structure-activity relationships at GPR17. We identified several agonists with significantly higher potency than 1.
Fig. 1 The first reported lead (1) and newly designed GPR17 agonists
References
1. Ciana P et al (2006) EMBO J 25:4615–4627
2. Hennen S et al (2013) Sci Signal 6:ra93
3. Köse M et al (2014) ACS Med Chem Lett 5:326–330
4. Baqi et al (2014) Med Chem Comm 5:86–92
D 081
P2Y-like orphan receptors: new antagonist for GPR18 and GPR55
Clara T. Schoeder1,*, Viktor Rempel1, Tadeusz Karcz2, Katarzyna Kieć-Kononowicz2 and Christa E. Müller1
1Pharma-Zentrum Bonn, Pharmazeutisches Institut, Pharmazeutisches Chemie I, An der Immenburg 4, D-53121 Bonn, Germany;2Department of Technology and Biotechnology of Drugs, Faculty of Pharmacy, Jagiellonian University, Medical College, 9 Medyczna St., 30-688 Kraków, Poland
P2Y purine receptors are nucleotide-activated G protein-coupled receptors (GPCRs) that belong to the class A or rhodopsin-like subfamily of GPCRs. Within this subfamily they belong to the δ-branch. That branch is characterized by GPCRs with very different ligands, including nucleotides, peptides and lipids, and by a considerable number of orphan receptors, of which the physiological agonist is still unknown. Two of these orphan receptors are GPR18 and GPR55, both of which may be modulated by cannabinoids. However, they are phylogenetically unrelated to the classical cannabinoid (CB) receptors. GPR18 was reported to play a role in migration of microglia and endometrial tissue [1]. GPR55 was found to be involved in pain and inflammatory responses, and is upregulated in many cancer cells [2]. To further elucidate the (patho)physiological roles of these orphan GPCRs and to investigate their potential as novel drug targets, potent and selective ligands are required. In a screening campaign using our compound library (www.mueller-group.pharma.uni-bonn.de/bibliothek) bicyclic imidazole-4-one derivatives synthesized by the group of K. Kiec-Kononowicz [3] (Cracow, Poland) were identified as a novel class of antagonists at both receptors. We subsequently investigated their structure-activity relationships at GPR18 and GPR55, and evaluated their selectivity versus related receptors, including CB1, CB2 and GPR35 [4]. (Z)-(2,3-difluorobenzylidene)-6,7-dihydro-2H-imidazo[2,1-b][1,3]thiazin-3(5H)-one was found to be a selective GPR55 antagonist with an IC50 value of 3.15 μM. (Z)-2-(3-(4-chlorobenzyloxy)benzylidene)-6,7-dihydro-2H-imidazo[2,1-b][1,3]thiazin-3(5H)-one was developed as a potent, selective GPR18 antagonist with an IC50 of 0.279 μM, >36-fold selectivity vs. CB1 and GPR55 and 14-fold vs. CB2. It represents the most potent and selective GPR18 antagonist known to date.
References
1. McHugh D, Page J, Dunn E, Bradshaw HB (2012) Br J Pharmacol 165:2414–2424
2. Rempel V, Volz N, Hinz S, Karcz T, Meliciani I, Nieger M, Wenzel W, Bräse S, Müller CE (2012) J Med Chem 5:7967–7977
3. Kieć-Kononowicz K, Karolak-Wojciechowska J, Michalak B, Pękala E, Schumacher B, Müller CE (2004) Eur J Med Chem 39:205–218
4. Rempel V, Atzler K, Behrenswerth A, Karcz T, Schoeder C, Hinz S, Kaleta M, Thimm D, Kieć-Kononowicz K, Müller CE (2014) Med Chem Comm 5:632–649
D 082
Magnolol and derivatives as lead structures for the development of GPR55 antagonists
Alexander Fuchs*, Viktor Rempel, Clara Schoeder and Christa E. Müller
PharmaCenter Bonn, Pharmaceutical Institute, An der Immenburg 4, 53121 Bonn, Germany
The orphan G protein-coupled receptor (GPCR) GPR55 is widely expressed in different cells and organs including high expression on several cell types of the immune system, and in the brain. Furthermore, it is up-regulated in many cancer cells. GPR55 may therefore qualify as a new drug target for the treatment of neuropathic/inflammatory pain or for cancer therapy [1]. Potent and selective antagonists are urgently required for target validation.
Recently we discovered that a bark extract of Magnolia officinalis, which is used in traditional Chinese medicine (TCM), exhibits cannabinoid (CB) receptor agonistic effects. The main active constituents of the extract were shown to be the biphenylic neolignans magnolol, honokiol and to a minor extent 4′-O-methylhonokiol. We could show that these biphenylic compounds and the main metabolite of magnolol, tetrahydromagnolol, activate CB receptors and show a preference for the CB2 subtype [2]. Tetrahydromagnolol was additionally found to be a weak antagonist at the orphan G protein-coupled receptor GPR55.
The simple and drug-like structure of the magnolol derivatives prompted us to synthesize analogs and study their structure-activity relationships with regard to CB receptors and the CB-like orphan receptors GPR18 and GPR55 [3]. Although the natural product magnolol showed no interaction with the two orphan receptors, we could develop a GPR55 antagonist with an IC50 value of 3.25 μM based on the magnolol structure. This derivative may serve as a new starting point for the development of potent and selective antagonists for GPR55.
References
1. Henstridge CM, Balenga NA, Kargl J, Andradas C, Brown AJ, Irving A, Sanchez C, Waldhoer M (2011) Mol Endocrinol 25:1835–1848
2. Rempel V, Fuchs A, Hinz S, Karcz T, Lehr M, Koetter U, Müller CE (2013) ACS Med Chem Lett 4:41–45
3. Fuchs A, Rempel V, Müller CE (2013) PLOS One 8:e77739
D 083
Probing the putative pocket: mutagenesis studies at the rat adenine receptor
Anke C. Schiedel1,*, Melanie Knospe1, Mohamad W. Alnouri1, Ivar von Kügelgen2 and Christa E. Müller1
1PharmaCenter Bonn, Pharmaceutical Chemistry I, University of Bonn, An der Immenburg 4, 53121 Bonn, Germany;2PharmaCenter Bonn, Department of Pharmacology, University of Bonn, Sigmund-Freud-Straße 25, 53127 Bonn, Germany
A new family of purinergic G protein-coupled receptors activated by the nucleobase adenine has recently been described, which constitutes at least two different subtypes [1,2]. The first cloned and characterized member of the adenine receptor (AdeR) family is the rat AdeR [3]. In the present study we took this receptor as a model receptor to analyze its putative binding pocket. Based on a tentative homology model [4,5], alignment studies, and structure-activity relationships several amino acid residues were predicted to be part of the putative binding pocket. A set of nine amino acid residues was exchanged for alanine, and the mutant receptors were expressed in Spodoptera frugiperda (Sf9) insect cells. [3H]Adenine binding studies performed with membrane preparations revealed only minor effects indicating that none of the exchanged amino acids is part of the ligand binding pocket, at least in the inactive state of the receptor. To probe for receptor activation the mutant AdeRs were coexpressed with mammalian Gi proteins in Sf9 insect cells and analyzed using the [35S]GTPγS assay. Two residues, Asn1945.40 and Leu2015.47, both located at the extracellular half of transmembrane domain 5 were found to be crucial for receptor activation since their alanine mutants did not respond to adenine. Tyr2687.32, located at the extracellular end of transmembrane domain 7 appeared to be involved in stabilizing a certain receptor conformation, since at its alanine mutant adenine showed significantly increased efficacy.
Furthermore, using a newly developed specific antibody against rAdeRs we were able to show that the extended N-terminal sequence of the rAdeR constitutes a putative signal peptide of yet unknown function, that is cleaved off in the mature receptor. Our results provide important insights into this new, still poorly investigated family of purinergic receptors.
References
1. von Kügelgen I, Schiedel AC, Hoffmann K, Alsdorf BB, Abdelrahman A, Müller CE (2008) Mol Pharmacol 73:469–477
2. Thimm, D, Knospe, M, Abdelrahman, A, Moutinho, M, Alsdorf, BBA, von Kügelgen, I, Schiedel, AC, Müller, CE (2013) Purinergic Signal. DOI: 10.1007/s11302-013-9360-9
3. Bender E, Buist A, Jurzak M, Langlois X, Baggerman G, Verhasselt P, Ercken M, Guo HQ, Wintmolders C, Van den Wyngaert I, Van Oers I, Schoofs L, Luyten W (2002) Proc Nat Acad Sci USA 99:8573–8578
4. Heo J, Vaidehi N, Wendel J, Goddard 3rd WA (2007) J Mol Graph Model 26:800–812
5. Knospe, M, Müller, CE, Rosa, P, Abdelrahman, A, Thimm, D, Schiedel, AC (2013) Purinergic Signal. DOI: 10.1007/s11302-013-9355-6
E: Transport of nucleobases, nucleosides and nucleotides
E 084
Activation of ATP secretion via volume-dependent anion channels by sphingosine-1-phosphate in RAW macrophages
Philipp Burow*, Manuela Klapperstück and Fritz Markwardt
MLU Halle-Wittenberg, JBI for Physiology, Halle (Saale), Germany
We report about the activation of outwardly rectifying anion currents by sphingosine-1-phosphate (S1P) in the murine macrophage cell line RAW 264.7. The current reversal potential was shifted by replacement of extracellular Cl− by glutamate− but not when extracellular Na+ was substituted by Tris+ revealing that S1P-induced current is mainly carried by anions. The inhibition of the currents by hypertonic extracellular or hypotonic intracellular solution as well as the inhibitory effects of NPPB, tamoxifen and glibenclamide indicate that the anion current is mediated by volume-regulated anion channels (VRAC). The S1P effect was blocked by intracellular GDPβS and W123 which points to a signalling via the S1P receptor 1 (S1PR1) and G-proteins. As cytochalasin D diminished the action of S1P we conclude that the actin cytoskeleton is involved in the stimulation of VRAC. S1P and hypotonic extracellular solution induced a secretion of ATP from the macrophages which was blocked in both cases in a similar way by typical VRAC blockers. We suppose that the S1P-induced ATP secretion in macrophages via activation of VRAC constitutes a functional link between sphingolipid and purinergic signalling in essential processes such as inflammation, migration of leukocytes as well as phagocytosis and killing of intracellular bacteria.
E 085
ATP release in human erythrocytes—is hemolysis a primary release mechanism?
Ryszard Grygorczyk1,*, Jacek Sikora1,2, Sergei N. Orlov1,3 and Kishio Furuya4
1University of Montreal, Medicine, Montreal, Russian Federation;2Poznan University of Medical Sciences, Poznan, Poland;3Moscow State University, Moscow, Russian Federation;4Nagoya University, Nagoya, Japan
The hypothesis that regulated ATP release from red blood cells (RBCs) contributes to nitric oxide-dependent control of local blood flow has sparked much interest in the underlying release mechanisms. Several stimuli including shear stress and hypoxia have been found to induce significant RBC ATP release attributed to activation of ATP conducting channels. In this study we evaluated different experimental approaches for studying stimulated ATP release from RBCs and quantifying hemolysis, then measured ATP and hemoglobin content in the same RBC supernatant samples to directly assess the contribution of hemolysis to ATP release. We tested several stimuli that are known to induce ATP release from RBCs, including hypotonic shock, shear stress, cAMP agonists and hypoxia. All these stimuli, except cAMP agonists, significantly enhanced ATP release. In each case, however, we observed a tight, linear relationship between extracellular ATP and free hemoglobin in RBC supernatants, indicating that lysis was responsible for most, if not all, ATP release. This finding was further confirmed in experiments in which stimulated ATP release was investigated by luminescence imaging combined with simultaneous infrared cell imaging, to identify ATP-releasing cells, Figure 1. Experiments showed that ATP was released exclusively from lysing cells with no contribution from intact cells. In summary, for all stimuli tested, we found no evidence of regulated ATP release from intact RBCs other than cell lysis. Nevertheless, such a release mechanism might be physiologically relevant in vivo, e.g., during exercise and hypoxia where intravascular hemolysis is augmented.
Supported by the Canadian Institutes of Health Research (MOP64364, RG).
Fig. 1 Luminescence imaging of ATP release from RBC induced by hypotonic shock
An example of overlayed infrared images of RBCs (shown in green color) and extracellular ATP-dependent luminescence (in red). 20 % hypotonic shock-induced luminescence response (center image) can be seen shortly before lysis of single RBC (indicated by white arrow). No ATP release from intact RBCs is evident. Images were acquired with 20× water-immersion objective at 500-ms exposure time. The elapsed time (min:s) for the three images was 4:51, 4:54 and 5:27 respectively.
E 086
Inhibitors of ATP release inhibit vesicular nucleotide transporter
Yuri Kato1,2,*, Hiroshi Omote1 and Takaaki Miyaji2
1Okayama University, Graduate School of Medicine, Dentistry and Pharmaceutical Sciences, Okayama, Japan;2Okayama University, Advanced Science Research Center, Okayama, Japan
Previous studies involving various inhibitors suggested that various proteins (transporter, channel and receptor) involves in the mechanisms of ATP release, although ATP is accumulated in secretory vesicles and exocytosed into the extracellular space from ATP-secreting cells. Recently we found that SLC17A9 protein acts as a vesicular nucleotide transporter (VNUT) and is responsible for vesicular ATP storage in ATP-secreting cells [1]. There are several compounds known to inhibit ATP release. Here we investigated whether such ATP release inhibitors affect VNUT.
Human VNUT was overexpressed in E. coli, solubilized and purified to near homogeneity. The proteoliposomes containing purified human VNUT actively took up radiolabeled ATP by imposing an inside positive membrane potential (Δψ) through valinomycin-dependent K+ diffusion. The ATP transport activity was inhibited by glibenclamide (an inhibitor of ATP-binding cassette proteins), 18 α-glycyrrhetinic acid, flufenamic acid (inhibitors of hemichannels), arachidonic acid (an inhibitor of maxi anion channel) and A438079 (an inhibitor of P2X7 receptor) without the formation of Δψ as a driving force being affected. The IC50 values of glibenclamide, 18 α-glycyrrhetinic acid, flufenamic acid, arachidonic acid and A438079 were 1.6, 0.5, 36, 6.5 and 0.3 μM, respectively. Thus, various inhibitors of ATP release inhibit VNUT activity and subsequent ATP release. Careful consideration will be necessary to characterize the mechanism of ATP release [2].
References
1. Sawada K et al (2008) Proc Natl Acad Sci USA 105:5683–5686
2. Kato Y et al (2013) Biol Pharm Bull 36:1688–1691
E 087
Expression and localization of VNUT in intestinal L cells
Yuika Harada* and Miki Hiasa
Okayama University, Graduate School of Medicine, Dentistry and Pharmaceutical Science, Okayama, Japan
Introduction: Nucleotides such as ATP are major chemical transmitters in purinergic chemical transmission and involved in a variety of physiological functions. Vesicular nucleotide transporter (VNUT) is a secretory vesicle protein that is responsible for vesicular storage and subsequent exocytosis of ATP [1]. Thus, we can ask when, where and how is secreted ATP using VNUT as a probe. In this study, we studied expressing of VNUT in intestinal L cell. Intestinal L cells are gastrointestinal endocrine cells that secrete glucagon-like peptide-1 (GLP-1), one of the proglucagon-derived peptides that maintain blood glucose homeostasis through GLP-1 receptor on pancreatic β cells.
Results and Discussion: Westernblotting and RT-PCR analysis indicated that VNUT was expressed in rodent intestine. Immunohistochemical microscopy indicated that VNUT is expressed in GLP-1 containing cells in rat ileum.
VNUT immunoreactivity is not co-localized with GLP-1, a marker for secretory granules, and synaptophysin, a marker for synaptic-like microvesicles (SLMVs). Similar results were obtained from GLUTag cells. Sucrose density gradient analysis in membrane fraction from GLUTag cells revealed that VNUT is presented in the light fraction, unlike secretory granules. These results demonstrate that intestinal L cells express VNUT in the unidentified organelles at light density fraction other than secretory granules or SLMVs, and suggest that L cells are purinergic in nature and secrete nucleotides independent of GLP-1 secretion.
Reference
1. Sawada et al (2008) Proc Natl Acad Sci USA 105:5683–5686
E 088
Divalent cation transport by vesicular nucleotide transporter
Takaaki Miyaji1,*, Keisuke Sawada2, Hiroshi Omote2 and Yoshinori Moriyama1,2
1Okayama University, Advanced Science Research Center, Okayama, Japan;2Okayama University Graduate School, Pharmaceutical Sciences, Okayama, Japan
The vesicular nucleotide transporter (VNUT, SLC17A9) is responsible for the vesicular storage and subsequent exocytosis of ATP in purinergic chemical transmission. Previous biochemical analysis indicated that VNUT actively transports nucleotides, including ATP, ADP and GTP, in a membrane potential (Δψ)- and Cl−-dependent manner irrespective of divalent cations such as Mg2+ and Ca2+. Although the fact indicated that VNUT recognizes free ATP as a transport substrate, it is unknown whether VNUT transports chelating complexes with divalent cations. Here, we report that proteoliposomes containing purified VNUT actively took up Mg2+ and Ca2+ in the presence of ATP upon imposing Δψ but not ΔpH. The Δψ-driven Ca2+ uptake required ATP and a millimolar concentration of Cl−, which was inhibited by Evans blue, a specific inhibitor of SLC17-type transporters. The VNUT mutant possessing R119A, which is the essential amino acid residue of the SLC17 family, lost both ATP and Ca2+ transport activities. Kinetic analysis indicated that Ca2+ or Mg2+ did not affect the apparent affinity for ATP. RNAi of the VNUT gene in PC12 cells decreased the vesicular Mg2+ content as well as ATP. These results indicate that VNUT transports both nucleotides and divalent cations as chelating complexes and suggest that VNUT functions as a divalent cation importer in secretory vesicles under physiological conditions.
References
1. Proc Natl Acad Sci USA 105:5683–5686 (2008)
2. J Biol Chem 286:42881–42887 (2011)
E 089
Pannexin1: a tale of two channels
Gerhard Dahl1, Junjie Wang1,*, Cinzia Ambrosi2, David Jackson1 and Gina Sosinsky2
1University of Miami, Physiology and Biophysics, Miami, USA;2University of California, Neuroscience, San Diego, USA
There is abundant evidence that the Pannexin1 (Panx1) protein forms a large ATP release channel. However, this view has been challenged by the observation of a low conductance, small anion selective channel associated with Panx1 expression. We found that within the same cellular environment, Panx1 membrane channels can function in two distinct modes with different conductances and permeabilities. Panx1 formed a high conductance channel of ~500 pS and permeability to ATP when stimulated by potassium ions. Various physiological stimuli can induce this ATP permeable conformation of the channel. In contrast, exclusive activation of the channel by voltage led to a low conductance (50 pS) channel with no detectable ATP permeability. The two channel conformations were associated with different reactivities of the terminal cysteine of Panx1 to thiol reagents. Single particle electron microscopic analysis revealed a larger pore diameter induced by potassium stimulation. These data suggest that different stimuli lead to disparate channel structures with distinct functions.
E 090
NPT homologue (SLC17A4) is an intestinal urate exporter
Natsuko Togawa1,*, Takaaki Miyaji2, Hiroshi Omote1 and Yoshinori Moriyama1,2
1Okayama University, Graduate School of Medicine, Dentistry and Pharmaceutical Science, Okayama, Japan;2Okayama University, Advanced Science Research Center, Okayama, Japan
Vesicular nucleotide transporter (VNUT), the 9th member of SLC17 type transporters, is responsible for vesicular storage of nucleotides in neuronal synaptic vesicles and secretory granules in the neuroendocrine cells. Function of other members except NPT homologue (SLC17A4) and NPT3 (SLC17A2) are well known. NPT1 (SLC17A1) and NPT4 (SLC17A3) act as exporters of urate, which is the end product of purine metabolism in human. Function of two other members remain to be solved. Here, we investigated the expression and transport function of NPT homologue.
Immunohistochemical experiments using specific antibodies indicated the presence of NPT homologue at brush border membrane of small intestine. Proteoliposomes containing the purified protein took up radiolabeled p-aminohippuric acid and urate in a membrane potential driven. The urate uptake activity required Cl− and was inhibited by a low concentration of DIDS. These transport properties are similar to those of ATP uptake by VNUT.
We concluded that NPT homologue is an intestinal urate exporter. Since NPT1 and NPT4 are responsible for renal urate extrusion, our results reveal the possible involvement of NPT homologue in urate extrusion from the intestinal duct.
F: Purine metabolism
F 091
Methionine and methionine sulfoxide treatment induces M1/classical macrophage polarization and modulates extracellular ATP metabolism
Lien Mapelli*, Tatiane Silva, Juliana Azambuja, Priscila Ramos, Fernanda Teixeira, Marta Gazal, Francielli stefanello, Roselia Sapavanello and Elizandra Braganhol
Ufpel, Capão do Leão, Brazil
Hypermethioninemia is an inborn error of metabolism characterized by a persistent increase of methionine (Met) levels and their metabolite methionine sulfoxide (MetO) which favors oxidative stress and tissue damage with inflammatory conditions (Mudd, 2011). Macrophages are key elements of inflammatory process, whereas depending on the microenvironmental stimulation they exhibit a pro-inflammatory (classical/M1) or an anti-inflammatory (alternative/M2) phenotype (Martinez and Gordon, 2010). Extracellular ATP and adenosine are well known components of immune response and inflammation and changes in ectonucleotidase activities were described to modulate the macrophage spectrum activation by controlling the extracellular nucleotide/nucleoside levels (Zanin et al., 2012). Here we evaluated whether Met and/or MetO treatment modulates the polarization and the extracellular ATP metabolism of mouse macrophage cultures. Macrophages were collected by lavage of swiss mice (6–8 weeks) peritoneal cavity and treated for 24 h with Met (1 mM), MetO (0.5 mM) or Met (1 mM)/MetO (0.5 mM) in combination. Macrophages exposed to LPS (10 ng/mL) or IL-4 (10 ng/mL) for 24 h were applied as positive controls for classical/M1 and alternative/M2 macrophage phenotypes, respectively. Nitrite concentrations were measured using the Greiss reaction (Stuehr and Nathan, 1989) and arginase activity was determined according to Corraliza et al. (1994). Oxidative stress parameters were analyzed by evaluating the superoxide dismutase (SOD), glutathione peroxidase (GPx) and catalase activities as described in Gazal et al. (2014). The ectonucleotidase activities were determined by the malachite green method (Chan et al., 1986). The results indicate that the treatment with Met, MetO and Met/MetO in combination promoted an overall increase by 3.0 times of iNOS activity when compared to control. In contrast, arginase activity remained unchanged, which suggest that the Met, MetO and Met/MetO exposition induced a M1/classical macrophage polarization. Moreover, the treatments promoted a parallel increase by 5.0 and 6.0 times of SOD and catalase activities, respectively. Finally, Met, MetO and Met/MetO treatments resulted in an increase by 1.5 and 1.8 times of ATP and ADP hydrolysis, respectively, while AMPase activity remained unchanged. These data indicate that the classical/M1 macrophage polarization induced by Met, MetO and Met/MetO treatment can contribute to tissue damage observed in hypermethioninemia patients, and the alterations observed in stress oxidative parameters and purinergic signaling concur with that condition.
F 092
Evidence for purinergic system involvement in the release of IL-6, IL-10 and MCP-1 by macrophages exposed to glioma-conditioned medium
Letícia Scussel Bergamin1,*, Elizandra Braganhol2, Fabiana Manica1, Fabrício Figueiró1, Emerson Casali3, Rafael Fernandes Zanin4, Jean Sévigny5 and Ana Maria Oliveira Battastini1
1Universidade Federal do Rio Grande do Sul, Departamento de Bioquímica, Porto Alegre, Brazil;2Universidade Federal de Pelotas, Centro de Ciências Químicas, Farmacêuticas e de Alimentos, Pelotas, Brazil;3Universidade Federal do Rio Grande do Sul, Departamento de Ciências Morfológicas, Porto Alegre, Brazil;4Pontifícia Universidade Católica do Rio Grande do Sul, Instituto de Toxicologia e Farmacologia, Porto Alegre, Brazil;5Université Laval & Centre de recherche du CHU de Québec, Département de microbiologie-infectiologie et d’immunologie, Québec, Canada
Gliomas are the most common brain tumors. The presence of immune cells in the glioma microenvironment is an essential component of tumor cell proliferation and survival. Distinct macrophage populations have been related to cancer inhibition or progression and exhibit a spectrum of activation ranging from M1/pro-inflammatory to M2/anti-inflammatory phenotypes. Extracellular nucleotides modulate a variety of biological actions via purinergic receptors. ATP increases the release of cytokines, which is important for macrophages and neutrophils recruitment to tumor sites. Adenosine (ADO) favors tumor progression. Here, we investigated whether P2X7 and A2A receptors are involved in IL-6, MCP-1 and IL-10 secretion by macrophages exposed to glioma-conditioned medium. Glioma-conditioned medium (GL-CM) was prepared by seeding GL261 cell line in six multiwell plates. Subconfluent cultures were exposed to fresh DMEM/10 % FBS and the GL-CM were collected following 24 h. Primary macrophages were isolated from mouse peritoneal cavity and exposed to DMEM/10 % FBS (control; Mo) or GL-CM for 24 h (Mo-CM). Macrophage phenotype was evaluated by arginase and iNOS activities and by IL-10, TNF-α and IL-12 production. The involvement of ATP and ADO on IL-10, IL-6 e MCP-1 release was performed by CBA method in the presence or absence of ATP (1 mM), ADO (100 μM), A740003 (antagonist selective to P2X7; 10 μM), caffeine (general P1 antagonist; 30 μM and 100 μM) and SCH58261 (antagonist selective to A2AR; 50nM). GL-CM did not alter the release of IL-6 by Mo, however the addition of ATP promoted an increase in the IL-6 release, which was prevented by P2X7 antagonist. The release of MCP-1 was increased in Mo exposed to GL-CM, and the addition of ATP did not promote a further increase in MCP-1, but the P2X7 antagonist decreased the liberation of MCP-1. The production of MCP-1 by Mo-CM was further increased by ADO treatment, which was prevented by P1 antagonists. The release of IL-10 by Mo-CM was increased and the addition of ATP caused an additional increase in IL-10 release. The addition of P2X7 antagonist prevented the ATP-stimulated increase of IL-10. ADO treatment did not cause any additional increase in the IL-10 release by Mo-CM. Moreover, treatment with P1 antagonists reversed the secretion of this cytokine. In conclusion, macrophages exposed to GL-CM showed an M2-like phenotype, providing further evidence for the involvement of the purinergic system in the inflammatory process associated with tumor progression. Supported by: CAPES, CNPq, FAPERGS, FIPE-HCPA, CIHR
F 093
Direct signaling between astrocytes and glioma cells increases iNOS and ectonucleotidase activities
Carlus Augustu do Couto*, Juliana H. Azambuja, Gabriela N. Debom, Fernanda Cardoso Teixeira, Roselia M. Spanevello and Elizandra Braganhol
1Universidade Federal de Pelotas, Centro de Ciências Químicas, Farmacêuticas e de Alimentos, Capão do Leão, Brazil
Glioblastoma multiforme (GBM), the most common and aggressive brain tumor diagnosed in adults, is characterized by neoplastic undifferentiated cells with high invasive and proliferative potential [2]. Increased activity of inducible nitric oxide synthase (iNOS) [3] and changes in purinergic signaling [1,4] have been reported as positive factors of GBM growth. Although the presence of a favorable microenvironment rich in growth factors and inflammatory mediators be related to tumor development, the cross-talk between glioma and astrocyte cells and its consequence on tumor progression is still poorly investigated. Therefore, the aim of the present work was to evaluate whether glioma cells or its conditioned-medium modulate the iNOS and ectonucleotidase activities in astrocyte cultures. To this end, astrocyte cultures were exposed to C6-conditioned medium or co-cultured with rat C6 glioma cells. To produce glioma conditioned medium (C6-CM), C6 cells were incubated in DMEM/10 % FBS for 24 h. Following incubation, the medium was collected centrifuged at 1,000g for 10 min and snap frozen until use. Cortical astrocytes were isolated from newborn Wistar rats in according to Gottfried et al. [1999]. Confluent astrocyte cultures were exposed to C6-CM or directly co-cultured with C6 cells for 24 and 48 h, respectively. After this period, the supernatant was collected for iNOS activity measurement applying Greiss reagent and ectonucleotidase activity was evaluated by malachite green method. C6-astrocyte co-culture conditions promoted a 12.4 times increase of iNOS activity as well as 2.3 and 1.4 times increase of ATP and AMP hydrolysis, respectively, when compared to C6 and astrocytes cultured isolated. In addition, the exposition of astrocytes to C6-CM increased by four times ADP hydrolysis, when compared to astrocytes exposed to DMEM/10 % FBS. These results support a relationship between glioma and astrocyte, suggesting induction of malignancy via iNOS activity stimulation and tumor immune suppression by adenosine generation in the tumor microenvironment.
References
1. Braganhol E, Morrone FB, Bernardi A, Huppes D, Meurer L, Edelweiss MI, Lenz G, Wink MR, Robson SC, Battastini AM (2009) Selective NTPDase2 expression modulates in vivo rat glioma growth. Cancer Science 100(8):1434–1442
2. Maher CO, Raffel C. Neurosurgical treatment of brain tumors in children. Ped Clinics North America 51(2):327–357
3. Yin LT, Fu YJ, Xu QL, Yang J, Liu ZL, Liang AH, Fan XJ et al (2007) Potential biochemical therapy of glioma cancer. Biochem Biophys 362:225–229
4. Wink MR, Lenz G, Braganhol E, Tamajusuku AS, Schwartsmann G, Sarkis JJ, Battastini AM (2003) Altered extracellular ATP, ADP and AMP catabolism in glioma cell lines. Cancer Letters 198(2):211–218
F 094
IMP–GMP specific cytosolic 5′-nucloetidase as mediator of cell resistance to anticancer chemotherapy
Cividini Federico1, Filoni Daniela1,2, Pesi Rossana1, Allegrini Simone2, Camici Marcella1 and Tozzi Maria Grazia1,*
1Dipartimento di Biologia, Unità di Biochimica, Università di Pisa, Via San Zeno 51, 56127, Pisa, Italy,2Dipartimento di Chimica e Farmacia, Università di Sassari, Via Muroni 23°, 07100, Sassari, Italy
Cell resistance to anticancer drugs is one of the major concerns determining a very poor outcome of therapies in several patients, which is very difficult to predict and counteract. Recently, a number of papers referring results obtained with cultured cells, demonstrated the involvement of type II cytosolic 5′-nucleotidase (cN-II) in the mechanisms of resistance to antitumoral drugs such as cytarabine, gemcitabine and fludarabine. In fact, hyper-expression of cN-II appeared to be associated to a decrease of cytotoxic effect exerted by these drugs in different cell models. Furthermore, cN-II is involved in drug resistance in patients affected by hematological malignancies influencing the clinical outcome. cN-II catalyses the hydrolysis of 6-hydroxypurine monophosphates and, to some extent, of pyrimidine monophosphates. Curiously, high cN-II expression strongly correlates with poor outcome of therapies utilizing analogs whose monophosphates are poor or to no extent substrates in in vitro tests. More recently, the expression of genetic variants of cN-II was found to drive chemical resistance both in acute lymphoblastic leukemia and in acute myeloid leukemia. Although the implication of cN-II expression and/or activity appears to be correlated with drug resistance and poor prognosis, the molecular mechanism by which cN-II mediates drug resistance is still to be unravelled. To address this important issue we utilized a human embryonic kidney cell model in which the expression and activity of cN-II could be modulated by external signals. Our results demonstrate that a hyper-expression of cN-II causes a drop of intracellular nucleoside triphosphate concentration that correlates with drug resistance.
F 095
Analysis of ecto-nucleotide pyrophosphatase/phosphodiesterase (E-NPP) activity in Neuro-2a neuroblastoma cells
Javier Gualix1,*, Rosa Gomez-Villafuertes1, Jesus Pintor2 and M. Teresa Miras-Portugal1
1Universidad Complutense de Madrid, Veterinary Faculty, Biochemistry, Madrid, Spain;2Universidad Complutense de Madrid, Faculty of Optics and Optometry, Biochemistry, Madrid, Spain
Diadenosine polyphosphates (ApnAs) comprise a group of compounds formed by two adenosine moieties linked by a phosphate chain of variable length. ApnAs fulfill with the requirements to be considered as signaling molecules in the CNS: they are co-stored with ATP and other neurotransmitters in storage granules of neural and neuroendocrine cells. The exocytotic release of these compounds permits them to interact with P2 receptors, both metabotropic and ionotropic. Moreover, ApnAs can also activate specific receptors termed dinucleotide receptors. Ap3A, Ap4A and Ap5A have been recently identified in microdialysis samples from the cerebellum of conscious freely moving rats by means of a HPLC method [1]. The concentrations of the ApnAs in the dyalisates are in the range that allows the activation of the presynaptic dinucleotide receptors. However, a possible interaction of these dinucleotides with other purinergic receptors cannot be ruled out. Extracellular actions of ApnAs are finished by ectonucleotidases that degrade these compounds yielding adenosine as the final product.
N2a cells display an ectoenzymatic hydrolytic activity able to degrade diadenosine polyphosphates. The ApnA-cleaving activity has been analyzed with the use of the fluorogenic compound BODIPY-FL-GTPγS. Hydrolysis of this dinucleotide analog showed a hyperbolic kinetic with a Km value of 4.9 ± 1.3 μM. Ap5A, Ap4A, Ap3A as well as AMP behaved as inhibitors of BODIPY-FL-GTPγS extracellular degradation. Ectoenzymatic activity shared the typical characteristics of the E-NPP family, as hydrolysis reached maximal activity at alkaline pH and was dependent on the presence of divalent cations, being strongly inhibited by EDTA and activated by Zn2+ ions. Both NPP1 and NPP3 isozymes are expressed in N2a cells, their expression levels substantially changing when cells differentiate into a neuronal-like phenotype. It is relevant to point the expression pattern of the NPP3 protein, whose levels were drastically reduced in the differentiated cells, being almost completely absent after 24 h of differentiation. Enzymatic activity assays carried out with differentiated N2a cells showed that NPP1 is the main isozyme involved in the extracellular degradation of dinucleotides in these cells.
Reference
1. Gualix J et al (2013) Presence of diadenosine polyphosphates in microdialysis samples from rat cerebellum in vivo: effect of 8023 mild hyperammonemia on their receptors. Purinergic Signal. doi:10.1007/s11302-013-9382-3
F 096
Epicardium-derived cells effectively degrade nucleotides to adenosine and tenascin-C—mediated inhibition of CD73 stimulates their migration
Julia Hesse*, Elisabeth Boden, Daniela Friebe, Bodo Steckel, Zhaoping Ding and Jürgen Schrader
Heinrich Heine University of Düsseldorf, Department of Molecular Cardiology, Düsseldorf, Germany
Epicardium-derived cells (EPDC) play a fundamental role in heart development, contributing to coronary vascular precursors, fibroblasts and cardiomyocytes. In the adult heart, EPDC can be reactivated in response to tissue injury like myocardial infarction (MI). EPDC are therefore considered as endogenous cell source with the potential to mediate cardiac regeneration after MI. EPDC are of mesenchymal origin and express high CD73. We thus explored the extracellular purine metabolism of EPDC and the impact of the matricellular protein tenascin-C (TNC) on CD73.
EPDC were purified from rat hearts 5 to 7 days after MI by an enzymatic procedure and thereafter analyzed in vitro. Extracellular purine metabolism was assessed by high performance liquid chromatography (HPLC). Expression of ATP and adenosine receptors was measured by quantitative RT-PCR. Migration of EPDC was assessed in a transwell assay.
Measurement of nucleotide degradation over time revealed that EPDC equally well degrade both extracellular ATP and NAD via AMP to adenosine. Thus, CD73 appears to be the critical bottleneck of both degradation pathways, leading to the formation of adenosine. Quantitative RT-PCR analysis showed that EPDC express a distinct set of both adenosine (A2A > A2B > A3 > A1) and ATP (P2X4 > P2X7 > P2X5; P2Y2 > P2Y4 > P2Y12) receptors.
CD73 is known to directly bind to proteins of the extracellular matrix, which in turn may modulate its enzymatic activity. The matricellular protein TNC is highly expressed during embryogenesis, but is absent from healthy adult tissues. However, its expression becomes reactivated after myocardial infarction. TNC expression therefore resembles the activation pattern of EPDC formed after MI. We found that EPDC showed strong TNC expression on their own, as measured by quantitative RT-PCR and immunofluorescence. We also found that TNC dose-dependently (2–20 μg/ml) inhibited the CD73 activity of EPDC. In addition, the CD73-TNC interaction strongly stimulated migration of EPDC in a transwell assay.
Together our data demonstrate that EPDC avidly degrade ATP and NAD via CD73 to adenosine. EPDC themselves produce TNC so that self-inhibition of CD73 by TNC may serve as a novel mechanism to stimulate migration of EPDC into the injured myocardium.
F 097
Exercise training prevents ecto-nucleotidases alterations in platelets and reduce cardiovascular risks in metabolic syndrome patients
Vera Morsch1,*, Caroline Curry Martins1, Andréia Machado Cardoso1, Diéssica Padilha Dalenogare1, Daniela Zanini1, Margarete Dulce Bagatini2 and Maria Rosa Chitolina Schetinger1
1University Federal of Santa Maria, Chemistry, Santa Maria, Brazil;2Federal University of Southern Boundary, Nursing Course, Chapecó, SC, Brazil
Metabolic Syndrome (MetS) is a condition characterized by a set of cardiovascular risks factors. The adenine nucleotides and nucleoside (ATP, ADP and adenosine) have been implicated in a great number of cardiovascular diseases. In addition, regular exercise training has shown beneficial effects in the cardiovascular diseases and on prevention of platelets ecto-nucleotidase alterations. Then, in this study we investigated the effect of physical exercise on platelet ecto-nucleotidase activities of patients with MetS and analyzed the prevalence of cardiovascular risks before and after an exercise intervention. We studied 38 MetS patients performing regular moderate aerobic resistance and muscle hypertrophy exercises for 15 weeks. Anthropometric measurements, lipid profile, glucose level, hydrolysis of adenine nucleotides in platelets, and platelet aggregation were determined pre and post the exercise intervention and in control group. MetS patients had a decrease in the cardiovascular risks at the end of the exercise intervention (P < 0.001). In addition, the hydrolysis of adenine nucleotides (ATP, ADP and AMP) in platelets of MetS patients was increased before exercise intervention, however this alteration in ecto-nucleotidase activities was prevented by training in MetS (P < 0.001). On the other hand, a decrease in the ADA activity and an increase in platelet aggregation were observed in MetS (P < 0.001, P < 0.05), but the exercise training was able to prevent these alterations. In conclusion, our results clearly indicated a protector action of moderate intensity exercise on nucleotides and nucleoside hydrolysis and on platelet aggregation, which highlights the exercise training effect to avoid MetS complications related to ecto-nucleotidase activities.
Acknowledgements The authors wish to thank Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Fundação de Amparo à Pesquisa do Rio Grande do Sul (FAPERGS), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), FINEP research grant “Rede Instituto Brasileiro de Neurociência (IBN-Net)” and “Instituto Nacional de Ciência e Tecnologia” (INCT) for financial support.
F 098
Lung cancer alters the hydrolysis of adenine nucleotides and nucleoside in human platelets and lymphocytes
Maria Schetinger1,*, Luana Pelinson1, Roberta Schmatz1, Andréia Cardoso1, Víctor Pimentel1, Maria do Carmo Araújo2, Liliane Oliveira2, Juarez Chiesa2, Daniela Leal1 and Vera Morsch1
1Federal University of Santa Maria, Deparment of Chemistry, Santa Maria, Brazil;2Hospital from the Federal University of Santa Maria, Hematology-Oncology, Santa Maria, Brazil
Recent studies have shown that lung cancer has been the most commonly diagnosed cancer as well as the main cause of cancer death in human beings worldwide. Patients affected by neoplastic diseases present a series of physiological changes, including the occurrence of thrombotic and inflammatory processes. The adenine nucleotides ATP, ADP and AMP and their corresponding nucleoside, adenosine, are signaling molecules related to thromboregulation, pro-inflammatory process and modulation of immune responses in patients with malignancies. Thus, these studies aimed to determine NTPDase activity in platelets and lymphocytes, 5′-nucleotidase and ectonucleotide pyrophosphatase/ phosphodiesterase (E-NPP) activities in platelets as well as adenosine deaminase (ADA) activity in the platelets and serum of patients with lung cancer. Blood samples were collected from patients (n = 31) previously treated for lung cancer with chemotherapy. Patients were classified as stage IIIb and IV according to the Union for International Cancer Control (UICC). Patients showed a significant decrease in the hydrolysis of adenosine diphosphate (ADP) and adenosine degradation in platelets (55 and 52 %, respectively), whereas the adenosine monophosphate (AMP) hydrolysis was significantly increased (38 %) in this group. The expression of CD 39 was 50 % lower in platelets of patients with lung cancer compared to the control group. Furthermore, the results showed a significant increase in the hydrolysis of ATP (40 %) and ADP (35 %) in lymphocytes and adenosine degradation (26 %) in serum in patients with lung cancer when compared with the control group. The percentage of CD39 positive cells in lymphocytes from lung cancer patients group was significantly increased (25 %) when compared with the control group. While analyzing these results it may be suggested that ectonucleotidases as well as ADA are enzymes involved in thromboembolic, inflammatory and immune processes. And especially in this study, where it can be observed that the adenine nucleotides and nucleoside are directly involved in the promotion and progression of lung cancer and its complications.
Acknowledgments The authors wish to thank all the lung cancer patients and the professionals at the Hematology/Oncology Laboratory (HUSM) and CNPq, FAPERGS and CAPES for their financial support.
F 099
Adenosine deaminase inhibition alterates glucose metabolism in a human astrocytoma cell line
Mercedes Garcia-Gil1, Maria Grazia Tozzi2, Stefano Varani 1, Lorenza Della Verde1, Edoardo Petrotto1, Francesco Balestri2, Laura Colombaioni3 and Marcella Camici2
1Dipartimento di Biologia, Unità Fisiologia Generale, Via S. Zeno 31, Pisa, Italy;2Dipartimento di Biologia, Unità Biochimica, Via S. Zeno 51, Pisa, Italy;3CNR, Istituto di Neuroscienze, Via Giuseppe Moruzzi 1, Pisa, Italy
Adenosine deaminase, which catalyzes the deamination of adenosine and deoxyadenosine, plays a central role in purine metabolism. Indeed, its deficiency is associated with severe immunodeficiency and abnormalities in the functioning of many organs, including nervous system. We have mimicked an adenosine deaminase-deficient situation by incubating a human astrocytoma cell line (ADF) in the presence of deoxycoformycin, a strong adenosine deaminase inhibitor, and deoxyadenosine, which accumulates in vivo when the enzyme is deficient. We have previously demonstrated that, after 15 h of treatment, this combination increases both mitochondrial reactive oxygen species and mitochondrial mass, induces apoptosis as indicated by cytochrome c release from mitochondria and activation of caspase-3. These events are preceded by reduction in lactacte release in the medium [1]. In this work we demonstrate that after 8 h of incubation with deoxyadenosine and deoxycoformycin, caspase-8 is activated, mitochondrial mass increases and mitochondrial reactive oxygen species decrease. The addition of baicalein to the incubation medium reduces cell death and caspase-3 activity induced by deoxycoformycin and deoxyadenosine in combination. This protective effect is correlated to an increase of lactate released in the medium, a decrease in the intracellular levels of dATP, and an increase in ATP levels, as compared to the cells subjected to the treatment with deoxycoformycin and deoxyadenosine in combination without any further addition. The effect of baicalein appears to be related to an inhibition of deoxyadenosine phosphorylation, rather than or in addition to the well known antioxidant activity of the compound.
Reference
1. Garcia-Gil M, Tozzi MG, Allegrini S, Folcarelli S, Della Sala G, Voccoli V, Colombaioni L, Camici M (2012) Neurochem Int 60:523–532
G: Purinergic signaling in the cardiovascular system
G 100
Adenosine signaling through the A2Badenosine receptor contributes to pathological remodeling following myocardial infarction
Jason Maas1, Tina Wan1, Shraddha Nayak1, Elizabeth Gizewski1, Robert Thompson2, Robert Figler3 and John Auchampach1,*
1Medical College of Wisconsin, Pharmacology, Milwaukee, USA;2Lewis and Clark Pharmaceuticals, Pharmacology, Charlottesville, USA;3University of Virginia, Molecular Physiology and Biological Physics, Charlottesville, USA
The interstitial concentration of adenosine is elevated during acute myocardial infarction (MI) in both the ischemic and non-ischemic regions, and remains elevated to a lesser extent as the heart undergoes remodeling and progresses to heart failure. The influence of adenosine signaling during pathological post-MI remodeling has not been investigated in detail. In this study, we observed that less interstitial fibrosis develops in regions of the myocardium remote from the infarcted area in mice subjected to 8 weeks of permanent coronary artery ligation when the A2B adenosine receptor (AR) subtype is genetically deleted or pharmacologically inhibited with the specific A2BAR antagonist ATL-801. In association with a reduction in fibrosis, the left ventricles in these mice remained more compliant and showed less impairment in diastolic performance. Administration of the A2BAR agonist BAY 60-6583 to wild-type mice, but not to A2B gene knock-out mice, increased expression of collagen transcripts in the myocardium, which was associated with increased production of interleukin-6 (IL-6). Further study identified abundant expression of the A2BAR in cardiac fibroblasts, and determined that activation of the A2BAR increases fibroblast production of IL-6 as well as the chemokine CXCL1. Taken together, these data suggest that adenosine functions as a fibrotic mediator in the heart during chronic disease states by signaling via the A2BAR, potentially by increasing production of IL-6 by fibroblasts. The A2BAR may present as a novel target to limit pathological remodeling following MI and thereby reduce the risk for progression to heart failure.
G 101
Assessment of cardioprotective efficacy of A3adenosine receptor agonists using mice with cardiac-specific deletion of the A3adenosine receptor gene
Tina Wan1, Akihito Tampo2 and Wai-Meng Kwok2 and John Auchampach1,*
1Medical College of Wisconsin, Pharmacology, Milwaukee, USA;2Medical College of Wisconsin, Anesthesiology, Milwaukee, USA
Treatment with selective A3 adenosine receptor (AR) agonists is effective at reducing myocardial ischemia/reperfusion injury, although the mechanism of protection remains unclear and is likely to be multifactorial. Since A3AR agonists protect aginst ischemic injury in isolated heart systems devoid of blood components and a complete inflammatory response, it has been speculated that A3ARs coupled to cardioprotective signaling pathways may be expressed in cardiac muscle. In this study, we generated a mouse line in which the A3AR gene (Adora3) was selectively deleted in cardiac myocytes (Adora3fl/fl/Myh6Cre+) by breeding a mouse line in which the first coding exon of Adora3 was flanked by loxP sites (Adora3fl/fl) with a mouse line carrying a Cre recombinase gene whose expression is driven by the murine α-myosin heavy chain gene (Myh6Cre) promoter. Adora3fl/fl mice were created from targeted ES cells using standard procedures. After generation of Adora3fl/fl/Myh6Cre+ mice and their respective littermate controls (Adora3fl/fl/Cre−), tolerance to ischemic injury was assessed using an isolated heart model of global ischemia/reperfusion injury. We observed that treatment with the A3AR agonist CP-532,903 (100 nM) significantly improved functional recovery (LV developed pressure, +dP/dt, -dP/dt) of hearts subjected to 20 min of global ischemia and 45 min of reperfusion isolated from Adora3fl/fl/Myh6Cre− mice where Adora3 remains undisturbed. The extent of protection provided by CP-532-903 was similar to that observed in experiments with wild-type C57Bl/6J control mice. In contrast, treatment with CP-532,903 provided no improvement in post-ischemic functional recovery of hearts obtained from Adora3fl/fl/Myh6Cre+ mice in which Adora3 is ablated in cardiomyocytes. In subsequent electrophysiological studies utilizing the whole-cell recording technique and myocytes isolated from global A1 and A3AR gene knock-out mice, direct evidence for coupling of both A1 and A3ARs to opening of KATP channels in cardiomyocytes was obtained. These results support the idea that benefit provided by A3AR agonists during myocardial ischemia/reperfusion injury occurs at least in part via a direct cardioprotective mechanism.
G 102
Purinergic receptor regulation in activated endothelial cells of obesity triggered early atherosclerosis
Patrick Babczyk*, Dorothee Schipper, Yu Zhang, Silvana Knapp, Anne Seifert and Edda Tobiasch
Department of Natural Sciences, University of Applied Science Bonn-Rhine-Sieg, von-Liebig –Str. 20, 53359 Rheinbach, Germany
During the process of gaining weight mesenchymal stem cells (MSC) are differentiated towards adipocytes and thus secrete adipokines influencing the endothelial cell (EC) layer within blood vessels. EC activation due to a variety of stimuli such as e.g. cytokines is a pivotal part in the development of early atherosclerosis and can be evaluated by EC migration. However the underlying molecular mechanisms of this process have not yet been fully understood. Purinergic receptors are known to play a crucial role in vascular tone modulation. They are also important in endothelial cell regulation and therefore might be involved in EC migration as well.
The migration of primary bovine aortic ECs (BAEC) was investigated under the influence of conditioned medium collected from MSC differentiating towards adipocytes. Medium collected in week three of adipogenic differentiation had the highest impact on BAEC migration. Interestingly P2X4 receptor gene expression was upregulated under the same conditions in activated BAECs.
Taken together P2X4 seems to play a role in EC activation during the development of early atherosclerosis which is the underlying cause of cardiovascular diseases accounting for the majority of deaths in industrialised countries. Therefore P2X4 is an interesting candidate for further research with respect to future drug development.
G 103
P2X4 receptors regulate shear stress- and ATP-induced atheroprotective gene expression in vascular endothelial cells
Ramasri Sathanoori1,*, Federica Rosi2, Ben Gu3, James Wiley3, Christa E. Müller2, Björn Olde1 and David Erlinge1
1Lund University, Cardiology, Lund, Sweden;2University of Bonn, Pharmaceutical Institute, Bonn, Germany;3University of Melbourne, Florey Neuroscience Institutes, Melbourne, Australia
Vascular endothelial cells are in direct contact with blood flow and are exposed to fluid shear stress. The role of P2X4 receptors in shear stress-mediated mechanotransduction is well documented [1]. Studies have shown endothelial cells to release ATP in response to shear stress modulating cellular functions via vascular P2 receptors [1, 2]. In fact, a recent study shows that the inheritance of a loss-of-function polymorphism (rs28360472) resulting in a Tyr315 > Cys mutation in the human P2X4 receptor is associated with increased pulse pressure and impaired endothelium-dependent vasodilation [3]. However, the molecular mechanisms governing ATP-mediated P2 receptor signaling downstream of shear stress in endothelial cells remains largely unknown. Although shear stress-induced KLF2 expression implicated in atheroprotection is well studied [4], whether ATP regulates KLF2 remains unanswered and is the aim of this study.
At the outset, we validated the in vitro orbital shaker model (5, 6) for shear stress (10 dyn/cm2) using human umbilical vein endothelial cells (HUVECs) showing cell alignment in the direction of flow and upregulation of KLF2 and NOS3. Next, exposing HUVECs to apyrase and the selective P2X4 antagonist (PSB-12253) inhibited KLF2 and NOS3 expression under shear stress. Furthermore, the siRNA knockdown of P2X4 implicated its role in regulating shear stress-induced KLF2. The exogenous administration of a non-hydrolysable analog of ATP (ATPγS) to static cultures led to an increase in KLF2 mRNA as early as 3 h, which was inhibited by antagonizing and knocking down P2X4. Moreover, transient transfection of static HUVEC cultures with the P2X4 mutant construct (Y315C) blocked ATP-mediated KLF2 expression and its targets. Collectively, these results suggest that shear stress-induced KLF2 is dependent on ATP released by endothelial cells acting via the P2X4 receptor.
References
1. Yamamoto K et al (2006) Nature Med 12(1):133–137
2. Bodin P et al (1991) British J Pharmacol 103(1):1203–1205
3. Stokes L et al (2011) Hypertension 58(6):1086–1092
4. Atkins GB, Jain MK (2007) Circ Res 100(12):1686–1695
5. Dardik A et al (2005) J Vasc Surg 41(5):869–880
6. dela Paz NG (2011) J Cell Sci 125(0):1–13
G 104
Activation of cardiac P2X4 receptors reduces heart rate by driving the Na+/Ca2+exchanger in the reverse mode while keeping the ventricular function
Bruno Bragança*, Sílvia Nogueira-Marques, Tânia Rodrigues, Nádia Oliveira-Monteiro, Ana Patrícia Fontes-Sousa and Paulo Correia-de-Sá
Instituto de Ciências Biomédicas Abel Salazar (ICBAS), Universidade do Porto, Laboratório de Farmacologia e Neurobiologia / UMIB e MedInUP, Porto, Portugal
Question: The sinoatrial node (SAN) is a heterogeneous structure that sets cardiac rhythm, being under neurohormonal control. ATP released near the SAN produces a global decrease of heart rate (HR) through mechanisms not completely elucidated. Decreasing SAN activity and, therefore, the cardiac work load is desirable in many cardiovascular diseases. Ivabridine, a pacemaker channel inhibitor, remains the only drug available to specifically decrease HR, without altering other cardiac parameters. Interestingly, the P2X4R receptor has a cardioprotective role, being also upregulated in SAN from animals with heart failure, but its effects on HR have been overlooked.
Methods: Experiments were performed on isolated spontaneously beating right atria and on 2 Hz-paced right ventricle strips from Wistar rats superfused with gassed Tyrode’s solution. Isometric muscle tension was monitored with a PowerLab data acquisition system.
Results: On spontaneously beating rat atria, ATP (100 μM) and ATPγS (100 μM) caused a rapid and sustained decrease of HR. This effect of ATP was potentiated by POM-1 (100 μM), a ecto-NTPDase inhibitor. Selective blockade of P2X4 with 5′-BDBD (10 μM) prevented the negative chronotropic effect of ATP. Moreover, stimulation of P2X4R with CTP (1 mM) produced a rapid decrease of HR. Inhibition of Na+ efflux through the Na+/Ca2+ exchanger (NCX) with KB-R7943 (3 μM) also prevented the negative chronotropic response of ATP. ATP (100 μM) produced a mild decrease in the magnitude of paced ventricular tension, which was further potentiated (approximately by 2-fold) in the presence of 5′-BDBD (10 μM); KB-R7943 (3 μM) mimicked the effect of the P2X4 antagonist.
Conclusions: Data suggest that P2X4R activation decreases the SAN rate while increasing the ventricular contractile force. The mechanism underlying these dual effects of ATP may involve bolstering of the NCX function in the reverse mode. Na+ influx via P2X4 might inhibit/revert the electrogenic forward mode of the NCX decreasing chronotropy. Likewise, intracellular Ca2+ accumulation due to inhibition and/or reversal of the NCX might explain the positive inotropic effect due to P2X4 activation on paced right ventricular strips. Thus, drugs targeting the P2X4 activation or underlying NCX-based mechanisms may afford cardioprotection by slowing down HR without compromising the ventricular function.
G 105
P2 purinoceptors activation shift in patients with diabetic erectile dysfunction
Miguel Faria1, Maria Alexandrina Timóteo1, José Maria LaFuente-de-Carvalho1,2 and Paulo Correia-de-Sá1,*
1ICBAS - University of Porto, Lab. Farmacologia e Neurobiologia, UMIB, Porto, Portugal;2Serviço de Urologia, Centro Hospitalar do Porto, Porto, Portugal
ATP is a potent relaxant agent in the human corpus cavernosum (HCC) acting either directly, through P2 receptors located on endothelium and smooth muscle fibers, or indirectly, via adenosine generated by ecto-nucleotidases (Faria et al., 2008, Nucleos. Nucleot. Nucleic Acids27: 761–768). Diabetes mellitus causes systemic cardiovascular unbalance with a high prevalence of drug-resistant erectile dysfunction in men. This unmet therapeutic need lead us to investigate the role of purines acting via P2 purinoceptors responsible for relaxation of the HCC in samples collected from organ donors (control group) and from impotent men with Diabetes mellitus submitted to surgery for penile prosthesis implantation.
In the control group, the rank potency order for relaxation of HCC strips pre-contracted with 1 μM phenylephrine (PE) was as follows (IC50 μM): ADP(30) ~ ADPbetaS(30) > ATPgammaS(100) ~ 2-MeSATP(100) > ATP(300). In diabetic patients, relaxation of HCC strips by adenine nucleotide analogues, ADPbetaS (30 μM) and ATPgammaS (30 μM), was decreased to a maximum of 20 %. The concentration-response curve of ADP (0.3–3,000 μM) was significantly (P < 0.05) shifted to the right in the presence of the selective P2Y12 receptor antagonist, MRS 2395 (10 μM), yet this effect only occurred in control but not in diabetic tissues. Selective blockade of P2Y1 (with MRS 2179, 0.3 μM) and P2Y13 (with MRS 2211, 10 μM) receptors were virtually ineffective in control individuals, but these drugs attenuated relaxation caused by both ADP and ADPbetaS in diabetics.
Data indicate that relaxation of the HCC by P2 purinoceptor agonists is severely attenuated in impotent men with Diabetes mellitus. Relaxation of the HCC from control subjects may be mediated by ADP-sensitive P2Y12 receptors, while we observed a yet unexplained shift towards the activation of P2Y1 (and perhaps P2Y13) receptors in diabetics.
Work supported by FCT (Pest-OE/SAU/UI215/2014), Univ. Porto/Santander Totta and Soc. Port. Andrologia.
G 106
UTP signaling in cardiac myocytes
Ulrich Gergs1,*, Daniel Rothkirch1, Stefan Dhein2, Christa E. Müller3, Bernard Robaye4 and Joachim Neumann1
1Institute for Pharmacology and Toxicology, Medical Faculty, Martin-Luther-University Halle-Wittenberg, Halle/Saale, Germany;2Clinic for Cardiac Surgery, Heart Centre, University of Leipzig, Leipzig, Germany;3Pharmaceutical Institute, Pharmaceutical ChemistryI, University of Bonn, Bonn, Germany;4Institute of Interdisciplinary Research in human and molecular Biology, Charleroi, Belgium
Extracellular UTP can be released from the mammalian heart during pathological conditions such as ischemia, hypoxia or reperfusion. In humans, UTP levels are increased during myocardial infarction. UTP is supposed to act mainly via P2Y2 and P2Y4 purinoceptors and to lesser extent via P2Y6 receptors. We previously demonstrated that UTP can induce inotropic effects in cardiac preparations of mice and man. The aim of the present work was to study the signal transduction pathway involved. Therefore, we studied the effects of UTP on p38 MAPK and ERK1/2 phosphorylation in isolated adult mouse cardiac myocytes, in neonatal rat cardiac myocytes and in human atrial tissue using phosphorylation-specific antibodies. UTP increased time and concentration-dependently phosphorylation of ERK1/2 and p38 up to 300 %. The maximum effect was reached 5 to 10 min after application of UTP in cardiac myocytes. Moreover, 100 μM UTP increased ERK1/2 and p38 MAPK-phosphorylation in isolated human atrial tissue obtained by bypass surgery. In adult mouse cardiac myocytes, the UTP-induced phosphorylations were blocked by the P2 purinoceptor antagonist PPADS (Pyridoxalphosphate-6-azophenyl-2′,4′-disulfonic acid) but not by the adenosine receptor antagonist DPCPX (8-Cyclopentyl-1,3-dipropylxanthine), the P2 purinoceptor antagonist reactive blue, the adenylyl cyclase inhibitor SQ22536 (9-(Tetrahydro-2-furanyl)-9H-purin-6-amine), or the phospholipase C inhibitor U73122 (1-[6-((17β-3-Methoxyestra-1,3,5(10)-trien-17-yl)amino)hexyl]-1H-pyrrole-2,5-dione). In contrast, phosphorylation of ERK1/2 (not p38) in neonatal rat cardiac myocytes was decreased by reactive blue and suramine but not by PPADS. Interestingly, the inotropic effects of UTP were sensitive to SQ22536 and U73122 in mouse but not in human preparations. Additionally, in atrial preparations from P2Y2 knockout mice, the positive inotropic effect of UTP was still present compared to wild type (p < 0.05 vs. control, n = 5). Moreover, a selective P2Y2 antagonist (AR-C118925) did not suppress the inotropic effect of UTP in wild type atria, supposing a P2Y4-dependent pathway in mice. In summary, not only in animal cardiac myocytes but also in human cardiac tissue, UTP induced a transient phosphorylation of MAP kinases. Interestingly, the inotropic effects lasted much longer than MAPK phosphorylation. Moreover, signaling pathways seem to be species dependent. This indicates that further downstream phosphorylations are involved in the sustained positive inotropic effect and that additional studies are necessary to elucidate the subsequent signaling pathways.
G 107
Characterization of the contractile P2Y14receptor in mouse coronary and cerebral arteries
Kristian Agmund Haanes* and Lars Edvinsson
Copenhagen University Hospital, Clinical Experimental Research, Glostrup, Denmark
Nucleotides have a well-established physiological role in vascular smooth muscle cells, where the purinergic receptors can modulate both contraction and relaxation. The recently characterized purinergic receptor, P2Y14 can be activated by extracellular UDP-glucose. The present study was designed to examine the physiological importance of the P2Y14 receptor in the vasculature, specifically in the brain (basilar artery) and heart (left anterior descending artery). Since UDP-glucose has been shown to also activate P2Y2 receptors, experiments were performed on wild type (WT) and on P2Y2 receptor knock out (KO) mice
Our study shows that UDP-glucose causes contraction in mouse coronary and basilar arteries. There was no significant difference between the WT and P2Y2 receptor KO arteries, suggesting minor influence of UDP-glucose binding to the P2Y2 receptor. The EC50 values and immunohistochemistry illustrated strongest P2Y14 receptor expression in the basilar artery. The relation between endothelial function and Emax values for UDP-glucose contraction was investigated but there was no relation between the amount of functional endothelium and the UDP-glucose induced contraction in these experiments.
Pertussis toxin was used to inhibit Gi proteins. In the presence of pertussis toxin, UDP-glucose inhibited contraction in coronary arteries and in the basilar artery it surprisingly caused relaxation.
We have previously revealed that endothelin receptors, in particular the ETB receptor is upregulated in the coronary artery downstream of an induced heart attack, and that similar effects can be seen after organ culture for 24 h. After 24 h of incubation of the coronary arteries, we observed a clear leftward shift in the concentration-response curve. Increased staining for the P2Y14 receptor was also observed, showing receptor plasticity.
In conclusion, we have demonstrated that UDP-glucose can induce endothelium independent contraction through the P2Y14 receptor in both mouse coronary and basilar arteries. After 24 h of organ culture, P2Y14 is upregulated.
G 108
Endothelial cell dysfunction in pulmonary artery hypertension is manifested with attenuated CD39/ENTPD1 activity in remodeled vessels
Mikko Helenius1,*, Sanna Vattulainen1, Joonas Aho2, Anne Komulainen1, Pekka Taimen3, Vinicio A. de Jesus Perez4, Mark Orcholski4, Juha W. Koskenvuo2,5 and Tero-Pekka Alastalo1
1University of Helsinki and Helsinki University Central Hospital, Children’s Hospital Helsinki, Pediatric Cardiology, Helsinki, Finland;2University of Turku, Research Centre of Applied and Preventive Cardiovascular Medicine, Turku, Finland;3University of Turku and Turku University Hospital, Department of Pathology, Turku, Finland;4Stanford University, Division of Pulmonary and Critical Care Medicine, Stanford, California, USA;5Turku University Hospital, Department of Clinical Physiology and Nuclear Medicine, Turku, Finland
Occlusive intimal hyperplasia in pulmonary arterial hypertension (PAH) leads to devastating consequences in pulmonary circulation and contributes to accompanying high morbidity and mortality. A phenotypic switch in pulmonary endothelial cells (EC) has been suggested to play an important role in the pathobiology of occlusive vasculopathy. We hypothesized that purinergic signaling that modulates cell metabolism and phenotype, could play a role in regulating EC fate during PAH progression. Ecto-enzymes such as CD39 and CD73 play an essential role in regulating the level of extracellular purine nucleotides.
With a magnet bead-based ex vivo EC profiling strategy we identified a 45 % decrease (p < 0.001) in ATP-hydrolyzing CD39 activity in rat monocrotaline model of PAH. Similarly, CD39 activity was decreased 60 % (p < 0.05) in cultured pulmonary ECs from an idiopathic PAH (iPAH) patient. Attenuated CD39 activity was partly mediated through down-regulation of protein expression. Immunohistochemistry on human iPAH patient lung confirmed that CD39 was significantly down-regulated in the endothelium of small arteries with intimal and media disease. Suppression of CD39 in vitro, leading to increased ATP microenvironment, initiated an EC phenotypic switch with apoptosis resistant and hyperproliferative phenotype, similar to iPAH ECs.
In addition to changes in EC phenotype increased ATP niche stimulates pulmonary artery smooth muscle cell proliferation (50 %, p < 0.005) and migration (50 %, p < 0.05) and, thus, could also mediate SMC pathology in PAH. Furthermore, attenuated CD39 activity in ECs could be at least partly restored with apelin in vitro and thereby suggests a novel mechanism for apelin-mediated responses. In search for relevant receptor for the observed ATP-mediated cell proliferation and survival, we identify that expression of EC surface ATP receptor P2Y11 is essential for ATP-mediated EC proliferation and survival. We conclude that sustained attenuation of CD39 activity is tightly linked to vascular dysfunction and remodeling in PAH and could represent a novel target for therapy (Fig. 1).
G 109
Markers of purinergic signaling give new insight on vascular inflammation, thrombosis, tobacco smoke and peripheral artery disease
Juho Jalkanen1,*, Gennady Yegutkin2, Maija Hollmen3, Sirpa Jalkanen2 and Harri Hakovirta1
1Turku University Hospital, Vascular Surgery, Turku, Finland;2Turku University, Microbiology and Immunology, Turku, Finland;3ETH Zürich, Pharmaceutical Sciences, Zürich, Switzerland
Question: Atherosclerosis is an inflammatory process. Although purinergic signaling plays an important role in inflammation and vascular integrity, little is known about purinergic mechanisms involved in the pathogenesis of atherosclerosis in humans. Our aim was to study markers of purinergic signaling in a cohort of patients with peripheral artery disease (PAD).
Methods and Results: Plasma ATP and ADP levels and serum nucleoside triphosphate diphosphohydrolase-1 (NTPDase1/CD39) and ecto-5′-nucleotidase/CD73 activities were measured in 226 patients with a stable state of PAD who came for non-urgent invasive imaging and/or treatment. The major findings were that ATP, ADP, CD39, CD73 tended to be higher in atherosclerotic patients than in healthy controls. CD39 was significantly elevated amongst young patients with proximal (aorta-iliac) PAD related to smoking (P = 0.003) while high CD73 was related to severe COPD and chronic hypoxia (p
Conclusions: Elevated levels of circulating ATP and ADP may be considered as risk factors accounting for cardiovascular diseases, especially for atherosclerotic diseases related to younger age and smoking. The prothrombotic nature of tobacco smoke leads to elevated ADP and this may explain why smoking cardiovascular patients benefit from platelet P2Y12 receptor antagonists more than their non-smoking peers.
Fig. 1 Plasma ATP, ADP and CD39 by age group. ATP, ADP and CD39 are significantly higher in younger age groups, especially in men, and steadily decline by age. Statistical significance (p-value) between age group compared against healthy controls calculated using the Wilcoxon rank sum test* for non-parametric data, and Student’s t-test** for normally distributed data. In the box plots the line represents median and the diamond represents the mean
Fig. 2 Plasma ATP, ADP and CD39 expression by distribution of PAD. ATP, ADP and CD39 are higher in patients with proximal peripheral artery disease and tend to be more close to “normal” in a distal disease. Statistical significance (p-value) between PAD distribution group and healthy controls calculated using the Wilcoxon rank sum test* for non-parametric data, and Student’s t-test** for normally distributed data. In the box plots the line represents median and the diamond represents mean
Fig. 3 The relationship of CD39 with smoking status. CD39 steadily increases with the activity of smoking. Each category is compared against the healthy control group using the Wilcoxon rank sum test* for non-parametric data, and Student’s t-test** for normally distributed data. In the box plots the line represents median and the diamond represents mean
G 110
Signaling by extracellular nucleotides in resistance arteries—focus on myogenic tone
Gilles Kauffenstein1,*, Linda Grimaud1, Audrey Ayer1, Bertrand Toutain1, Laurent Loufrani1, Marie Billaud2, Brant Isakson2, Jean Sévigny3, Jean-Marie Boeynaems4, Bernard Robaye4 and Daniel Henrion1
1Angers University, UMR CNRS 6214 INSERM U1083, Angers, France;2University of Virginia, Cardiovascular research Center, Charlottesville, US;3Laval University, CRCHUL, Québec, Canada;4Université libre de Bruxelles, IRIBHM, Gosselies, Belgium
Extracellular nucleotides promote vascular constriction through cell membrane P2 receptors. This effect involves neurogenic activation of vascular smooth muscle cell P2X1 (ATP) and some pyrimidine-sensitive (UDP, UTP) P2Y receptors. We used knockout mouse models to unravel the role of extracellular nucleotides in resistance arteries myogenic tone (Bayliss effect).
The contractile effects of exogenous UDP and UTP are abrogated in P2ry6−/− arteries suggesting that P2Y6 receptor fully underlies vascular contraction to uracyl nucleotides in mouse. Moreover, the deletion of nucleoside triphosphate diphosphohydrolase-1 (NTPDase1 or CD39), the dominant ectoenzyme hydrolysing nucleotides at the smooth muscle surface (Entpd1−/− mice), unmasks potent constrictor effect of UDP and UTP in conductance (thoracic aorta) and resistance (mesenteric) arteries. Mirroring these observations, myogenic tone (responsive contraction to pressure increase) was diminished in P2ry6−/− while it was exaggerated in Entpd1−/− resistance arteries. This suggests that stretch-induced release of extracellular nucleotides occurs and participates autocrine P2Y6 receptor activation and vascular contraction. Involvement of hemichannel in this release is likely and was corroborated by pharmacological approach as well as dye uptake experiments.
We propose that this signaling pathway, which contributes to resistance arteries autoregulation, also participates to blood pressure increase in pathological conditions since P2ry6−/− mice are resistant to experimental hypertension.
G 111
Adenosine receptors transcriptomic profiling in leukocytes and cardiac fibroblast of patients with valvular disease undergoing prosthetic implantation
Manuela Cabiati1, Veronica Della Latta1,2,*, Stefania Zimbone3, M Natale3, Daniela Gentile3, Francesco Diciolla4, PierLeopoldo Capecchi3, Franco LaghiPasini3, Maria Aurora Morales1 and Silvia Del Ry1
1Institute of clinical Physiology, CNR, Pisa, Italy;2University of Siena, Siena, Italy;3University of Siena, Department of Medical Sciences, Surgery and Neurosciences, Siena, Italy;4University of Siena, Department of Heart, Vessels and Thorax, Siena, Italy
Beckground: Adenosine is a potent extracellular messenger produced in high concentrations under metabolically unfavourable conditions. It restores tissue homeostasis through the interaction with its G-protein coupled membrane receptors (A1R, A2aR, A2bR and A3R), all with a cardioprotective role. ARs are expressed on multiple cardiac cells, including fibroblasts, endothelial cells, smooth muscle cells and leukocytes however the modulation of these receptors is still not fully understood. Aim of this study was to assess changes of transcriptomic profiling of A1R, A2aR, A2bR and A3R in human leukocytes of patients with HF due to valvular disease (HF-V) as compared to healthy subjects (C) and in cardiac fibroblast (CF) obtained by human atrium (auricle) fragments of patients undergoing cardiac surgery for prosthetic implantation. A human CF atrial cell line (NHCF-A) was used as control (cc).
Methods: Total RNA was extracted from leukocytes of C (n = 7) and of Vlv pts (n = 6, NYHA III-IV) with PAXgene Blood RNA Kit andby acid guanidinium thiocyanate-phenol-chloroform from CF (n = 13). To avoid changes in the original phenotype, the experiments were conducted at the third passage in fibroblast cultures. Real-time PCR was performed and optimized for each AR primer. CD39 and CD73 mRNA expression was also evaluated.
Results: For each receptor analyzed higher levels of mRNA expression were observed in patients with respect to C both at peripheral and cardiac level: Leukocytes A1R: C = 1.97 ± 0.72, HF-V = 10.9 ± 3.27, p = 0.0098; A2aR: C = 0.45 ± 0.15, HF-V = 3.19 ± 0.94, p = 0.0057; A2bR: C = 0.43 ± 0.16, HF-V = 5.00 ± 2.98, p = 0.05; A3R: C = 1.39 ± 0.26, HF-V = 2.9 ± 0.71, p = 0.04; CD39:C = 0.33 ± 0.05, HF-V = 2.37 ± 0.40 pCF A1R: cc = 0.33 ± 0.004, CF = 4.24 ± 1.72; A2aR: cc = 1.01 ± 0.006, CF = 2.77 ± 0.67; A2bR: cc = 0.07 ± 0.03, CF = 3.04 ± 1.3; A3R: cc = 0.74 ± 0.26, CF = 2.9 ± 0.71, p = 0.04; CD39 cc = 0.66 ± 0.002 CF = 9.6 ± 3.06; CD73: cc = 0.32 ± 0.01, CF = 4.71 ± 2.22. In the subgroup of patients (n = 4) in whichsimultaneouscardiac biopsyand blood sample were obtained, ARleukocytestranscriptomic profilemimed that ofCF showing the previously reported trend.
Conclusions: These results demonstrated that all ARs are activated both in peripheral leukocytes and at tissue cardiac level inHF-V pts. These preliminary data show, for the first time, that human circulating blood cells may express the same alterations occurring in the heart, thus suggesting their application for the study of the adenosinergic system in greater populations and in different pathologic conditions.
G 112
Metabolic pathway of 4-pyridone-3-carboxamide-1β-D-ribonucleoside revealed by gene silencing
Iwona Pelikant-Małecka*, Alicja Sielicka, Ewa Kaniewska, Adrianna Radulska, Ryszard T. Smolenski and Ewa M. Słominska
Department of Biochemistry, Medical University of Gdansk, Gdansk, Poland
We identified recently that endogenously produced nucleoside 4-pyridone-3-carboxamide-1β-D-ribonucleoside (4PYR), a compound related to nicotinamide could be converted in the cell to nucleosides phosphates—4PYTP, 4PYMP and NAD analogue 4PYRAD. The aim of this study was to test whether adenosine kinase (AK), cytosolic 5′nucleotidase type II (NT5C2) and nicotinamide nucleotide adenylyltransferase type III (NMNAT3) were involved in metabolic pathway of 4PYR.
HEK 293T cells were incubated for 48 h with 100 μM to allow for accumulation of 4PYMP and 4PYRAD. siRNA for AK, NT5C2, NMNAT3 genes and non-targeting negative control siRNA was introduced using Lipofectamine RNAiMAX. Cells transfected with AK siRNA were supplemented with 100 μM adenine and 2.5 mM ribose to provide alternative substrates to maintain adenine nucleotide pool. After 48 h of incubation, cells and incubation medium were separated and cellular 4PYMP and 4PYRAD were measured by HPLC.
Silencing of AK resulted in reduction of 4PYMP concentration from 1.47 ± 0.17 to 0.50 ± 0.14 nmol/mg prot (mean ± SEM) while 4PYRAD concentration was reduced from 0.93 ± 0.12 to 0.28 ± 0.06 nmol/mg prot. Silencing of NT5C2 resulted in increases of PYMP from 3.64 ± 0.24 to 10.95 ± 0.44 nmol/mg prot as compared to control while 4PYRAD level was not different (1.08 ± 0.15 vs. 1.18 ± 0.23 nmol/mg prot). Silencing of NMNAT3 resulted in decrease of 4PYRAD concentration from 1.17 ± 0.16 to 0.66 ± 0.08 nmol/mg protein while 4PYMP concentration remained the same.
Our results suggest that adenosine kinase and nicotinamide nucleotide adenylyltransferase are involved in upstream pathway leading to formation of 4PYR phosphates and 4PYRAD while cytosolic 5′nucleotidase II is involved in degradation of 4PYMP back to 4PYR.
G 113
Changes in resistance arteries expression of extracellular nucleotides signaling partners during arterial hypertension
Charlotte Roy*, Bertrand Toutain, Daniel Henrion and Gilles Kauffenstein
UMR CNRS 6214 INSERM U1083, Angers University, Angers, France
Cardiovascular diseases are the leading cause of mortality in industrialized countries and their prevalence increases with aging populations. Resistance arteries constitute the main site of peripheral vascular resistance and play a key role in the regulation of blood pressure. Vascular tone is exacerbated in hypertension and accompanied by an hypertrophy of the arterial wall. Although signaling by extracellular nucleotides is important in vascular homeostasis its contribution to vascular pathologies remains poorly understood. We evaluated here the expression pattern of nucleotides signaling elements in resistance arteries in mice including P2 receptors, ectonucleotidases (CD39, CD73) and hemi channels (Connexins, Pannexin1) by quantitative RT-PCR. Their expression in resistance arteries was assessed in Angiotensin II-treated mice, spontaneously hypertensive rats (SHR) and, since high blood pressure is related to age, in 24-month old mice.
Our results showed that several genes are specific (P2rx1, Gja4/Connexin 37) or preferentially expressed (P2ry6) in resistance arteries. The latest might be involved in pathologies affecting these arteries. Indeed, we found that P2X1 and P2Y6 expression level increased in SHR rats and with aging respectively. Connexin 37 expression level decreased in Angiotensin II-treated mice and with aging while Connexin 45 tremendously increased (30 fold) in SHR arteries. Interestingly, CD39 (regulator of arterial tone) decreased in the two models of hypertension and with aging. Such decrease in nucleotidase activity may enhance P2 receptors activation. In combination with an increased P2Y6 expression (arterial contraction) with aging, this inbalance may increase vascular contractility/tone.
Further studies may help to evaluate the contribution of these partners in the development of resistance arteries defect in aging associated or not with high blood pressure. Signaling by extracellular nucleotides may represent new therapeutic targets in the treatment of hypertension.
G 114
Thrombin and related peptides increased extracellular release of ATP from endothelial cells elicited by mechanical stimulation
J. Pablo Huidobro-Toro*, Maria Veronica Donoso, Shantal Raso and Rosario Gajardo
Universidad de Santiago de Chile, Quimica y Biologia, Santiago, Chile
Prior work from our laboratory demonstrated that mechanical stimulation by gentle pipetting of the cell culture media elicited a significant increase in basal levels of extracellular ATP. Pipetting of the cell media of endothelial cells isolated from the rat mesenteric bed, caused within 1 min, a 6 fold increase in extracellular ATP from 50.2 ± 6.5 (n = 11) to 307 ± 30 pmol/mg protein (n = 13, P < 0.001). No cell damage was apparently associated with this procedure as assessed by the lack of lactic dehydrogenase secretion to the cell media; no visual cell damage was observed. The mechanism of release involves pannexin hemichannels since the extracellular ATP released by pipetting was considerably attenuated when cells were incubated with 20 μM carbenexolone (203 ± 11, n = 14, p < 0.01) or 100 μM probenecid (40.9 ± 24.4, n = 7, p < 0.001). These drugs also reduced, although modestly, the basal nucleotide release. Application of 0.3U/ml thrombin (PAR-1 agonist) which per se increased 1.8 fold basal extracellular ATP. Pipetting in the presence of thrombin, increased within 1 min 9-fold extracellular ATP (876 ± 32 pmol/mg protein, n = 4, P < 0.01). The thrombin effect was mimicked by 10 μM peptide TRAP-6 (SFLLRN), which increased basal ATP release (Fig A) and that stimulated following pipetting (Fig B). ATP release was not modified by 10 μM TFLLRN, evidencing receptor selectivity (Fig. A–B). Likewise, 20 μM carbenexolone reduced the ATP released elicited by pipetting in the presence of 0.3U/ml thrombin, further supporting the involvement of pannexins in the release of ATP. A Rho kinase inhibitor H-1152 (100 nM) significantly blocked the release of ATP elicited by the combined use of 10 μM TRAP-6 plus mechanical stimulation (p < 0.05), suggesting the involvement of the Rho kinase pathway in the signaling mechanism of PAR-1 receptor activation. In sum, the combination of a mechanical stimuli plus PAR-1 activation increased the secretion of extracellular ATP suggesting a possible role of both stimuli in the pathophysiology of vascular diseases.
Funded by Fondecyt 1141132, and NuBEs P10-035F.
G 115
Caveolar membrane lipid order affects ATP-mediated shear stress mechanotransduction in vascular endothelial cells
Kimiko Yamamoto1 and Joji Ando2
1The University of Tokyo, Biomedical Engineering, Graduate School of Medicine, Tokyo, Japan;2Dokkyo Medical University, School of Medicine, Tochigi, Japan
Vascular endothelial cells (ECs) respond to shear stress, a mechanical force generated by flowing blood, by changing their morphology, functions, and gene expression, and these EC responses play important roles in maintaining the homeostasis of the circulatory system. Impairment of EC responses to shear stress leads to a variety of vascular diseases, including hypertension, aortic aneurysms, and atherosclerosis, however, it remains unknown how ECs sense and transduce shear stress into biochemical signals that ultimately regulate cellular functions. Our previous studies showed that ATP-mediated Ca2+ signaling plays an important role in shear stress mechanotransduction. Shear stress evokes a rapid ATP release from ECs, and the ATP activates P2X4 receptors, a subtype of P2X purinoceptors, causing an influx of extracellular Ca2+ and increasing the intracellular Ca2+ concentration dose-dependently. We analyzed the mechanisms of ATP-mediated shear stress Ca2+ signaling by visualizing ATP release at the cell surface in real time by means of a novel chemiluminescence imaging method that we developed, and we investigated the relationship between shear-stress-induced changes in the physical properties of plasma membranes and ATP release by performing Laurdan two-photon imaging and making FRAP measurements. When subjected to shear stress, cultured human pulmonary artery ECs simultaneously released ATP in two distinct manners, a weakly concentrated, diffuse manner and a highly concentrated, localized manner. The localized ATP release occurred at caveolar membrane domains, and the ATP concentration rose to more than 10 mM, which is sufficient to activate purinoceptors. Ca2+ imaging with Fluo-4 combined with ATP imaging revealed that shear stress evoked an increase in intracellular Ca2+ concentration and subsequent Ca2+ wave that originated from the same sites as the localized ATP release and propagated throughout the entire cell. Laurdan imaging showed that the lipid order of the EC membranes rapidly decreased in response to shear stress and that the caveolar membrane domains changed from the liquid-ordered state to the liquid-disordered state. A similar decrease in lipid order occurred when the artificial membranes of giant unilamellar vesicles were exposed to shear stress, suggesting that the membrane lipid order change is a physical phenomenon. Membrane fluidity increased over the entire EC membranes in response to shear stress. Addition of cholesterol to ECs abolished the effects of shear stress on membrane lipid order and fluidity and markedly suppressed shear-stress-induced ATP release. These findings indicate that EC membranes directly respond to shear stress by rapidly decreasing the order of their lipid phase and increasing their fluidity; these changes are linked to ATP-mediated shear stress Ca2+ signaling.
G 116
The role of purinergic signaling in endothelial and smooth muscle cells under simulated microgravity
Yu Zhang1 4,*, Patrick Lau2, Andreas Pansky1, Matthias Kassack4, Ruth Hemmersbach3 and Edda Tobiasch1
1Department of Natural Sciences, Bonn-Rhine-Sieg University of Applied Sciences, von-Liebig-Straße 20, 53359 Rheinbach, Germany;2Space Physiology Division, Institute of Aerospace Medicine, German Aerospace Center, Linder Höhe, 51147 Cologne, Germany;3Gravitational Biology Division, Institute of Aerospace Medicine, German Aerospace Center, Linder Höhe, 51147 Cologne, Germany;4Institute of Pharmacology and Medical Chemistry, University of Düsseldorf, Universitätsstraße 1, 40225 Düsseldorf, Germany
Astronauts suffer from cardiovascular deconditioning during space flight where they are exposed to microgravity. Alterations under real and simulated microgravity have been found e.g. in the cytoskeleton and apoptosis in endothelial cells (ECs) and smooth muscle cells (SMCs) [1,2]. P2 receptors play an important role in a variety of vascular functions of ECs and SMCs. However, the functional role of purinergic signalling in ECs and SMCs under microgravity is still unclear.
In this study primary ECs and SMCs were isolated from bovine aorta and characterized using specific markers. Additionally, EC growth medium collected during culture under normal gravity was used as conditioned medium for SMCs and vice versa to mimic a co-culture model. Here we show for the first time that the P2-receptor expression pattern is altered in ECs and SMCs under simulated microgravity achieved by a clinostat. Interestingly, conditioned medium compensated the alterations in the expression of specific P2-receptors. P2X7 was down-regulated in ECs after 24 h clinorotation but recovered to the gene and protein expression level found under normal gravity when cultured in conditioned medium from SMCs.
Our results showed an altered P2-receptor expression pattern under simulated microgravity. The paracrine effect between ECs and SMCs seems to be an important regulator of cell behaviour under altered gravity conditions. Several artificial P2-receptor ligands are already utilized as drugs. Thus it might be reasonable to consider them for drug development for astronaut treatment of cardiovascular deconditioning in the future.
References
1. Zhang Y, Sang C, Paulsen K et al (2010) Acta Astronautica 69:1073–1080
2. Grosse J, Hemmersbach R, Grimm D et al (2012) FASEB J 26:639–655
G 117
The effect of CD73 knock-out and high-fat diet in mice on development of aortic valve dysfunction
Paulina Żukowska1,*, Iwona Rybakowska2, Barbara Kutryb-Zając1, Agnieszka Jaszta4, Marta Toczek1, Tomasz Borkowski1, Paweł Romaszko1, Marcin Lipiński3, Stefan Chłopicki4, Ewa M. Słominska1 and Ryszard T. Smolenski1
1Department of Biochemistry,2Department of Biochemistry and Clinical Physiology,3Department of Pharmaceutical Biochemistry, Medical University of Gdansk;4Jagiellonian Center for Experimental Therapeutics, Jagiellonian University, Krakow, Poland
Aortic valve stenosis involves inflammation that could be controlled by nucleotides and adenosine. The aim of the study was to investigate the effect of high-fat diet and CD73 knock out (CD73−/−) on aortic valve function in mice.
Mice were fed special diets for 15 weeks in four groups: low-fat diet CD73−/−, low-fat diet Wild Type (WT), high-fat diet CD73−/− and high-fat diet WT with monitoring weight, blood glucose concentration and aortic flow with Doppler ultrasound. Aortic roots were collected for histology and analysis of ecto-nucleoside triphosphate diphosphohydrolase (eNTPD), ecto-5′-nucleotidase (e5′NT) and ecto-adenosine deaminase (eADA) activities.
CD73−/− had lower weight and higher glucose level than WT (27.7 ± 1.4 vs. 31.3 ± 2.3 g and 156.6 ± 7.5 vs. 113.3 ± 7.7 mg/dl, respectively). High-fat diet increased blood glucose level in both groups. Activity of e5′NT in CD73−/− was below 20 % of that in WT. Activity of eADA was lower in the CD73−/− as compared to WT (0.72 ± 0.14 vs. 1.30 ± 0.14 nmol/cm2/min, respectively) while eNTPD was not different. High-fat diet had no effect on ecto-enzymes. High-fat diet in WT caused an increase in peak aortic flow as compared to low-fat diet (3.69 ± 0.32 vs. 2.85 ± 0.25 m/s). CD73−/− on low-fat diet led to increase in peak aortic flow (3.73 ± 0.21 m/s) as compared to WT. Peak aortic flow was highest in CD73−/− on high-fat diet (5.04 ± 0.37 m/s). Histological changes in aortic valve correlated with aortic flows.
Our results indicate that knock out of CD73 leads to aortic dysfunction in mice similar to that induced by high-fat diet. The effect of high-fat diet and CD73 knock out is additive.
Abstracts—Posters
H: Purinergic signaling in renal, gastrointestinal, muscosceletal system and cellular metabolism
H 118
[Ca2+]ioscillations and Il-6 release induced by α-haemolysin fromEscherichia colirequire P2 receptor activation in renal epithelia
Mette G Christensen*, Steen K Fagerberg, Randi G Bjaelde, Jens Leipziger and Helle A Praetorius
Aarhus University, Department of Biomedicine, Aarhus, Denmark
Urinary tract infections are commonly caused by α-haemolysin (HlyA)-producing E. coli. HlyA forms pores in cell membranes that renders the attacked cells permeable to ions and water. In erythrocytes, the effect of HlyA is strongly amplified by P2X receptor activation and our recent data support that ATP pass through the HlyA pore itself as an early event after pore insertion into the membrane. We therefore hypothesise that HlyA-induced [Ca2+]i oscillations in renal epithelia is mediated by ATP release and following P2 receptor activation. Here, we confirm that HlyA, added at a concentration that cause 50 % lysis of human erythrocytes after 60 min, initiate marked [Ca2+]i oscillatory activity in renal epithelia. This was quantified by live cell fluorescence microscopy in both MDCK cells and freshly isolated medullary thick ascending limb (mTAL) from mice. The HlyA-induced oscillations were reversible and did not cause permanent cell damage. HlyA clearly triggered ATP release from MDCK cells and accordingly the HlyA-induced [Ca2+]i oscillations were completely prevented by non-selective P2 receptor antagonists and by ATP-scavenging in MDCK cells. To confirm these results, we tested the effect of HlyA on ATP biosensor cells. In native 132-1N1 astrocytoma cells that do not express any P2 receptors, HlyA barely caused any changes in [Ca2+]i. Transfection of the cells with a hP2Y2 receptor resulted in an extensive increase in the HlyA-induced [Ca2+]i oscillatory activity, which were sensitive to P2 receptor inhibition. Moreover, the HlyA-induced [Ca2+]i oscillations were markedly reduced in mTAL isolated from P2Y2 receptor deficient mice compared to wild type. Interestingly, the HlyA-induced interleukin 6 (Il-6) release was absent in the P2Y2 receptor knockout (P2Y2−/−) mice, whereas it was readily detectable in the wild type (P2Y2+/+) mice. These results suggest that HlyA trigger ATP release from renal epithelia, which via P2 receptor activation is responsible for the HlyA-induced [Ca2+]i oscillations and following Il-6 release.
H 119
Extracellular ATP metabolism is altered in kidney of experimental diabetic rats
Robert Kowalski*, Ewelina Kreft and Mirosława Szczepańska-Konkel
Medical University of Gdansk, Department of Therapy Monitoring and Pharmacogenetics, Gdansk, Poland
Extracellular nucleotides metabolism has been implicated as important regulators of intrarenal microcirculation and tubular function in diabetes. Here we evaluated the concentration of ATP and its metabolite, adenosine and the nucleotidase activity in the renal cortical tissue of rats with streptozotocin (STZ) induced diabetes.
Experiments were performed on male Wistar rats 10 days after STZ injection (65 mg/kg, ip). Blood glucose concentration in experimental group was 393 ± 27 mg/dl and 119 ± 10 mg/dl in control. Using microdialysis technique interstitial concentration of ATP and adenosine, product of its metabolism in renal cortex was measured. In diabetic rats ATP concentration was lower than in control rats, 1.34 ± 0.21 nM vs 2.12 ± 0.18 nM, p < 0.05. On the other hand, adenosine concentration was higher in diabetic rats than in control, 237 ± 40 nM vs 98 ± 28 nM, p < 0.05. In next series of experiments we assessed adenine nucleotides hydrolysis by kidney cortex homogenates, measuring inorganic phosphate liberation from ATP, ADP and AMP. We observed a raise in ATP (by 62 %, p < 0.05) and ADP (by 41 %, p < 0.01) hydrolysis 10 days after STZ diabetes induction. Our results are consistent with previously published findings on serum adenine nucleotides metabolism in diabetic rats (Rucker B., 2010).
Data presented in this study suggest that increased hydrolytic activity in diabetic kidney may results in imbalance between ATP and adenosine and in a consequence, increased the role of adenosine in the regulation of glomeruli contractility and intrarenal microcirculation in early STZ-induced diabetes.
This project was supported by the Ministry of Science and Higher Education Republic of Poland: N N405 024940, ST-47, MN 01-0052/08 and quality promoting subsidy under the Leading National Research Centre (KNOW) programme 2012–2017.
H 120
Contribution of P2X7 receptors in renal hemodynamics in streptozotocine-induced diabetic rats
Robert Kowalski*, Ewelina Kreft and Mirosława Szczepańska-Konkel
Medical University of Gdansk, Department of Therapy Monitoring and Pharmacogenetics, Gdansk, Poland
In the early phase of diabetic nephropathy microcirculation deregulation and glomerular filtration rate (GFR) disturbances are seen. ATP is emerging as an important factor in the regulation of renal hemodynamics. The role of purinergic system in the regulation of the intrarenal microcirculation in the diabetic kidney is not well established.
In diabetic rats kidney increased P2X7 receptors abundance and its potential role in apoptosis is well documented. However its role in regulation of renal microcirculation and excretory function is not elucidated. In the present study the effect of intravenous administration of benzoylbenzoyl adenosine triphosphate (BzATP), P2X7 receptor agonist on renal cortical and medullar blood flow, GFR and urinary excretions were investigated.
Clearance experiments were performed on male Wistar rats 4 weeks after STZ injection (65 mg/kg, ip), blood glucose concentration was 389 ± 21 mg/dl. Control group were normoglycemic rats corresponding in age. In STZ rats BzATP (0.2 μmol/kg bw + 2 nmol/kg/min.) increased cortical blood flow (laser Doppler measurement) by 12 %, p < 0.05 and reduced GFR (3H-inulin clearance) by 20 %, p < 0.01 without significant changes in medullar blood flow and urinary excretion. In control group BzATP did not change nor renal hemodynamics nor excretory functions. Mean arterial pressure was not affected by BzATP infusion in both STZ and control rats.
We conclude that ATP via P2X7 receptors might be involved in the regulation of intrarenal microcirculation and GFR in animals with experimentally induced hyperglycemia.
This project was supported by the Ministry of Science and Higher Education Republic of Poland: N N405 024940, ST-47 and quality promoting subsidy under the Leading National Research Centre (KNOW) programme 2012–2017.
H 121
Differences in renal physiology between P2X7KO and WT animals
Patricia Santana*, Celso Caruso-Neves and Robson Coutinho-Silva
IBCCF - UFRJ, Rio de Janeiro, Brazil
Question: The kidney is a key organ in the regulation of homeostasis of organisms. Extracellular nucleotides released by cells, such as ATP, activate purinergic receptors to exert their functions. The P2X7 receptor is often related to inflammatory responses but it also participates in physiological events in the nervous system and the gut. Therefore the aim of this work is to analyze the participation of the purinergic receptor P2X7 in the renal physiology.
Methods: Wild type (WT) and P2X7 receptor knockout (P2X7KO) mice were individually housed in metabolic cages for 24 h and then urine and blood samples were collected. Analysis of urine volume, urinary flow rate and renal function parameters such as levels of urinary protein (UP), urinary and serum creatinine (Cr and SCr, respectively) and UPCr were measured by colorimetric assays (Doles Reagents Kit, Brazil).
Results: The levels of protein in the urine and the urinary flow rate of P2X7KO animals were reduced (*p ≤ 0.05) when compared to WT animals. We also measured the serum and urinary levels of creatinine and it was observed that P2X7KO animals presented greater urinary creatinine levels (*p ≤ 0.05), whereas serum levels did not change between the groups. The ratio between urinary protein and urinary creatinine was then obtained and it is significantly lower in P2X7KO animals.
Conclusion: Taken together these data suggest that P2X7KO animals have a better renal performance when compared to WT animals.
Finantial Support: CNPq, FAPERJ, PRONEX, InPETAM-CNPq
H 122
Histological analysys and sulphate-reducing bacteria (SRB) detection in experimental colitis induced by TNBS on P2X7 knockout mice
Alessandra A. Abalo1,*, Vanessa R. F. da Paz1,2, Hayandra F. Nanini1,2,3, Robson Coutinho-Silva2, Heitor S. P. de Souza3 and Cláudia M. L. M. Coutinho1,4
1Laboratório de Inovações em Terapias, Ensino e Bioprodutos—LITEB, Instituto Oswaldo Cruz, FIOCRUZ, Rio de Janeiro, RJ, Brasil;2Laboratório de Imunofisiologia, Instituto de Biofísica Carlos Chagas Filho, Universidade Federal do Rio de Janeiro, RJ, Brasil;3Laboratório Multidisciplinar de Pesquisa, Hospital Universitário Clementino Fraga Filho, Universidade Federal do Rio de Janeiro, RJ, Brasil;4Departamento de Biologia Celular e Molecular, Instituto de Biologia, Universidade Federal Fluminense, Niterói, RJ, Brasil
P2X7 receptors are important components in the immune response against Gram-negative bacteria. Sulphate-reducing bacteria (SRB) are a unique mixed group of anaerobic microorganisms found colonizing the human gut and they have been associated with the development of intestinal bowel disease (IBD). In the present study, we investigated histological aspects and also SRB growth from colon biopsies in experimental colitis induced by 2,4,6-trinitrobenzene sulfonic acid (TNBS) on P2X7 knockout (KO) and C57BL/6 wild type (WT) mice. Colitis was chemically induced by intracolonic instillation (in anesthetized mice) of 0.1 ml of a solution containing 25 mg of TNBS in 40 % ethanol. On experimental day seven, body weight significantly decreased in WT mice treated with TNBS compared with both TNBS- or ethanol-treated KO mice. Analysis of intestinal HE-stained sections revealed an increase in the microscopic damage in WT mice treated with TNBS compared with control animals (ethanol-treated). TNBS- and ethanol-treated KO animals did not present significant histological differences. Biopsies (0.5 cm3) collected from distal intestinal colon were transferred for penicillin sealed vials containing 10 ml of VNMI medium (SRB growth medium), degassed under sterile N2 to create anaerobic condition and maintained at 37 °C. SRB growth was daily observed based on the blackening of the vials indicating iron sulphide formation, result of reaction of the iron present in the medium with H2S produced by SRB metabolism. The results showed that SRB derived from ethanol-treated KO mice presented faster growth compared with the counterpart WT group. We did not observe SRB growth differences between TNBS-treated KO and WT groups. Taken together, our data suggest that P2X7 receptor may be involved on the regulation of SRB population in the intestinal colon and also may have a role on IBD development.
H 123
Growth evaluation of sulphate-reducing bacteria (SRB) in different compartments from the gastrointestinal tract of P2X7 knockout mice
Hayandra F. Nanini1,2,3,*, Vanessa R. Figliuolo da Paz1,2, Ângela Santos1, Alessandra A. Abalo1, Robson Coutinho-Silva2, Heitor S. P. de Souza3 and Cláudia M. L. M. Coutinho1,4
1Laboratório de inovações em Terapias, Ensino e Bioprodutos—LITEB, Instituto Oswaldo Cruz, FIOCRUZ, Rio de Janeiro, RJ, Brasil;2Laboratório de Imunofisiologia, Instituto de Biofísica Carlos Chagas Filho, Universidade Federal do Rio de Janeiro, RJ, Brasil;3Laboratório Multidisciplinar de Pesquisa, Hospital Universitário Clementino Fraga Filho, Universidade Federal do Rio de Janeiro, RJ, Brasil;4Departamento de Biologia Celular e Molecular, Instituto de Biologia, Universidade Federal Fluminense, Niterói, RJ, Brasil
P2X7 receptors are important components in the immune responses against Gram-negative bacteria, such as M. tuberculosis and C. trachomatis. Anaerobic sulphate-reducing bacteria (SRB) are Gram-negative bacteria present in the flora of the mammals gastrointestinal tract and are associated with the development of inflammatory bowel diseases. Our goal was to study SRB population recovered from different compartments of the gastrointestinal tract of P2X7 knockout (P2X7 KO) and C57BL/6 wild type (WT) control mice. Recovery protocol for SRB was performed using biopsies (0.5 cm3) collected from the stomach, duodenum and distal intestinal colon of P2X7 KO and C57BL/6 WT control mice. Gastrointestinal biopsies were transferred to penicillin sealed vials containing 10 ml of VNMI medium especially designed for SRB growth, which were purged for 40 min under sterile N2 to create anaerobic condition. SRB population were then cultivated at 37 °C for 96 h and blackening of vials containing VMNI medium indicates iron sulphide formation, result of reaction of the iron present in the medium with H2S produced by SRB metabolism. Our results showed that SRB population derived from stomach biopsies of P2X7 KO mice showed greater and faster growth compared with C57BL/6 WT control mice. Similar results were found in SRB population recovered from duodenum and distal intestinal colon biopsies that showed increased and faster growth compared with C57BL/6 WT control mice. It is possible that P2X7 receptor is involved in the regulation of SRB population in different compartments of gastrointestinal tract and also that purinergic signaling may be implicated in the pathogenesis of inflammatory bowel diseases.
H 124
Modulatory role of adenosine A2Breceptors in fibrotic gut wall remodeling associated with experimental colitis
Luca Antonioli1,2,*, Matteo Fornai1, Carolina Pellegrini1, Deborah Sacco1, Balázs Csóka2, Cristina Segnani1, Chiara Ippolito1, Nunzia Bernardini1, György Haskó2, Corrado Blandizzi1 and Rocchina Colucci1
1University of Pisa, Department of Clinical and Experimental Medicine, Pisa, Italy;2Rutgers-New Jersey Medical School, Department of Surgery and Center for Immunity and Inflammation, Newark (USA), USA
Introduction: The adenosine system is now emerging as a major pathway involved in the regulation of the balance between tissue repair and/or fibrosis in lung, liver, skin and kidney. However, the role played by adenosine in the pathophysiology of gut fibrotic remodeling has been scarcely investigated. The present study was aimed at evaluating the role of adenosine A2B receptors on pro-fibrotic signalling in experimental colitis.
Methods: Colitis was induced in rats by intrarectal 2,4-dinitrobenzenesulfonic acid (DNBS, 30 mg/rat in 0.25 ml ethanol 50 %). After 6 days, systemic [body and spleen weight] and tissue inflammatory parameters [macroscopic and microscopic damage, myeloperoxidase (MPO)] were assessed. Three days before colitis assessment, the animals were treated daily with BAY 60-6583 (BAY, selective A2B receptor agonist, 2 mg/kg) or MRS1754 (MRS, selective A2B receptor antagonist, 1 mg/kg) by intraperitoneal injection. At the time of sacrifice, the expression of molecular factors involved in tissue fibrotic remodelling (collagen I and III; fibronectin; TGF-beta; phosphorylated SMAD2) was analyzed by western blot. Collagen fibers (Sirius red) and elastic fibers (orcein) were examined by histochemistry.
Results: DNBS-induced bowel inflammation was associated with a decrease in body weight and an increase in spleen weight, tissue MPO as well as macroscopic and microscopic tissue alterations. Western blot analysis, performed on inflamed/fibrotic colonic tissues, revealed an increased expression of collagen I and III, fibronectin, TGF-beta and pSMAD2, as compared with control animals. Histochemistry confirmed an increment of collagen deposition and a loss of elastic fibers.
In this setting, treatment with MRS counteracted the inflammatory indexes, while it did not affect the altered fibrotic parameters. By contrast, the pharmacological activation of A2B receptors with BAY failed to modify both the systemic and tissue inflammatory indexes. However, BAY increased the expression of the pro-fibrotic markers collagen I and fibronectin, despite it significantly downregulated the TGF-beta/ pSMAD2 pathway.
Conclusions: Colonic fibrotic remodelling associated with bowel inflammation was not affected by A2B receptor antagonism, suggesting a marginal role for endogenous adenosine in regulating fibrotic remodelling via A2B receptors. By contrast, the pharmacological stimulation of adenosine A2B receptors appears to enhance the colonic fibrotic remodelling through pathways independent from TGF-beta signalling.
H 125
Secretion of ATP along the loop of henle in response to increased luminal flow rate: implications for sodium transport
Grégory Jacquillet1, Luciana Morla2 and Robert J. Unwin1,*
1UCL Centre for Nephrology, Royal Free Campus, University College London, London, UK;2Centre de recherche des Cordeliers, Université Pierre et Marie Curie, Université Paris-Descartes, UMRS872 et ERL7226, Paris, France
Introduction: It has now become generally accepted that extracellular nucleotides such as ATP and ADP can influence a wide variety of physiological functions, including epithelial transport, via purinergic receptors. In the kidney these nucleotide receptors have been found along the whole length of the nephron, and evidence is accumulating that nucleotides can significantly affect ion and water transport in several nephron segments, as well as being implicated in renal pathology. Recent evidence from in vitro studies indicates that the cells of the thick ascending limb (TAL) of the loop of Henle (LOH) can secrete nucleotides into the lumen and that this secretion is stimulated by an increase in tubular flow and shear stress or intraluminal pressure. Furthermore, in vitro studies also suggest that ATP can inhibit sodium (Na+) reabsorption in the TAL.
The purpose of the present investigation was to assess in vivo whether significant amounts of ATP are secreted by the LOH into the tubular lumen and if this is affected by tubular flow rate, and might influence Na+ reabsorption.
Methods: Rats were anaesthetised and prepared surgically for renal micropuncture. To assess the contribution of increased luminal flow rate to the levels of ATP and Na+ measured in the loop of Henle, we compared, in the same animals, ATP and Na+ concentrations in pooled collected tubular fluid samples (n = 1 to 4 collections) at 20 and/or 40 nl min−1.
Results: When loops were perfused at 20 nl min−1 and 40 nl min−1 (n = 19 pairs of pooled collections) the ATP concentration in the collected fluid was 80 ± 15 and 96 ± 26 nM, respectively (NS), and the ATP secretion rate was 1.1 ± 0.2 and 3.0 ± 0.8 fmol min−1, respectively (P < 0.01, paired t-test). In these experiments, the Na+ concentration in the collected fluid was 90 ± 6 and 108 ± 3 mM, respectively (P < 0.01, paired t), and the fractional reabsorption of Na+ was 53 ± 3 and 35 ± 3 %, respectively (P < 0.05, paired t-test).
Our data indicate that physiologically significant amounts of ATP can be secreted by loops of Henle in vivo. Furthermore, the amount secreted is affected by changes in tubular fluid flow rate. These results also suggest a possible link between ATP secretion and reduced flow-dependent fractional reabsorption of Na+. However, when we perfused at 40 nl min−1 with apyrase, an enzyme that catalyses the hydrolysis of ATP, we did not find any changes in the fractional reabsorption of Na+ compared with the control period (31 ± 5 and 32 ± 5 %, n = 7).
Conclusion: Thus, although fractional reabsorption Na+ is flow-dependent, this does not seem to depend on flow-mediated ATP secretion. What effect, if any, increased ATP may have on LOH function is still an open question.
H 126
The topology and enzymatic activity of distinct E-NTPDases as fine-tuning regulators of purinergic signaling in the rat ileum
Margarida Duarte-Araújo1,*, Salomé Gonçalves-Monteiro1, Carla Tavares1, Maria Teresa Magalhães-Cardoso1, Ana Alves-Laço1, Fátima Ferreirinha1, Jean Sévigny2 and Paulo Correia-de-Sá1,*
1ICBAS—UP, IMFF, UMIB, MEDinUP, Porto, PORTUGAL, Portugal;2Centre Hospitalier Universitaire de Québec, Centre de Recherche en Rheumatologie et Immunologie, Québec, CANADA, Canada
ATP transiently increases spontaneous acetylcholine (ACh) release at the myenteric neuromuscular synapse. The facilitatory effect of ATP is cut-short by its rapid breakdown into ADP leading to activation of inhibitory P2Y1 receptors [1]. On the other hand, endogenously generated adenosine exerts a dominant tonic facilitatory action on evoked ACh release via A2A receptors expressed on myenteric nerve terminals [2]. The close proximity between A2A receptors and muscle-bound ecto-5′-nucleotidase (E-5′-NTase) [3] explains the dominant facilitatory action of the nucleoside. In this study, we decided to go depth into the regional distribution, enzymatic activity and functional relevance of ecto-NTPDases in the myenteric plexus of the rat ileum.
Confocal microscopy studies demonstrate that the rat ileum lacks immunoreactivity against E-NTPDase1, except a few blood vessels. E-NTPDase2 immunoreactivity is most evident in GFAP-positive glial cells at myenteric ganglia, whereas E-NTPDase3 co-localizes predominantly with synaptophysin in myenteric nerve terminals and with vimentin-positive intramuscular interstitial cells. E-NTPDase8 labels some, yet not all, neuronal cell bodies present in myenteric ganglia.
The extracellular catabolism of ATP (30 μM, t½ = 7 ± 1 min, n = 6) was delayed in the presence of POM-1 (100 μM, t½ = 12 ± 3 min, n = 4), a non-selective E-NTPDases inhibitor, and of ARL67156 (100 μM, t½ = 14 ± 1 min, n = 4), a preferential E-NTPDase1 and 3 inhibitor. The two inhibitors decreased the formation of ADP, AMP and ADO, as well as tissue deposition of phosphates (Wachstein-Meisel histochemical reaction), when ATP was used as a substrate. Inhibition of E-5′-NTase with α,β-methyleneADP (AOPCP, 200 μM) prevented the deposition of phosphates in enteric muscular layers following incubation with AMP (100 μM). ARL67156 and AOPCP reduced [3H]ACh release from stimulated (5Hz, 1,350 pulses) myenteric neurons by 42 ± 4 % (n = 4) and 36 ± 5 % (n = 3), respectively. The inhibitory effect of ARL67156 was prevented by blocking A2A receptors (with ZM 241385, 50 nM), but not with P2Y1 antagonist MRS2179 (300 nM). Data suggest that the predominant localization of E-NTPDase2 in myenteric glial cells favours ADP accumulation in close proximity to inhibitory P2Y1 receptors. Localization of E-NTPDase3 at the myenteric neuromuscular synapse catalyses the rapid breakdown of ATP into AMP, which is subsequently converted into adenosine by muscle-bound E-5′-NTase near prejunctional facilitatory A2A receptors. Thus, co-localization of the various components of the “purinome” dictates which signals are to be translated into intestinal cells function.
Work supported by FCT (FEDER funding, PEst-OE/SAU/UI0215/2014)
References
1. Duarte-Araújo et al (2009) Br J Pharmacol 156:519–533
2. Vieira et al (2011) Neurochem Int 59:1043–1055
3. Nitahara et al (1995) Neuroscience 67:159–168
H 127
Feed-forward inhibition of CD73 and upregulation of adenosine deaminase underlie adenosine neuromodulation failure in the chronic inflamed ileum
Cátia Vieira1, Maria Teresa Magalhães-Cardoso1, Fátima Ferreirinha1, Isabel Silva1, Ana Sofia Dias1, Julie Pelletier2, Jean Sévigny2 and Paulo Correia-de-Sá1,*
1ICBAS—University of Porto, Lab. Farmacologia e Neurobiologia, UMIB, Porto, Portugal;2Centre de Recherche du CHU de Québec, Québec, QC, Canada
Purinergic signaling undergoes remarkable plastic modifications during gastrointestinal (GI) inflammation [1]. Thus, selective drugs targeting the “purinome” (e.g. release sites, ectoenzymes, receptors) may be helpful for inflammatory GI diseases. Considering that myenteric neuromuscular transmission is fine-tuned controlled by adenosine via A1 inhibitory and A2A excitatory receptors [2], we now investigated the catabolism of adenine nucleotides and adenosine in TNBS-inflamed longitudinal muscle—myenteric plexus of the rat ileum.
Chronic inflammation detected by H&E stained infiltrates at post-operative day 7 led to a global loss of adenosine modulation of acetylcholine release, which may contribute to accelerate gastrointestinal transit. Impairment of adenosine neuromodulation is predominantly due to a deficient accumulation of the nucleoside at the inflamed myenteric synapse despite that increases in ATP release were observed. Discrepancy between ATP outflow and adenosine deficiency can be ascribed to feed-forward inhibition of ecto-5′-nucleotidase/CD73 by high extracellular ATP and/or ADP, as detected by HPLC kinetic analysis. Interestingly, redistribution of NTPDase2, but not of NTPDase3, from ganglion cell bodies to myenteric nerve terminals leads to preferential ADP accumulation from released ATP, thus contributing to prolong inhibition of muscle-bound ecto-5′-nucleotidase/CD73 and to delay adenosine formation at inflamed neuromuscular synapses. On the other hand, depression of endogenous adenosine accumulation may also occur due to enhancement of ADA activity. We observed a remarkably increase in the activity of soluble forms of ecto-5′-nucleotidase/CD73 and ADA in the chronic inflamed ileum.
Data suggest that pharmacological inhibition of ADA, and/or the use of AMP-derived prodrugs that selectively activate P1 receptors localized in the close proximity of ecto-5′-nucleotidase/CD73, represent promising therapeutic strategies to ameliorate motility disturbances of inflammatory enteric disorders.
This work was partially supported by FCT (FEDER funding, Pest-OE/SAU/UI215/2011); CV is in receipt of a PhD studentship by FCT (SFRH/BD/79091/2011).
References
1. Roberts et al (2012) Curr Op Pharmacol 12:659–666
2. Duarte-Araújo et al (2004) Br J Pharmacol 141:925–934
H 128
Neurogenic purines in colons from human and non-human primates
Violeta Mutafova-Yambolieva and Leonie Durnin
University of Nevada School of Medicine, Physiology and Cell Biology, Reno, USA
Background: Enteric purinergic motor neurotransmission is the dominant inhibitory neural control of the human colon via release of purines from motor nerve terminals and activation of P2Y1 purinergic receptors and Ca2+-activated small-conductance K+ (SK) channels on target cells. ATP has traditionally been considered the purine responsible for inhibitory responses; however, nicotinamide adenine dinucleotide (NAD+) and ADP-ribose (ADPR) are also agonists of P2Y1 receptors and SK channels in the colon and might be enteric neurotransmitters.
Objective: The present study analyzed the basal and stimulus-evoked release of purines in human and monkey (Macaca fascicularis) colons.
Methods: Use of human colon tissues was approved by Human Subjects Research Committees at Renown hospital, and the Biomedical Institutional Review Board at University of Nevada, Reno. Likewise, the experimental procedures with monkey colon tissues were approved by the Institutional Animal Care and Use Committee at University of Nevada. A small-volume superfusion assay and high-performance liquid chromatography with fluorescence detection were used to evaluate ATP, NAD+, and ADPR released spontaneously and by electrical field stimulation of enteric nerves in colonic tunica muscularis. To discriminate between sites of release of ATP and NAD+/ADPR, nicotinic acetylcholine receptors (nAChR) and serotonergic 5-HT3 receptors (5-HT3R) on cell bodies were stimulated and purine release was evaluated in the absence and presence of neurotoxins.
Results: ATP, NAD+ and ADPR were released from human and monkey colonic muscles spontaneously and during nerve stimulation. NAD+ was the dominant purine released. Release of NAD+ and ADPR, but not of ATP, was frequency-dependent, occurred in muscles that had nerve processes but no ganglia, and was blocked by neurotoxins. Activation of nACh and 5-HT3 receptors on myenteric nerve cell bodies evoked release of ATP and NAD+, and release of NAD+, but not of ATP, was inhibited by blockers of neuronal fast Na+ channels and N-type Ca2+ channels. Therefore, ATP was likely released from ganglionic sources, possibly from the cell bodies of motor neurons, whereas NAD+ and ADPR were likely released from motor nerve terminals and varicosities, which is consistent with the idea that NAD+ and ADPR, but not ATP, are likely enteric neurotransmitters (Figure 1).
Conclusion: In the primate colon, NAD+ and ADPR meet presynaptic criteria for enteric neurotransmitter better than ATP.
Fig. 1 In the primate colon, ATP is likely released from myenteric ganglia and extraneuronal sources, whereas NAD+ and ADPR are likely released from motor nerve varicosities. (Drawing adapted from Smith K, Colonic motor neurotransmission-is β-NAD+ in control? Nat Rev Gastroenterol Hepatol. 2013, 10(2):64)
H 129
Effect of diadenosine tetraphosphate and PPADS in an animal model of dwarfism
Patricia Loma, Javier Ortin and Jesus Pintor*
Faculty of Optometry, Universidad Complutense, Biochemistry, Madrid, Spain
Different mutations in FGFR1-3 have been shown to result in congenital skeletal disorders with FGFR3 being of high importance for regulating the development of the endochondral skeleton. The main skeletal disorders due to FGFR3 mutations are hypochondroplasia, achondroplasia and thanatophoric dysplasia These pathologies depict similar morphological features including short limb skeletal development and skull abnormalities. Achondroplasia, also known as dwarfism, is the most common one being an orphan disease till date.
We had former evidences that purinergic agonists and anatagonists were able to rescue achondroplastic chondrocytes in culture from its pathological state when they were challenged with both, diadenosine tetraphosphate, Ap4A, and PPADS.
By means of a murine model of achondroplasia we have investigated the role of these two compounds to see whether or not their effect may help to increase the animal size.
Subcutaneous injections of either Ap4A or PPADS (100 ul, 1 mM) were performed once every 2 days during 1 month. Achondroplastic mice length and weight were measured and compared with a wild type mouse. Radiographic studies were also performed to verify changes in the bones.
The most relevant result was that of the two substances that have been working in vitro, Ap4A and PPADS, only the second has yielded positive results. In this sense, the effects could be seen in special at the end of treatment, since mice treated with PPADS have a size greater than the untreated, but slightly lower than the healthy mice. In particular achondroplastic treated mice were 30 % bigger than the ones treated with saline. On the other hand, associated skeletal problems such as lordosis and kyphosis were significantly attenuated. Finally, it was possible to see a reduction in another typical sign such is hydrocephaly.
In summary, purinergic agonists and antagonists can positively rescue a murine model of human achondroplasia opening the possibility of using these molecules as tools for the treatment of this orphan disease.
Supported by Areces Foundation
H 130
Effect of Ap4A on aquaporin-1 present in human achondroplastic chondrocytes
Maria Jesus Perez de Lara and Jesus Pintor
Faculty of Optometry, Universidad Complutense, Biochemistry, Madrid, Spain
Achondroplasia, Thanatophoric Dysplasia I and Cartilage-Hair Hypoplasia, (CHH), are pathologies characterized by different degrees of short-limb dwarfism. ACH and TD result from mutations in the fibroblast growth factor receptor type 3 (FGFR3) gene, one of the key regulators of endochondral ossification. There is no treatment for dwarfism, surgery being the only possible approach for bone elongation.
Experiments performed in our laboratory have demonstrated that the distribution of aquaporin-1 (AQ1) in achondroplastic chondrocytes in culture change with the development of the pathology. Since we had previous evidences that diadenosine tetraphosphate (Ap4A) had effect rescuing these chondrocytes from the pathological features, we have decided to investigate the possible role of these purinergic substances in the AQ1 distribution.
Immortalized human chondrocytes carrying the heterozygous achondroplasia mutation (G380R) were generated and characterized by Dr. Laurence Legeai-Mallet. Cells were cultured in DMEM supplemented with 10 % fetal calf serum, 1 % penicillin/streptomicyn and 500 ng/ml geneticin. Immunocytochemical studies were performed by means of an antibody raised against aquaporin-1 (AQP1). Previously, cells were challenged with single doses of Ap4A, time-course of AQP1 after treatment and aquaporin distribution were studied.
In normal chondrocytes there is a slightly increase in the levels of AQ1 although there appears to be no difference in the distribution of this protein during chondrocyte maturation. In achondroplastic chondrocytes we observe not only a substantial increase in the presence of AQ1 as the chondrocyte matures, but also there is a higher presence in the plasma membrane of the achondroplastic cell. This fact is responsible in part of the accelerated death of these cells. In this sense, we have been able to see how the dinucleotide Ap4A, is able to prevent this phenomenon, by inhibiting the trafficking of AQ1 from the intracellular structures to the membrane. This mechanism is basically due to the stimulation of a P2Y2 receptor. These results are promising because it allows us to continue investigating Ap4A for the treatment of this pathological condition.
Supported by Areces Foundation
H 131
Aberrant bone density in aging mice lacking the adenosine transporter ENT1
David Hinton*, Meghan McGee-Lawrence, Moonnoh Lee, Hoi Kwong, Jennifer Westendorf and Doo-Sup Choi
Mayo Clinic College of Medicine, Rochester, USA
Adenosine is known to regulate bone production and resorption in humans and mice. Type 1 equilibrative nucleoside transporter (ENT1) is responsible for the majority of adenosine transport across the plasma membrane and is ubiquitously expressed in both humans and mice. However, the contribution of ENT1-mediated adenosine levels has not been studied in bone remodeling. With the recent identification of the importance of adenosine signaling in bone homeostasis, it is essential to understand the role of ENT1 to develop novel therapeutic compounds for bone disorders. Here we examined the effect of ENT1 deletion on bone density using X-ray, dual energy X-ray absorptiometry and micro-computerized tomography analysis. Our results show that bone density and bone mineral density is reduced in the lower thoracic and lumbar spine as well as the femur of old ENT1 null mice (>7 months) compared to wild-type littermates. Furthermore, we found increased mRNA expression of tartrate-resistant acid phosphatase (TRAP), an osteoclast marker, in isolated long bones from 10 month old ENT1 null mice compared to wild-type mice. In addition, aged ENT1 null mice displayed severe deficit in motor coordination and locomotor activity, which might be attributed to dysregulated bone density. Overall, our study suggests that ENT1-regulated adenosine signaling plays an essential role in lumbar spine and femur bone density.
H 132
Adenosine A2Areceptor as a potential new therapeutic target for the prevention/treatment of osteoarthritis
Carmen Corciulo*, Aranzazu Mediero*, Tuere Wilder* and Bruce Cronstein*
NYU School of Medicine, Division of Translational Medicine, Department of Medicine, New York, USA
Purpose: Osteoarthritis results from trauma, mechanical factors or metabolic changes in bone and cartilage. Adenosine, acting via the A2AR, inhibits inflammation and plays a critical role in regulating bone metabolism. Aging A2AKO mice experience difficulty in movement, taking food and walking. We determined whether changes in their bone or joint structure or function could explain these changes.
Methods: Four month old C57Bl/6 wild type (WT) and A2AKO mice (n = 4) were sacrificed and knee joints were prepared for microCT and histology. PAS (Periodic Acid Staining), Safranin-O and Trichrome staining were carried out. MicroCT analysis of knees was performed on the distal femur below the growth plate. Immunostaining for MMP-13 and Collagen-X were performed. Human immnortalized chondrocytes (TC28a2) and primary osteoarthirtic chondrocytes were treated with A2AR agonist CGS21680 1 μM in the presence/absence of the A2AR antagonist ZM241385 1 μM. Collagen-X, MMP-13 and b-catenin were analyzed by WesternBlot and ELISA.
Results: microCT analysis of A2AKO mice knees showed osteophyte formation together with remodeling and subchondral sclerosis when compared to WT. Bone volume/total volume was significantly decreased in A2AKO mice when compared to WT (33.324 ± 0.56 vs 35.782 ± 0.78, respectively, p < 0.01). Trabecular thickness was also decreased (0.0685 ± 0.0035 vs 0.081 ± 0.002, p < 0.05) together with bone mineral density (0.3795 ± 0.003 vs 0.4265 ± 0.01, respectively, p < 0.5). Trichrome, Safranin-O and PAS staining of A2AKO articular cartilage showed chondrocyte hyperthrophy with a reduction of collagen and proteoglycans. Immunostaining for MMP-13 and Collagen-X showed an increased in these biomarkers in A2AKO mice. In both TC28a2 and primary human chondrocytes, activation of A2AR by CGS21680 induced a significant decrease in Collagen-X and MMP-13 expression and release, with an increase in b-catenin expression. These effects are reversed by pretreatment with ZM241385.
Conclusions: Deficiency in adenosine A2AR leads to spontaneous osteoarthritis and suggests that A2AR may be novel targets for development of therapies to ameliorate or prevent osteoarthitis.
H 133
Adult bone marrow stromal cells expressing ecto-NTPDase3 may be a novel therapeutic target for defective osteogenesis and bone mineralization
José Bernardo Noronha-Matos1, Isabel Calejo1, Maria Teresa Magalhães-Cardoso1, Isabel Silva1, Fátima Ferreirinha1, Rui Rocha2, José Marinhas2, Rolando Freitas2, Maria Adelina Costa1,3, Jean Sévigny4 and Paulo Correia-de-Sá1,*
1ICBAS—University of Porto, Lab. Farmacologia e Neurobiologia, UMIB, Porto, Portugal;2Centro Hospitalar de Gaia-Espinho, Serv. Ortopedia e Traumatologia, V.N. Gaia, Portugal;3ICBAS—University of Porto, Departmento Química, Porto, Portugal;4Centre de Recherche du CHU de Québec, Québec, QC, Canada
Bone marrow stromal cells (BMSCs) are able to differentiate into osteoblasts, the bone-forming cells. This phenomenon is significantly impaired in elderly individuals resulting in bone loss. Nucleotides, via P2 purinoceptors, are important regulators of osteogenic differentiation of BMSCs, which action is balanced by their enzymatic degradation by ectoenzymes. Our group demonstrated that enzymatic inactivation of extracellular nucleotides leads to a loss of function of P2Y6 and P2X7 purinoceptors in BMSCs from postmenopausal women (56–83 years old, n = 12) as compared to younger females (13–40 years old, n = 9) [1]. We also showed that ecto-NTPDase3 is overexpressed in BMSCs from elderly women. This prompted us to investigate whether osteogenic differentiation of postmenopausal BMSCs could be improved by inhibitors of ecto-NTPDase3.
Inhibition of ecto-NTPDase3 with PSB06126 (3 μM) increased by 3.5-fold the endogenous levels of ATP in BMSC cultures from postmenopausal woman (n = 3). PSB06126 (3 μM) increased the alkaline phosphatase activity by 43 ± 17 % at culture day 21 and promoted the expression of osteogenic differentiation markers, such as Runx2 (2 to 5-fold increase) and Osterix (1.4 to 1.7-fold increase), throughout the culture period (n = 3). PSB 06126 (3 μM) also increased the mineralization of bone-nodules by 261 ± 33 % as evaluated by the Alizarin-red test. The mineralization effect of PSB 06126 was mimicked by a monoclonal mouse anti-human NTPDase3 antibody (hN3-B3S, 0.5 μg/ml), which specifically inhibits the activity of this enzyme [2]. The osteogenic effect of PSB 06126 (3 μM) was prevented by apyrase (2.5 U/ml, ecto-diphosphohydrolase or CD39), as well as by the selective blockade of P2X7 and P2Y6 receptors with A438079 (3 μM) and MRS 2578 (100 nM) (n = 3), respectively.
Data suggest that overexpression of ecto-NTPDase3 negatively modulates P2 purinoceptors activity in BMSCs from postmenopausal woman. Thus, we propose ecto-NTPDase3 as a previously unrecognized therapeutic target for disorders related to bone mass loss, such as osteoporosis.
Work supported by FCT (FEDER funding and COMPETE, projects PTDC/SAU-OSM/73576/2006 and Pest-OE/SAU/UI215/2014). JBNM is in receipt of a PhD Scholarship from FCT (SRFH/BD/68584/2010).
References
1. Noronha-Matos et al (2012) J Cell Physiol 227:2694
2. Munkonda et al (2009) FEBS J 276:479
H 134
P2Y4and P2Y14receptors weaken the osteogenic differentiation potential of bone chip-derived stem cells
Constanze Kaebisch1,*, Martin Winter2, Roua Zodi1, David Werheid1 and Edda Tobiasch1
1Bonn-Rhine-Sieg University of Applied Sciences, Department of Natural Sciences, Rheinbach, Germany;2Oral Surgical Practice, Rheinbach, Germany
The demographic change in industrialized countries and a risky lifestyle of the young generation create a strong need for bone reconstructions. Autologous mandibular bone chips contain ecto-mesenchymal stem cells with a stronger osteogenic commitment compared to adipose tissue-derived stem cells, which makes them ideal grafting material for Regenerative Dentistry.1 During the stem cell maturation into osteoblasts the P2 receptor gene expression profile is altered.2 However, the impact of P2 receptor signalling in osteogenesis has not been investigated so far.
Human ecto-mesenchymal stem cells were isolated from bone chip material harvested during oral surgery intervention. Their stem cell character was demonstrated by the expression of the specific surface markers CD73, CD90 and CD105. The osteogenic differentiation was monitored by Alizarin Red S staining of calcium deposits in the extracellular matrix. During the mineralization process P2Y4 and P2Y14 are down-regulated. In addition, the administration of the artificial P2Y4 agonist MRS 4062 triethylammonium salt and P2Y14 agonist MRS 2690 resulted in a dose-dependent reduction of extracellular calcium deposition.
Taken together, mandibular bone chips contain ecto-mesenchymal stem cells capable to differentiate into osteoblasts. Application of potent P2 receptor ligands confirmed the functional role of P2Y4 and P2Y14 during osteogenesis. In the future, selective P2Y4 and P2Y14 receptor antagonists might be applied to further reduce negative side-effects such as tumour formation thus improving bone tissue engineering approaches.
References
1. Kaebisch C, Winter M, Kleinfeld C, Khan D, Oligschleger C, Tobiasch E (submitted)
2. Zippel N, Limbach CA, Ratajski N, Urban C, Luparello C, Pansky A, Kassack MU, Tobiasch E (2012) Stem Cells Dev 21(6):884–900
H 135
Adenosine receptors stimulate bone regeneration by targeting osteoclasts.
Aranzazu Mediero*, Tuere Wilder* and Bruce Cronstein*
NYU School of Medicine, Division of Translational Medicine, Department of Medicine, New York, USA
Purpose: Various types of orthopedic procedures, including spinal fusion and repair of bone defects due to trauma, infection or metastatic disease, require formation of new bone. Adenosine, generated from the catabolism of adenine nucleotides, modulates cell function by interacting with specific cell-surface receptors (A1R, A2AR, A2BR, A3R). We have previously reported that A1R receptor blockade and A2AR stimulation inhibit osteoclast (OC) differentiation but only A2BR stimulation affects osteoblast (OB) differentiation or function. We determined whether A1R blockade, A2AR stimulation or enhancing adenosine concentrations via blockade of purine transport into cells via ent1 with dipyridamole regulates bone formation in a murine calvarial model.
Methods: Male C57Bl/6 mice were anesthetized; a 3 mm trephine defect was formed and covered with a collagen scaffold soaked in saline or 1 mM adenosine receptor agonists/antagonists. Animals received appropriate treatment daily until sacrifice. Bone Morphogenetic Protein 2 (BMP-2) 200 ng was used as bone formation control. At 0, 2, 4, 6 and 8 weeks calvarias were harvested and prepared for microCT and histology. XenoLight Rediject Bone Probe 680 was injected intravenously at different time points.
Results: Eight weeks after surgery microCT examination of mouse calvaria demonstrate that an A1R antagonist (DPCPX), A2AR (CGS21680M) or dipyridamole markedly enhances bone regeneration in a similar way as BMP-2 (77 ± 0.2 %, 60 ± 2 %, 79 ± 2 % and 75 ± 1 % bone regeneration, respectively, vs. 32 ± 4 % in control, p < 0.001, n = 5 mice per condition) whereas an A3R agonist (IB-MECA) had no effect (32 ± 3 % regeneration, n = 5). Both CGS21680 and Dipyridamole effects were abrogated by ZM241385 (A2AR antagonist, 20 ± 3 % and 26 ± 4 % bone regeneration, respectively, vs. 32 ± 4 % in control, p = ns, n = 5 mice per condition). In DPCPX-, CGS21680- and dipyridamole-treated mice there is increased immunostaining for osteoblast or bone formation markers (Alkaline Phosphatase, Osteocalcin and Osteonectin) in the bony defects (Alkaline Phosphatase positive cells/hpf increased from 15 ± 1 for control to 22 ± 1 for DPCPX, 21 ± 1 for CGS21680 and 24 ± 1 for Dipyridamole, p < 0.001), and diminished inmunostaining for macrophages (CD163, TNfa) and osteoclasts (RANKL, RANK) in treated defects when compared to control. TRAP staining revealed fewer OCs in DPCPX-, CGS21680- and Dipyridamole-treated defects (14 ± 1, 17 ± 1 and 16 ± 1 OC/hpf respectively vs. 24 ± 1 Osteoclast/hpf for control, p < 0.001) 8 weeks after defect formation. In vivo imaging with XenoLight Rediject Bone Probe 680 (a marker of bone formation) reveals a strong fluorescent signal in treated animals (DPCPX, CGS21680 and Dipyridamole), equivalent to BMP-2 signal, when compared to control as soon as 1 week after bone defect formation and lasting for at least 7 weeks.
Conclusions: Inhibition of OC formation via A2AR stimulation, A1R blockade or increasing local adenosine concentration stimulates new bone formation, in a similar was as BMP2, and represents a novel approach to stimulating bone regeneration.
H 136
Stimulation of the adenosine A2Areceptor (A2AR) regulates the expression of Netrin1 and their receptors (Unc5b, DCC) and inhibits osteoclast differentiation and wear particle-induced (UHMWPE) inflammatory osteolysis
Aranzazu Mediero1,*, Bhama Ramkhelawon2, Miguel Perez-Aso1, Kathryn Moore2 and Bruce Cronstein1
1NYU School of Medicine, Division of Translational Medicine, Department of Medicine, New York, USA;2NYU School of Medicine, Leon H. Charney Division of Cardiology, Department of Medicine, New York, USA
Purpose: A variety of molecules mediate communication between osteoclasts and osteoblasts during bone remodeling. Netrin1 is a member of the family of axonal guidance proteins, that regulates inflammation and macrophage function. Because osteoclasts are derived from myeloid precursors we asked whether osteoclasts expressed Netrin-1 and whether A2AR activation, which diminishes osteoclast differentiation, might regulate Netrin-1 expression and inflammatory osteolysis.
Methods: One centimeter midline sagittal incision was made over the calvaria in C57Bl/6 mice age 6–8 weeks. Mice received no particles (Control) or 3 mg of UHMWPE with 20 μl of saline 0.9 % or CGS21680 (A2AR agonist, 1 μM, n = 4 each) at the surgical site every day up to 14 days. After sacrifice, calvaria were prepared for immunostaining for Netrin1/Unc5b/DCC, sema3A/neuropilin 1. Protein and mRNA expression were studied by RT-PCR and WB in mouse and human primary bone marrow-derived OC and OB in the presence/absence of CGS21680 and ZM241385 (A2AR antagonist) 1 μM each.
Results: UHMWPE increased expression of Netrin1 and Unc5b but not DCC, an effect which was reversed by CGS21680. RANKL induced 25 ± 4 and 3 ± 0.5 fold change respectively, in Netrin1 and Unc5b mRNA, changes that were completely blocked by CGS21680 (1.17 ± 0.1, p < 0.001, n = 4). In contrast, DCC mRNA expression was not significantly changed during osteoclast differentiation and was unaffected by CGS21680 (1.16 ± 0.2 fold increase vs 1.9 ± 0.2 for RANKL, p = ns, n = 4). There was no change in Netrin1 or Unc5b during OB differentiation. Similar changes were observed in protein expression and secretion.
Conclusions: UHMWPE-induced osteolysis is regulated by Netrin1 activation. Adenosine A2AR normalizes expression of Netrin1 and inhibit the bony destruction found at sites of UHMWPE-induced osteolysis. These results suggest that targeting Netrin1 directly or via stimulation of adenosine A2AR may be a novel approach to preventing osteolysis and joint prosthesis loosening.
H 137
Adenosine A2Areceptor (A2AR) diminishes wear particle (UHMWPE)-mediated osteolysis, increases bone formation and regulates expression of axonal guidance proteins (AGP) by macrophages, osteoclasts (OC) and osteoblasts (OB)
Aranzazu Mediero*, Miguel Perez-Aso, Tuere Wilder and Bruce Cronstein
NYU School of Medicine, Division of Translational Medicine, Department of Medicine, New York, USA
Purpose: Communication between OC and OB is critical for maintenance of bone homeostasis. Among these signaling molecules are semaphoring 3A and 4D (sema3A and sema4D). During inflammatory osteolysis bone destruction is regulated by RANKL, M-CSF and TNF. A2AR ligation diminishes osteolysis and expression of RANKL, M-CSF and TNF. We asked whether A2AR activation modulates OB-OC crosstalk by regulating sema3A, sema4D or their receptors PlexinA1/Neuropilin1 and PlexinB1 respectively, in a model of inflammatory osteolysis.
Methods: 6–8 weeks old C57Bl/6 mice calvaria were exposed to 20 μl of PBS containing 3 mg of UHMWPE followed by daily injections of vehicle or CGS21680 (A2AR agonist) 1 μM (n = 4) for 14 days. XenoLight Rediject Bone Probe was injected IV and fluorescence of calvaria measured (IVIS) to assay bone formation. Immunostaining for sema3A, Sema4D and their receptors was performed. OC and OB differentiation were studied in primary murine bone marrow as the number of TRAP-positive or Alizarin Red-positive cells respectively, after challenge with CGS21680 and ZM241385 (A2AR antagonist) 1 μM each. Sema3A, Sema4D and their receptors expression were studied by RT-PCR and WesternBlot. RANKL and Osteoprotegerin (OPG) levels were studied by RT-PCR. β-catenin activation was studied in primary OB culture.
Results: XenoLight imaging revealed a 53 ± 6 % reduction in bone formation after exposure to UHMWPE (p < 0.001) and CGS21680 completely reversed this effect (11 ± 5 % greater than control bone formation, p = ns). In UHMWPE-exposed calvaria there was a decreased number of cells expressing Sema3A and PlexinA1 but not Neuropilin1, effects reversed by CGS21680. Sema4D/PlexinB1 expressing cells, primarily macrophages and OC, were increased in UHMWPE-exposed calvaria and CGS21680 reversed these changes as well. RANKL induced a 2.5 ± 0.1 fold increase in Sema4D mRNA (p < 0.001) which was blocked by CGS21680 (1.3 ± 0.3 fold change, p < 0.00). PlexinA1 mRNA was enhanced by CGS21680 (9 ± 0.7 fold increase vs 5 ± 0.6 for RANKL, p < 0.001) but Neuropilin-1 mRNA was unchanged. Sema3A mRNA increased 3.5 ± 0.5 fold during OB differentiation and CGS21680 enhanced this increase (9 ± 0.2 fold, p < 0.001); PlexinB1 mRNA was increased 2 fold during OB differentiation and was not altered by CGS21680. Similar changes were observed at the protein level. CGS21680 decreased RANKL expression and increased OPG expression in OB. Total and nuclear β-catenin expression were increased in OB after CGS21680 and this increase was abrogated by ZM241385.
Conclusions: UHMWPE-induced inflammatory osteolysis involves bone destruction and reduction of new bone formation. A2AR activation diminishes secretion of Sema4D by OC and enhances secretion of Sema3A by OB, leading to an increase in OB differentiation and function, than in combination with the suppressive effects of A2AR on OC differentiation and function, improves bone production.
H 138
Role of purinergic signaling and hypoxia in cellular bioenergetics and angiogenic responses of pulmonary arteryvasa vasorumendothelial cells
Martin Lapel1, Petr Paucek2, Tasas Lyubchenko3, Nana Burns1, Kurt Stenmark1 and Evgenia Gerasimovskaya1,*
1University of Colorado Denver, Pediatrics, Aurora, USA;2University of Colorado Boulder, Intergative Physiology, Boulder, USA;3University of Colorado Denver, Medicine, Aurora, USA
Cell proliferation and differentiation are energy demanding processes, however, the role of cellular metabolism in angiogenically active endothelial cells, as well as the regulation of cellular energy pathways by extracellular stimuli remain unexplored. Extracellular purines are widely recognized as important regulators of endothelial cell function [1–3]. Using a neonatal bovine model of hypoxia-induced pulmonary hypertension, we previously showed that ATP, an endogenous ligand for purinergic receptors P2Y and P2X, is released by endothelial cells and fibroblasts in response to hypoxia [4], and plays an autocrine/paracrine role in pulmonary artery vasa vasorum angiogenesis [5–7]. In this study we demonstrated that glycolysis and oxidative phosphorylation (OXPHOS) are both vital for ATP- stimulated VVEC mitogenesis, by using pharmacological inhibitors of glycolysis and mitochondrial respiration (2-DG, Rotenone and Oligomycin), as well as the pyruvate dehydrogenase kinase (PDK) inhibitor DCA. We also showed that vasa vasorum endothelial cells (VVEC) isolated from control animals (VVEC-Co) exhibited higher rates of OXPHOS compared to VVEC isolated from their chronically hypoxic counterparts (VVEC-Hx). Measurement of OXPHOS in digitonin-permeabilized VVEC demonstrated that chronic hypoxia, in vivo and in vitro, significantly decreased basal, Complex I, and Complex II mitochondrial respiratory activity. A decreased expression level of F1Fo ATP synthase b-subunit and cytochrome c oxidase subunit IV accompanied those results, suggesting hypoxia-induced persistent changes in VVEC bioenergetic phenotype. Culturing VVEC in the presence of galactose (20 mM, 24 h) resulted in significant increases in maximal respiratory rate (state III uncoupled) in VVEC-Co but not in VVEC-Hx. Additionally, stimulation with ATP, MeSADP, and adenosine increased basal and maximal respiratory rates in VVEC cultured for 24 h in medium containing either glucose (25 mM) or galactose (20 mM). Lastly, our data revealed that extracellular ATP induced transient increases of Ca2+ in VVEC mitochondria, which is consistent with an increased OXPHOS rate. Together, our data demonstrated that purinergic and hypoxic regulation of VVEC angiogenesis requires functional glycolysis and OXPHOS cellular energy pathways and can be modulated by exogenous metabolic substrates. In perspective, these studies may suggest novel therapeutic approaches for regulation of hypoxia-induced angiogenesis via simultaneous targeting of purinergic receptors and cellular metabolic pathways.
References
1. Burnstock G (2002) Arterioscler Thromb Vasc Biol 22:364–373
2. Erlinge D and Burnstock G (2008) Purinergic Signal 4:1–20
3. Bours MJ et al (2006) Pharmacol Ther 112:358–404
4. Gerasimovskaya EV et al (2002) J Biol Chem 277:44638–44650
5. Gerasimovskaya EV et al (2008) Angiogenesis 11:169–182
6. Woodward HN et al (2009) Am J Physiol Lung Cell Mol Physiol 297:L954–L964
7. Lyubchenko T et al (2011) Am J Physiol Cell Physiol 300:C266–C275
Support R01 HL086783-05 (E.V. Gerasimovskaya)
H 139
Release of adenosine in brown adipose tissue during sympathetic activation
Saskia Scheibler1,2,*, Thorsten Gnad1, Ivar von Kügelgen1 and Alexander Pfeifer1
1Institute of Pharmacology and Toxicology, Sigmund-Freud-Str. 25, D-53105 Bonn, Germany;2Research Training Group 1873, University of Bonn, 53127 Bonn, Germany
Brown adipose tissue (BAT) plays an important role in the energy expenditure in mammals due to its property to induce non-shivering thermogenesis. Since the recent discovery of metabolically active BAT in human adults [1] much attention has been paid to the tissue as a pharmacological target to the world-wide obesity problem.
So far the major focus of research was on the neurotransmitter noradrenaline (NE), which is released from sympathetic neurons. NE activates brown adipocytes (BA), but also purinergic co-transmitter might play a role in regulation of lipolysis. Previous studies [2] have demonstrated a regulatory role of adenosine in BAT. Here, we wanted to study the source of adenosine.
BAT was isolated from neonatal mice. To study NE release BAT was incubated with 3H-NE. Electrical field stimulation (10 Hz, EFS) was applied to stimulate sympathetic nerves in BAT. Subsequently the flow-through was collected, radioactivity was counted and ATP was measured using luciferase bioluminescence assay. Adenosine was measured as described by Helenius [3]. EFS induced a 5-fold (+/− 0.5 fold) increase of 3H-NE, concomitantly with a 7-fold (+/− 0.5 fold) increase of ATP release.
Importantly, EFS induced a 7-fold (+/− 0.5 fold) increase of adenosine concentrations in the flow-through of BAT. Release of NE, ATP and adenosine was inhibited when tetrodotoxin (TTX), which blocks voltage-gated sodium channels and neuronal action potentials, was added to BAT.
In conclusion, the EFS data indicate purinergic co-transmission during sympathetic stimulation in BAT; adenosine is released together with NE and ATP in BAT.
References
1. Virtanen KA et al (2009) Functional brown adipose tissue in healthy adults. N Engl J Med 360:1518–1525
2. Schimmel RJ et al (1984) Role of adenosine as an endogenous regulator of respiration in hamster brown adipocytes. Am J Physiol 246:C301–C307
3. Helenius et al (2012) Enzyme-coupled assays for simultaneous detection of nanomolar ATP, ADP, AMP, adenosine, inosine and pyrophosphate concentrations in extracellular fluids. Biochim Biophys Acta 1823(10):1967–1975
J: Purinergic signaling in immunology and inflammation
J 140
ATP evoked intracellular calcium dynamics in human monocytes and polarised macrophages
Lisa Burrows* and Samuel Fountain
University of East Anglia, Biosciences, Norwich, UK
Extracellular ATP is a damage-associated molecular pattern molecule (DAMP) that can stimulate cells of the innate immune system via activation of cell surface P2 receptors for ATP. P2 receptor activation can elicit intracellular calcium signals either through direct permeation (P2X ionotropic receptors) or via mobilisation of intracellular calcium stores (P2Y metabotropic receptors). Here we investigate ATP-evoked calcium signals in human monocytic cells and polarised macrophages. Monocytes (THP-1) cells were stimulated with PMA (320nM, 48 h) to derived macrophages (M0). M0 macrophages were polarised to M1 inflammatory or M2 anti-inflammatory phenotypes by further treatment with LPS (100 ng/mL) and IFNγ (20 ng/mL), or IL-4 (20 ng/mL) for 24 h, respectively. Intracellular calcium measurements were made in Fura-2 loaded cells using a Flexstation III reader. ATP evoked concentration-dependent calcium responses in all leukocytes but with varying potency; monocytes (EC50 0.1 μM) > M1 macrophage (EC50 7 μM) > M0 macrophage (EC50 16 μM) > M2 macrophage (EC5061μM). The magnitude of calcium response at maximal ATP concentrations (100 μM) also varied with all macrophage phenotypes displaying smaller responses than monocytes; 41 ± 3 (M0), 59 ± 2 (M1) and 21 ± 1 (M2) percentage peak response compared to monocytes (p < 0.001).
Furthermore we explored the dependency of ATP-evoked calcium signals on extracellular calcium. ATP responses in monocytes were highly dependent upon extracellular calcium with its removal causing a 92 % reduction in peak response to ATP (100 μM). Interestingly, responses in M1 and M0 macrophages showed no dependency on extracellular calcium, indeed responses showed a small increase by 14 % and 16 %, respectively. In contrast responses in M2 macrophages at 100 μM ATP were significantly reduced (16 %, P < 0.05) in the absence of extracellular calcium. In the absence of extracellular calcium ATP evoked responses with varying potency; monocytes (EC50 0.1 μM) > M1 macrophage (EC50 19 μM) > M0 macrophage (EC50 32 μM) > M2 macrophage (EC50 92 μM).
This study illustrates the response to extracellular ATP of monocytes and monocyte-derived cells is highly dynamic and displays a varied dependency on calcium entry to shape the response, with monocytes being most sensitive to ATP but highly dependent on calcium influx. These data also support observations of a dampened ATP response in M2 anti-inflammatory phenotypes versus pro-inflammatory cell types (monocytes and M0, M1 macrophages).
J 141
The combination of ATP and potassium ions is deadly for astrocytes
Gerhard Dahl1,*, David Jackson1, Junjie Wang1, Robert Keane1 and Eliana Scemes2
1University of Miami, Physiology and Biophysics, Miami, USA;2Albert Einstein College of Medicine, Neuroscience, New York, USA
The ATP release channel Pannexin1 (Panx1) is inhibited by its own permeant, i.e. ATP inhibits the channel from the extracellular space. The affinity of the ATP binding site is lower than that of the purinergic P2X7 receptor allowing a transient activation of Panx1 by ATP through P2X7R. We have found that the inhibition of Panx1 by ATP is abrogated by increased extracellular potassium ion concentration ([K+]o) in a dose-dependent manner. Since increased [K+]o is also a stimulus for Panx1 channels, it can be expected that a combination of ATP and increased [K+]o would be deadly for cells. Indeed, astrocytes did not survive exposure to these combined stimuli. The death mechanism, although involving P2X7R, does not appear to strictly follow a pyroptotic pathway. Instead, caspase-3 was activated, a process inhibited by Panx1 inhibitors. These data suggest that Panx1 plays an early role in the cell death signaling pathway involving ATP and K+ ions. Additionally, Panx1 may play a second role once cells are committed to apoptosis, since Panx1 is also a substrate of caspase-3.
J 142
Inhibitors of the 5-lipoxygenase arachidonic acid pathway induce ATP release and ATP-dependent organic cation transport in macrophages
Hercules Antonio Da Silva-Souza1,2, Maria Nathalia Lira2, Helio Miranda Costa-Junior1, Cristiane Monteiro Da Cruz1, Jorge Silvio Silva Vasconcellos1, Anderson Nogueira Mendes1, Gabriela Pimenta-Reis1,2, Cora Lilia Alvarez1,2, Lucia Helena Faccioli3, Carlos Henrique Serezani4, Julieta Schachter1,2 and Pedro Muanis Persechini1,2
1Federal University of Rio de Janeiro, Institute of Biophysics Carlos Chagas Filho, Rio de Janeiro, Brazil;2INPeTAm, National Institute of Science and Technology of Translational Research in Health and Enviroment of the Amazon Region, Rio de Janeiro, Brazil;3Faculty of Pharmaceutical Sciences of Ribeirão Preto, Department of Clinical and Toxicological Analysis Bromatological, Ribeirão Preto, Brazil;4Indiana University School of Medicine, Department of Microbiology and Immunology, Indianapolis, Brazil
Text: We have previously described that arachidonic acid (AA)-5-lipoxygenase (5-LO) metabolism inhibitors such as NDGA and MK886, inhibit cell death by apoptosis, but not by necrosis, induced by extracellular ATP (ATPe) binding to P2X7 receptors in macrophages. ATPe binding to P2X7 also induces large cationic and anionic organic molecules uptake in these cells, a process that involves at least two distinct transport mechanisms: one for cations and another for anions In this study we have examined the effects of NDGA and MK886 on the membrane murine macrophage.
Methods and Results: We have used intracellular Ca2+ measurements, flow cytometry and fluorescent dye uptake assays to study the transport of large organic dyes in thioglycollate-elicited murine peritoneal macrophages. We also have measured the release of ATP in these cells with a luminometer. Our results show that inhibitors of the AA-5-LO pathway induce two new transport phenomena in macrophages: a cation-selective uptake of fluorescent dyes and the release of ATP. The release of ATP was blocked by brefeldin A, suggesting the involvement of an exocytic process. The cation organic uptake requires secreted ATPe and was blocked by apyrase, but, differently from the P2X7/ATPe-induced phenomena, it is also present in macrophages derived from mice deficient in the P2X7 gene. Furthermore, the mechanism induced by NDGA and MK886 do not inhibit the ATPe-induced uptake of fluorescent anionic dyes related to activation of P2X7 receptors in macrophages. In addition, we describe that inhibitors of phospholipase A2 and of the AA-cyclooxygenase pathway did not induce the cation uptake. Finally, the uptake of non-organic cations was investigated by measuring the free intracellular Ca2+ concentration ([Ca2+]i) by Fura-2 fluorescence. NDGA, but not MK886, induced an increase in [Ca2+]i. Chelating Ca2+ ions in the extracellular medium suppressed the intracellular Ca2+ signal without interfering in the uptake of cationic dyes.
Conclusion: We conclude that inhibitors of the AA-5-LO pathway do not block P2X7 receptors, trigger the release of ATP and induce an ATP-dependent uptake of organic cations by a Ca2+- and P2X7-independent transport mechanism in macrophages.
Reference
1. Da Silva-Souza, HA et al (2014) Inhibitors of the 5-lipoxygenase arachidonic acid pathway induce ATP release and ATPdependent organic cation transport in 8139 macrophages, BBA - Biomembranes. doi: 10.1016/j.bbamem.2014.04.006
J 143
Immuno-suppression by pharmacological preconditioning with adenosine A1receptor agonist
Oshri Naamani, Cidio Chaimovitz and Amos Douvdevani*
Soroka University Medical Center, Clinical Biochemistry and Pharmacology, Beer-Sheva, Israel
Background: Under stressful conditions such as ischemia, sepsis, and severe trauma, adenosine levels are elevated and protect the tissue by interaction with its G coupled receptors. In a model of peritonitis, we previously shown that pharmacological preconditioning (PPC) of mice with a selective adenosine A1 receptor (A1R) agonist, 2-chloro-N(6)-cyclopentyladenosine (CCPA), induced the A2AR which reduces cytokine secretion and leukocyte recruitment. In our present study we determined whether mice PPC will moderate cellular immune response and attenuates the immune reaction to allograft challenge.
Methods: For PPC, mice (BALB/c) were injected with the A1R agonist (CCPA 0.1 mg/kg) twice (24 & 12 h booster) with or w/o daily IP injection of suboptimal dose of cyclosporine A (CsA, 5 mg/Kg). In some experiments Balb/c mice were challenged with allogeneic subcutaneous graft (Pectoralis Major Muscle) from C57Bl/6 mice in the nape. Following PPC or 10 days post PPC and allogeneic challenge, spleens were removed for mitogen T-cell response or mixed lymphocyte reaction (MLR).
Results: Similar to the effect on inflammation, PPC reduced the response to lymphocytes mitogens and allogeneic MLR response. Combined treatment with PPC and suboptimal CsA (5 mg/Kg) yield additive effect and suppressed proliferation more than sole treatment with high dose of CsA (20 mg/Kg).
The inhibitory effect of PPC on the immune response was A1R and A2AR dependent as illustrated by experiments with antagonists of these receptors and mice with knock out (KO) receptors. In MLR with PPC splenocytes we found reduced levels of pro-inflammatory cytokines (IFN-γ, IL-15, TNF-α) and elevation of IL-10, as well as elevation of regulatory T-cell.
Conclusion: Our data indicate that PPC is able to remarkably suppress cellular immune response due to the sensitization A2AR. This effect of PPC sheds light on the protective role of adenosine in ischemic preconditioning and makes this treatment candidate for prevention of graft rejection.
J 144
Adenosine A2Areceptor agonist, CGS21680, inhibits inflammation and increases FGF-2 tissue expression in carrageenin induced rat paw edema
Elisabetta Caiazzo1, Alfredo Carmineri1, Silvana Morello2, Armando Ialenti1 and Carla Cicala1,*
1University of Naples Federico II, Department of Pharmacy, Naples, Italy;2University of Salerno, Department of Pharmaceutical and Biomedical Sciences, Fisciano, Salerno, Italy
Adenosine A2A receptor has been described to be antinflammatory in several models of acute and chronic inflammation. Furthermore, the same receptor is also involved in adenosine-mediated wound healing in vivo; in vitro, in primary human dermal fibroblasts, there is evidence that A2A receptor mediates the collagen-inducing effect of adenosine. Fibroblast growth factor-2 (FGF-2) is a growth factor playing a role in tissue proliferation, wound healing and also in inflammation. High levels of FGF-2 have been found in bronchoalveolar lavage fluids (BALFs) from asthmatic subjects; moreover, in a murine model of asthma, recombinant FGF-2 has been shown to reduce airway responsiveness; furthermore, FGF-2 has also been shown to reduce acute inflammation.
In the present study we have investigated on the possible involvement of FGF-2 in the antinflammatory effect of the A2A agonist, CGS21680.
All experiments were performed on male Wistar rats (Charles River; 120–150). Rats were slightly anaesthetized with enflurane and treated with the selective A2A receptor agonist, CGS21680 (2 mg/kg ip.) or with an equal volume of vehicle (DMSO) just before carrageenin (1 % w/v in saline, 100 μl) was injected in the left hind paw. Edema was measured by the means of a hydroplethismometer at time zero and each hour for the following 6 h.
Western blot and immunofluorecence analysis for A2A and FGF-2 expression and localization were performed on inflamed tissues excised at different time following edema induction. Controlateral, not injected paws, were also excised as control tissues
We found that rats treatment with CGS21680 inhibited in a dose related manner carrageenin-induced paw edema. The effect of CGS21680 was reverted by co-administration of A2A antagonist, ZM241385 (3 mg/kg ip.) .
Western blot analysis performed on paw excised at different time after edema induction showed an increased A2A protein expression starting 1 h following oedema induction, and peaking between 3 and 4 h, that was reduced to control values by CGS21680 treatment. Conversely, FGF-2 expression in excised rat paws was increased each hour following treatment with CGS21680. Immunofluorescence analysis showed increased immunostaining for FGF-2 localized in the derma of paws from CGS21680 treated animals.
Our results suggest that FGF-2 might play a role in the antinflammatory effect mediated by adenosine A2A receptor activation.
J 145
CD73-dependent adenosine formation restores neuromuscular transmission and immunological competence in experimental autoimmuneMyasthenia gravisvia A2Areceptors activation
Laura Oliveira1, Cristina Costa1, Sónia Guerra-Gomes1, Alexandra Correia2, Fátima Ferreirinha1, Maria Teresa Magalhães-Cardoso1, Manuel Vilanova2 and Paulo Correia-de-Sá1,*
1ICBAS—University of Porto, Lab. Farmacologia e Neurobiologia, UMIB, Porto, Portugal;2ICBAS—University of Porto, Lab. Imunologia and IBMC, Porto, Portugal
Myasthenia gravis (MG) is a B-cell-mediated T-cell dependent neuromuscular disease characterized by use-dependent muscle weakness. Adenosine (ADO) is a ubiquitous molecule acting as a potent modulator of both neuromuscular and immunological responses via the activation of A2A receptors (A2AR) [1,2]. AMP dephosphorylation via ecto-5′-nucleotidase/CD73 is the rate limiting step to generate extracellular ADO from released adenine nucleotides. The immunosuppressive and neuroexcitatory properties of A2AR and the pivotal role of ecto-5′-nucleotidase/CD73 in controlling extracellular ADO formation, prompted us to investigate their participation in the pathogenesis of autoimmune MG.
In this study, we used a model of experimental autoimmune myasthenia gravis (EAMG) in Wistar rats. Experimental animals were immunized with the R97-116 peptide, a synthetic peptide corresponding to a specific region on the α subunit of the rat nicotinic AChR, made up in Complete Freund’s Adjuvant (CFA) [3]. Control animals received the CFA emulsion without the peptide; animals from the naive group were not submitted to treatment.
Flow cytometry analysis demonstrate that CD4+CD25+FoxP3+ regulatory T cells express lower amounts of ecto-5′-nucleotidase/CD73 and A2AR as compared to control animals. Moreover, deficits in endogenous ADO formation might contribute to neuromuscular transmission failure in EAMG rats since restoration of the A2AR-mediated facilitation of transmitter release was observed upon incubation with the nucleoside precursor, AMP.
These findings, together with the well-known increase in serum adenosine deaminase activity that is characteristic of MG, strengthen the hypothesis that the adenosinergic pathway may be dysfunctional in EAMG. Given that endogenous ADO formation is balanced by ecto-5′-nucleotidase/CD73 activity and that A2AR exert a dual role to restore use-dependent neurocompetence and immune suppression in myasthenics, one may hypothesize that stimulation of the two mechanisms might have therapeutic impact in MG.
Work supported by FCT (PTDC/SAU-FCF/108462/2008 and PEst-OE/SAU/UI0215/2011) and by U. Porto/Santander Totta (PP-IJUP2011-232). We also thank Mrs. M. Júlia Reis from CHP-HSA for technical support on ADA activity analysis and Dr. Barbara Oliveira for animal handling
References
1. Correia-de-Sá et al (1991) Br J Pharmacol 103:1614–1620
2. Csoka et al (2008) FASEB J 22:3491–3499
2. Baggi et al (2004) J Immunol 172:2697–2703
J 146
Activation of P2X7R and downstream effects in bleomycin treated lung epithelial cells
Robert Bläsche*, Georg Ebeling, Michael Kasper and Kathrin Barth
Institute of Anatomy, Medical Faculty “Carl Gustav Carus”, TU Dresden
BLM induces a first wave of cell damage with ATP release leading to lung inflammation [1]. Lung epithelial cells have several receptors that could interact with extracellular ATP, including P2X4R and P2X7R. The P2X7R, an ATP-gated ion channel, is expressed in alveolar epithelial type I cells. Its biological function in this epithelium is unknown.
Our previous studies have shown that the N-glycosylated P2X7R is localized in the plasma membrane and that they are partly associated with caveolae, containing the structure protein caveolin-1. We have shown that the P2X7R interact with caveolin-1 in alveolar epithelial type I cells [2].
Here we report that the treatment of lung epithelial cells with BLM resulted in elevated intracellular Ca2+ levels. BLM further increased P2rx7 mRNA expression and P2X7R protein levels, paralleled by increased PKC-b1 levels. BLM treatment or stimulation of the P2X7R with the P2X7R agonist BzATP induced translocation of PKC-b1 from the cytoplasm to the membrane. The expression of PKC-b1 was repressed by the P2X7R inhibitor oxATP, suggesting that PKC-b1 is downstream of P2X7R activation. Furthermore, cells exposed to BLM contained increased amounts of P2X7R and PKC-b1 in caveolin-1 containing lipid raft fractions.
The present experiments demonstrated that the increased expression of P2X7R influences PKC-b1. We predict that increased Ca2+ concentration stimulates PKC-b1, whereas the prerequisite for activating PKC-b1 after P2X7R increase remained to be determined. Our findings suggest that PKC-ß1 is involved in the establishment of lung inflammation and subsequent lung injury leading to fibrotic processes.
References
1. O'Neill CA, Giri SN (1994) Exp Lung Res 20:41–56
2. Weinhold K, Krause-Buchholz U, Rodel G, Kasper M, Barth K (2010) Cell Mol Life Sci 67:2631–2642
J 147
P2X7 receptor-deficient mice are susceptible toLeishmania amazonensisinfection due to decreased leukotriene B4production
Mariana Chaves*, Vanessa Figliuolo, Camila Marques-da-Silva, Cláudio Canetti and Robson Coutinho-Silva
Federal University of Rio de Janeiro, Biophysics Institute Carlos Chagas Filho, Rio de Janeiro, Brazil
Aims: P2X7 receptors activation has been reported as key component of host response against intracellular parasites. Our group has shown that extracellular ATP treatment controls Toxoplasma gondii, Chlamydia trachomatis and Leishmania amazonensis infection. Recently, we demonstrated that P2X7 activation leads to leukotriene B4 (LTB4) production and consequent L. amazonensis elimination. Furthermore, P2X7 receptor-deficient (P2X7−/−) mice were more susceptible to L. amazonensis infection when compared wild type (WT) mice (unpublished data). Thus, we evaluated if the susceptibility of P2X7−/− mice to L. amazonensis infection is due to an impaired LTB4 production.
Methods: C57Bl/6, SV129, P2X7−/−, and 5-lipoxygenase (LO)-deficient mice (5-LO−/−) were infected in the right paw with 106L. amazonensis promastigotes. After 7 days, P2X7−/− mice were treated with 5 ng of LTB4 in infect footpad twice a week for 3 weeks. Infection in 5-LO−/− mice was accompanied for 28 days and paw thickness and parasitic load were determined. Paw thickness was accompanied with thickness gauge and the parasitic load was established by limiting dilution assay (LDA). LTB4 levels were measured by EIA and nitric oxide (NO) levels were measured by Griess assay.
Results: P2X7−/− mice infected and treated with LTB4 showed more resistance to infection, since we found they present lower parasite load (difference between means 1 × 109 ± 5 × 108 parasites; n = 8) and lower lesion (difference between means 38 ± 11 mm, n = 8) when compared to P2X7−/−mice untreated. There was no difference in NO levels between treated and untreated animals. Culture of drain lymph nodes from P2X7−/−mice produced lower LTB4 levels when compared with those obtained from WT mice. The paw thickness and NO production in infected 5-LO−/− and WT mice were similar, despite of the more pronounced parasitic load in 5-LO−/− (difference between means 8×108 ± 5×107, n = 5). Therefore, these data suggest that P2X7−/− mice susceptibility to L. amazonensis infection is due to a decreased in LTB4 production, independently of NO secretion.
Funds: CNPq, FAPERJ and INPeTAm.
J 148
Major contribution of the P2X1 receptor in transfusion-related acute lung injury
Blandine Maitre1, Henri de la Salle1, Cecile Leguay1, Veronique Heim1, Stephanie Magnenat1, Richard J Evans2, Christian Gachet1 and Beatrice Hechler1,*
1Etablissement Français du Sang-Alsace, INSERM UMR_S949, STRASBOURG, France;2University of Leicester, Cell Physiology & Pharmacology, Leicester, UK
Question: Transfusion-related acute lung injury (TRALI) is the most prevalent remaining cause of transfusion-associated mortality and results from a combination of two events. One is related to the patient’s clinical condition which causes recruitment of neutrophils to the lungs, while the other involves transfusion of a blood product containing antibodies and/or substances released during storage which activate the primed neutrophils and lead to tissue damage. Since extracellular nucleotides and their P2 receptors are potentially involved in neutrophil functions, we investigated the role of the ATP-gated P2X1 cation channel in antibody-mediated TRALI in a mouse model.
Methods: TRALI was provoked by a lipopolysaccharide priming step followed by a challenge with an MHC I antibody. TRALI was estimated by (i) the rate of mortality of mice after 2 h, (ii) the measure of the lung edema, assessed by protein content in bronchoalveolar fluids and, (iii) the analysis lung inflammation, by quantifying the presence of neutrophils. The role of the P2X1 receptor was checked using the NF449 P2X1 antagonist (10 mg/kg) administered i.v. before LPS and also before the MHC I antibody injection.
Results: Within 2 h after MHC I injection, 70 % of control mice died, whereas only 30 % of NF449-treated mice died (P1 receptor in each event leading to TRALI), NF449 was administered either only before LPS sensitization or only before MHC I injection. In both cases, the rate of mortality was only slightly decreased, suggesting that the P2X1 receptor is involved both in the recruitment of neutrophils and in their activation. Indeed, we observed that P2X1 receptor inhibition impaired both LPS-induced recruitment of neutrophils to lung tissues and antibody-mediated activation of neutrophils, as demonstrated by their reduced CD11b exposure.
Conclusions: This study highlights a key role of the P2X1 receptor in TRALI and suggests that it represents a potential pharmacological target to prevent ALI related to transfusion or other neutrophil-dependent pathogenic events.
J 149
P2X7 receptor stimulation in mouse T lymphocytes triggers different signaling pathways according to their state of activation
Hanaa Safya*, Amine Mellouk and Pierre Bobé
INSERM U757, Université Paris-Sud, 91405 Orsay, France
Extracellular ATP plays a key role in innate immunity as a danger signal. It causes through receptor P2X7 (P2X7R), the activation of the inflammasome, enhancement of immune cell infiltration, cell death and resolution of inflammation. The role of the ATP/P2X7R pathway in adaptative immunity remains underestimated, although it has been reported that P2X7R regulates signalling events involved in T-cell activation, proliferation, and differentiation into effector lineages. ATP sensitivity of T lymphocytes varies among the different subsets, with CD8+ T cells being less sensitive than CD4+ T cells. Moreover, we have previously shown that effector T lymphocytes (either CD4+ or CD8+) that express the B220 isoform of CD45 at the plasma membrane during the process of activation-induced cell death are totally resistant to ATP stimulation due to loss of P2X7R membrane expression. In the present study, we investigate whether the different signaling pathways known to be triggered by P2X7R are similarly expressed in T lymphocytes as they differentiate from naive to effector/memory cells. Thus, CD69-negative CD62L+ T lymphocytes shed their CD62L molecules following ATP treatment, but loose this ability after the expression of the very early activation marker CD69. However, activated CD69+ T lymphocytes maintain their ability to externalize phosphatidylserine and form pore. Metalloproteases activity, which is responsible for CD62L shedding, is not defective in CD69+ T lymphocytes. The expression of CD69 occurs on antigen-stimulated B220−CD90+ T lymphocytes with low or high levels of CD44. Interestingly, CD62L shedding is induced in ATP-treated CD44low T lymphocytes (either naïve or recently activated), but not in effector/memory CD44high T lymphocytes (either CD69− or CD69+). Phosphatidylserine exposure and caspase 9 activation occur in both CD44low and CD44high T lymphocytes upon ATP treatment, but more efficiently on CD69+ T lymphocytes. Pore formation happens also in all stages of T-cell activation, but more efficiently in CD69+ CD44low T lymphocytes. We show herein that P2X7R signaling in T cells is far more complex than previously suspected, and the expression of each cellular response induced by the ATP/P2X7 pathway varies according to the stage of T-cell activation.
J 150
P2X7 receptor controls extracellular ATP during skin graft allogenic rejection
Maria Barbera-Cremades, Carlos Manuel Martinez, Alberto Baroja-Mazo, Joaquín Amores-Iniesta and Pablo Pelegrin*
Murcia BioHealth Research Institute, Murcia, Spain
Extracellular ATP acting on P2X7 receptors activates the NLRP3 inflammasome and the subsequent release of IL-1beta. P2X7 receptor activation requires high concentrations of ATP, which in vivo are found after severe tissue injury or stress. ATP activating the P2X7 receptor is considered a danger signal aimed to restore homeostasis by eliciting an inflammatory response. P2X7 receptor function has been implicated in different pathophysiological scenarios, including fever, contact hypersensitivity, graft-vs-host disease, lung inflammation or irritable bowel syndrome. Our recent work has investigated the release of ATP and P2X7 receptor signalling in allogeneic graft rejection, where immunity develops recognizing non-self antigens in the absence of microbes. In vivo models of skin transplantation showed that extracellular ATP is accumulated in response to allogeneic skin, but not syngeneic skin grafts. The release of ATP occurred before the adaptive immune response is established and the allogeneic tissue is destroyed and rejected. The mechanism for ATP release in vivo in response to allogeneic skin transplantation was highly dependent on macrophages acting as antigen presenting cells and involved the hemichannel pannexin-1, but not the NLRP3 inflammasome. Mechanistically, we found that the activation of the alloantigen presentation pathway in macrophages via the major histocompatibility complex type II was responsible to induce the release of ATP by the opening of pannexin-1 hemichannels and then positively amplified via P2X7 receptor. In vivo, P2X7 receptor deficiency or specific antagonism with A438079 reduced the levels of ATP release and delayed the rejection of allogeneic skin grafts. All this data suggest that P2X7 receptor controls the production of endogenous danger signals that modulate the immune system after the recognition of “non-pathogenic” and “non-self” signals.
This work was supported by grants from PN I+D+I 2008–2011-Instituto Salud Carlos III-FEDER (EMER07/049, PI09/0120, PI13/00174), Fundación Séneca (11922/PI/09).
J 151
P2X7 receptor induces the release of NLRP3 inflammasome to propagate inflammation
Alberto Baroja-Mazo, Fatima Martin-Sanchez, Ana Isabel Gomez, Maria Barbera-Cremades, Joaquín Amores-Iniesta, Carlos Manuel Martinez and Pablo Pelegrin*
Murcia BioHealth Research Institute, Murcia, Spain
The purinergic P2X7 receptor signal the assembly of the NLRP3-inflammasome that activates caspase-1 and mediates the processing and release of the leaderless cytokine IL-1beta, playing a central role in the inflammatory response and in diverse human diseases. The NLRP3-inflammasome is a multiprotein complex formed of the sensing protein NLRP3 (Nucleotide-binding domain and Leucine rich repeat Receptor containing a Pyrin domain 3), the adaptor protein ASC (Apoptotic Speck-like protein with a Caspase activating domain) and the effector protein caspase-1. Here we report that upon P2X7 receptor activation, both IL-1beta and inflammasome particles are secreted from macrophages. Extracellularly, inflammasome particles are able to recruit soluble ASC and upon internalization by surrounding macrophages stimulate caspase-1 activation. Our findings support a model whereby P2X7 receptor induces the release of inflammasome particles, acting as extracellular complexes that amplify the inflammatory response.
This work was supported by grants from PN I+D+I 2008–2011-Instituto Salud Carlos III-FEDER (EMER07/049, PI09/0120, PI13/00174), Fundación Séneca (11922/PI/09).
J 152
Impaired P2X1 receptor function in eosinophils from asthmatic patients
Andrew Wardlaw1, Martyn Mahaut-Smith2, Fiona Symon1, Adam Wright1, Nicolas Sylvius3, Mona Bafadhel1, Michelle Muessel1, Peter Bradding1 and Catherine Vial2,*
1University of Leicester, Institute for Lung Health, Respiratory Biomedical Unit, Hospitals of Leicester NHS Trust, Leicester, UK;2University of Leicester, Cell Physiology and Pharmacology, Leicester, UK;3University of Leicester, Genetics, Leicester, UK
Eosinophils contribute to the pathogenesis of asthma. They can be activated by extracellular nucleotides released following cell damage and inflammation. This study aimed at identifying the ATP-gated P2X receptor(s) present on eosinophils and determining their contribution to eosinophil biology.
Human eosinophils were isolated from the peripheral blood of healthy and asthmatic volunteers. qPCR and Western blots were used to determine respectively eosinophil P2X receptor mRNA and protein expression level. Conventional whole cell patch-clamp experiments were performed to identify eosinophil functional P2X receptor subtypes. Eosinophil CD11b cell surface expression was measured by flow cytometry.
qPCR showed that P2X1, P2X4 and P2X5 receptor transcripts were expressed in eosinophils from 3 healthy and 3 asthmatic donors. ATP (100 μM) elicited a rapidly activating and rapidly desensitizing inward current (75.0 ± 18.6 pA/pF; 6 donors, n = 15 cells) in healthy human eosinophils which was abolished by 1 μM of the selective P2X1 receptor antagonist NF449 (2.7 ± 0.5 pA/pF; 6 donors, n = 11 cells, p = 0.0313). The P2X1 receptor agonist α,β-meATP (10 μM) also induced a fast transient current in eosinophils (18.4 ± 3.2 pA/pF; 3 donors, n = 24 cells) which was totally inhibited by 1 μM NF449 (2.7 ± 1.8 pA/pF; 8 donors, n = 17 cells, p = 0.0078). These P2X1-like currents were markedly reduced (66 %) in eosinophils from asthmatic donors compared to healthy donors (10 μM α,β-meATP-induced currents of 8.4 ± 1.7 pA/pF [12 donors, n = 44 cells] and 24.4 ± 3.6pA/pF [18 donors, n = 41 cells], for asthmatic and healthy eosinophils respectively, p < 0.0001). P2X1 transcript and protein levels were similar in asthmatic and healthy eosinophils. Interestingly, preincubation of the cells with a substantial apyrase concentration (10 IU/ml) rescued P2X1 receptor activity in asthmatic eosinophils [α,β-meATP (10 μM)-induced currents of 17.1 ± 4.5 pA/pF for 10 IU/ml and 11.4 ± 2.6 pA/pF for 0.32 IU/ml of apyrase, 6 donors, 7–9 cells/donor, p = 0.0313]. In healthy eosinophils, P2X1 receptor activation (10 μM α,β-meATP for 2 h) had no effect on CD11b cell surface expression (+4.8 ± 5.1 %, 8 donors) but increased CD11b activated form expression by 42.9 ± 20.5 % compared to control (13 donors, P = 0.0409). No change in the cell surface expression of CD11b or CD11b activated form was observed in asthmatic eosinophils (−0.6 ± 2.6 %, 12 donors and +8.4 ± 10.9 %, 10 donors, respectively).
In conclusion, healthy human eosinophils express functional P2X1 receptors. While the receptor activity is significantly reduced in asthmatic eosinophils, it can be rescued by the presence of high apyrase concentration, supporting receptor desensitisation. P2X1 receptor activation increases cell surface expression of CD11b (activated form) in the healthy eosinophil, suggesting a role for P2X1 receptors in eosinophil adhesion and migration.
J 153
The P2X7 receptor contributes to protection and to ameliorate the clinical manifestations of blood-stagePlasmodium chabaudiAS malaria
Érika Machado de Salles1,*, Maria Nogueira de Menezes1, Alexandra dos Anjos Cassado1, Flávia Sarmento Vieira1, Eduardo Pinheiro Amaral1, Sheyla Castillo Mendéz1, Henrique Borges da Silva1, José Maria Álvarez1, Robson Coutinho Silva2 and Maria Regina D’Império Lima1
1University of São Paulo, São Paulo, Brazil;2Federal University of Rio de Janeiro, Rio de Janeiro, Brazil
Introduction: Malaria, a disease caused by the protozoan parasite Plasmodium, remains a serious healthcare problem in developing countries. The blood stage of infection is responsible for all symptoms associated with malaria, which are mostly related to excessive activation of the immune system. Recently, it has been shown that innate immune receptors are able to detect signals released by damaged cells as ATP. P2X7 receptor (P2X7R) detects extracellular ATP and therefore could contribute to activate the immune response against Plasmodium.
Methods and Results: Six-to-eight-week-old C57BL/6 (B6) and P2X7R−/− (B6 background) female or male mice were infected by intraperitoneal injection with 106 parasitized erythrocytes. Serum samples of B6 mice were collected before and after the erythrocyte rupture and the ATP was quantified using ATP Bioluminescence Assay Kit. ATP levels were significantly higher after the erythrocyte rupture. Erythrocytes were removed from blood cell preparations by Percoll gradient separation, to assess whether the ATP released from infected and non-infected erythrocytes is able to permeabilize spleen cells. Supernatants from lysed infected erythrocytes induced an increase in permeabilization of CD4+ T cells and CD11c+ cells. We observed that infection by Plasmodium is capable of up-modulating the ATP-induced permeabilization of CD4+ T cells and CD11c+ cells. P2X7R-/- mice showed a deficient expansion of splenic populations of DCs, macrophages, T cells and B cells and impaired production of IFN-γ and antibodies-producing cells on day 7 p.i.. Additionally, the immune response in the liver is affected by the absence of the P2X7R in acutely infected mice. Moreover, in females and males, the absence of the P2X7R resulted in the difficulty to control parasitemia and to reestablish the clinical parameters of acute malaria.
Conclusion: This study provides a new insight into the pathogenesis of malaria by indicating that recognition of damage signals released during Plasmodium infection contributes to activate the immune system.
J 154
P2X7 receptor signaling contributes to hepatic necrosis caused by blood-stagePlasmodium chabaudias malaria
Flávia Sarmento Vieira1,*, Ana Paula Freitas do Rosário1, Érika Machado de Salles1, Robson Coutinho-Silva2 and Maria Regina D’Império Lima1
1University of São Paulo, Immunology, São Paulo, Brazil;2University of Rio de Janeiro, IBCCF, Rio de Janeiro, Brazil
The intense activation of the immune system during the erythrocytic stage of Plasmodium is responsible for several syndromes associated with the disease. The purinergic P2X7 receptor detects extracellular ATP and their interaction induces inflammasome activation in macrophages and consequent production of proinflammatory cytokines and cell death. In malaria, ATP is released upon erythrocyte rupture and leukocyte activation. In the murine model of malaria caused by P. chabaudi AS, infected erythrocytes preferentially adhere to the liver endothelium. Therefore, it is believed that the rupture of parasitized erythrocytes (PE) and consequent release of ATP mainly occurs in that organ.
Methods and Results: To study the role of P2X7R in hepatic necrosis, C57BL/6, P2X7R−/−, Nalp3−/−, ASC−/− and caspase 1/11−/− mice were infected intraperitoneally with 106 PE and 7 days later the livers were collected for histopathological analysis. Differently from infected C57BL/6 mice, infected P2X7R−/− mice did not show liver necrosis. Infected Nalp3−/−, ASC−/− and caspase 1/11−/− mice presented smaller areas of liver necrosis if compared to infected C57BL/6 mice. In another approach, we infected C57BL/6 mice, which were treated with P2X7-antagonist Brilliant Blue-G (BBG) (45.5 mg/Kg) every 48 h for 5 days. Seven days after infection the livers were collected for histopathological analysis. Our results indicate that BBG administration prevents hepatic necrosis in C57BL/6 mice, and similar results were observed using DBA/2 mice treated with BBG. The inhibition of P2X7R in DBA/2 mice not only prevented the tissue damage but also had an impact on the survival of mice.
Conclusions: These data demonstrate that the absence of P2X7 receptor or the administration of BBG ameliorate the severity of hepatic necrosis.
J 155
The P2X1 receptor is required for neutrophil extravasation during LPS-induced lethal endotoxemia
Blandine Maitre1, Stephanie Magnenat1, Veronique Heim1, Catherine Ravanat1, Richard J Evans2, Henri de la Salle1, Christian Gachet1 and Beatrice Hechler1,*
1Etablissement Français du Sang-Alsace, INSERM UMR_S949, STRASBOURG, France;2University of Leicester, Cell Physiology & Pharmacology, Leicester, UK
Question: Extracellular adenosine 5′-triphosphate (ATP) regulates inflammation via the activation of a limited number of P2 receptor subtypes. Since paradoxical observations have been reported concerning the role of the ATP-gated P2X1 cation channel in endotoxemia, we here reevaluated whether this receptor participates to acute systemic inflammation provoked by LPS-induced endotoxemia.
Methods: Wild-type (WT) mice and mice deficient for the P2X1 receptor (P2X1−/− mice) were compared in an experimental model of lethal endotoxemia. Lipopolysaccharide (LPS, 10 mg/kg) was injected intraperitoneally and survival was recorded over the five following days. The activation of coagulation was checked, the concentrations of blood cytokines and chemokines were measured and, lung histology was examined.
Results: Within the 5 days following LPS administration, 70 % of WT mice, vs 15 % of P2X1−/− mice, died (P = 0.0003, n = 20). As compared to WT mice, P2X1−/− mice displayed much less pronounced endotoxemia-associated features, namely lower activation of coagulation, reflected by lower TAT concentrations in plasma (35.0 ± 2.4 ng/mL in P2X1−/− mice vs 78.6 ± 11.9 ng/mL in WT mice, P = 0.0015, n = 10), diminished neutrophil accumulation in the lung (429 × 103 ± 41 × 103 pixels in P2X1−/− mice vs 660 × 103 ± 57 × 103 pixels in WT mice, P = 0.0017, n = 6), and reduced tissue damages. P2X1 receptor deficiency was also associated with a marked reduction in blood concentration of main pro-inflammatory cytokines (TNFα, IL6) and chemokines (MIP1α, MIP1β, RANTES), known to be induced by LPS. Interestingly, isolated macrophages and neutrophils from WT and P2X1−/− mice produced similar levels of pro-inflammatory cytokines when stimulated with LPS in vitro. Using intravital microscopy, we observed a defect in LPS-induced neutrophil emigration from cremasteric venules into tissue of the P2X1−/− mice. Using adoptive transfer of immunofluorescently-labeled neutrophils from WT and P2X1−/− mice in WT mice, we could demonstrate that the absence of the P2X1 receptor on neutrophils per se was responsible of this defect.
Conclusions: This study reveals a major role of the P2X1 receptor in LPS-induced lethal endotoxemia through critical involvement in neutrophil emigration out of venules. The use of selective antagonists of the P2X1 receptor might be a promising pharmacological mean to limit the damages caused during acute systemic endotoxemia.
J 156
Purinergic signaling on leukocytes infiltrating the LPS-injured lung
Daniela Friebe*, Tao Yang, Timo Schmidt, Nadine Borg, Bodo Steckel, Zhaoping Ding and Jürgen Schrader
Heinrich-Heine-University Düsseldorf, Department of Cardiology, Düsseldorf, Germany
Extracellular nucleotides and nucleosides have been implicated as important signaling molecules in the pathogenesis of acute lung injury (ALI). While adenosine is known to inhibit T cell activation, little information is available as to ATP and NAD degrading enzymes, the expression of ATP and adenosine receptors/transporters in different T cell subsets. ALI was induced by challenging mice with intra-tracheal instillation of 60 μl (3 μg/g) LPS. After 3 and 7 days blood, lung tissue and bronchoalveolar lavage was collected and immune cells were analyzed using flow cytometry. The transcriptional phenotype of T helper cells, cytotoxic and regulatory T cells sorted by FACS was assessed by measuring the expression profile of 28 genes related to purinergic signaling using TaqMan Array Micro Fluidic Cards. Catabolism of ATP, NAD and cAMP by activated CD4+ T cells was evaluated by HPLC. CD73 was found to be highly abundant on lymphoid cells with little abundance on myeloid cells, while the opposite was true for CD39. After ALI, the abundance of CD39 and CD73 significantly increased on all T cell subsets derived from lung tissue and bronchoalveolar space. Expression analysis in T cell subsets of the lung revealed ATP (Cd39, Cd73) and NAD (Cd38, Cd157, Cd296, Pc-1) degrading enzymes. However, only transcription of Cd38, Cd39, Cd73, Ent1 and A2a receptor was significantly upregulated after ALI in T helper cells. CD4+ T cells from injured lung rapidly metabolized extracellular ATP to AMP and adenosine but not NAD or cAMP. These findings show that lung T cells—the dominant cell fraction in the later phase of ALI—exhibit a unique expression pattern of purinergic signaling molecules. Adenosine is formed by T cells at an enhanced rate from ATP but not from NAD and together with upregulated A2a receptor is likely to modulate the healing process after acute lung injury.
J 157
Inhibition of purinergic P2Y signaling by PGE2in macrophages. Implications in the regulation of the innate immune response
Esmerilda García Delicado1,*, Paqui G. Través1,2, María Pimentel-Santillana2, Luz María G. Carrasquero2, Raquel Pérez-Sen1, Alfonso Luque3, Manuel Izquierdo2, Paloma Martín-Sanz2, María Teresa Miras-Portugal1 and Lisardo Boscá1,2
1Universidad Complutense de Madrid, Dpto. Bioquímica y Biología Molecular IV, Facultad de Veterinaria, Madrid, Spain;2CSIC-UAM, Instituto de Investigaciones Biomédicas Alberto Sols, Madrid, Spain;3Centro de Investigación en Sanidad Animal-Instituto Nacional de Investigación y Tecnología Agraria y Alimentaria, Valdeolmos, Madrid, Spain
The innate immune system acts as a primary sensor for the presence of pathogens and different cell stressors, and macrophages play a key role in the initiation, execution and resolution of these responses. Under the inflammatory conditions prevailing in the early stages of many diseases different bioactive lipids accumulate at sites of tissue damage due to the rapid expression of cyclooxygenase 2, the high throughput enzyme involved in the synthesis of most of these molecules, among them prostaglandin E2 (PGE2). This bioactive lipid activates specific membrane receptors belonging to the G-protein coupled receptor family: EP1-EP4, being EP2 EP3 and EP4 highly expressed in macrophages and other immune cells. In addition to this, nucleotides can be released in the course of inflammatory responses and immune cells, such as monocytes, macrophages or lymphocytes, exerting several effects that include cytoskeleton reorganization, cell migration, phagocytosis and exocytosis. There are several evidences of cross-regulation between purinergic system and inflammatory molecules. In macrophages, it has been described that UTP potentates PGE2 release which is involved in the enhancement of NOS-2 expression induced by LPS. In the current study the effect of PGs on P2 receptors expressed in murine and human macrophages was investigated. In resting and alternatively activated macrophages PGE2 inhibited [Ca2+]i increases elicited by ATP, UTP, or UDP in a reversible manner. In contrast, the response triggered by BzATP (P2X7 agonist) was not affected by PGE2 pretreatment. Other PGs with cyclopentenone structure (PGA1, PGD2, and 15dPGJ2) failed to decrease significantly UTP-dependent [Ca2+]i increases, revealing that PGE2 selectively impairs P2Y but not P2X7 signaling. This effect is absent in LPS-activated cells and is specific for PGE2 as it cannot be reproduced by other PGs with cyclopentenone structure. We have proved that the inhibition of P2Y responses by PGE2 is independent on membrane receptors and involves the activation of nPKCs (PKCε) and PKD that can be abrogated by selective inhibitors or by the expression of dominant negative forms of PKD. The inhibition of P2Y signaling by PGE2 has an impact on the cell migration elicited by P2Y-agonists (ATP/UTP) in resting and alternatively activated macrophages, which provides new clues to understand the resolution phase of inflammation, when accumulation of PGE2, anti-inflammatory and pro-resolving mediators occurs [1].
Reference
1. Través PG, Pimentel-Santillana M, Carrasquero LM, Pérez-Sen R, Delicado EG, Luque A, Izquierdo M, Martín-Sanz P, Miras-Portugal MT, Boscá L (2013) Selective impairment of P2Y signaling by prostaglandin E2 in macrophages: implications for Ca2+-dependent responses. J Immunol 190(8):4226–35. doi: 10.4049/jimmunol.1203029. Epub 2013 Mar 11
J 158
Purinergic signaling involving P2Y1receptors and NTPDase 2 and 3 in mesenteric endothelial cells during schistosomiasis-related vascular inflammation
Suellen D’arc S Oliveira1,2, Nathália F. Oliveira1, José Roberto Meyer-Fernandes3, Luiz Eduardo B. Savio2, Flavia G. Ornelas4, Zulma S. Ferreira4, Robson Coutinho-Silva2 and Claudia Lucia M Silva1,*
1Universidade Federal do Rio de Janeiro, Pharmacology and Inflammation, Rio de Janeiro, Brazil;2Universidade Federal do Rio de Janeiro, Institute of Biophysics Carlos Chagas Filho, Rio de Janeiro, Brazil;3Universidade Federal do Rio de Janeiro, Institute of Medical Biochemistry, Rio de Janeiro, Brazil;4Universidade São Paulo, Institute of Bioscience, São Paulo, Brazil
Introduction and Objective: Schistosomiasis is a chronic inflammatory disease associated with alterations of vascular physiology (Oliveira et al., 2011, Plos One 6:e23547) and purinergic signaling (Oliveira et al., 2013, Purinergic Signal. 9: 81–89). The objective of the present work was to evaluate the role of P2Y1 receptors (P2Y1R) and NTPDases to leukocyte adhesion in mesenteric endothelial cells (MECs) during schistosomiasis.
Methods: Male S. mansoni-infected and control Swiss mice (60–80 days old; protocol approved by UFRJ ethics committee under the license DFBCICB011) were used to obtain primary cultures of MECs (Oliveira et al., Plos One 6:e23547, 2011) in order to evaluate NTPDase activity, expression and mononuclear cell (MNC) adhesion. The number of adherent MNC per field was determined by microscopy (400× magnification). MEC P2Y1R and NTPDases expressions were accessed by Western blotting or real time RT-PCR analysis.
Results: In the control group, the P2Y1R agonist 2-MeSATP (60 μM, 4 h) increased MNC adhesion to MEC from 9.4 ± 1 to 24.5 ± 2 cells/field (n = 26–36, P < 0.01) and the P2Y1R antagonist MRS2179 (0.3 μM) blocked its effect (9.2 ± 1 cells/field, n = 36). In fluorimetric assays using fura-2 AM (5 μM), 2-MeSATP also increased endothelial intracellular Ca2+. In the infected group, the basal MNC adhesion was higher (22 ± 2 cells/field, n = 33, P < 0.05) than in the control, and the agonist 2-MeSATP had a marginal effect. We did not observe any alteration of MEC P2Y1R expression. On the other hand, [32P]ATP hydrolysis was higher in the infected (17 ± 3 pmol Pi/μg protein, P < 0.05) than in the control group (7 ± 1 pmol Pi/μg protein, n = 16−14). Real time RT-PCR data revealed an increased expression of mRNA Entpdases 2 and 3 (Fig. 1, P < 0.05). Endothelial cells release ATP and as these enzymes have higher affinity for ATP rather than ADP they might increase extracellular ADP availability. Accordingly, we observed an increased extracellular ADP content in the infected group and the antagonist MRS2179 reduced basal MNC adhesion (9.6 ± 1 cells/field, n = 33) to values similar to control (P < 0.05).
Conclusion: Our data suggest that the P2Y1R-mediated increased basal leukocyte adhesion to MEC in schistosomiasis may be due to an autocrine loop where the increased expression of NTPDases 2 and 3 could favor the formation of ADP, an endogenous P2Y1R agonist. This purinergic signaling may contribute to the chronic vascular inflammation and mesenteric dysfunction observed in the disease.
Fig. 1 NTPDases 2 (A) and 3 (B) expression in mesentheric endothelial cells from control (white bars) and S. mansoni-infected mice (black bars) (n=4-5 animals to each condition; *p < 0.05; **p < 0.01, Student's t test)
Financial support: CNPq, FAPERJ-PRONEX, FAPERJ, INCT-INPeTAm/CNPq/MCT
J 159
A 312R>S polymorphism in the human P2Y2receptor affects nucleotide-induced CCL2 secretion in monocytes and macrophages
Kathryn Higgins1, William Kovacevic1, James Wiley1,2 and Leanne Stokes1,3,*
1University of Sydney, Nepean Clinical School, Penrith, Australia;2Florey Neurosciences Institute, Melbourne, Australia;3RMIT University, Health Innovations Research Institute, Bundoora, Australia
Monocyte chemoattractant protein -1 (MCP-1/CCL2) is an important inflammatory chemokine involved in monocyte recruitment to inflamed tissues. The extracellular nucleotide signalling molecule UTP acting via the P2Y2 receptor has been previously demonstrated to induce CCL2 production and secretion in macrophages. We have confirmed this in the human monocytic cell line THP-1 showing that UTP is as efficient as LPS at inducing CCL2 at early timepoints (2–6 h) but not at later timepoints (24 h). Expression and calcium experiments confirm the presence of functional P2Y2 receptors on THP-1 cells. We then determined whether UTP stimulation of peripheral CD14 positive human monocytes would induce CCL2 secretion. Responses to LPS were low following 4 h stimulation but significantly increased above background levels following 6 h LPS treatment. The response to UTP was variable and not significantly different from background after 4 h (n = 11 donors). After 6 h of UTP treatment human monocytes showed some increase in CCL2 detected (n = 5 donors). With such variability in response we looked for polymorphisms in P2RY2 that may affect the functional response. Sequencing of P2RY2 from THP-1 monocytes revealed the presence of a SNP altering amino acid 312 Arg>Ser. Genotyping confirmed this SNP rs3741156 to be relatively common at a frequency of 0.276 (n = 404 subjects). Finally we investigated CCL2 secretion from IFNγ/LPS differentiated THP-1 macrophages or 7 day monocyte-derived human macrophages in response to LPS or UTP. Macrophages expressing 312S-P2Y2 had higher UTP-induced CCL2 secretion compared to 312R-P2Y2 expressing macrophages (n = 4–5 donors).
J 160
Melatonin inhibits P2Y1receptor-mediated leukocyte adhesion to endothelial cell
Tassya Cataldi Cardoso1, Suellen D’arc S. Oliveira1,2 and Claudia Lucia M Silva1,*
1Universidade Federal do Rio de Janeiro, Pharmacology and Inflammation, Rio de Janeiro, Brazil;2Universidade Federal do Rio de Janeiro, Institute of Biophysics Carlos Chagas Filho, Rio de Janeiro, Brazil
Introduction: Besides the role of melatonin in the circadian rhythms control, the hormone (1–100 nM) reduces leukocyte adhesion stimulated by leukotriene B4 to rat microcirculation in vivo [1]. Similar results were observed in vitro [2]. It was also shown that melatonin reduces the endothelial adhesion molecule expression [3]. P2Y1 receptors (P2Y1R) increases leukocyte rolling to the endothelium [4] having an important role in inflammation [5]. Thus, our aim was to evaluate the effect of melatonin on P2Y1R-induced leukocyte adhesion to mesenteric endothelial cells (MEC).
Methods: The protocol was approved by the institutional ethics committee (license DFBCICB011). MEC were obtained from male Wistar rats accordingly to Oliveira et al. [6]. MEC were incubated for 4 h with vehicle (basal) or the P2Y1R agonist 2MeSATP (60 μM) in the absence or presence of melatonin 30 nM. Alternatively, the P2Y1R antagonist MRS2179 (0.3 μM) or the melatonin MT receptor antagonist luzindole 10 μM were used. MEC were also used for Western blot assays. Rat mononuclear cells (MNC) were isolated from blood and purified by Ficoll-Paque Plus gradient (Oliveira et al., Plos One 6:e23547, 2011). At the end of the fourth hour, 1E4 MNC/well were added and incubated for 30 min. Non-adherent cells were removed and four fields per well were randomly chosen and the number of adherent MNC was determined by microscopy (400× magnification).
Results: Western blot assay performed with total protein extract from endothelial cells revealed a band in the expected molecular mass range for P2Y1R. 2MeSATP (60 μM) increased MNC adhesion to MECs when compared with basal condition (33.1 ± 2.6 and 15.1 ± 1.1 cells/field, n = 33, respectively, P < 0.05). Pre-treatment with MRS2179 blocked the effect induced by 2MeSATP (7.5 ± 1 cells/field, n = 12, P < 0.05). Melatonin did not alter basal MNC adhesion but it inhibited the effect induced by 2MeSATP (from 33.1 ± 2.6 cells/field, n = 33, to 16.4 ± 1.5 cells/fields, n = 34, P < 0.001, Fig. 1). Additionally, melatonin’s anti-inflammatory action was mediated by MT receptors since the antagonist luzindole prevented its effect (13.4 ± 1.1 cells/field, n = 12 and 26.8 ± 3.6 cells/field, n = 12, in the absence or presence of luzindole, respectively, P < 0.05).
Conclusion: Our data indicate that melatonin has an inhibitory effect on the stimulation of endothelial-leukocyte adhesion promoted by P2Y1R, having therefore a wider range of anti-inflammatory action.
Fig. 1 Melatonin inhibits rat mononuclear cells adhesion to mesenteric endothelial cells in response to P2Y1R activation by 2MeSATP. ***p < 0.001 (one way ANOVA followed by Newman-Keuls test)
References
1. Lotufo et al (2001) Eur J Pharmacol 430:351
2. Lotufo et al (2006) Eur J Pharmacol 534:258
3. Marçola et al (2013) J Pineal Res 54:162
4. Zerr et al (2011) Circulation 123:2404
5. Burnstock and Ralevic (2014) Pharmacol Rev 66:102
6. Oliveira et al (2011) Plos One 6:e23547
J 161
Hydrolysis of extracellular nucleotides attenuates vascular inflammation via modulating miR-142-3p levels in plasma microparticles
Stephanie Kuhn1,*, Katrin Splith1, Ines Kämmerer1, Cindy Hegewald1, Linda Feldbrügge1,2,3, Jan Schulte am Esch4, Sven Jonas1,2, Simon C. Robson3 and Moritz Schmelzle1,2,3
1University of Leipzig, Translational Centre of Regenerative Medicine (TRM), Leipzig, Germany;2University Hospital Leipzig, Department of Visceral-, Transplantation-, Thoracic- and Vascular Surgery, Leipzig, Germany;3Harvard University, The Transplant Institute and Division of Gastroenterology, Beth Israel Deaconess Medical Centre, Boston, Germany;4University Hospital Düsseldorf, Department of Surgery, Düsseldorf, Germany
Question: Hematopoietic stem cell (HSC) subsets express functional active CD39 and are recruited after liver injury to promote liver regeneration. HSC also shed plasma microparticles (MP) in a CD39-dependent manner. Besides serving as novel biomarkers in liver disease, MP carry microRNA (miR) and play essential roles in intercellular communication. We here hypothesized that regulated hydrolysis of extracellular nucleotides attenuates vascular inflammation via modulating levels of miR-142-3p in plasma MP.
Methods: Partial hepatectomy (70 %) was performed in C57Bl/6 wild type, Cd39 null and Cd73 null mice. MP were obtained from the plasma and cell culture supernatants, respectively, by a 2-step ultracentrifugation. Total RNA from MP and cells was isolated, reverse transcribed and analyzed by qPCR. Anti-inflammatory effects of miR-142-3p were investigated after transfection of endothelial cells, in vitro.
Results: Wild type and mutant mice respond to partial hepatectomy by shedding of miR-142-3p bearing MP. Significantly decreased miR-142-3p levels are noted in Cd39 null and Cd73 null MP, when compared to wild type MP (p < 0.05). This associates miR-142-3p MP levels with ectonucleotidase surface profiles. Mechanistic studies in vitro revealed that miR-142-3p levels in MP, but not in the cell of origin, are modulated by purinergic signaling and purinergic receptors. Successful transfection of predicted endothelial target cells with miR-142-3p leads to decreased mRNA levels of pro-inflammatory cytokines.
Conclusions: Regulated hydrolysis of extracellular ATP to adenosine by CD39 and CD73 modulates levels of non-coding miR-142-3p in plasma MP after experimental liver resection. Transfection of endothelial cells with miR-142-3p attenuates sterile inflammation of endothelial cells, which might have implications for hepatocyte proliferation and liver regeneration. These observations have implications for monitoring and indicate future therapeutic avenues.
J 162
Purinergic cell death in T lymphocytes—a clue to understanding the etiology of narcolepsy
Karin Dreisig* and Birgitte Kornum
Glostrup Hospital, Molecular Sleep Laboratory, Glostrup, Denmark
Background and Aim: The narcolepsy-associated P2RY11 gene encodes a purinergic G-protein coupled receptor, P2Y11, that is activated by ATP. Increasing concentrations of ATP induce cell death in white blood cells, an effect likely mediated by another purinergic receptor i.e. an ion-channel termed P2X7. It is hypothesised that P2Y11 mitigates the effect of P2X7, and that immune-cell death in the presence of high ATP is controlled by a balance of activation of the two purinergic receptors.
Results: In HEK-293 cells transfected with P2RX7, incubation with bzATP (stable ATP analogue) showed increased cell death dose-dependently measured as Lactate Dehydrogenase (LDH) activity. None of the tested bzATP concentrations significantly increased cell death compared to control when P2RY11 and P2RX7 were co-expressed; demonstrating that expression of P2RY11 is able to counteract the effect of P2RX7.
CD8+ T lymphocytes express more P2RY11 over P2RX7 compared to CD4+ T lymphocytes. I found that bzATP caused significantly higher degree of cell death in CD4+ T lymphocytes compared to CD8+ T lymphocytes, indicating that expression of more P2RY11 over P2RX7 makes cells more resistant to high concentrations of bzATP.
Finally, 2 days of activation with CD3/CD28 Dynabeads changed the bzATP response in CD4+ T lymphocytes. The activation of CD4+ T lymphocytes caused an increase in P2RY11 gene expression correlating with a decrease in sensitivity to cell death induction by high bzATP levels.
Conclusion and Perspectives: My data provide evidence that the P2Y11 receptor is involved in the rescue of T lymphocytes from P2X7-mediated cell death induced by high bzATP concentrations. Currently, I carry out studies testing the mechanism for P2Y11-P2X7 interaction.
J 163
Changes in microRNAs expression disclose novel links between purinergic signalling and neuroinflammation
Chiara Parisi1,*, Ivan Arisi2, Nadia D’Ambrosi1,3, Andrea Ennio Storti2, Rossella Brandi2, Mara D’Onofrio2,4 and Cinzia Volonté1,5
1CNR, IBCN, Rome, Italy;2EBRI Foundation, Rome, Italy;3Università Cattolica del Sacro Cuore, Rome, Italy;4CNR, IFT, Rome, Italy;5Fondazione Santa Lucia, Rome, Italy.
MicroRNAs (miRNAs) are endogenous small non-coding RNAs that regulate gene expression by acting at the post-transcriptional level. In the past few years, they emerged as key modulators of immune system whose dysfunction contributes to the progression of several neurodegenerative diseases including Amyotrophic Lateral Sclerosis (ALS) [1]. ALS is a non-cell-autonomous disease targeting both motor neurons and neighbouring glia, with microgliosis substantially contributing to neurodegeneration. P2X7 receptor has a recognized role in neuroinflammation in ALS pathogenesis [2], but its mechanistic signalling is only partially known. While several studies have identified a prominent and complex role of miRNAs as key modulators of signal propagation, there are only a few works on miRNAs regulation of purinergic systems [3]. With the aim to identify those miRNAs implicated in ALS and P2X7-mediated neuroinflammation, we examined the transcriptional profiling of miRNAs in primary microglia cultures from SOD1-G93A mice in resting conditions and after activation of P2X7 receptor. We identified a strong upregulation of immune-enriched miRNAs transcriptome in ALS microglia and particularly after P2X7 receptor stimulation, recognizing miR-22, miR-155, miR-125b and miR-146b as key mediators. Moreover, we proved that miR-365 and miR-125b, suppressing the IL-6/STAT3 pathway and enhancing NF-kB activation, determine an increase of TNFalpha transcription. Inhibition of miR-125b then abolishes BzATP-induced TNFalpha production. Since also TNFalpha up regulates miR-125b, we might recognize the induction of miR-125b as the gateway of a vicious cycle culminating in abnormal TNFalpha release in ALS. These results not only strengthen the impact of microRNAs in modulating genes linked to inflammation and ALS, but also identify specific miRNAs implicated in purinergic signalling and P2X7 mediated microglia activation.
References
1. Kiernan MC, Vucic S, Cheah BC et al (2011) Lancet 377:942–955
2. Apolloni S, Parisi C, Pesaresi MG et al (2013) J Immunol 190:5187–5195
3. Volonté C, Parisi C, Burnstock G (2012) CNS Neurol Disord Drug Targets 11:751–767
J 164
Generation and characterization ofEntpd8−/−mice
Mabrouka Salem1,2,*, Patrick Luyindula1,2, Joanna Lecka1,2, Alain Tremblay2, Julie Pelletier2 and Jean Sévigny1,2
1Laval University, Microbiology-Immunology, Quebec, Canada;2Centre de recherche du CHU de Québec, Rhumatology-Immunology, Quebec, Canada
Background: Nucleoside triphosphate diphosphohydrolase-8 (NTPDase8) is the last member of the E-NTPDase family. NTPDases hydrolyze extracellular nucleotides at the cell surface, thus have the ability to regulate the activation of nucleotide receptors, namely P2X and P2Y. NTPDase8 is the major ATPase of the liver [1,2]. In order to elucidate the role of this enzyme recently discovered, cloned and characterized in our laboratory [2], we generated mice deficient in the expression of NTPDase8 (Entpd8−/−).
Material and Method: To generate the Entpd8−/− mice, deficient embryonic stem cells for the gene encoding the full NTPDase8 mouse were injected into mouse blastocysts B6 (Cg)-Tyrc-2j/J. The chimeras were backcrossed with C57/BL6 mice to obtain homozygous Entpd8−/− mice. The deletion of NTPDase8 gene was verified by PCR on mouse genomic DNA and liver RNA level. The absence of NTPDase8 protein was tested by Western blot and immunohistochemistry with our newly generated antibodies and the activity of NTPDase8 was verified in situ in liver sections by enzyme histochemistry. Histological and proliferation analysis of liver sections were evaluated by hematoxylin eosin staining and Ki67 antibody respectively. Leukocyte infiltration was focused on macrophages using F4/80 antibody.
Results: PCR confirmed the deficiency of NTPDase8 in the generated mice. Immunohistochemistry showed that NTPDase8 was localized on liver canaliculi in wild type mice (WT). Liver canaliculi of Entpd8−/− mice did not hydrolyze nucleotide confirming that NTPDase8 is the only NTPDase present in this structure. The Entpd8−/− mice breed and grow normally and we did not observe any apparent phenotypes. Further characterization at the cellular level was carried out with liver sections. Histological analysis of Entpd8−/− mice liver showed increase in inflammatory sites and proliferation level compared to WT. Quantitative evaluation of macrophages exhibited their higher presence in Entpd8−/− mice liver compared to WT.
Conclusion: we have developed a mouse line deficient in NTPDase8 expression as confirmed at the DNA, RNA, protein and enzyme activity level. We also demonstrated the localization of NTPDase8 in liver canaliculi as we previously observed in rat. We are presently investigating the role of NTPDase8 helped with these mice.
References
1. Fausther M, Lecka J, Kukulski F, Lévesque SA, Pelletier J, Zimmermann H, Dranoff JA, Sévigny J (2007) Am J Physiol Gastrointest Liver Physiol 292(3):785–795
2. Bigonnesse F, Lévesque SA, Kukulski F, Lecka J, Robson SC, Fernandes MJG, Sévigny J (2004) Biochemistry 43(18):5511–5519
J 165
Adenine nucleotide catabolism on the surface of calcified aortic valve
Barbara Kutryb-Zajac1, Patrycja Sommerfeld1, Marta Toczek1, Paulina Zukowska1, Romuald Lango2, Jan Rogowski3, Ewa M. Słominska1 and Ryszard T. Smolenski1
1Department of Biochemistry,2Department of Cardiac Anesthesiology,3Chair and Clinic of Cardiac and Vascular Surgery, Medical University of Gdansk, Poland
Enzymes of extracellular nucleotide metabolism controls function of immune system, thrombosis, bone remodeling and cell proliferation—the processes that are involved in heart valve dysfunction and calcification. The purpose of this study was to investigate the changes in extracellular nucleotide metabolism in pathology of human aortic valves.
Stenotic aortic valves were obtained from patients after aortic valve replacement (n = 100) and non-stenotic controls were obtained after heart transplantation or Bentall procedure (n = 20). Valves were analyzed for ecto-nucleoside triphosphate diphosphohydrolase (eNTPD), ecto-5′-nucleotidase (e5NT) and ecto-adenosine deaminase (eADA) activities by exposing specific area of fibrosa or ventricularis side to medium with nucleotide substrates and tracing conversions by high perfomance liquid chromatography. These measurements were correlated with clinical data.
Activities of eNTPD, e5NT and eADA on the fibrosa of stenotic aortic valve were: 1.80 ± 0.09, 1.11 ± 0.06, 0.95 ± 0.07 nmol/min/cm2, respectively, whereas, in the non-stenotic valves these activities were higher: 2.60 ± 0.32 (eNTPD) and 2.11 ± 0.21 (e5NT) or lower: 0.55 ± 0.09 nmol/min/cm2 (eADA). No differences between stenotic and non-stenotic valves were observed on the ventricular side. Negative correlations were found between: eNTPD and e5NT activities and plasma low density lipoproteins as well as between eADA activity and ejection fraction. Positive correlation was demonstrated beetween eADA activity and left ventricular end-systolic diameter.
These results demonstrated that extracellular nucleotide breakdown in the aortic valve is adversely modified in aortic stenosis in the areas prone to calcification. Diminished activities of eNTPD and e5NT with increase in eADA activity may affect extracellular nucleotide concentrations in the way that favors valve inflammation and calcification.
J 166
Pore-forming a-haemolysin fromE. coli—implications of erythrocytal adenosine receptors
Mette Bak Brogård*, Marianne Skals and Helle Praetorius
Aarhus University, Institute for Biomedicine, Aarhus, Denmark
It has previously been shown that haemolysis inflicted by pore-forming toxins such as a-haemolysin (HlyA) from E-coli is completely depended on ATP release and activation of P2X-receptors on erythrocyte membrane. ATP is rapidly degraded to ADP and AMP by ectoATPases bound to the erythrocyte membranes, however, erythrocytes has little if any ecto-5-prime-nucleotidase (CD73)-activity. On the contrary, CD73 is heavily expressed endothelial cells and thus, able to convert AMP to adenosine. The endothelial conversion of ATP to adenosine is exceedingly important for erythrocyte physiology in vivo, for example for generation of 2,3 DPG in response to hypoxia. With regard to the toxin-induced haemolysis a potential effect of adenosine has not been settled. This issue is particularly pending for the evaluation of in vivo effect of HlyA in itself and sepsis inflicted by HlyA producing E. coli.
The aim of this project is to investigate the impact of adenosine signalling on HlyA-induced haemolysis. All experiments are performed on human blood from healthy volunteers. Haemolysis is determined as the OD540 of the erythrocyte supernatant and ATP release is measured with an online luciferine/luciferase method.
Addition of adenosine in itself did not influence the HlyA-induced haemolysis, neither did any of the more selective agonist for the adensine receptors A1, A2a, A2b and A3 (in concentrations from 10−10 to 10−6). Blocking of A1, A2a, A2b and A3 receptors with specific antagonist (in concentrations from 10−10 to 10−6) showed no effect on HlyA-induced haemolysis either in the absence or presence of additional adenosine. Stimulation of the adenosine receptors was, however, able to stimulate ATP release from the erythrocytes, similar to other procedures that enhance cAMP in erythrocytes. Currently, responsible adenosine receptor and the specific pathway for the cAMP-induced ATP release is investigated. Extracellular adenosine does not seem to have an effect HlyA-induced haemolysis. Thus, the conversion of AMP to adenosine on endothelial cells is very unlikely to influence the HlyA-induced cell lysis in vivo.
J 167
Bacterial RTX toxins allow acute ATP release from human erythrocytes directly through the toxin pore
Helle Praetorius*, Randi Bjaelde, Jesper Reinholdt, Knud Poulsen, Brian S. Vad, Daniel E. Otzen, Jens Leipziger and Marianne Skals
Aarhus University, Department of Biomedicine, Physiology, Aarhus C, Denmark
ATP is as an extracellular signaling molecule able to amplify the cell lysis inflicted by certain bacterial toxins including the two RTX toxins a-hemolysin (HlyA) from E. coli and leukotoxin A (LtxA) from Aggregatibacter actinomycetemcomitans. Inhibition of P2X receptors completely blocks the RTX toxin-induced hemolysis over a larger concentration range. It is, however, at present not known how the ATP that provides the amplification is released from the attacked cells.
Here we show that both HlyA and LtxA trigger acute release of ATP from human erythrocytes that preceded and were not caused by cell lysis. This early ATP-release did not occur via previously described ATP-release pathways in the erythrocyte. Both HlyA and LtxA were capable of triggering ATP release in the presence of the pannexin 1 blockers carbenoxolone and probenecid, and the HlyA-induced ATP-release was found to be similar in erythrocytes from pannexin 1 wild type and knockout mice. Moreover, the voltage dependent anion channel (VDAC) antagonist TRO19622 had no effect on ATP-release by either of the toxins. Finally, we showed that both HlyA and LtxA were able to release ATP from ATP-loaded lipid (POPC) vesicles devoid of any erythrocyte channels or transporters. Again we were able to show that this happened in a non-lytic fashion, using calcein-containing vesicles as controls. These data show that both toxins incorporate into lipid vesicles and allow ATP to be released. We suggest that both toxins cause acute ATP-release by letting ATP pass the toxin pores in both human erythrocytes and artificial membranes.
K: Purinergic signaling in the tumor growth and metastasis
K 168
Identification of a murine monoclonal antibody specific for human CD73 and inhibiting extracellular adenosine production generated by the CD38/CD203a/CD73 ectoenzymatic pathway
Antonella Chillemi1,*, Alberto L. Horenstein1,2, Valeria Quarona1, Fabio Morandi3, Gianluca Zaccarello1, Andrea Zito1, Valentina Mariani1, Vito Pistoia3 and Fabio Malavasi1,2,4
1University of Torino, Medical Sciences, Torino, Italy;2University of Torino, Centro di Ricerca in Medicina Sperimentale (CeRMS), Torino, Italy;3Instituto Giannina Gaslini, Department of Experimental and Laboratory Medicine, Genova, Italy;4“Città della Salute e della Scienza” Hospital, Transplantation Immunology Service, Torino, Italy
Question: One of the functions of adenosine (ADO) is the modulation of anti-tumor immune responses [1]. Monoclonal antibodies (mAbs) targeting CD73, the surface 5′-nucleotidase (5′NT) producing ADO, are reported as limit tumor growth through i) inhibition of the catalytic activity of CD73, or by ii) inducing internalization of the ectoenzyme. For these reasons, CD73 has been recognized as a potentially useful target in immunotherapy, thus prompting the search for mAbs specific for human CD73 endowed with the ability to block (or interfere with) its enzymatic functions.
Methods: Anti-CD73 mAbs were selected for of their ability to interfere with the 5′NT activity and ADO generation. Proliferation analysis was performed both in co-cultures and in trans-well systems.
Results: The CB73 mAb (IgG1) identified following these criteria inhibits the ectoenzymatic functions of the CD73 molecule on human EBV lines from healthy donors, where it blocks ADO generation. At least in these conditions, proliferation is unaffected, while ADO synthesis from adenine nucleotides was inhibited in modalities dependent from mAb concentration (75 % ± 5) and from epitope constitution.
ADO is canonically produced by the concerted action of CD39 and CD73. This network is flanked by a unique CD38/CD203a/CD73 pathway [2]. The original observations in human T lymphocytes was lately confirmed in different models, among which human melanoma lines, where ADO is produced from NAD+, ATP and AMP. Co-culture of human melanoma cells with CD4+ T cells was followed by inhibition of the lymphocyte proliferation. These effects are reverted when fresh melanoma cells or lines are pre-treated with APCP, an inhibitor of CD73. Identical results were obtained using the CB73 mAb as a 5′NT inhibitor. Similar results were generated from mixed lymphocyte cultures obtained from healthy donors, taken as representative of alloimmune response.
Conclusions: Different tumors exploit CD73 to drive an escape mechanism from immune defenses: however, growth and metastasis may be blocked by mAb treatment. Ongoing efforts focus on the use of an inhibitory CB73 mAb to determine whether pharmacological inhibition of ADO can be used in therapy to avoid tumor evasion.
References
1. Chillemi A et al 2014 Front Biosci (Landmark Ed) 19:152–62
2. Horenstein AL et al (2013) Oncoimmunology 2(9):e26246
K 169
CD73 plays a key role in B16F10 melanoma tumor growth, its vascularization and metastasis formation
Patrycja Koszałka1,*, Monika Golunska, Aleksandra Urban, Marceli Majewski, Andrzej C. Skladanowski and Jacek Bigda
Medical University of Gdansk, Department of Medical Biotechnology, Intercollegiate Faculty of Biotechnology UG-MUG, Gdansk, Poland
The importance of the role of ecto-5′-nucleotidase (CD73), an enzyme providing interstitial adenosine, was investigated in B16F10 melanoma progression.
CD73 activity in B16F10 cells was decreased both by chemical inhibitor AOPCP (adenosine 5′-α,β-methylene diphosphate) or RNAi (MISSION shRNA, Sigma). Tumor cells were injected into both WT and CD73-deficient C57BL/6 mice, s.c. to analyze tumor growth or i.v. to analyze lung metastases formation.
Tumor growth in CD73-deficient mice was significantly decreased by both AOPCP treatment or CD73 silencing of tumor cells compared to the control (noninhibited/nonmodified cells in WT animals), with significantly stronger effect of RNAi. To induce significant decrease in the formation of lung metastases, reduction of CD73 on tumor cells was needed, as its reduction on host cells was not sufficient. Immunohistochemical analysis of tumor tissues have shown that AOPCP induced visible reduction in microvessel density (anti-CD31 staining) and in expression of angiogenic markers such as VEGFR2 compared to the control. Macrophage (anti-F4/80 staining) infiltration of these tumors was also strongly reduced with macrophages forming a compact inflammatory zone at the interface between the dermis and tumor. No such changes were observed in CD73-deficient mice when AOPCP was not used. On the contrary, RNAi of CD73 induced no reduction in microvessel density and expression of VEGFR2, and inhibition of macrophage infiltration was visibly decreased. Additionally, in lysates of tumors with RNAi of CD73 an increase in IL-2 was detected with ELISA assay, when such increase was not observed for other tumors. One of the more significant molecular changes in tumor tissues was a strong decrease in activation of MAP-kinase ERK (pERK) in tumors from CD73-deficient mice but only when CD73 on tumor cells was inhibited or silenced. Other more significant changes were observed, e.g., in an expression of proteins involved in apoptosis pathways.
We can conclude, that CD73 has an important role in the vascularization of the tumor bed by influencing the process of angiogenesis. It is in an agreement with our previously published data showing inhibition of angiogenesis by AOPCP in Matrigel plugs (Koszalka et al. Oncol Rep 31(2):819–827, 2014). CD73 activity can also be important for the infiltration of monocytes/macrophages that secrete angiogenic factors and metalloproteinases into tumor tissues. The effect can be abrogated by the slight chronic expression of CD73 on the tumor cells (as RNAi induces knock-down rather than a knock-out), which can also increase immune response induced by a T-helper lymphocytes. Additionally CD73 is also important for activation of MAP kinase proliferative pathway in the tumor tissue and to regulate the process of apoptosis in tumor tissues. The role of CD73 on tumor cells is also important in the process of metastasis.
K 170
The A2Badenosine receptor in MDA-MB-231 breast cancer cells mediates inhibition of ERK1/2 phosphorylation by activation of MAPK-phosphatase-1
Marthe Koussémou*, Kristina Lorenz and Karl-Norbert Klotz
Universitaet Wuerzburg, Pharmacology, Wuerzburg, Germany
MAP-kinase signaling is associated with the control of growth, proliferation and differentiation of cells and as such might serve as a promising target for tumor treatment. Stimulation of the Gs-coupled A2B adenosine receptors mediates an activation of adenylyl cyclase and in addition may also trigger a Ca2+ signal. In the estrogen-receptor negative breast cancer cell line MDA-MB-231, which is expressing A2B adenosine receptors as the sole adenosine receptor subtype, inhibition of ERK1/2 phosphorylation was found as an additional A2B receptor-mediated signal. Therefore, in cells with high expression of A2B receptors their stimulation should result in growth inhibition.
Similar to the A2B-mediated inhibition of ERK1/2 phosphorylation a reduced amount of phopho ERK1/2 was also observed after stimulation of adenylyl cyclase with forskolin or application of ‘caged’ cAMP (cAMP-AM) in the presence of the PDE inhibitor Ro-20-1724. The inhibition of ERK1/2 phosphorylation was abolished with the PLC inhibitor U-73122 or by chelating intracellular Ca2+ with BAPTA-AM. These results point to an important role for both cAMP and Ca2+ signaling in the pathway leading to a decrease in ERK1/2 phosphorylation. Such a decrease might occur as the result of inhibition of a kinase or stimulation of a phosphatase. Therefore, we investigated a potential role for MAP-kinase phosphatase-1 (MKP-1) which is an important enzyme controlling the phosphorylation state of ERK1/2. Our results show that stimulation of A2B adenosine receptors in MDA-MB-231 cells indeed caused an increase in MKP-1 expression. This effect provides a novel mechanism for the receptor-mediated inhibition of ERK1/2 phosphorylation seen after stimulation of A2B adenosine receptors. The A2B receptor and the downstream MKP-1 might therefore be interesting targets for the inhibition of proliferation fast-growing cancer cells.
K 171
Targeting adenosine A2b receptor reduces melanoma progression in mice: role of tumor-associated MDSCs
Silvana Morello*, Maria Teresa Loffredo, Claudia Sorrentino and Aldo Pinto
University of Salerno, Pharmacy, Salerno, Italy
Question: Adenosine is a potent immune suppressive mediator in the tumor microenvironment. A2b adenosine receptor subtype (A2bR) contributes to adenosine-mediated effets in tumor tissue. The aim of this work was to dissect the mechanism/s through which A2bR induces immune suppression in the tumor tissue, focusing on the relationship between myeloid-derived suppressor cells (MDSCs) and other key mediators in the tumor microenvironment.
Methods: Using a melanoma mouse model we performed in vivo and ex vivo experiments to study the molecular and cellular events occurring upon A2bR stimulation. Antibody neutralization or cell depletion experiments were performed to evaluate the functional relevance of mediators or cells, respectively, under investigation. To reinforce the translational relevance of targeting A2bR in cancer, experiments with pharmacological blockers of A2bR were conducted.
Results: We demonstrated that selective blockade of A2bR, with the antagonist PSB1115, stimulates T cell-mediated immunosurveillance by impairing the influx of MDSCs into the tumor microenvironment, that results in robust antineoplastic effects. These data suggest that A2bR plays a pivotal role in promoting immune suppression in the tumor microenvironment. Accordingly, the percentage of tumor-associated MDSCs, identified as CD11b+Gr1+CD11c- cells, was significantly increased in tumor tissue harvested from mice treated with the A2bR agonist Bay60-6583 compared with controls. Conversely, the levels of TGF-b and T regulatory cells (Tregs) were unchanged, suggesting that the pro-tumor activity of A2bR relies on its ability to increase the accumulation of MDSCs and not Tregs within tumor tissues. This effect appears to be selective for CD11b+Gr1+cells, because the number of dendritic cells (DC) or CD11b+Gr1- cells were not significantly altered, suggesting that the maturation of these cells is not affected. Depletion of MDSCs completely reversed the effect induced by Bay60-6583. The MDSCs accumulation within tumor tissue was associated with increased tumor levels of CCL-2, IL-10, VEGF, MMP-9 and IL-23. These factors are critically involved in the MDSCs recruitment to cancer. Moreover MDSCs can themselves produce these pro-angiogenic and inflammatory factors. Administration of PSB1115 significantly reverses immune-suppression in the tumor tissue and limits tumor progression in mice.
Conclusions: Therefore targeting A2bR could be a new therapeutic strategy to overcome immune suppression in melanoma tissue.
K 172
Role of ATP on proliferation and viability of esophageal cancer cells lines
Fernanda B. Morrone1,2, André A. Santos Jr.1, Luis Felipe Ribeiro Pinto3, Juliano D. Paccez4 and Luiz F. Zerbini4
1Programa de Pós-Graduação em Biologia Celular e Molecular,2Faculdade de Farmácia, Pontifícia Universidade Católica do Rio Grande do Sul, Porto Alegre, Brazil,3Instituto Nacional do Cancer, Rio de Janeiro, Brazil,4International Centre for Genetic Engineering and Biotechnology (ICGEB), Cancer Genomics Group, Cape Town, South Africa.
Oesophageal cancer is one of the most aggressive cancers and is the sixth leading cause of cancer death worldwide [1]. ATP is known to inhibit the growth of cancer cells in a variety of models and through several mechanisms, including the activation of P2X7 purinergic receptors [2].
Aim: To investigate the role of ATP on the proliferation and viability of oesophageal cancer cells lines and to investigate the expression of P2X7 receptor in cancer cells lines.
Methods: The viability of the cell lines OE21 (oesophageal squamous cell carcinoma), OE19 and OE33 (oesophageal adenocarcinoma), was evaluated through MTT assay, the proliferation was analysed by cell counting, the expression of P2X7 protein was analysed by Western Blot in EPC2, KYSE30, KYSE450, KYSE520 and WHCO1. Results: The treatment with ATP (3 and 5 mM) along 24 h was able to reduce the viability of OE19 (17 ± 4 % and 27 ± 5 %, respectively), OE21 cells (38 ± 4 % and 47 ± 4 %, respectively) and OE-33 (19 ± 6 % e 23 ± 3 %, respectively) (Figure 1 A–C), after 48 h, ATP treatment (3 and 5 mM) reduced cell viability on OE19 (18 ± 1 % and 22 ± 1 %, respectively), OE21 (62 ± 3 % and 72 ± 5 %) and OE33 cells (32 ± 5 % and 31 ± 4 %). The treatment with ATP 1 mM for 48 h also reduced OE21 cells viability (31 ± 3 %) (Figure 1 D–F). ADP treatment (50, 100 and 200 μM) was not able to alter viability of these cells (see figure 1G–I). The treatment with ATP (1, 3 and 5 mM) reduced the number of OE21 cells after 24 h (36 ± 5 %, 69 ± 3 % and 79 ± 6 % respectively) (see figure 1J). We also found a higher immunoreactivity of P2X7 protein in KYSE450, KYSE520 and WHCO1 cells (Figure 1K) when compared to normal cells (EPC2).
Conclusion: The preliminary findings allow us to infer that ATP, through P2X receptors, has a role in the control of proliferation and viability of OE cells, and that this effect is time and concentration-dependent. Furthermore, the presence of P2X7 protein in KYSE450, KYSE520 and WHCO1 cells, can lead us to further investigate the effect of this receptor in type of cancer.
Fig. 1 Effect of 24 h ATP treatment on viability of OE19, OE21 and OE33 cells (A–C). Effect of 48 h ATP treatment on viability of OE19, OE21 and OE33 cells (D–F). Effect of 24 h ADP treatment on viability of OE19, OE21 and OE33 cells (G–I). Effect of 24 h ATP treatment on proliferation of OE21 cells (J). P2X7 Western blot immunoreactivity (K)
Acknowledgements CAPES, FINEP (PUCRSINFRA) # 01.11.0014-00, PUCRS.
References
1. Kamangar F, Dores GM, Anderson WF (2006) J Clin Oncol 24(14):2137–2150
2. Chueh SH Kao LS (1993) J Neurochem 61:1782–1788
K 173
Extracellular ATP suppresses cell proliferation and increases cisplatin-induced cytotoxicity in the human ovarian cancer cell line HEY
Nicole Brockmann*, Alexandra Hamacher and Matthias M. Kassack
Heinrich Heine Universität Düsseldorf, Pharm. Biochemie, Düsseldorf, Germany
Introduction: Extracellular ATP mediates multiple physiological effects through cell signaling such as proliferation, differentiation, apoptosis, chemotaxis, cytokine production and generation of reactive oxygen species [1, 2]. Activation of P2RY11 in neutrophils may for example delay apoptosis [3]. In endothelial cells, P2RY11 activation impairs cellular proliferation by induction of cell cycle arrest [1]. In cancer cells, depending on the P2R expression pattern, ATP and other nucleotides are able to regulate proliferation, differentiation and apoptosis by proinflammatory, immunosuppressant and tumor growth-promoting or -inhibiting effects [2,4]. Stimulation of P2RY1 or P2RY2 can lead to an increase or decrease in cell proliferation depending on the tumor type. Stimulation of P2RY11 in cancer cells shows an antiproliferative effect and leads to a more differentiated state [4].
Aim: Are nucleotide receptors suitable targets to modulate proliferation and apoptosis in ovarian cancer cells and furthermore, to increase chemosensitivity of cytotoxic agents?
Methods: The human ovarian cancer cell line HEY, natively expressing P2RYs, was used to investigate the effects of ATP on cell proliferation, apoptosis, cell cycle, and cisplatin-induced cytotoxicity (MTT assay). The P2 receptor expression profile was determined by PCR and immunohistochemistry (IHC). Functional integrity of P2RYs was confirmed by intracellular calcium measurements.
Results: P2RY1 and P2RY2 are expressed in HEY ovarian cancer cells as found by PCR and IHC. Further, both receptors are functionally responsive (calcium mobilization studies). EC50-values were determined as follows: ATP = 107 nM, UTP = 64 nM, ADP = 5.2 μM and UDP = 1.8 μM. ATP inhibited viability of HEY cells with an IC50 of 145 μM as estimated by MTT assay. Furthermore, ATP increased cisplatin-induced cytotoxicity if ATP was pre-incubated prior to addition of cisplatin. IC50 of cisplatin (MTT assay) was reduced 2-fold by 100 μM ATP and reduced 4.5-fold by 200 μM ATP pre-incubation. The combination of cisplatin and ATP was found to be synergistic as combination indices were <0.9 (calculated according to Chou-Talalay [5]). A recently discovered P2RY2 antagonist was then used to inhibit functional effects (calcium mobilization) in HEY cells induced by ATP. Furthermore, the P2RY2 antagonist was able to inhibit the chemosensitizing effect of ATP on cisplatin.
Conclusions: In the ovarian cancer cell line HEY, P2RY2 modulation may be a useful tool to reduce cellular proliferation and to increase the potency of cytostatic agents such as cisplatin.
References
1. Xiao Z et al (2011) J Cell Biochem 122(9):2257–2265
2. Di Virgilio F (2012) Cancer Res 72(21):5441–5447
3. Vaughan KR et al (2007) J Immunol 179:8544–8553
4. White N, Burnstock G (2006) Trends Pharmacol Sci 27(4):211–217
5. Chou T (2010) Cancer Res 70:440–446
K 174
Reduction in P2X7 receptor expression is a marker of resistance to ATP treatment in human cervical carcinoma cell line
Andreia Buffon1,*, Paola Andrade Mello1, Eduardo Cremonese Filippi Chiela2, Jessica Nascimento 1, Francieli Kipper2, Aline Beckenkamp1, Danielle Bertodo Santana1, Alessandra Nejar Bruno3 and Guido Lenz2
1Federal University of Rio Grande do Sul- UFRGS, Department of Analysis, Porto Alegre, Brazil;2Federal University of Rio Grande do Sul- UFRGS, Department of Biophysics, Porto Alegre, Brazil;3Instituto Federal de Educação, Ciência e Tecnologia—IFRS, Porto Alegre, Brazil
Cervical cancer is the third most commonly diagnosed malignancy and the fourth leading cause of cancer death in females worldwide. The high mortality rate is largely due to the lack of effective therapies for eliminating disease in women with high-grade cervical cancer and the lack of response to chemotherapy of inoperable disease. Thus, to understand the mechanisms involved on cervical cancer development and find a molecular target to prevent it is strongly desirable. Until now, researchers show that HPV infection and suppression of cell death mechanisms like apoptosis are the most important factors involving of cervical carcinogenesis. Anyway, the mechanism that leads cells infected by HPV escape to apoptosis remains unknown. A role for purinergic signaling on control of cell growth and death, mainly through P2X7 receptor activation by extracellular ATP, has been described. We hypothesized that a disruption in this signaling could be involved with cervical cancer resistance to apoptosis and development. Previous work, using human carcinoma cell line SiHa, showed that ATP 5 mM induces cell death through apoptosis by P2X7 activation. However, the mechanism involved in this cytotoxic effect remained unclear. To answers this question, we investigate the role of P2X7 on ATP induce cell death. We started analyzing P2X7 protein expression in cells resistant to ATP treatment. In this case, SiHa cells were treated with ATP 5 mM for 24, 48 and 72 h, and the adherent survival cells were removed and analyzed by Western Blot. After, we knockdown P2X7 receptor in SiHa cell line and evaluate this effect on cell viability after ATP treatment. Adding, we study the mechanistic way involved in this cell death pathway using EGTA and caspase-3 inhibitor previous to ATP treatment. For our surprise, SiHa wild type (WT) cells that remained adherent after treatment with ATP showed less expression of P2X7, suggesting a defense way to apoptosis. Corroborating with this, SiHa cells knockdown (KD) for P2X7 showed increased cell viability when compared to SiHa WT and SiHa KD control, after exposure to ATP 5 mM for 24, 48 and 72 h. The mechanism of ATP induces apoptosis through P2X7 don’t seems to be by increase intracellular calcium and caspase-3 activation, but in an independent caspase way. These findings suggest that P2X7 receptor could be involved with death and resistance cell mechanisms, indicating a possible new target on therapeutical research in cervical cancer and on tumor aggressiveness.
K 175
Consequences of P2X7 receptor host deficiency on cancer growth
Marina Capece*, Alessia Franceschini, Elena Adinolfi and Francesco Di Virgilio
University of Ferrara, Department of Morphology, Surgery and Experimental Medicine, Ferrara, Italy
The P2X7 receptor (P2X7R) is an ATP-gated ion channel mainly expressed by immune cells of monocyte-macrophage origin. Due to its distribution P2X7R has a key role in inflammation and immunity as it mediates secretion of mature interleukin 1 beta (IL-1β), is involved in inflammatory response in transplantation [1] and its function is required to support an efficient antigen presentation and CD4+ cell stimulation by tumor-associated dendritic cells (DCs) [2].
Beside its physiological role, P2X7R has a relevant function also in tumor growth [3] and metastatization [4]. In our previous study we have shown that P2X7R expression accelerates tumor growth in vivo [3], also stimulating secretion of the angiogenic factor VEGF [3].
Question: The reported dual role of P2X7R in tumor growth and in host immune defense raises the question to what extend this receptor is important to control cancer development. To clarify this issue we investigated the impact of genetic deletion of host P2X7R on subcutaneous tumor growth and metastatic dissemination of B16 murine melanoma cells.
Methods: B16 were subcutaneously or intravenously inoculated into wild type (wt) and P2X7R-KO syngeneic C57Bl/6. Tumor size was assessed by in vivo caliper measurement, while cell transfection with intracellular luciferase allowed us to follow metastatic spreading by a small animal total body luminometer (IVIS Lumina).
Results: In P2X7R-KO mice, tumor growth and metastatic diffusion were strongly accelerated compared to P2X7R-wt mice. Ex-vivo analysis of experimental melanomas revealed that lymphocytic and macrophagic infiltrate (CD3+ or F4/80 positive cells, respectively) was almost completely abrogated in P2X7R-KO mice. Furthermore intra-tumor IL-1β and VEGF release, analyzed by ELISA, were drastically reduced in P2X7R deficient host. Lack of robust inflammatory infiltration of tumor from P2X7R-KO mice could be due to impaired ability of P2X7R-less cells to respond to stimulation with cancer cells and thus penetrate tumor mass. Moreover IL-1β release from DCs of P2X7R-KO mice co-cultured with B16 cells was significantly reduced compared to that of DCs from wt mice.
Conclusion: Our data show that lack of host P2X7R expression handicaps anti-tumor immune response and favors tumor growth and metastatic diffusion.
References
1. Vergani A et al (2013) Circulation 127(4):463–475
2. Aymeric L et al (2010) Cancer Res 70:855–858
3. Adinolfi E et al (2012) Cancer Res 72:2957–2969
4. Jelassi B et al (2011) Oncogene 30(18):2108–2122
K 176
P2X7 receptor supports tumorigenesis trough PI3K/Akt/HIF1α/GSK3β pathway in neuroblastoma
Francesca Saveria Amoroso1,*, Alessandra Rotondo1, Marina Capece1, Davide Cangelosi2, Alessia Franceschini1, Luigi Varesio2 and Elena Adinolfi1
1University of Ferrara, Dpt. of Morphology, Surgery and Experimental Medicine, Ferrara, Italy;2G. Gaslini Institute, Laboratory of Molecular Biology, Genova, Italy
P2X7 receptor for extracellular ATP has been focus of interest for decades as a classic cytotoxic receptor, but over the last years, robust evidence demonstrated P2X7 can drive cell proliferation, tumor growth, engraftment and vascularization [1]. Moreover, P2X7 was shown to sustain tumor growth, and to stimulate aerobic glycolysis [2]. To support findings, expression of P2X7 was reported to be increased in several tumors, including breast and skin cancers, neuroblastoma and leukemia [3–5].
Question: An in depth investigation on P2X7 receptor participation in neuroblastoma is still missing. Neuroblastoma is a common and aggressive extra cranial solid tumor in childhood and still causes death in 40 % of the cases.
Methods: To investigate the role of P2X7 receptor in neuroblastoma cells, we took advantage of P2X7 expressing ACN human neuroblastoma cell line, that release VEGF in a P2X7 dependent fashion1, and Neuro-2a murine neuroblastoma cell line. We injected them respectively in nude/nude mice, for allogeneic in vivo model, and Albino J mice, for syngeneic in vivo model.
Results: We show here that basal P2X7 receptor expression supports ACN and Neuro-2a tumor growth in vivo. P2X7 silencing and antagonism with AZ10606120 and A740003 causes a down-modulation of PI3K/Akt/HIF1α/GSK3β pathway, both in vitro and in vivo. Moreover, we found a correlation between P2X7 mRNA expression and a decreased overall survival of stage IV neuroblastoma patients.
Results: Altogether, these data show that P2X7 receptor plays an important role in neuroblastoma growth by modulating PI3K/Akt/HIF1α/GSK3β pathway. Thus, we suggest P2X7 receptor as a possible pharmacological target for neuroblastoma treatment.
References
1. Adinolfi E, Raffaghello L, Giuliani AL et al (2012) Cancer Res 72(12):2957–2969
2. Amoroso F, Falzoni S, Adinolfi E, Ferrari D, Di Virgilio F (2012) Cell Death Dis 3:e370
3. Adinolfi E, Amoroso F, Giuliani AL (2012) J Osteoporosis 2012:637863
4. Raffaghello L, Chiozzi P, Falzoni S, Di Virgilio F, Pistoia V (2006) Cancer Res 66(2):907–914
5. Solini A, Cuccato S, Ferrari D et al (2008) Endocrinology 149(1):389–96
K 177
Extracellular ATP modulates myeloid derived suppressor cells functions via P2X7 and CD39
Marta Vuerich1,*, Giovanna Bianchi2, Patrizia Pellegatti3, Danilo Marimpietri2, Laura Emionite2, Ilaria Marigo4, Vincenzo Bronte5, Francesco Di Virgilio1, Vito Pistoia2, Lizzia Raffaghello2 and Simon C Robson6
1Università di Ferrara, Morfologia, Chirurgia e Medicina Sperimentale, Ferrara, Italy;2Instituto Giannina Gaslini, Genova, Italy;3Università di Ferrara, Scienze della vita e Biotecnologie, Ferrara, Italy;4Instituto Oncologico Veneto, Padova, Italy;5Università di Verona, Verona, Italy;6Beth Israel Deaconess Medical Center, Harvard Medical School, Gastroenterology, Boston, Italy
Question: The main obstacle to the success of immunotherapy is the potent tumor-induced tolerance, resulting in the inability of the host immune system to effectively destroy the malignancy. Over the last years the important role played by Myeloid Derived Suppressor Cells (MDSCs) was well established, but the mechanisms leading to their recruitment and activation are not completely understood. ROS, Arg-1 and TGF-β are clearly involved but many other factors may have a role. In this regard we focused our investigation on the tumor microenvironment where deviated host-tumor interaction takes place. This highly inflammatory milieu, rich in nucleotides and nucleosides such as ATP and adenosine, provides favorable conditions for immune system inhibition and tumor progression.
Methods: In this study we performed an extensive characterization of the purinergic signaling, mainly focusing on P2X7 receptor, in primary and immortalized MDSCs cultures. Moreover we investigated the tumor-promoting activity of MDSCs in a murine neuroblastoma model.
Results: In all MDSCs subsets we verified functional P2X7R expression, and P2X7R-mediated release of TGF-β, ROS and ARG-1. Quite surprisingly, P2X7R activation in MDSC was uncoupled from cytotoxicity. In a murine neuroblastoma model, we observed a specific membrane localization of P2X7R in CD11b/Gr1low MDSCs (M-MDSC), the subset endowed with the strongest immunosuppressive activity. This particular localization was coupled to an increased functional response of M-MDSCs compared to the CD11b/Gr1high subset, where P2X7 was restricted in the cytosol. All these results were supported by the observation that M-MDSCs stimulated a more potently in vivo tumor growth as compared to the granulocytic subset. We also explored the functional expression of CD39 and CD73, and observed that the stimulation with ATP and adenosine increased the in vitro suppressive activity of MDSCs on CD4+ and CD8+ T eff cells.
Conclusions: Based upon all the results, we propose a scenario whereby extracellular ATP, via P2X7 and CD39, plays a crucial role in the modulation of the MDSCs immunosuppressive function.
K 178
Extracellular ATP Induces Apoptosis through P2X7 Receptor activation in MDA-MB 231 cells
Marycruz Flores Flores1,2,* and Eduardo Monjaraz1
1Instituto de Fisiología, BUAP, Laboratorio de Neuroendocrinología, Puebla, México, Mexico;2Facultad de Medicina, BUAP, Biomedicina, Puebla, México, Mexico
Extracellular ATP and other nucleotides act through specific cell surface receptors and regulate a wide variety of cellular responses in many cell types and tissues. The present study aims to explore the effect of ATP on the apoptosis of MDA-MB 231 cells and the underlying mechanism. We demonstrate that MDA-MB 231 cells express several P2Y and P2X receptor subtypes including P2X7, and also we determined the description of functional responses of these cells to extracellular ATP.
Given that the P2X7 receptor not only plays a major role in apoptosis, necrosis, cytokine secretion, but also is involved in cell proliferation and survival as well. We found that extracellular ATP inhibited the proliferation and induces apoptosis in MDA-MB 231 cells. The apoptosis of MDA-MB 231 cells was induced under the treatment of 1 mM ATP over 72 h period which was confirmed by the following techniques such as: a) cell counting (Handheld automated cell counter, Scepter 2.0); b) protein concentration, and c) propidium iodide staining. The effects of ATP were mimicked by micromolar concentrations of 3-O-(4′-benzoyl)-benzoyl-benzoyl-ATP, (Bz-ATP, specific agonist of P2X7 receptor) and were inhibited by pretreatment of MDA-MB 231 cells with a selective blocker of human P2X7 receptor BBG, as well as oxidized ATP.
The nucleotide selectivity and pharmacological profile data support the role for P2X7 receptor as the mediator of the ATP-induced responses. Given the importance of breast cancer in health public, the ability of extracellular ATP to induce the P2X7-mediated apoptosis in breast cancer cell lines may facilitate the development of new strategies to modulate proliferation and invasiveness of breast cancer cells.
K 179
Role of P2X7R in pancreatic ductal adenocarcinoma
Andrea Giannuzzo* and Ivana Novak
University of Copenhagen, biology, Copenhagen, Denmark
The P2X7 receptor is well known for its dual role in promoting cell death and cell proliferation [1]. This unusual role of the P2X7R is very relevant in cancer field, as it is proposed that nucleotide/side concentrations increase in cancer microenvironment [2]. One of the cancers with the worst prognosis is the pancreatic ductal adenocarcinoma (PDAC) with a 5 year survival rate less than 5 % and lack of effective treatment [3]. The aim of this study was to elucidate the possible role of P2X7R in PDAC behavior.
First, we determined the expression of P2X7R in human PDAC cell lines (AsPC-1, BxPC-3, Capan-1, MiaPaCa-2, Panc-1) and a “normal” human pancreatic duct epithelial cell line (HPDE). This was done at the mRNA (qPCR) and the protein levels (WB, immunocytochemistry). For WB antibodies for the C-terminal and extracellular epitopes were used, which allowed distinction between A, D, F, H and A, B, E, G, H, J isoforms, respectively. The data show a down-regulation of mRNA in four cancer cell lines compared to HPDE. Nevertheless, there was a 2 to 4 fold up-regulation of the P2X7R protein (isoform A) in PDAC compared to HPDE cells. Confocal microscopy revealed that the isoforms recognized by the C-terminal antibody localized mostly to the cytoplasmic compartment; the isoforms detected by extracellular antibody were also located on the plasma membrane.
The chronic effect of the activation/inhibition of P2X7R on cell proliferation was estimated with a BrdU assay. The assay showed, after 60 h, there was 30 to 65 % decrease in BrdU incorporation with AZ10606120 inhibitor in all cell lines, indicating inhibition of cell proliferation. Interestingly, with 100 μM of ATP, there was 40 to 70 % decrease in BrdU incorporation; the decrease was higher with 1–5 mM ATP. These data indicate that ATP induces cell death. In addition, we show that there was 60 % decrease with 100 μM of BzATP in HPDE and AsPC-3; other cells required 1 mM of BzATP for the same effect. The combination of different concentrations of ATP and AZ10606120 resulted in a greater decrease in BrdU incorporation.
The acute effects of P2X7R stimulation were evaluated with the pore formation assay (YoPro-1 uptake). ATP, 5 mM, showed the highest fluorescence after 30–60 min in HPDE, AsPc-1, BxPC-3 and Panc-1. A-438079 inhibitor reduced pore formation in these cells; the AZ10606120 inhibitor reduced pore formation only in HPDE and BxPC-3.
In conclusion, the study shows the presence of P2X7R in PDAC cell lines and its involvement in PDAC behavior. We confirm the versatility of this receptor in tumor growth/death, and propose that it could be exploited for drug design for pancreatic cancer therapy.
References
1. Di Virgilio F, Ferrari D, Adinolfi E (2009) doi: 10.1007/s11302-009-9145-3
2. Burnstock G, Di Virgilio F (2013) doi: 10.1007/s11302-013-9372-5
3. Hidalgo M (2010) doi: 10.1056/NEJMra0901557
K 180
The P2X7/NLRP-3 axis: a new possible target in chronic lymphocytic leukemia
Erica Salaro1,*, Alessia Rambaldi1, Simonetta Falzoni1, Francesca Amoroso1, Alessia Franceschini1, Francesco Cavazzini2, Antonio Cuneo2 and Francesco Di Virgilio1
1University of Ferrara, Department of Morphology, Surgery and Experimental Medicine, Ferrara, Italy;2Azienda Ospedaliero-Universitaria, Arcispedale Sant’Anna, Department of Medical Sciences, Section of Hematology, Ferrara, Italy
The P2X7 receptor is overexpressed in some tumors [1], such as B-cell Chronic Lymphocytic Leukemia [2] and thought to be involved in tumor growth. Alterations in P2X7 expression and function are mainly responsible for CLL outcome and have been associated with the clinical course [3]. It has been demonstrated that P2X7 can support both proliferation and apoptosis in hematopoietic cells [4], depending on its trophic [5] or tonic stimulation [6]. Furthermore, the gene encoding P2X7 is located on chromosome 12 and one of the most common cytogenetic aberration in CLL is chromosome 12 trisomy [7]. P2X7 is known as one of the most potent activators of intracellular multiprotein complex, NLRP-3 Inflammasome, that acts as platform for the maturation and secretion of the proinflammatory cytokines IL-1b and IL-18 [8]. Recently, experimental evidence showed that NLRP-3 Inflammasome is deregulated in hepatic parenchymal cells during liver cancer progression [9]. Moreover patients affected by CLL have lower plasma levels of IL-1 b than healthy subjects. Chronic lymphocytic leukemia is the most frequent type of adult leukemia in the western world, resulting in the accumulation of CD5+ B lymphocytes in the blood and the bone marrow.
Question: The aim of this study is to verify the existence of a correlation between trisomy 12 and P2X7 expression in CLL patient, evaluate the role of the P2X7/NLRP-3 axis in CLL patients, and finally understand the role of NLRP-3 Inflammasome in cell proliferation.
Methods: We have analysed PBMC from 20 CLL patients, determined P2X7, NLRP-3 and ASC mRNA and protein levels, LPS-stimulated IL-1b release. Finally, THP-1 cell line silenced for NLRP-3 was used as an in vitro model.
Results: Here we highlight the link between trisomy 12 and P2X7 expression in CLL patients. Higher levels of P2X7 mRNA were expressed by trisomy 12 positive CLL patients. Furthermore, there is an inverse correlation between P2X7 and NLRP-3 expression, together with a lower levels of released IL-1b. This intriguing link between P2X7 and NLRP-3 was further investigated in the THP-1 cell model, silenced for NLRP-3. In agreement with the patients data, lower levels of NLRP-3 supported cell proliferation and increased P2X7 expression also in the THP-1 model.
Conclusion: In conclusion, this study suggests that the P2X7/NLRP-3 axis may be a new possible prognostic marker in CLL and a possible therapeutic target.
References
1. Burnstock G, Di Virgilio F (2013) Purinergic Signal 9(4):491–540
2. Adinolfi E et al (2002) Blood 99:706–708
3. Cabrini G et al (2005) J Immunol 175:82–89
4. Baricordi OR et al (1999) J Biol Chem 274(47):33206–33208
5. Adinolfi E et al (2010) FASEB J 24(9):3393–3404
6. Adinolfi E et al (2009) J Biol Chem 284(15):10120–10128
7. Zhang LYet al (2003) Leukemia 17(11):2097–2100
8. Di Virgilio F (2007) Trends Pharmacol Sci 28:465–472
9. Wei Q et al (2014) Lab Invest 94(1):52–62
10. Hulkkonen J et al (2000) Haemat 85:600–606
K 181
Role of P2X7 receptor splice variants in osteosarcoma
Anna Lisa Giuliani1,*, Elena Adinolfi 1, Carlotta Roncato1, Davide Colognesi1, Francesca Amoroso1, Tommaso Zordan1, Qi Guang Wang2, Marina Capece1, Elena De Marchi1, Alison Gartland3 and Francesco Di Virgilio1
1University of Ferrara, Morphology, Surgery and Experimental Medicine, Ferrara, Italy;2The University of Manchester, Centre for tissue injury and repair, Manchester, UK;3The University of Sheffield, The Mellanby Centre for Bone Research, Sheffield, UK
Osteosarcoma (OS) is the most common primary bone cancer, accounting for approximately six percent of all new paediatric tumors per year. Despite progress in therapy few treatments are currently available to counteract pathologic bone remodelling and alleviate the associated pain. The P2X7 receptor (P2X7R) has recently been shown to have a central role in carcinogenesis as it enhances in vivo tumor cell growth [1], as well as in vitro cancer-associated angiogenesis and invasiveness [2]. Among the nine different naturally occurring P2X7R splice variants known, P2X7RA is the well-characterized full-length isoform, able to form both a cation selective plasma membrane channel and a large conductance pore, permeable to solutes of molecular mass up to 900 Da. P2X7RB is, on the contrary, a shorter isoform, widely expressed in human tissues, lacking pore forming ability while retaining channel properties. Co-expression of the A and B isoforms in HEK293 cells has been demonstrated to potentiate receptor activities [3].
Question: Aim of this study was to throw light on the role of P2X7R in human osteosarcoma and to clarify if the A and B isoforms of the receptor might differently contribute to tumor growth and metastatic spread.
Methods: P2X7RA and B expression was thus investigated in a 54 human OS samples tissue array while their function was assessed in the human OS Te85 cell line transfected with the two isoforms, separately or jointly.
Results: Tissue array analysis showed that 44 tumors (81.4 %) stained positive for both P2X7RA and B, whereas only 31 (57.4 %) were positive with an anti-P2X7RA antibody. Finally, 16 of these OS (27.7 %) expressed only P2X7RB, highlighting for the first time expression of this isoform in cancer. When expressed in Te85 cells, P2X7R was a powerful stimulus for cell growth, the most efficient growth-promoting isoform being P2X7RB alone. Growth stimulation was combined with an increase of both Ca2+ mobilization and NFATc1 activity, the latter being a marker of osteoblast proliferation. RANK-L expression was down modulated in all transfected clones leading to an overall decreased RANK-L/OPG (osteoprotegerin) ratio, indicating reduced signalling to osteoclasts for bone resorption. Interestingly, osteodeposition appeared differently regulated by P2X7R expression since a reduction was caused by P2X7RB alone whereas an increase was produced by co-tranfection of the two isoforms.
Conclusions: All these data highlight a role of P2X7R in OS, identify specific contribution of the A and B isoforms to growth and bone remodelling, and point to P2X7R and related intracellular signalling pathways as potential targets for OS therapy.
Fig. 1 P2X7RA and B increase cell proliferation rate of Te85 transfected cells
References
1. Adinolfi E, Raffaghello L, Giuliani AL, Cavazzini L, Capece M, Chiozzi P, Bianchi G, Kroemer G, Pistoia V, Di Virgilio F (2012) Cancer Res 72(12):2957–2969
2. Adinolfi E, Amoroso F, Giuliani AL (2012) J Osteoporosis. doi: 10.1155/2012/637863
3. Adinolfi E, Cirillo M, Woltersdorf R, Falzoni S, Chiozzi P, Pellegatti P, Callegari MG, Sandonà D, Markwardt F, Schmalzing G, Di Virgilio F (2010) FASEB J 24(9):3393–3404
K 182
Adenosine and ectoenzymes in myeloma life and survival in the bone marrow niche
Alberto Horenstein1,2,*, Valeria Quarona2, Antonella Chillemi2, Gianluca Zaccarello2, Fabio Morandi3, Vito Pistoia3, Nicola Giuliani4 and Fabio Malavasi1,2
1University of Torino, CeRMS, Torino, Italy;2University of Torino, Department of Medical Sciences, Torino, Italy;3Instituto Giannina Gaslini, Department of Experimental and Laboratory Medicine, Genova, Italy;4University of Parma, Hematology and Blood and Marrow Transplantation Center, Parma, Italy
Question: The bone marrow (BM) provides a protected environment for generating a vast array of cell types and of soluble factors used locally or at a distance from their site of production. The question was whether the myeloma subvert the cell components of the BM niche where they grow and expand. These may happen by using products generated by the concerted action of ectoenzymes leading to the production of adenosine (ADO), a regulator of local immunological tolerance.
Methods: We analyzed the expression and roles of set of ectoenzymes in the BM niche, where human myeloma grows. Our working hypothesis is that CD38 and CD157, both NAD+-consuming enzymes, are active in the BM niche, and lead a chain of ectoenzymes, whose final product is exploited by the neoplastic plasma cell as part of its local survival strategy. The pathway identified includes CD203a and CD73, which control the production of ADO. The enzymatic activities and ADO generation were measured by reverse-phase HPLC assays.
Results: The results obtained indicate that myeloma cells usurp the normal organization of BM, replacing it with a niche that maximizes local growth and protection from immune defenses. In line with this hypothesis is the observation that myeloma and surrounding cells are endowed with an ectoenzymatic pathway leading to ADO production in a discontinuous way, where the components do not need to be expressed by the same cell but operating in a closed system. The CD38 (CD157)/CD203a/CD73 pathway appears to be highly efficient in the myeloma niche.
Conclusions: The proposed molecular circuit relies upon proteins which do not need to be ectopically expressed, a major difference from other models of immune evasion observed in breast or colon cancers, which are dependent on de novo expression of CD73. Unlike these tumor models, the myeloma niche represents a closed system, which defines an environment that protects the neoplastic cells and also consents exchange of ectoenzyme substrates and products. A hypothesis relevant for translational medicine is that ADO levels in the myeloma niche might be an early indicator of an aggressive form of disease. Consequently, ADO levels could become a valuable prognostic marker in myeloma. Lastly, the presence of ADO lends to support the use of anti-CD38 mAbs in myeloma therapy. In this case, the mAb would exert cytotoxic functions on cells expressing the molecule, simultaneously depressing the enzymatic activity of CD38 to lead to the production of ADO.
K 183
Ketoprofen-loaded polymeric nanocapsules selectively inhibit cancer cell growth and modulate extracellular ATP metabolism in lymphocytes in a preclinical model of glioblastoma multiforme
Fernanda Teixeira*, Elita Silveira, Juliana Azambuja, Gabriela Debom, Leticia Cruz, Rosélia Spanevello and Elizandra Braganhol
UFPEL, Pelotas, Brazil
Glioblastoma-multiforme (GBM) is the worst and most common brain tumor. Alterations in purinergic signaling and COX-2 overexpression may contribute to inflammatory environment, which favor tumor progression and angiogenesis. Therefore AINEs, like Ketoprofen, have emerging as alternative to cancer therapy. Here, we investigated the effect of ketoprofen-loaded nanocapsules (Keto-NC) treatment on in vivo glioma progression and in the ectonucleotidase activity in lymphocytes from glioma-implanted rats. C6 glioma was implanted in rat brain and 05 days after surgery, animals were divided into four groups: (1) Control (DMSO-treated); (2) NC (drug-unloaded-nanocapsules); (3) Keto-free; (4) Keto-NC (3 mg/Kg/day). Following 15 days of treatment, rats were decapitated and the brain was analyzed by HE. Blood samples were collected for lymphocyte isolation and ectonucleotidase and adenosine deaminase activities were determined as described previously. Keto-NC treatment decreased the in vivo glioma growth by ~80 % as well as reduced the malignity characteristics of implanted tumors. Keto-free and Keto-NC treatment altered the ectoenzyme activities in lymphocytes from glioma-bearing rats. ATP and ADP hydrolysis were significantly increased in lymphocytes from rats treated with Keto-free (2 and 4 times increase for ATP/ADP hydrolysis, respectively) and Keto-NC (1.5 and 2.4 times increase for ATP/ADP hydrolysis, respectively). In contrast, Keto-free and Keto-NC significantly decreased ADA activity in 2 and 3.8 times, respectively, suggesting that antiinflammatory effects of ketoprofen could also be associated with the modulation of the adenine nucleotide metabolism in lymphocytes. Data indicate the potential of Keto-NC as a therapeutic alterative to GBM treatment.
K 184
Gene expression profile comparison between bone metastatic and non-metastatic prostate cancer cell lines
Freyja Docherty, Anne Fowles, Colby Eaton and Ning Wang*
The University of Sheffield, Human Metabolism, Sheffield, UK
Prostate cancer is currently the most commonly diagnosed cancer and the second leading cancer death in man in western countries. About 90 % of patients with advanced prostate cancer have metastases in bone which is currently untreatable and is the main cause of morbidity in these patients. It has been suggested only the so called metastasis initiating cells are responsible for the formation of bone metastases, via acquisition of a mesenchymal phenotype through epithelial to mesenchymal transfer (EMT). Studies have shown that P2 receptors were involved in prostate cancer cell growth. A recent study has suggested that alterations in the expression of P2 receptors were involved in EMT in MDA-MB-468 breast cancer cells. Similar gene expression alterations of P2 receptors may also present in bone metastasis initiating prostate cancer cells. In this study, we performed Taqman quantitative RT-PCR to reveal the gene expression profile of three human prostate cancer cell lines with different ability to form bone metastases, including LNCaP (non-bone metastatic to bone), C4 2B4 (derivative of LNCaP but forming osteoblastic metastasis), and PC3 (forming osteolytic metastasis). Total RNA was isolated from cultured cells using the Promega ReliaPrep RNA Cell Miniprep system. First strand cDNA was synthesized from 200 ng RNA in a 20 μl reaction system containing 250 ng Promega random primer, 100 mM dNTP, and 200U Invitrogen SuperScript III. Individual qRT-PCR was performed using equal amount of synthesized first strand cDNA. Results showed that RNA of P2X4, X5, X7, Y1, Y4, Y13, and Y14 receptors were expressed (CT value < 35) in all the three cell lines. Among these P2 receptors, P2RX4 is the most highly expressed (CT value ~ 24), while P2RY14 receptor is the least expressed (CT value ~32). The relative gene expressions (ΔCT) were also compared between the non-bone metastatic cell line LNCaP and bone metastatic cell lines C4 2B4 and PC3, using GAPDH as the endogenous control. Results showed that both C4 2B4 and PC3 expressed significantly higher level of P2RX3 but lower level or no expression of P2RX2, P2RY6, and P2RY11 compared to LNCaP, while the more mesenchymal-like PC3 cells express significantly higher levels of P2RY1 (~8 fold) than both LNCaP and C4 2B4. In conclusion, this study has provided consistent and further detailed gene expression profile of prostate cancer cell line compared to previous studies. It also highlighted that alterations in gene expression of certain P2 receptor subtypes (P2X2, P2X3, P2Y6, and P2Y11) may be correlated with the ability of prostate cancer cells to metastasize to bone and a mesenchymal phenotype.
M: Purinergic signaling in nervous system, glial function, development and pain
M 185
The ectonucleotidase NTPDase2 reduces progenitor cell proliferation in the dentate gyrus of the adult mouse brain
Jennifer Stefani1,*, Kristine Gampe1, Klaus Hammer1, Peter Brendel1, Alexandra Poetzsch1, Grigori Enikolopov2, Keiichi Enjyoji3, Amparo Acker-Palmer1, Simon C. Robson2, Herbert Zimmermann1
1Institute of Cell Biology and Neuroscience, Goethe-University, Frankfurt, Germany;2Cold Spring Harbor Laboratory, Cold Spring Harbor, NY 11724, USA;3Department of Medicine, Beth Israel Deaconess Medical Center, Harvard Medical School, Boston, Mass, USA
Neural stem cells reside in the dentate gyrus of the hippocampus which has been identified as a major site of neurogenesis in the adult rodent brain. Increasing evidence suggests that extracellular purine and pyrimidine nucleotides are involved in the control of both embryonic and adult neurogenesis [1]. Ectonucleotidases hydrolyze extracellular nucleotides and thus control their functionality. We have previously shown that stem/progenitor cells in the adult dentate gyrus express high levels of the plasma membrane-located ectonucleotidase nucleoside triphosphate diphosphohydrolase 2 (NTPDase2) [2]. NTPDase2 catalyzes the dephosphorylation of nucleoside triphosphates and diphosphates, thus modulating their effect on nearby nucleotide receptors. Deletion of the ectonucleotidase would increase local extracellular nucleotide concentrations and might indicate roles of purinergic signaling in neurogenesis.
We demonstrate that genetic deletion of NTPDase2 abrogates ectonucleotidase activity in the neurogenic hippocampus but does not affect levels of activity and protein of other ectonucleotidases. It causes increased progenitor cell proliferation without affecting long-term survival and new neuron formation. Lack of NTPDase2 leads to expansion of the hippocampal stem cell pool as well as of the intermediate progenitor cell types-2a and -2b. Cell expansion is lost at the level of maturing doublecortin-expressing young neurons, paralleled by decreases in CREB phosphorylation. Our data suggest that NTPDase2 functions as a brake on nucleotide-mediated progenitor cell proliferation and expansion. The enzyme thus functions as a homeostatic regulator of hippocampal neurogenesis at the level of progenitor cell expansion.
References
1. Zimmermann H (2011) Semin Cell Dev Biol 22:194–204
2. Shukla V, Zimmermann H, Wang L, Kettenmann H, Raab S, Hammer K, Sévigny J, Robson SC, Braun N (2005) J Neurosci Res 80:600–610
M 186
P2X7 receptors mediate phagocytosis by human neural precursor cells and neuroblasts in the early developing CNS
Michael D. Lovelace1,2, Ben Gu3,4,*, Steven Eamegdool1,2, Michael W. Weible II1,2,5, James S. Wiley4, David G. Allen2,6 and Tailoi Chan-Ling1,2
1Discipline of Anatomy and Histology, Sydney Medical School, University of Sydney, Sydney, Australia;2Bosch Institute, The University of Sydney, Sydney, Australia;3Nepean Campus, Sydney Medical School, The University of Sydney, Sydney, Australia;4Florey Institute of Neuroscience and Mental Health, University of Melbourne, Melbourne, Australia;5Biomolecular and Physical Sciences, Griffith University, Brisbane, Australia;6Discipline of Physiology, Sydney Medical School, The University of Sydney, Sydney, Australia
During early human neurogenesis there is overproduction of neuroblasts and neurons accompanied by widespread programmed cell death (PCD). Whilst it is understood that CD68+ microglia and astrocytes mediate target-dependent PCD and subsequent phagocytosis that takes place much later in embryonic development, little is known of the cell identity or the scavenger molecules utilized to remove apoptotic corpses during the earliest stages of human neurogenesis. Recently, studies have found that the P2X7 receptor can serve as a scavenger receptor for apoptotic debris in the absence of extracellular ATP or serum, both of which inhibit this function (1–2). As the early developing human central nervous system (CNS) does not contain microglia or astrocytes, and has an established blood–brain barrier which prevents the extravasation of serum proteins across into the brain parenchyma, we hypothesized that the P2X7 receptor could play a role in removing the corpses of dying cells during neurodevelopment.
Methods: This study used a combination of immunocytochemical staining to identify distinct cellular populations from primary cultures of human foetal telencephalon(3), P2X7 receptor functional assays, and flow cytometry/time lapse microscopy of phagocytotic uptake of latex beads, apoptotic human neural ReNcells and autologous cells (human neural precursor cells (hNPCs) and neuroblasts).
Results: We show convincing evidence that doublecortin+ (DCX) neuroblasts and human neural precursor cells (hNPCs) can clear apoptotic cells by innate phagocytosis mediated via the P2X7 receptor (P2X7R). Both P2X7RhighDCXlow hNPCs and P2X7RhighDCXhigh neuroblasts phagocytose targets including latex beads, apoptotic ReNcells and apoptotic hNPC/neuroblasts. Pretreatment with 1 mM ATP, 100 μM OxATP (P2X7R antagonist), human serum or siRNA knockdown of P2X7R inhibited phagocytosis of the targets.
Conclusions: This is the first demonstration that hNPCs and neuroblasts participate in clearance of apoptotic corpses during pre target-dependent neurogenesis and mediate phagocytosis using P2X7R as a scavenger receptor. The data support the novel concept that hNPCs and neuroblasts may regulate their own numbers by phagocytosis in early CNS development.
References
1. Gu BJ, Saunders BM, Petrou S, Wiley JS (2011) P2X7 Is a Scavenger Receptor for Apoptotic Cells in the Absence of Its Ligand, Extracellular ATP. J Immunol 187:2365–2375
2. Gu BJ et al (2012) P2X7 receptor-mediated scavenger activity of mononuclear phagocytes toward non-opsonized particles and apoptotic cells is inhibited by serum glycoproteins but remains active in cerebrospinal fluid. J Biol Chem 287:17318–17330
3. Weible MW, Chan-Ling T (2007) Phenotypic characterization of neural stem cells fromhuman fetal spinal cord: synergistic effect of LIF and BMP4 to generate astrocytes. Glia 55:1156–1168
M 187
Human mesenchymal stem cells display a distinct P1 receptor expression pattern during differentiation into several lineages
Dilek Gueneri*, Dorothee Schipper, Andreas Pansky and Edda Tobiasch
Department of Natural Sciences, Bonn-Rhine-Sieg University of Applied Sciences, Von-Liebig-Str. 20, Rheinbach, Germany
Mesenchymal stem cells have the capacity for self-renewal and differentiation into various lineages. Of major interest are the differentiations towards the adipogenic, osteogenic, and the two vascular lineages namely endothelial and smooth muscle cells. For a better understanding of the differentiation pathways it is of interest to identify key players and signal transduction involved in these processes. Purinergic receptors play a role in many physiological processes, including proliferation and differentiation.
In this project mesenchymal stem cells were isolated from human adipose tissue and differentiated towards the above mentioned lineages. The differentiations were confirmed using cell lineage specific markers as well as cell specific staining. The gene and protein expression pattern of P1A1, P1A2A, P1A2B, and P1A3 receptors was investigated.
Interestingly the specific regulation of P1A3 receptor was found to divide the vascular lineages from the other two. The second step of branching is linked to the up- vs. down-regulation of the P1A1 receptor in all four differentiation lineages. This finding is in line with the specific expression pattern of the P2 receptors which was found to define the same cell lineages also with the up- vs. down-regulation of one specific P2 receptor.
Here we showed for the first time that next to P2 also specific P1 receptors seem to play a role in adult stem cell differentiation lineages. This is of major interest because a better understanding of the differentiation processes of stem cells could improve regenerative medicine approaches for tissue engineering.
M 188
Comparative evaluation of GPR17 ligands in silico and in vitro on cultured primary oligodendrocyte precursor cells
Maria P. Abbracchio1,*, Chiara Parravicini1, Simona Daniele3, Elisabetta Bonfanti1, Elisa Zappelli3, Marta Fumagalli1, Cristina Sensi2, Claudia Martini3, Ivano Eberini2 and Maria Letizia Trincavelli3
1Department of Pharmacological and Biomolecular Sciences, Via Balzaretti 9;2Via Trentacoste 2, University of Milan, 20100 Milan, Italy;3Dipartimento di Farmacia, Università di Pisa, Via Bonanno 6, 56126 Pisa, Italy
The membrane GPR17 receptor responsive to uracil nucleotides and cysteinyl-leukotrienes [1–4] is a key regulator of oligodendrogenesis and a new target for remyelinating diseases [1,4,5]. GPR17 is transiently expressed in early oligodendrocyte precursor cells (OPCs) in transition to pre-immature phenotypes, followed by marked downregulation in mature cells expressing myelin basic protein (MBP) [1,2]. Several GPR17 agonists, such as UDPglucose (UDPglu) and LTD4 [1,2] promote, while the recently reported synthetic GPR17 agonist MDL29,951 has been described to inhibit OPC maturation [6]. Here, we compared some putative endogenous and synthetic compounds in molecular docking experiments and in GPR17 activation in cultured OPCs. In silico, all compounds were able to bind to the orthosteric binding site of GPR17 corresponding to the nucleotide binding site [7], with decreasing affinity and pKi values of 8.80, 7.65, 7.23 and 4.97 for cangrelor, Asinex 1 (ASN1), UDPglu and MDL29,951, respectively. In cultured OPCs, where GPR17 activation is linked to a Gi protein [1,2,6], UDPglu, ASN1 and MDL29,951 concentration-dependently inhibited forskolin-stimulated cAMP formation, with IC50 values of 530 nM, 6.5 nM and 2.5 μM, respectively. As previously reported for UDPglu [1], activation of GPR17 by MDL29,951 was competitively antagonized by the P2Y antagonist cangrelor. ASN1 was also tested in vitro on OPC differentiation, in parallel with UDP, LTD4 and LTE4. All agonists significantly stimulated the formation of mature MBP-expressing cells. Finally, preliminary data show that, in OPC-dorsal root ganglion neuron co-cultures, similarly to UDP and UDPglu, ASN1 markedly stimulated the formation of MBP-positive myelin tracts aligning neuronal axons, indicated as neurofilament (NF)-positive axons in the Figure, confirming that this compound could efficiently promote myelination. Sponsored by Italian FISM, Grant N. 2010/R/2 and 2013/R/1 to MPA.
References
1. Fumagalli M, Daniele S, Lecca D et al (2011) J Biol Chem 286:10593–10604
2. Daniele S, Trincavelli ML, Fumagalli M et al (2014) Cell Signal 26:1310–1325
3. Ciana P, Fumagalli M, Trincavelli ML et al (2006) Embo J 25:4615–4627
4. Lecca D, Trincavelli ML, Gelosa P et al (2008) PLoS ONE 3:e3579
5. Chen Y, Wu H, Wang S et al (2009) Nat Neurosci 12:1398–1406
6. Hennen S, Wang H, Peters L et al (2013) Sci Signal 6:ra93
7. Eberini I, Daniele S, Parravicini C et al (2011) J Comput Aided Mol Des 25:743–752
M 189
L-glutamate and [60] fullerene modulates gene expression of adenosine and metabotropic glutamate receptors in rat’s neuronal cells
Davide Giust1, Tatiana Da Ros2, Mairena Martin3 and Jose Luis Albasanz3
1University of Manchester, Institute of Inflammation and Repair, Manchester, UK;2University of Trieste, Dipartimento di Scienze Chimiche e Farmaceutiche, Trieste, Italy;3Universidad de Castilla-La Mancha, Quimica Inorgánica, Orgánica Y Bioquímica, Ciudad ReAL, Spain
Adenosine is a nucleoside which modulates neurotransmitter release through specific receptors in membrane. A1 receptor inhibits glutamate release while A2A stimulates this release. L-Glutamate (L-Glu) is the main excitatory neurotransmitter which is involved in learning and memory processes through ionotropic and metabotropic receptors. However, at high concentrations L-Glu acts a neurotoxic causing degeneration and neuronal death which are related to neurodegenerative diseases. The aim of the present work was to study the effect of L-Glu on the cell’s structural architecture and the expression of adenosine and metabotropic glutamate receptors. In addition, the possible protective effect exhibited by t3ss a C60 hydro soluble fullerene derivative was also analyzed. To this end, rat’s neuronal cells were treated with L-Glu (100 nM, 1 and 10 μM) and/or t3ss and microtubule associated protein-2 (MAP-2) and neurofilament heavy polypeptide (NEFH), natively associated proteins to the dendritic shape maintenance were determined by Immunocitochemistry. Expression of mGluR and AdoR was analyzed by qPCR. Results showed that C60 fullerene derivative t3ss decreased cell death exhibited by glutamate exposure, as demonstrated by MTT assay. In addition, t3ss exerted a different effect on adenosine and metabotropic glutamate receptors analyzed. Interestingly, A2A and mGlu1 mRNAs were significantly decreased in conditions in which t3ss neuroprotected cortical neurons from L-Glu toxicity. These results suggest that fullerene derivatives protect neurons against glutamate toxicity in a process that appears to be associated with the modulation of the gene expression of adenosine and metabotropic glutamate receptors.
M 190
Nuclear location of P2X6 subunit in mouse hippocampal neurons
Juan Ignacio Díaz-Hernández*, Álvaro Sebastián-Serrano, Rosa Gomez-Villafuertes, Miguel Díaz-Hernández and María Teresa Miras-Portugal
P2X6 subunit expression and immunoreactivity is observed throughout the central nervous system, especially in hippocampus and cerebellum [1]. The ability if this subunit to form homomeric receptors is very inefficient, but its presence associated with P2X2 and P2X4 subunits is well documented. The abundance of mRNA and protein expression of P2X6 in hippocampus is relatively high in neuronal bodies of CA1, CA2, CA3 regions and dentate gyrus.
In this work, we described the age-dependent increase of P2X6 levels in mice hippocampus, which was not correlated with an increase of P2X2 and P2X4 subunits. Moreover, this increase in P2X6 levels was more evident as a dotted pattern in perinuclear and nuclear areas of neuronal bodies, as observed either with immunohistochemistry or electron microscopy. In order to clarify this unexpected distribution, we developed several molecular tools for exogenous expression of P2X6 subunit. Initially, P2X6 subunit was cloned in-frame with yellow fluorescent protein (YFP) at N-terminal end (P2X6-YFP). This chimeric protein allowed us to confirm the presence of P2X6 in perinuclear and nuclear areas of transfected hippocampal neurons. However P2X6 does not co-localize with either fibrillarin (nucleoli marker), NeuN (neuronal-only transcription factor) or DAPI (condensed chromatin). Moreover, when we overexpress P2X6-YFP protein without the first N-terminal 14 aminoacids, deletion required for P2X6 to be able of reach extracellular membrane [2], no fluorescence is observed in perinuclear and nuclear location. Thereafter, different regions from P2X6 sequence were amplified by PCR and cloned joined with the c-myc flag in C-terminal end of each fragment, and interestingly, we found that the extracellular loop of P2X6 subunit is the responsible for the nuclear and perinuclear location.
All these data, opens a new field of questions and mechanisms for P2X subunit processing, trafficking and, perhaps, new functions, especially for those whose are less known or defined such as P2X6.
References
1. García-Lecea M, Pérez-Sen R, Soto F, Miras-Portugal MT, Castro E (2001) P2 Receptors in cerebellar neurons: Molecular diversity of ionotropic ATP receptors in Purkinje cells. Drug Dev Res 52:104–113
2. Ormond SJ et al (2006) An uncharged region within the N terminus of the P2X6 receptor inhibits its assembly and exit from the 10847 endoplasmic reticulum. Mol Pharmacol 69:1692–1700. doi:10.1124/mol.105.020404
M 191
Functional characterization of P2X7 receptors from mouse cerebellar granule neurons at the axodendritic and somatic regions
Jesús Sánchez-Nogueiro1, Patricia Marín-García2, Diego Bustillo1, Luis Alcides Olivos-Oré1, María Teresa Miras-Portugal1 and Antonio R. Artalejo1,*
1Universidad Complutense, Faculty of Veterinary, Madrid, Spain;2Universidad Rey Juan Carlos, Faculty of Health Sciences, Madrid, Spain
The subcellular distribution and early signalling events of P2X7 receptors were studied in mouse cerebellar granule neurons. Whole-cell patch-clamp recordings evidenced inwardly directed non-desensitizing currents following adenosine 5′-triphosphate (ATP; 600 μM) or 2′-3′-o-(4-benzoylbenzoyl)-adenosine 5′-triphosphate (BzATP; 100 μM) administration to cells bathed in a medium with no-added divalent cations (Ca2+ and Mg2+). Nucleotide-activated currents were inhibited by superfusion of 2.5 mM Ca2+, 1.2 mM Mg2+ or 100 nM Brilliant Blue G (BBG), hence indicating the expression of ionotropic P2X7 receptors. Fura-2 calcium imaging showed [Ca2+]i elevations in response to ATP or BzATP at the somas and at a small number of axodendritic regions of granule neurones. Differential sensitivity of these [Ca2+]i increases to three different P2X7 receptor antagonists (100 nM BBG, 10 mM 4-[(2S)-2-[(5-isoquinolinylsulfonyl) methylamino]-3-oxo-3-(4-phenyl-1-piperazinyl)propyl] phenyl isoquinolinesulfonic acid ester, KN-62, and 1 mM 3-(5-(2,3-dichlorophenyl)-1H-tetrazol-1-yl)methyl pyridine hydrochloride hydrate, A-438079) revealed that P2X7 receptors are co-expressed with different P2Y receptors along the plasmalemma of granule neurons. Finally, experiments with the fluorescent dye YO-PRO-1 indicated that prolonged stimulation of P2X7 receptors does not lead to the opening of a membrane pore permeable to large cations. Altogether, our results emphasize the expression of functional P2X7 receptors at both the axodendritic and somatic levels in mouse cerebellar granule neurons, and favour the notion that P2X7 receptors might function in a subcellular localization-specific manner: presynaptically, by controlling glutamate release, and on the cell somas, by supporting granule neuron survival against glutamate excytotoxicity.
M 192
Evidence for a role of the P2X7 receptor in myelination of peripheral nerves
Giovanna Sociali, Karina Kaczmarek-Hájek, Anika Saul, Wiebke Möbius, Annette Nicke and Santina Bruzzone*
University of Genova, Department of Experimental Medicine, Section of Biochemistry, Genova, Italy
Question: Charcot-Marie-Tooth 1A (CMT1A) is a progressive hereditary motor and sensory neuropathy, characterized by progressive demyelination resulting in distal muscle wasting and weakness, foot deformities, and severe slowing of nerve conduction. It is caused by overexpression of the peripheral myelin associated protein (PMP22). Recently, a PMP22-dependent overexpression of the P2X7R was observed in Schwann cells isolated from a transgenic rat CMT1A model and shown to causally impair their function (i.e. migration, ciliary neurotrophic factor release and myelination). In this study, we aim at estimating the effect of a PMP22-independent P2X7R overexpression on myelination of peripheral nerves.
Methods: To this end, we used a novel P2X7 BAC transgenic mouse model in which a EGFP-tagged P2X7 receptor is overexpressed under the control of its endogenous promoter. A strain with a particular high transgene expression (X7EGFP-61) develops motor deficits already at a very young age and was used in this study.
Results: Preliminary electron microscopy analysis of sciatic nerve fibers from these mice suggest a higher (approximately two-fold) frequency of axons surrounded by non-compact myelin in P2X7-overexpressing mice, compared to wild-type mice.
Next, we performed RT-PCR analysis to compare the expression level of Schwann cell differentiation/de-differentiation/proliferation markers in sciatic nerves from P2X7-overexpressing or wild-type mice. Our data show that mRNA levels of the proliferation markers Mki67 and the dedifferentiation markers Oct6, cJun and p75, were increased two- to three-fold in sciatic nerves from P2X7-overexpressing mice. In contrast, P2X7R overexpression did not significantly affect the expression of any of the differentiation markers (i.e. Krox20, PRX, P0 and PMP22).
Conclusion: These data are in line with a role of P2X7Rs in myelination.
M 193
The diadenosine homodinucleotide P18 improves in vitro myelination in experimental Charcot-Marie-Tooth type 1A
Lucilla Nobbio, Giovanna Sociali, Davide Visigalli, Elena Mannino, Laura Sturla, Fulvia Fiorese, Matthias Kassack, Michael Sereda, Angelo Schenone and Santina Bruzzone*
University of Genova, Department of Experimental Medicine, Section of Biochemistry, Genova, Italy
Question: Charcot-Marie-Tooth 1A (CMT1A) is a demyelinating hereditary neuropathy whose pathogenetic mechanisms are still poorly defined and an etiologic treatment is not yet available. An abnormally high intracellular Ca2+ concentration ([Ca2+]i) occurs in Schwann cells from CMT1A rats (CMT1A SC) and is caused by overexpression of the purinoceptor P2X7. Normalization of the Ca2+ levels through down-regulation of P2X7 appears to restore the normal phenotype of CMT1A SC in vitro.
We recently demonstrated that the diadenosine 5′,5″′-P1, P2-diphosphate (Ap2A) isomer P18 behaves as an antagonist of the P2X7 purinergic receptor, effectively blocking channel opening induced by ATP. In addition, P18 behaves as a P2Y11 agonist, inducing cAMP overproduction in P2Y11-overexpressing cells. Here we investigated the in vitro effects of P18 on CMT1A SC.
Methods: We compared the levels of intracellular cAMP ([cAMP]i) in CMT1A SC and in SC from wild-type (wt) rats, in basal conditions and upon stimulation with P18. The levels of myelin protein zero, a marker of myelin production, were evaluated in CMT1A and wt organotypic dorsal root ganglia (DRG) cultures by Western blot analysis. We evaluated the effect of P18 on the expression levels of SC differentiation/de-differentiation markers by RT-PCR.
Results: We observed that [cAMP]i, a known regulator of SC differentiation and myelination, are significantly lower in CMT1A SC than in wt cells. P18 increased [cAMP]i in both CMT1A and wt SC, and this effects was blunted by NF157, a specific P2Y11 antagonist. Prolonged treatment of DRG cultures with P18 significantly increased expression of myelin protein zero in both CMT1A and wt cultures. Interestingly, P18 decreased the content of non-phosphorylated neurofilaments, a marker of axonal damage, only in CMT1A DRG cultures.
In addition, we evaluated the effect of P18 on the expression levels of SC differentiation/de-differentiation markers. DRG isolated from wild type and CMT1A rat embryos were treated with P18 (200 nM for 1 week). RT-PCR analysis showed a significant increase in the mRNA levels of P0, Krox20 and PRX (all differentiation markers) in CMT1A DRG cultures treated with P18, compared to vehicle treated CMT1A DRG cultures. On the contrary, P18 did not affect mRNA levels of SC de-differentiation markers.
Conclusions: These results suggest that P2X7 antagonists, in combination with [cAMP]i-increasing agents, could represent a therapeutic strategy aimed at correcting the molecular derangements causing the CMT1A phenotype.
M 194
Reciprocal interaction between purinergic and glutamatergic network modulation in mouse cerebellum
Ramona Rudolph1, Hannah M. Jahn2, Frank Kirchhoff2 and Joachim W. Deitmer1,*
1FB Biologie, University of Kaiserslautern;2Molecular Physiology, University of Saarland, Homburg, Germany
We have studied the relevance of purinergic modulation of neural circuits in the cerebellar cortex of rodents. Spontaneous inhibitory input to Purkinje neurons has been studied with respect to its modulation by purinergic and glutamatergic inputs, by means of patch-clamp recordings from acute brain slices from wild-type and genetic models deficient in cell-specific receptor subtypes. In the presence of ADP or AMPA, the frequency of spontaneous evoked postsynaptic currents in Purkinje neurons increased two- and threefold, respectively. Surprisingly, prior activation of one pathway suppressed the activation of the other pathway, although repetitive activation of either pathway was not impaired. Employing Bergmann glia/astrocyte-specific transgenic mice, deficient in either P2Y1 or AMPA receptors, indicated the receptor types and the cells involved in this modulation of the cerebellar circuit. Our results suggest that both purinergic and glutamatergic modulation of the inhibitory input to Purkinje neurons were mediated to a large part by Bergmann glial cells via P2Y1 and AMPA-type glutamate receptors, presumably resulting in the release of glutamate. Glutamate released from Bergmann glia would then activate NMDA-type glutamate receptors in local inhibitory interneurons, stellate and/or basket cells, thereby increasing the firing rate of these neurons and hence augmenting the inhibitory input to Purkinje neurons. We propose that this glial-mediated modulation of the cerebellar circuit contributes to the information processing in the cerebellar cortex leading to fine motor coordination.
Supported by the Deutsche Forschungsgemeinschaft.
M 195
The regulation and function of the ATP-gated P2X7 receptor in epilepsy
A. Jimenez Pacheco1,*, G. Mesuret1, A. Sanz1, M. Diaz-Hernandez2, T. Engel1 and D. C. Henshall1
1Department of Physiology & Medical Physics, Royal College of Surgeons in Ireland;2Department of Biochemistry and Molecular Biology of Universidad Complutense, Madrid, Spain
Epilepsy is a common and disabling neurologic disorder which affects about 1 % of the population. Seizures are the result of abnormal electrical activity in the brain and can be profoundly disabling, affecting work, social activity and increasing risk of harm. In at least 30–40 % of patients, treatment is inadequate, with patients continuing to experience seizures; therefore, there is an important need to develop new anti-epileptogenic drugs. Increased P2X7 receptor expression has been reported in the hippocampus and cortex after prolonged seizures in animal models and P2X7 receptor antagonists reduced seizure-induced damage to these brain regions. Here we show that P2X7 receptor mRNA and protein level is up-regulated in the hippocampus and cortex of epileptic mice. Using GFP-expressing P2X7 receptor reporter mice we localized the increased expression mainly to neuron and microglia in epileptic animals. In isolated synaptosomes, we observed increased presence and, using FURA-2 Ca+ imaging, functional activation of P2X7R in epileptic mice. These findings support a role for P2X7R in the pathophysiology of chronic epilepsy and suggest P2X7 receptor antagonists may have therapeutic effects against recurrent seizures or progression of disease pathology.
This work was supported by a studentship from the Irish Research Council (A.J.) and funding from the Health Research Board Ireland (HRA_POR/2012/56, HRA_POR/2010/123) is acknowledged.
M 196
Investigation of anticonvulsant and antiepileptogenic effects of P2X7 receptor antagonists in selected seizure models
Wolfgang Fischer1,*, Ute Krügel1, Heike Franke1, Klaus Dinkel2 and Michael Letavic3
1Rudolf-Boehm-Institute of Pharmacology and Toxicology, University of Leipzig, Germany;2Affectis Pharmaceuticals AG, Dortmund, Germany;3Janssen Research and Development, LLC, San Diego, CA, USA
The ATP-gated P2X7 receptor (P2X7) is a non-selective cation channel that senses high extracellular ATP concentrations. In the CNS, P2X7 activation contributes to microglia stimulation, astrogliosis and neuroinflammatory responses. Accordingly, this receptor has attracted interest as a modulator of various disorders in the nervous system, including epilepsy. Recent data indicate that P2X7 may be implicated in epileptogenic phenomena, neuronal loss and gliosis, and provide support for the use of P2X7 antagonists for the treatment of status epilepticus and seizure control1, but contradictory results exist due to the use of different animal models. However, direct anticonvulsant properties of P2X7 antagonists were not examined to date. In this regard, we started studies with three CNS-permeable P2X7 antagonists (Brilliant Blue G, AFC-5128, JNJ-47965567) and a natural compound (tanshinone IIA sulfonate) to investigate the anticonvulsant and antiepileptogenic effects in experimental seizure models. At first, we tested and compared the potency of the antagonists using HEK293 cell lines stably expressing human, rat and mouse P2X7 and Ca2+ fluorometric analysis, at which JNJ-47965567 revealed the highest blocking potency at low nanomolar concentrations for the rat and mouse P2X7. Second, in the maximal electroshock seizure threshold test and in the pentylenetetrazol seizure threshold test in mice, used as standard models for generalized tonic-clonic seizures and myoclonic (and absence) seizures, respectively, the investigated P2X7 antagonists showed no marked anticonvulsant activity. Third, in the pentylenetetrazol kindling model in rats, a useful model for epileptogenesis as well as primary generalized seizures, in which repeated injections of an initial subconvulsant dose of the chemoconvulsant induces the gradual development of clonic-tonic seizures, Brilliant Blue G and tanshinone exhibited modest, yet significant antiepileptogenic effects, at the beginning of the kindling process. On the other hand, the selective and potent P2X7 antagonist JNJ-47965567 did not modulate significantly the progression of seizure development. Studies with AFC-5128 are in progress. Finally, we have started immunohistochemical studies with focus on changes in P2X7 expression, neuronal damage and gliosis. In summary, our preliminary findings did not indicate remarkable anticonvulsant or antiepileptogenic effects of the investigated P2X7 antagonists. We conclude that P2X7 inhibition alone may not be sufficient to suppress epileptogenic processes in vivo.
Reference
1. Engel T, Jimenez-Pacheco A, Miras-Portugal MT, Diaz-Hernandez M, Henshall DC (2012) Int J Physiol Pathophysiol Pharmacol 4:174–187
M 197
The involvement of methionine exposure in some parameters of purinergic system in zebrafish (Danio rerio) brain
Fernanda Vuaden1, Luiz Eduardo Savio1, Maurício Bogo2, Carla Bonan2 and Angela Wyse1,*
1UFRGS, Biochemistry, Porto Alegre, Brazil;2PUC-RS, Porto Alegre, Brazil
Aim: Hypermethioninemic patients exhibit a wide range of clinical manifestations from completely asymptomatic toward neurological dysfunctions, but the mechanisms involved in these alterations have not been completely clarified. ATP exerts important roles in synaptic transmission while adenosine, the final product of ATP breakdown, has been described as an endogenous neuroprotective agent. Ectonucleotidases constitute the predominant way to control extracellular nucleotides and nucleoside levels. Ectonucleotidases E-NTPDase hydrolyzes extracellular ATP and ADP to AMP and ecto-5′-nucleotidase hydrolyzes AMP to adenosine. Parameters of purinergic signaling and adenosine receptors subtypes (A1, A2A1, A2A2, A2B) have already been characterized in zebrafish brain. Since adenine nucleotides play crucial roles in neurotransmission and neuromodulation, in the present study we investigated the effects of long-term methionine exposure on nucleotide catabolism, as well as the gene expression pattern of E-NTPDases (1, 2, and 3), ecto-5′-nucleotidase, and adenosine receptors in zebrafish brain.
Methods: Animals were exposed to methionine in tank water during 7 days (long-term treatment) at two different concentrations (1.5 and 3.0 mM). For enzyme assays, brain membranes were prepared fresh daily and maintained at 0–4 °C throughout preparation. For molecular experiments, the animals were euthanized and the brains were removed for total RNA extraction with Trizol® and the cDNA species were synthesized according manufacturer’s instructions.
Results: We observed a significant decrease in both concentrations tested for ATP (16 and 22 %, respectively) and ADP (37 and 41 %, respectively) catabolism in zebrafish brain membranes (n = 6). However, ntpd1 gene expression pattern increased significantly (38 % at 1.5 mM and 50 % at 3.0 mM), while ntpd2mg (24 %), ntpd2mv (29 %), and ntpd3 (44 %) mRNA transcript levels increased significantly at 3.0 mM (n = 5). AMP hydrolysis and Nt5e gene expression were not significant altered after long-term methionine exposure. However, we observed a significant increase in A1 (21 % at 1.5 mM and 33 % at 3.0 mM) and A2A1 (31 % at 3.0 mM) transcript levels after 7 days of treatment (n = 5).
Conclusion: Methionine exposure decreased ATP and ADP hydrolysis in zebrafish brain, leading to ATP accumulation on synaptic cleft, which can be potentially toxic for nervous system. On the other hand, ntpd gene expression pattern was increase after methionine treatment, which could be acting as a compensatory effect in zebrafish brain in order to efficient removal the excessive ATP. Adenosine receptors presented changes on their expression pattern after methionine exposure, which can modulate the effects of hypermethioninemia. These results may contribute to better understand the pathophysiological mechanisms that increase the susceptibility to neurological symptoms observed in hypermethioninemic patients.
Sources of research support CNPq; CAPES, PROPESQ/UFRGS
M 198
Role of adenosine signaling on PTZ-induced seizures in zebrafish
Anna Maria Siebel*, Katiucia Marques Capiotti, Juliana Zanetti Frantz, Rosane Souza da Silva and Carla Denise Bonan
Pontifícia Universidade Católica do Rio Grande do Sul, Laboratório de Neuroquímica e Psicofarmacologia, Porto Alegre, Brazil
Adenosine is a well known endogenous modulator of neuronal excitability with anticonvulsant properties. Thus, the modulation exerted by adenosine might be an effective tool to control seizures. In this study, we investigated the effects of drugs able to modulate adenosinergic signaling on pentylenetetrazole (PTZ)-induced seizures in adult zebrafish. The adenosine A1 receptor antagonist 8-cyclopentyl-1,3-dipropylxanthine (DPCPX) decreased the latency to tonic-clonic seizure stage onset. The adenosine A1 receptor agonist cyclopentyladenosine (CPA) increased the latency to reach the tonic-clonic seizure stage. Both adenosine A2A receptor agonist and antagonist, CGS 21680 and ZM 241385, respectively, did not promote changes in seizure parameters. Pretreatment with the ecto-5′nucleotidase inhibitor adenosine 5′-(α,β-methylene)diphosphate (AMPCP) decreased the latency to tonic-clonic seizure stage onset. However, when pretreated with the adenosine deaminase (ADA) inhibitor erythro-9-(2-hydroxy-3-nonyl-adenine (EHNA) or with the NT inhibitor dipyridamole, animals showed longer latency to reach the tonic-clonic seizure status. Our findings indicate that adenosine A1 receptors activation is an important mechanism to control the development of seizures in zebrafish. Furthermore, the action of ecto-5′-nucleotidase, ADA, and NTs are directly involved with the control of extracellular adenosine levels and has important role on the development of seizure episodes in zebrafish.
M 199
Characterization of the nucleotide vesicular transporter (VNUT) and changes in ATP and P2X7 receptor levels in glaucomatous retina
Maria J Pérez de Lara*, Ana Guzman-Aranguez and Jesús Pintor
Complutense University of Madrid, Spain, Spain
ATP-mediated excitotoxicity via activation of the P2X7 receptor (P2X7R) is suggested to be involved in neuronal degeneration and loss of visual function in glaucomatous retinas. The aim of this study was to assess the changes in the VNUT, P2X7R expression and extracellular ATP levels in a murine model of glaucoma during the development of the disease. Retinas were obtained from the glaucomatous DBA/2J mice at 3 and 15 months together with C57BL/6J mice used as age-matched controls. In order to investigate VNUT expression, sections of mouse retinas were evaluated with immunohistochemistry and western blots studies using newly developed antibodies against VNUT and P2X7 receptor. All images were examined and photographed under a fluorescence microscope. For the study of retinal nucleotide release retinas were dissected and prepared as flattened whole-mounts and stimulated in Ringer buffer with or without 59 mM KCl. ERG recordings were performed on C57BL/6J and DBA/2J mice to analyze the changes in the electrophysiological response. Glaucomatous mice exhibited changes in retinal ATP release as long as the pathology progressed. Changes occurred when compared to those animals which basal retinal ATP level was 1.02 ± 0.31 pmol/retina while the stimulated with KCl was 2.62 ± 0.42 pmol/retina at 15 months. Concomitantly, VNUT and P2X7R expression was significantly increased along glaucoma progression in the DBA/2J mice (58 and 36 %, respectively) by immunohistochemical and western-blot techniques. Our results may indicate a possible correlation of retinal dysfunction with the increased levels of extracellular ATP and nucleotide transporter. This altered cellular environment could contribute to increase the P2X7R expression and it may related, together with other factors, to the changes in visual processing in the retina and the concomitant death of retinal ganglion cells. This functional and biochemical analysis allows reliable assessment of the development of the pathology.
M 200
Regulation of spontaneous, transient adenosine release in the caudate-putamen of the brain
Michael Nguyen*, Matt Ryals and B. Jill Venton
1University of Virginia, Chemistry, Charlottesville, USA
Adenosine regulates blood flow and neurotransmission in the brain. Traditionally, adenosine changes have been studied in the brain on a slower time scale of minutes to hours, following events such as ischemia [1]. However, more recently, there is evidence that adenosine regulates physiological processes on a faster time scale, in the second to sub-second time range. Recently, our lab developed an electrochemical method, called fast-scan cyclic voltammetry (FSCV), to quantitate changes in extracellular adenosine concentrations on the sub-second time scale [2]. Using FSCV at carbon-fiber microelectrodes we discovered spontaneous, transient adenosine in vivo that was released without external stimulation. In the caudate-putamen of anesthetized rats, spontaneous adenosine release lasts about 3 s, with an average peak concentration of 0.17 μM [3]. To further characterize spontaneous transient adenosine, we examined modes of adenosine clearance from the extracellular space: equilibrative nucleoside transporters and metabolic breakdown with adenosine deaminase. The drug NBTI, which blocks nucleoside transporters, was administered in vivo and significantly increased the duration of transient adenosine release from 3.1 ± 0.1 to 3.8 ± 0.1 s. EHNA, an adenosine deaminase inhibitor, also significantly increased the duration of transient adenosine release from 3.2 ± 0.1 to 3.9 ± 0.1 s. The adenosine decay curve was fit with an exponential decay to the rate of clearance before and after the drugs. EHNA significantly decreased the decay rate, while NBTI did not have a significant effect. This suggests with spontaneous transient adenosine release, that metabolic breakdown and nucleoside tranporters both clear extracellular adenosine; however, metabolism has more of an effect on clearance rate. We also examined the effect of adenosine receptor antagonists on adenosine modulation. DPCPX, an A1 antagonist, increased the frequency of transient adenosine release and CPA, an A1 agonist, decreased the frequency of spontaneous adenosine release. This suggests that adenosine is self-regulated by A1 receptors. These studies demonstrate that transient adenosine is spontaneously released on a rapid time scale, cleared by metabolic breakdown, and regulated by A1 receptors.
References
1. Villarreal F et al (2003) Mol Cell Biochem 251(1–2):17–26
2. Swamy BEK et al (2007) Anal. Chem 79(2):744–750
3. Nguyen MD et al (2014) PLoS ONE 9(1):e87165
M 201
Systemic administration of diadenosine tetraphosphate (AP4A) improves locomotor recovery in murine models of traumatic spinal cord injury
David Reigada*, Rosa Navarro-Ruiz, Marcos Javier Caballero-López, Angela del Águila, Teresa Muñoz-Galdeano, Manuel Nieto-Díaz and Rodrigo Martínez-Maza
National Hospital for Paraplegics, Molecular Neuroprotection Group, Toledo, Spain
Traumatic spinal cord injury (SCI) is one of the main causes leading disability in developed countries [1]. After a first acute phase, with a predominant necrotic death of neural cells, it follows a secondary phase when there is an activation of apoptotic cell death that implies the exacerbation and spatial and temporal extension of the injury [1]. Excytotoxic processes are important in this phase due to massive release of ATP and glutamate [2].
It has been reported that purinergic system has neuroprotective and neurorregenerative properties in neurodegenerative trauma and disorders [3]. Diadenosine polyphosphates (APnAs) are purinergic molecules formed by the union of two adenosine moieties through a variable number of phosphates. Particularly, diadenosine tetraphosphate (AP4A) is a quite selective agonist of several types of P2 receptors and it has been used as candidate molecule to reduce neurodegeneration [4,5].
The main goal of the present work is to study the protective effects of AP4A, to evaluate its capacity to ameliorate deleterious effects of the traumatic SCI and to determine the molecular and physiological basis of these protective effects.
We evaluated the degree of motor function recovery in murine models of contusive SCI after intraperitoneal administration of AP4A. We detect the expression of several P2 receptors in mice and rat spinal cord, localized in neurons (P2X2,3,7; P2Y1), astrocytes (P2X1,3; P2Y2, 4) and oligodendrocytes (P2X2,3,7; P2Y1,2,11). Intraperitoneal injection of AP4A in both rats (50 mg/Kg) and mice (20 mg/Kg) reduces tissue damage and improves several key parameters of locomotion.
In addition, we used human (SH-SY5Y) and murine (Neuro2a) neuroblastoma cell lines to evaluate the molecular basis of the cytoprotective effect of AP4A against a pro-apoptotic insult and the modulation of caspase cascade activitation. AP4A (100 mM) reduces the expression of P2X2,4 and 7 and P2Y2 receptors in Neuro2a cells. Also, AP4A reduces the excytotoxic increase in intracellular calcium in response to ATP (300 mM). Finally, AP4A protects against staurosporine pro-apoptotic stimuli (25nM) in Neuro2a and SH-SY5Y cells, reducing the activation of caspases 3 and 7 and the cleavage of PARP.
In conclusion, AP4A reduces the harmful effects of the SCI reducing the excytotoxic effects of a massive release of ATP, calcium mobilization and activation of caspase cascades. This modulation improves motor and histological deficits resulting from trauma.
Fig. 1 The BMS scale and the coordination subscore indicates that the intraperitoneal AP4A treatment ameliorate the locomotor deficits. Also, this treatment increases the latency time in the rotarod test
References
1. Rowland JW (2008)
2. Ray SK et al (2003)
3. Burnstock G et al (2006)
4. Wang X et al (2004)
5. Hoyle CH (2010)
M 202
Characterisation of BAC transgenic TagRFP P2Y1reporter mice
Michaela Schumacher1,*, Marcus Grohmann2, Heike Franke2, Günther Schmalzing1 and Ralf Hausmann1
1Department of Molecular Pharmacology, RWTH Aachen University, Aachen, Germany;2Rudolf-Boehm-Institute of Pharmacology and Toxicology, University of Leipzig, Leipzig Germany
P2Y1 receptors (P2Y1Rs) are widely expressed throughout the body and are involved in several physiological processes. For instance, P2Y1Rs are expressed on sensory neurons or platelets where they modulate sensory neurotransmission and pain sensation or induce platelet aggregation, respectively. Furthermore, the P2Y1R expression is up regulated on astrocytes following acute injury of the brain. However, it is not clear whether P2Y1R expression and activation after brain injury exerts protective or detrimental effects. Thus, tracking of the gene expression of the P2Y1R in health and disease is of significant interest. To facilitate the analysis of the gene expression of the P2Y1R in neurons and glial cells, we have generated a C57BL/6J BAC-transgenic reporter mouse expressing the red fluorescent reporter protein TagRFP under control of the promoter of the P2RY1 gene. P2Y1R TagRFP-reporter mice showed native TagRFP fluorescence in hippocampal neurons and glial cells and in platelets. Immunostaining with a monoclonal TagRFP antibody revealed P2Y1R gene expression in various neuronal cells including neurons of the dorsal horn of the spinal cord, cerebellar Purkinje neurons, the hippocampus, dopaminergic and non-dopaminergic cells of the VTA and in hepatocytes.
The P2Y1R TagRFP-reporter mice may be a valuable tool to analyze the P2Y1R gene expression profile and may thus improve our understanding of the role of P2Y1R-mediated purinergic signaling under physiological and pathophysiological conditions.
M 203
Comorbidities in psychiatry and neurology: focus on astrocytes and adenosine dysregulation
Hai-Ying Shen1,*, Eleonora Aronica2,3 and Detlev Boison1,4
1Legacy Research Institute, Neurobiology, Portland, USA;2Academic Medical Center/Swammerdam Institute for Life Sciences, Center for Neuroscience, University of Amsterdam, (Neuro)Pathology, Amsterdam, Netherlands;3Stichting Epilepsie Instellingen Nederland, SEIN, Heemstede, Netherlands;4Oregon Health and Science University (OHSU), Neurology, Portland, USA
Question: Clinically, comorbid psychiatric and cognitive impairments occur in epilepsy whereas seizures are comorbid with schizophrenia (SZ). Also, cognitive impairment occurs in Alzheimer’s disease (AD), Parkinson’s disease (PD) and amyotrophic lateral sclerosis (ALS) while above conditions may share certain aspects of psychosis, depression, and sleep dysfunction. The remarkable overlap of symptoms across seemingly unrelated psychiatric and neurological conditions suggests the existence of common pathological mechanisms that may link to disruptions in inflammation, glial activation and neurotransmission in the brain. Adenosine (ADO) is a homeostatic regulator of the brain that affects dopaminergic, glutamatergic, and GABAergic neurotransmission, neuroinflammation via ADO receptor (AR)-dependent effects and exerts epigenetic and bioenergetic functions via AR-independent effects. ADO is subject to metabolic clearance via astrocytic adenosine kinase (ADK), an enzyme overexpressed in epilepsy and several neurodegenerative disorders along with astrocyte dysfunction in the brain. Here we hypothesize that ADO dysregulation may be an explanation for comorbidities in psychiatry and neurology and aim to address the ‘adenosine hypothesis of comorbidities’.
Methods: This study is based on the characterization of genetically engineered mice with brain-wide overexpression of ADK, and the analysis of brain specimens from TLE, AD, PD and ALS patients.
Results: 1) Astrogliosis is a common pathological hallmark of human TLE, AD, PD, and ALS, where we found marked overexpression of ADK implicating common ADO deficiency. 2) We generated a transgenic ‘co-morbidity model’ by brain-wide overexpression of ADK (Adk-tg) in mice, which decreases ambient ADO. These mice displayed a comorbid behavioral spectrum of deficits in working memory, expression of conditioned freezing, attentional impairment, altered dopaminergic function, sleep regulation, and recurrent electrographic seizures. This suggests that ADO deficiency per se can cause a wide range of comorbid symptoms. 3) Using a model of prepulse inhibition we showed that augmenting ADO by pharmacological ADK inhibition facilitated sensory gating, i.e., yielded an antipsychotic-like effect. In addition, focal ADO augmentation by cellular implants to the hippocampus improved cognitive performance of Adk-tg mice, whereas the same cells grafted into the striatum restored dopaminergic function. Furthermore, focal ADO augmentation therapies are highly effective in the prevention of epileptic seizures.
Conclusions: ADO dysfunction is common in psychiatric and neurological conditions, which can explain co-morbid phenotypes shared among seemingly unrelated psychiatric and neurological conditions. Therapeutic ADO augmentation might be a highly effective approach for the treatment of comorbidities in multiple psychiatric and neurological conditions.
M 204
A P2X7 antagonist improves muscle strength and motor distal amplitude in CMT1A rats: preliminary results from an in vivo trial
Giovanna Sociali1,*, Thomas Prukop2, Ilaria Cervellini2, Lucilla Nobbio3, Santina Bruzzone1, Angelo Schenone3 and Michael W. Sereda2
1University of Genova, Department of Experimental Medicine, Section of Biochemistry, Genova, Italy;2Max Planck Institute of Experimental Medicine, Department of Neurogenetics, Göttingen, Germany, Germany;3University of Genova, Department of Neurosciences, Rehabilitation, Ophthalmology, Genetics and Mother and Child Sciences and CEBR, Genova, Italy
Question: Charcot-Marie-Tooth disease (CMT) is the most common inherited neuropathy, and a duplication of the Pmp22 gene causes the most frequent subform CMT1A. An abnormally high intracellular Ca2+ concentration ([Ca2+]i) occurs in Schwann cells (SC) from CMT1A rats and is caused by overexpression of the P2X7 purinoceptor. Normalization of Ca2+ levels through down-regulation and pharmacological inhibition of P2X7 appears to restore the normal phenotype of CMT1A SC in vitro (Nobbio L et al., J Biol Chem 2009). Here, we investigated whether treatment with a P2X7 inhibitor (A438079) improves CMT1A disease in vivo.
Methods: We applied placebo-controlled A438079 therapy (3 mg/Kg) to CMT1A rats for 11 weeks and performed the grip strength analysis every 2 weeks to assess the motor phenotype and electrophysiology (at the end of the treatment).
Results: After 6 weeks of treatment, A438079 significantly increased muscle strength of CMT1A rats, whereas it did not affect muscle strength of wild-type rats. Moreover, a preliminary analysis indicates that compound action potentials (CMAP) were increased in treated CMT1A rats indicating that axonal loss may be ameliorated.
Conclusions: Our preliminary results suggest that antagonizing P2X7 could represent a promising strategy for CMT1A. Morphological analysis on nerves from treated and control animals are currently being performed, together with RT-PCR analysis of SC differentiation markers.
M 205
Sp1 mediates transcriptional regulation of P2X7 receptors in the nervous system: implications in neural cell differentiation
Rosa Gomez-Villafuertes*, Paula Garcia-Huerta, Juan Ignacio Diaz-Hernandez, Miguel Diaz-Hernandez, Esmerilda G. Delicado and M. Teresa Miras-Portugal
University Complutense of Madrid, Veterinary School, Biochemistry and Molecular Biology, Madrid, Spain
In the nervous system P2X7 receptors are involved not only in physiological functions, including cell growth, differentiation or apoptosis, but also in brain pathologies. Although an increasing number of findings indicate that altered receptor expression has a causative role in neurodegenerative diseases and cancer, little is known about how expression of P2rx7 gene is controlled. Here we reported the first molecular and functional evidence that specificity protein 1 (Sp1) transcription factor plays a pivotal role in the transcriptional regulation of P2X7 receptor in the nervous system. We delimited a minimal region in the murine P2rx7 promoter containing four SP1 sites, two of them being highly conserved in mammals. The functionality of this SP1 sites was confirmed by site-directed mutagenesis, and Sp1 overexpression/downregulation in neuroblastoma cells. Inhibition of Sp1-mediated transcriptional activation by mithramycin A reduced endogenous P2X7 receptor levels in primary cultures of cortical neurons and astrocytes. Using P2rx7-EGFP transgenic mice that express enhanced green fluorescent protein under the control of P2rx7 promoter, we found a high correlation between reporter expression and Sp1 brain levels. In addition, we found that Sp1 mediates upregulation of native P2X7 receptors in Neuro-2a neuroblastoma cells following serum withdrawal. Pharmacological inhibition of P2X7 receptor or its knockdown by shRNA interference resulted in increased neuritogenesis in neuroblastoma cells cultured in low serum-containing medium, whereas P2X7 overexpression significantly reduced the formation of neurites. In conclusion, these data demonstrate that Sp1 is a key element in the transcriptional regulation of P2X7 receptor in the nervous system and support the involvement of P2X7 receptors in the maintenance of neural cells in a non-differentiated state.
References
1. García-Huerta P, Díaz-Hernandez M, Delicado EG, Pimentel-Santillana M, Miras-Portugal MT, Gómez-Villafuertes R (2012) The specificity protein factor Sp1 mediates transcriptional regulation of P2X7 receptors in the nervous system. J Biol Chem 287(53):44628–44644
2. Gómez-Villafuertes R, del Puerto A, Díaz-Hernández M, Bustillo D, Díaz-Hernández JI, Huerta PG, Artalejo AR, Garrido JJ, Miras-Portugal MT (2009) Ca2+/calmodulin-dependent kinase II signalling cascade mediates P2X7 receptor-dependent inhibition of neuritogenesis in neuroblastoma cells. FEBS J. 276(18):5307–5325
M 206
Purines released from astrocytes inhibit excitatory synaptic transmission in the ventral horn of the spinal cord
Eva Meier Carlsen* and Jean-François Perrier
Department of Neuroscience and Pharmacology, University of Copenhagen
Spinal neuronal networks are essential for motor function. They are involved in the integration of sensory inputs and the generation of rhythmic motor outputs. They continuously adapt their activity to the internal state of the organism and to the environment. This plasticity can be provided by different neuromodulators. These substances are usually thought of being released by dedicated neurons. However, in other networks from the central nervous system synaptic transmission is also modulated by transmitters released from astrocytes. The star-shaped glial cell responds to neurotransmitters by releasing gliotransmitters, which in turn modulate synaptic transmission. Here we investigated if astrocytes present in the ventral horn of the spinal cord modulate synaptic transmission. We evoked synaptic inputs in ventral horn neurons recorded in a slice preparation from the spinal cord of neonatal mice. Neurons responded to electrical stimulation by monosynaptic EPSCs. We used mice expressing the enhanced green fluorescent protein under the promoter of the glial fibrillary acidic protein to identify astrocytes. Chelating calcium with BAPTA in a single neighboring astrocyte increased the amplitude of synaptic currents. In contrast, when we selectively stimulated astrocytes by activating PAR-1 receptors with the peptide TFLLR, the amplitude of EPSCs evoked by a paired stimulation protocol was reduced. The paired-pulse ratio was increased, suggesting an inhibition occurring at the presynaptic side of synapses. In the presence of blockers for extracellular ectonucleotidases, TFLLR did not induce presynaptic inhibition. Puffing adenosine reproduced the effect of TFLLR and blocking adenosine A1 receptors with DPCPX prevented it. Altogether our results show that ventral horn astrocytes are responsible for a tonic and a phasic inhibition of excitatory synaptic transmission by releasing ATP, which gets converted into adenosine that binds to inhibitory presynaptic A1 receptors.
M 207
ATP-mediated signaling plays a key role in generation of BOLD fMRI responses
Isabel N Christie1,*, Jack W Wells1, Patrick S Hosford1, Melina F Figueiredo2, Sergey Kasparov2, Mark F Lythgoe1 and Alexander V Gourine 1
1University College London, London, UK;2Bristol University, Bristol, UK
Introduction: Cellular mechanisms underlying the blood oxygen-level dependent (BOLD) signal measured in fMRI are poorly understood. There is evidence that astrocytes—the most numerous glial cells of the CNS—are responsible for triggering changes in local blood flow following changes in neuronal activity. Here we present data suggesting that the principal astrocytic signalling molecule ATP is critically involved in the mechanisms of the BOLD response.
Methods: To interfere with ATP-mediated signalling we generated a lentiviral vector to drive the expression of a potent ectonucleotidase—transmembrane prostatic acid phosphatase (TMPAP) on the cell surface membranes to accelerate the breakdown of extracellular ATP. Prior to MRI experiments, rats underwent bilateral viral injections targeted to the forepaw region of the somatosensory (SSFP) cortex. One hemisphere was transduced to express TMPAP-GFP while the other hemisphere was transduced to express green fluorescent protein (GFP).
Results: Bilateral electrical forepaw stimulation triggered significantly smaller BOLD responses in the cortex transduced to express TMPAP-GFP in comparison to the hemisphere transduced to express GFP (n = 11 Fig 1a). We thought that adenosine accumulation (following facilitated ATP breakdown) may be affecting neuronal activity, however systemic treatment with A1 adenosine receptor antagonist DPCPX (1#mgkg−1) further reduced the magnitude of fMRI signal in the hemisphere expressing TMPAP (n = 9 Fig 1b). To determine if neuronal responses are affected by TMPAP expression, electrophysiological experiments were performed. We assessed the minimum amplitude of stimulation required to evoke multiunit activity in the SSFP cortex and evaluated the amplitude of this response under the same stimulation parameters used in the fMRI protocol; no significant differences were observed between cortices.
Conclusions: Our novel approach allows effective suppression of ATP-mediated communication between astrocytes. The result is a marked suppression of the BOLD response suggesting that purinergic signalling plays an important role in neurovascular coupling.
Fig. 1 a) Group wise activation maps and mean BOLD responses within the SSFP cortical region triggered by 20s bilateral forepaw stimulation (shaded region represents SEM across all subjects and colour bar represent the t-score from SPM mixed effects analysis, p)
M 208
P2X7 receptors regulate engulfing activity of non-stimulated resting astrocytes
Kazuki Nagasawa*, Mina Yamamoto, Yosuke Kamatsuka, Takahiro Furuta, Akihiro Ohishi and Kentaro Nishida
Kyoto Pharmaceutical University, Department of Environmental Biochemistry, Kyoto, Japan
Objective: We previously demonstrated that P2X7 receptors (P2X7Rs) expressed by cultured mouse astrocytes were activated without any exogenous stimuli, but its roles in non-stimulated resting astrocytes remained unknown. It has been reported that astrocytes exhibit engulfing activity, and that the basal activity of P2X7Rs expressed by macrophages regulates their phagocytic activity. In this study, therefore, we investigated whether P2X7Rs regulate the engulfing activity of mouse astrocytes.
Materials and Methods: Primary astrocyte cultures were prepared from the cortices of 1-day old ddY mice and SJL mice. Astrocytic P2X7R channel/pore and engulfing activity were assessed by their uptakes of YO-PRO-1 dye and non-opsonized yellow-green carboxylate latex beads (1 μm), respectively. Western blot and immunocytochemical analyses were performed by the previously reported methods. siRNA for P2X7R was transfected into cultured astrocytes by a lipofection methods. Astrocyte death was quantified by measuring LDH activity in the lysates of surviving cells.
Results and Discussion: Uptake of beads by resting astrocytes derived from ddY-mouse cortex time-dependently increased, and the uptaken beads were detected in the intracellular space. The bead uptake was inhibited by cytochalasin D (CytD), an F-actin polymerization inhibitor, and agonists and antagonists of P2X7Rs apparently decreased the uptake. Spontaneous YO-PRO-1 uptake by ddY-mouse astrocytes was reduced by the agonists and antagonists of P2X7Rs, but not by CytD. Down-regulation of P2X7Rs using siRNA decreased the bead uptake by ddY-mouse astrocytes. In addition, compared to in the case of ddY-mouse astrocytes, SJL-mouse astrocytes exhibited higher YO-PRO-1 uptake activity, and their bead uptake was significantly greater. These findings suggest that resting astrocytes exhibit engulfing activity and that the activity is regulated, at least in part, by their P2X7Rs.
M 209
Overactive human bladder: tandem activation of P2Y6and P2X3 purinoceptors operated by ATP released from the urothelium via pannexin-1 hemichannels
Isabel Silva1,*, Fátima Ferreirinha1, Miguel Silva-Ramos1,2 and Paulo Correia-de-Sá1
1UMIB / MedInUp / ICBAS-UP, Lab. Farmacologia e Neurobiologia, Porto, Portugal;2Hospital Geral de Santo António—Centro Hospitalar do Porto, Serviço de Urologia, Porto, Portugal
Urothelium distension during bladder filling causes the release of ATP, by several possible mechanisms, which initiates the voiding reflex via activation of P2X3 receptors on sensory nerve fibers. Increases in the purinergic tone are associated with aging and several pathological bladder conditions (e.g. interstitial cystitis, neurogenic bladder, outlet obstruction). Our group demonstrated that urinary ATP may be a dynamic biomarker of detrusor activity in overactive bladder syndromes [1].
Besides the influence of ionotropic P2X3 receptors, we demonstrated that UDP-sensitive P2Y6 receptors increases the voiding frequency indirectly by releasing ATP from the urothelium via pannexin-1 hemichannels [2]. Co-expression of P2Y6 and P2X3 in the urothelium has been shown by confocal microscopy [3]. Therefore, we sought it was important to investigate the crosstalk between these two receptor subtypes regarding the release of signaling molecules (ATP and ACh) from the human urothelium. Human bladder samples were collected from cadaveric organ donors. All procedures were approved by the Ethics Committees of CHP-HGSA and ICBAS-UP.
The selective P2Y6 receptor agonist, PSB0474 (100nM), increased the release of ATP and [3H]ACh respectively by 50 ± 12 % (n = 3) and 21 ± 6 % (n = 3) from the human urothelium, an effect that was blocked by MRS2578 (50nM, a P2Y6 antagonist) and by carbenoxolone (10 μM, a pannexin-1 inhibitor). The effect of PSB0474 on bladder urothelium contrasts with the inhibitory (~50 %) responses on [3H]ACh release from stimulated cholinergic nerves terminals in the detrusor. The P2X3 receptor antagonist, A317491 (100nM), prevented PSB0474-induced facilitation of evoked ATP and [3H]ACh release from the human urothelium, but not from the detrusor layer.
Data indicate that activation of UDP-sensitive urothelial P2Y6 receptors triggers the release of ATP (as well as ACh) via pannexin-1 hemichannels, which may subsequently activate P2X3 receptors on neighboring urothelial cells and suburothelial sensory nerve fibres to increase bladder activity. Thus, targeting tandem activation of urothelial P2Y6 and P2X3 receptors triggered by ATP released from the urothelium may have therapeutic relevance in overactive bladder syndromes.
Work supported by FCT (FEDER funding, PTDC/SAU-OSM/104369/2008 and Pest-OE/SAU/UI215/2014), Assoc. Port. Urologia and Univ. Porto / Caixa Geral de Depósitos. IS is in receipt of a PhD fellowship by FCT (SFRH/BD/88855/2012).
M 210
The selective antagonism of P2X7and P2Y1receptors prevents synaptic failure and affects cell proliferation induced by oxygen and glucose deprivation in rat dentate gyrus
Anna Maria Pugliese1, Giovanna Maraula1, Daniele Lana1, Elisabetta Coppi1, Tommaso Mello2, Alessia Melani1, Andrea Galli2, Maria Grazia Giovannini3 and Felicita Pedata1,*
1University of Florence, Department of Neuroscience, Psychology, Drug Research and Child Health, NEUROFARBA, Division of Pharmacology and Toxicology, Florence, Italy;2University of Florence, Department of Experimental and Clinical Biomedical Sciences, Florence, Italy;3University of Florence, Department of Health Sciences, Clinical Pharmacology and Oncology Unit, Florence, Italy
In this work we investigated the role of P2X7 and P2Y1 receptors on the synaptic and proliferative response of the dentate gyrus (DG) to severe oxygen and glucose deprivation (OGD) in acute rat hippocampal slices. Extracellular field excitatory post-synaptic potentials (fEPSPs) in granule cells of the DG were recorded from rat hippocampal slices. Nine-min OGD elicited an irreversible loss of fEPSP and was invariably followed by the appearance of anoxic depolarization (AD). MRS2179 (selective antagonist of P2Y1 receptor) and BBG (the most used antagonist of P2X7 receptor), applied before and during OGD, prevented AD appearance and allowed a significant recovery of neurotransmission after 9-min OGD.
The effects of 9-min OGD on proliferation and maturation of cells localized in the subgranular zone (SGZ) of DG of slices prepared from rats treated with 5-Bromo-2′-deoxyuridine (BrdU) were investigated. Slices were further incubated with an immature neuronal marker, doublecortin (DCX). The number of BrdU+ cells in the SGZ was significantly decreased 6 h after OGD. This effect was antagonized by BBG. Conversely, MRS2179 reduced the number of BrdU+ cells. Twenty-four hours after 9-min OGD, the number of BrdU+ cells returned to control values and an increased arborisation of tertiary dendrites of DCX+ cells was observed. This phenomenon was still evident in the presence of BBG, but not MRS2179.
Results demonstrate that P2X7 and P2Y1 receptors contribute to early damage induced by OGD in the DG. Later after OGD, P2Y1 receptors might play an additional and different role promoting cell proliferation and maturation in the DG.
M 211
Inhibition of ecto-5′-nucleotidase activity facilitates short-term and inhibits long-term plasticity in two different mouse brain regions
Francisco Queiroz Gonçalves1,2,*, Daniel Rial1, Henrique Silva1 and Rodrigo Cunha1,3
1Center for Neuroscience and Cell Biology, Neuromodulation, Coimbra, Portugal;2Faculty of Life Sciences—University of Coimbra, Coimbra - Portugal, Portugal;3Faculty of Medicine—University of Coimbra, Coimbra - Portugal, Portugal
ATP is stored in synaptic vesicles and released together with neurotransmitters. ATP acts as an extracellular signal, either directly activating P2 receptors or indirectly activating adenosine A1 or A2A receptors (A2AR) after its extracellular catabolism into adenosine by ecto-nucleotidases, the final step being mediated by ecto-5′-nucleotidase (e5′N). Since it is proposed that e5′N is selectively responsible for the formation of the ATP-derived adenosine activating A2AR (Augusto. E, J. Neuroscience, 2013) which selectively control synaptic transmission (Constela. R, European J. Neuroscience, 2011), we now tested the impact of e5′N inhibition (with 100 mM a,b-methylene ADP, AOPCP) on synaptic transmission and plasticity. We recorded synaptic transmission, short term plasticity (paired pulse stimulation with inter pulse intervals between 20 and 80 ms) and long term plasticity (high frequency stimulation, 100 Hz for 0.1 ms) in brain slices in two glutamatergic synapses: cortico-striatal synapses and Schaffer fiber-CA1 pyramid synapses. AOPCP did not change basal synaptic transmission neither in the hippocampus nor in the striatum but it enhanced short-term plasticity both in the hippocampus (n = 5) and in the striatum (n = 3). In contrast, AOPCP inhibited long-term potentiation by 12.7 ± 2.1 % (n = 4; P < 0.05) in hippocampus and by 37.0 ± 1.7 % (n = 3; *P < 0.05) in the striatum. When AOPCP was tested in the continuous perfusion on top of the selective A2AR antagonist (SCH 58261, 50 nM) it depressed LTP in a cumulative way effect both in the hippocampus (15.8 ± 6 %; n = 3; *p < 0.05) and striatum (16.2 ± 3 %; n = 3; *p < 0.05). However, application of SCH 58261 prevented the facilitatory effect of AOPCP in the hippocampus and striatum short-term potentiation. It seems that the endogenous extracellular adenosine responsible for the tonic inhibition of synaptic transmission might not be originated from the extracellular catabolism of ATP and the synaptic plasticity impact of e5′N inhibition is not only due to the decrease of adenosine that activates A2AR.
Supported by DARPA, FCT and CNPq.
M 212
Purinergic modulation is required for development of tuning properties of central auditory brainstem neurons
Tamara Radulovic1,2,*, Sasa Jovanovic1,*, Rudolf Rübsamen1 and Ivan Milenkovic2
1Faculty of Biosciences, Pharmacy and Psychology, University of Leipzig, Institute of Biology, Leipzig, Germany;2Faculty of Medicine, University of Leipzig, Carl Ludwig Institute for Physiology, Leipzig, Germany
Spontaneous neuronal activity arises in the developing auditory system before the onset of hearing independently of any systemic input. This activity is believed to influence axonal pathfinding, dendritic morphogenesis and the segregation of axonal terminal arbours. Respective developmental processes contribute to establishment of nuclear tonotopic maps, i.e. the orderly arrangement of neurons differing in the sound frequency to which they are most sensitive. Purinergic signaling attunes neuronal activity in the cochlea and in the brainstem during this critical developmental period. Endogenous release of ATP facilitates action potential generation in the inner hair cells and enhances glutamate-driven firing of bushy cells in the ventral cochlear nucleus, the first central station along the afferent auditory pathway. In bushy cells, activation of P2X2/3R increases action potential firing through cytosolic calcium signaling and protein kinase C activity. The modulatory effects of ATP diminish with maturity, both in the periphery and in the central auditory neurons.
We used in vivo and ex vivo-electrophysiological recordings to determine the developmental time course of purinergic modulation referring to the tonotopic organization of the cochlear nucleus. The responses mediated by P2X2/3R were examined in bushy cells at topographically distinct nuclear positions during postnatal development of Mongolian gerbils. Additional data were acquired from P2X2/3RDbl−/− mice to investigate the properties of bushy cells deprived of purinergic modulation during development.
In vivo extracellular recordings during the initial period of auditory experience (postnatal days P13–16) conducted in combination with iontophoretic drug applications showed P2X2/3R-mediated enhancement of spontaneous and sound evoked neuronal activity restricted to the low-frequency regions of the ventral cochlear nucleus. This data were confirmed with slice recordings demonstrating progressive reduction of the area engaged with P2X2/3R signaling during postnatal development. The P2X2/3RDbl−/− mice had significantly impaired sound frequency selectivity of bushy cells compared to wild type (P20–26). Moreover, the maximum firing rates evoked by high intensity sound stimulation were lower in P2X2/3RDbl−/− suggesting either weaker excitatory input or increased inhibition. Corresponding slice recordings excluded the second option by showing no alterations of inhibitory synaptic inputs on bushy cells.
In summary, we conclude that purinergic signaling follows a defined developmental pattern related to the topographic position of bushy cells within the ventral cochlear nucleus. By attuning neuronal activity during early postnatal development, P2X2/3R might be necessary for the development of tuning properties, particularly for a proper stimulus coding in high sound intensity environment.
M 213
A2Breceptor activation decreases short-term plasticity and A1-mediated responses in the mice hippocampus
Johny Pires*, Francisco Queiroz, Rodrigo Cunha and Daniel Rial
Center for Neuroscience and Cell Biology, Life Science, Coimbra, Portugal
The A2B receptor (A2BR) is a low affinity receptor for adenosine without a properly described function in the CNS. Our aim was to investigate its role in the control of hippocampal basal synaptic transmission (BST) and short-term plasticity (STP). Hippocampal slices from C57/Bl6 mice were used to record BST and STP using paired-pulse stimulation in Schaffer fiber-CA1 synapses. We report that the activation of A2BR with the selective agonist BAY 60-6583 (300 nM and 3 μM) decreased the PPS ratio (P2/P1 = −21 % and −19 %, n = 4; p < 0.05) without interfe ring with BST. The selective antagonist, MRS 1754 (20 nM, 200 nM and 2 μM n = 4) did not modify PPS or BST, but blocked the alteration of PPS by BAY 60-6583. In view of the role of A1R in controlling BST, we next explored if A2BR might control A1R-mediated responses. DPCPX (100 nM), an A1R antagonist, increased BST (~50 % n = 4) while BAY 60-6583 (300 nM) ‘re-normalized’ the response (~5 % n = 4). An effect abrogated in the presence of MRS 1754 (200 nM) (~45 %, n = 4). DPCPX also decreased the PPS ratio (P2/P1 ratio = −20 %, n = 4), a response unaffected by the presence of BAY 60-6583. 2-Chloroadenosine (CADO, 0.1–1 μM), a non-selective A1R agonist, concentration-dependently decreased BST (~−35 % at 0.1 μM, ~−50 % at 0.3 μM and ~−70 % at 1 μM, n = 4) and BAY 60-6583 attenuated this inhibitory effect of CADO (~−20 %, ~−35 % and ~−55 % respectively, n = 4). Our results suggest that A2BR directly control STP and functionally interact with A1R to control BST in the mouse hippocampus.
M 214
Modulation of dendrodendritic synaptic transmission in olfactory bulb mitral cells by adenosine
Kristina Schulz*, Natalie Rotermund, Christian Lohr and Daniela Hirnet
Universitiy of Hamburg, Biocenter Grindel, Division of Neurophysiology, Hamburg, Germany
Besides their predominant role in energy metabolism, purines such as ATP, ADP and adenosine play a major part in the communication between cells in various areas throughout the whole nervous system. In the olfactory bulb (OB), ATP is released as a neurotransmitter and stimulates neuronal network activity as well as glial calcium signaling. In addition, ATP-degrading enzymes are highly expressed in the OB, highlighting a pivotal role of purinergic modulation in olfactory information processing.
In this study, we analyzed adenosine-mediated modulation of signal transmission between neurons of the OB in acute mouse brain slices. We studied dendrodendritic inhibition (DDI) in mitral cells (MCs), the principal neurons projecting to higher brain centers, by using whole-cell patch-clamp recordings. DDI plays a major role in the processing of olfactory information and is thought to be mediated mostly by recurrent synapses between glutamatergic MCs and GABAergic granule cells (GCs). DDI was evoked by a brief depolarizing voltage step and was greatly enhanced by magnesium-free extracellular media and application of cyclothiazide to enhance NMDAR- and AMPAR-mediated synaptic transmission, respectively. Bath application of adenosine led to a 20 % reduction of DDI in MCs as well as a 35 % reduction of MC self-excitation, the latter used as a quantification of glutamate release from mitral cell dendrites. Adenosine also reduced isolated AMPAR-mediated DDI currents by 15 %. These currents were blocked by 80 % using NASPM, a specific antagonist of calcium-permeable AMPARs, which are particularly expressed on parvalbumin interneurons (PVNs). This illustrates an impact of adenosine not only at GC-MCs synapses, but also at PVN-MC synapses. Additionally, adenosine produced a 20 % decrease in MC calcium currents, suggesting a presynaptic modulation of synaptic transmission involving inhibition of presynaptic Ca2+ influx and thus reducing the release of glutamate at MC lateral dendrites. Thus, adenosine influences the performance of dendrodendritic synapses between mitral cells and different classes of interneurons, thereby modulating processing of olfactory information.
M 215
The role of P2Y12receptors in a chronic, CFA-induced inflammatory pain model
Katinka Beko1,*, Balint Botz2,3, Zsuzsanna Helyes2,3, Gergely Horvath1,4, Beata Sperlagh1 and Christa E. Müller5
1IEM HAS, Laboratory of Molecular Pharmacology, Budapest, Hungary;2University of Pecs, School of Medicine, Department of Pharmacology and Pharmacotherapy, Pecs, Hungary;3Janos Szentagothai Research Centre, Molecular Pharmacology Research Team, Pecs, Hungary;4Semmelweis University School of Ph.D Studies, Janos Szentagothai School of Neurosciences, Budapest, Hungary;5PharmaCenter Bonn, Pharmaceutical Institute, University of Bonn, Bonn, Germany
Question: Our previous studies indicate that genetic deletion and pharmacological antagonism of P2Y12 receptors alleviate mechanical allodynia in acute inflammatory pain. In the present project we investigated the role of P2Y12 receptors in chronic inflammatory pain.
Methods: By the injection of Complete Freund’s Adjuvant (CFA) in the plantar surface of the right hind paw, we induced local inflammation and mechanical hyperalgesia that was persisted up to 14 days after the CFA injection. During this 2 week period, mechanical sensitivity was evaluated at several time points using dynamic plantar von Frey aesthesiometer. To follow up the course of CFA-induced inflammation, myeloperoxidase induced luminol bioluminescence was monitored, at two different time points of the experiment using in vivo imaging technique.
Using this model the effect of two different P2Y12 antagonists were tested: cangrelor, the non-pro-drug P2Y12/P2Y13 receptor antagonist (3 mg/kg, i.p.) and PSB-0739, the selective and potent P2Y12 receptor antagonist (0.3 mg/kg, i.t.). To obtain further evidence on the role of P2Y12 receptors in chronic inflammatory pain, in a set of experiment P2Y12R gene deficient (P2Y12−/−) mice were used.
Results: Both tested compounds were able to attenuate the inflammatory pain in wild type mice in the acute and chronic phase too.
After CFA injection, mechanical allodynia developed in P2Y12 receptor gene deficient mice, but the decline in the paw withdrawal threshold (PWT) was much lower than in wild type mice. Interestingly in P2Y12−/− mice, whilst no effect by cangrelor was detected during the acute phase of hyperalgesia, cangrelor increased, but not decreased mechanical hypersensitivity at the chronic phase indicating the activation of non-P2Y12 receptors at this time point.
In the in vivo imaging experiments CFA treatment induced a robust increase in luminol bioluminescence in wild type mice on the 3rd day after the CFA injection, which was attenuated, but was still detectable at the 10th day. The CFA induced increase in luminol bioluminescence was significantly less intensive in P2Y12−/− mice at the 3rd day, whereas it. injection of PSB-0739 did not affect this parameter.
Conclusion: In conclusion our findings indicate that P2Y12 receptor inhibition is a potential therapeutic approach in chronic inflammatory pain, although its exact mechanism of action needs further investigation.
M 216
P2X7 receptor inhibition attenuates cancer-induced bone pain in rats
Sarah Falk1,*, Rasmus P. Clausen1, Anthony H. Dickenson2 and Anne-Marie Heegaard1
1University of Copenhagen, Department of Drug Design and Pharmacology, Copenhagen, Denmark;2University College London, Department of Neuroscience, Physiology and Pharmacology, London, UK
Aim: Pain is a common and debilitating complication for cancer patients significantly compromising their quality of life. Cancer-induced bone pain involves a complex interplay of mechanisms including inflammatory and neuropathic processes but also unique changes. The P2X7 receptor is involved in a variety of cellular functions especially in immune and glia cells. The function of the receptor is linked to both inflammatory and neuropathic pain and the expression is speculated to correlate with glia activation, a feature often seen in models of cancer-pain. The aim of the study was to study the role of the P2X7 receptor in cancer-induced bone pain.
Methods: MRMT-1 cancer cells were implanted into tibia of 7 weeks old Sprague Dawley rats. Electrophysiological recordings from wide dynamic rage (WDR) neurons in lamina V were performed in isoflurane anaesthetized rats on day 12–16 post surgery. The effect of spinal application of 0.2 mg/kg, 0.4 mg/kg and 1.2 mg/kg A839977, a selective P2X7 antagonist, was tested on evoked responses to repeated peripheral mechanical, thermal and electrical stimulation. Pain behavior was tested on day 7, 10 and daily from day 12 to detect onset of pain behavior (reduction in 50 % withdrawal threshold, Von frey) and late stage pain behavior (onset of weight-bearing and limb use deficits). The effect of 40 mg/kg and 120 mg/kg (i.p.) A839977 was tested at onset and at late stage pain behavior by measuring changes in behavior outcome 30 min post injection.
Results: In cancer-bearing animals A839977 attenuated the neuronal responses in a modality and intensity specific way. With the exception of an increase in the input and C-fibre evoked response no changes in baseline values were observed. However, 0.4 and 1.2 mg/kg A839977 significantly reduced the evoked response to high intensity mechanical and thermal stimulation (26 g, 60 g and 48 °C), whereas no effect was seen in response to low intensity mechanical and thermal stimulations (brush, 2 g, 8 g, 15 g, 35 °C, 42 °C, 45 °C) or electrical stimulation. In contrast, A839977 had no effect on the tested parameters in naïve or sham animals. In addition, 0.2 mg/kg A839977 has no effect.
In awake animals, 40 mg/kg A839977 significantly reduced pain behavior in both the early phase and late phase. A839977 had no effect on pain threshold in sham operated or vehicle treated animals. High doses (120 mg/kg) of systemic A839977 demonstrated toxic systemic effects.
Conclusions: The results suggest that the P2X7 receptor is involved in cancer-induced bone pain. A839977 significantly attenuated both neuronal responses and pain behavior indicating that P2X7 receptor antagonism might be a useful analgesic target. As no effect was observed in sham operated or naïve animals P2X7 receptor inhibition may be associated with fewer adverse effects than traditional analgesic used in cancer pain treatment, ex. opioids.
M 217
Effect of genetic deletion and P2X7 receptor antagonism in an animal nitroglycerin-induced hyperalgesia in mouse model of migraine
Flora Goloncser*, Agnes Kittel, Romeo Ando and Beata Sperlagh
IEM HAS, Laboratory of Molecular Pharmacology, Budapest, Hungary
Background: Recent studies have revealed that purine receptors participate in peripheral and central sensitization and are associated with migraine headache. The main aims of our study was to examine the role of P2X7 receptor (P2rx7) and test the analgesic effects of the P2X7 receptor antagonists in nitroglycerin(NTG)-induced mouse model of migraine.
Methods: We have used the increased temperature hot plate test (ITHT) to measure thermal nociceptive threshold. Intraperitoneal NTG injection (15 mg/kg) triggered thermal and mechanical hyperalgesia in the paws of wild-type C57BL/6J mice, followed by the induction of c-fos in upper cervical spinal cord and trigeminal nucleus caudalis. The effect of genetic deletion of P2rx7 and the administration of P2rx7 antagonists (BBG, AZ10606120, A438079) were examined on hyperalgesia and c-fos induction.
Results: NTG decreased the paw withdrawal threshold in both wild-type and P2rx7 knockout mice. Nevertheless, subacute BBG treatment (50 mg/kg/day i.p.) completely prevented the effect of NTG in wild-type, but not in knockout mice in the ITHT. Whereas P2rx7 deficiency differentially affected the expression of c-fos, the average number of fos-reactive neurons in trigeminal nucleus caudalis, but not in upper cervical spinal cord was lower in BBG-treated wild-type mice after NTG treatment.
Conclusion: Our results show that P2rx7 receptors might participate in the pathogenesis of migraine, although upregulation of other P2X receptors probably compensate the loss of its action in the knockout mice. The data also suggest therapeutic potential of P2rx7 antagonists for the treatment of migraine.
M 218
The role of alpha7-nicotinic acetylcholine receptors in the mediation of antihyperalgesic effect of P2Y12receptor inhibition in an acute inflammatory pain model
Gergely Horvath1,2,*, Beata Sperlagh1, Cecilia Csölle1,2, Bence Koványi1,2 and Christa E. Müller3
1IEM-HAS, Pharmacology, Budapest, Hungary;2Semmelweis University, Budapest, Hungary;3University of Bonn, PharmaCenter Bonn, Pharmaceutical Institute, Bonn, Germany
Our previous studies showed that pharmacological inhibition P2Y12 receptor (P2Y12R) leads to the alleviation of mechanical hyperalgesia in animal models of inflammatory, neuropathic pain and of acute thermal nociception. The aim of the present study was to confirm these findings on P2Y12R deficient animals and to identify the mechanism of action of P2Y12R antagonists in an acute inflammatory pain model.
The hindpaws of rats/wild-type and P2Y12R knockout (P2Y12R−/−) mice were injected by Complete Freund adjuvant (CFA) and hyperalgesia was tested 48 h/96 h later. Hot-plate test was applied to investigate acute thermal nociception, whereas partial ligation of the sciatic nerve was applied as a model of neurpathic pain (PSNL, Seltzer model). Furthermore, the effect of the selective P2Y12 receptor antagonist PSB-0739 was also examined. The production of pro-inflammatory cytokines (IL-1 beta, IL-6 and TNF-alpha) in the spinal cord and in the inflamed hindpaw was assessed by a Luminex xMAP platform.
Genetic deletion of P2Y12Rs significantly attenuated of mechanical hyperalgesia following intraplantar CFA injection and in the PSNL model and increased thermal nociceptive threshold. Similar effects were obtained by intrathecal application of PSB-0739 (0.3 mg/kg), when compared to an identical saline treatment. CFA treatment caused a time-dependent elevation in the level of pro-inflammatory cytokines in the hindpaw and spinal cord 48 and 96 h after injection, respectively. PSB-039 almost completely abolished IL-beta induction in the spinal cord and the elevation of cytokine levels in the hindpaw at these time points. Because PSB-0739 is unlikely able to penetrate the blood brain barrier, to further elucidate the mechanism of this latter effect, 6-OHDA pretreatment and subdiaphragmatic vagotomy was performed to identify potential efferent pathways. Whereas 6-OHDA pretreatment was ineffective, subdiaphragmatic vagotomy prevented the the antihyperalgesic effect of PSB-0739. Moreover systemic treatment with the a7-receptor nicotinic receptor antagonist Methyllycaconitine (MLA, 3 mg/kg i.p.) reproduced this effect and also prevented the effect of PSB-1739 on peripheral cytokine production.
These results suggest that central inhibition of P2Y12Rs leads to the alleviation of inflammatory hyperalgesia. The antihyperalgesic effect of PSB-0739 could be mediated by the inhibition of both central and peripheral cytokine production and involves a7-receptor mediated efferent pathways.
M 219
VNUT contributes to the pathogenesis of neuropathic pain after nerve injury
Takahiro Masuda1,*, Yui Ozono1, Satsuki Mikuriya1, Yuta Kohro1, Hidetoshi Tozaki-Saitoh1, Ken Iwatsuki2, Hisayuki Uneyama2, Reiko Ichikawa2, Makoto Tsuda1 and Kazuhide Inoue1
1Kyushu University, Department of Molecular and System Pharmacology, Graduate School of Pharmaceutical Sciences, Fukuoka, Japan;2Ajinomoto Co., Inc., Institute for Innovation, Kawasaki, Japan
Neuropathic pain is one of the most debilitating pain syndromes, which is refractory to currently available treatments. We have previously shown that activation of ATP-gated purinergic P2X4 receptor in the spinal cord, the expression of which is upregulated in reactive microglia following peripheral nerve injury (PNI), is crucial for producing neuropathic pain. However, the mechanism of ATP supply in the spinal cord remains unknown. In this study, we examined the role of vesicular nucleotide transporter (VNUT), which is required for accumulation of ATP in the secretory vesicles of cells. In the spinal cord of normal mice, VNUT expression was detectable but low; however, the expression of which was markedly increased in the ipsilateral spinal cord after PNI. Interestingly, PNI-induced pain hypersensitivity was markedly suppressed in VNUT-deficient mice compared to wild-type littermates. Furthermore, knockdown of VNUT expression by spinal administration of small interference RNA alleviated pain hypersensitivity after PNI. Together, these findings suggest that the VNUT may play an important part in forming ATP-enriched vesicles crucial for generating neuropathic pain.
M 220
Searching for analgesics targeted at P2X4 receptors by screening of well-established drugs
Tomohiro Yamashita*, Makoto Tsuda, Hidetoshi Tozaki-Saitoh and Kazuhide Inoue
Graduate School of Pharmaceutical Sciences, Kyushu University, Molecular and System Pharmacology, Fukuoka, Japan
Purinergic receptors are widely expressed throughout the human body, it is known that involved in various physiological response. We have previously demonstrated that activation of P2X4 receptors (ionotropic P2X receptors) in spinal microglia have a crucial role in chronic neuropathic pain. Neuropathic pain associated with cancer, diabetic neuropathy and postherpetic neuralgia is one of the intractable pains and is also characterized by mechanical allodynia, abnormal pain hypersensitivity evoked by innocuous stimuli. However this disorder has no specific treatment, so patients expect development of novel therapeutics. Therefore, the present study was to search for antagonists of P2X4 receptors from among well-established drugs of 1,979 compounds. In this analysis, we discovered the selective norepinephrine reuptake inhibitor (SNRI) duloxetine as a potent inhibitor of rat P2X4 receptors function. Duloxetine blocked calcium responses of human P2X4 receptors as well as rat P2X4 receptors. Further we found that various antidepressants blocked P2X4 receptors function, but duloxetine was effective and selective to block it. In microglial cells, duloxetine also selectively inhibited P2X4R-mediated calcium responses. These results suggest that duloxetine are able to efficiently suppress P2X4 receptors function and to be new analgesics targeted at P2X4 receptors.
N: Purinergic signaling in other functions and plants
N 221
Endocytic vesicle recycling on root apex of plant regulated by extracellular ATP via new eATP receptor DORN1
Tomoko Kagenishi1,*, Ken Yokawa1,2, Gary Stacey3 and František Baluška1
1University of Bonn, Institute of Cellular and Molecular Botany IZMB, Bonn, Germany;2Tokyo Metropolitan University, Department of Biological Sciences, Tokyo, Germany;3University of Missouri, Divisions of Biochemistry and Plant Sciences, Christopher S. Bond Life Sciences Center, Columbia, USA
Extracellular ATP (eATP) is known as a signal molecule both in mammal and plant cells. In mammals, eATP studies have accelerated since the purinoreceptor (eATP receptor) was cloned. In contrast, the plant purinoreceptor was reported only recently (Choi et al. 2014). Nevertheless, several studies demonstrated that eATP is involved in the regulation of plant growth and in their adaptation to the environment. For example, eATP was shown to inhibit root gravitropism and cell/organ growth in Arabidopsis (Tang et al. 2003). We have analyzed the expression of eATP receptor gene At5G60300 by microarray database of the Genenvestigator (https://www.genevestigator.com/). It shows higher expression in root tissues in comparison to other tissues. Moreover, it was also reported that the Arabidopsis root apex shows high activity of eATP release induced by touch stress (Dark et al. 2011). It emerges that eATP may play important roles in environmental sensing and adaptation. The aim of our present study is to investigate the mechanisms of eATP signaling in root apex. Results of our study show that eATP controls endocytic vesicle recycling in cells of the root apex transition zone. In addition, we checked the endocytosis of dorn1-1 mutant (Does not Respond to Nucleotides 1-1) with observing BFA compartment. In the result, dorn1-3 did not inhibit the endocytosis by eATP. Therefore, we hypothesized that eATP controls endocytic vesicle recycling via DORN. Moreover, root behavior (crawling growth and tropisms) is modified by eATP and this response requires ROS generation by NADPH oxidase AtRBOHC.
N 222
Ap4A increases corneal epithelial permeability inducing tight junction disassembly
Patricia Loma Lozano*, Ana Guzman Aranguez, Maria J Perez de Lara and Jesus Pintor
Complutense University of Madrid, Biochemistry and Molecular Biology IV, Madrid, Spain
The corneal epithelium is sealed with intercellular tight junctional complexes and constitutes a defensive barrier preventing the entrance of potentially harmful substances but also limiting drug absorption. The aim of this work was to determine the effect of the diadenosine polyphosphate Ap4A on corneal barrier function conferred by TJ proteins and its involvement in ocular drug delivery and therefore in therapeutic efficiency. Human corneal epithelial cells (HCLE) were treated with Ap4A (100 μM) for 5 min. After nucleotide removal, cells were incubated to different times for tight junction protein analysis by western blot. Transepithelial electrical resistance (TEER) measurements were made to evaluate barrier permeability. In in vivo assays the hypotensor compound 5-methoxycarbonylamino-N-acetyltryptamine (5-MCA-NAT) and the glaucoma drug timolol maleate were topically applied to New Zealand rabbit eyes alone, simultaneously and 2 h after Ap4A pre-treatment. The presence of both compounds in the aqueous humour was assessed by high-performance liquid chromatography and their effect on intraocular pressure was measured using a Tonopen® tonometer. Treatment with Ap4A induced a significant decrease in tight junction proteins (ZO-1 (39 ± 8 % of reduction), occludin (47 ± 8 % of reduction) and claudin-7 (43 ± 5 % of reduction)) when compared with non-treated (control) cells. Concomitantly, TEER values were dramatically reduced at 2 and 4 h (68 and 52 % of reduction, respectively). TJ protein reduction and ERK activation were blocked by the ERK inhibitor U0126 and P2Y2 siRNAs, indicating that Ap4A reduces TJ through P2Y2 stimulating and subsequently ERK activation. In vivo assays showed that topical application of Ap4A disrupts the membrane distribution of ZO-1 along cell-cell contact regions. The presence of 5-MCA-NAT and timolol maleate in the aqueous humour was 2.75 higher when Ap4A was previously instilled and its hypotensive effect was also increased. This hypotensive action was reversed by three P2 receptor antagonists and P2Y2 siRNA. When Ap4A was applied 2 h before the mentioned drugs significantly increased their reducing IOP. Treatment with Ap4A increased corneal barrier permeability by modifying tight junction protein levels and function. Ap4A application can contribute to improving ocular drug delivery and consequently therapeutic efficiency.
N 223
IL-6, A1 and A2aR: a crosstalk that induces neuroprotection
Rafael Perígolo-Vicente1,2,*, Karen Ritt3, Cassiano Gonçalves-de-Albuquerque4, Hugo Castro-Faria-Neto4, Roberto Paes-de-Carvalho2 and Elizabeth Giestal-Araujo2
1Blizard Institute—Queen Mary, University of London, Neuroscience and Trauma, London, Brazil;2Federal Fluminense University, Rio de Janeiro, Brazil;3Universidade Federal Fluminense, Neurociencias, Rio de Janeiro, Brazil;4Oswaldo Cruz Foundation, Rio de Janeiro, Brazil
Previously our group showed that IL-6 induces neuroprotection on axotomized Retinal Ganglion cells (RGC) in vitro [1]. Recently we demonstrated that the trophic effect of IL-6 is mediated by A1R activation [2]. Here we show that there is a crosstalk between IL-6, A1R and A2aR in retinal cell cultures that results in neuroprotection on RGC.
The survival of RGC was analyzed in monolayers of mixed retinal cells kept in culture for 48 h. Western blot technique was used to evaluate the levels of A1R, A2aR and IL-6. The levels of extracellular IL-6 were quantified by ELISA technique.
Our data show that IL-6 trophic effect depends on both A1 and A2a receptors activation since the antagonists of these receptors inhibited the IL-6 effect. Accordingly, treatment with 1nM CHA (A1R agonist) or 10nM CGS (A2aR agonist) rescued RGC from cell death. Surprisingly, when extracellular IL-6 was neutralized with anti-IL-6 antibody, both CHA and CGS trophic effect were inhibited. After treatment with either CGS or CHA for 48 h the levels of IL-6 were increased. Nonetheless, CHA treatment decreased the release of IL-6 whilst CGS treatment increased it. Interestingly, IL-6 treatment for 48 h increased the levels of A1R and decreased the levels of A2aR. In a different way, treatment with IL-6 for 24 h decreased A1R and increased A2aR levels.
Our data indicate that a complex signalling pathway is triggered after treatment with IL-6, which plays an important role regulating the adenosinergic phenotype in retinal cell cultures.
References
1. Torres PMM, Araujo EG (2001) Interleukin-6 increases the survival of retinal ganglion cells in vitro. J Neuroimmunol 117:43–50
2. Perígolo-Vicente R, Ritt K, Pereira MR, Torres P, Paes-de-Carvalho R, Giestal-de-Araujo E (2013) Interleukin-6 increases the survival of retinal ganglion cell: The role of adenosine A1 receptors. Biochem Byophys Res Commun 430(2):512–518
N 224
Adenosinergic transcriptomic profile in murine lung fibroblasts treated or not with bleomycin
Veronica Della Latta1,2,*, Antonella Cecchettini3, Manuela Cabiati1, Silvia Burchielli4, Maria Aurora Morales1 and Del Ry Silvia1
1Institute of clinical Physiology, CNR, Pisa, Italy;2University of Siena, Siena, Italy;3University of Pisa, Unit of Experimental Biology and Genetics, Department of Clinical and Experimental Medicine, Pisa, Italy;4Gabriele Monasterio Foundation, Pisa, Italy
Background: Adenosine (ADO) is an endogenous nucleoside ubiquitously expressed in the organism. It mediates its functions interacting with specific G-protein coupled membrane receptors: A1R, A2aR, A2bR and A3R. During stress or injury conditions, ADO is produced and released in excess: ATP is released to extracellular level, dephosphorylated to ADO, by two extracellular nucleotidases, CD39 and CD73. Unfortunately it is difficult to measure ADO concentrations since this nucleoside is rapidly degraded into inosine by adenosine deaminase (ADA). Fibrotic lung diseases represent a broad spectrum of pulmonary pathologies characterized by different degrees of inflammation and fibrosis. A possible link between the adenosinergic system and lung fibrosis -more specifically idiopathic pulmonary fibrosis- can be supported by the role of ADO and its receptors in lung diseases with an extensive fibrotic component. Experimental studies on animal models use bleomycin, a chemotherapic agent, to induce pulmonary fibrosis and to assess the biological pathways involved in the development of fibrosis. Aim of the study was to evaluate the possible changes of the adenosinergic transcriptomic profile (receptors, CD39, CD73 and ADA) in fibroblasts with or without bleomycin treatment, isolated from mice lungs.
Methods: To isolate lung fibroblasts, five lungs of C57bl/j mice were explanted. From lung fragments fibroblast cultures were isolated and experiments conducted at the third passage. A part of fibroblast cultures was treated with 2 mg/ml of bleomycin. Total RNA was extracted from cells (about 400.000) treated with bleomycin (BLEO, n = 5) and from untreated cells (C, n = 5) by specific extraction assays. Real-time PCR was performed and optimized for each ARs primer, CD39, CD73 and ADA. The experimental data were normalized with the three most stably expressed genes (HPRT1, PPiA, RPL13a).
Results: For each analyzed receptor higher, although not significantly, levels of mRNA expression were observed in fibroblasts untreated as compared to fibroblasts treated with bleomycin: A1R: C = 0.48 ± 0.11, BLEO = 0.30 ± 0.11, p = 0.324; A2aR: C = 0.23 ± 0.09, BLEO = 0.11 ± 0.03, p = 0.220; A2bR: C = 0.41 ± 0.18, BLEO = 0.32 ± 0.14, p = 0.682; CD73:C = 0.07 ± 0.02, BLEO = 0.05 ± 0.02, p = 0.511. In contrast CD39 and ADA analysis showed a higher trend towards levels of mRNA expression in BLEO as compared to C (CD39: C = 0.90 ± 0.08, BLEO = 1.02 ± 0.16 p = 0.55; ADA: C = 0.44 ± 0.25, BLEO = 0.63 ± 0.28, p = 0.624).
Discussion: The higher level of expression of CD39 and ADA compared to ARs and CD73 mRNA expression could represent a condition of desensitization and internalization of ARs in response to high concentrations ofADO released by lung fibroblasts after bleomycin induced damage.
N 225
P2X4receptor modulates secretion and fluid transport in the lung
Kristin E. Thompson*, Pika Miklavc, Nina Hobi, Paul Dietl and Manfred Frick
Institute of General Physiology, University of Ulm, Ulm Germany
Secretion via exocytosis of secretory vesicles is a fundamental, tightly regulated cellular process. In several cell types, purinergic P2 receptors, have been shown to play a role in regulating exocytosis and secretion. Our recent findings demonstrated the presence of P2X4 receptors in alveolar type II epithelial (ATII) cells. Specifically, P2X4 receptors are expressed on lamellar bodies (LBs), large, lysosome-related organelles storing pulmonary surfactant. Surfactant is a lipidic substance which is not easily secreted from fused LBs. Upon exocytosis of LBs P2X4 receptors are translocated to the plasma membrane and activation of the receptors by extracellular ATP induces a “fusion-activated cation entry” (FACE) localized to the fusing LB. FACE leads to a localized increase in cytoplasmic Ca2 surrounding the LB. This localized Ca2+ increase drives fusion pore dilation and widening of the fusion pore is essential for surfactant secretion. However, controlling the rate of surfactant release is not the only function of P2X4 in the alveoli. As a non-specific cation channel, a large sodium influx via the receptor at the time of LB fusion occurs. Using a combined atomic force and fluorescence microscopy technique, we demonstrate that influx of sodium via the P2X4 receptor at the time of fusion leads to cell volume increase. Correlative confocal microscopy experiments demonstrated that FACE modulates water resorption in the alveoli at the time of surfactant secretion. By regulating transepithelial fluid transport, FACE likely plays a part in integrating surfactant into the alveolar surface liquid following secretion. This is necessary to functionalize the surfactant and promote its key role in preventing collapse of the alveoli by reducing surface tension. This role for the P2X4 receptor in modulating secretion via the fusion pore, and additionally promoting water resorption may likely be extended to the goblet cells of the upper airways as well.
N 226
Bile acid induced Ca2+responses are mediated in part by ATP release and purinergic signalling in pancreatic exocrine cells
Justyna Magdalena Kowal*, Kristian Agmund Haanes, Nynne M Christensen and Ivana Novak
University of Copenhagen, Department of Biology, Copenhagen, Denmark
In recent years, it has been recognized that bile acids (BA) have positive effects on physiological functions of the pancreas [1]. Research has shown BA increase insulin sensitivity and insulin release, and some of these effects are mediated via the bile acid TGR5 receptor. The pancreas could be exposed to systemic BA, as well as to intra-ductal BA from bile reflux. In low concentrations, BA can possibly regulate pancreatic duct secretion; in contrast, in high concentrations they could impair the function of acinar cells by increasing intracellular [Ca2+]i levels and eventually depleting intracellular ATP (ATPi), both processes can lead to pancreatitis. Since purinergic signalling is important in physiology and pathophysiology of pancreas [2], we asked whether there was any interplay between ATP signalling and BA effects.
The aim of our study was to investigate whether the primary BA, chenodeoxycholic acid (CDCA), induces ATP release and affects Ca2+ signalling in exocrine pancreas and whether purinergic signaling is involved. Furthermore, we aimed to determine the expression and involvement of TGR5 in those responses.
Mg-Green indicator, ATP sensor (AT1.03YEMK) and luciferase/luciferin assays were used to determine the ATPi changes and concentrations of ATP released from acinar cells (AR42J) and pancreatic duct cells (Capan-1) in response to various stimulants. Fura-2AM was used for detecting [Ca2+]i changes. Additionally, RT-PCR and Western Blot were applied to detect TGR5 expression. Results are given as mean changes from basal values ± S.E.M.
CDCA caused fast and dose-dependent ATP release from AR42J and Capan-1 cells; estimated EC50 values were 0.44 and 0.43 mM, respectively. Interestingly, there was a fast decrease of ATPi detected with Mg-Green fluorescence and AT1.03YEMK FLIM-FRET. In Capan-1 monolayers, we show that ATP was released mainly from the luminal membrane and it involves ion channels and exocytosis. CDCA evoked slow changes in [Ca2+]i (177 ± 21 nM, n = 5), which were inhibited 41 % by a P2 inhibitor cocktail (90 ± 9 nM, n = 5). The expression of TGR5 has been detected on luminal membrane of pancreatic ducts (Capan-1 cells) for the first time. Activation of TGR5 by CDCA and specific agonist GPBAR-A resulted in fast and significant decrease of [Ca2+]i by 81 ± 1 % and 49 ± 7 % respectively (n = 3, 4).
In conclusion, our study shows that part of the CDCA effects can be explained by simultaneous purinergic effects. CDCA releases ATP, which then via purinergic receptors can elicit Ca2+ responses alongside/or in addition to the CDCA effects on the TGR5 receptor. Whether BA and purinergic signalling interaction contribute to pancreatic physiology or pathophysiology, and whether these are relevant to other cells remains to be investigated.
References
1. Chiang JY (2013) Compr Physiol 10.1002/cphy.c120023
2. Novak I (2008) Purinergic Signal 10.1007/s11302-007-9087-6
N 227
Loss or blockade of adenosine A2B receptor prevents fatty liver by down-regulating microRNA expression
Hailing Liu*, Bruce Cronstein and Tuere Wilder
NYU School of Medicine, Medicine, New York, USA
Background: Non-alcoholic fatty liver is a common medical problem affecting more than 5 % of the US population and as many as 20 % of affected individuals will develop cirrhosis. MicroRNAs (miRs) are small, noncoding RNAs that negatively regulate gene expression and some miRs are overexpressed in NAFLD. Adenosine, acting at A2B receptors, promotes development of fatty liver after chronic alcohol abuse and we postulated that adenosine might play a similar role in the pathogenesis of non-alcoholic fatty liver (NAFLD). We therefore explored the effect of A2B receptor blockade on miR expression in the HepG2 liver cell line and tested the effect of A2B receptor blockade on development of steatosis and steatohepatitis in a murine model.
Methods: Fatty liver was induced by feeding methionine choline-deficient diet (MCD Diet) (protein 14.6 %, fat 22.1 %, and CHO 63.2 % of total kcal) for 4 weeks vs standard chow. Hepatic steatosis was graded semiquantitatively based on percentage of lipid-laden hepatocytes in H&E stained slides and confirmed by oil red O staining and direct measurement of hepatic triglyceride content. miR16, 33b, 34a (Qiagen) expression levels in HepG2 liver cells after being treated by A2B receptor antagonist PSB1115 were detected by miScript miRNA PCR Arrays.
Results: Wild type (WT) developed severe hepatic steatosis after MCD diet with obvious lipid droplets present in both periportal and pericentral areas of hepatocytes, but A2BKO mice suffered only minimal fatty change (Steatosis grade: 3.70 ± 0.17 vs 1.55 ± 0.3, n = 4, p < 0.05). Consistent with the histological appearance, the hepatic triglycerides were much lower in A2BKO mice than those in WT (10.75 ± 3.75 mg/g, n = 4 vs 51.20 ± 10.64 mg/g, n = 4, p < 0.05). miR16, 33b and 34a expression in human hepatic HepG2 cells were decreased significantly by A2B receptor antagonist in 6 h (miR16: 0.32 ± 0.08, n = 5, p < 0.001; miR33b: 0.27 ± 0.16, N = 4, p < 0.01; and miR34a: 0.34 ± 0.08, n = 5, p < 0.001 compared with its expression in control as 1.00 respectively).
Conclusion: These results indicate that adenosine A2B receptor plays a role in hepatic steatosis induced by MCD diet and that diminished expression of miRNAs associated with steatosis plays a role in this effect.
N 228
Adenosine receptors and beige adipocytes
Thorsten Gnad1,*, Christa E. Müller2 and Alexander Pfeifer1
1Institute of Pharmacology and Toxicology, Sigmund-Freud-Str. 25, D-53105 Bonn, Germany;2Pharma Center Bonn, Pharmaceutical Institute, Pharmaceutical Chemistry I, An der Immenburg 4, D-53121 Bonn
Pandemic obesity has become one of the biggest burdens of modern health systems and there is a growing need for pharmacological prevention and treatment. White adipose tissue (WAT) is the major site of energy storage, whereas brown adipose tissue (BAT) is responsible for basal and inducible energy expenditure in mammals. In addition, a third type of fat cell has been described in white fat depots, so-called beige or brite (brown-in-white) adipocytes. These beige adipocytes resemble classical brown adipocytes and they also express uncoupling protein 1 (UCP1).
UCP1 is activated by free-fatty acids and uncouples mitochondrial respiration, which results in the generation of heat instead of ATP [1]. Thereby, BAT possesses enormous energy-consuming potential. Different stimuli have been identified that induce beige adipocytes. These include, among others, Irisin, fibroblast growth factor 21, natriuretic peptides, norepinephrine, or microRNA 155 [2–5]. Noteworthy, induction of beige adipocytes protects from diet-induced obesity [6].
The purinergic signaling molecule adenosine mediates intracellular signaling via four different G protein-coupled receptors. Adenosine has previously been described to have an inhibitory role on white adipocytes’ lipolysis [7]. Accordingly, we found the Gi-coupled adenosine receptor A1 to be much more highly expressed in murine inguinal WAT compared to BAT. Moreover, A1 is strongly upregulated during in vitro differentiation of primary murine white adipocytes (WA). Next, we analysed the effect of the A1-specific antagonist PSB-36 on the induction of brown-adipocyte marker genes UCP1 and PGC1α in WA. Acute treatment of WA with PSB-36 significantly increased gene expression of UCP1 and PGC1α 3-fold and 2.2-fold, respectively. Consistently, blockade of A1 in WA caused an increase of intracellular cAMP concentration by 70 %. Additionally, cellular lipolysis was doubled after blocking the A1 receptor.
Hence, we propose the adenosine receptor A1 as a promising target for the induction of beige adipocytes in WAT.
References
1. Cannon B, Nedergaard J (2004) Brown adipose tissue: function and physiological significance. Physiol Rev 84:277–359
2. Bostrom P et al (2012) A PGC1-alpha-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature 481:463–468
3. Fisher FM et al (2012) FGF21 regulates PGC-1alpha and browning of white adipose tissues in adaptive thermogenesis. Genes Dev 26:271–281
4. Bordicchia M et al (2012) Cardiac natriuretic peptides act via p38 MAPK to induce the brown fat thermogenic program in mouse and human adipocytes. J Clin Invest 122:1022–1036
5. Chen Y et al (2013) miR-155 regulates differentiation of brown and beige adipocytes via a bistable circuit. Nat Commun 1769
6. Schulz TJ et al (2013) Brown-fat paucity due to impaired BMP signalling induces compensatory browning of white fat. Nature 495:379–383
7. Johansson SM, Lindgren E, Yang JN, Herling AW, Fredholm BB (2008) Adenosine A1 receptors regulate lipolysis and lipogenesis in mouse adipose tissue-interactions with insulin. Eur J Pharmacol 597:92–101




